Wetlands as Climate-Sensitive Hotspots: Evaluating Greenhouse Gas Emissions in Southern Chhattisgarh
Abstract
1. Introduction
2. Materials and Methods
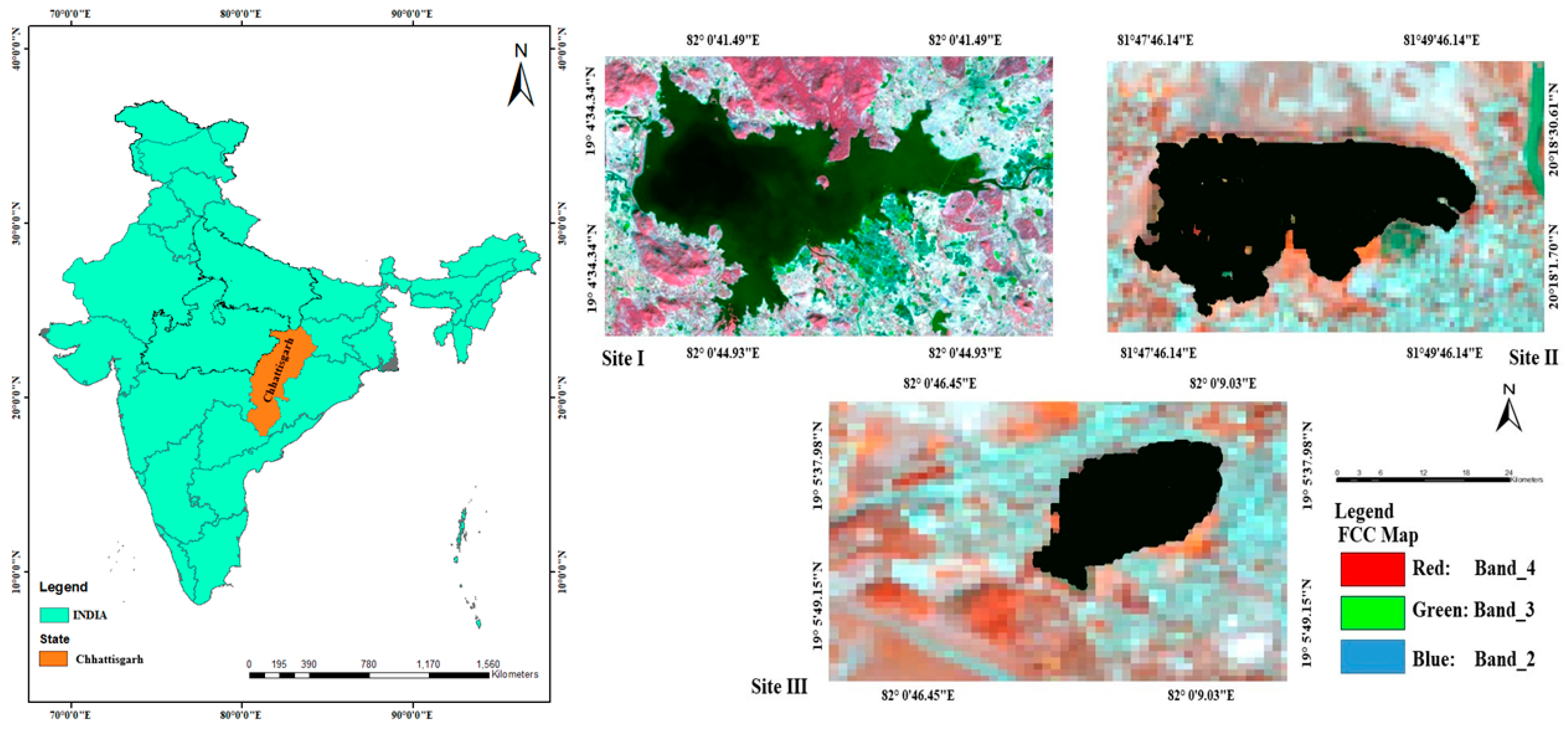
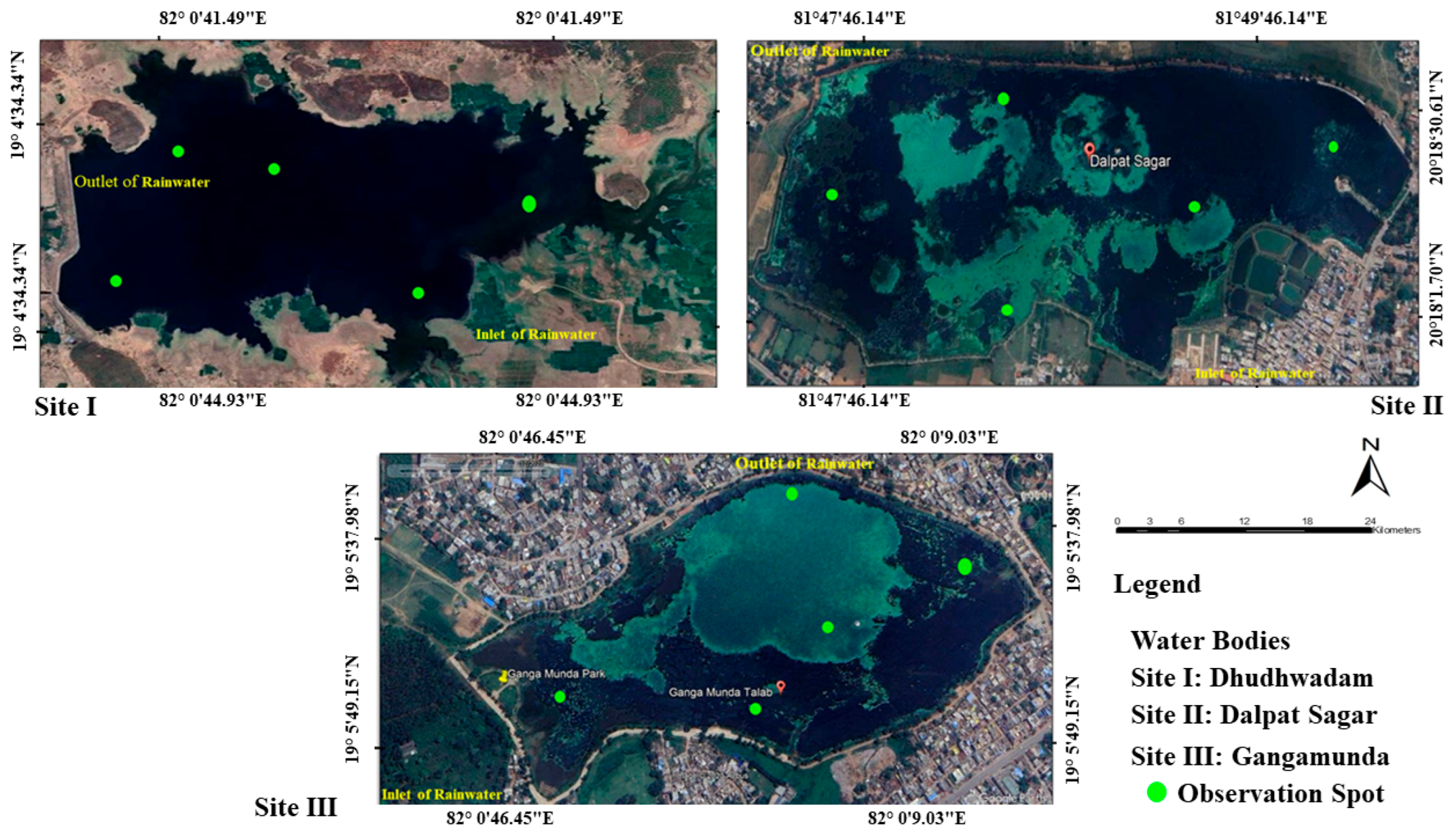
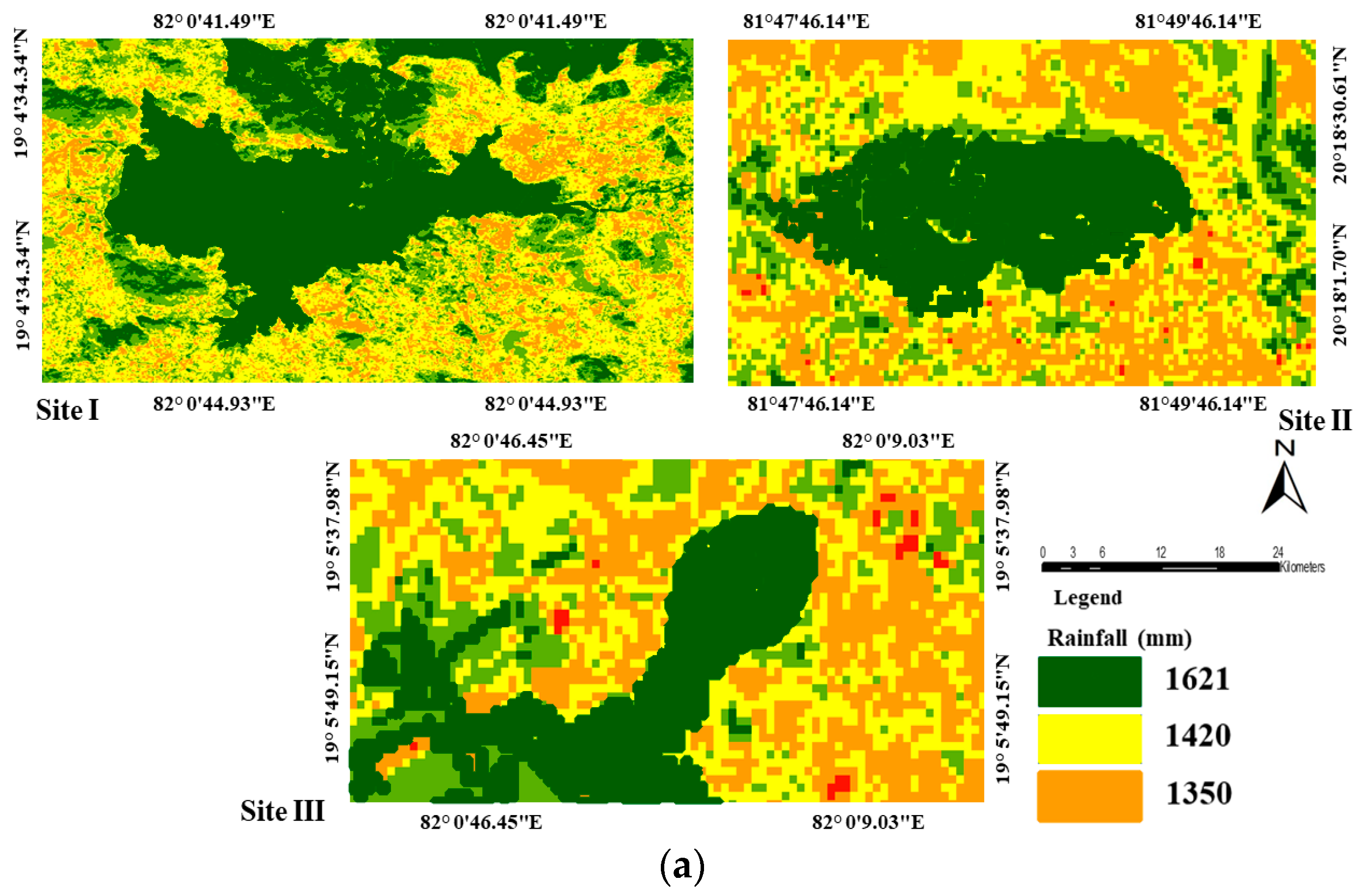
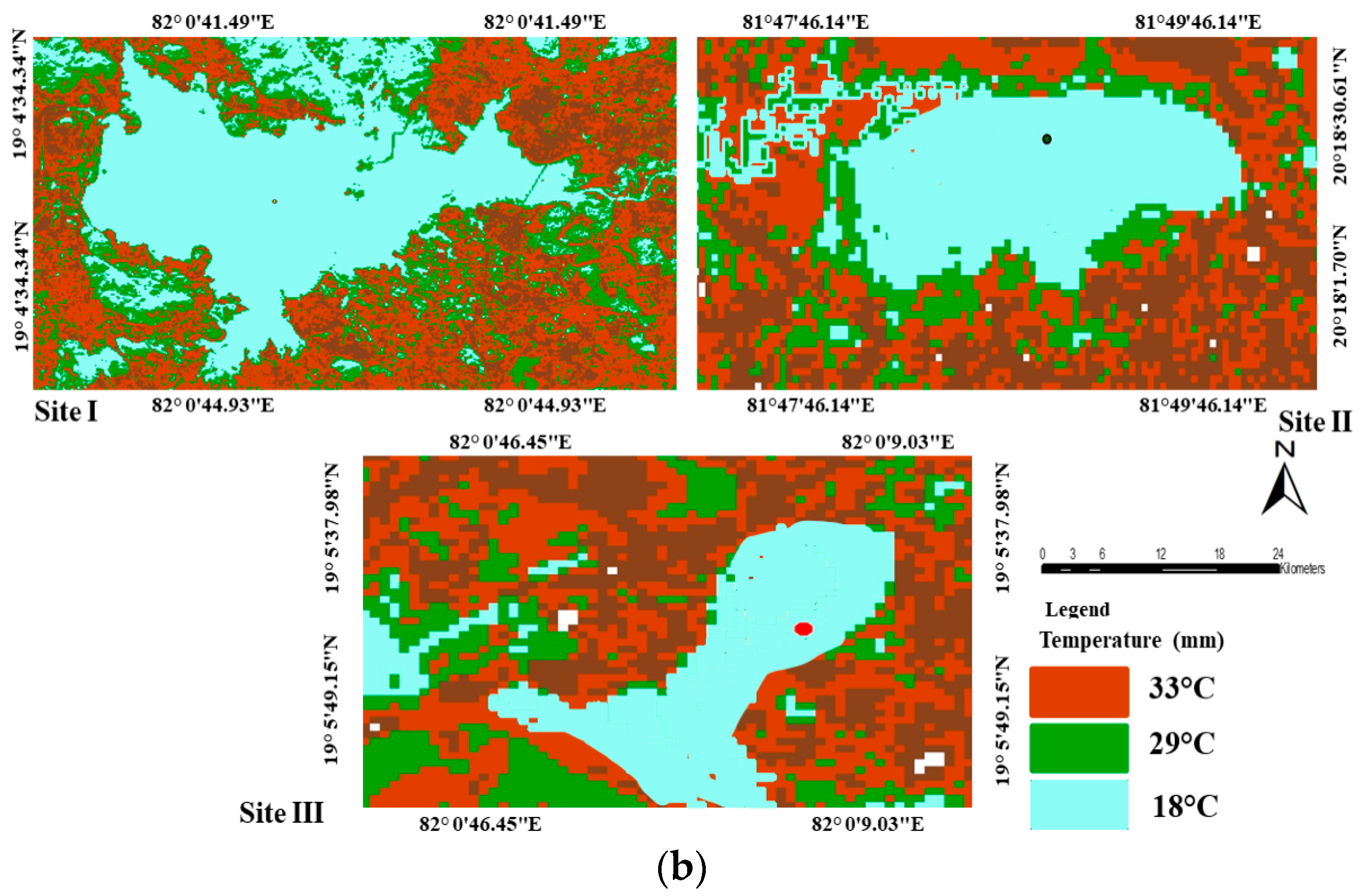
2.1. Study Area
2.2. Mapping of Wetlands
2.3. Greenhouse Gas Analysis
2.4. Estimation of Global Warming Potential
2.5. Statistical Analysis
3. Results
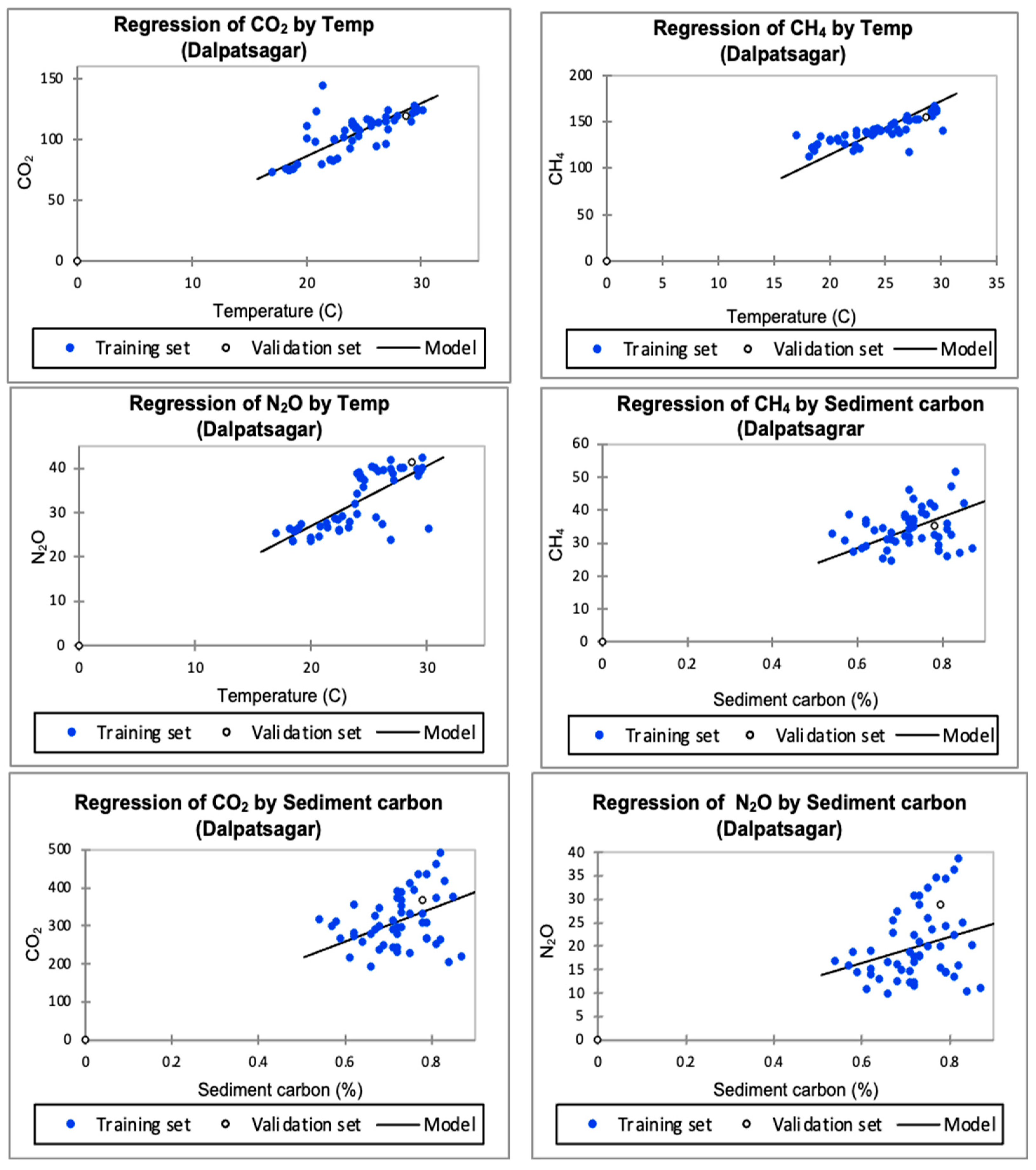
3.1. Dalpatsagar Water Body
3.1.1. Greenhouse Gases Influenced by Temperature
3.1.2. Greenhouse Gases Influenced by Sediment Carbon
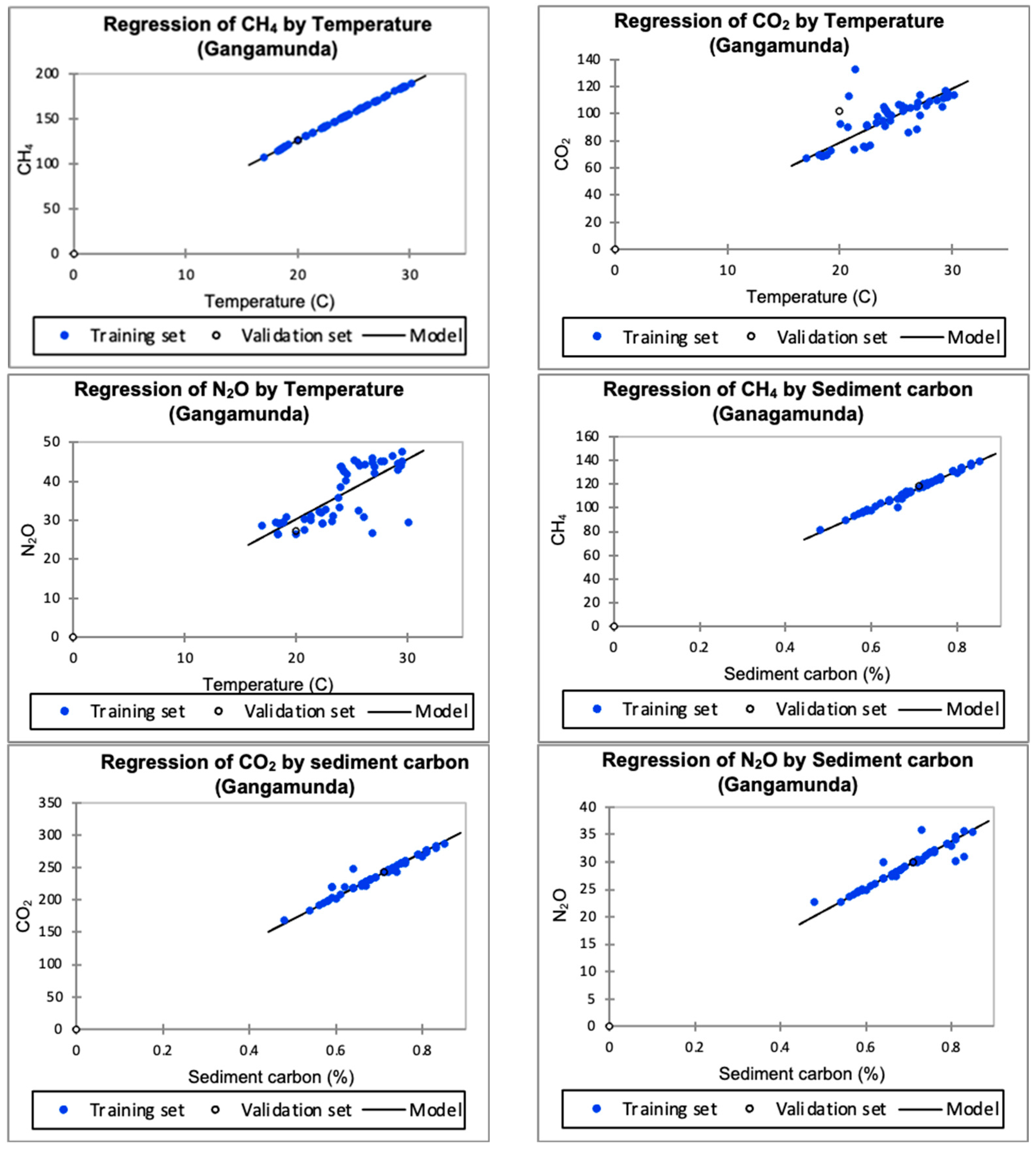
3.2. Gangamunda Water Body
3.2.1. Greenhouse Gases Influenced by Temperature
3.2.2. Greenhouse Gases Influenced by Sediment Carbon
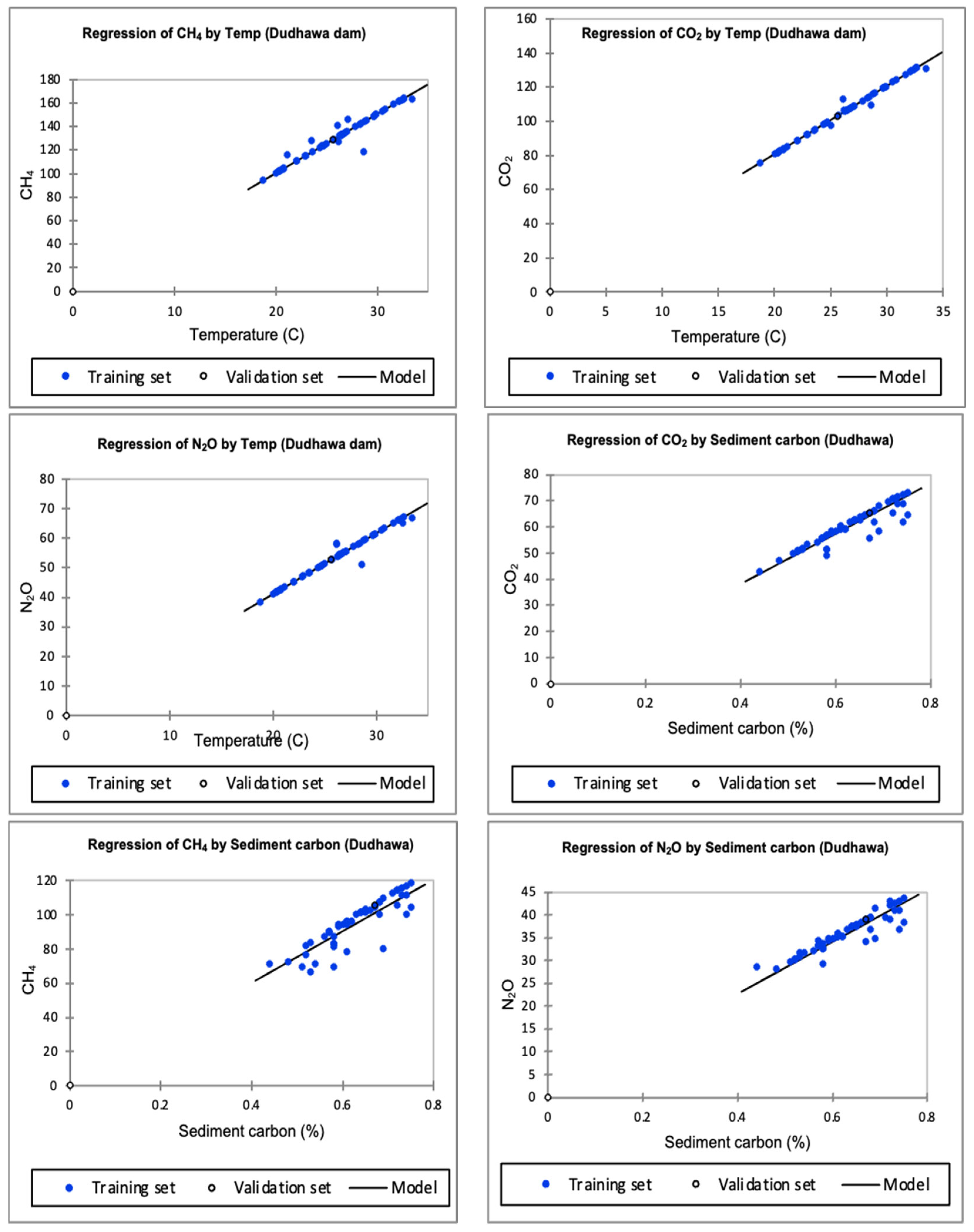
3.3. In Dudhawa Dam
3.3.1. Greenhouse Gases Influenced by Temperature
3.3.2. Greenhouse Gases Influenced by Sediment Carbon
3.4. Global Warming Potential of Water Bodies
4. Discussion
4.1. Dalpatsagar Water Body
4.1.1. Greenhouse Gases Affected by Temperature
4.1.2. Greenhouse Gases Affected by Sediment Carbon
4.2. Greenhouse Gases: Temperature and Sediment in the Gangamunda Water Body
4.3. Sediment Carbon in the Gangamunda Water Body
4.4. Greenhouse Gases Influenced by Temperature and Sediment at Dudhawa Dam
4.5. Water Bodies’ Global Warming Potential
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacock, M.; Audet, J.; Jordan, S.; Smeds, J.; Wallin, M.B. Greenhouse gas emissions from urban ponds are driven by nutrient status and hydrology. Ecosphere 2019, 10, e02643. [Google Scholar] [CrossRef]
- Jensen, S.; Leavitt, P.R.; Baulch, H.M.; Badiou, P.; Cordeiro, M.R.C.; Webb, J.R. Differential Controls of Greenhouse Gas (CO2, CH4, and N2O) Concentrations in Natural and Constructed Agricultural Waterbodies on the Northern Great Plains. J. Geophys. Res. Biogeosci. 2023, 128, e2022JG007261. [Google Scholar] [CrossRef]
- Yang, P. Fluxes of carbon dioxide and methane across the water-atmosphere interface of aquaculture shrimp ponds in two subtropical estuaries: The effect of temperature, substrate, salinity and nitrate. Sci. Total Environ. 2018, 635, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- IPCC. Summary for Policymakers. In Climate Change 2013: The Physical Science Basis Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; 1535p. [Google Scholar] [CrossRef]
- IPCC. Climate Change (2014). Fifth Assessment Synthesis Report (Longer Report) of Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Olefeldt, D. Net carbon accumulation of a high-latitude permafrost palsa mire similar to permafrost-free peatlands. Geophys. Res. Lett. 2012, 39, L03501. [Google Scholar] [CrossRef]
- ICAR. Handbook of Fisheries and Aquaculture; ICAR: New Delhi, India, 2011. [Google Scholar]
- Dusek, J.; Darenova, E.; Pavelka, M.; Marek, M.V. Methane and Carbon Dioxide Release from Wetland Ecosystems. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 509–553. [Google Scholar]
- Deemer, B.R.; Harrison, J.A.; Li, S.; Beaulieu, J.J.; Delsontro, T.; Barros, N.; Bezerra-Neto, J.F.; Powers, S.M.; Dos Santos, M.A.; Vonk, J.A. Greenhouse gas emissions from reservoir water surfaces: A new global synthesis. BioScience 2016, 66, 949–964. [Google Scholar] [CrossRef]
- Rosentreter, J.A.; Borges, A.V.; Deemer, B.R.; Holgerson, M.A.; Liu, S.; Song, C.; Melack, J.; Raymond, P.A.; Duarte, C.M.; Allen, G.H.; et al. Half of Global Methane Emissions Come from Highly Variable Aquatic Ecosystem Sources. Nat. Geosci. 2021, 14, 225–230. [Google Scholar] [CrossRef]
- Bastviken, D.; Cole, J.J.; Pace, M.; Tranvik, L. Methane emissions from lakes: Dependence of lake characteristics, two regional assessments, and a global estimate. Glob. Biogeochem. Cycles 2004, 18, GB4009. [Google Scholar] [CrossRef]
- Kumar, S.; Bijalwan, A. Comparison of Carbon Sequestration Potential of Quercus leucotrichophora Based Agroforestry Systems and Natural Forest in Central Himalaya, India. Water Air Soil Pollut. 2021, 232, 350. [Google Scholar] [CrossRef]
- Alin, S.R.; Johnson, T.R. Physical controls on carbon dioxide transfer velocity and flux in low-gradient river systems and implications for regional carbon budgets. J. Geophys. Res. 2007, 116, G01009. [Google Scholar] [CrossRef]
- Wei, D.; Tarchen, T.; Dai, D.; Wang, Y.; Wang, Y. Revisiting the role of CH4 emissions from alpine wetlands on the Tibetan Plateau: Evidence from two in situ measurements at 4758 and 4320 m above sea level. J. Geophys. Res. Biogeosci. 2015, 120, 1741–1750. [Google Scholar] [CrossRef]
- Bevelhimer, M.; Stewart, A.; Fortner, A.; Phillips, J.; Mosher, J. CO2 is Dominant Greenhouse Gas Emitted from Six Hydropower Reservoirs in Southeastern United States during Peak Summer Emissions. Water 2016, 8, 15. [Google Scholar] [CrossRef]
- Adams, D.D. Diffuse Flux of Greenhouse Gases—Methane and Carbon Dioxide—At the Sediment-Water Interface of Some Lakes and Reservoirs of the World. In Greenhouse Gas Emissions—Fluxes and Processes; Garneau, M., Tremblay, A., Roehm, C., Varfalvy, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 129–153. [Google Scholar]
- Pradhan, A.; Chandrakar, T.; Nag, S.K.; Dixit, A.; Mukherjee, S.C. Crop planning based on rainfall variability for Bastar region of Chhattisgarh. India J. Agrometeorol. 2017, 22, 509–517. [Google Scholar] [CrossRef]
- Hutchinson, G.L.; Mosier, A.R. Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Liu, C.; Holst, J.; Brueggemann, N.; Butterbach-Bahl, K.; Yao, Z.; Yue, J.; Han, S.; Han, X.; Zheng, X. Winter-grazing reduces methane uptake by soils of a typical semi-arid steppe in Inner Mongolia, China. Atmos. Environ. 2016, 145, 105–113. [Google Scholar] [CrossRef]
- Yvon-Durocher, G.; Allen, A.P.; Bastviken, D.; Conrad, R.; Gudasz, C.; St-Pierre, A.; Thanh-Duc, N.; del Giorgio, P.A. Methane fluxes show consistent temperature dependence across microbial to ecosystem scales. Nature 2014, 507, 488–491. [Google Scholar] [CrossRef]
- Pathak, H.; Upadhyay, R.C.; Muralidhar, M.; Bhattacharyya, P.; Venkateswarlu, B. Measurement of Greenhouse Gas Emission from Crop, Livestock and Aquaculture; Indian Agricultural Research Institute: New Delhi, India, 2013; p. 101. [Google Scholar]
- Lal, R. Soil carbon sequestration in India. Clim. Change 2004, 65, 277–296. [Google Scholar] [CrossRef]
- DelSontro, T.; Beaulieu, J.J.; Downing, J.A. Greenhouse Gas Emissions from Lakes and Impoundments: Upscaling in the Face of Global Change. Limnol. Oceanogr. Lett. 2018, 3, 64–75. [Google Scholar] [CrossRef]
- Lamers, P.M.; van Roozendaal, M.E.; Roelofs, G.M. Acidification of freshwater wetlands: Combined effects of non- airborne sulfur pollution and desiccation. Water Air Soil Pollut. 1998, 105, 95–106. [Google Scholar] [CrossRef]
- Rawat, S.; Khanduri, V.P.; Singh, B.; Riyal, M.K.; Thakur, T.K.; Kumar, M.; Cabral-Pinto, M.M. Variation in carbon stock and soil properties in different Quercus leucotrichophora forests of Garhwal Himalaya. CATENA 2022, 213, 106210. [Google Scholar] [CrossRef]
- Vizza, C.; West, W.E.; Jones, S.E.; Hart, J.A.; Lamberti, G.A. Regulators of coastal wetland methane production and responses to simulated global change. Biogeosciences 2017, 14, 431–446. [Google Scholar] [CrossRef]
- Cheng, S.; Kumar, A.; Lan, G.; Zhang, W.; Yu, Z.; Zhang, S.; Yu, Z.-G.; Yao, M.; Lin, J. Thermal sensitivity and rising greenhouse gas emissions in riparian zone soils: Implications for ecosystem carbon dynamics. J. Env. Manag. 2025, 381, 125194. [Google Scholar] [CrossRef] [PubMed]
- Mondal, B.; Bauddh, K.; Kumar, A.; Bordoloi, N. India’s Contribution to Greenhouse Gas Emission from Freshwater Ecosystems: A Comprehensive Review. Water 2022, 14, 2965. [Google Scholar] [CrossRef]
- Malyan, S.K.; Singh, O.; Kumar, A.; Anand, G.; Singh, R.; Singh, S.; Yu, Z.; Kumar, J.; Fagodiya, R.K.; Kumar, A. Greenhouse Gases Trade-Off from Ponds: An Overview of Emission Process and Their Driving Factors. Water 2022, 14, 970. [Google Scholar] [CrossRef]
- Zhao, Y.; Lin, J.; Cheng, S.; Wang, K.; Kumar, A.; Yu, Z.; Zhu, B. Linking soil dissolved organic matter characteristics and the temperature sensitivity of soil organic carbon decomposition in the riparian zone of the Three Gorges Reservoir. Ecol. Indic. 2023, 154, 110768. [Google Scholar] [CrossRef]
- Upadhyay, P.; Prajapati, S.K.; Kumar, A. Impacts of riverine pollution on greenhouse gas emissions: A comprehensive review. Ecol. Indic. 2023, 154, 110649. [Google Scholar] [CrossRef]
- Tranvik, L.J.; Downing, J.A.; Cotner, J.B.; Loiselle, S.A.; Striegl, R.G.; Ballatore, T.J.; Dillon, P.; Finlay, K.; Fortino, K.; Knoll, L.B.; et al. Lakes and reservoirs as regulators of carbon cycling and climate. Limnol. Oceanogr. 2009, 54, 2298–2314. [Google Scholar] [CrossRef]
- Bastviken, D.; Tranvik, L.J.; Downing, J.A.; Crill, P.M.; Enrich-Prast, A. Freshwater methane emissions offset the continental carbon sink. Science 2011, 331, 50. [Google Scholar] [CrossRef] [PubMed]
- Nazaries, L.; Murrell, J.C.; Millard, P.; Baggs, L.; Singh, B.K. Methane, microbes and models: Fundamental understanding of the soil methane cycle for future predictions. Environ. Microbiol. 2013, 15, 2395–2417. [Google Scholar] [CrossRef]
- Beaulieu, J.J.; Tank, J.L.; Hamilton, S.K.; Wollheim, W.M.; Hall, R.O., Jr.; Mulholland, P.J.; Peterson, B.J.; Ashkenas, L.R.; Cooper, L.W.; Dahm, C.N.; et al. Nitrous oxide emission from denitrification in stream and river networks. Proc. Natl. Acad. Sci. USA 2019, 116, 9802–9807. [Google Scholar] [CrossRef]
- Borges, A.V.; Darchambeau, F.; Teodoru, C.R.; Marwick, T.R.; Tamooh, F.; Geeraert, N.; Omengo, F.O.; Guérin, F.; Lambert, T.; Morana, C.; et al. Globally significant greenhouse-gas emissions from African inland waters. Nat. Geosci. 2018, 11, 637–642. [Google Scholar] [CrossRef]
- IPCC. Sixth Assessment Report; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2021. [Google Scholar]
- Bărbulescu, A. Modeling Greenhouse Gas Emissions from Agriculture. Atmosphere 2025, 16, 295. [Google Scholar] [CrossRef]
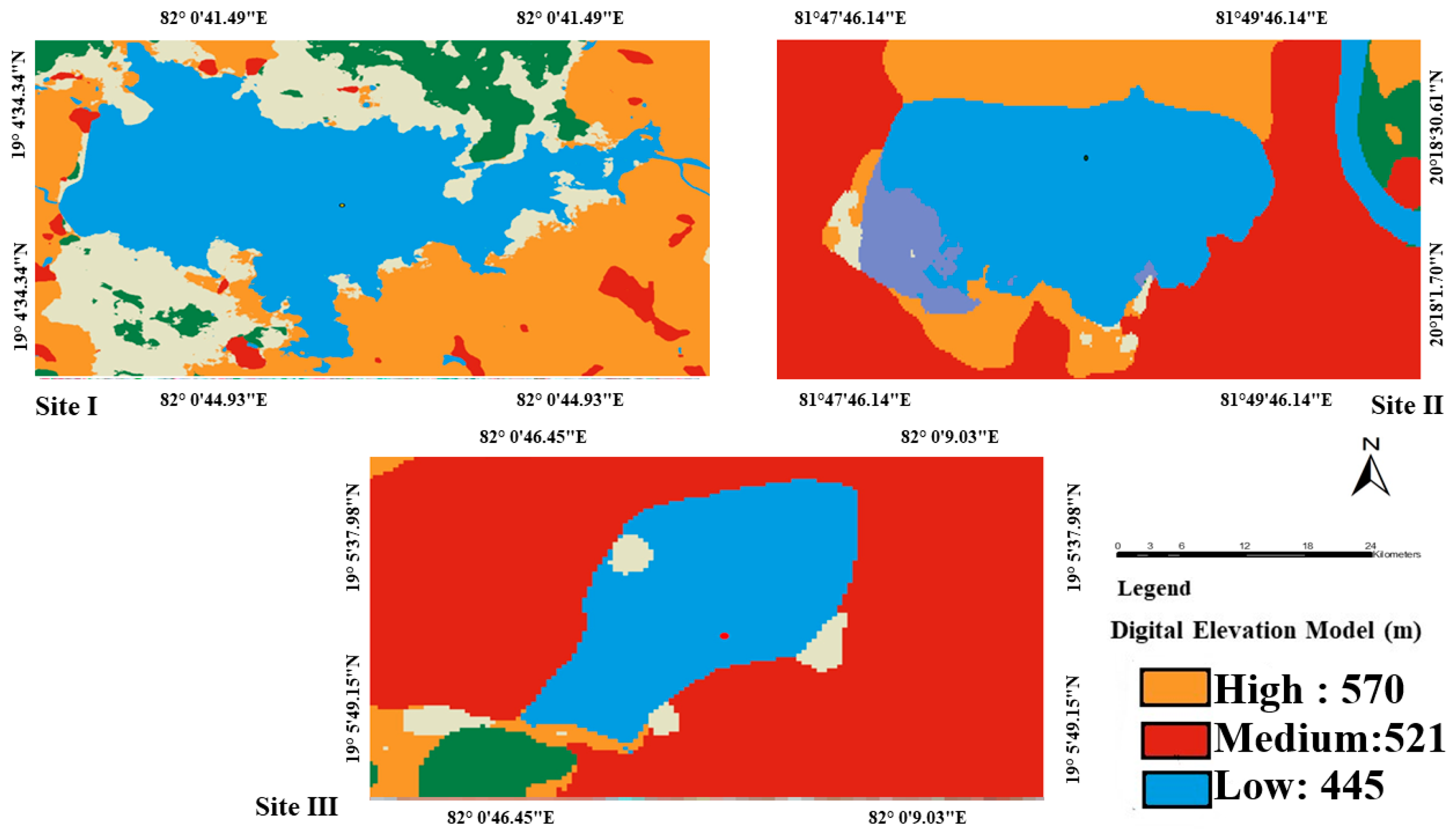
| Characteristic Parameter | Dalpatsagar | Gangamunda | Dudhawa Dam |
|---|---|---|---|
| Area (ha) | 143.90 | 30.95 | 1882.10 |
| Mean depth (m) | 1.85 | 2.01 | 3.58 |
| Secchi Depth (m) | 0.55 | 2.13 | 1.64 |
| pH | 6.12 | 6.77 | 7.68 |
| Surface DO (mg L−1) | 3.89 | 4.78 | 6.53 |
| Bottom DO (mg L−1) | 0.19 | 0.24 | 0.41 |
| Surface temperature (°C) | 21.75 | 22.18 | 22.46 |
| Bottom temperature (°C) | 17.35 | 18.07 | 19.28 |
| Parameter | Data Type | Resolution/Scale | Sources |
|---|---|---|---|
| Geological | Polygon | 1:25,000 | Geological survey of India/ Data.gov |
| Rainfall | Gridded | India Meteorological Department | |
| Temperature | Gridded | India Meteorological Department | |
| Elevation | Raster | 1 arc second (30 m) SRTM data | Dem (USGS 1 arc second), UTM-45, WGS 1984 |
| Slope | Raster | 1 arc second (30 m) SRTM data | Dem (USGS 1 arc second), UTM-45, WGS 1984 |
| Satellite | Landsat 8 2022 | OLI (Operational Land Imager)/TIRS (Thermal Infrared Sensor) 30 m | USGS |
| Water Body | CO2 (mg m−2 h−1) | N2O (mg m−2 h−1) | CH4 (mg m−2 h−1) | Global Warming Potential (mg m−2 h−1) | Carbon Equ. Emission (mg m−2 h−1) |
|---|---|---|---|---|---|
| Dalpatsagar | 59.48 ** | 36.92 ** | 90.76 ** | 13,410 ** | 3657 ** |
| Gangamunda | 47.06 | 34.90 ** | 85.03 ** | 12,658 ** | 3452 ** |
| Dudhawa Dam | 40.48 | 23.38 | 62.31 | 8597 | 2344 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pradhan, A.; Sao, A.; Thakur, T.K.; Anderson, J.T.; Chandel, G.; Kumar, A.; Paramesh, V.; Jinger, D.; Kumar, R. Wetlands as Climate-Sensitive Hotspots: Evaluating Greenhouse Gas Emissions in Southern Chhattisgarh. Water 2025, 17, 1553. https://doi.org/10.3390/w17101553
Pradhan A, Sao A, Thakur TK, Anderson JT, Chandel G, Kumar A, Paramesh V, Jinger D, Kumar R. Wetlands as Climate-Sensitive Hotspots: Evaluating Greenhouse Gas Emissions in Southern Chhattisgarh. Water. 2025; 17(10):1553. https://doi.org/10.3390/w17101553
Chicago/Turabian StylePradhan, Adikant, Abhinav Sao, Tarun Kumar Thakur, James T. Anderson, Girish Chandel, Amit Kumar, Venkatesh Paramesh, Dinesh Jinger, and Rupesh Kumar. 2025. "Wetlands as Climate-Sensitive Hotspots: Evaluating Greenhouse Gas Emissions in Southern Chhattisgarh" Water 17, no. 10: 1553. https://doi.org/10.3390/w17101553
APA StylePradhan, A., Sao, A., Thakur, T. K., Anderson, J. T., Chandel, G., Kumar, A., Paramesh, V., Jinger, D., & Kumar, R. (2025). Wetlands as Climate-Sensitive Hotspots: Evaluating Greenhouse Gas Emissions in Southern Chhattisgarh. Water, 17(10), 1553. https://doi.org/10.3390/w17101553












