Adsorption Combined with Electrocoagulation Process for Ketoprofen Removal from Aqueous Solution: Optimization Using Central Composite Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Activated Carbon Characterization
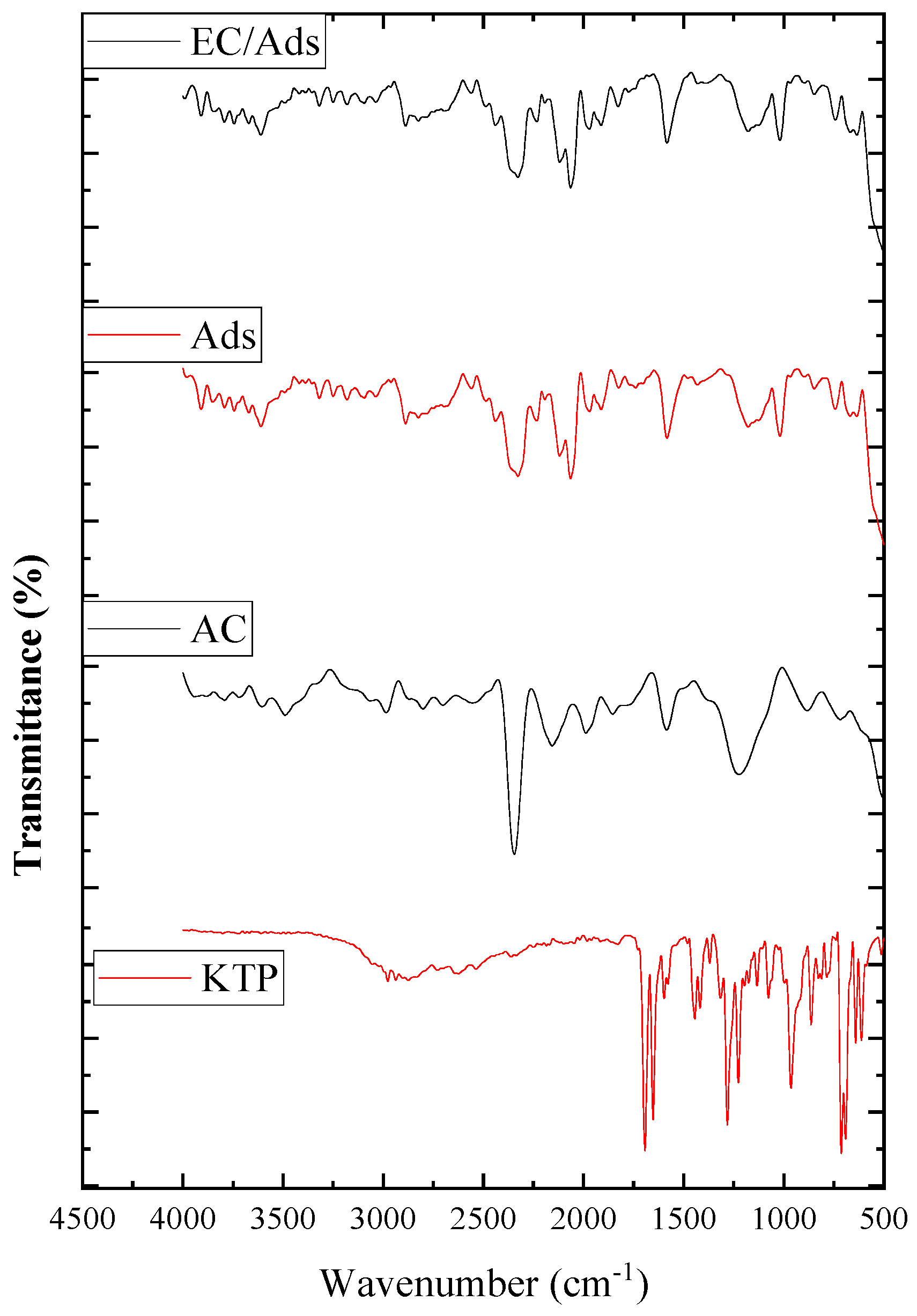
2.2.1. Infrared Spectroscopy
2.2.2. Activated Carbon Texture Characterization
2.3. pHPZC Determination
2.4. Experimental Procedure
2.4.1. Adsorption Experiments
2.4.2. Electrocoagulation
2.4.3. Combined System—Electrocoagulation/Adsorption
2.4.4. Analysis Technique
2.4.5. Electrodes
2.5. Experimental Design
- Predicted yield (ŷ);
- The coefficients of the response model ();
- Linear terms, corresponding to the variables ();
- Squared terms, corresponding to the variables (, , …, );
- First-order interaction terms for each paired combination (, , …, ).
3. Results
3.1. Characterization Study
3.1.1. FTIR Analysis
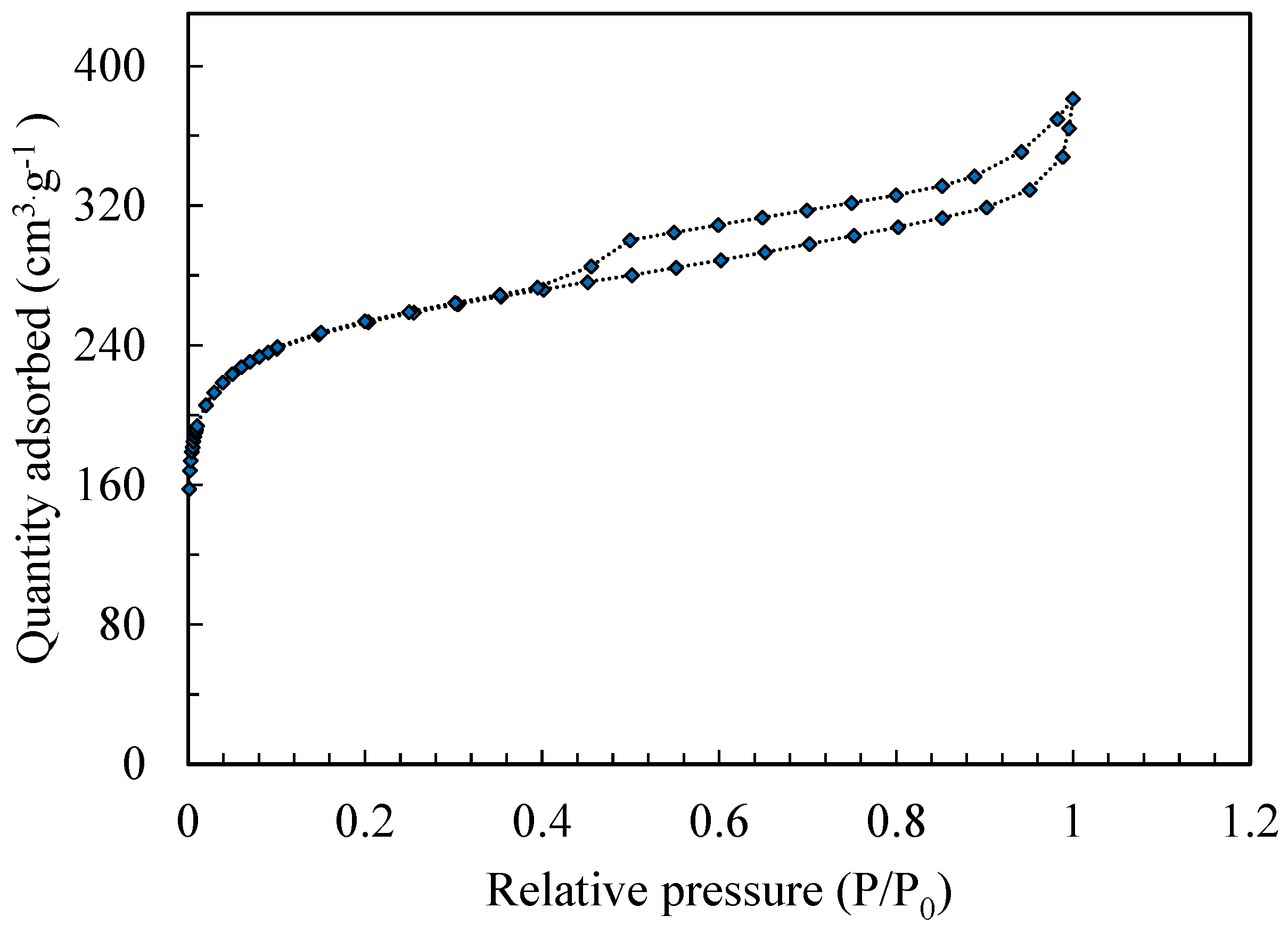
3.1.2. BET Analysis
- Nitrogen isotherm and surface area: Figure 3 illustrates the nitrogen adsorption–desorption isotherms for the AC. The amount of N2 adsorbed is plotted as a function of the relative pressure (P/P0), where P is the vapor pressure and P0 is the saturation vapor pressure of nitrogen. The lower branch of the isotherm corresponds to the incremental adsorption of N2 on the adsorbent surface, while the upper branch reflects the desorption process as nitrogen is progressively released. These curves provide insights into the pore structure and adsorption behavior of the activated carbon material. The N2 adsorption–desorption isotherms corresponding to the AC type H3 are related to having a high affinity for the adsorbent. At very low concentrations, curves do not start at zero but at a positive value on the y-axis corresponding to the amounts adsorbed; this isotherm is encountered when there is chemisorption of the solute.
- Surface area and pore volume: The physical characteristics of the analyzed AC derived from the N₂ adsorption–desorption isotherms, including the total surface area (SBET), total pore volume (Vtot), external surface area (Sext), microporous surface area (Smic), mesoporous surface area (Smes), microporous volume (Vmic), mesoporous volume (Vmes) and average pore diameter (Dp), are summarized in Table 5.
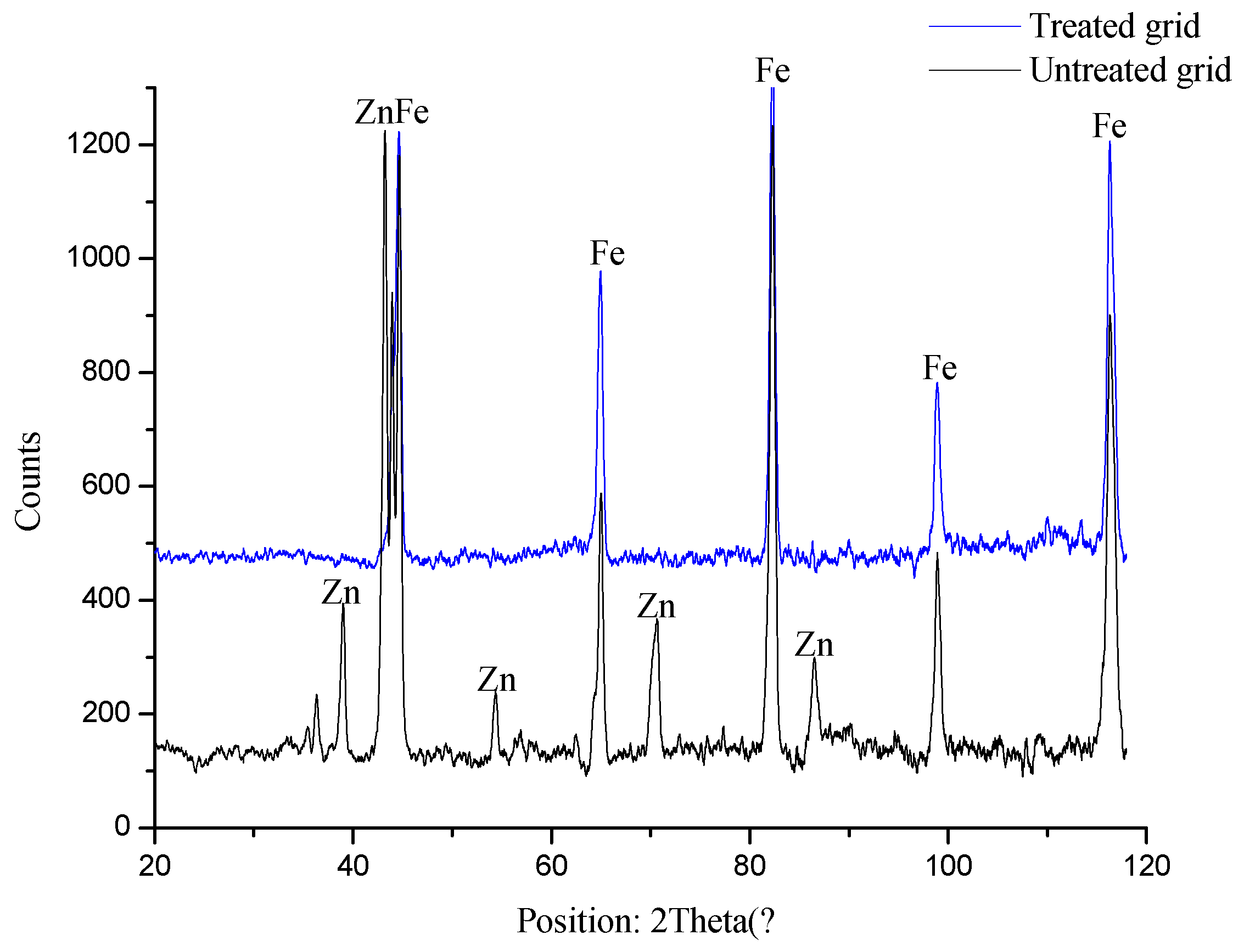
3.1.3. X-Ray Diffraction Analysis
3.2. Preliminary Tests
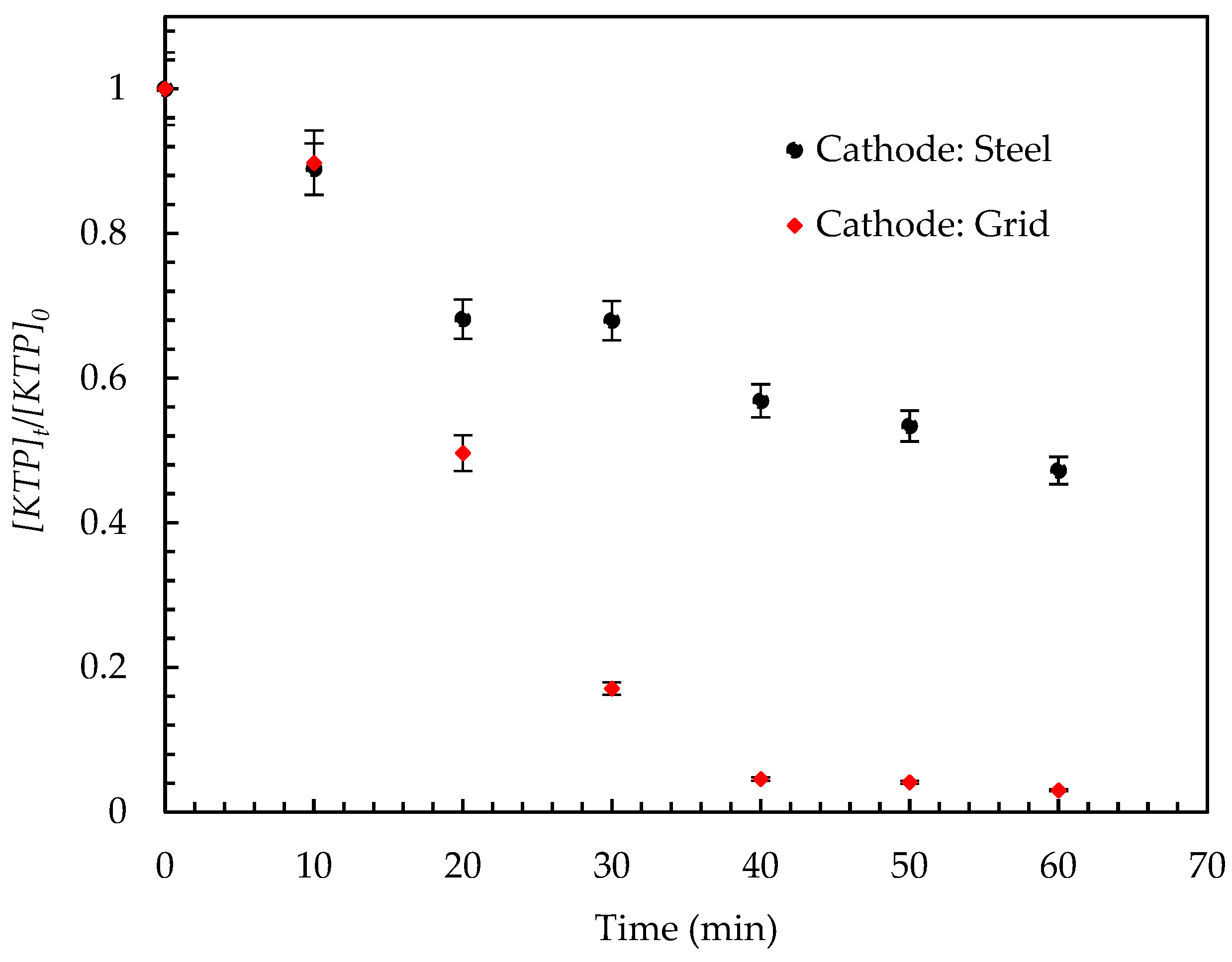
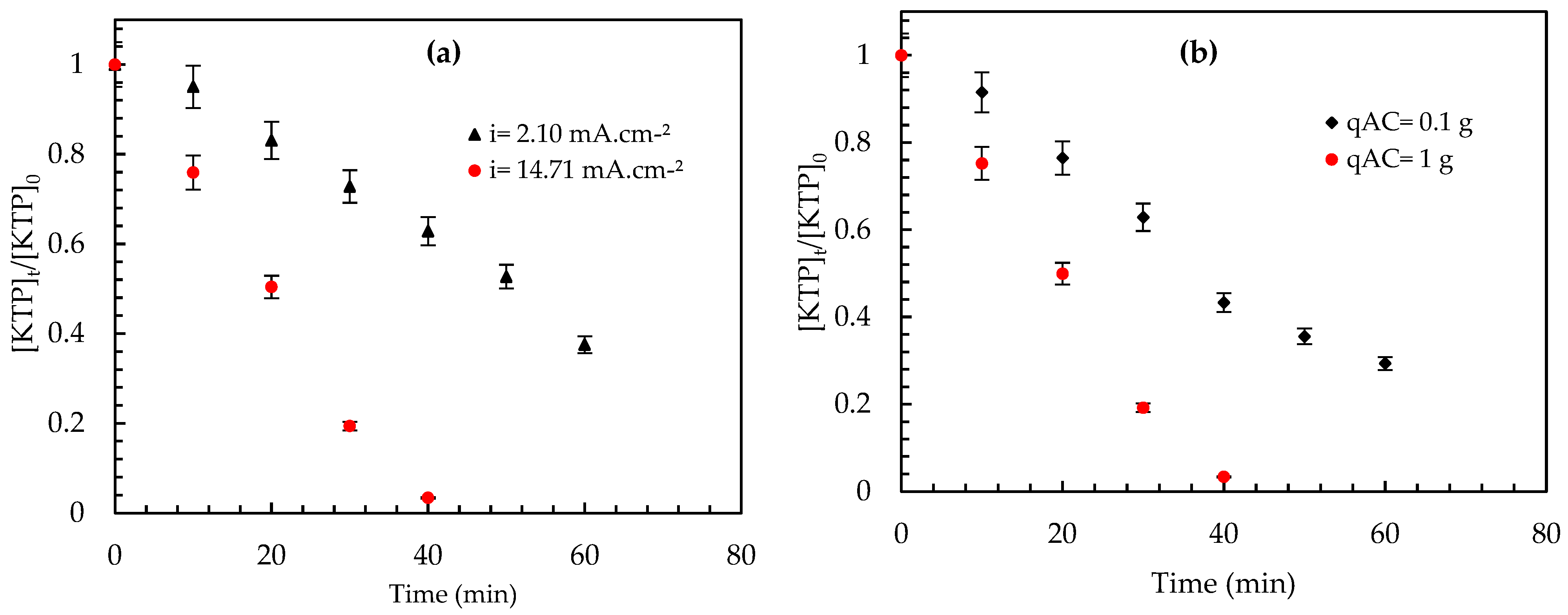
- The performance of the EC process is significantly impacted by the various electrode materials and electrode combinations used; to this end, two electrode materials were considered: steel and iron grids. The KTP removal efficiency as a function of time and electrode type is shown in Figure 7. The results shows that using an iron grid cathode is more efficient than using a steel cathode. After 40 min of electrolysis, a remarkable elimination in the case of an iron grid cathode was observed, with a yield of 95% compared to 43.13% when using a steel cathode. The superior performance of the iron grid cathode can be explained by the electrochemical properties of iron, which exhibit a higher reduction capacity compared to other metals. This allows iron to produce more hydroxide ions (OH⁻) and hydrogen bubbles (H₂), crucial for efficient pollutant removal. Combined with its large surface area and stability, these properties enhance the overall electrochemical reactions, making iron the optimal choice for water treatment applications. Its non-toxic and recyclable nature further supports its suitability for use in sustainable processes.
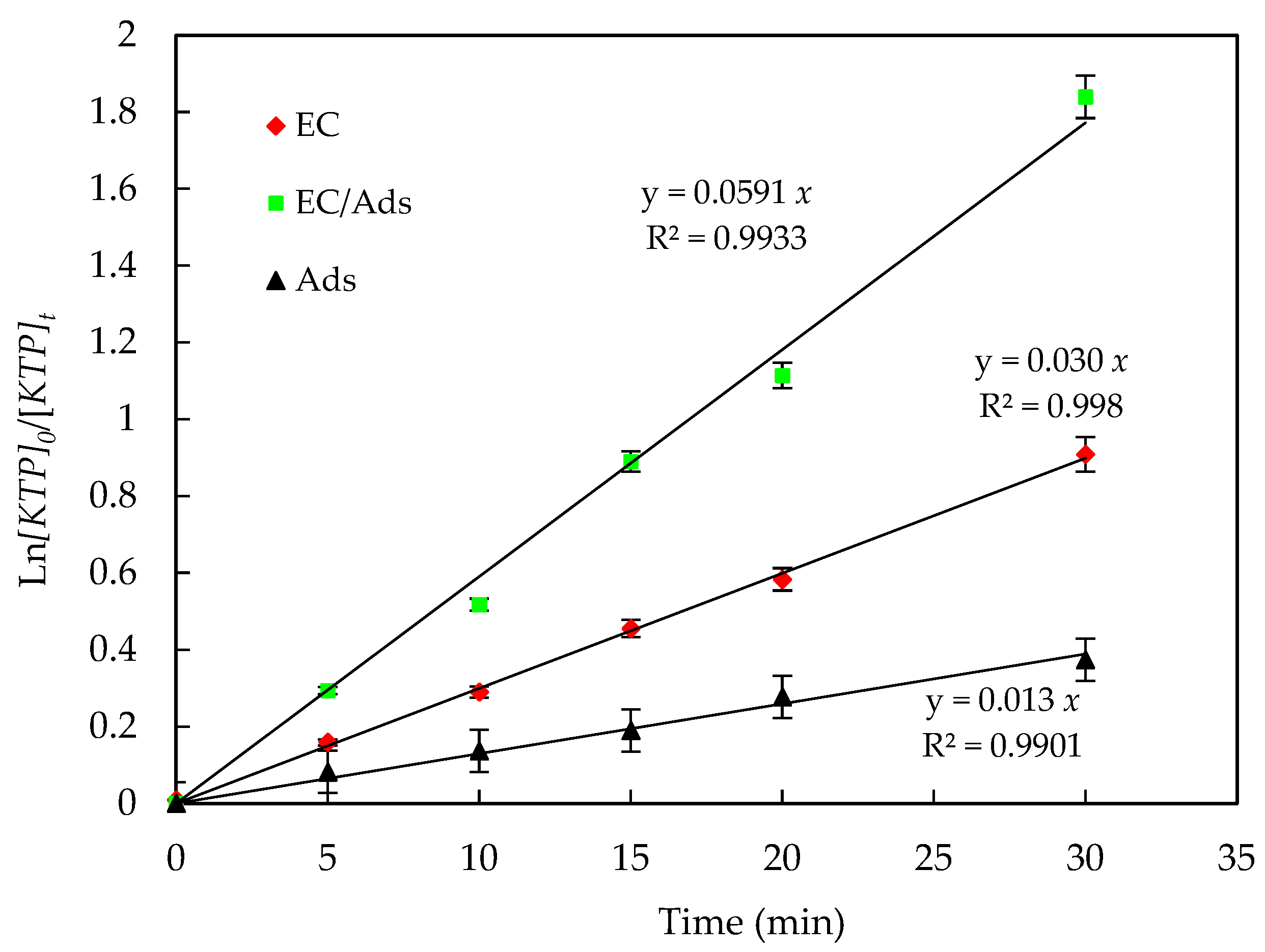
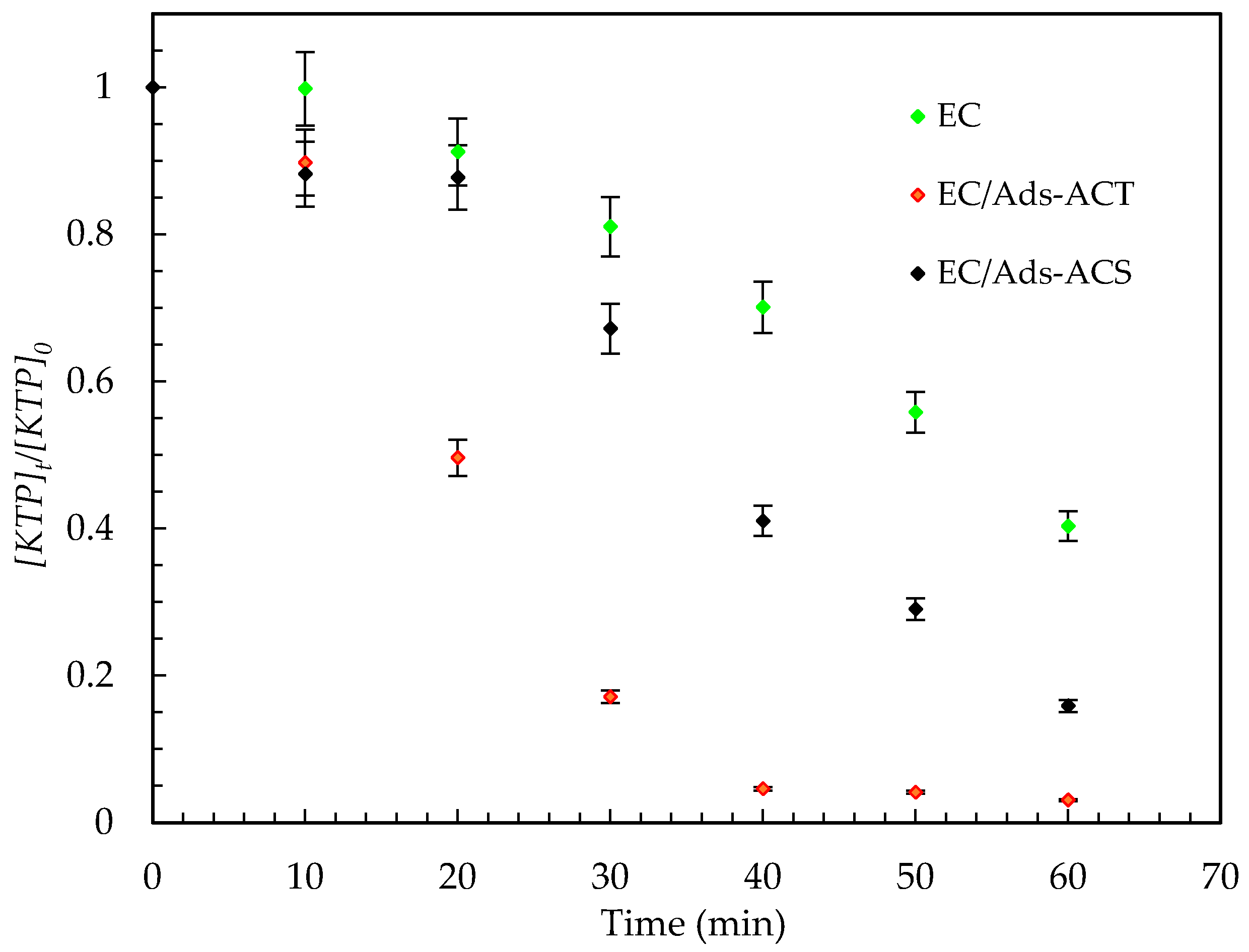
- The performances of the three configurations—electrocoagulation alone, electrocoagula-tion with suspended activated carbon (EC/Ads-ACS) and electrocoagulation with an-ode-immobilized activated carbon (EC/Ads-ACT)—were evaluated in terms of KTP re-moval efficiency over different treatment durations (Figure 8).
- After 10 min of treatment, the electrocoagulation process alone exhibited a very low removal efficiency (0.179%). In contrast, the addition of activated carbon, either suspended (ACS) or immobilized within the anode (ACT), led to a significant improvement, achieving removal efficiencies of 11% and 10.23%, respectively, with comparable performance at this early treatment stage.
- After 30 min, the differences between the configurations became more pronounced. The EC process reached a removal efficiency of 18.95%, while the EC/Ads-ACS system achieved 32.81%. Notably, the EC/Ads-ACT configuration showed a substantial enhancement, reaching 82.90% removal efficiency.
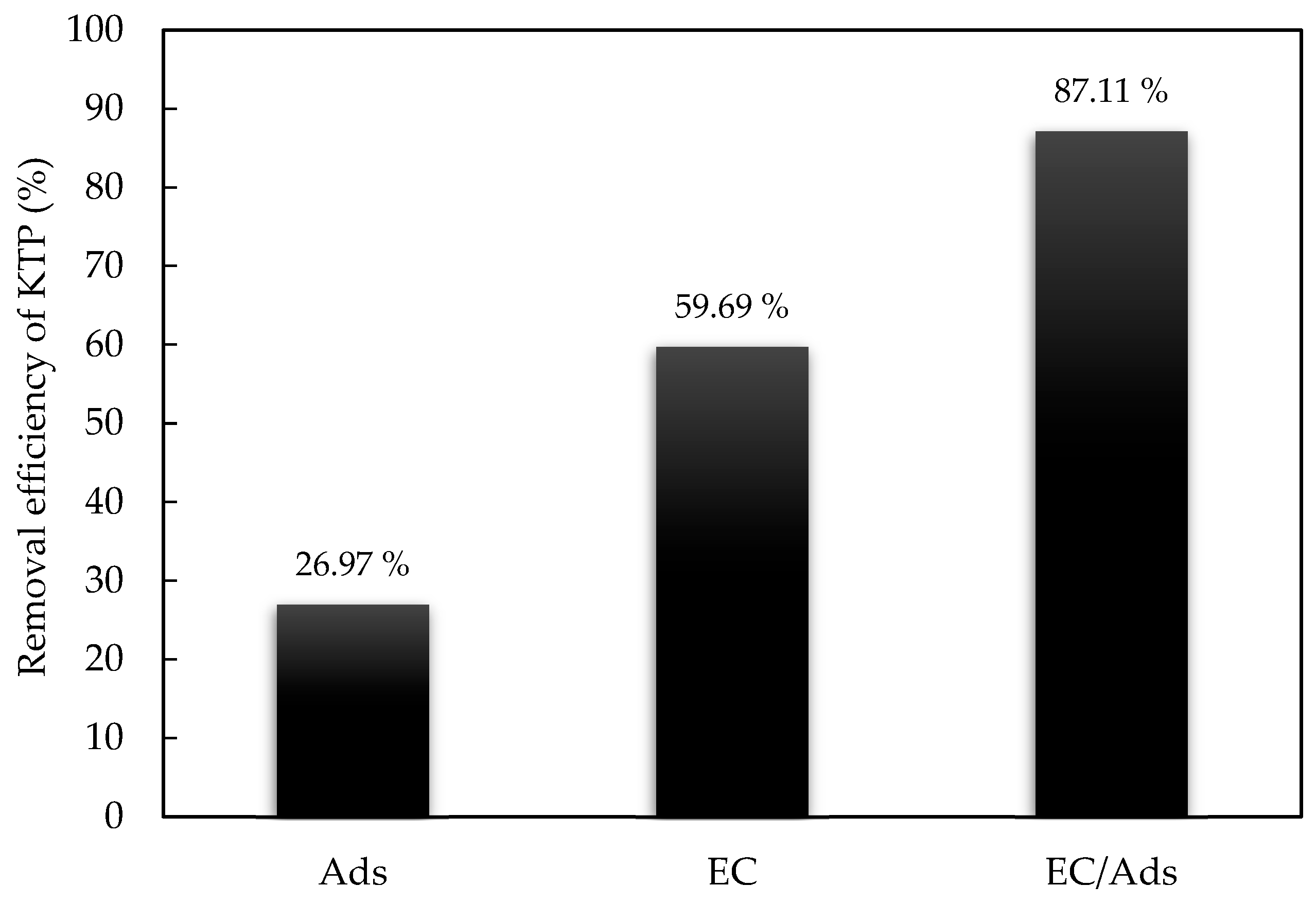
- At 60 min, all configurations demonstrated improved performance, with EC/Ads-ACT remaining the most effective, achieving a removal efficiency of 96.96%, followed by EC/Ads-ACS (84.15%) and EC alone (59.69%).
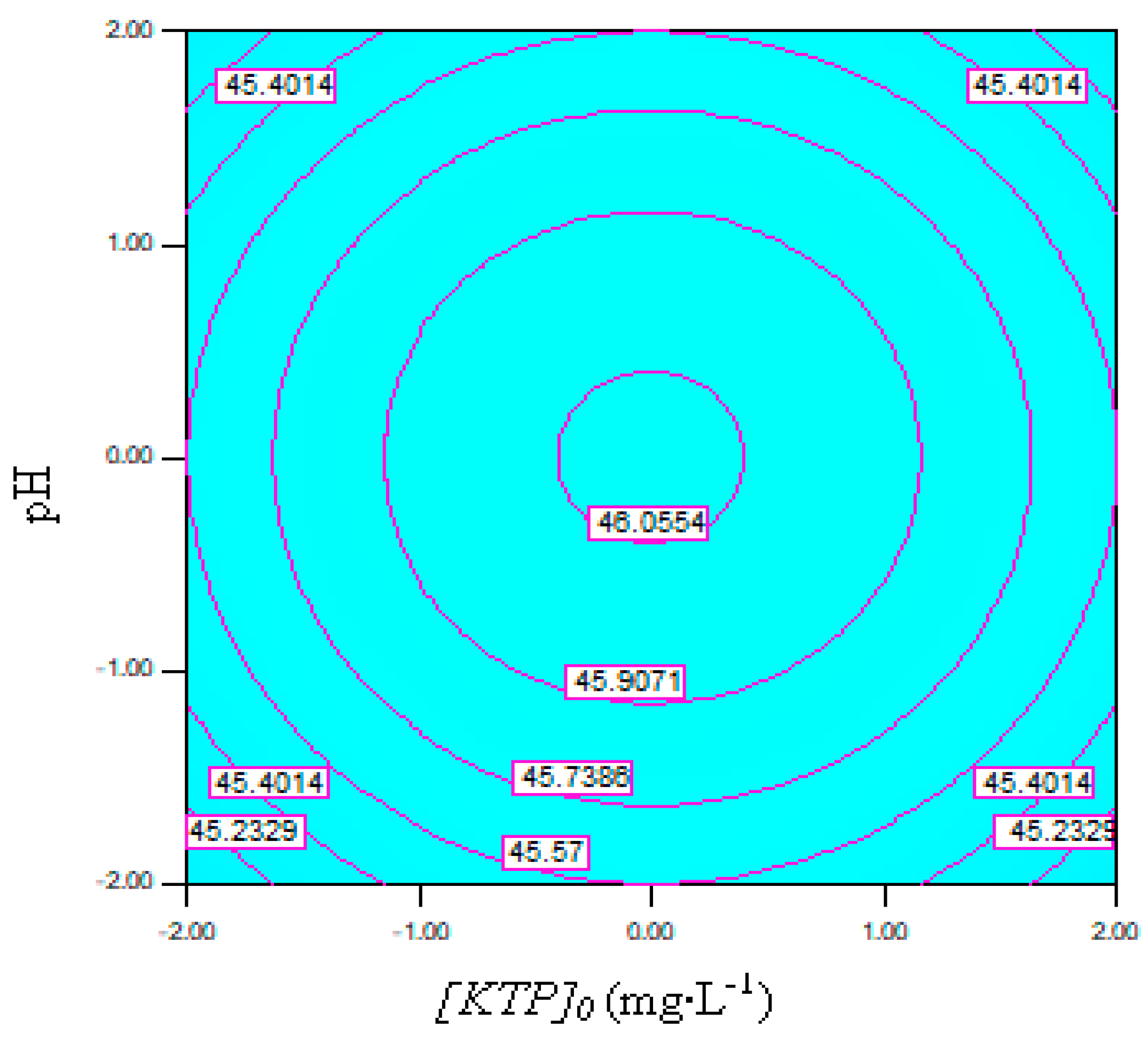
3.3. Modeling and Optimization by CCD
4. Comparative Review
5. Conclusions
- FTIR analysis confirmed the successful adsorption of ketoprofen (KTP) onto activated carbon without any significant modification of the adsorbent surface due to the electrocoagulation process.
- The combined EC/Ads approach demonstrated superior efficiency compared to the individual processes. A synergy factor of 1.37 was calculated, highlighting a significant interactive effect between electrocoagulation and adsorption.
- A comparative study of cathode types revealed that the iron grid cathode outperformed the steel plate cathode in terms of process efficiency.
- The electrocoagulation (EC) process can be significantly improved by integrating an adsorption mechanism, particularly through a configuration in which activated carbon is immobilized within the anode. This combined EC/Ads-ACT approach not only enhances overall pollutant removal efficiency but also allows for the reuse of activated carbon, thereby reducing operational costs and streamlining the treatment process.
- The model derived from the central composite design (CCD) optimization identified the following optimal conditions for complete ketoprofen removal (100%): an activated carbon dose (qAC) between 0.63 and 0.99 g/500 mL, a current density (i) ranging from 12.32 to 14.68 mA·cm−2, a pH of 6.5 and an initial KTP concentration of 22.5 mg·L−1.
- The statistical analysis of the model revealed a strong interaction between current density and activated carbon dosage, confirming their combined influence on removal efficiency.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef]
- Smiljanić, D.; de Gennaro, B.; Izzo, F.; Langella, A.; Daković, A.; Germinario, C.; Rottinghaus, G.E.; Spasojević, M.; Mercurio, M. Removal of emerging contaminants from water by zeolite-rich composites: A first approach aiming at diclofenac and ketoprofen. Microporous Mesoporous Mater. 2020, 298, 110057. [Google Scholar] [CrossRef]
- Alothman, Z.A.; Badjah, A.Y.; Alduhaish, O.M.; Rathinam, K.; Panglisch, S.; Ali, I. Synthesis, characterization, kinetics and modeling studies of new generation pollutant ketoprofen removal in water using copper nanoparticles. J. Mol. Liq. 2021, 323, 115075. [Google Scholar] [CrossRef]
- Perneger, T.V.; Whelton, P.K.; Klag, M.J. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. N. Engl. J. Med. 1994, 331, 1675–1679. [Google Scholar] [CrossRef] [PubMed]
- Georgin, J.; Franco, D.S.P.; Martinello, K.D.B.; Lima, E.C.; Silva, L.F.O. A review of the toxicology presence and removal of ketoprofen through adsorption technology. J. Environ. Chem. Eng. 2022, 10, 107798. [Google Scholar] [CrossRef]
- Fan, H.; Ren, G.; Yan, D.; Wu, T.; Gao, Y.; Zhu, Z.; Zhao, G. Modification of dispersive soil by physical adsorption method using activated carbon. J. Rock Mech. Geotech. Eng. 2025, 17, 1213–1226. [Google Scholar] [CrossRef]
- Zeng, L.; Yi, Q.; Peng, X.; Huang, Z.; Van der Bruggen, B.; Zhang, Y.; Kuang, Y.; Ma, Y.; Tang, K. Modelling and optimization of a new complexing retardant-enhanced polymer inclusion membrane system for highly selective separation of Zn2+ and Cu2+. Sep. Purif. Technol. 2022, 292, 121056. [Google Scholar] [CrossRef]
- Hamiche, A.; Yahiaoui, I.; Khenniche, L.; Amrane, A.; Aissani-Benissad, F. Degradation of paracetamol by sulfate radicals using UVA-irradiation/heat activated peroxydisulfate: Kinetics and optimization using Box–Behnken design. React. Kinet. Mech. Cat. 2024, 137, 433–451. [Google Scholar] [CrossRef]
- Taoufik, N.; Boumya, W.; Achak, M.; Sillanpää, M.; Barka, N. Comparative overview of advanced oxidation processes and biological approaches for the removal of pharmaceuticals. J. Environ. Manag. 2021, 288, 112404. [Google Scholar] [CrossRef]
- Balouchi, H.; Baziar, M.; Dehghan, A.; Alidadi, H.; Shams, M. Combination of electrocoagulation and MOF adsorption systems for EBT removal from water. Int. J. Environ. Anal. Chem. 2020, 102, 1307–1317. [Google Scholar] [CrossRef]
- Graça, N.S.; Rodrigues, A.E. The combined implementation of electrocoagulation and adsorption processes for the treatment of wastewaters. Clean Technol. 2022, 4, 1020–1053. [Google Scholar] [CrossRef]
- Liew, W.L.; Kassim, M.A.; Muda, K.; Loh, S.K.; Affam, A.C. Conventional methods and emerging wastewater polishing technologies for palm oil mill effluent treatment: A review. J. Environ. Manag. 2015, 149, 222–235. [Google Scholar] [CrossRef]
- Tahreen, A.; Jami, M.S.; Ali, F. Role of electrocoagulation in wastewater treatment: A developmental review. J. Water Process Eng. 2020, 37, 101440. [Google Scholar] [CrossRef]
- Madi-Azegagh, K.; Yahiaoui, I.; Boudrahem, F.; Aissani-Benissad, F.; Vial, C.; Audonnet, F.; Favier, L. Applied of central composite design for the optimization of removal yield of the ketoprofen (KTP) using electrocoagulation process. Sep. Sci. Technol. 2019, 54, 3115–3127. [Google Scholar] [CrossRef]
- Wang, X.; Ni, J.; Pang, S.; Li, Y. Removal of malachite green from aqueous solutions by electrocoagulation/peanut shell adsorption coupling in a batch system. Water Sci. Technol. 2017, 75, 1830–1838. [Google Scholar] [CrossRef]
- Sorayyaei, S.; Raji, F.; Rahbar-Kelishami, A.; Ashrafizadeh, S.N. Combination of electrocoagulation and adsorption processes to remove methyl orange from aqueous solution. Environ. Technol. Innov. 2021, 24, 102018. [Google Scholar] [CrossRef]
- Yang, G.C.C.; Tang, P.L.; Yen, C.H. Removal of micropollutants from municipal wastewater by graphene adsorption and simultaneous electrocoagulation/electrofiltration process. Water Sci. Technol. 2017, 75, 1882–1888. [Google Scholar] [CrossRef] [PubMed]
- Gomez, J.G.; Garcia, A.M.; Diez, M.A.D.; Garcia, C.G.; Rey, E.S. Preparation and characterization of activated carbons from impregnation pitch by ZnCl2. Appl. Surf. Sci. 2006, 252, 5976–5979. [Google Scholar] [CrossRef]
- Leofanti, G.; Padovan, M.; Tozzola, G.; Venturelli, B. Surface area and pore texture of catalysts. Catal. Today 1998, 41, 207–219. [Google Scholar] [CrossRef]
- Khalili, N.R.; Campbell, M.; Sandi, G.; Gola, J. Production of micro- and mesoporous activated carbon from paper mill sludge: I. Effect of zinc chloride activation. Carbon 2000, 38, 1905–1915. [Google Scholar] [CrossRef]
- Brahmi, A.; Agsous, M.; Benkhaoula, B.N.; Ziani, S.; Khireddine, H.; AitAli, S.; Abdullah, M.M.S.; Chai, B.X.; Belaadi, A. Fluoro-Hydroxyapatite/Chitosan composites as an eco-friendly adsorbent for Direct Red 23 dye removal: Optimization through Response Surface Methodology. J. Mol. Struct. 2025, 1321, 140212. [Google Scholar] [CrossRef]
- Yahiaoui, I.; Belattaf, A.; Aissani-Benissad, F.; Yahia Cherif, L. Full Factorial Design applied to a Biosorption of Lead(II) Ions from Aqueous Solution Using Brewer’s Yeast (Saccharomyces cerevisiae). J. Chem. Eng. Data 2011, 56, 3999–4005. [Google Scholar] [CrossRef]
- Boudrahem, F.; Yahiaoui, I.; Saidi, S.; Yahiaoui, K.; Kaabache, L.; Zennache, M.; Aissani-Benissad, F. Adsorption of pharmaceutical residues on adsorbents prepared from olive stones using mixture design of experiments model. Water Sci. Technol. 2019, 80, 998–1009. [Google Scholar] [CrossRef]
- Narukulla, S.; Bogadi, S.; Tallapaneni, V.; Sanapalli, B.K.R.; Sanju, S.; Khan, A.A.; Malik, A.; Barai, H.R.; Mondal, T.K.; Karri, V.V.S.R.; et al. Comparative study between the Full Factorial, Box–Behnken, and Central Composite Designs in the optimization of metronidazole immediate release tablet. Microchem. J. 2024, 207, 111875. [Google Scholar] [CrossRef]
- Essekri, A.; Aarab, N.; Hsini, A.; Ajmal, Z.; Laabd, M.; El Ouardi, M.; Addi, A.A.; Lakhmiri, R.; Albourine, A. Enhanced adsorptive removal of crystal violet dye from aqueous media using citric acid modified red-seaweed: Experimental study combined with RSM process optimization. J. Dispers. Sci. Technol. 2022, 43, 1359–1372. [Google Scholar] [CrossRef]
- Ghelich, R.; Jahannama, M.R.; Abdizadeh, H.; Torknik, F.S.; Vaezi, M.R. Central composite design (CCD)-response surface methodology (RSM) of effective electrospinning parameters on PVP-B-Hf hybrid nanofibrous composites for synthesis of HfB2-based composite nanofibers. Compos. Part B Eng. 2019, 166, 527–541. [Google Scholar] [CrossRef]
- Iqbal, M.; Iqbal, N.; Bhatti, I.A.; Ahmad, N.; Zahid, M. Response surface methodology application in optimization of cadmium adsorption by shoe waste: A good option of waste mitigation by waste. Ecol. Eng. 2016, 88, 265–275. [Google Scholar] [CrossRef]
- Granato, D.; Calado, V.M.A. The use and importance of design of experiments (DOE) in process modelling in food science and technology. In Mathematical and Statistical Methods in Food Science and Technology; Wiley: Hoboken, NJ, USA, 2014; pp. 1–18. [Google Scholar] [CrossRef]
- Ioannidi, A.; Arvaniti, O.S.; Nika, M.C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Removal of drug losartan in environmental aquatic matrices by heat-activated persulfate: Kinetics, transformation products and synergistic effects. Chemosphere 2022, 287, 131952. [Google Scholar] [CrossRef]




| Commercial Name | Raw Formula | λmax (nm) | Molar Weight (g·mol−1) | Purity (%) | pKa | Solubility in Water (mg·L−1) at 25 °C |
|---|---|---|---|---|---|---|
| ketoprofen | C16H14O3 | 260 | 254.28 | ≥98% | 4.4 | 51 |
| Chemical structure | ||||||
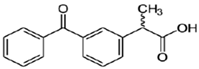 | ||||||
| Variables | Levels | ||||
|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | |
| pH: | 3.00 | 4.75 | 6.50 | 8.25 | 10.00 |
| [KTP]0 (mg·L−1): | 5.00 | 13.75 | 22.5 | 31.25 | 40.00 |
| i (mA·cm−2): | 2.10 | 5.25 | 8.41 | 11.56 | 14.71 |
| qAC (g): | 0.10 | 0.325 | 0.550 | 0.775 | 1.00 |
| Run | Real Variables | Coded Variables | Response | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| pH | [KTP]0 (mg·L−1) | i (mA·cm−2) | qAC (g) | y (%) | ||||||
| 1 | 4.75 | 13.75 | 5.25 | 0.325 | 1 | −1 | −1 | −1 | −1 | 40.91 |
| 2 | 4.75 | 13.75 | 5.25 | 0.775 | 1 | −1 | −1 | −1 | 1 | 42.62 |
| 3 | 4.75 | 13.75 | 11.56 | 0.325 | 1 | −1 | −1 | 1 | −1 | 47.72 |
| 4 | 4.75 | 13.75 | 11.56 | 0.775 | 1 | −1 | −1 | 1 | 1 | 77.53 |
| 5 | 4.75 | 31.25 | 5.25 | 0.325 | 1 | −1 | 1 | −1 | −1 | 40.91 |
| 6 | 4.75 | 31.25 | 5.25 | 0.775 | 1 | −1 | 1 | −1 | 1 | 42.62 |
| 7 | 4.75 | 31.25 | 11.56 | 0.325 | 1 | −1 | 1 | 1 | −1 | 47.72 |
| 8 | 4.75 | 31.25 | 11.56 | 0.775 | 1 | −1 | 1 | 1 | 1 | 77.53 |
| 9 | 8.25 | 13.75 | 5.25 | 0.325 | 1 | 1 | −1 | −1 | −1 | 40.91 |
| 10 | 8.25 | 13.75 | 5.25 | 0.775 | 1 | 1 | −1 | −1 | 1 | 42.62 |
| 11 | 8.25 | 13.75 | 11.56 | 0.325 | 1 | 1 | −1 | 1 | −1 | 47.72 |
| 12 | 8.25 | 13.75 | 11.56 | 0.775 | 1 | 1 | −1 | 1 | 1 | 77.53 |
| 13 | 8.25 | 31.25 | 5.25 | 0.325 | 1 | 1 | 1 | −1 | −1 | 40.91 |
| 14 | 8.25 | 31.25 | 5.25 | 0.775 | 1 | 1 | 1 | −1 | 1 | 42.62 |
| 15 | 8.25 | 31.25 | 11.56 | 0.325 | 1 | 1 | 1 | 1 | −1 | 47.72 |
| 16 | 8.25 | 31.25 | 11.56 | 0.775 | 1 | 1 | 1 | 1 | 1 | 77.53 |
| 17 | 6.50 | 22.50 | 8.41 | 0.55 | 1 | 0 | 0 | 0 | 0 | 45.70 |
| 18 | 6.50 | 22.50 | 8.41 | 0.55 | 1 | 0 | 0 | 0 | 0 | 46.57 |
| 19 | 6.50 | 22.50 | 8.41 | 0.55 | 1 | 0 | 0 | 0 | 0 | 45.96 |
| 20 | 3.00 | 22.50 | 8.41 | 0.55 | 1 | −2 | 0 | 0 | 0 | 45.57 |
| 21 | 10.00 | 22.50 | 8.41 | 0.55 | 1 | 2 | 0 | 0 | 0 | 45.57 |
| 22 | 6.50 | 5.00 | 8.41 | 0.55 | 1 | 0 | −2 | 0 | 0 | 45.57 |
| 23 | 6.50 | 40.00 | 8.41 | 0.55 | 1 | 0 | 2 | 0 | 0 | 45.57 |
| 24 | 6.50 | 22.5 | 2.10 | 0.55 | 1 | 0 | 0 | −2 | 0 | 51.21 |
| 25 | 6.50 | 22.5 | 14.71 | 0.55 | 1 | 0 | 0 | 2 | 0 | 92.94 |
| 26 | 6.50 | 22.5 | 8.41 | 0.10 | 1 | 0 | 0 | 0 | −2 | 29.81 |
| 27 | 6.50 | 22.5 | 8.41 | 1.00 | 1 | 0 | 0 | 0 | 2 | 61.33 |
| Band | Assignment | KTP | AC | EC/Ads and Ads |
|---|---|---|---|---|
| ~1700 cm−1 | (C=O) | strong | absent | appeared after adsorption |
| ~1600–1500 cm−1 | (C=C aromatic) | present | present | present, intensified |
| ~1250–1000 cm−1 | (C–O) | strong | weak | appeared/intensified |
| SBET (m2·g−1) | Vtot (cm3·g−1) | Sext (m2·g−1) | Smic (m2·g−1) | Vmic (cm3·g −1) | Vmes (cm3·g −1) | Dp (nm) |
|---|---|---|---|---|---|---|
| 947.251 | 0.509 | 564.438 | 382.812 | 0.430 | 0.079 | 2.149 |
| Response | Model | F_Value | p_Value | R2 | R2Adj |
|---|---|---|---|---|---|
| Ketoprofen removal | quadratic | 12,969.48 | <0.0001 | 0.9999 | 0.9999 |
| Authors | Pollutant | Method | Experimental Conditions | Reaction Time (min) | Removal Efficiency (%) |
|---|---|---|---|---|---|
| Balouchi et al. [11] | Eriochrom black T (Azo dye) | Adsorption | pH = 3, qAC = 0.3 g·L−1 | 60 | 75 |
| Electrocoagulation | pH = 7, i = 20 mA·cm−2 | 60 | 80 | ||
| EC/Ads | pH = 7, qAC = 0.3 g·L−1, i = 20 mA·cm−2 | 60 | 95 | ||
| Wang et al. [16] | Malachite Green | Adsorption | pH = 5, qAC = 2 g·L−1 | 30 | 70 |
| Electrocoagulation | pH = 5, i = 20 mA·cm−2 | 30 | 82 | ||
| EC/Ads | pH = 5, qAC = 0.3 g·L−1, i = 20 mA·cm−2 | 30 | 97 | ||
| Yang et al. [18] | Sulfamethoxazole Cephalexin | Adsorption | pH = 6, 0.5 g·L−1 | 45 | 35 * 82 ** |
| EC/filtration | pH = 6, i = 15 mA·cm−2 | 45 | 97 * 96 ** | ||
| EC/Ads | pH = 6, qAC = 0.5 g·L−1, i = 15 mA·cm−2 | 45 | 98 * 100 ** | ||
| Present Study | Ketoprofen | Adsorption | pH = 6, qAC = 1 g·L−1 | 30 | 27 |
| Electrocoagulation | pH = 6, i = 18.60 mA·cm−2 | 30 | 59.69 | ||
| EC/Ads | pH = 6, qAC = 1.26–1.98 g·L−1, i = 12.32–14.68 mA·cm−2 | 30 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madi-Azegagh, K.; Yahiaoui, I.; Arfi, R.; Benkerrou, L.; Khenniche, L.; Lebik, H.; Assadi, A.A.; Khezami, L.; Kriaa, K.; Aissani-Benissad, F. Adsorption Combined with Electrocoagulation Process for Ketoprofen Removal from Aqueous Solution: Optimization Using Central Composite Design. Water 2025, 17, 1679. https://doi.org/10.3390/w17111679
Madi-Azegagh K, Yahiaoui I, Arfi R, Benkerrou L, Khenniche L, Lebik H, Assadi AA, Khezami L, Kriaa K, Aissani-Benissad F. Adsorption Combined with Electrocoagulation Process for Ketoprofen Removal from Aqueous Solution: Optimization Using Central Composite Design. Water. 2025; 17(11):1679. https://doi.org/10.3390/w17111679
Chicago/Turabian StyleMadi-Azegagh, Katia, Idris Yahiaoui, Rima Arfi, Lydia Benkerrou, Lamia Khenniche, Hafida Lebik, Amine Aymen Assadi, Lotfi Khezami, Karim Kriaa, and Farida Aissani-Benissad. 2025. "Adsorption Combined with Electrocoagulation Process for Ketoprofen Removal from Aqueous Solution: Optimization Using Central Composite Design" Water 17, no. 11: 1679. https://doi.org/10.3390/w17111679
APA StyleMadi-Azegagh, K., Yahiaoui, I., Arfi, R., Benkerrou, L., Khenniche, L., Lebik, H., Assadi, A. A., Khezami, L., Kriaa, K., & Aissani-Benissad, F. (2025). Adsorption Combined with Electrocoagulation Process for Ketoprofen Removal from Aqueous Solution: Optimization Using Central Composite Design. Water, 17(11), 1679. https://doi.org/10.3390/w17111679










