Abstract
Species belonging to the crustacean infraorder Astacoidea represent taxa of particular interest from a conservation point of view, such as the threatened European crayfish (i.e., Austropotamobius pallipes), and at the same time include invasive taxa having highly negative impacts where they are introduced. Among the latter, some freshwater-dwelling species seem to show some abilities to tolerate high salinity levels, such as Procambarus clarkii Girard, 1852. By using metadata and field observation, this review will investigate whether the alien P. clarkii can threaten coastal waters. Specifically, we will shed light on P. clarkii’s (1) invasiveness, (2) its dispersal pattern, (3) its tolerance to salinity, and (4) its ecological plasticity as an invasive species in relation to estuaries. This new habitat colonization is also possible as P. clarkii has been observed to survive up to 20 ppt of water salinity and a maximum of 30–35 ppt with its lifetime drastically reduced. As a result, P. clarkii colonizes different ecosystems globally, reaching estuarine and coastal ecosystems due to active and passive transport by human and animal vectors. Due to recent discoveries of alien crayfish in estuarine and coastal waters, monitoring activities have become mandatory to preserve coastal habitats and all the aquatic resources (e.g., limicolous birds, endemic fish, fishery and aquaculture activities) inhabiting therein.
1. Introduction
Astacoideans include species of particular concern from a conservation point of view as many crayfish are listed as threatened or endangered (see Austropotamobius pallipes in [1]), but also many taxa amongst the most threatening introduced worldwide [2,3,4] driving detrimental constraints to the native biodiversity shaping [5,6].
Indeed, once they became established in the new habitat, introduced crayfish began to receive great attention [4]. Here, they can pose several threats to the native biota due to their (i) tolerance to extreme environmental conditions, (ii) rapid spread, and (iii) parasite transmission [7,8], significantly altering the food web structure [9]. For these reasons, astacoideans are the largest and long-lived invertebrates in temperate areas, playing an important role in the community structure [10]. They are indeed keystone consumers, feeding on diverse living and non-living resources (e.g., [11,12]), and constitute a new resource for native taxa as well [13,14]. Additionally, alien crayfish are able to induce a decline in the species richness [15] and detrimental habitat changes also in industrial and agricultural areas [16,17,18,19,20].
In this context, the ecological plasticity of non-indigenous crayfish may represent a new unexpected threat to brackish and transitional aquatic ecosystems despite their habit of inhabiting freshwaters. This could also have an impact on fragile and ephemeral ecosystems, such as the Mediterranean temporary ponds (MTPs, hereafter). Particularly, Mediterranean coasts are characterized by these temporary habitats that are listed in the Habitat Directive according to the Natura 2000 network of the European Union (i.e., Natura code 3170* on MTPs, the 3120 habitats with Isoeto-NanoJuncetea and the 3130 containing Littorelletea uniflorae and/or IsoetoNano-Juncetea, Habitats Directive 92/43/EEC). The importance of those MTPs is widely recognized, being refugia and unique transitional habitats for biodiversity and protected species. Among the latter, many migratory bird and reptile species use MPTs as biological corridors (e.g., Ardea spp. and Emys orbicularis listed in the Habitat Directive and IUCN). MTPs also show several important ecosystem functions [14], such as being hotspots for cryptobiont species, unique organisms living in those habitats. Being a disappearing habitat, MTPs have great importance as they contain rare, endangered, and endemic species, which are going to disappear with the degradation of these ponds due to several threats, among these also alien species [14].
Among all alien crayfish species, this review investigates whether any crayfish species, with a focus on the most dangerous (i.e., Procambarus clarkii (Girard, 1852)), can threaten coastal waters. Specifically, we shed light on the adaptive features of crayfish as an invasive species of estuaries. In particular, the (1) invasiveness of P. clarkii, (2) its dispersal pattern, (3) its tolerance to salinity and (4) its ecological plasticity. Throughout the review, we will use metadata from the literature, and a particular field observation of P. clarkii in sea and coastal ecosystems.
2. Crayfish Invasiveness
European freshwaters are inhabited by several self-sustaining populations of diverse introduced species [15,21]. Regarding the Italian peninsula, the species actually found refer to the following: the narrow-clawed crayfish Astacus leptodactylus (Eschscholtz, 1823), the yabby Cherax destructor (Clark, 1936), the redclaw C. quadricarinatus (von Martens, 1868) (observed in rearing systems) [22], the spiny-cheek crayfish Faxonius limosus (Rafinesque, 1817), the signal crayfish Pacifastacus leniusculus (Dana, 1852), the red-swamp crayfish Procambarus clarkii (Girard, 1852), and a free-living population of marmorkrebs (marbled crayfish) P. virginalis Lyko, 2017 [23,24,25]. In central Italian freshwaters, five non-native crayfish occur: A. leptodactylus, C. destructor, F. limosus, P. clarkii, and P. virginalis [23,26,27].
Concerning P. clarkii, the red swamp crayfish is a pan-global taxon, found on every continent except Australia and Antarctica [28]. Its invasiveness is mainly due to the properties of an r-selected species facilitating the extensive colonization of diversified environments [29,30,31]. A long list of studies has demonstrated its detrimental effects (reviewed in [32]). Moreover, its habit of burrowing aids in holding out against dehydration [20], but at the same time may cause bank collapse and increased water turbidity as well [14]. Then, its aggressiveness and predatory ability allow it to threaten native flora and fauna [33,34], including endangered European indigenous crayfish [35]. These mechanisms add to the dangers posed by P. clarkii as a vector of Aphanomyces astaci Schikora 1922, responsible for outbreaks of the so-called crayfish plague (see [36]).
3. Dispersal Pattern of Procambarus clarkii
Regarding the pattern of introduction and dispersal, the spread of P. clarkii may happen with several transport mechanisms. P. clarkii can be transported by active and passive processes by both human and animal vectors, facilitated by its ability to survive outside the water [32]. For example, Anatidae ducks play an important role as vectors of crayfish passive dispersal [37]. In addition, P. clarkii may survive long-distance transportation, being carried by moving vehicles or by waterbird passive dispersal [37,38]. Recently, fishways and fish lifts have been highlighted as new dispersal ways used by P. clarkii [39]. Thus, crayfish can be successfully dispersed in new habitats, transported by several mechanisms, potentially posing a risk also for new areas (e.g., transitional and marine ecosystems).
Concerning its distribution pattern, P. clarkii is dispersed in Italy as well as in the rest of Europe with similar rates according to studies in the literature [32,40,41]. In general, P. clarkii follows the same pattern as other crayfish, such as faster movement at night due to high water temperature, followed by an intense wide-spreading period, alternating to stasis phases during colder periods [32,40]. This active dispersal pattern allows P. clarkii to cover a long-distance range, also exploiting its ability to move over land up to a 1 km distance [40,42,43,44,45].
4. Tolerance to Water Salinity in Captivity
Although the ancestor of Astacoidea colonized inland waters in the Triassic Period [46], some recent species retain the ability to tolerate high salinity levels [47]. This stimulated many studies in captivity on this topic, since they have many implications from diverse viewpoints in the fields of evolution, biology conservation and applied ecology [48,49,50,51,52,53,54,55]. Regarding the latter, farming and aquaculture activities provided insights on tolerance to water salinity [56,57,58,59]. For instance, Casellato and Masiero [58] conducted laboratory tests and in situ farming to assess P. clarkii adaptation to gradually increasing salinity on the Northeastern Adriatic Coast [58].
In this regard, Loyacano [56] discovered that freshly hatched P. clarkii directly exposed to 15 ppt of salinity died in less than one week, whereas adults survived at concentrations of 20 and 30 ppt. Interestingly, Sharfstein and Charfin [57] found that adults exposed to a gradual salt application could effectively survive and grow at 12 ppt. Additionally, Casellato and Masiero [58] pointed out that, when directly transferred into saltwater, individuals survived well up to 20 ppt, but at 30–33 ppt, their lifetime was drastically reduced. Finally, Bissattini et al. [59] discovered that crayfish had a decreased mortality rate at 35 ppt water salinity due to gradual acclimation in salt water rather than shock occurrence of specimens in water with high salt concentrations.
Information on the tolerance to salt water by some crayfish has recently been collected by Dobrzycka-Krahel and Fidalgo [60], who reported a long list of articles highlighting the great overall plasticity of P. clarkii, which, however, requires further field studies to understand the real threats for natural environments (Table 1).

Table 1.
Studies reporting captive or field observations reporting salinity (‰ or PSU or “not reported”) by habitat (coastal, sea). Bibliographic references are available in Dobrzycka-Krahel and Fidalgo [60].
5. Procambarus clarkii in Transitional Waters and Coastal Habitats
The ecological features of P. clarkii allow it to colonize a wide range of habitats including coastal brackish waters in the Iberian Peninsula [63,64], Italy [61,62,65,66], and France [67] (Figure 1). This has aroused great interest among many conservation biologists since the salt tolerance of P. clarkii was observed in captivity studies for many years but was neglected in research in situ (Table 1).
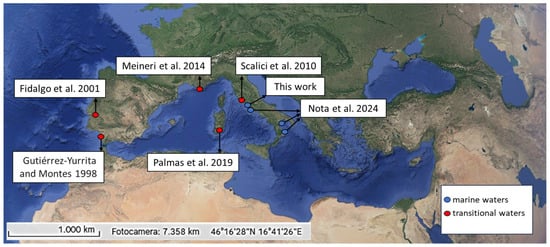
Figure 1.
Sites with Procambarus clarkii present in European coastal habitats. The blue and red dots indicate marine and transitional waters, respectively. The image was obtained by Google Earth and Copernicus. Data on the presence of P. clarkii were obtained by studies like Gutiérrez-Yurrita and Montes (1998) [41], Fidalgo et al. (2001) [63], Scalici et al. (2010) [61], Meineri et al. (2014) [67], Palmas et al. (2019) [66], Nota et al. (2024) [62], and Scalici and Gallitelli (2025, this work).
Although a recent review on decapods highlighted that P. clarkii could potentially be found in the sea [60], to date, only one (to be verified) reference on P. clarkii occurrence in the sea is reported (see below). The species currently appears to be limited to European transitional areas in Portugal, Spain, and France ([41,61,62,63,64,65,66], Figure 1), even if it was recently more and more frequently observed in Tyrrhenian central Italian coasts. Indeed, during a sampling session of a monitoring project on native and non-native crayfish populations’ health evaluation, we observed 544 individuals (from 29 in Marina di Pescia, the northernmost locality, to 177 in Sabaudia, the southernmost locality) of the red swamp crayfish. They were caught using baited traps on eight coastal water localities with salinity values ranging from 14 to 21‰. In detail, the traps were baited using cat food and set for 2–5 nights at a height of 1.5–2 m above sea level, positioned 10–25 m from the shoreline. The eight study sites were characterized by a sandy littoral, typical of the central Italian coast. Along the sea–land gradient, the sites were characterized by a backdune landscape surrounded by agricultural areas with irrigation ditches and canals. At only one site, we observed two individuals of P. clarkii emerging from the sea near the locality Passoscuro, Prov. Rome (Figure 2). Here, in Mediterranean marine ecosystems, water salinity may reach 35‰ (near the locality studied by Scalici et al. [61]). However, salinity was not measured here. Considering that the river mouth is a transitional area with fluctuating salinity, we tend to consider the water salinity in our study area to be characteristic of marine habitats, based on previous studies outlined in Table 1.
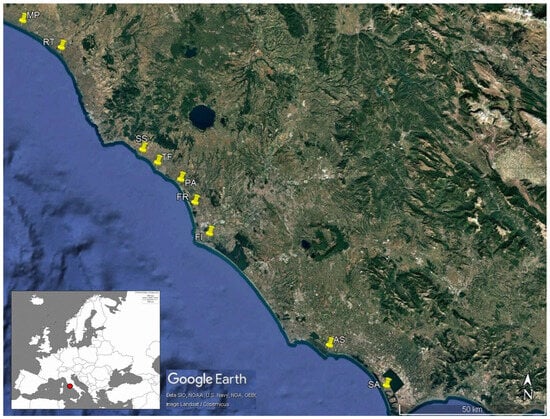
Figure 2.
Records of the red swamp crayfish Procambarus clarkii in transitional and marine waters of Tyrrhenian central Italian coasts. The red dot indicates the study area in central Italy, while the yellow points indicate the specific locations. Specifically, Marina di Pescia, Santa Severa, and Astura are brackish temporary ponds; Riva dei Tarquini and Fiumicino are estuarine zones; Torre Flavia and Sabaudia are brackish wetlands; and Passoscuro is the marine locality where P. clarkii was found. The image was obtained from Google Earth and Copernicus. MP = Marina di Pescia, RT = Riva dei Tarquini, SS = Santa Severa, TF = Torre Flavia, PA = Passoscuro, FR = Fregene, FI = Fiumicino, AS = Astura, and SA = Sabaudia.
These findings do not represent the unique report of the red swamp crayfish in the sea. In the literature, Nota et al. [62] recently published findings of P. clarkii in Italian coastal habitats in three different regions (Figure 1), exploiting information noted on social media. Particularly, the authors reported red swamp crayfish caught by professional fishermen in marine habitats at depths up to 20 m, suggesting that this species may disperse into marine environments for extended periods [62]. Reaching a depth of –20 m implies a significant distance from the shoreline, indicating that individuals must be able to survive in marine conditions for some time before being captured by nets at such depths. Although the precise duration of survival remains uncertain, previous studies [47,48,49,50,62] suggest that red swamp crayfish may survive in marine environments for approximately 2–3 days.
Although some detrimental effects on P. clarkii emerged after the colonization of transitional habitats, such as the growth rate and the expected longevity [61], it is likely that crayfish may initially benefit from colonizing transitional waters, probably due to the absence of ecological resistance (natural competitors/predators), which has a pivotal role in alien crayfish establishment and surviving. Its physiological plasticity may facilitate transitional habitat colonization because the energetic costs of maintaining salt and water balance are reduced since less than 10% of the total metabolism is sufficient for osmoregulatory purposes [68,69].
As P. clarkii is able to invade ephemeral waterbodies, as observed by Gherardi et al. [33] and Aquiloni et al. [40], we expected that crayfish might move from a river to another watercourse using ephemeral temporary habitats (i.e., MTPs) and canals as steppingstones. Survival in unfavorable aquatic habitats may depend on the anatomical and physiological responses facilitating certain forms of character displacement in colonized habitats. Particularly, the salinity increase affects the antennal gland organization in the red swamp crayfish, driving the acclimation to detrimental osmotic and hydric conditions [58]. Such structural alterations make the crayfish antennal glands similar to those of marine decapods, which have no nephridial tubule and produce urine iso-osmotic with the hemolymph [70], although mechanisms of ultrafiltration, secretion, and reabsorption involved in salt water colonization need to be clarified [71].
Given these findings, new research is needed to assess the effects of invasive crayfish on temporary aquatic habitats, as studies have so far mainly focused on the spread of P. clarkii among rivers or in the same river. Considering the fragile habitats listed in the Habitat Directive (3120, 3130, and 3170*), conservation efforts should be provided through specific monitoring in the coming years to preserve these habitats, with the possibility to control and eventually eradicate alien species.
6. Potential Threats to Coastal and Transition Habitats Due to Crayfish Stepping
Since some crayfish are able to survive high salinities, this adaptative process ought to be better investigated in order to understand (i) whether crayfish select transitional waters as a new elective habitat or merely use them as a biological corridor (given that salt tolerance, combined with their dispersal ability, may facilitate such movement—see Aquiloni et al. [40]) (Figure 3), and (ii) which driving environmental and evolutionary forces may facilitate the colonization of transitional waters within the area of introduction (such as the absence of natural competitors and/or predators and/or parasites). Beyond the adaptive aspects, this represents an issue of concern in the habitat conservation field, particularly with regard to (1) the massive introduction of non-indigenous crayfish in many European countries [23,26,27,72]; (2) the control of alien threats in coastal habitats (see [61,63,64,67]); and (3) their increasingly frequent presence in transitional waters.
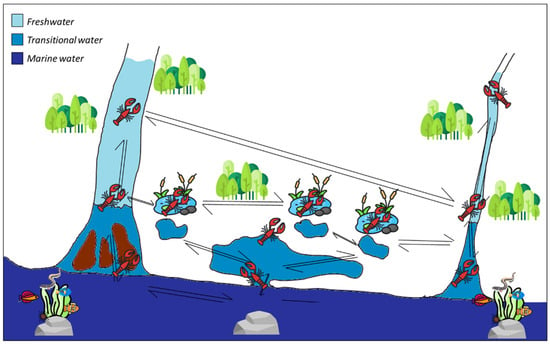
Figure 3.
The potential theoretical dispersion routes of the red swamp crayfish Procambarus clarkii, exploiting and potentially impacting three aquatic habitat types: freshwater, transitional, and marine waters. The image was freely obtained from flaticon.com.
The potential dispersal routes of P. clarkii presented in Figure 3 are possible scenarios since it can act as a native decapod competitor, macroinvertebrate predator, pathogen vector, habitat and biodiversity shaper, native vertebrate diet supporter, ecosystem services injurer, and native food web influencer [8,11,32]. In light of this, crayfish represent a dangerous threat to the biodiversity of transition zones, wetlands, estuarine and temporary waters, and are likely to damage human livelihoods through their impact on fishing and aquaculture. However, although the threats posed by P. clarkii on freshwater habitats are well-known globally, their impact on the local fauna and flora in transitional waters is not yet evident. Considering the damage that invasive crayfish can cause in various regions worldwide where they have been introduced [15,18,19], the possibility of this species to impact fragile ecosystems such as coastal waters seems certain. This could be since transitional and coastal habitats offer important refuge zones for limicolous birds and endemic fishes confined to this type of transition area [58,61].
Regarding the threats posed by crayfish entering marine habitats, they can be several and of different ecological values. In a first remote scenario, the potential threat may not even occur since P. clarkii would use the sea as a biological corridor to go elsewhere (e.g., ponds, coastal lakes, river mouths, etc.; see Figure 3). In the second scenario, P. clarkii could threaten marine ecosystems through direct impacts (e.g., acting as opportunistic omnivores in the food web, consuming remaining Posidonia banquettes) or indirect impacts (e.g., reducing several ecosystem services by reducing biodiversity, carbon storage, and oxygen production by eating Posidonia, or by eating juvenile fish, which could impact fisheries). These impacts could be the same impacts posed by P. clarkii in freshwaters but translated to transitional, coastal, and marine habitats. P. clarkii could also become a food resource of invasive and native predators in marine habitats, which could regulate crayfish populations.
Taking the previously described experiments of acclimatization to salinity increasing values into account, the colonization of brackish waters by P. clarkii could represent a pre-adaptation to the use of sea waters as a possible subsequent biological corridor (see [73,74]), as evidenced by the discovery of several individuals on the beach that came out of the sea in the locality Passoscuro. This is why the tolerance of crayfish to salt waters should be further investigated in situ as a fundamental management activity for the conservation of transitional and marine habitats.
Finally, regarding the diffusion of the species, particular attention should be given to parasites that could be carried by P. clarkii [75,76,77,78,79]—in particular, Aphanomyces astaci, mycoflora, and fungus pathogens (e.g., Phoma gomerata, [73]). Consequently, the high ecological plasticity of P. clarkii indicates the need to intervene before the species and its parasites [73,74,75,76,77] spread further in transitional waters, in order to conserve Mediterranean coastal habitats—particularly to protect Posidonia oceanica seagrass beds, a priority marine habitat under the 1120* sensu Habitats Directive 92/43 EEC.
Author Contributions
Conceptualization, M.S.; methodology, M.S. and L.G.; software, M.S. and L.G.; validation, L.G.; formal analysis, M.S. and L.G.; investigation, M.S. and L.G.; resources, M.S.; data curation, M.S. and L.G.; writing—original draft preparation, M.S.; writing—review and editing, M.S. and L.G.; visualization, L.G.; supervision, M.S. and L.G.; project administration, M.S.; funding acquisition, M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Grant of Excellence Departments (“Departments of Excellence 2023-27”, “Dipartimenti d’Eccellenza 2023-27”), MUR-Italy (Italian Minister of University and Research), and the National Biodiversity Future Center (NBFC).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank the Editor, the Special Issue Editors, and Reviewers for their valuable feedback.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Taylor, C.A. Taxonomy and conservation of native crayfish stocks. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science and Iowa State University Press: Ames, IA, USA, 2002; pp. 236–257. [Google Scholar]
- Hobbs, I.H.H.; Jass, J.P.; Huner, J.V. A review of global crayfish introductions with particular emphasis on two North American species (Decapoda, Cambaridae). Crustaceana 1989, 56, 299–316. [Google Scholar] [CrossRef]
- Horwitz, P. The translocation of freshwater crayfish in Australia: Potential impact, the need for control and global relevance. Biol. Conserv. 1990, 54, 291–305. [Google Scholar] [CrossRef]
- Lodge, D.M.; Taylor, C.A.; Holdich, D.M.; Skurdal, J. Non indigenous crayfishes threaten North American freshwater biodiversity: Lessons from Europe. Fisheries 2000, 25, 7–20. [Google Scholar] [CrossRef]
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sannwald, E.; Huenneke, L.; Jackson, R.B.; Kinzig, A.; et al. Global Biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Holdich, D.M.; Noël, P.Y.; Reynolds, J.D.; Haffner, P. Atlas of Crayfish in Europe; Museum National d’Histoire Naturelle: Paris, France, 2006. [Google Scholar]
- Gherardi, F. Understanding the impact of invasive crayfish. In Biological Invaders in Inland Waters: Profiles, Distribution, and Threats; Gherardi, F., Ed.; Invading Nature: Springer Series in Invasion Ecology; Springer: Dordrecht, The Netherlands, 2007; pp. 507–542. [Google Scholar]
- Covich, A.P.; Palmer, M.A.; Crowl, T.A. The role of benthic invertebrate species in freshwater ecosystems. Bioscience 1999, 49, 119–127. [Google Scholar] [CrossRef]
- Usio, N.; Townsend, C.R. Roles of crayfish: Consequences of predation and bioturbation for stream invertebrates. Ecology 2004, 85, 807–822. [Google Scholar] [CrossRef]
- Nyström, P.; Brönmark, C.; Granéli, W. Patterns in benthic food webs: A role for omnivorous crayfish? Freshw. Biol. 1996, 36, 631–646. [Google Scholar] [CrossRef]
- Whitledge, G.W.; Rabeni, C.F. Energy sources and ecological role of crayfishes in an Ozark stream: Insights from stable isotopes and gut analysis. Can. J. Fish. Aquat. Sci. 1997, 54, 2555–2563. [Google Scholar] [CrossRef]
- Slater, F.M.; Rayner, G. Austropotamobius pallipes in otter diet in the mid-Wye catchment of central Wales. Freshw. Crayfish 1993, 9, 365–367. [Google Scholar]
- Rodríguez, C.F.; Bécares, E.; Fernández-Aláez, M.; Fernández-Aláez, C. Loss of diversity and degradation of wetlands as a result of introducing exotic crayfish. Biol. Invasions 2005, 7, 75–85. [Google Scholar] [CrossRef]
- Gherardi, F. Crayfish invading Europe: The case study of Procambarus clarkii. Mar. Freshw. Behav. Physiol. 2006, 39, 175–191. [Google Scholar] [CrossRef]
- Nyström, P. Ecological impact of introduced and native crayfish on freshwater community: European perspectives. In Crayfish in Europe as Alien Species. How to Make the Best of a Bad Situation? Gherardi, F., Holdich, D.M., Eds.; A.A Balkema: Rotterdam, The Netherlands, 1999; pp. 63–85. [Google Scholar]
- Svärdson, G.; Hammar, J.; Nyberg, P.; Degerman, E. Human impact on the fish diversity in the four largest lakes of Sweden. Ambio 2001, 30, 522–528. [Google Scholar]
- Anastácio, P.M.; Correia, A.M.; Menino, J.P. Processes and patterns of plant destruction by crayfish: Effects of crayfish size and developmental stages of rice. Arch. Hydrobiol. 2005, 162, 37–51. [Google Scholar] [CrossRef]
- Anastácio, P.M.; Parente, V.S.; Correia, A.M. Crayfish effects on seeds and seedlings: Identification and quantification of damage. Freshw. Biol. 2005, 50, 697–704. [Google Scholar] [CrossRef]
- Barbaresi, S.; Tricarico, E.; Gherardi, F. Factors inducing the intense burrowing activity by the red swamp crayfish, Procambarus clarkii, an invasive species. Naturwissenschaften 2004, 91, 342–345. [Google Scholar] [CrossRef]
- Gherardi, F.; Bertolino, S.; Bodon, M.; Casellato, S.; Cianfanelli, S.; Ferraguti, M.; Lori, E.; Mura, G.; Nocita, A.; Riccardi, N.; et al. Animal xenodiversity in Italian inland waters: Distribution, modes of arrival, and pathways. Biol. Invasions 2008, 10, 435–454. [Google Scholar] [CrossRef]
- Gherardi, F.; Baldaccini, G.N.; Barbaresi, S.; Ercolini, P.; De Luise, G.; Mazzoni, D.; Mori, M. Alien crayfish in Europe: The situation in Italy. In Crayfish in Europe as Alien Species. How to Make the Best of a Bad Situation? Gherardi, F., Holdich, D.M., Eds.; A.A Balkema: Rotterdam, The Netherlands, 1999; pp. 107–128. [Google Scholar]
- Nonnis Marzano, F.; Scalici, M.; Chiesa, S.; Gherardi, F.; Gibertini, G. The first record of the marbled crayfish adds further threats to freshwater in Italy. Aquat. Invasions 2009, 4, 401–404. [Google Scholar] [CrossRef]
- Scholtz, G.; Braband, A.; Tolley, L.; Reimann, A.; Mittmann, B.; Lukhaup, C.; Steuerwald, F.; Vogt, G. Parthenogenesis in an outsider crayfish. Nature 2003, 421, 806. [Google Scholar] [CrossRef]
- Jones, J.P.G.; Rasamy, J.R.; Harvey, A.; Toon, A.; Oidtmann, B.; Randrianarison, M.H.; Raminosoa, N.; Ravoahangimalala, O.R. The perfect invader: A parthenogenic crayfish poses a new threat to Madagascar’s freshwater biodiversity. Biol. Invasions 2008, 11, 1475–1482. [Google Scholar] [CrossRef]
- Chiesa, S.; Scalici, M.; Gibertini, G. Occurrence of allochthonous freshwater crayfishes in Latium (Central Italy). Bull. Fr. Pêche Piscic. 2006, 380–381, 883–902. [Google Scholar] [CrossRef]
- Scalici, M.; Chiesa, S.; Gherardi, F.; Ruffini, M.; Gibertini, G.; Nonnis Marzano, F. The new threat to Italian inland waters from the alien crayfish ‘‘gang’’: The Australian Cherax destructor Clark, 1936. Hydrobiologia 2009, 632, 341–345. [Google Scholar] [CrossRef]
- Huner, J.V. Procambarus. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell: Oxford, UK, 2002; pp. 541–584. [Google Scholar]
- Scalici, M.; Gherardi, F. Structure and dynamics of an invasive population of the red swamp crayfish (Procambarus clarkii) in a Mediterranean wetland. Hydrobiologia 2007, 583, 309–319. [Google Scholar] [CrossRef]
- Scalici, M.; Pitzalis, M.; Gibertini, G. Crayfish distribution updating in central Italy. Knowl. Manag. Aquat. Ecosyst. 2009, 394–395, 1–8. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Scalici, M. Revisiting reproduction and population structure and dynamics of Procambarus clarkii eight years after its introduction into Lake Trasimeno (Central Italy). Knowl. Manag. Aquat. Ecosyst. 2013, 408, 10. [Google Scholar] [CrossRef]
- Souty-Grosset, C.; Anastácio, P.M.; Aquiloni, L.; Banha, F.; Choquer, J.; Chucholl, C.; Tricarico, E. The red swamp crayfish Procambarus clarkii in Europe: Impacts on aquatic ecosystems and human well-being. Limnologica 2016, 58, 78–93. [Google Scholar] [CrossRef]
- Gherardi, F.; Renai, B.; Corti, C. Crayfish predation on tadpoles: A comparison between a native (Austropotamobius pallipes) and an alien species (Procambarus clarkii). Bull. Fr. Peche Piscic. 2001, 361, 659–668. [Google Scholar] [CrossRef]
- Renai, B.; Gherardi, F. Predatory efficiency of crayfish: Comparison between indigenous and non-indigenous species. Biol. Invasions 2004, 6, 89–99. [Google Scholar] [CrossRef]
- Gherardi, F.; Cioni, A. Agonism and interference competition in freshwater decapods. Behaviour 2004, 141, 1297–1324. [Google Scholar]
- Diéguez-Uribeondo, J.; Söderhäll, K. Procambarus clarkii Girard as a vector for the crayfish plague fungus, Aphanomyces astaci Schikora. Aquac. Fish. Manag. 1993, 24, 761–765. [Google Scholar] [CrossRef]
- Anastácio, P.M.; Ferreira, M.P.; Banha, F.; Capinha, C.; Rabaça, J.E. Waterbird-mediated passive dispersal is a viable process for crayfish (Procambarus clarkii). Aquat. Ecol. 2014, 48, 1–10. [Google Scholar] [CrossRef]
- Banha, F.; Marques, M.; Anastácio, P.M. Dispersal of two freshwater invasive macroinvertebrates, Procambarus clarkii and Physella acuta, by off-road vehicles. Aquat. Conserv. 2014, 24, 582–591. [Google Scholar] [CrossRef]
- Santos, J.M.; Amaral, S.D.; Pádua, J. Can fish lifts aid upstream dispersal of the invasive red swamp crayfish (Procambarus clarkii) past high-head hydropower plants? River Res. Appl. 2021, 38, 1519–1523. [Google Scholar] [CrossRef]
- Aquiloni, L.; Ilhéu, M.; Gherardi, F. Habitat use and dispersal of the invasive crayfish Procambarus clarkii in ephemeral water bodies of Portugal. Mar. Freshw. Behav. Physiol. 2005, 38, 225–236. [Google Scholar] [CrossRef]
- Gutiérrez-Yurrita, P.J.; Montes, C. Environmental factors controlling crayfish Procambarus clarkii activity in the Doñana National Park freshwater marsh (SW-Spain). Comp. Biochem. Physiol. A. 1998, 120, 713–721. [Google Scholar] [CrossRef]
- Bernardo, J.M.; Costa, A.M.; Bruxelas, S.; Teixeira, A. Dispersal and coexistence of two non-native crayfish species (Pacifastacus leniusculus and Procambarus clarkii) in NE Portugal over a 10-year period. Knowl. Manag. Aquat. Ecosyst. 2011, 401, 28. [Google Scholar] [CrossRef]
- Anastácio, P.M.; Banha, F.; Capinha, C.; Bernardo, J.M.; Costa, A.M.; Teixeira, A.; Bruxelas, S. Indicators of movement and space use for two co-occurring invasive crayfish species. Ecol. Indic. 2015, 53, 171–181. [Google Scholar] [CrossRef]
- Kerby, J.L.; Riley, S.P.D.; Kats, L.B.; Wilson, P. Barriers and flow as limiting factors in the spread of an invasive crayfish (Procambarus clarkii) in southern California streams. Biol. Conserv. 2005, 126, 402–409. [Google Scholar] [CrossRef]
- Cruz, M.J.; Rebelo, R. Colonization of freshwater habitats by an introduced crayfish, Procambarus clarkii, in Southwest Iberian Peninsula. Hydrobiologia 2007, 575, 191–201. [Google Scholar] [CrossRef]
- Scholtz, G. Phylogeny and evolution. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 10–16. [Google Scholar]
- Newsom, J.E.; Davis, K.B. Osmotic responses of haemolymph in red swamp crayfish (Procambarus clarkii) and white river crayfish (P. zonangulus) to changes in temperature and salinity. Aquaculture 1994, 126, 373–381. [Google Scholar] [CrossRef]
- Bazer, C.E.; Preston, R.L.; Perry, W.L. Increased salinity affects survival and osmotic response of rusty crayfish Orconectes rusticus and northern clearwater crayfish O. propinquus (Decapoda: Astacoidea: Cambaridae) as salinity increases: The potential for estuarine invasions. J. Crustac. Biol. 2016, 36, 607–614. [Google Scholar] [CrossRef]
- Ali, M.Y.; Pavasovic, A.; Dammannagoda, L.K.; Mather, P.B.; Prentis, P.J. Comparative molecular analyses of select pH-and osmoregulatory genes in three freshwater crayfish Cherax quadricarinatus, C. destructor and C. cainii. PeerJ 2017, 5, e3623. [Google Scholar] [CrossRef][Green Version]
- Graczyk, R.; Chachaj, B.; Stanek, M.; Dąbrowski, J.; Gackowski, G. Fertility of spiny-cheek crayfish (Orconectes limosus Raf.) from the Vistula Lagoon. Bull. Environ. Contam. Toxicol. 2019, 102, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Sun, Y. Identification and characterization of salt-tolerance relative miRNAs in Procambarus clarkii by high-throughput sequencing. ExRNA 2019, 1, 18. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Medina-Villar, S. Alien species of Mediterranean origin in the Baltic Sea Region: Current state and risk assessment. Environ. Rev. 2020, 28, 339–356. [Google Scholar] [CrossRef]
- Dörr, A.J.M.; Scalici, M.; Caldaroni, B.; Magara, G.; Scoparo, M.; Goretti, E.; Elia, A.C. Salinity tolerance of the invasive red swamp crayfish Procambarus clarkii (Girard, 1852). Hydrobiologia 2020, 847, 2065–2081. [Google Scholar] [CrossRef]
- Liu, S.; Qi, C.; Jia, Y.; Gu, Z.; Li, E. Growth and intestinal health of the red claw crayfish, Cherax quadricarinatus, reared under different salinities. Aquaculture 2020, 524, 735256. [Google Scholar] [CrossRef]
- Rida, R.; Zein-Eddine, R.; Kreydiyyeh, S.; Garza de Yta, A.; Saoud, I.P. Influence of salinity on survival, growth, hemolymph osmolality, gill sodium potassium ATPase activity, and sodium potassium chloride co-transporter expression in the redclaw crayfish Cherax quadricarinatus. J. World Aquac. Soc. 2021, 52, 466–474. [Google Scholar] [CrossRef]
- Loyacano, H. Some effects of salinity on two populations of red swamp crawfish, Procambarus clarkii. In Proceedings of the 21st Annual Conference, New Orleans, LA, USA, 24–27 September 1967; Southeastern Association of Game and Fish Commissioners: Maggie Valley, NC, USA, 1967; Volume 21, pp. 423–434. [Google Scholar]
- Sharfstein, B.A.; Chafin, C. Red swamp crayfish: Short-term effects of salinity on survival and growth. Prog. Fish-Cult. 1979, 41, 156–157. [Google Scholar] [CrossRef]
- Casellato, S.; Masiero, L. Does Procambarus Clarkii(Girard, 1852) Represent a Threat for Estuarine Brackish Ecosystems of Northeastern Adriatic Coast(Italy)? J. Life Sci. 2011, 5, 549–554. [Google Scholar]
- Bissattini, A.M.; Traversetti, L.; Bellavia, G.; Scalici, M. Tolerance of increasing water salinity in the red swamp crayfish Procambarus clarkii. J. Crustac. Biol. 2015, 35, 682–685. [Google Scholar] [CrossRef]
- Dobrzycka-Krahel, A.; Fidalgo, M.L. Euryhalinity and geographical origin aid global alien crayfish invasions. Water 2023, 15, 569. [Google Scholar] [CrossRef]
- Scalici, M.; Chiesa, S.; Scuderi, S.; Celauro, D.; Gibertini, G. Population structure and dynamics of Procambarus clarkii (Girard, 1852) in a Mediterranean brackish wetland (Central Italy). Biol. Invasions 2010, 12, 1415–1425. [Google Scholar] [CrossRef]
- Nota, A.; Santovito, A.; Gattelli, R.; Tiralongo, F. From Fresh to Salt Waters: First Reports of the Red Swamp Crayfish Procambarus clarkii (Girard, 1852) in Mediterranean Marine Waters. Hydrobiology 2024, 3, 1–10. [Google Scholar] [CrossRef]
- Fidalgo, M.L.; Carvalho, A.P.; Santos, P. Population dynamics of the red swamp crayfish, Procambarus clarkii (Girard, 1852) from the Aveiro region, Portugal (Decapoda, Cambaridae). Crustaceana 2001, 74, 369–375. [Google Scholar]
- Sousa, R.; Freitas, F.E.; Mota, M.; Nogueira, A.J.; Antunes, C. Invasive dynamics of the crayfish Procambarus clarkii (Girard, 1852) in the international section of the River Minho (NW of the Iberian Peninsula). Aquat. Conserv. 2013, 23, 656–666. [Google Scholar] [CrossRef]
- Battisti, C.; Scalici, M. First records of the red swamp crayfish Procambarus clarkii (Girard, 1852)(Decapoda Cambaridae) from a small circum-Sardinian island (central Mediterranean Sea). BioInvasions Rec. 2020, 9, 333–339. [Google Scholar] [CrossRef]
- Palmas, F.; Podda, C.; Frau, G.; Cau, A.; Moccia, D.; Peddio, S.; Solari, P.; Pusceddu, A.; Sabatini, A. Invasive crayfish (Procambarus clarkii, Girard, 1852) in a managed brackish wetland (Sardinia, Italy): Controlling factors and effects on sedimentary organic matter. Estuar. Coast. Shelf Sci. 2019, 231, 106459. [Google Scholar] [CrossRef]
- Meineri, E.; Rodriguez-Perez, H.; Hilaire, S.; Mesleard, F. Distribution and reproduction of Procambarus clarkii in relation to water management, salinity and habitat type in the Camargue. Aquat. Conserv. 2014, 24, 312–323. [Google Scholar] [CrossRef]
- Hill, L.C.; Cancienne, E.A. Grow Crawfish in Rice Fields; Louisiana State University Agricultural and Mechanical College: Baton Rouge, LA, USA, 1963. [Google Scholar]
- Potts, W.T.; Parry, G. Osmotic and Ionic Regulation in Animals; Pergamon Press: Oxford, UK, 1964. [Google Scholar]
- Sarver, R.G.; Flynn, M.A.; Holliday, C.W. Renal Na, K-ATPase and osmoregulation in the crayfish, Procambarus clarkii. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1994, 107, 349–356. [Google Scholar] [CrossRef]
- Vogt, G. Functional anatomy. In Biology of Freshwater Crayfish; Holdich, D.M., Ed.; Blackwell Science: Oxford, UK, 2002; pp. 53–151. [Google Scholar]
- Aquiloni, L.; Tricarico, E.; Gherardi, F. Crayfish in Italy: Distribution, threats and management. Int. Aquat. Res. 2010, 2, 1–14. [Google Scholar]
- Jaffer, Y.D.; Bhat, I.A.; Mir, I.N.; Bhat, R.A.H.; Sidiq, M.J.; Jana, P. Adaptation of cultured decapod crustaceans to changing salinities: Physiological responses, molecular mechanisms and disease implications. Rev. Aquac. 2024, 16, 1520–1543. [Google Scholar] [CrossRef]
- Zhao, Y.; Luo, L.; Liu, D.L.; Huang, J.H.; Jiang, S.G.; Zhou, F.L.; Yang, Q.B.; Jiang, S.; Li, Y.D.; Tan, L.Q. Effect of Acute Salinity Stress on Metabolism, Antioxidant Status, and Histological Structure of Procambarus clarkii. Aquac. Res. 2023, 2023, 2748257. [Google Scholar] [CrossRef]
- Dörr, A.J.; Rodolfi, M.; Scalici, M.; Elia, A.C.; Garzoli, L.; Picco, A.M. Phoma glomerata, a potential new threat to Italian inland waters. J. Nat. Conserv. 2011, 19, 370–373. [Google Scholar] [CrossRef]
- Dörr, A.J.; Elia, A.C.; Rodolfi, M.; Garzoli, L.; Picco, A.M.; D’Amen, M.; Scalici, M. A model of co-occurrence: Segregation and aggregation patterns in the mycoflora of the crayfish Procambarus clarkii in Lake Trasimeno (central Italy). J. Limnol. 2012, 71, 135–143. [Google Scholar] [CrossRef][Green Version]
- Dörr, A.J.M.; Rodolfi, M.; Elia, A.C.; Scalici, M.; Garzoli, L.; Picco, A.M. Mycoflora on the cuticle of the invasive crayfish Procambarus clarkii. Fundam. Appl. Limnol. 2012, 180, 77–84. [Google Scholar] [CrossRef]
- Chiesa, S.; Scalici, M.; Lucentini, L.; Marzano, F.N. Molecular identification of an alien temnocephalan crayfish parasite in Italian freshwaters. Aquat. Invasions 2015, 10, 209–216. [Google Scholar] [CrossRef]
- Martín-Torrijos, L.; Correa-Villalona, A.J.; Pradillo, A.; Diéguez-Uribeondo, J. Coexistence of Two Invasive Species, Procambarus clarkii and Aphanomyces astaci, in Brackish Waters of a Mediterranean Coastal Lagoon. Front. Ecol. Evol. 2021, 8, 503. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).