Degradation of Micropollutants in Wastewater Using Photocatalytic TiO2@Ag-NPs Coatings Under Visible Irradiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of TiO2@Ag-NPs Coatings via MOCVD
2.3. Photocatalytic Degradation Experiments
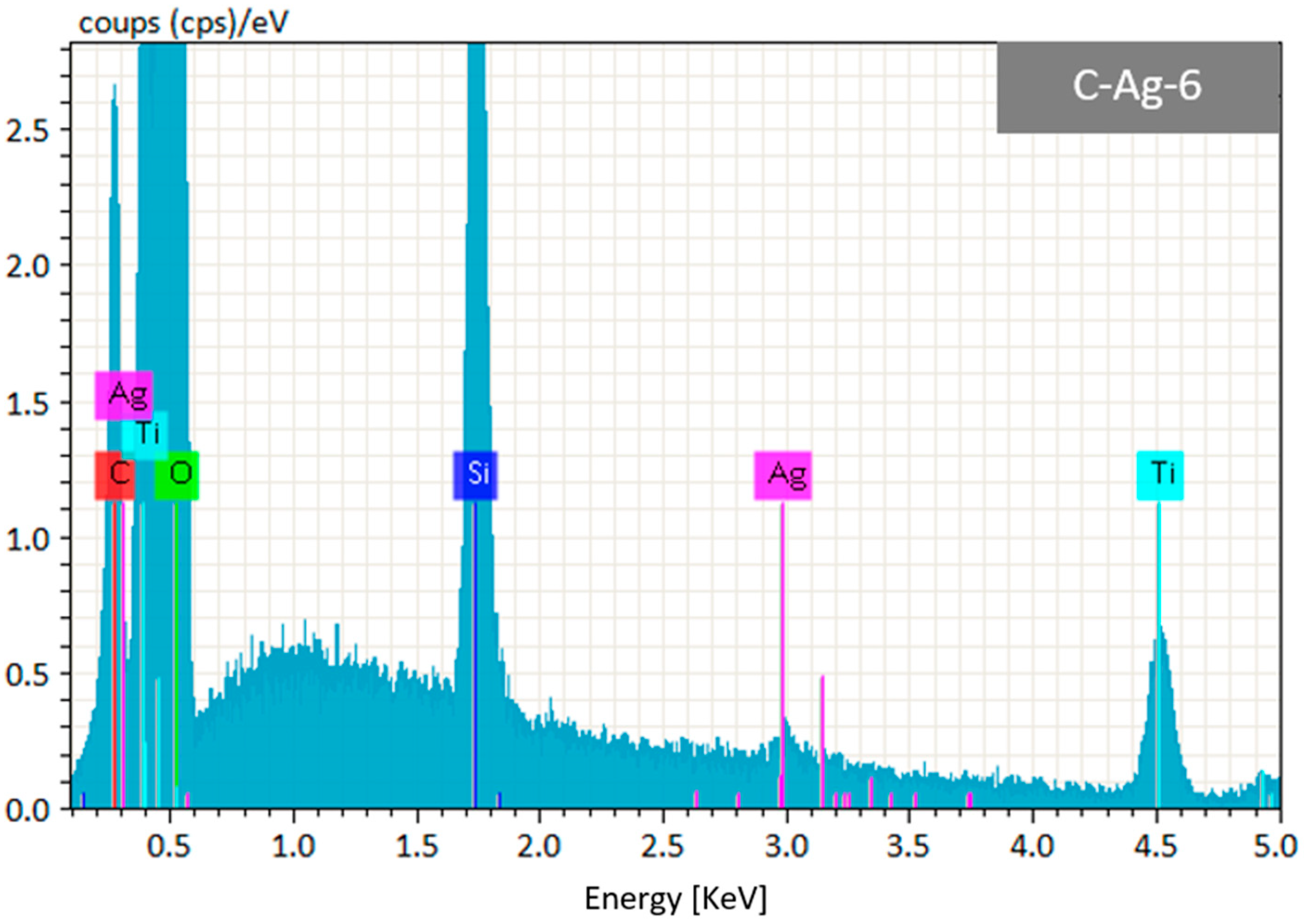
2.4. Characterisation of TiO2@Ag-NPs Coatings
2.5. Analytical Methods
3. Results and Discussion
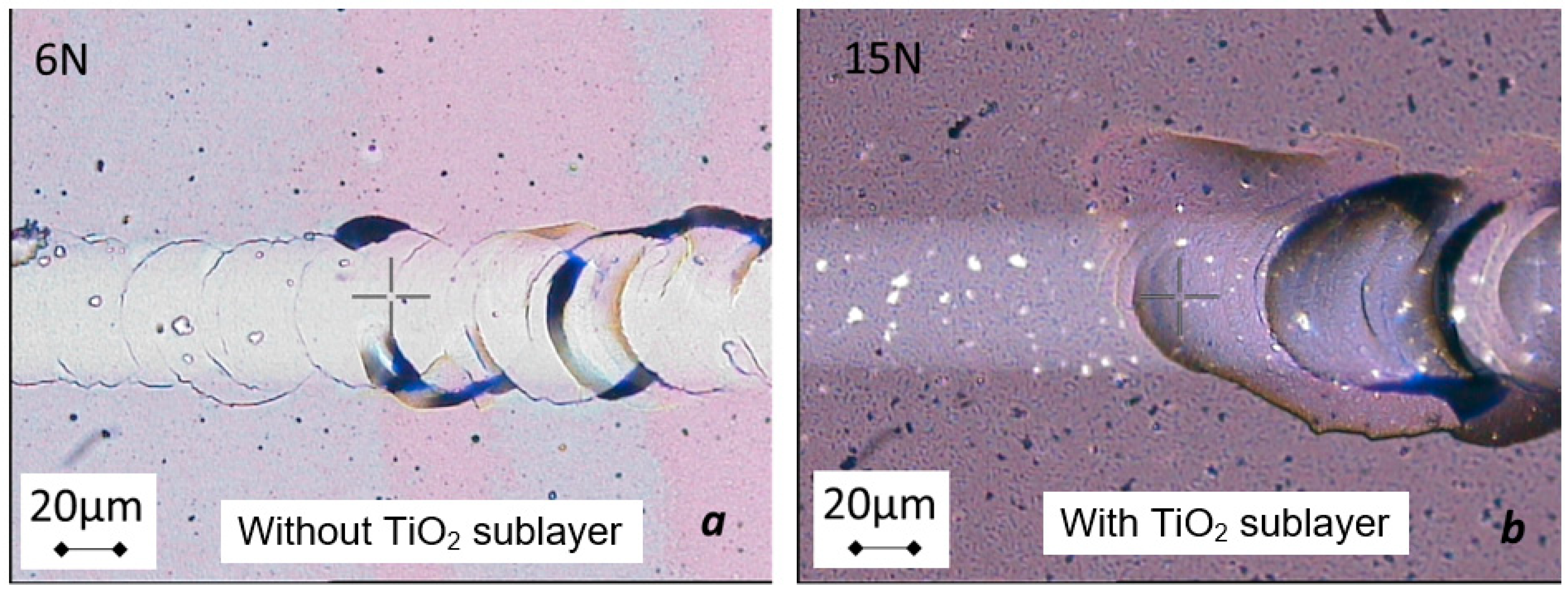
3.1. Adhesion of the Coating at the TiO2@Ag-NPs/Pyrex® Interface
3.2. Effects of the Adhesion Sublayer on the TiO2@Ag-NPs Coating Morphology
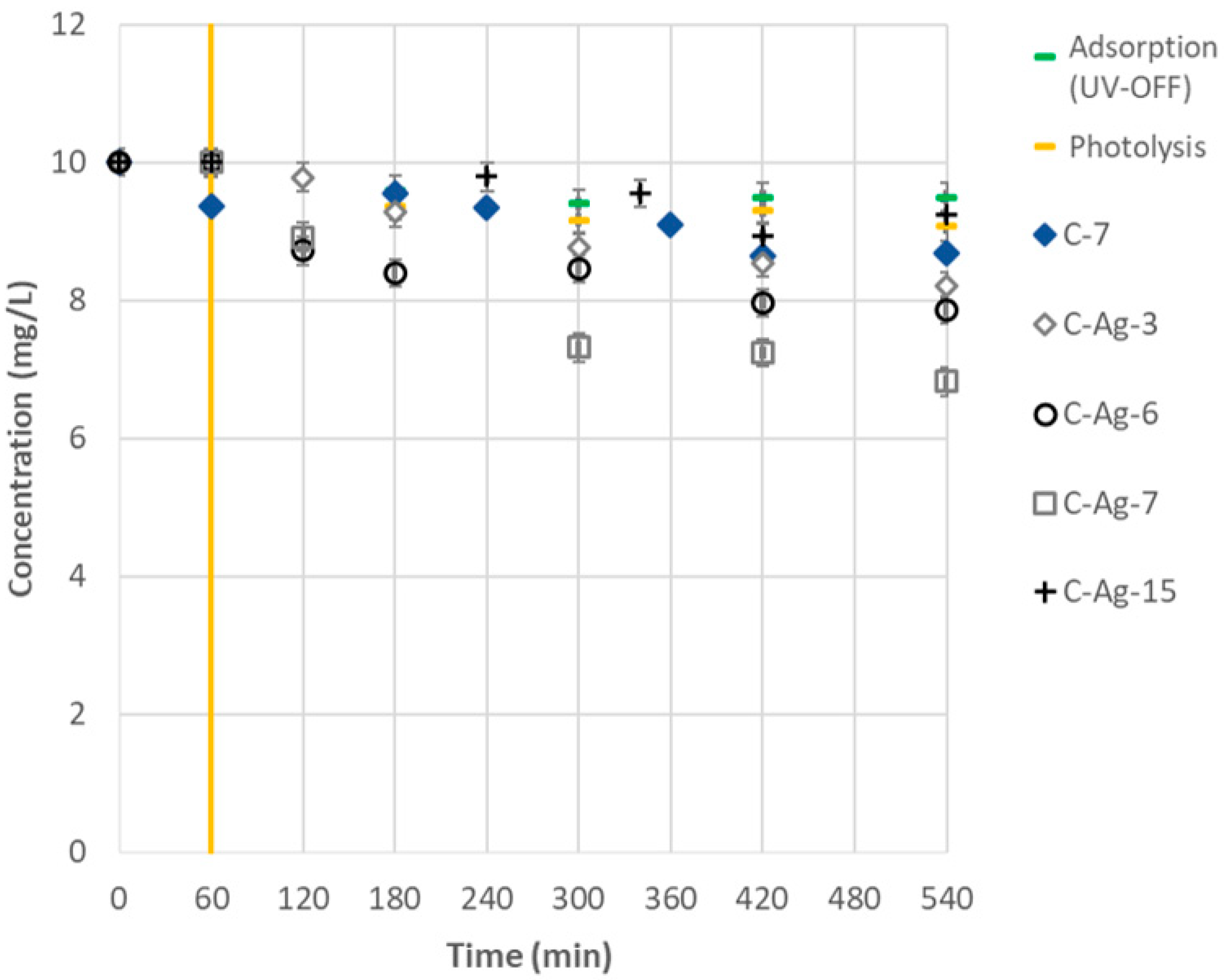
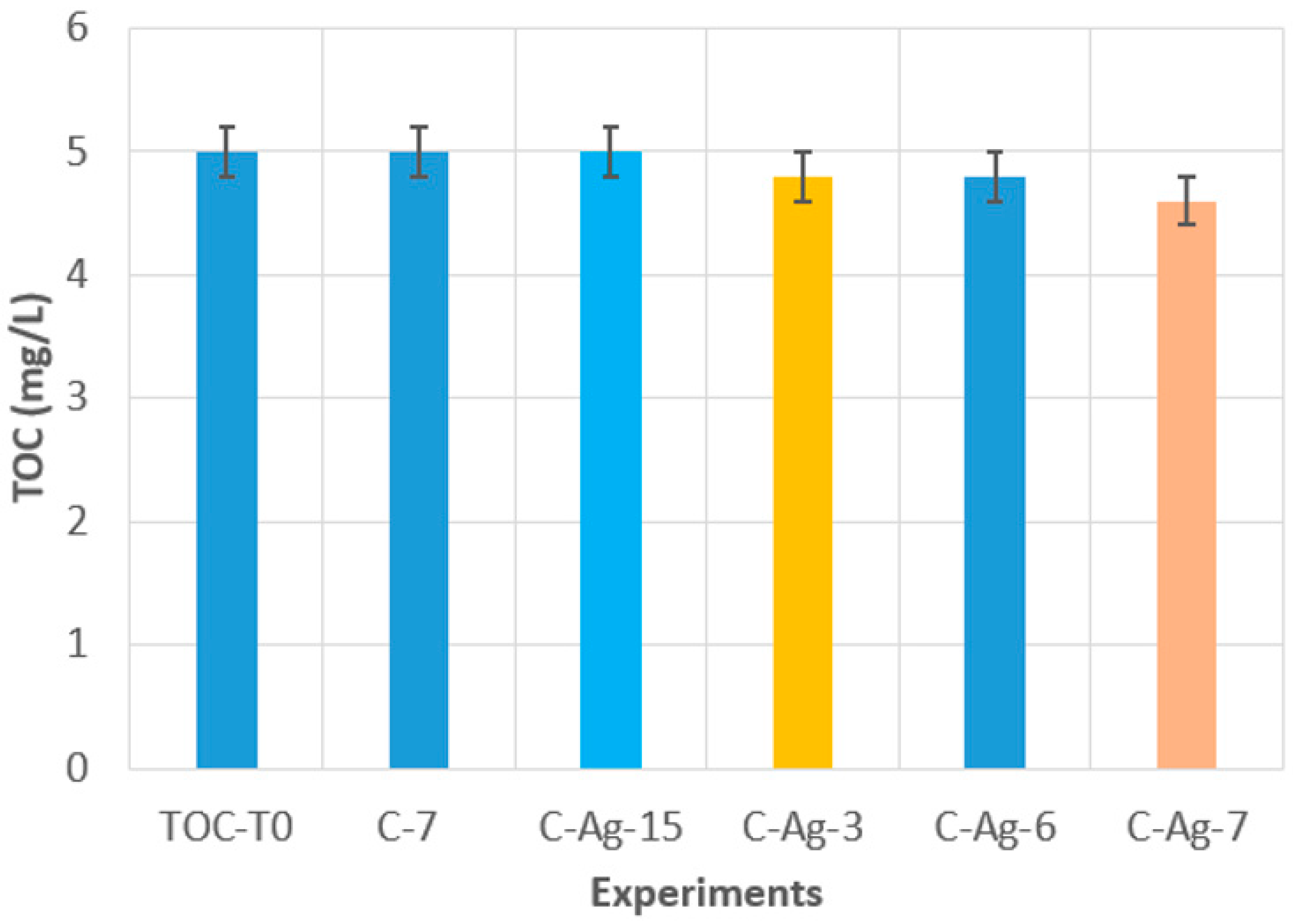
3.3. Photocatalytic Activity Under Visible Irradiation
- The ability of Ag doping to trigger photocatalytic activity is confirmed because neat TiO2 is inactive, whereas doped TiO2 contributes to decreasing the initial amount of diuron (C-7 and C-Ag-7).
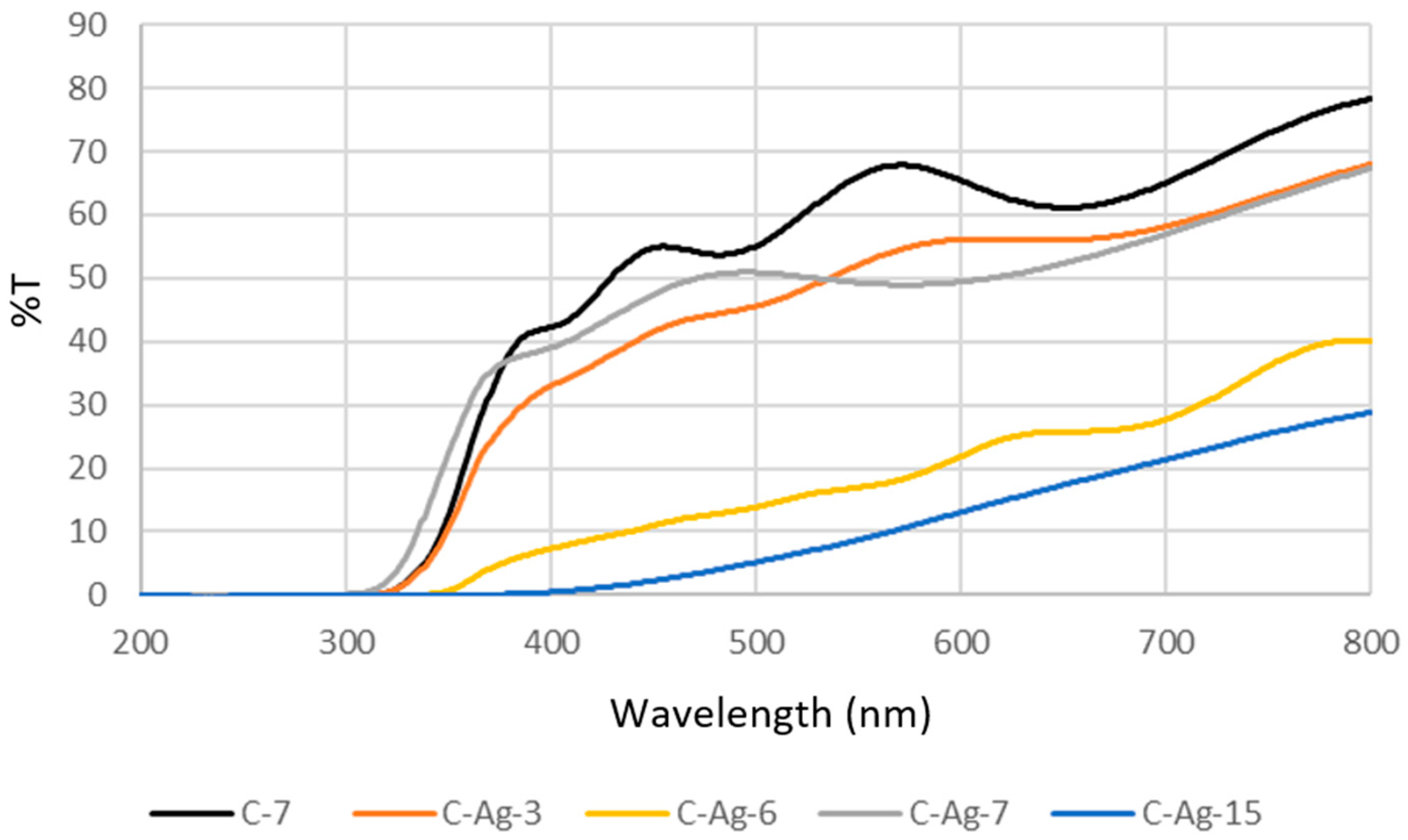
- The coating thickness is important, particularly in the back-side illumination mode (C-Ag-3, C-Ag-7, and C-Ag-15). The existence of an optimal photocatalyst thickness is confirmed; below and above this optimum thickness, the photocatalytic activity decreases as demonstrated in Ref. [39].
- The dispersion and the growth mode of Ag particles considerably affect the attenuation of the incident radiation, particularly in the back-side illumination mode (C-Ag-6 and C-Ag-7). The presence of the TiO2 adhesion sublayer promotes the dispersion of Ag-NPs within the matrix and limits the formation of large aggregates, ensuring the lowest radiation attenuation and enabling the photocatalytic activity in the visible range.
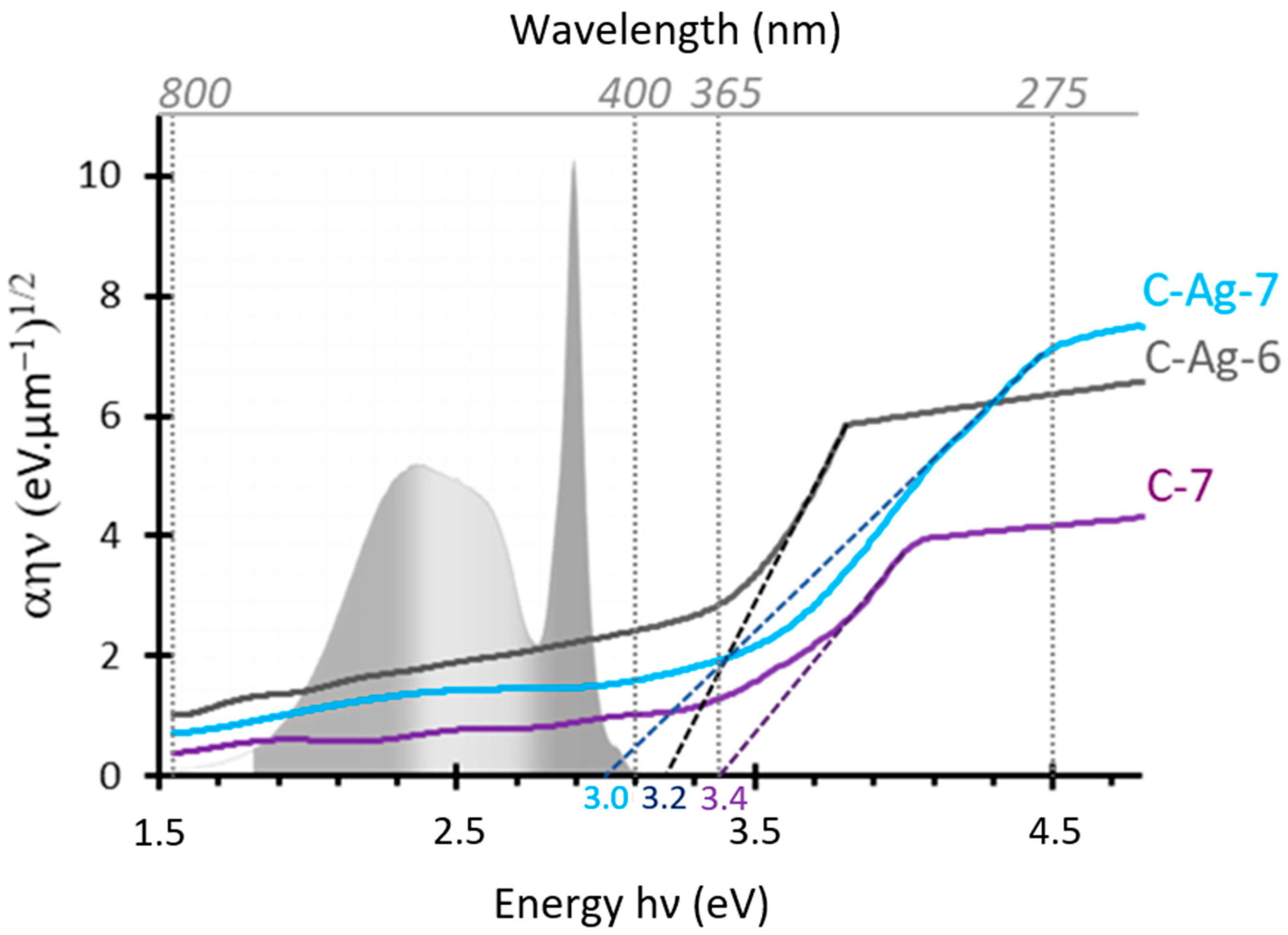
3.4. Effects of Doping on the Bandgap of Photocatalysts
4. Conclusions
5. Recommendations
- Increasing the incident photon flux by concentrating the solar radiation;
- The determination of the illumination mode (front-side illumination vs. back-side illumination) that can overcome the attenuation of the incident radiation;
- The optimisation of the coating architecture by investigating the effects of different Ag particle contents or a multilayer organisation;
- Costs, life cycle, and long-term stability should be studied in depth for a possible change of scale.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| Ag-NPs | Silver nanoparticles |
| LEDs | Light-emitting diodes |
| UV | Ultraviolet |
| MOCVD | Metal–organic chemical vapour deposition |
| CVD | Chemical vapour deposition |
| DLI | Direct liquid injection |
| XRD | X-ray diffraction |
| SEM | Scanning electron microscopy |
| EDS | Energy-dispersive spectroscopy |
| HPLC | High-performance liquid chromatography |
| TOC | Total organic carbon |
| JCPDS | Joint Committee on Powder Diffraction Standards |
References
- UNESCO. The United Nations World Water Development Report 2023: Partnerships and Cooperation for Water; UNESCO Biblioteca Digital: Paris, France, 2023; Available online: https://unesdoc.unesco.org/ark:/48223/pf0000384655 (accessed on 2 October 2023).
- Alexa, E.T.; Bernal-Romero del Hombre Bueno, M.d.l.Á.; González, R.; Sánchez, A.V.; García, H.; Prats, D. Occurrence and Removal of Priority Substances and Contaminants of Emerging Concern at the WWTP of Benidorm (Spain). Water 2022, 14, 4129. [Google Scholar] [CrossRef]
- Paijens, C.; Frère, B.; Caupos, E.; Moilleron, R.; Bressy, A. Determination of 18 Biocides in Both the Dissolved and Particulate Fractions of Urban and Surface Waters by HPLC-MS/MS. Water. Air. Soil Pollut. 2020, 231, 210. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A Review on the Occurrence of Micropollutants in the Aquatic Environment and Their Fate and Removal during Wastewater Treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Atasoy, M.; Zweers, H.; Rijnaarts, H.; Langenhoff, A.; Fernandes, T.V. Impact of Wastewater Characteristics on the Removal of Organic Micropollutants by Chlorella Sorokiniana. J. Hazard. Mater. 2023, 453, 131451. [Google Scholar] [CrossRef]
- Quintero-Castañeda, C.Y.; Acevedo, P.A.; Hernández-Angulo, L.R.; Tobón-Vélez, D.; Franco-Leyva, A.; Sierra-Carrillo, M.M. Wastewater Treatment by Coupling Adsorption and Photocatalytic Oxidation: A Review of the Removal of Phenolic Compounds in the Oil Industry. Eng 2024, 5, 2441–2461. [Google Scholar] [CrossRef]
- Triquet, T.; Tendero, C.; Latapie, L.; Manero, M.-H.; Richard, R.; Andriantsiferana, C. TiO2 MOCVD Coating for Photocatalytic Degradation of Ciprofloxacin Using 365 Nm UV LEDs - Kinetics and Mechanisms. J. Environ. Chem. Eng. 2020, 8, 104544. [Google Scholar] [CrossRef]
- Andriantsiferana, C.; Mohamed, E.F.; Delmas, H. Photocatalytic Degradation of an Azo-Dye on TiO2/Activated Carbon Composite Material. Environ. Technol. 2014, 35, 355–363. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mangalaraja, R.V.; Anandan, S. Review on the Recent Improvements in Sonochemical and Combined Sonochemical Oxidation Processes—A Powerful Tool for Destruction of Environmental Contaminants. Renew. Sustain. Energy Rev. 2016, 55, 426–454. [Google Scholar] [CrossRef]
- Weng, B.; Qi, M.-Y.; Han, C.; Tang, Z.-R.; Xu, Y.-J. Photocorrosion Inhibition of Semiconductor-Based Photocatalysts: Basic Principle, Current Development, and Future Perspective. ACS Catal. 2019, 9, 4642–4687. [Google Scholar] [CrossRef]
- Anaya-Rodríguez, F.; Durán-Álvarez, J.C.; Drisya, K.T.; Zanella, R. The Challenges of Integrating the Principles of Green Chemistry and Green Engineering to Heterogeneous Photocatalysis to Treat Water and Produce Green H2. Catalysts 2023, 13, 154. [Google Scholar] [CrossRef]
- Abd Rahman, N.; Choong, C.E.; Pichiah, S.; Nah, I.W.; Kim, J.R.; Oh, S.-E.; Yoon, Y.; Choi, E.H.; Jang, M. Recent Advances in the TiO2 Based Photoreactors for Removing Contaminants of Emerging Concern in Water. Sep. Purif. Technol. 2023, 304, 122294. [Google Scholar] [CrossRef]
- Gopalan, A.-I.; Lee, J.-C.; Saianand, G.; Lee, K.-P.; Sonar, P.; Dharmarajan, R.; Hou, Y.; Ann, K.-Y.; Kannan, V.; Kim, W.-J. Recent Progress in the Abatement of Hazardous Pollutants Using Photocatalytic TiO2-Based Building Materials. Nanomaterials 2020, 10, 1854. [Google Scholar] [CrossRef]
- Ahmadpour, N.; Nowrouzi, M.; Madadi Avargani, V.; Sayadi, M.H.; Zendehboudi, S. Design and Optimization of TiO2-Based Photocatalysts for Efficient Removal of Pharmaceutical Pollutants in Water: Recent Developments and Challenges. J. Water Process Eng. 2024, 57, 104597. [Google Scholar] [CrossRef]
- Wetchakun, K.; Wetchakun, N.; Sakulsermsuk, S. An Overview of Solar/Visible Light-Driven Heterogeneous Photocatalysis for Water Purification: TiO2- and ZnO-Based Photocatalysts Used in Suspension Photoreactors. J. Ind. Eng. Chem. 2019, 71, 19–49. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with Solar Energy at a Pilot-Plant Scale: An Overview. Appl. Catal. B Environ. 2002, 37, 1–15. [Google Scholar] [CrossRef]
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large Solar Plant Photocatalytic Water Decontamination: Degradation of Pentachlorophenol. Chemosphere 1993, 26, 2103–2119. [Google Scholar] [CrossRef]
- Rengifo-Herrera, J.A.; Pulgarin, C. Why Five Decades of Massive Research on Heterogeneous Photocatalysis, Especially on TiO2, Has Not yet Driven to Water Disinfection and Detoxification Applications? Critical Review of Drawbacks and Challenges. Chem. Eng. J. 2023, 477, 146875. [Google Scholar] [CrossRef]
- Fkiri, A.; Saidani, M.A.; Chmangui, A.; Smiri, L.S. One-Pot Synthesis of Copper-Doped ZnO Nanorods Photocatalysts: Degradation of Diuron Herbicide Under Simulated Solar Light. J. Inorg. Organomet. Polym. Mater. 2023, 33, 2523–2530. [Google Scholar] [CrossRef]
- Li, N.; Gao, X.; Su, J.; Gao, Y.; Ge, L. Metallic WO2-Decorated g-C3N4 Nanosheets as Noble-Metal-Free Photocatalysts for Efficient Photocatalysis. Chin. J. Catal. 2023, 47, 161–170. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Vasishth, A.; Kumar, A.; Pattnaik, S.S. Emerging Materials in Advanced Oxidation Processes for Micropollutant Treatment Process. In Advanced Oxidation Processes for Micropollutant Remediation; CRC Press: Boca Raton, FL, USA, 2023; pp. 133–155. ISBN 978-1-00-090654-7. [Google Scholar]
- Bensouici, F.; Souier, T.; Dakhel, A.A.; Iratni, A.; Tala-Ighil, R.; Bououdina, M. Synthesis, Characterization and Photocatalytic Behavior of Ag Doped TiO2 Thin Film. Superlattices Microstruct. 2015, 85, 255–265. [Google Scholar] [CrossRef]
- Çifçi, D.İ. Decolarization of Methylene Blue and Methyl Orange with Ag Doped TiO2 under UV-A and UV-Visible Conditions: Process Optimization by Response Surface Method and Toxicity Evaluation. Glob. NEST J. 2016, 18, 371–380. [Google Scholar] [CrossRef]
- de la Cruz, D.; Arévalo, J.C.; Torres, G.; Margulis, R.G.B.; Ornelas, C.; Aguilar-Elguézabal, A. TiO2 Doped with Sm3+ by Sol–Gel: Synthesis, Characterization and Photocatalytic Activity of Diuron under Solar Light. Catal. Today 2011, 166, 152–158. [Google Scholar] [CrossRef]
- Horovitz, I.; Avisar, D.; Baker, M.A.; Grilli, R.; Lozzi, L.; Di Camillo, D.; Mamane, H. Carbamazepine Degradation Using a N-Doped TiO2 Coated Photocatalytic Membrane Reactor: Influence of Physical Parameters. J. Hazard. Mater. 2016, 310, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Luna-Sanguino, G.; Tolosana-Moranchel, A.; Duran-Valle, C.; Faraldos, M.; Bahamonde, A. Optimizing P25-RGO Composites for Pesticides Degradation: Elucidation of Photo-Mechanism. Catal. Today 2019, 328, 172–177. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, Z.; Herout, R.; Liu, C.; Yu, K.; Lange, D.; Godin, R.; Kizhakkedathu, J.N.; Troczynski, T.; Wang, R. Axial Suspension Plasma Sprayed Ag-TiO2 Coating for Enhanced Photocatalytic and Antimicrobial Properties. Surf. Interfaces 2024, 45, 103856. [Google Scholar] [CrossRef]
- Borrego Pérez, J.A.; Morales, E.R.; Paraguay Delgado, F.; Meza Avendaño, C.A.; Alonso Guzman, E.M.; Mathews, N.R. Ag Nanoparticle Dispersed TiO2 Thin Films by Single Step Sol Gel Process: Evaluation of the Physical Properties and Photocatalytic Degradation. Vacuum 2023, 215, 112276. [Google Scholar] [CrossRef]
- Pawar, R.C.; Lee, C.S. Chapter 1-Basics of Photocatalysis. In Heterogeneous Nanocomposite-Photocatalysis for Water Purification; Pawar, R.C., Lee, C.S., Eds.; William Andrew Publishing: Boston, MA, USA, 2015; pp. 1–23. ISBN 978-0-323-39310-2. [Google Scholar]
- Huang, B.-S.; Chang, F.-Y.; Wey, M.-Y. Photocatalytic Properties of Redox-Treated Pt/TiO2 Photocatalysts for H2 Production from an Aqueous Methanol Solution. Int. J. Hydrogen Energy 2010, 35, 7699–7705. [Google Scholar] [CrossRef]
- Katsumata, H.; Sada, M.; Nakaoka, Y.; Kaneco, S.; Suzuki, T.; Ohta, K. Photocatalytic Degradation of Diuron in Aqueous Solution by Platinized TiO2. J. Hazard. Mater. 2009, 171, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ansari, S.A.; Lee, J.; Cho, M.H. Enhanced Optical, Visible Light Catalytic and Electrochemical Properties of Au@TiO2 Nanocomposites. J. Ind. Eng. Chem. 2013, 19, 1845–1850. [Google Scholar] [CrossRef]
- Schaefer, B.T.; Cheung, J.; Ihlefeld, J.F.; Jones, J.L.; Nagarajan, V. Stability and Dewetting Kinetics of Thin Gold Films on Ti, TiOx and ZnO Adhesion Layers. Acta Mater. 2013, 61, 7841–7848. [Google Scholar] [CrossRef]
- Shi, Y.; Ma, J.; Chen, Y.; Qian, Y.; Xu, B.; Chu, W.; An, D. Recent Progress of Silver-Containing Photocatalysts for Water Disinfection under Visible Light Irradiation: A Review. Sci. Total Environ. 2022, 804, 150024. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Dikici, T.; Yurddaskal, M.; Gultekin, S.; Toparli, M.; Celik, E. Synthesis and Characterization of Ag Doped TiO2 Heterojunction Films and Their Photocatalytic Performances. Appl. Surf. Sci. 2016, 390, 591–601. [Google Scholar] [CrossRef]
- Guillén-Santiago, A.; Mayén, S.A.; Torres-Delgado, G.; Castanedo-Pérez, R.; Maldonado, A.; de la, L. Olvera, M. Photocatalytic Degradation of Methylene Blue Using Undoped and Ag-Doped TiO2 Thin Films Deposited by a Sol–Gel Process: Effect of the Ageing Time of the Starting Solution and the Film Thickness. Mater. Sci. Eng. B 2010, 174, 84–87. [Google Scholar] [CrossRef]
- Yu, J.; Xiong, J.; Cheng, B.; Liu, S. Fabrication and Characterization of Ag–TiO2 Multiphase Nanocomposite Thin Films with Enhanced Photocatalytic Activity. Appl. Catal. B Environ. 2005, 60, 211–221. [Google Scholar] [CrossRef]
- Panis, C.; Candiotto, L.Z.P.; Gaboardi, S.C.; Gurzenda, S.; Cruz, J.; Castro, M.; Lemos, B. Widespread Pesticide Contamination of Drinking Water and Impact on Cancer Risk in Brazil. Environ. Int. 2022, 165, 107321. [Google Scholar] [CrossRef]
- Quintero-Castañeda, C.Y.; Tendero, C.; Triquet, T.; Acevedo, P.A.; Latapie, L.; Sierra-Carrillo, M.M.; Andriantsiferana, C. Towards a Better Understanding of the Back-Side Illumination Mode on Photocatalytic Metal–Organic Chemical Vapour Deposition Coatings Used for Treating Wastewater Polluted by Pesticides. Water 2024, 16, 1. [Google Scholar] [CrossRef]
- Chin-Pampillo, J.S.; Perez-Villanueva, M.; Masis-Mora, M.; Mora-Dittel, T.; Carazo-Rojas, E.; Alcañiz, J.M.; Chinchilla-Soto, C.; Domene, X. Amendments with Pyrolyzed Agrowastes Change Bromacil and Diuron’s Sorption and Persistence in a Tropical Soil without Modifying Their Environmental Risk. Sci. Total Environ. 2021, 772, 145515. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.S.; Araújo de Medeiros, R.C.; Silva, D.V.; de Freitas Souza, M.; das Chagas, P.S.F.; Lins, H.A.; da Silva, C.C.; Souza, C.M.M.; Mendonça, V. Interaction between Herbicides Applied in Mixtures Alters the Conception of Its Environmental Impact. Environ. Sci. Pollut. Res. 2022, 29, 15127–15143. [Google Scholar] [CrossRef]
- Mercurio, P.; Mueller, J.F.; Eaglesham, G.; O’Brien, J.; Flores, F.; Negri, A.P. Degradation of Herbicides in the Tropical Marine Environment: Influence of Light and Sediment. PLoS ONE 2016, 11, e0165890. [Google Scholar] [CrossRef]
- Sarantopoulos, C. Photocatalyseurs à Base de TiO2 Préparés Par Infiltration Chimique En Phase Vapeur (CVI) Sur Supports Microfibreux. Ph.D. Thesis, INPT, Toulouse, France, 2007. [Google Scholar]
- Miquelot, A.; Youssef, L.; Villeneuve-Faure, C.; Prud’homme, N.; Dragoe, N.; Nada, A.; Rouessac, V.; Roualdes, S.; Bassil, J.; Zakhour, M.; et al. In- and out-Plane Transport Properties of Chemical Vapor Deposited TiO2 Anatase Films. J. Mater. Sci. 2021, 56, 10458–10476. [Google Scholar] [CrossRef]
- Shetty, A.; Goyal, A. Total Organic Carbon Analysis in Water—A Review of Current Methods. Mater. Today Proc. 2022, 65, 3881–3886. [Google Scholar] [CrossRef]
- Jeong, E.; Zhao, G.; Lee, S.-G.; Bae, J.-S.; Yu, S.M.; Mun, C.; Han, S.Z.; Lee, G.-H.; Ikoma, Y.; Choi, E.-A.; et al. Exploring SiOx as an Effective Adhesion Promoter for Ag on Glass and Polymer Substrates. Appl. Surf. Sci. 2025, 688, 162342. [Google Scholar] [CrossRef]
- Abe, N.; Otani, Y.; Miyake, M.; Kurita, M.; Takeda, H.; Okamura, S.; Shiosaki, T. Influence of a TiO2 Adhesion Layer on the Structure and the Orientation of a Pt Layer in Pt/TiO2/SiO2/Si Structures. Jpn. J. Appl. Phys. 2003, 42, 2791. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic Activity Improvement and Application of UV-TiO2 Photocatalysis in Textile Wastewater Treatment: A Review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Galenda, A.; Natile, M.M.; El Habra, N. Large-Scale MOCVD Deposition of Nanostructured TiO2 on Stainless Steel Woven: A Systematic Investigation of Photoactivity as a Function of Film Thickness. Nanomaterials 2022, 12, 992. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, R. Shape Effect on the Elastic Properties of Ag Nanocrystals. Micro Nano Lett. 2018, 13, 312–315. [Google Scholar] [CrossRef]
- Ali, M.H.; Azad, M.A.K.; Khan, K.A.; Rahman, M.O.; Chakma, U.; Kumer, A. Analysis of Crystallographic Structures and Properties of Silver Nanoparticles Synthesized Using PKL Extract and Nanoscale Characterization Techniques. ACS Omega 2023, 8, 28133–28142. [Google Scholar] [CrossRef]
- Ling, L.; Feng, Y.; Li, H.; Chen, Y.; Wen, J.; Zhu, J.; Bian, Z. Microwave Induced Surface Enhanced Pollutant Adsorption and Photocatalytic Degradation on Ag/TiO2. Appl. Surf. Sci. 2019, 483, 772–778. [Google Scholar] [CrossRef]
- Guitoume, D.; Achour, S.; Sobti, N.; Boudissa, M.; Souami, N.; Messaoudi, Y. Structural, Optical and Photoelectrochemical Properties of TiO2 Films Decorated with Plasmonic Silver Nanoparticles. Optik 2018, 154, 182–191. [Google Scholar] [CrossRef]
- Castañeda, C.Y.Q. Photocatalyse Supportée à Base de TiO2: Vers une Meilleure Compréhension de la Dégradation du Diuron en Milieu Aqueux. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2024. [Google Scholar]
- Kusdianto, K.; Jiang, D.; Kubo, M.; Shimada, M. Fabrication of TiO2-Ag Nanocomposite Thin Films via One-Step Gas-Phase Deposition. Ceram. Int. 2017, 43, 5351–5355. [Google Scholar] [CrossRef]



| Operating Conditions | Coatings | ||||
|---|---|---|---|---|---|
| C-7 | C-Ag-3 | C-Ag-6 | C-Ag-7 | C-Ag-15 | |
| Deposition time (min) * (TiO2) ** (TiO2@Ag-NPs) | * (2 × 80) | * (2 × 10) + ** (2 × 35) | ** (2 × 80) | * (2 × 10) + ** (2 × 60) | * (2 × 10) + ** (2 × 90) |
| Temperature of the thermostatic bath of the TTIP bubbler (°C) | 37 | ||||
| Deposition temperature (°C) | 475 | ||||
| Deposition pressure (Torr) | 5 | ||||
| Carrier gas (N2) flow rate (cm3·min−1) | 8 | ||||
| Dilution gas (N2) flow rate (cm3·min−1) | 320 (Kemstream injector dilution line) + 210 (classic TiO2 dilution line) = 530 | ||||
| Injector frequency (Hz) | - | 1.5 | |||
| Injector opening time (ms) | - | 1 | |||
| Composition of the injectable solution | - | 0.012 M of silver pivalate in a mesitylene/dipropylamine mixture 90/10 vol. | |||
| Deposited mass (mg) | 7.7 ± 0.2 | 2.9 ± 0.2 | 5.9 ± 0.2 | 6.5 ± 0.2 | 15.3 ± 0.2 |
| Deposit Method | Target Molecule | Irradiation Source | Efficiency (%) Undoped TiO2 | Efficiency (%) TiO2@Ag-NPs | Effective (%) Enhancement | Reference |
|---|---|---|---|---|---|---|
| Sol-Gel | Methylene blue | UV + visible lamp | 36 | 54 | 18 | [35] |
| Sol-Gel | Rhodamine B | UVC lamp | 50 | 80 | 30 | [22] |
| Sol-Gel | Methylene blue | UV lamp | 25 | 35 | 10 | [36] |
| Sol-Gel | Methylene blue | 38 | 98 | 60 | [28] | |
| LPD | methyl orange | UV lamp | 63 | [37] | ||
| PECVD | Methylene blue | UV lamp | 60 | 95 | 35 | [55] |
| MOCVD | Diuron | Visible LED | 0 | 32 | 32 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintero-Castañeda, C.Y.; Tendero, C.; Triquet, T.; Villegas-Andrade, A.I.; Sierra-Carrillo, M.M.; Andriantsiferana, C. Degradation of Micropollutants in Wastewater Using Photocatalytic TiO2@Ag-NPs Coatings Under Visible Irradiation. Water 2025, 17, 1632. https://doi.org/10.3390/w17111632
Quintero-Castañeda CY, Tendero C, Triquet T, Villegas-Andrade AI, Sierra-Carrillo MM, Andriantsiferana C. Degradation of Micropollutants in Wastewater Using Photocatalytic TiO2@Ag-NPs Coatings Under Visible Irradiation. Water. 2025; 17(11):1632. https://doi.org/10.3390/w17111632
Chicago/Turabian StyleQuintero-Castañeda, Cristian Yoel, Claire Tendero, Thibaut Triquet, Arturo I. Villegas-Andrade, María Margarita Sierra-Carrillo, and Caroline Andriantsiferana. 2025. "Degradation of Micropollutants in Wastewater Using Photocatalytic TiO2@Ag-NPs Coatings Under Visible Irradiation" Water 17, no. 11: 1632. https://doi.org/10.3390/w17111632
APA StyleQuintero-Castañeda, C. Y., Tendero, C., Triquet, T., Villegas-Andrade, A. I., Sierra-Carrillo, M. M., & Andriantsiferana, C. (2025). Degradation of Micropollutants in Wastewater Using Photocatalytic TiO2@Ag-NPs Coatings Under Visible Irradiation. Water, 17(11), 1632. https://doi.org/10.3390/w17111632







