Impact of Diverse Calcite Vein Patterns on Dissolution Characteristics of Triassic Limestone in Three Gorges Reservoir Area
Abstract
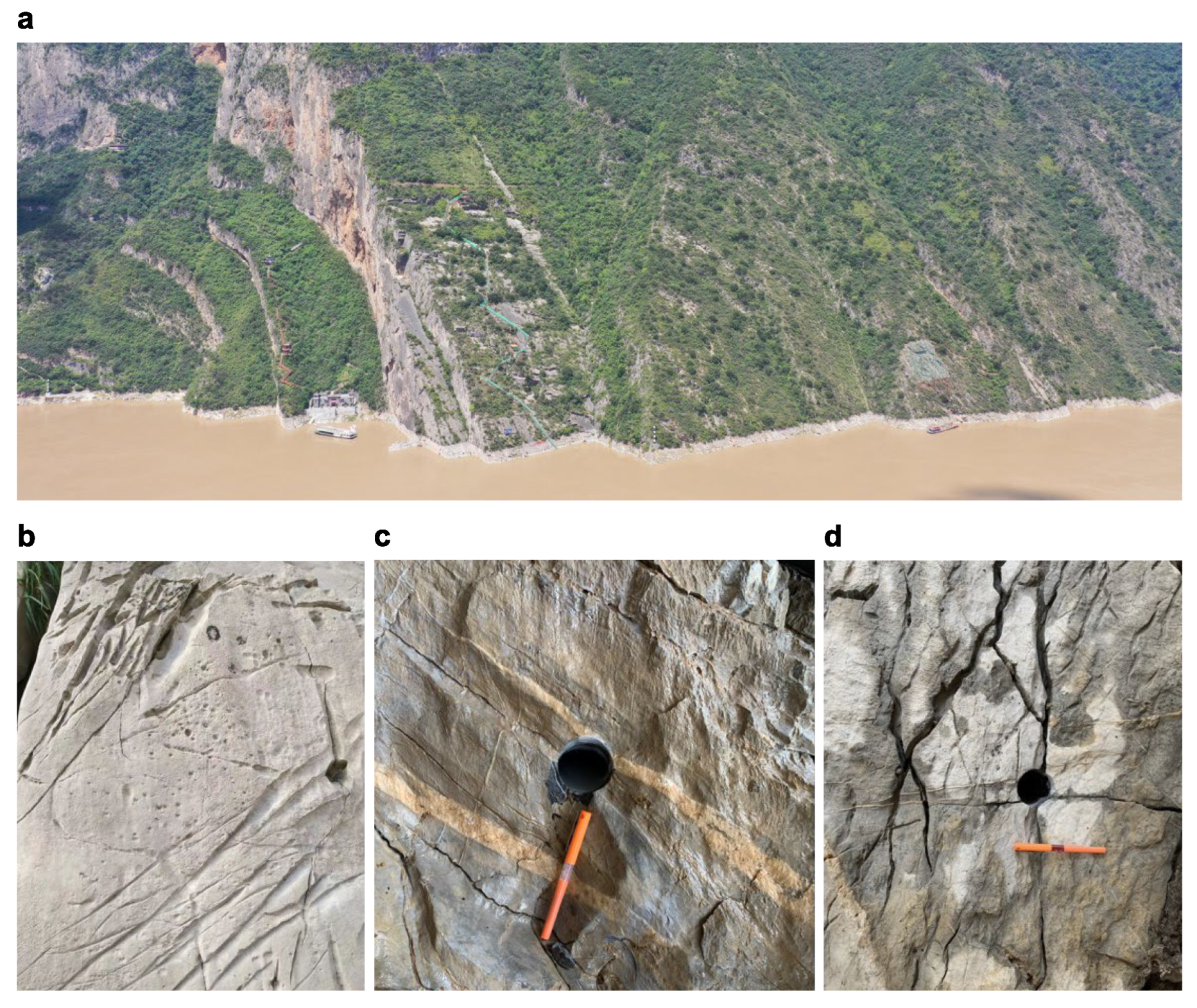
1. Introduction
2. Experiment
2.1. Limestone Sample Preparation
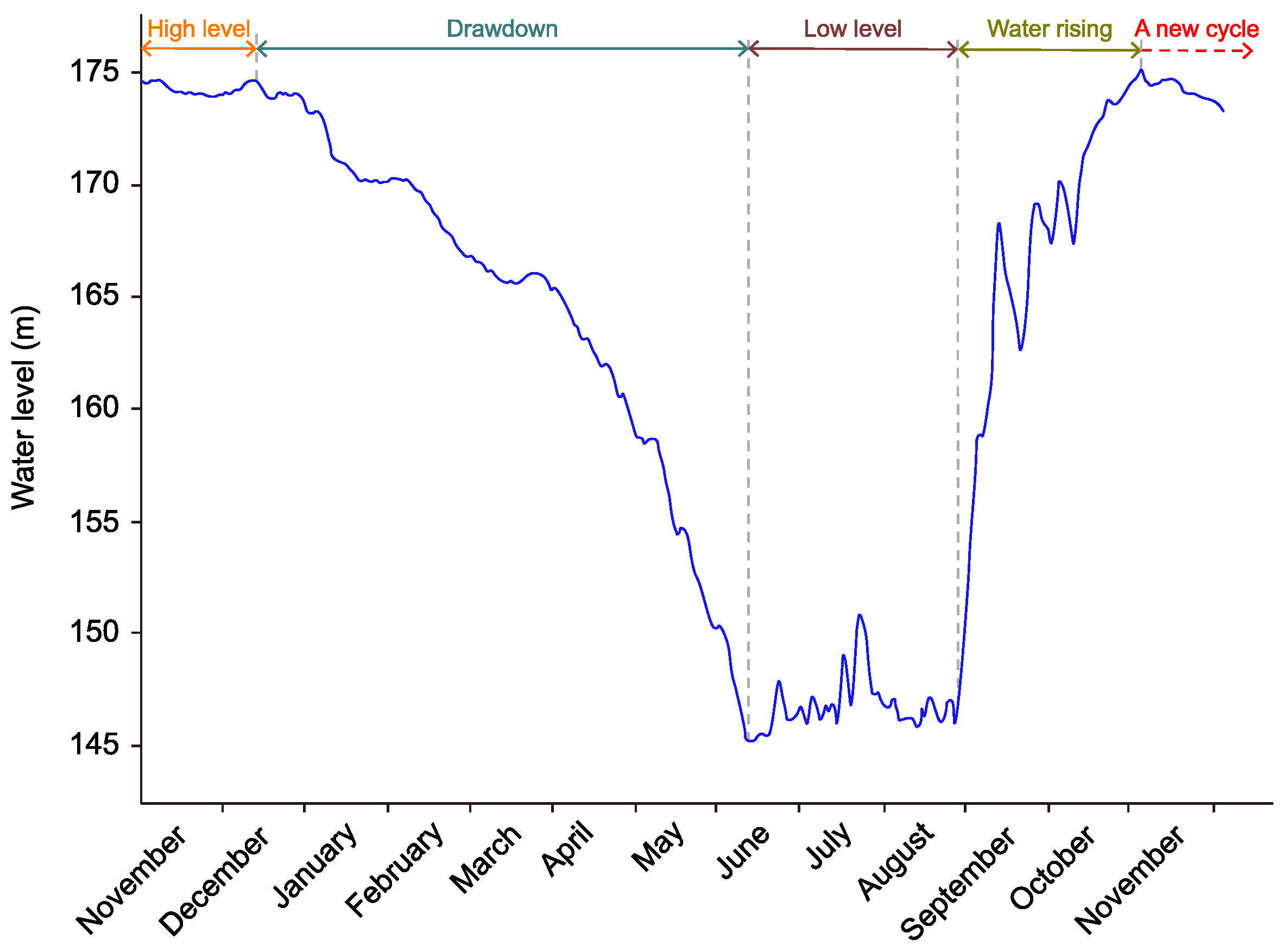
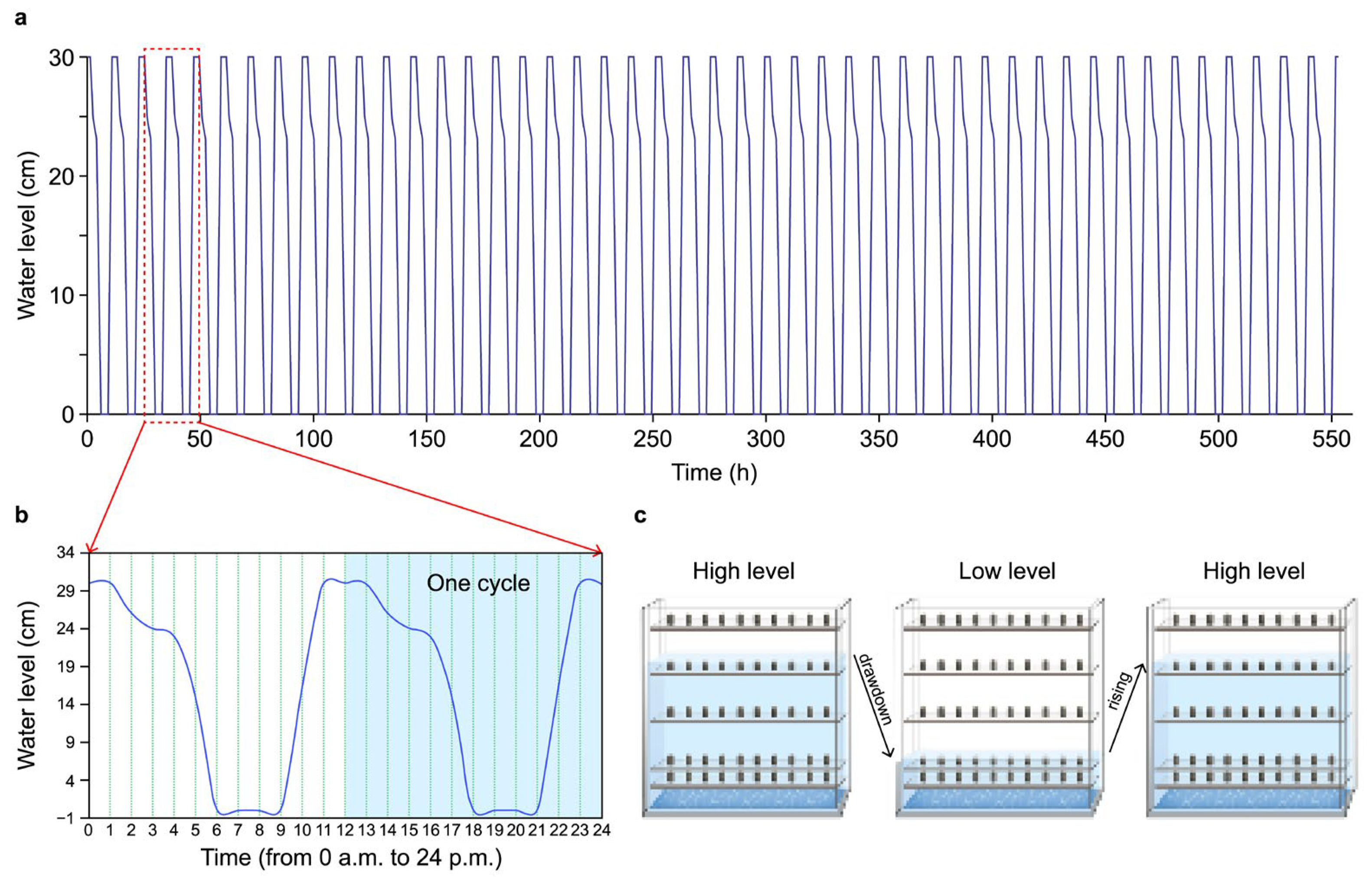
2.2. Testing Apparatus and Method
2.2.1. Sulfuric Acid Solution–Limestone Reaction Test
2.2.2. Water Absorption Test Under Normal Pressure and Temperature (NPT) Conditions
2.2.3. Microstructure Imaging
2.2.4. Mineral Composition Analysis
2.2.5. Fractal Dimension Analysis
2.2.6. Morphological Characterization and Roundness Quantification
3. Results and Analysis
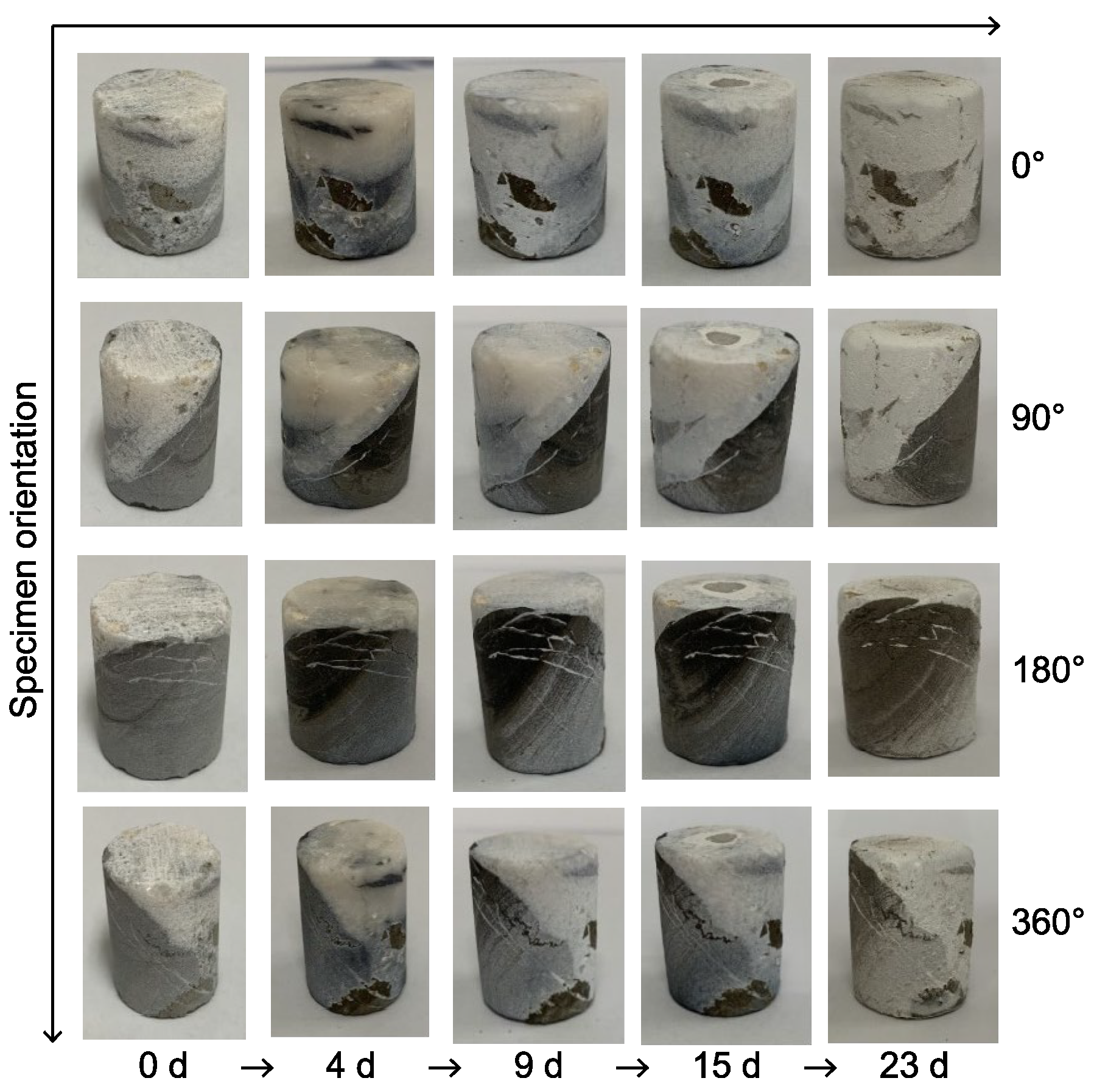
3.1. Comparative Analysis of Limestone’s Macrostructural Characteristics Before and After Acid-Induced Dissolution
3.1.1. Comparative Macrostructural Analysis of Limestone Without Veins Before and After Acid-Induced Dissolution
3.1.2. Comparative Macrostructural Analysis of Limestone with Sparse, Broad Calcite Veins Before and After Acid-Induced Dissolution
3.1.3. Comparative Macrostructural Analysis of Limestone with Dense, Fine Calcite Veins Before and After Acid-Induced Dissolution
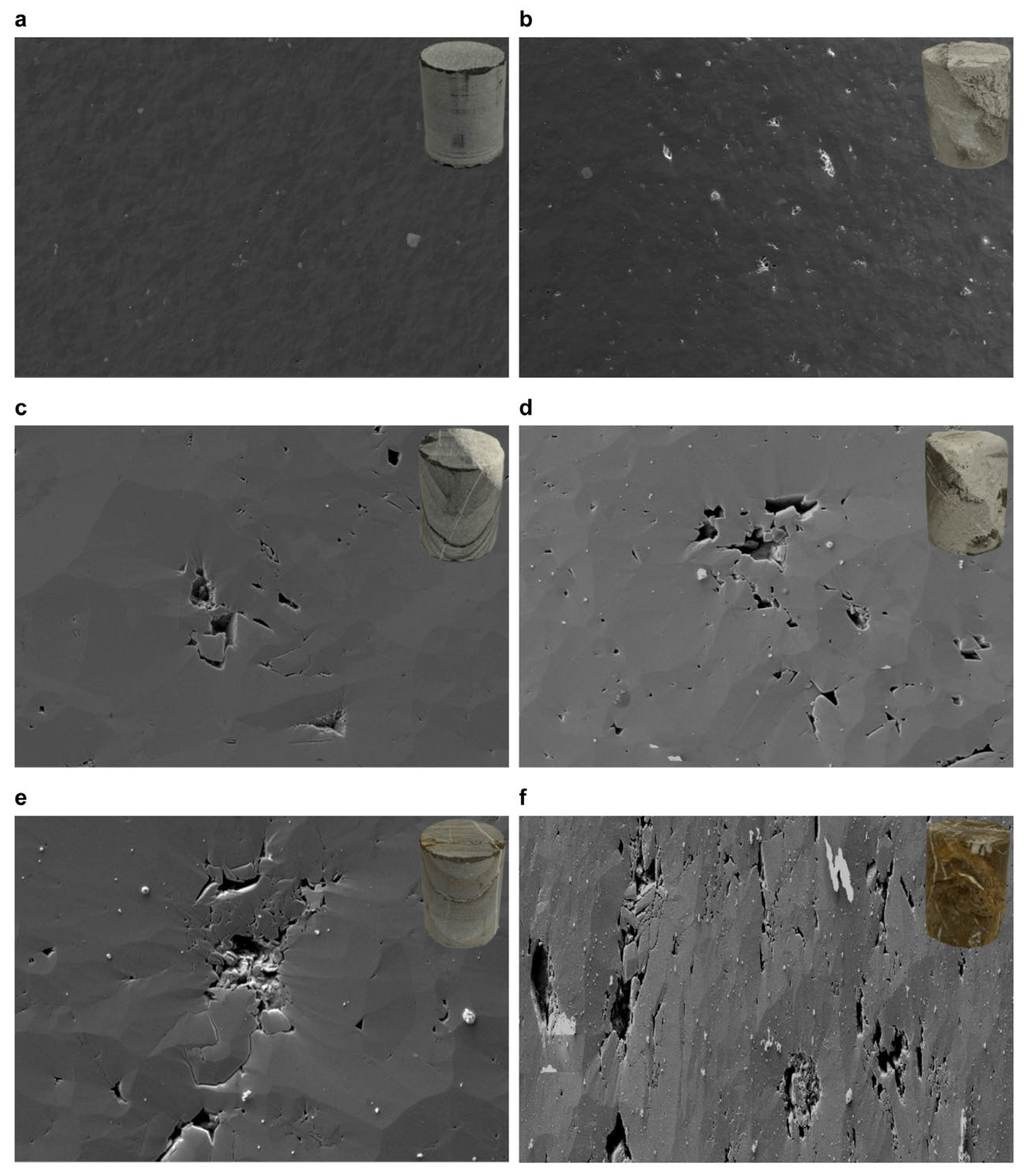
3.2. Comparative Microstructural Analysis of Limestone Before and After Acid-Induced Dissolution
3.2.1. Comparative Microstructural Analysis of Limestone Without Veins Before and After Acid-Induced Dissolution
3.2.2. Comparative Microstructural Analysis of Limestone with Sparse, Broad Calcite Veins Before and After Acid-Induced Dissolution
3.2.3. Comparative Microstructural Analysis of Limestone with Dense, Fine Calcite Veins Before and After Acid-Induced Dissolution
3.3. Evaluating Mineralogical Changes Pre- and Post-Dissolution
3.3.1. Mineralogical Analysis via Field Emission Scanning Electron Microscopy
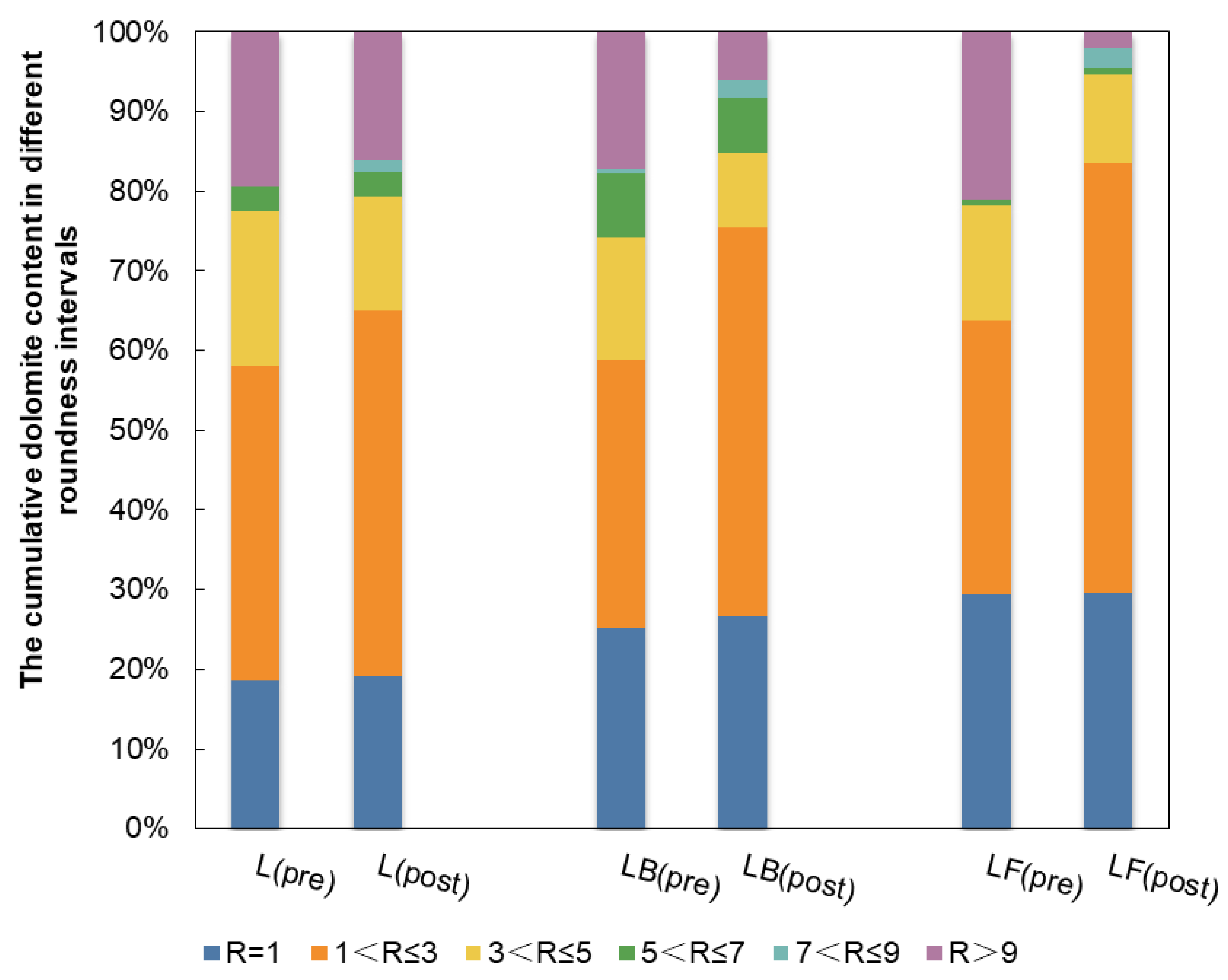
3.3.2. Assessing Dolomite Roundness in Three Varieties of Limestone
4. Discussion
5. Conclusions
- (1)
- Dissolution primarily occurs around microstructural planes, such as pores and cracks, altering their shapes, increasing their sizes, and generating extensive new dissolution fissures.
- (2)
- Calcite veins significantly affect the microscopic dissolution features of Triassic limestone. The contact surfaces between the host rock and calcite veins increase the dissolution areas between the limestone and sulfuric acid solution and intensify dissolution reactions. The dissolution intensity is more dependent on vein quantity than width.
- (3)
- Under normal temperature and pressure, Triassic limestone’s mineral types remain constant during dissolution. However, dolomite particles are gradually dissolved to approach roundness, and the presence of calcite veins promotes the dissolution of edges and corners.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- He, J.; Li, T.; Zheng, B.; Mao, T.; Wu, Y.; Li, S.; Li, X. Effect of Calcite Veins on the Mechanical Behavior and Fracture Propagation of Carboniferous Limestone. Eng. Geol. 2022, 300, 106592. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Teng, Z.; Ou, X.; Zhang, Q. Investigations on the effect of natural veined calcite on the mechanical properties of limestone. Sci Rep. 2024, 14, 5871. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Kaiser, P.K.; Uno, H. Estimation of Rock Mass Strength Including the Effect of Cracks and Fissures. Int. J. Rock Mech. Min. Sci. 2009, 46, 761–775. [Google Scholar]
- Yang, T.; Zhu, L.M.; Zhang, G.W.; Wang, F.; Lu, R.K.; Xia, J.C.; Zhang, Y.Q. Geological and Geochemical Constraints on Genesis of the Liziyuan Gold-Dominated Polymetal Deposit, Western Qinling Orogen, Central China. Int. Geol. Rev. 2012, 54, 1944–1966. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Q.; Larsen, C.; Zhao, C.; Sun, M.; Zhang, Y.; Zhang, T. Microfracture-Pore Structure Characterization and Water-Rock Interaction in Three Lithofacies of the Lower Eagle Ford Formation. Eng. Geol. 2021, 292, 106276. [Google Scholar] [CrossRef]
- Huang, X.; Pang, J.; Zou, J. Study on the Effect of Dry–Wet Cycles on Dynamic Mechanical Properties of Sandstone Under Sulfuric Acid Solution. Rock Mech. Rock Eng. 2022, 55, 1253–1269. [Google Scholar] [CrossRef]
- Thaysen, E.M.; Soler, J.M.; Cama, J. Laboratory-Scale Interaction Between CO2-Saturated H2SO4-Rich Brine and Reservoir Rocks (Limestone and Sandstone). Procedia Earth Planet. Sci. 2017, 17, 614–617. [Google Scholar] [CrossRef]
- Pearce, J.K.; Kirste, D.M.; Dawson, G.K.; Farquhar, S.M.; Biddle, D.; Golding, S.D.; Rudolph, V. SO2 Impurity Impacts on Experimental and Simulated CO2–Water–Reservoir Rock Reactions at Carbon Storage Conditions. Chem. Geol. 2015, 399, 65–86. [Google Scholar] [CrossRef]
- Ali, M.; Hascakir, B. Water/Rock Interaction for Eagle Ford, Marcellus, Green River, and Barnett Shale Samples and Implications for Hydraulic-Fracturing-Fluid Engineering. SPE J. 2016, 22, 162–171. [Google Scholar] [CrossRef]
- Ma, J.; Xie, S.; Tang, H.; Ma, M.; Carranza, E.J.M.; Han, J.; He, Z.; Jiao, C.; Zhang, H. Evolution Mechanisms of Carbonate Reservoirs Based on Dissolution Rates and Multifractal Analysis of Microscopic Morphology. Nat. Resour. Res. 2020, 29, 2843–2865. [Google Scholar] [CrossRef]
- Lai, J.; Guo, J.; Ma, Y.; Zhou, H.; Wang, S.; Liu, Y. Effect of Acid-Rock Reaction on the Microstructure and Mechanical Property of Tight Limestone. Rock Mech. Rock Eng. 2022, 55, 35–49. [Google Scholar] [CrossRef]
- Lyu, Q.; Wang, K.; Hu, C.; Dick, J.M.; Shi, J.; Tan, J. Experimental Study on the Mechanical Properties of Shale After Long-Term of Immersion in Fracturing Fluids with Different pH. Rock Mech. Rock Eng. 2022, 55, 5047–5061. [Google Scholar] [CrossRef]
- Chicco, J.M.; Vacha, D.; Mandrone, G. Thermo-Physical and Geo-Mechanical Characterization of Faulted Carbonate Rock Masses (Valdieri, Italy). Remote Sens. 2019, 11, 179. [Google Scholar] [CrossRef]
- Qiao, L.; Wang, Z.; Huang, A. Alteration of Mesoscopic Properties and Mechanical Behavior of Sandstone Due to Hydro-Physical and Hydro-Chemical Effects. Rock Mech. Rock Eng. 2017, 50, 255–267. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Feng, X.; Zhang, H.; Feng, J. Effects of Acid Sulfate and Chloride Ion on the Pore Structure and Mechanical Properties of Sandstone Under Dynamic Loading. Rock Mech. Rock Eng. 2021, 54, 6105–6121. [Google Scholar] [CrossRef]
- Li, H.; Zhong, Z.; Liu, X.; Sheng, Y.; Yang, D. Micro-Damage Evolution and Macro-Mechanical Property Degradation of Limestone Due to Chemical Effects. Int. J. Rock Mech. Min. Sci. 2018, 110, 257–265. [Google Scholar] [CrossRef]
- Liu, D.; Yan, W.; Yan, S.; Kang, Q. Study on the Effect of Axial and Hydraulic Pressure Coupling on the Creep Behaviors of Sandstone Under Multi-Loading. Bull. Eng. Geol. Environ. 2021, 80, 6107–6120. [Google Scholar] [CrossRef]
- Sisodiya, S.M.A.; Singh, S.M.A.; Thomas, P.E.D.; Zhang, Y.A.M.A. Effect of Water–Rock Interaction on the Axial Capacity of Drilled Caissons Socketed in Claystone Bedrock. J. Geotech. Geoenviron. Eng. 2021, 147, 04021113. [Google Scholar] [CrossRef]
- Tang, H.; Wasowski, J.; Juang, C.H. Geohazards in the Three Gorges Reservoir Area, China—Lessons Learned from Decades of Research. Eng. Geol. 2019, 261, 105267. [Google Scholar] [CrossRef]
- Delle Rose, M.; Federico, A.; Parise, M. Sinkhole Genesis and Evolution in Apulia, and Their Interrelations with the Anthropogenic Environment. Nat. Hazards Earth Syst. Sci. 2004, 4, 747–755. [Google Scholar] [CrossRef]
- Huang, D.; Gu, D.M.; Song, Y.X.; Cen, D.F.; Zeng, B. Towards a Complete Understanding of the Triggering Mechanism of a Large Reactivated Landslide in the Three Gorges Reservoir. Eng. Geol. 2018, 238, 36–51. [Google Scholar] [CrossRef]
- Tsykin, R.A. Karst Sibiri; Krasnoyarsk University Publishing House: Krasnoyarsk, Russia, 1990; pp. 2–10. [Google Scholar]
- Ford, D.; Williams, P.W. Karst Hydrogeology and Geomorphology; John Wiley & Sons: Chichester, UK, 2007; ISBN 978-0-470-84996-5. [Google Scholar]
- Dochez, S.; Laouafa, F.; Franck, C.; Guedon, S.; Martineau, F.; d’Amato, J.; Saintenoy, A. Multi-Scale Analysis of Water Alteration on the Rockslope Stability Framework. Acta Geophys. 2014, 62, 1025–1048. [Google Scholar] [CrossRef]
- Sun, H. Modeling Soil Water Retention Curves by Pore Fractal Dimension Based on Microstructure Image. Earth Sci. Inform. 2024, 17, 3589–3597. [Google Scholar] [CrossRef]
- Rauch, H.W.; White, W.B. Lithologic Controls on the Development of Solution Porosity in Carbonate Aquifers. Water Resour. Res. 1970, 6, 1175–1192. [Google Scholar] [CrossRef]







| Limestone Types | Calcite (%) | Feldspar (%) | Quartz (%) | Clay (%) | Dolomite (%) | Pyrite (%) |
|---|---|---|---|---|---|---|
| Limestone without veins | 66.4 | 21.3 | 4.6 | 4.6 | 2.8 | 0.2 |
| Limestone with sparse, broad calcite veins | 72.5 | 19.4 | 3.6 | 3.8 | 0.6 | 0.0 |
| Limestone with multiple fine calcite veins | 68.8 | 18.9 | 5.4 | 5.2 | 1.7 | 0.0 |
| Limestone Type | Specimen Types | Average Water Absorption (%) | Standard Deviation (%) |
|---|---|---|---|
| Limestone without veins | specimen without calcite veins | 0.07 | 0.01 |
| Limestone with sparse, broad calcite veins | specimen with a distinct broad calcite vein | 0.10 | 0.02 |
| Limestone with multiple fine calcite veins | specimen with multiple fine calcite veins | 0.13 | 0.02 |
| Limestone Types | Dolomite Content in Various Roundness Intervals | |||||
|---|---|---|---|---|---|---|
| R = 1 | 1 < R ≤ 3 | 3 < R ≤ 5 | 5 < R ≤ 7 | 7 < R ≤ 9 | R ≥ 9 | |
| L (pre) | 18.55% | 39.52% | 19.35% | 3.23% | 0 | 19.35% |
| L (post) | 19.21% | 45.79% | 14.29% | 3.21% | 1.43% | 16.07% |
| Gradient | 0.67% | 6.27% | −5.07% | −0.01% | 1.43% | −3.28% |
| LB (pre) | 25.14% | 33.71% | 15.43% | 8.00% | 0.57% | 17.14% |
| LB (post) | 26.62% | 48.92% | 9.35% | 6.91% | 2.16% | 6.04% |
| Gradient | 1.48% | 15.21% | −6.08% | −1.09% | 1.59% | −11.11% |
| LF (pre) | 29.41% | 34.45% | 14.29% | 0.84% | 0 | 21.01% |
| LF (post) | 29.61% | 53.99% | 11.05% | 0.82% | 2.55% | 1.98% |
| Gradient | 0.20% | 19.53% | −3.24% | −0.02% | 2.55% | −19.03% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, J.; Li, S.; He, J.; Zhang, Z.; Li, X. Impact of Diverse Calcite Vein Patterns on Dissolution Characteristics of Triassic Limestone in Three Gorges Reservoir Area. Water 2025, 17, 1550. https://doi.org/10.3390/w17101550
Guo J, Li S, He J, Zhang Z, Li X. Impact of Diverse Calcite Vein Patterns on Dissolution Characteristics of Triassic Limestone in Three Gorges Reservoir Area. Water. 2025; 17(10):1550. https://doi.org/10.3390/w17101550
Chicago/Turabian StyleGuo, Jingyun, Shouding Li, Jianming He, Zhaobin Zhang, and Xiao Li. 2025. "Impact of Diverse Calcite Vein Patterns on Dissolution Characteristics of Triassic Limestone in Three Gorges Reservoir Area" Water 17, no. 10: 1550. https://doi.org/10.3390/w17101550
APA StyleGuo, J., Li, S., He, J., Zhang, Z., & Li, X. (2025). Impact of Diverse Calcite Vein Patterns on Dissolution Characteristics of Triassic Limestone in Three Gorges Reservoir Area. Water, 17(10), 1550. https://doi.org/10.3390/w17101550








