Hydrochemical Characteristics and Dominant Controlling Factors of the Qujiang River Under Dual Natural–Anthropogenic Influences
Abstract
1. Introduction
2. Materials and Methods
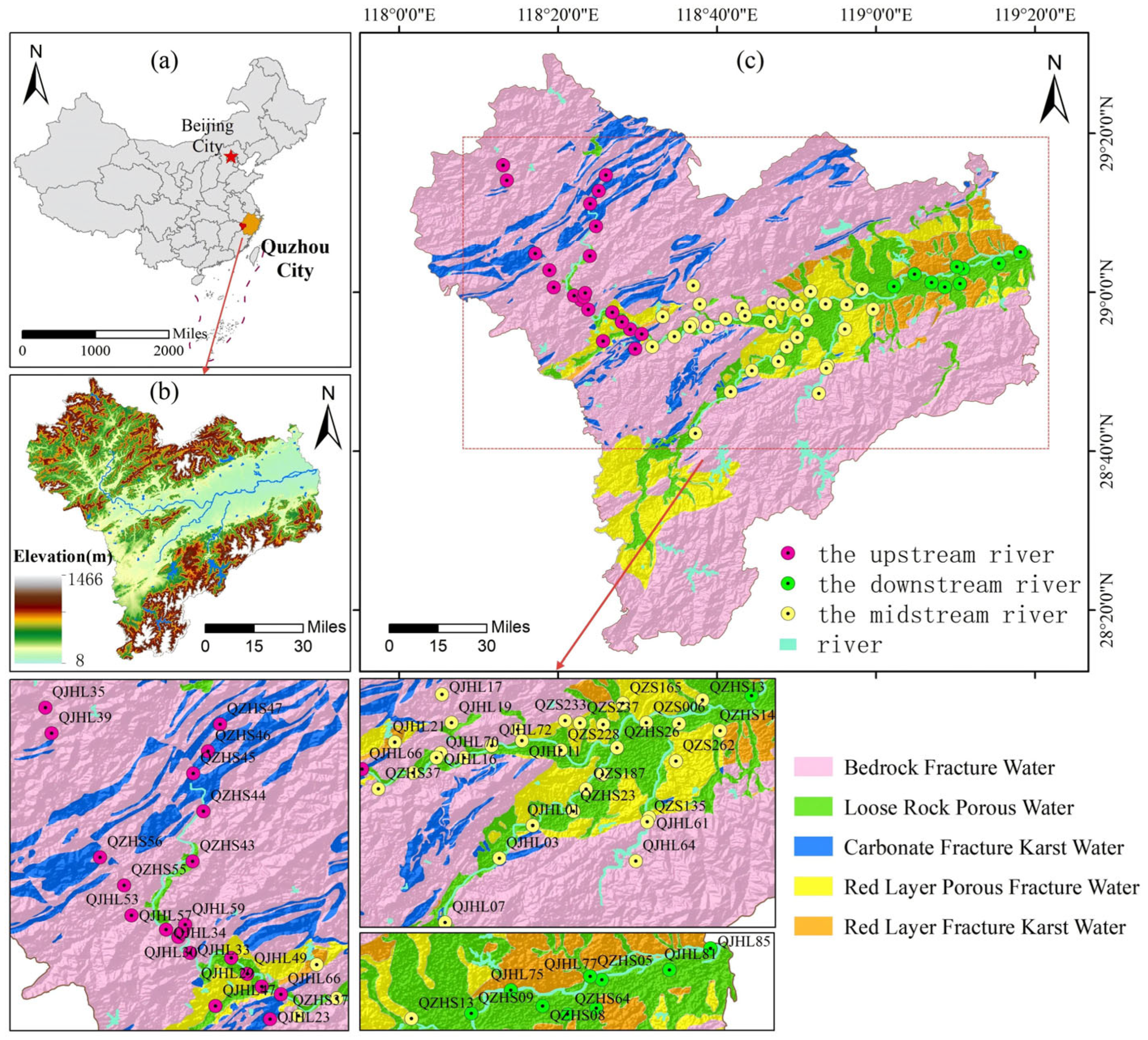
2.1. Overview of the Study Area
2.2. Sample Collection and Testing
3. Results and Discussion
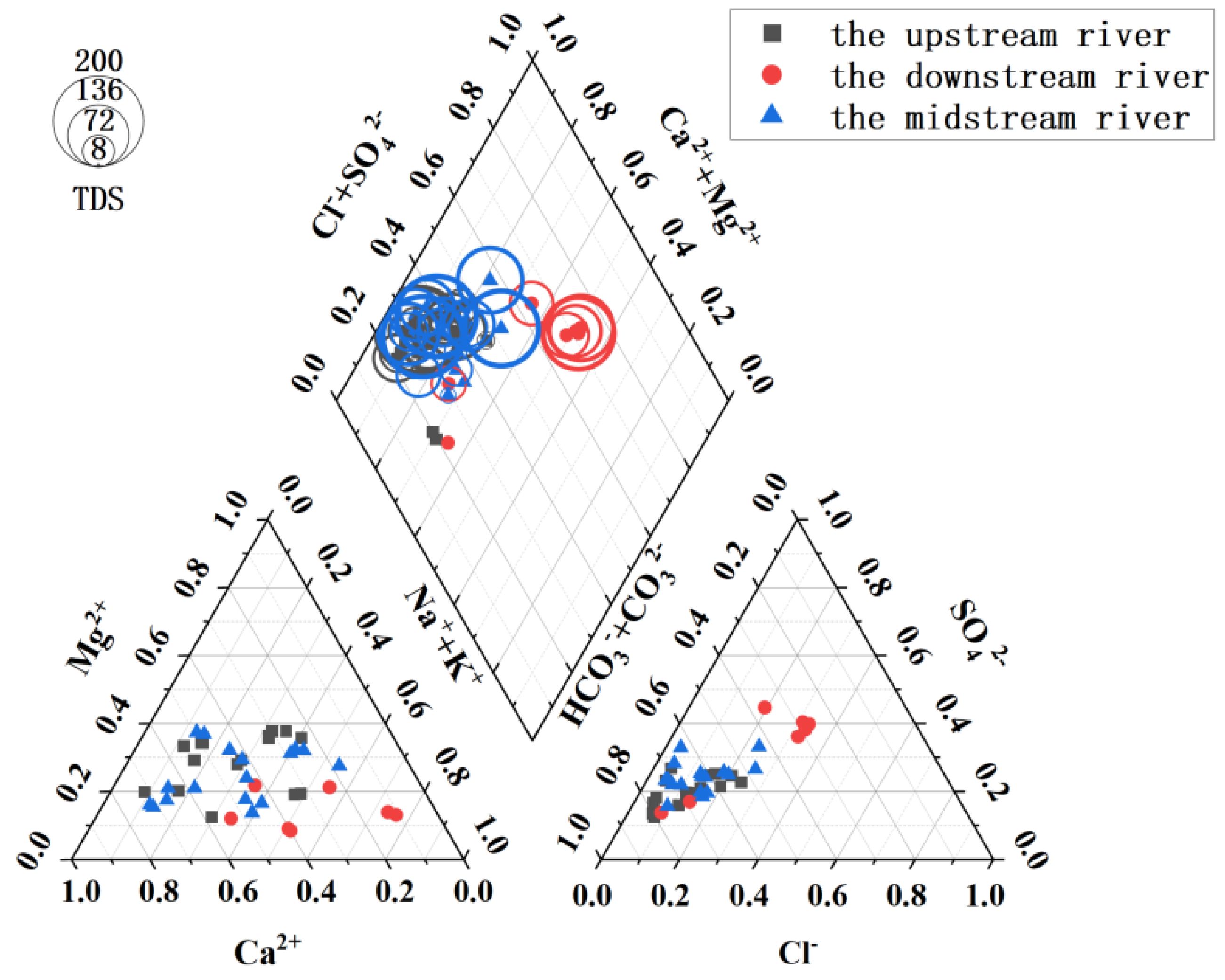
3.1. Hydrochemical Composition Characteristics of Qujiang River Basin
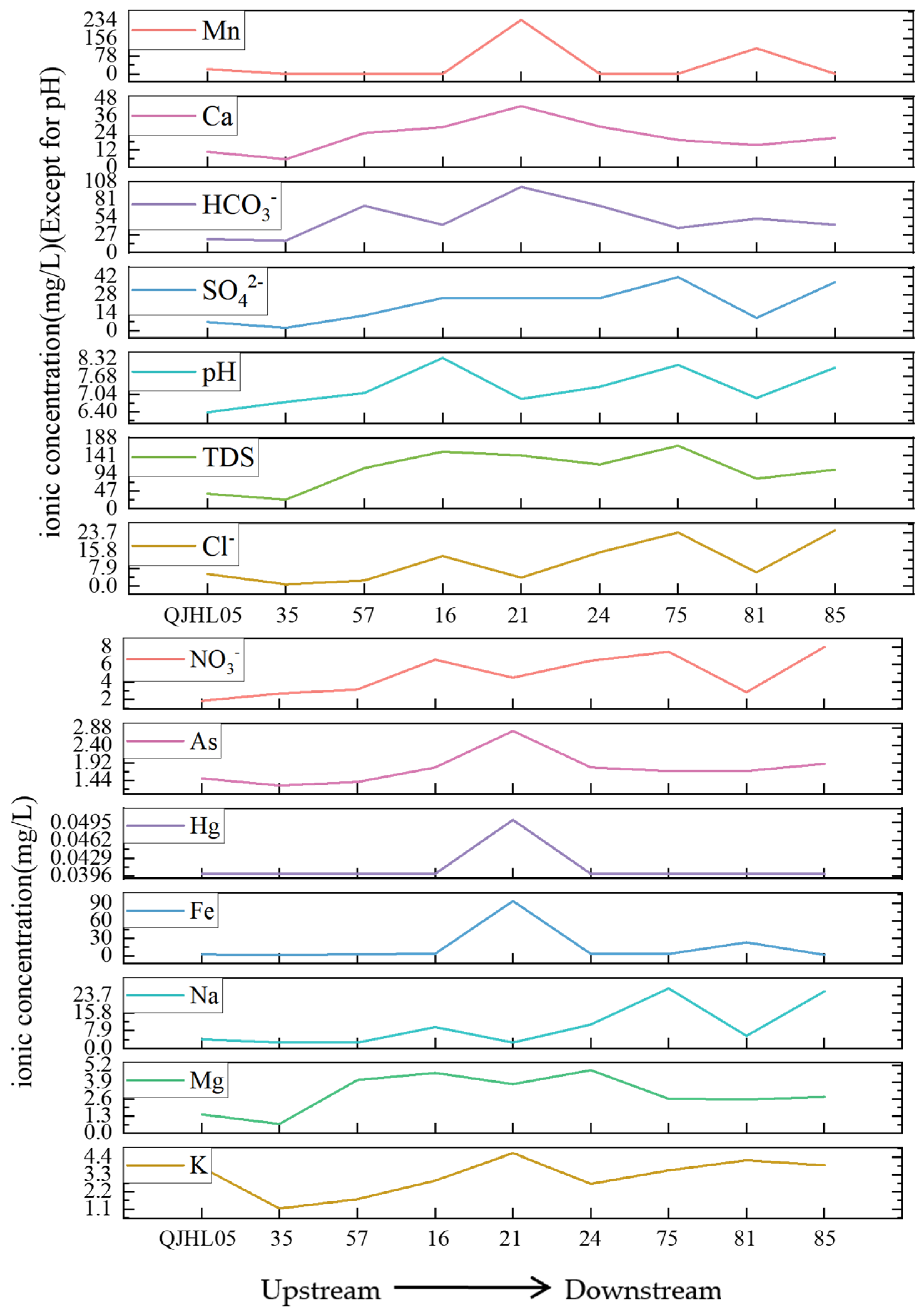
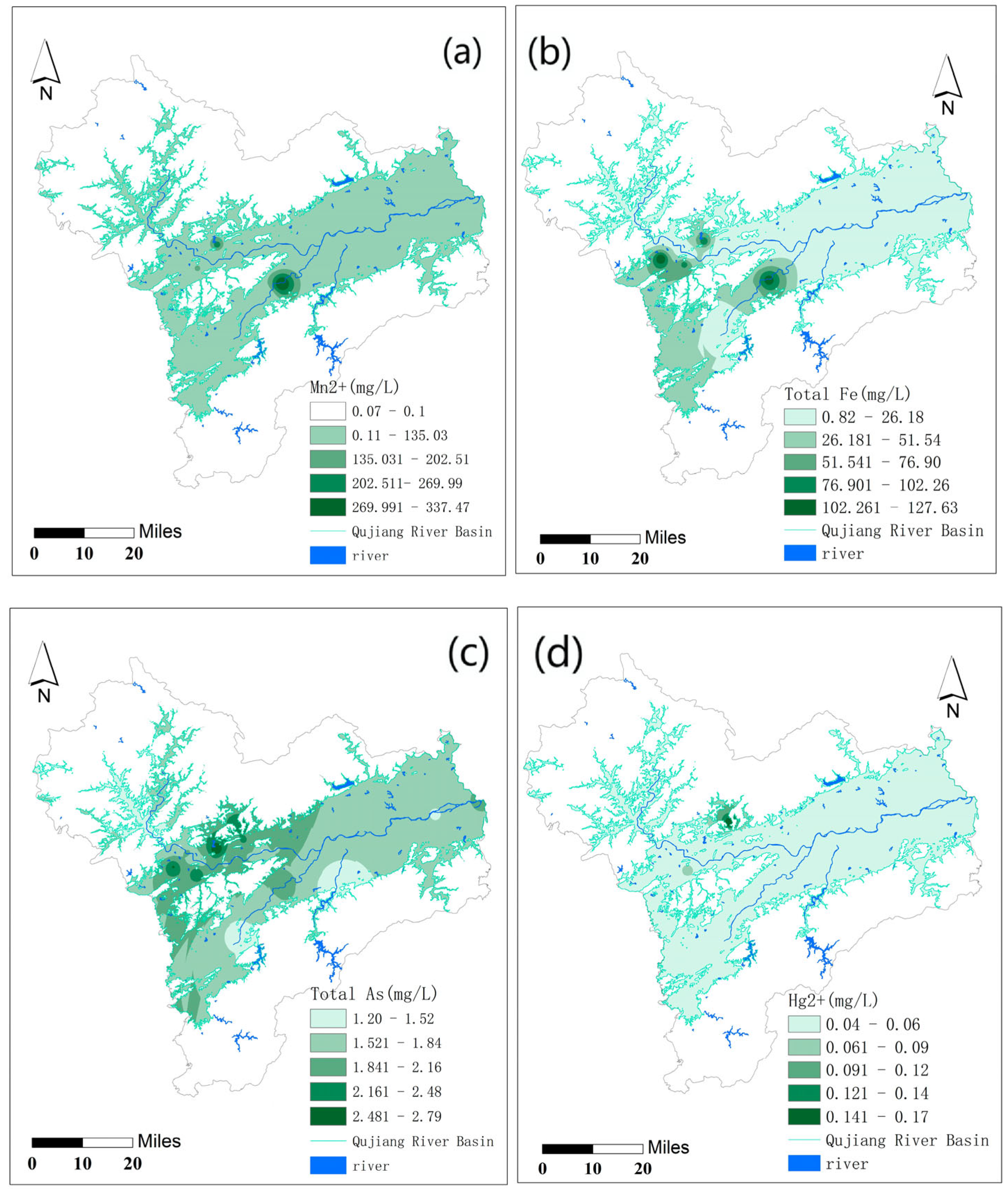
3.2. Spatial Distribution Characteristics of Hydrochemical Components in Qujiang River Water
3.3. Comparative Analysis of the Hydrochemical Composition in the Qujiang River and Global River Systems
3.4. Identification of Hydrochemical Component Sources in the Qujiang River
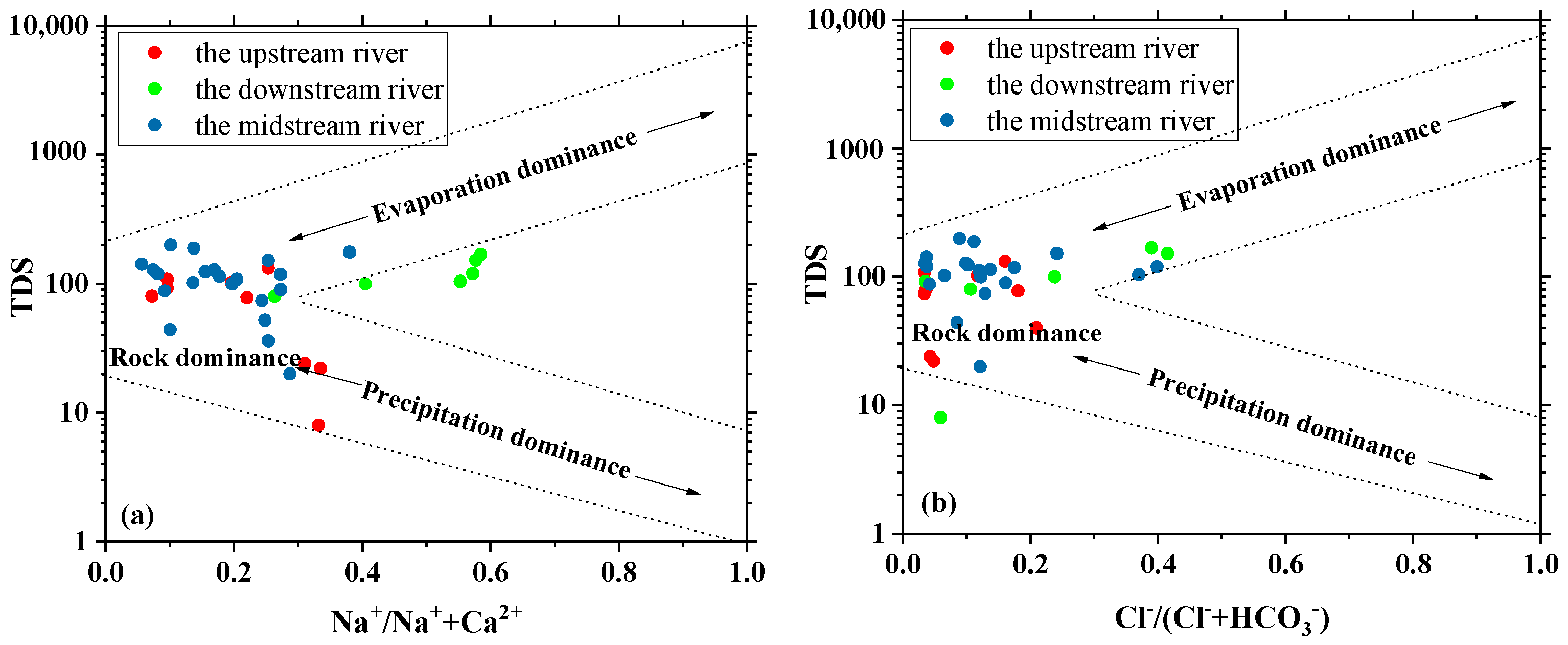
3.4.1. Source Discrimination via Gibbs Diagram Analysis
3.4.2. Hydrochemical Component Source Analysis via the Ion Ratio Method
3.5. Impacts of Anthropogenic Activities on Qujiang River Hydrochemistry
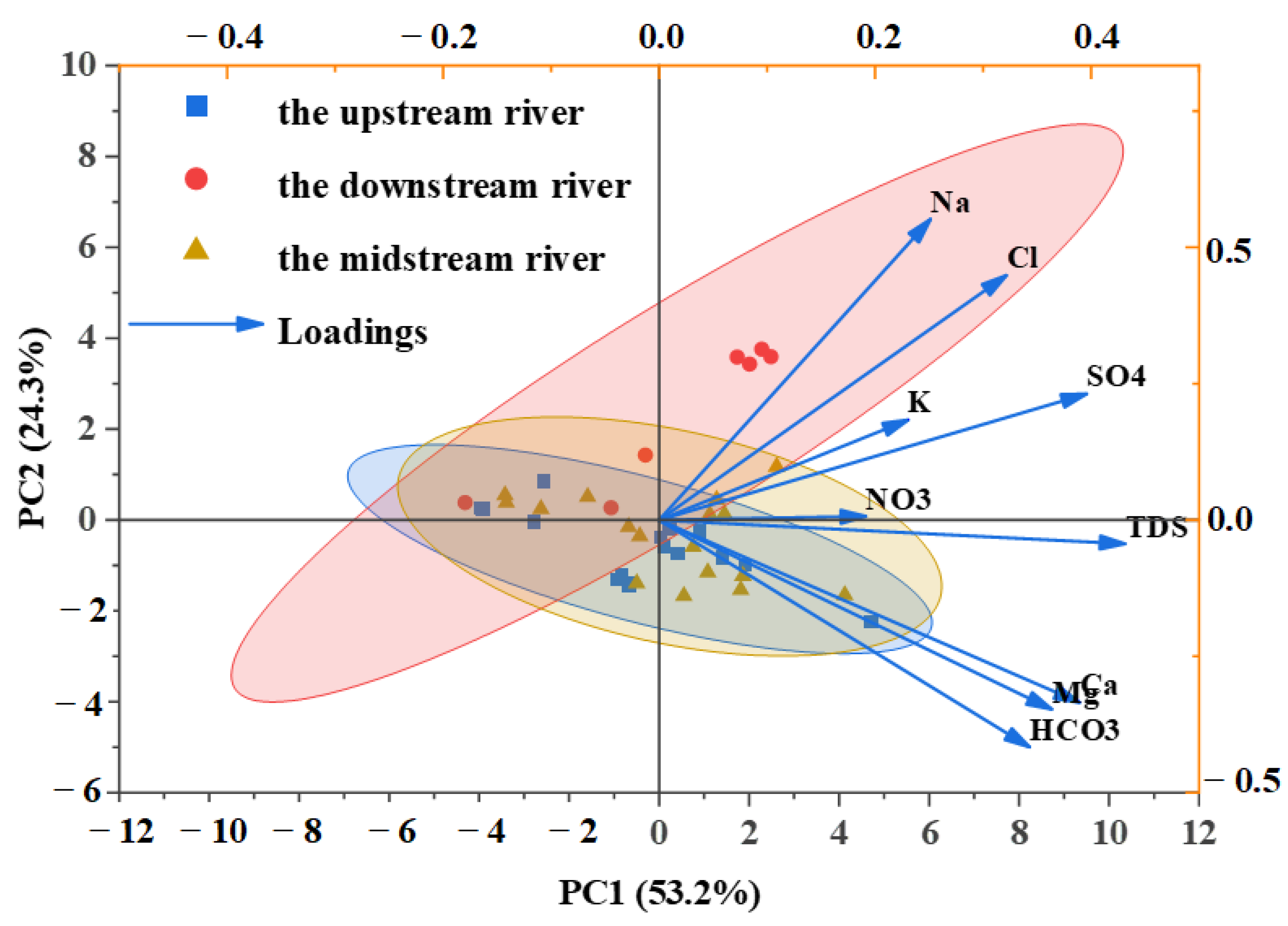
3.6. Identification of Dominant Factors Controlling Qujiang River Hydrochemistry
4. Conclusions
- The data only cover the wet season from July to September 2019, lacking data from the dry season and, thus, making it difficult to reveal the seasonal dynamics of hydrochemical characteristics.
- In the analysis of complex mixed pollution sources, the existing methods are unable to accurately distinguish the contributions from natural and anthropogenic sources, affecting the in-depth analysis of the sources of solutes and the pollution mechanisms.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, L.C.; Cun, D.X.; Tu, C.L.; Ma, Y.Q.; Liu, Z.N.; Yi, L.H.; He, C.G.; Pang, L.; Zhang, Q.D. Hydrochemical Characteristics and Controlling Factors of Surface Water in the Kuaize River Basin, Pearl River Source. China Environ. Sci. 2023, 44, 6025–6037. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, C.; Qiu, X.; Zhou, R.; Yang, Q.; Zhao, R. Hydrochemical Characteristics of Surface Water and Genesis Mechanism of High—Fluoride Water in the Qingshui River Basin, Ningxia. Earth Sci. Front. 2024, 31, 462–473. [Google Scholar] [CrossRef]
- Hu, H.; Wei, R.; Zerizghi, T.; Du, C.; Zhao, C.; Wang, Z.; Zhang, J.; Tan, Q.; Guo, Q. Control mechanisms of water chemistry based on long-term analyses of the Yangtze River. Sci. Total Environ. 2023, 892, 164713. [Google Scholar] [CrossRef]
- Antelmi, M.; Mazzon, P.; Höhener, P.; Marchesi, M.; Alberti, L. Evaluation of MNA in A Chlorinated Solvents-Contaminated Aquifer Using Reactive Transport Modeling Coupled with Isotopic Fractionation Analysis. Water 2021, 13, 2945. [Google Scholar] [CrossRef]
- Ríos-Villamizar, E.A.; Lopes, A.; da Silva, M.D.S.R.; dos Santos, N.A.S.; Oliveira, R.; Calazães, R.M.; Junk, W.J. Water chemistry of rivers and streams from the Jaú and Uatumã basins in central Brazilian Amazon. Sustain. Water Resour. Manag. 2022, 8, 117. [Google Scholar] [CrossRef]
- Khatita, A.M.A.; Geldern, R.V.; Bamousa, A.O.; Alexakis, D.E.; Ismail, E.; Abdellah, W.R.; Babikir, I.A.A. Combining Hydro-Geochemistry and Environmental Isotope Methods to Evaluate Groundwater Quality and Health Risk (Middle Nile Delta, Egypt). Hydrology 2025, 12, 72. [Google Scholar] [CrossRef]
- Zhang, J. Hydropolitics in the Nile River Basin: History and Reality. Arab World Stud. 2019, 42, 62–75. [Google Scholar] [CrossRef]
- Li, J.; Zheng, F.; Wu, C.; Huang, C.; Tang, C.; Peng, Y.; Xu, C.; Yang, H. Hydrochemical Characteristics of River in a Representative Silicate Rock Region under Natural Weathering and Anthropogenic Activities—A Case Study of the Fuhe River in the Middle and Lower Reaches of the Yangtze River. Earth Environ. 2021, 49, 347–357. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, H.; Liu, Z.; Bao, Q.; Chen, B.; Yang, M.; Yan, H.; Li, D.; He, H.; Wei, Y.; et al. Organic carbon source tracing and the BCP effect in the Yangtze River and the Yellow River: Insights from hydrochemistry, carbon isotope, and lipid biomarker analyses. Sci. Total Environ. 2021, 812, 152429. [Google Scholar] [CrossRef]
- Ya, N.; Jun, W.; Na, W.; Zi, H.; Lin, l.; Zhi, Q. Co-occurrence of elevated arsenic and fluoride concentrations in Wuliangsu Lake: Implications for the genesis of poor-quality groundwater in the (paleo-)Huanghe (Yellow River) catchment, China. Water Res. 2024, 258, 121767. [Google Scholar] [CrossRef]
- Cai, X.; Kang, C. Hydrochemical characteristics and water quality evaluation of groundwaterin lower reaches of the Qiantang River. Yangtze River 2023, 54, 27–33. [Google Scholar] [CrossRef]
- Luo, S.; Huang, Z. Systematic Analysis and Application of Spatiotemporal Variation of Water Resources Quality in the Minjiang River Basin. Hydraul. Sci. Technol. 2015, 38, 1–6. [Google Scholar]
- The Largest Area for Harmonious Development Between Humanity and Nature: Interpreting the Productivity Layout and Industrial Belt Development Plan for Jinqu-Lishui Region. Today’s Zhejiang 2005, 13, 26–28.
- Huang, X. Research on the Location Selection and Layout Characteristicsof Traditional Villages Under the Influence of Qujiang River. Master’s Thesis, Zhejiang A&F University, Hangzhou, China, 2024. [Google Scholar] [CrossRef]
- Shi, X.; He, T.; Zhang, Q.; Gao, G. Practice and Exploration of Comprehensive Improvement of the Whole Area and Comprehensive Development and Utilization of Mining Land in the Context of Ecological Civilization Construction—Taking Shang fang Town, Qujiang District, Quzhou City, Zhejiang Province as an Example. Sci. Technol. Innov. 2024, 28, 33–38. [Google Scholar]
- Significant Achievements in Pollution Control of Pig Farming in Qujiang, Zhejiang. Swine. Ind. Outlook 2015, 20, 10. Available online: https://kns.cnki.net/kcms2/article/abstract?v=fSCzX0TVvUg-fiWD8d8QG9LDLez5SrcYK1owmkfVfcMHB1e5WrUhZwZQFFn_Rj9qDMmd-Fk-UuR_4GFu8o4WaDIyigcjlRTZjvEqaBNpAiPpSjT8I_a57w2I9XWIwCU9PTETN4q9T4esWoTAEYPvpONwa0kd9HoDR8T2l-2V1y4=&uniplatform=NZKPT (accessed on 20 May 2025).
- Liu, Y.; Zhang, C.; Jiang, J.; Zhang, Y.; Wang, G.; Xu, L.; Qu, Z. Interaction between Groundwater and Surface Water in the Qujiang River Basin in China: Evidence from Chemical Isotope Measurements. Water 2023, 15, 3932. [Google Scholar] [CrossRef]
- Wang, G. Anomaly Identification and Traceability Analysis of Groundwaterchemical Components in Red Bed Area of Quzhou. Master’s Thesis, Institute of Disaster Prevention, Beijing, China, 2021. [Google Scholar] [CrossRef]
- Zhao, Z.; Gong, X.; Zhang, L.; Jin, M.; Cai, Y.; Wang, X. Riverine transport and water-sediment exchange of polycyclic aromatic hydrocarbons (PAHs) along the middle-lower Yangtze River, China. J. Hazard. Mater. 2021, 403, 123973. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Gui, Y.; Sun, R.; Li, J.; Wu, Q.; Ding, Y.; Chen, K. Chemical characteristics of groundwater and surface water affected by human activities in the upper Jinzi River Basin, China. Sci. Rep. 2025, 15, 9294. [Google Scholar] [CrossRef]
- Liu, H.; Wang, M.; Wang, X.; Su, C.; Jiang, G.; Li, Z.; Ren, F. Hydrochemical characteristics and formation mechanism of shallowgroundwater in Eastern Henan Plain. Saf. Environ. Eng. 2024, 31, 170–179. [Google Scholar] [CrossRef]
- GB 3838-2002; Environmental Quality Standards for Surface Water. China Environmental Science Press: Beijing, China, 2002.
- GB 5749-2022; Hygienic Standards for Drinking Water. Standards Press of China: Beijing, China, 2022.
- Ren, C.; Liu, L. Under the Strong Influence of Human Activities: The Patterns and Controlling Factors of River Water Chemistry Changes—A Case Study of the Lower Yellow River. Water 2024, 16, 1886. [Google Scholar] [CrossRef]
- Lin, L.; Zhang, Y.; Qian, X.; Wang, Y. Hydrochemical Characteristics and Human Health Risk Assessment of Surface Water in the Danjiang River Source Basin of the Middle Route of China’s South-to-North Water Transfer Project. Water 2023, 15, 2203. [Google Scholar] [CrossRef]
- Bai, J.; Yuan, Z.; Su, X. Evolution of river–aquifer disconnections and the migration and transformation of iron and manganese under non-time-varying/time-varying riverbed permeability. Sci. Total Environ. 2024, 949, 175156. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, S.; Che, F.; Dong, X.; Yang, X.; Chen, H.; Huang, W. Arsenic source-sink dynamics under phosphorus competition in sediments from river–lake connected systems. Environ. Sci. Process. Impacts 2025, 27, 706–717. [Google Scholar] [CrossRef]
- Mao, L.; Ren, W.; Liu, X.; He, M.; Lin, C.; Zhong, Y.; Tang, Y.; Ouyang, W. Tracking the multiple Hg sources in sediments in a typical river-lake basin by isotope compositions and mixing models. J. Hazard. Mater. 2023, 459, 132166. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.; Wang, H.; Zhai, T.; Liu, L.; Li, G.; Xu, Z. Spatial patterns in water quality and source apportionment in a typical cascade development river southwestern China using PMF modeling and multivariate statistical techniques. Chemosphere 2023, 311, 137139. [Google Scholar] [CrossRef]
- Neal, C.; Neal, M.; Hill, L.; Wickham, H. The water quality of the River Thame in the Thames Basin of south/south-eastern England. Sci. Total Environ. 2006, 360, 254–271. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.J. Mechanisms Controlling World Water Chemistry. Science 1970, 170, 1088–1090. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Xu, J.; Peng, J. Spatial Hydrochemical Characteristics and Controlling Factors of Surface Water in the Yancheng Area. China Environ. Sci. 2021, 42, 4772–4780. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Kang, C.; Xi, J.; Ding, L.; Zhao, Y.; Zhao, Z. Hydrochemical characteristics and genesis analysis of surface waterin the Jushui River basin (Hancheng City section). South North Water Transf. Water Sci. Technol. 2025, 23, 410–420. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Q.; Wang, H. Rapid Urbanization Has Changed the Driving Factors of Groundwater Chemical Evolution in the Large Groundwater Depression Funnel Area of Northern China. Water 2023, 15, 2917. [Google Scholar] [CrossRef]
- Wang, H.; Guo, X.; Zhang, Q.; Li, B. Evolution of groundwater hydrochemical characteristics and originanalysis in Hutuo River Basin. Environ. Chem. 2021, 40, 3838–3845. [Google Scholar]
- Xing, J.; Wang, L.; Zhao, J.; Zhai, T. Hydrochemical variation characteristics and driving factors of surface water in arid Areas—A case study of Beichuan River in Northwest China. Front. Environ. Sci. 2024, 12, 1493390. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, L. Research and Practice on Systematic Management of Mountains, Rivers, Forests, Fields, Lakes, Grasslands, and Sands in the Northwest Arid Zone—Taking the Tarim River Important Headwaters Area as an Example. Front. Environ. Sci. 2022, 35, 13–18+63. [Google Scholar] [CrossRef]




| Parameters | Determination Methods |
|---|---|
| pH, Eh, TDS, DO, EC | Portable multimeter (HQ40d, HACH, Shanghai, China) |
| HCO3− | Titration |
| K+, Na+, Ca2+, Mg2+, SO42−, NO3−, Cl− | Ion chromatography (IC, Metrohm 930 Compact) and UV-Vis spectrophotometry (Shimadzu UV-2600, Shimadzu Corporation, Kyoto, Japan) |
| TN | Alkaline persulfate digestion–UV spectrophotometry (HJ 636-2012) |
| TP | Ammonium molybdate spectrophotometry after sulfuric acid digestion (HJ 670-2013) |
| CODMn | Dichromate reflux method (HJ 828-2017) |
| Parameters | pH | K+ | Na+ | Ca2+ | Mg2+ | Cl− | SO42− | HCO3− | NO3− | TDS | Mn | Fe | As | Hg | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Upstream | Min (mg/L) | 6.39 | 1.13 | 2.04 | 5.64 | 0.70 | 0.81 | 2.45 | 18.10 | 1.86 | 22.00 | 0.12 | 0.82 | 1.30 | 0.04 |
| Max (mg/L) | 7.15 | 2.08 | 9.07 | 26.72 | 4.80 | 13.14 | 19.96 | 73.60 | 8.61 | 132.00 | 5.20 | 2.31 | 1.50 | 0.04 | |
| Mean (mg/L) | 6.91 | 1.68 | 4.42 | 20.52 | 3.55 | 5.44 | 12.90 | 54.28 | 4.05 | 79.75 | 1.23 | 1.83 | 1.45 | 0.04 | |
| SD (mg/L) | 0.23 | 0.32 | 2.58 | 8.65 | 1.65 | 4.75 | 6.53 | 21.93 | 2.08 | 38.95 | 1.74 | 0.63 | 0.08 | 0.00 | |
| CV (%) | 3.41% | 18.91% | 58.46% | 42.18% | 46.68% | 87.36% | 50.58% | 40.39% | 51.40% | 48.84% | 141.3% | 34.52% | 5.21% | 0.00% | |
| Midstream | Min (mg/L) | 6.31 | 1.63 | 1.34 | 7.18 | 0.68 | 1.35 | 4.96 | 18.10 | 0.02 | 20.00 | 0.07 | 0.82 | 1.20 | 0.04 |
| Max (mg/L) | 8.83 | 5.21 | 16.80 | 51.73 | 8.34 | 21.17 | 34.14 | 126.70 | 13.39 | 200.00 | 337.51 | 127.64 | 2.80 | 0.17 | |
| Mean (mg/L) | 7.04 | 3.01 | 5.57 | 25.85 | 3.90 | 7.52 | 18.35 | 62.31 | 5.24 | 109.71 | 42.76 | 22.83 | 1.84 | 0.05 | |
| SD (mg/L) | 0.64 | 1.04 | 3.68 | 11.57 | 1.78 | 5.26 | 9.00 | 28.05 | 3.63 | 47.96 | 85.21 | 39.56 | 0.40 | 0.03 | |
| CV (%) | 9.18% | 34.4% | 66.10% | 44.75% | 45.65% | 69.98% | 49.06% | 45.04% | 69.21% | 43.70% | 199.3% | 173.3% | 21.76% | 64.86% | |
| Downstream | Min (mg/L) | 6.85 | 3.36 | 5.53 | 15.45 | 2.57 | 6.16 | 10.08 | 30.20 | 2.34 | 80.00 | 0.09 | 0.94 | 1.20 | 0.04 |
| Max (mg/L) | 8.10 | 4.21 | 26.78 | 20.57 | 2.77 | 24.79 | 41.86 | 51.90 | 8.02 | 168.00 | 112.64 | 22.80 | 2.10 | 0.06 | |
| Mean (mg/L) | 7.46 | 3.76 | 17.19 | 17.82 | 2.66 | 16.07 | 29.83 | 40.43 | 5.17 | 113.00 | 33.29 | 6.32 | 1.68 | 0.04 | |
| SD (mg/L) | 0.68 | 0.37 | 10.55 | 2.40 | 0.08 | 9.65 | 14.12 | 9.10 | 2.30 | 38.14 | 43.87 | 8.18 | 0.28 | 0.01 | |
| CV (%) | 9.11% | 9.90% | 61.37% | 13.45% | 3.16% | 60.10% | 47.34% | 22.52% | 58.07% | 33.75% | 131.8% | 129.3% | 16.50% | 16.64% | |
| River | K+ + Na+ | Ca2+ | Mg2+ | SO42− | Cl− | NO3− |
|---|---|---|---|---|---|---|
| Qujiang | 10.21 | 23.41 | 3.59 | 19.32 | 8.70 | 4.89 |
| Yangtze River [8] | 8.20 | 34.1 | 7.60 | 11.7 | 2.90 | 3.44 |
| Lancang River [27] | 20.6 | 48.17 | 10.72 | 63.01 | 24.8 | 2.52 |
| Nile Delta [6] | 44.8 | 40.0 | 16.6 | 30.1 | 36.4 | 20.1 |
| Thames River [28] | 9.40 | 39.0 | 124 | 5.60 | 73.3 | 21.9 |
| Parameters | PC1 | PC2 | PC3 |
|---|---|---|---|
| K+ | 0.23 | −0.04 | 0.41 |
| Na+ | 0.24 | −0.44 | 0.29 |
| Ca2+ | 0.39 | 0.25 | −0.16 |
| Mg2+ | 0.37 | 0.20 | −0.31 |
| HCO3− | 0.35 | 0.29 | −0.24 |
| Cl− | 0.31 | −0.38 | 0.21 |
| SO42− | 0.39 | −0.18 | 0.12 |
| NO3− | 0.18 | −0.13 | −0.25 |
| Fe | −0.01 | 0.39 | 0.38 |
| Mn | 0.06 | 0.31 | 0.46 |
| Hg | 0.07 | 0.10 | −0.13 |
| As | 0.05 | 0.41 | 0.27 |
| TDS | 0.43 | 0.02 | 0.01 |
| Characteristic value | 4.82 | 2.64 | 1.75 |
| Contribution rate (%) | 37.10 | 20.29 | 13.47 |
| Cumulative contribution rate (%) | 37.10 | 57.39 | 70.86 |
| River Section | Principal Component Contribution | Proportion of Natural Factors | Proportion of Human Activities | Characteristic Ions |
|---|---|---|---|---|
| Upstream | PC2 (41%) | 78% | 22% | Ca2+, HCO3− |
| Midstream | PC1 (53%) | 52% | 48% | SO42−, NO3− |
| Downstream | PC1 (67%) | 34% | 66% | Na+, Cl− |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, Y.; Xu, L.; Jiang, J.; Zhang, C.; Wang, G.; Liu, Y.; Zhang, C.; Tian, D. Hydrochemical Characteristics and Dominant Controlling Factors of the Qujiang River Under Dual Natural–Anthropogenic Influences. Water 2025, 17, 1581. https://doi.org/10.3390/w17111581
Li X, Zhang Y, Xu L, Jiang J, Zhang C, Wang G, Liu Y, Zhang C, Tian D. Hydrochemical Characteristics and Dominant Controlling Factors of the Qujiang River Under Dual Natural–Anthropogenic Influences. Water. 2025; 17(11):1581. https://doi.org/10.3390/w17111581
Chicago/Turabian StyleLi, Xiao, Ying Zhang, Liangliang Xu, Jiyi Jiang, Chaoyu Zhang, Guanghao Wang, Yi Liu, Chengfu Zhang, and Dengke Tian. 2025. "Hydrochemical Characteristics and Dominant Controlling Factors of the Qujiang River Under Dual Natural–Anthropogenic Influences" Water 17, no. 11: 1581. https://doi.org/10.3390/w17111581
APA StyleLi, X., Zhang, Y., Xu, L., Jiang, J., Zhang, C., Wang, G., Liu, Y., Zhang, C., & Tian, D. (2025). Hydrochemical Characteristics and Dominant Controlling Factors of the Qujiang River Under Dual Natural–Anthropogenic Influences. Water, 17(11), 1581. https://doi.org/10.3390/w17111581





