Abstract
Interest in microalgae cultivation is continuously growing due to their tremendous potential for a broad spectrum of applications. The established units for the measurement of a crucial parameter for algae growth, i.e., the light dosage in photobioreactors, are susceptible to severe criticism. Various units are currently utilized without however accounting for the volume of the culture exposed to light, which might differ depending on the reactor volume. Two new units of light irradiation measurement are proposed, namely, (lux-hour) and . For the latter, , the parameters taken into account include the light illuminance, light exposure time, and volume and surface of the culture in the reactor, which are commonly measured. Cylindrical and flat-panel reactors are studied to determine the constant light illuminance and variant illuminance within a day period. It is shown that the unit is much more objective for expressing the light availability in photobioreactors than the current and most common expressions. The proposed parameter could be useful for comparisons of different experiments in a reactor or for up-scaling purposes.
1. Introduction
Microalgae are autotrophic organisms that can grow by using various nutrient substrates depending on the species; recently, efforts have been focused on the valorization of effluents as a low-cost nutrient source for microalgae. Algal biomass has a very broad range of applications, which span from wastewater treatment [1,2,3] to biodiesel production from cell lipids [4,5,6], food products, or high-added-value substances [7,8,9]. Therefore, microalgae cultivation in photobioreactors has received much research attention in recent decades. The active introduction of various microalgae species in the treatment of wastewater from diverse sources—such as municipal, industrial, and agricultural effluents—has garnered increasing attention due to their dual capability of effective bioremediation and biomass generation. Microalgae can assimilate excess nutrients, heavy metals, and organic pollutants, transforming wastewater into a more environmentally benign discharge while simultaneously producing a biomass rich in lipids, proteins, and pigments of commercial value [10,11]. Among the cultivation strategies, mixotrophic growth, combining autotrophic and heterotrophic modes, stands out for enhancing both pollutant removal and biomass yield. The light availability in mixotrophic systems is a critical factor that not only drives photosynthesis but also synergistically boosts metabolic activity and resource conversion [12]. Adequate illumination significantly increases the efficiency of bioremediation processes and supports the accumulation of high-value biomass components [13,14], making it a vital parameter in wastewater-based microalgal cultivation strategies.
Various parameters are taken into account for efficient cultivation in photobioreactors, such as the light conditions (the intensity of irradiation, time of exposure, etc.), CO2 availability, concentration of nutrients, and applied diet pattern. Numerous studies have focused on the effect of light irradiation on the growth rate of algae and/or the efficiency of the process used for wastewater treatment through the assimilation of various nitrogen forms [15,16]. Due to the importance of the light source and irradiation in the photosynthetic process, various light units have been proposed and are commonly used: the light irradiation dosage (often termed light intensity) and light exposure time are taken into account in light illuminance (expressed in lx) [17,18], photosynthetic photon flux density (PPFD, expressed in μmol/s/m2) [16,19,20,21], and light intensity (expressed in mW/cm2) [22,23]. In addition, the characteristics of artificial light sources may be given, such as the watts and amperes provided by a certain LED source [15]. However, the established expressions of light dosage in photobioreactors do not account for the active volume of the culture that is exposed to light. Depending on the reactor configuration, different volumes of biomass can be exposed to the same light source, therefore impeding the comparison of different experimental tests or introducing significant errors in the evaluation of the appropriate reactor size for up-scaling purposes.
The volume of the microalgae culture that is exposed to light is not considered in these parameters, although the corresponding area of the biomass is taken into account. Nevertheless, the area rather than the volume of a two-dimensional leaf is crucial for the estimation and assessment of the photosynthetic process in plants; however, the photosynthetic activity and the respective growth of microalgae in photobioreactors is three-dimensional, and, therefore, the active volume of the algal culture exposed to a light source is of great importance. Thus, the objective of this work is to suggest a new expression for the measurement of light availability in algal photobioreactors by taking into account the volume of the algal culture. Such a unit may facilitate the comparison of different experimental results and contribute to appropriate calculations for the up-scaling of lab-scale photobioreactors.
2. Theoretical Aspects
For the simultaneous incorporation of light illuminance and exposure time in a single parameter, a new expression is suggested, the lux-hour () factor, corresponding to (watt-hours) used to anticipate energy consumption in power calculations. For example, the irradiation of a sample for 2 under 5000 corresponds to 2 × 5000 = 10,000 . In addition, in order to take into account the irradiated surface and the volume of the culture, the parameter volume-to-surface ratio () of the culture can be utilized, given by the following equation:
where:
is the active biomass volume, i.e., the culture volume inside the reactor, m3.
is the area of the culture that is exposed to irradiation, m2.
The units of correspond to those of the length. Furthermore, the daily light exposure time () is required to anticipate the daily light exposure time in . For example, under a light-to-dark regime of 16:8 (16 of lighting and 8 of darkness), the .
Based on the above, a new parameter is suggested for the assessment of the light that is available to microalgae in a photobioreactor, the light irradiation availability () factor, expressed as follows:
It follows that the units of correspond to
Nevertheless, in a case where the light illuminance () is not constant within a day but represents a function of time (for example, when sunlight is used as a source), then , and the new parameter is calculated as
where:
is the lighting period in h/day.
When LI = constant, Equation (2) can be derived from Equation (4), by integrating values from 0 to the DLET.
The application potential of the new suggested parameter is justified and discussed in the following. However, it should be noted that the main advantage of the LID parameter is the incorporation of the volume-to-surface ratio into the light irradiation unit, corresponding to the light that is available to the microalgae. However, it is not related to the utilization of light by the microalgae, and it certainly does not reflect species growth, since several other factors usually affect cultivation, such as the strain of the photosynthetic cells, the cell density, and the mixing pattern in the photobioreactor. Nevertheless, such an approach is not considered in any light-related parameter used so far.
3. Results and Discussion
Microalgae cultivation in a vertically configured cylindrical photobioreactor is considered as an example, with illumination from two light sources installed at the perimeter of the reactor, as shown in Figure 1. The irradiated surface corresponds to the side area of the cylinder. For this geometry, the volume-to-surface ratio () of the microalgae culture can be expressed as
where:
: the diameter of the cylinder, m.
: the height of the cylinder, m.
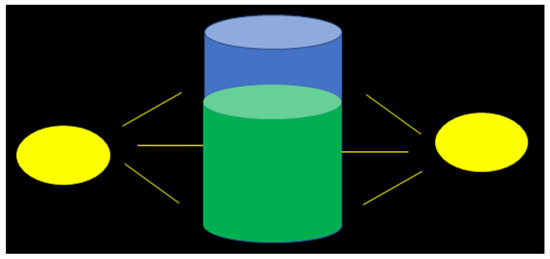
Figure 1.
Conceptual drawing of the irradiation of a cylindrical reactor from two light sources at the perimeter of the system.
In order to better illustrate the rationale behind the suggested parameter, four different (lab-scale and pilot-scale) experimental configurations are used, performed using different vertical cylindrical photobioreactors with various diameters and height dimensions, as shown in Table 1. The surface, volume, volume-to-surface ratio, light illuminance, exposure time, and values calculated for these reactor configurations are presented in Table 1, assuming the same light illumination and exposure time conditions.

Table 1.
Parameters calculated for four assumed configurations of algae culture photobioreactors.
As presented in Table 1, the diameter and height of the four reactors are chosen to ensure that the reactors exhibit the same side surface, A. However, the active volume of the biomass differs, almost by an order of magnitude, ranging from 1.57 L (considered typical of a lab-scale reactor) to 62.8 L (typical of a semi-pilot-scale reactor). The same surface A is available for irradiation in all reactor configurations, while the light illuminance and exposure time are identical in all four experiments, indicating that the algae are exposed to the same light conditions in all four experiments. When the more common parameters are used to identify the light irradiation and intensity, such as lx, W/m2, or μmol/s/m2 (photosynthetic photon flux density), the same value for each one is assigned in the four runs, although the light available to the microalgae differs due to the differences in the biomass volumes. As shown in Table 1, the microalgae in experiments 1 and 3 are exposed to much less light irradiation than those in experiments 2 and 4 as a result of the higher volume. This information, i.e., the active biomass volume, is not taken into account in the parameters usually applied for light irradiation assessment. On the contrary, this particular volume information is embedded in the parameter and the respective units; as can be seen in the last row of Table 1, the values reveal that the light available to the algae is quite different in these four experiments (the values are expressed in , that is, kilo-lux-hour per meter per day). From the above analysis, it is evident that the values and the respective units can better express the light that is available to the microalgae and properly distinguish among the different experimental conditions or photobioreactor configurations.
The concept of can be used in any type of photobioreactor (tubular, flat-panel, etc.) provided that the volume and surface of the reactor (which are occupied by the algal biomass phase) are known. For example, for the case of a flat-panel reactor, the calculation of the volume and surface can be readily accomplished: let us assume a laboratory-scale flat-panel photobioreactor with a 4 cm width, a 40 cm height, and a 30 cm length, where the algae liquor occupies a height of 35 cm, while the reactor is irradiated from one side, as shown in Figure 2.
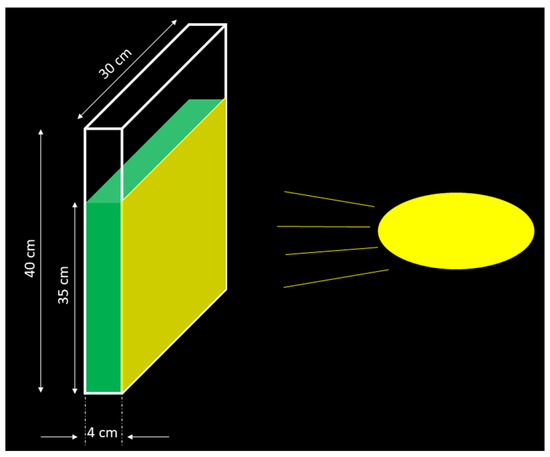
Figure 2.
A sketch showing the irradiation of a flat-panel reactor from one side.
The irradiated surface (highlighted in yellow in Figure 2) is 35 × 30 = 1050 cm2, while the volume of the algae liquor is 35 × 30 × 4 = 4200 cm3. It follows that the volume-to-surface ratio z is equal to 0.04 m. Assuming that the reactor is irradiated with 10,000 lx for 12 h/day, it follows that the value is equal to 10,000 × 12/0.04 = 3,000,000 = 3000. If the reactor is irradiated by two light sources from two sides, then the surface would be 2 × 35 × 30 = 2100 cm2, resulting in a twofold increase in LIA.
When LI is not constant but is rather a function of time, LI(t), the corresponding results are calculated using Equation (4). Let us assume that the flat-panel reactor configuration in Figure 2 is irradiated by a variable light intensity within a 24 h/day period. For example, in order to simulate natural sunlight, the following (Gaussian) function can be used to anticipate the intensity of the light source:
Parameter a corresponds to the maximum (height) of the peak. Parameter b determines the position of the center of the peak, and parameter c determines the width of the peak. By using a = 100,000 lxh/day, b = 14 h/day, and c = 2.1 h/day, the natural light over a 24 h/day period in a certain area during summertime, can be simulated. For example, as shown in Figure 3, where the light illuminance typical of a Mediterranean country is provided, the light intensity is zero during the time period from 12:00 to 7:00 (sunrise occurs between 6 and 7 a.m.), reaches its maximum at noon (14:00), and becomes zero again at around 21:00 (typical time for sunset in summertime). By performing graphical integration, it is calculated that LI(t) = 526,377.5 lxh/day. Thus, since a value of z = 0.04 is assumed for the flat-panel reactor, using Equation (4), it follows that LIA = 526,377.5/0.04 = 13,159,437.5 = 13,159.4.
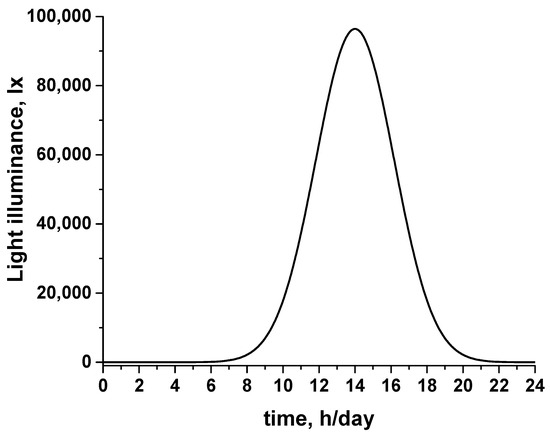
Figure 3.
The light illuminance of a light source as a function of time, simulating the typical variance of sunlight intensity within a 24 h/day period in summertime in a Mediterranean country.
Finally, it is worth briefly discussing the case of continuous-flow tubular reactors. Continuous-flow tubular reactors are widely used for microalgae cultivation due to their high surface-area-to-volume ratio, which enhances light availability—an essential factor for photosynthesis and biomass productivity. A proper light distribution along the reactor ensures efficient energy conversion, supporting faster growth rates and improved nutrient uptake. Optimizing the light intensity and exposure time in these systems is key to maximizing microalgal performance and product yield. The above concept can be easily extended in order to be applied to continuous-flow tubular reactors. Assuming a reactor module consisting of 10 cylindrical tubes that are 2 m long with a 0.04 m diameter, irradiated from one side, the total volume of the system is , while the overall side area equals . However, since the reactor is irradiated only from one side, half of the side area has to be used for the calculation of z, that is, . Therefore, under a light intensity of 15,000 lx for 14 h/day, the LIA value is estimated to be 15,000 × 14/0.04 = 5,250,000 = 5250.
4. Conclusions
The light conditions inside photobioreactors are crucial for the fine-tuning of algae cultivation. The established units for the expression of light availability inside reactors do not take into account the volume of the culture and can lead to misinterpretations. Although the area of a leaf is crucial to estimate photosynthetic activity in plant growth studies, the growth of algae in photobioreactors is three-dimensional. Therefore, light availability over the overall biomass volume must be taken into account. The incorporation of relevant factors into a single parameter, such as the biomass volume-to-surface ratio, light illuminance LI in lux-hours (), and exposure time over a day period, resulted in the definition of a gross parameter, the light irradiation availability (), with the unit . The use of the new suggested parameter, the , was extended to include constant and variant light illuminance within a day period. It was shown that values could vary from 1600 to 64,000 in four different vertical photobioreactor configurations, even though light illuminance was assumed to be the same in all four cases. values and the respective units provide objective information about the available light in a photobioreactor. These values can be used for a proper comparison of experiments conducted in various reactor configurations, including lab-scale and pilot-scale tests, while the parameter can be useful for up-scaling calculations.
Author Contributions
Conceptualization, C.T.; methodology, C.T.; investigation, C.T.; data curation, C.T. and P.S.; writing—original draft preparation, C.T.; writing—review and editing, P.S.; visualization, C.T.; supervision, P.S.; project administration, P.S.; funding acquisition, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the EU European Climate Infrastructure and Environment Executive Agency (CINEA), project FUELPHORIA PN 101118286. The views and opinions expressed are, however, those of the authors only and do not necessarily reflect those of the European Union or CINEA. Neither the European Union nor CINEA can be held responsible for them.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bang Truong, H.; Nguyen, T.H.T.; Ba Tran, Q.; Son Lam, V.; Thao Nguyen Nguyen, T.; Cuong Nguyen, X. Algae-constructed wetland integrated system for wastewater treatment: A review. Bioresour. Technol. 2024, 406, 131003. [Google Scholar] [CrossRef] [PubMed]
- Phyu, K.; Zhi, S.; Graham, D.W.; Cao, Y.; Xu, X.; Liu, J.; Wang, H.; Zhang, K. Impact of indigenous vs. cultivated microalgae strains on biomass accumulation, microbial community composition, and nutrient removal in algae-based dairy wastewater treatment. Bioresour. Technol. 2025, 426, 132349. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Qiang, X.; Song, Y.; Gu, W.; Ma, Z.; Wang, G. Design, construction and application of algae-bacteria synergistic system for treating wastewater. J. Environ. Manag. 2024, 366, 121720. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Chou, S.K.; Cao, S.; Wu, C.; Zhou, Z. An updated comprehensive techno-economic analysis of algae biodiesel. Bioresour. Technol. 2013, 145, 150–156. [Google Scholar] [CrossRef]
- Du, Y.; Schuur, B.; Samorì, C.; Tagliavini, E.; Brilman, D.W.F. Secondary amines as switchable solvents for lipid extraction from non-broken microalgae. Bioresour. Technol. 2013, 149, 253–260. [Google Scholar] [CrossRef]
- Samorì, C.; López Barreiro, D.; Vet, R.; Pezzolesi, L.; Brilman, D.W.F.; Galletti, P.; Tagliavini, E. Effective lipid extraction from algae cultures using switchable solvents. Green Chem. 2013, 15, 353–356. [Google Scholar] [CrossRef]
- Lo, C.; Wijffels, R.H.; Eppink, M.H.M. Lipid recovery from deep eutectic solvents by polar antisolvents. Food Bioprod. Process. 2024, 143, 21–27. [Google Scholar] [CrossRef]
- Zhu, J.; Xiao, X.; Du, W.; Cai, Y.; Yang, Z.; Yin, Y.; Wakisaka, M.; Wang, J.; Zhou, Z.; Liu, D.; et al. Leveraging microalgae as a sustainable ingredient for meat analogues. Food Chem. 2024, 450, 139360. [Google Scholar] [CrossRef]
- Fernandes, A.S.; Caetano, P.A.; Jacob-Lopes, E.; Zepka, L.Q.; de Rosso, V.V. Alternative green solvents associated with ultrasound-assisted extraction: A green chemistry approach for the extraction of carotenoids and chlorophylls from microalgae. Food Chem. 2024, 455, 139939. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Zhou, T.; Xie, Z.; Jiang, X.; Zou, X.; Cheng, J.; Chen, C.; Kuang, C.; Ye, J.; Wang, Y.; Liu, F. Efficient Solar-Powered Bioremediation of Hexavalent Chromium in Contaminated Waters by Chlorella sp. MQ-1. Water 2024, 16, 3315. [Google Scholar] [CrossRef]
- Li, T.; Zheng, Y.; Yu, L.; Chen, S. Mixotrophic cultivation of a Chlorella sorokiniana strain for enhanced biomass and lipid production. Biomass Bioenergy 2014, 66, 204–213. [Google Scholar] [CrossRef]
- Klamczynska, B.; Mooney, W.D. Chapter 20—Heterotrophic Microalgae: A Scalable and Sustainable Protein Source. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 327–339. [Google Scholar]
- Mehta, A.K.; Chakraborty, S. Multiscale integration of mixotrophic microalgal cultivation, lipid synthesis, rapid biomass harvesting, and nutrient recycling in pilot-scale photobioreactors. Algal Res. 2021, 53, 102146. [Google Scholar] [CrossRef]
- Amiri, R.; Ahmadi, M. Treatment of wastewater in sewer by Spirogyra sp. green algae: Effects of light and carbon sources. Water Environ. J. 2020, 34, 311–321. [Google Scholar] [CrossRef]
- Palikrousis, T.L.; Manolis, C.; Kalamaras, S.D.; Samaras, P. Effect of Light Intensity on the Growth and Nutrient Uptake of the Microalga Chlorella sorokiniana Cultivated in Biogas Plant Digestate. Water 2024, 16, 2782. [Google Scholar] [CrossRef]
- Jia, H.; Yuan, Q. Ammonium removal using algae–bacteria consortia: The effect of ammonium concentration, algae biomass, and light. Biodegradation 2018, 29, 105–115. [Google Scholar] [CrossRef]
- Satthong, S.; Saego, K.; Kitrungloadjanaporn, P.; Nuttavut, N.; Amornsamankul, S.; Triampo, W. Modeling the effects of light sources on the growth of algae. Adv. Differ. Equ. 2019, 2019, 170. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Bouterfas, R.; Belkoura, M.; Dauta, A. Light and temperature effects on the growth rate of three freshwater [2pt] algae isolated from a eutrophic lake. Hydrobiologia 2002, 489, 207–217. [Google Scholar] [CrossRef]
- Lee, M.-C.; Yeh, H.-Y.; Jhang, F.-J.; Lee, P.-T.; Lin, Y.-K.; Nan, F.-H. Enhancing growth, phycoerythrin production, and pigment composition in the red alga Colaconema sp. Through optimal environmental conditions in an indoor system. Bioresour. Technol. 2021, 333, 125199. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Song, X.; Jiang, M.; Zhao, X.; Cao, X. The inhibitory effects of simulated light sources on the activity of algae cannot be ignored in photocatalytic inhibition. Chemosphere 2022, 309, 136611. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, C.; Zhao, X.; Wang, Y.; Li, Z.; Zhou, Y.; Ren, G. Algae-Bacteria cooperated microbial ecosystem: A self-circulating semiartificial photosynthetic purifying strategy. Sci. Total Environ. 2023, 905, 167187. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).