Effects of Planting Density on Water Restoration Performance of Vallisneria spinulosa Yan Growth System Constructed by Enclosure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
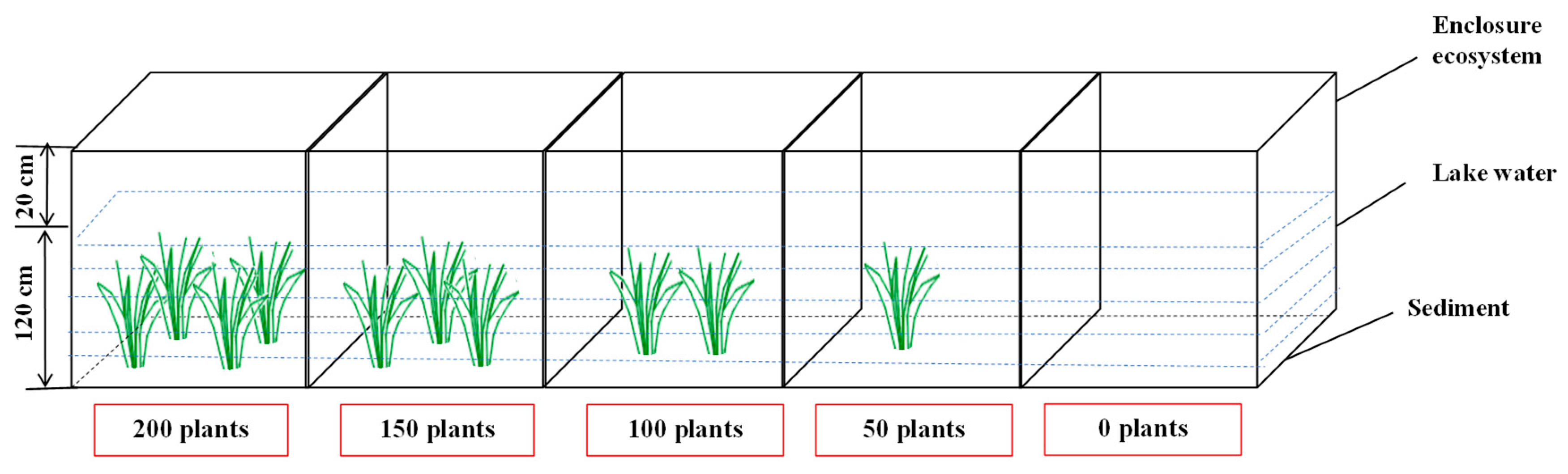
2.2. Experimental Design
2.3. Monitoring and Analysis Methods
2.3.1. Water Quality
2.3.2. Growth Characteristics of V. spinulosa
2.3.3. The Physiological Characteristics of V. spinulosa
2.4. Data Analysis
3. Results and Discussion
3.1. The Growth Characteristics of V. spinulosa
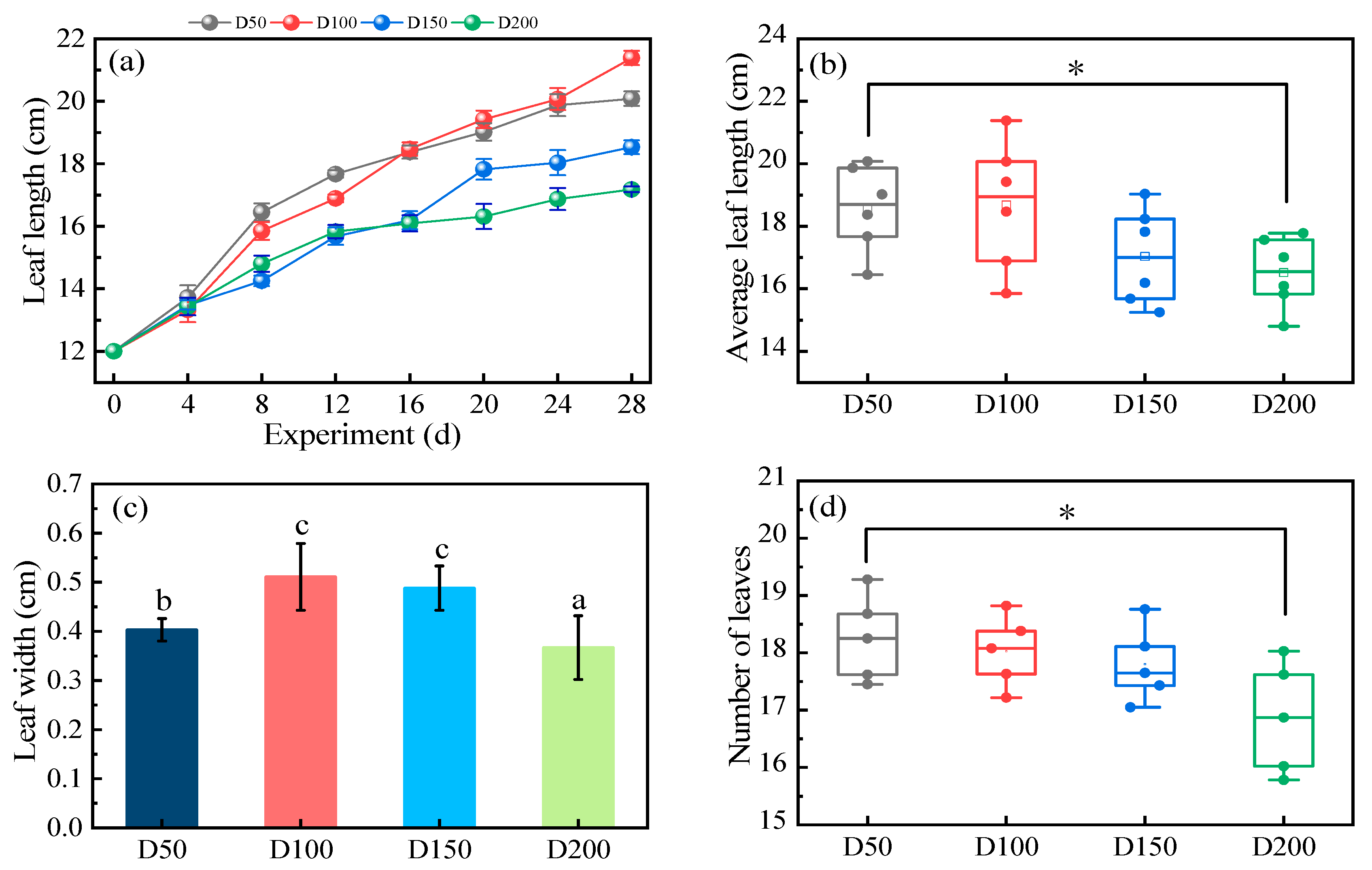
3.1.1. Leaf
3.1.2. Root Characteristics
3.2. The Physiological Characteristics of V. spinulosa
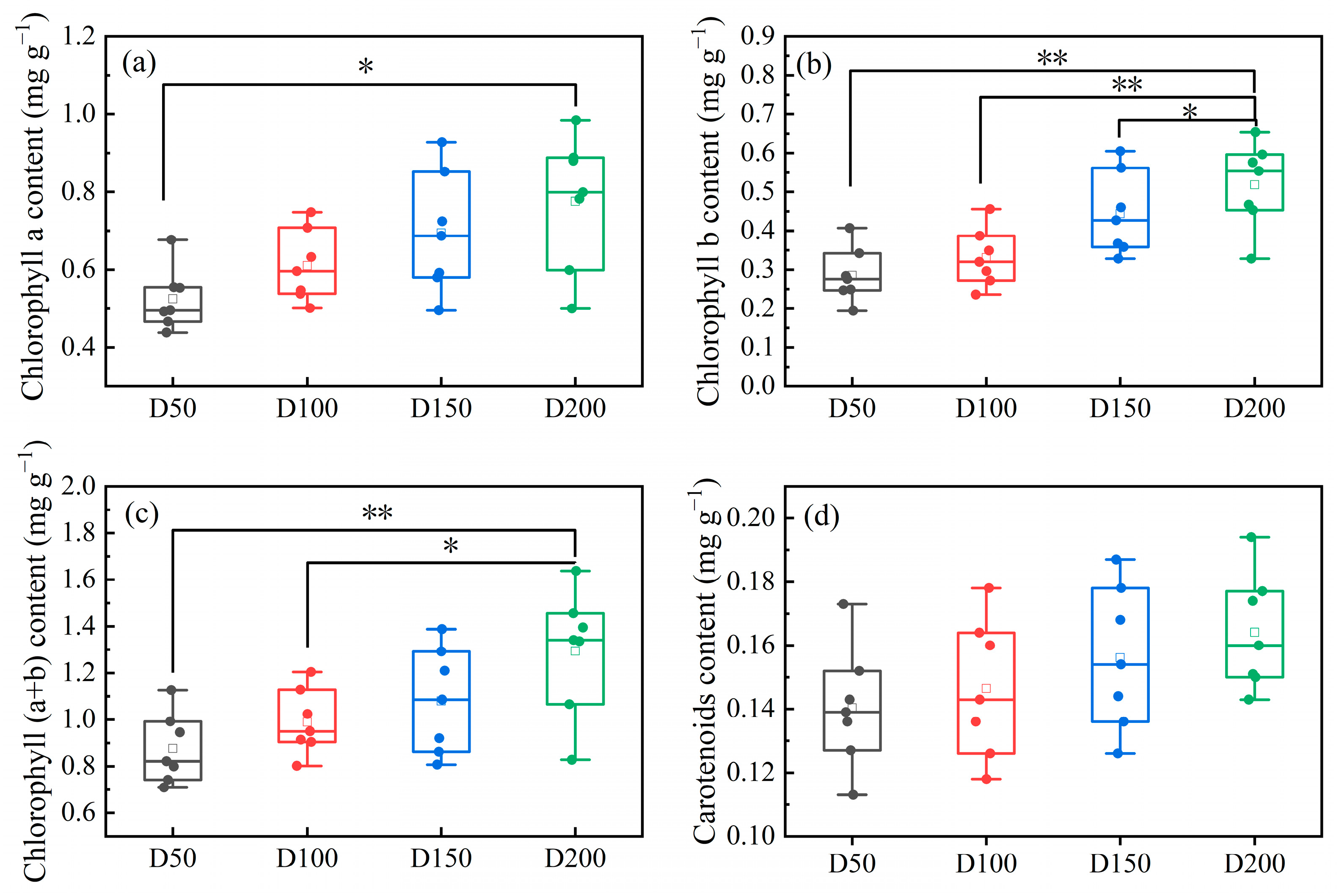
3.2.1. Photosynthesis in Leaves
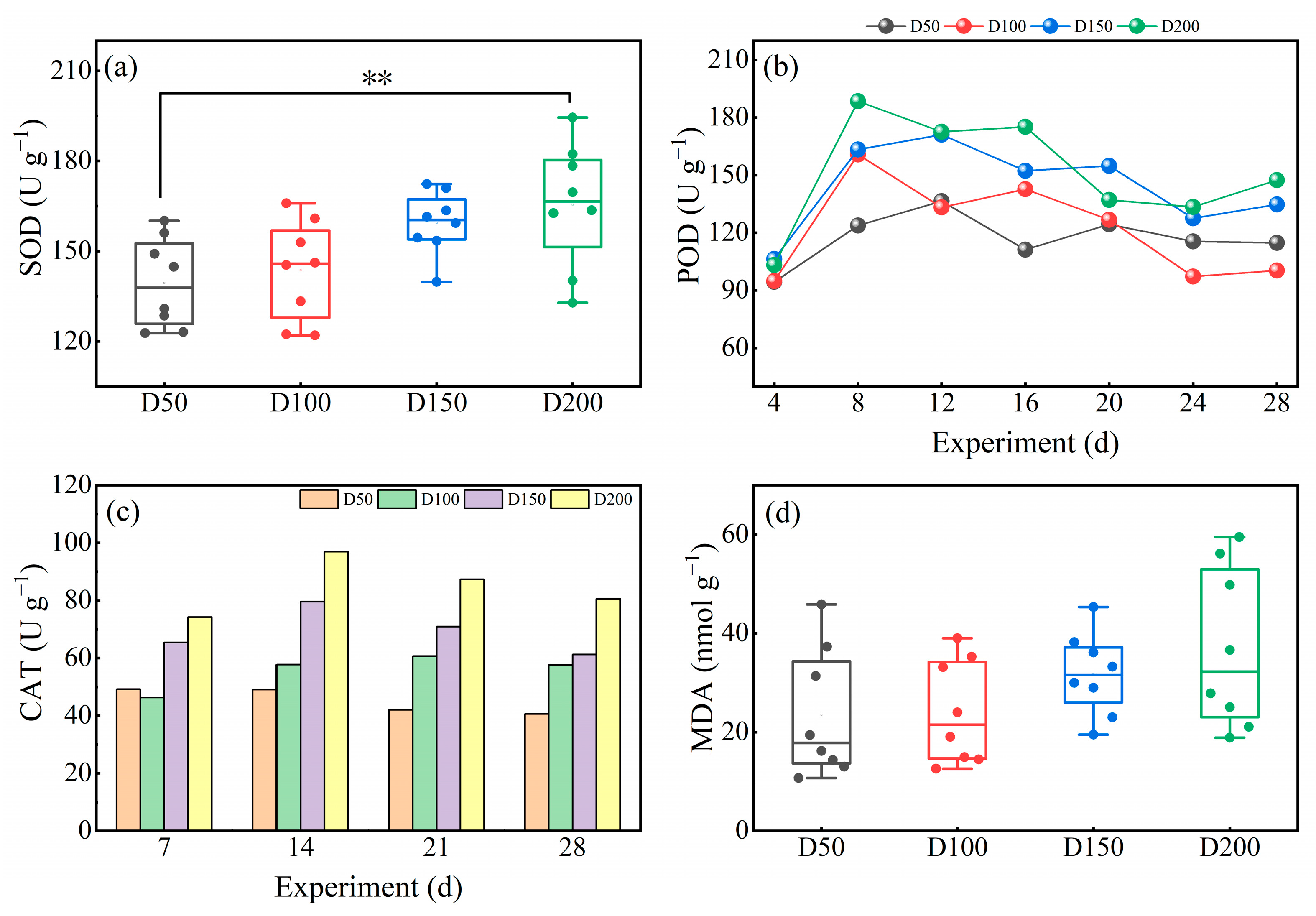
3.2.2. Antioxidant Enzyme Activity in Leaves
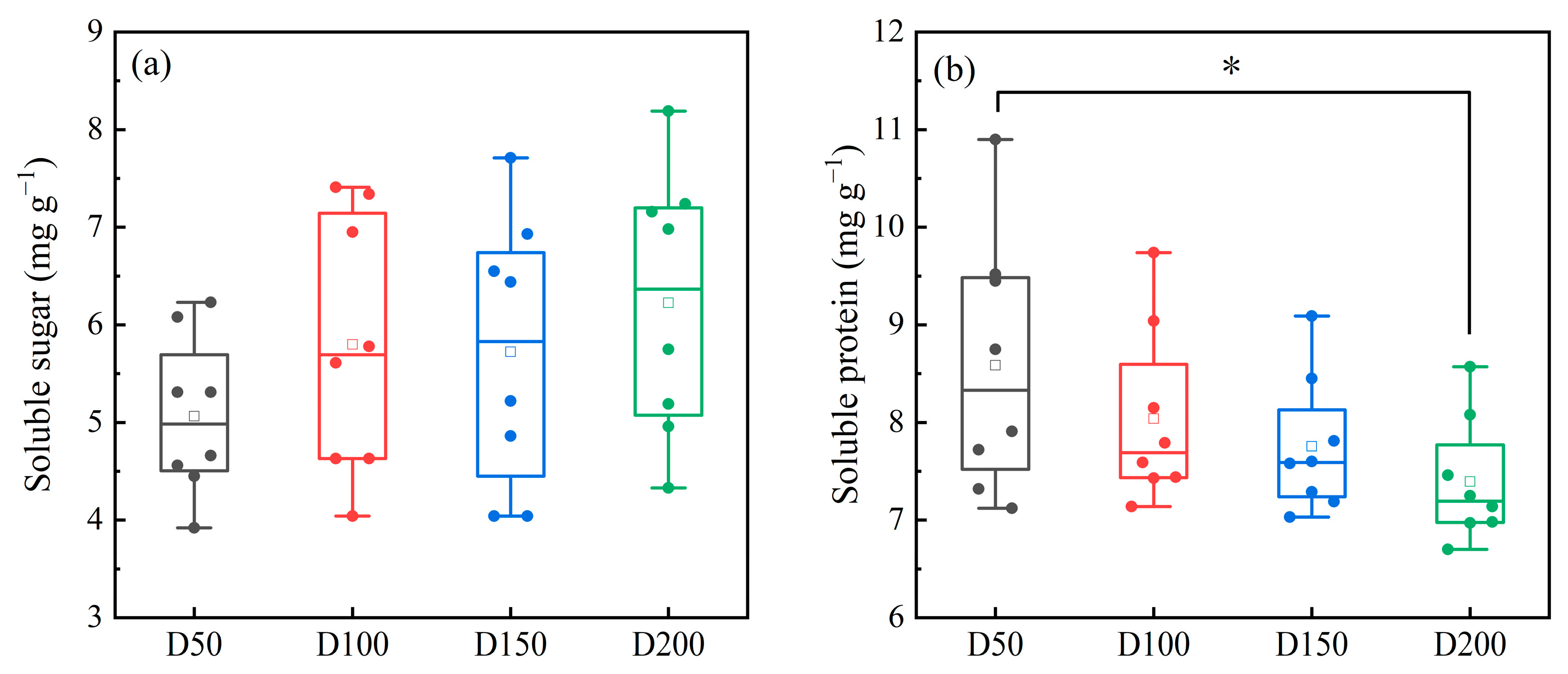
3.2.3. Leaf Secretions
3.3. The Water Restoration Effectiveness of the System
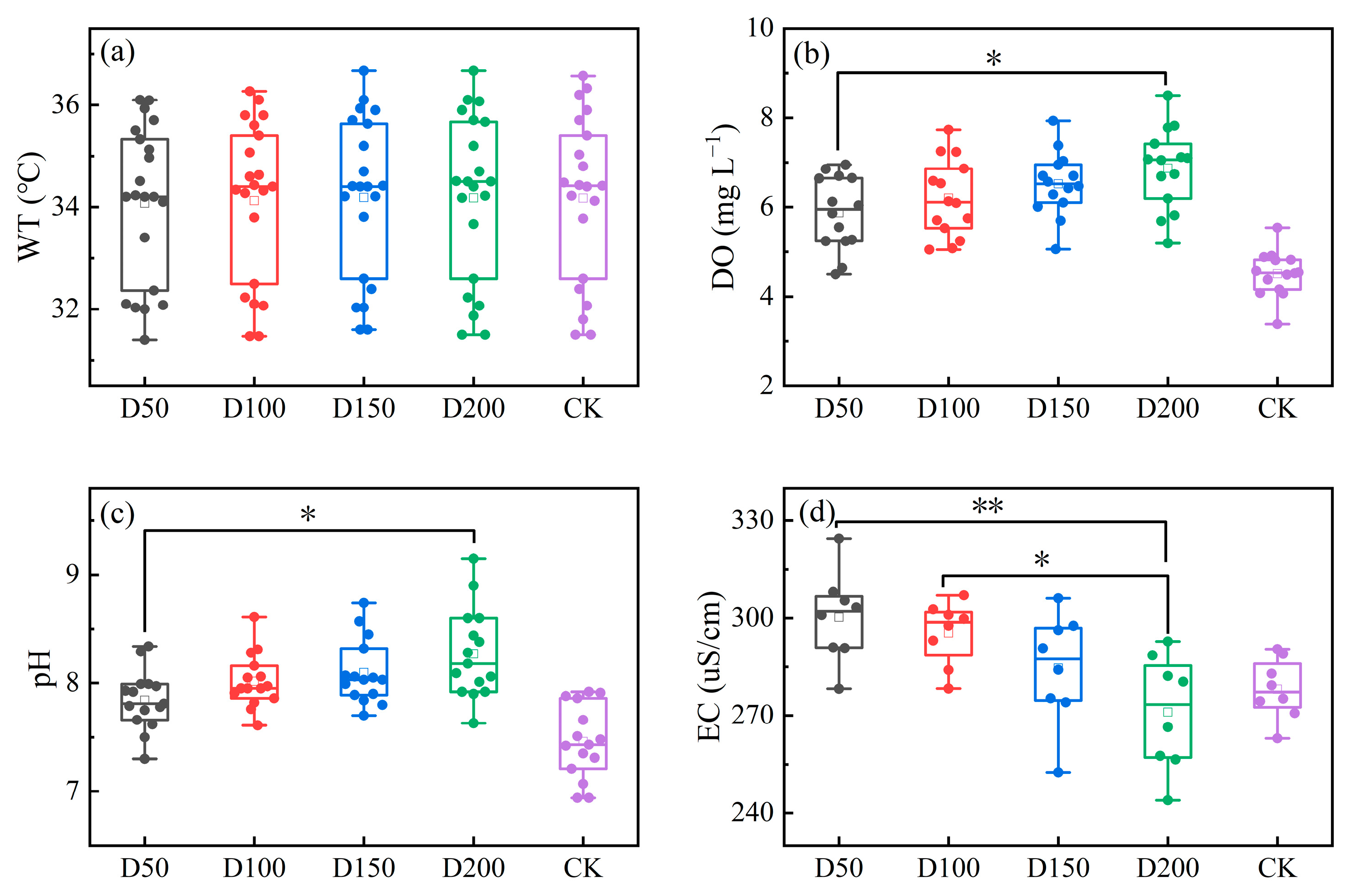
3.3.1. The Physicochemical Environment of the Water Body
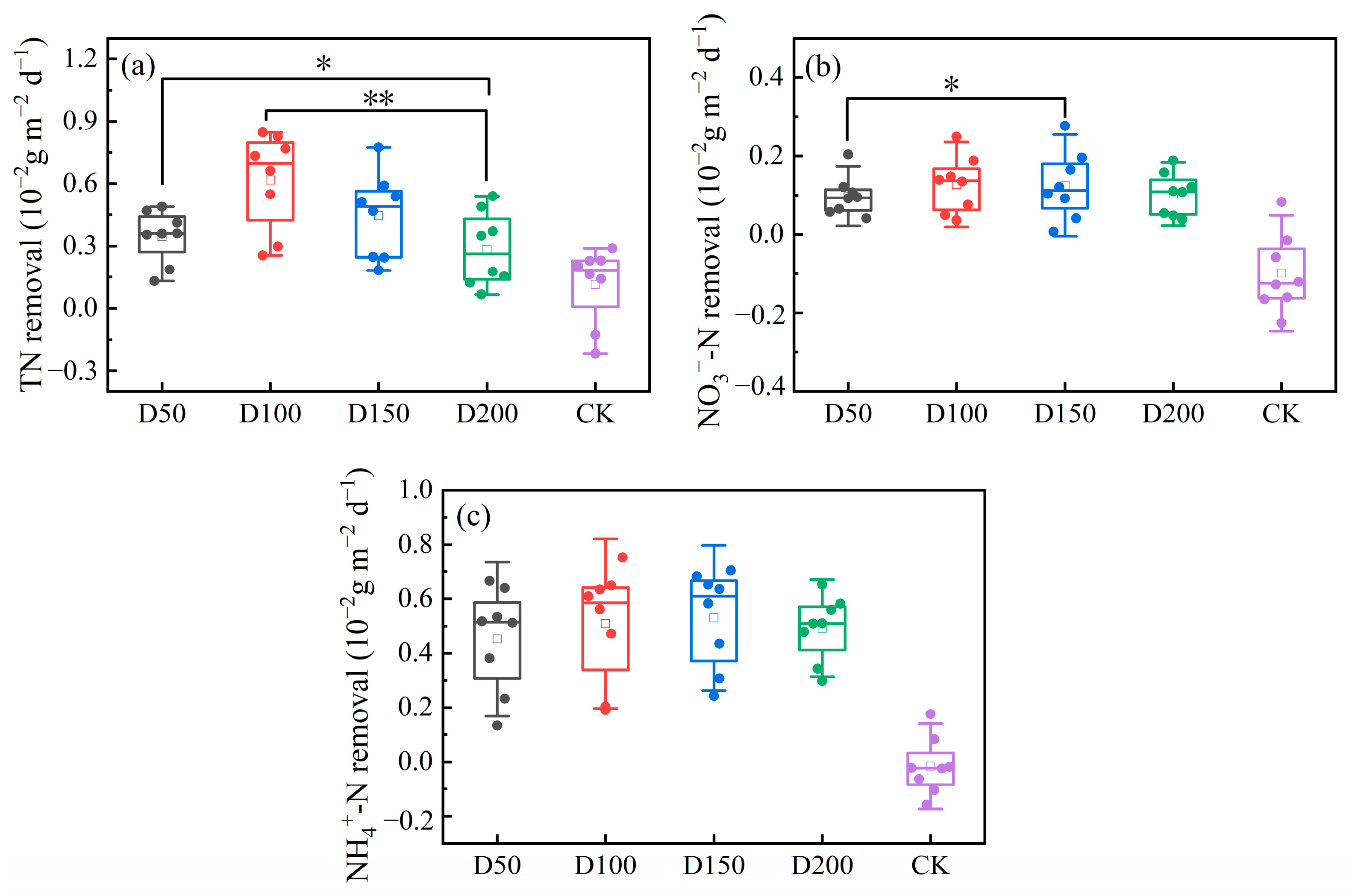
3.3.2. Phosphorus Removal from Water Bodies
3.3.3. Nitrogen Removal from Water Bodies
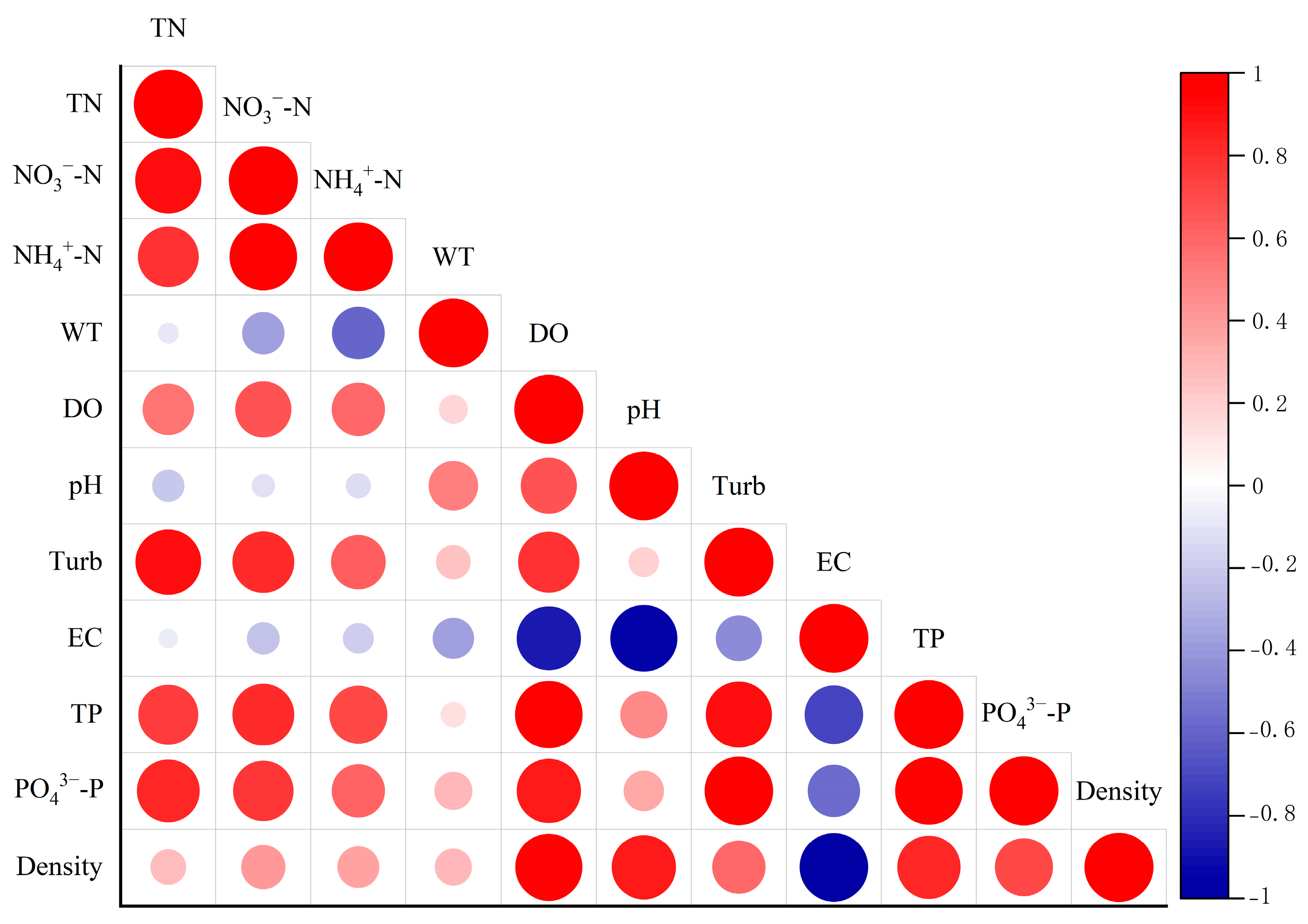
3.3.4. Correlation Between Planting Density and Water Restoration Efficiency
4. Conclusions and Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gomi, T.; Moore, R.; Hassan, M.A. Suspended sediment dynamics in small forest streams of the Pacific Northwest. J. Am. Water Resour. Assoc. 2010, 41, 877–898. [Google Scholar] [CrossRef]
- Walling, D.E.; Fang, D. Recent trends in the suspended sediment loads of the world’s rivers. Glob. Planet. Change 2003, 39, 111–126. [Google Scholar] [CrossRef]
- Barko, J.W.; Gunnison, D.; Carpenter, S.R. Sediment interactions with submersed macrophyte growth and community dynamics. Aquat. Bot. 1991, 41, 41–65. [Google Scholar] [CrossRef]
- Hu, S.; Chen, X.; Huang, X.; Wu, C. Submerged Macrophyte Restoration in Enclosure: A Proper Way for Ecological Remediation of Shallow Lakes? Water 2023, 15, 1317. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, D.; He, R.; Sun, X.; Zeng, J. Enclosure restoration regulates epiphytic microbial communities involved in carbon sequestration in a restored urban lake: A new insight from the stability of dissolved organic matter. J. Clean. Prod. 2025, 501, 145295. [Google Scholar] [CrossRef]
- Nan, J.; Yang, C.; Yu, H.; Shi, X.; Huang, Q.; Huang, J.; Li, J. Enclosure culture ban and its effects on water quality and phytoplankton community in a subtropical shallow lake. Aquac. Res. 2022, 53, 221–231. [Google Scholar] [CrossRef]
- ILEC (International Lake Environment Committee). World Lake Vision Report 2021: Pathways for Sustainable Lake Management; ILEC Foundation: Shiga-ken, Japan, 2021. [Google Scholar]
- Chen, K.N.; Bag, X.M.; Shi, L.X.; Chen, W.M. Ecological restoration engineering in Lake Wuli, Lake Taihu: A large enclosure experiment. J. Lake Sci. 2006, 18, 417–424. [Google Scholar]
- Chinese Academy of Environmental Sciences. Assessment Report on Ecological Restoration Technology of Chinese Lakes (2022); Ministry of Ecology and Environment: Beijing, China, 2023.
- Sondergaard, M.; Jeppesen, E.; Lauridsen, T.L.; Skov, C.; Vannes, E.H.; Roijackers, R.; Lammens, E.; Portielje, R. Lake restoration: Successes, failures and long-term effects. J. Appl. Ecol. 2010, 44, 1095–1105. [Google Scholar] [CrossRef]
- Lin, X.; Wu, X.; Chao, J.; Ge, X.; Tan, L.; Liu, W.; Sun, Z.; Hou, J. Effects of combined ecological restoration measures on water quality and underwater light environment of Qingshan Lake, an urban eutrophic lake in China. Ecol. Indic. 2024, 163, 112107. [Google Scholar] [CrossRef]
- Li, Q.; Wan, P.; Han, C.; Dai, X.; Hua, X.; Wang, Y.; Zhang, K.; Cai, S.; Tian, X. Ecological engineering in a eutrophic lake: A case study of large aquatic macrophyte enclosures in Baima Lake, China. Limnologica 2021, 90, 125907. [Google Scholar] [CrossRef]
- Yin, Y.; Gao, M.; Cao, X.; Wei, J.; Zhong, X.; Li, S.; Peng, K.; Gao, J.; Gong, Z.; Cai, Y. Restore polder and aquaculture enclosure to the lake: Balancing environmental protection and economic growth for sustainable development. Sci. Total Environ. 2024, 933, 173036. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Wang, L.; Li, Y.; Yan, Z.W.; Liu, H.M.; Yu, D.; Liu, C.H. Response of sediment and water microbial communities to submerged vegetations restoration in a shallow eutrophic lake. Sci. Total Environ. 2025, 801, 149634. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.; Finkler, R.; Crossetti, L.O.; David, M.L.; Cardoso, L.; Fragoso, C.R.; Egbert, H. The structuring role of submerged macrophytes in a large subtropical shallow lake: Clear effects on water chemistry and phytoplankton structure community along a vegetated-pelagic gradient. Limnologica 2018, 69, 142–154. [Google Scholar] [CrossRef]
- Xiao, L.T.; Wang, S.G. Experimental Techniques in Plant Physiology; China Agriculture Press: Beijing, China, 2023. [Google Scholar]
- Jia, J.; Wang, Y. Exploring cohesive sediment flocculation with surface heterogeneity: A theoretical Lagrangian-type flocculation model. Water Res. 2024, 250, 121045. [Google Scholar]
- Davies, R.J.; Smith, D.G. Turbidity, suspended sediment, and water clarity: A review. J. Am. Water Resour. Assoc. 2001, 37, 1085–1101. [Google Scholar] [CrossRef]
- Horppila, J.; Nurminen, L. Effects of submerged macrophytes on sediment resuspension and internal phosphorus loading in Lake Hiidenvesi. Hydrobiologia 2003, 506, 217–224. [Google Scholar] [CrossRef]
- Yang, J.Q. Solute flow and particle transport in aquatic ecosystems: A review on the effect of emergent and rigid vegetation. Environ. Sci. Ecotechnol. 2024, 21, 100412. [Google Scholar] [CrossRef]
- Nepf, H.M. Flow and transport in regions with aquatic vegetation. Annu. Rev. Fluid Mech. 2012, 44, 123–142. [Google Scholar] [CrossRef]
- Liu, S.W.; Li, D.X.; Liu, D.C.; Zhang, X.F.; Wang, Z.L. Characteristics of sedimentation and sediment trapping efficiency in the Three Gorges Reservoir, China. Catena 2022, 208, 105715. [Google Scholar] [CrossRef]
- Yusuf, A.; Lei, D.; Sun, Y.Q.; Duan, S.; Zhang, Y.Z. Nitrogen transformation and microbial community interactions in hydrodynamic heterogeneous hyporheic zone sediment: Insights for ecosystem sustainability. Limnologica 2025, 112, 126143. [Google Scholar] [CrossRef]
- Xiao, Y.; Yin, X.J.; Chen, L.J.; Wang, J.; Wang, Y.C.; Liu, G.L.; Hua, Y.M.; Wan, X.Q.; Xiao, N.D.; Zhao, J.W.; et al. Effects of illumination on nirS denitrifying and anammox bacteria in the rhizosphere of submerged macrophytes. Sci. Total Environ. 2020, 760, 143420. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, L.; Zhou, D.W. Root morphological responses to population density vary with soil conditions and growth stages: The complexity of density effects. Ecol. Evol. 2021, 11, 10590–10599. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, M.; Xie, P. Alterations in biomass allocation indicate the adaptation of submersed macrophytes to low-light stress. Ecol. Indic. 2020, 113, 106235. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.G.; Chao, C.X.; Yu, H.W.; Yu, D.; Liu, C.H. Submerged macrophytes successfully restored a subtropical aquacultural lake by controlling its internal phosphorus loading. Environ. Pollut. 2020, 267, 115494. [Google Scholar] [CrossRef] [PubMed]
- Bledsoe, B.P.; Shear, T.H. Vegetation along hydrologic and edaphic gradients in a North Carolina coastal plain creek bottom and implications for restoration. Wetlands 2000, 20, 126–147. [Google Scholar] [CrossRef]
- Wan, L.L.; Chen, X.Y.; Deng, Q.H.; Yang, L.; Li, X.W.; Zhang, J.Y.; Song, C.L.; Zhou, Y.Y.; Cao, X.Y. Phosphorus strategy in bloom-forming cyanobacteria (Dolichospermum and Microcystis) and its role in their succession. Harmful. Algae 2019, 84, 46–55. [Google Scholar] [CrossRef]
- Tóth, V.R. Vertical optical complexity shaped by submerged macrophytes. Sci. Rep. 2025, 14, 5100. [Google Scholar] [CrossRef]
- Lu, J.; Wang, Z.X.; Xing, W.; Liu, G.H. Effects of substrate and shading on the growth of two submerged macrophytes. Hydrobiologia 2013, 700, 157–167. [Google Scholar] [CrossRef]
- Yan, X.; Yu, D.; Wang, H.Y.; Wang, J.W. Response of submerged plant (Vallisneria spinulosa) clones to lead stress in the heterogenous soil. Chemosphere 2006, 63, 1459–1465. [Google Scholar] [CrossRef]
- Huang, Z.J.; Liu, Q.Q.; An, B.; Wu, X.J.; Sun, L.J.; Wu, P.F.; Liu, B.; Ma, X.Q. Effects of planting density on morphological and photosynthetic characteristics of leaves in different positions on Cunninghamia lanceolata saplings. Forests 2021, 12, 853. [Google Scholar] [CrossRef]
- Song, Y.Z.; Kong, F.F.; Xue, Y.; Qin, B.Q. Responses of chlorophyll and MDA of Vallisneria natans to nitrogen and phosphorus availability and epiphytic algae. J. Freshw. Ecol. 2015, 30, 103–110. [Google Scholar] [CrossRef]
- Szabó, S.; Csizmár, A.; Koleszár, G.; Oláh, V.; Birk, S.; Peeters, E.T.H.M. Density-dependent facilitation and inhibition between submerged and free–floating plants. Hydrobiologia 2024, 851, 2749–2760. [Google Scholar] [CrossRef]
- Xie, D.; Yu, D.; You, W.H.; Wang, G.L. Morphological and physiological responses to sediment nutrients in the submerged macrophyte Myriophyllum spicatum. Wetlands 2013, 33, 1095–1102. [Google Scholar] [CrossRef]
- Courbier, S.; Pierik, R. Canopy light quality modulates stress responses in plants. iScience 2019, 22, 441–452. [Google Scholar] [CrossRef] [PubMed]


| Index | TN (mg/L) | NH4+-N (mg/L) | NO3−-N (mg/L) | TP (mg/L) | pH | Turbidity (NTU) |
|---|---|---|---|---|---|---|
| Numeric value | 0.9–1.3 | 0.5–0.7 | 0.4–0.6 | 0.07–0.14 | 6.8–7.8 | 100–110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, W.; Xing, J.; Li, X.; Wu, S. Effects of Planting Density on Water Restoration Performance of Vallisneria spinulosa Yan Growth System Constructed by Enclosure. Water 2025, 17, 1603. https://doi.org/10.3390/w17111603
Sun W, Xing J, Li X, Wu S. Effects of Planting Density on Water Restoration Performance of Vallisneria spinulosa Yan Growth System Constructed by Enclosure. Water. 2025; 17(11):1603. https://doi.org/10.3390/w17111603
Chicago/Turabian StyleSun, Weiguang, Jia Xing, Xinyu Li, and Suqing Wu. 2025. "Effects of Planting Density on Water Restoration Performance of Vallisneria spinulosa Yan Growth System Constructed by Enclosure" Water 17, no. 11: 1603. https://doi.org/10.3390/w17111603
APA StyleSun, W., Xing, J., Li, X., & Wu, S. (2025). Effects of Planting Density on Water Restoration Performance of Vallisneria spinulosa Yan Growth System Constructed by Enclosure. Water, 17(11), 1603. https://doi.org/10.3390/w17111603





