Legionella in Urban and Rural Water, a Tale of Two Environments
Abstract
1. Introduction
2. Material and Methods
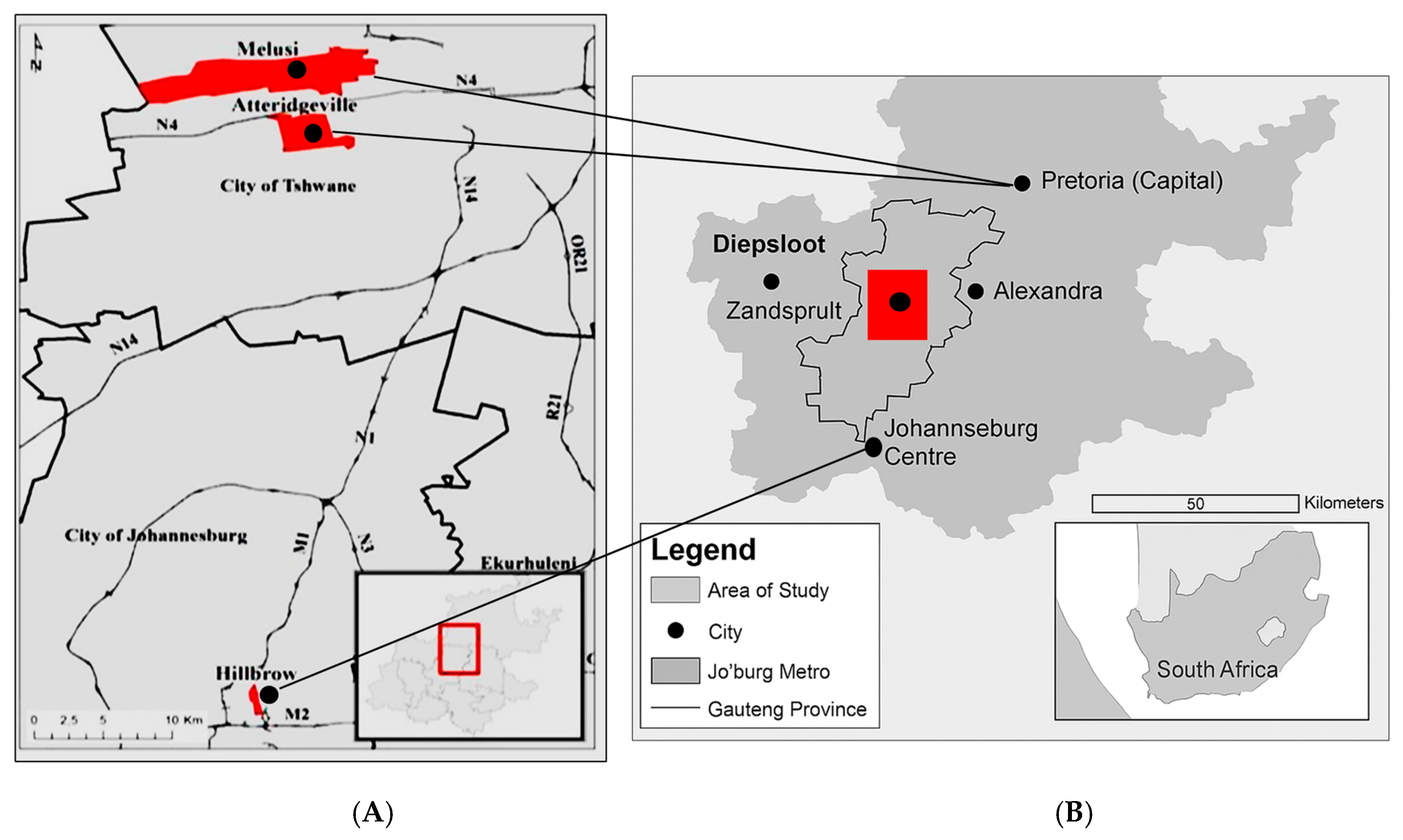
2.1. Study Design and Site Description
2.2. Ethical Clearance
2.3. Pre-Sampling Building Site Assessment
2.4. Sample Collection
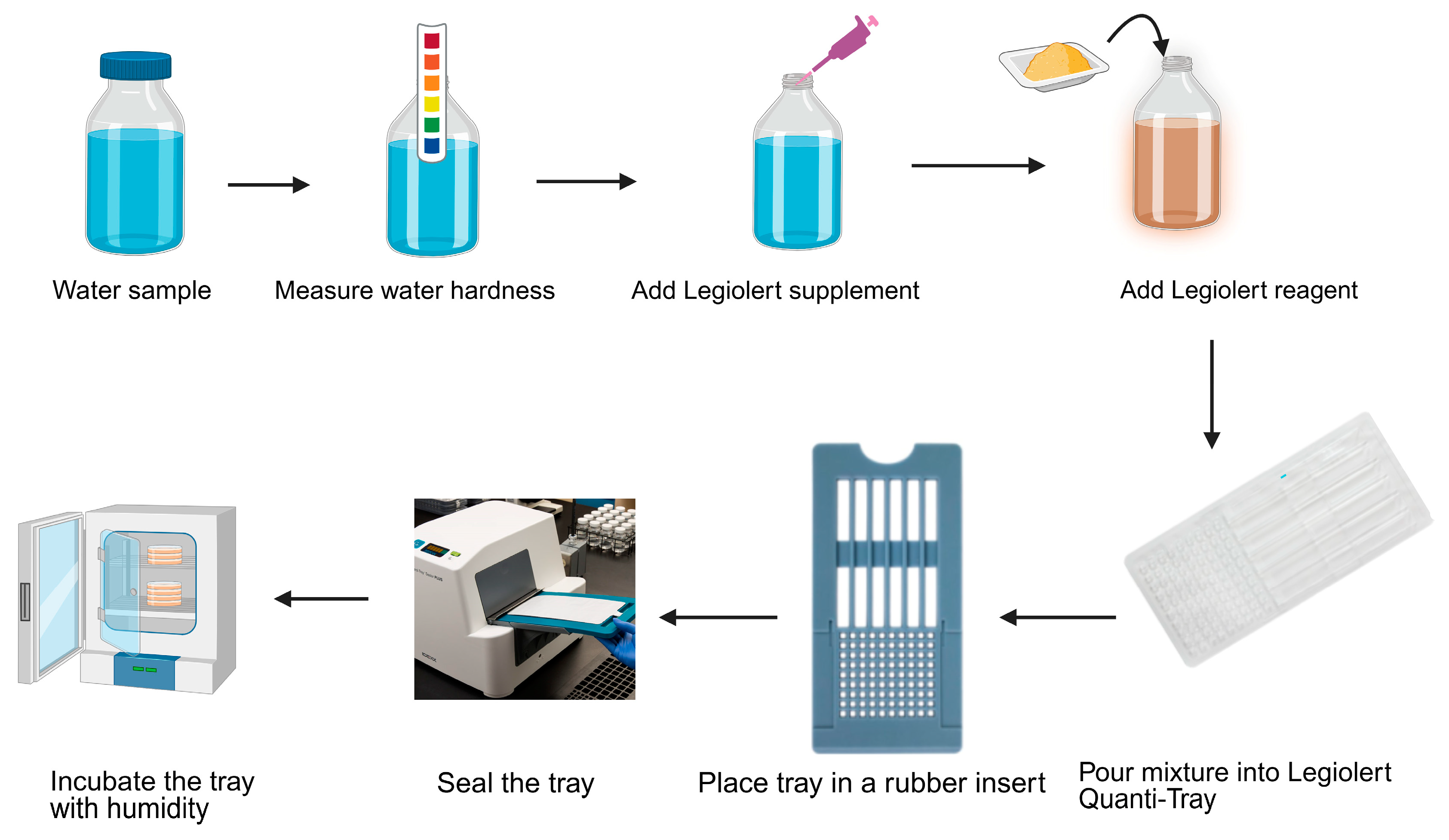
2.5. In-Field Water Analysis
2.6. Bacterial Control Strains
2.7. Detection of E. coli and Total Coliforms in Water Samples
2.8. Microbiological Detection of Legionella Species
2.8.1. Detection of Legionella pneumophila by IDEXX LegiolertTM
2.8.2. Amoeba Enrichment Technique
2.9. Molecular Detection of Legionella Species
2.9.1. DNA Extraction
2.9.2. Identification of Legionella by Polymerase Chain Reaction (PCR)
2.9.3. DNA Electrophoresis
2.9.4. Sequencing
2.9.5. Real-Time PCR
2.9.6. Data Analysis
3. Results
3.1. Study Site: Urban and Rural Building Classification
3.2. Pre-Sampling Walkthrough Site Assessment for Legionella spp. Exposure
3.3. In-Field Water Analysis
3.4. Detection of E. coli and Total Coliform
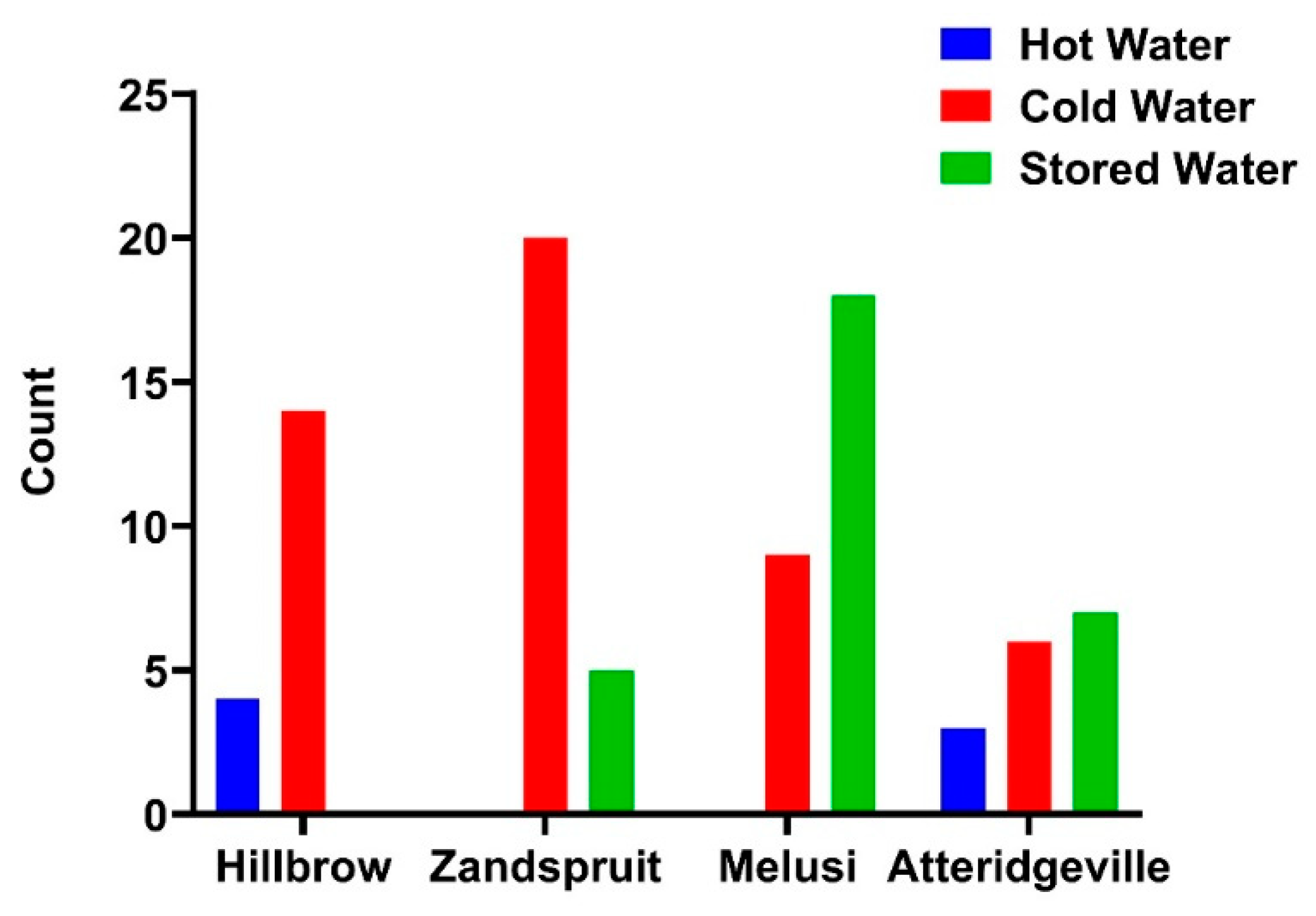
3.5. Legionella pneumophila Detected by IDEXX LegiolertTM
3.6. Microbial Isolation of Free-Living Amoeba and Amoeba-Associated Legionella
3.7. Molecular Detection of Legionella Species in Water Samples
3.8. Real-Time PCR
3.9. Sequencing
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Romano Spica, V.; Borella, P.; Bruno, A.; Carboni, C.; Exner, M.; Hartemann, P.; Gianfranceschi, G.; Laganà, P.; Mansi, A.; Montagna, M.T. Legionnaires’ Disease Surveillance and Public Health Policies in Italy: A Mathematical Model for Assessing Prevention Strategies. Water 2024, 16, 2167. [Google Scholar] [CrossRef]
- Viasus, D.; Gaia, V.; Manzur-Barbur, C.; Carratalà, J. Legionnaires’ disease: Update on diagnosis and treatment. Infect. Dis. Ther. 2022, 11, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Staff, M.; Nyinawingeri, A. The increasing health burden of Legionella pneumophila in NSW. Infect. Dis. Health 2024, 29, 137–143. [Google Scholar] [CrossRef]
- Cullom, A.C.; Martin, R.L.; Song, Y.; Williams, K.; Williams, A.; Pruden, A.; Edwards, M.A. Critical review: Propensity of premise plumbing pipe materials to enhance or diminish growth of Legionella and other opportunistic pathogens. Pathogens 2020, 9, 957. [Google Scholar] [CrossRef]
- Ha, R.; Heilmann, A.; Lother, S.A.; Turenne, C.; Alexander, D.; Keynan, Y.; Rueda, Z.V. The Adequacy of Current Legionnaires’ Disease Diagnostic Practices in Capturing the Epidemiology of Clinically Relevant Legionella: A Scoping Review. Pathogens 2024, 13, 857. [Google Scholar] [CrossRef]
- Graham, F.F.; Finn, N.; White, P.; Hales, S.; Baker, M.G. Global perspective of Legionella infection in community-acquired pneumonia: A systematic review and meta-analysis of observational studies. Int. J. Environ. Res. Public health 2022, 19, 1907. [Google Scholar] [CrossRef]
- Wolter, N.; Carrim, M.; Walaza, S.; Chandu, L.; Morifi, M.; Lawrence, C.; Cohen, C.; von Gottberg, A. Notified Legionnaires’ disease in South Africa, 2018–2020. NICD Bull. 2020, 18, 159–164. [Google Scholar]
- Shetty, S.; Kenjar, A.; Raj, J.R.M.; Akhila, D.; Karunasagar, I.; Vittal, R. Prevalence and Characterization of Legionella pneumophila and Related Species from Water-Based Recreational Sites. J. Health Allied Sci. NU 2024, 14, 260–266. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, D. Legionella. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1547–1557. [Google Scholar]
- Cavallaro, A. The Diversity of Interactions Between Legionella Species and Aquatic Bacteria; ETH Zurich: Zürich, Switzerland, 2024. [Google Scholar]
- Xi, H.; Ross, K.E.; Hinds, J.; Molino, P.J.; Whiley, H. Efficacy of chlorine-based disinfectants to control Legionella within premise plumbing systems. Water Res. 2024, 259, 121794. [Google Scholar] [CrossRef]
- Monistero, V.; Vicari, N.; Prati, P.; Bragoni, R.; Gazzola, A.; Sala, L.; Maisano, A.; Moroni, P.; Bronzo, V.; Luini, M.V. A rapid and reliable method for early Legionella pneumophila identification and characterization in support of the epidemiology study. Front. Microbiol. 2024, 15, 1452861. [Google Scholar] [CrossRef]
- Reinares Ortiz, J.; Pérez-Serrano, J.; González-Rubio, J.M.; González-Camacho, F. Two Sporadic Cases of Legionellosis Associated with the Use of Domestic Ultrasonic Humidifiers. Microorganisms 2024, 12, 2139. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Allam, C.; Ruiz-Spinelli, A.; Jarraud, S. Severe Legionnaires’ disease. Ann. Intensive Care 2024, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Harte, D. Literature Review: Legionella in Recycled Wastewater and Greywater; ESR Environmental Health Report; Client Report No. FW24021; Health New Zealand: Wellington, New Zealand, 2024.
- La Sorda, M.; Palucci, I.; Natalini, D.; Fillo, S.; Giordani, F.; Paglione, F.; Monte, A.; Lista, F.; Mancini, F.; Girolamo, A. Case report: First report of Legionella pneumophila and Bordetella bronchiseptica coinfection in an immunocompromised patient. Front. Med. 2024, 11, 1470567. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Chu, R.; Wu, Q.; Sheng, J.; Liu, P.; Ke, J.; Duan, M.; Li, Q. Evaluation of clinical characteristics of Legionella pneumophila pneumonia diagnosed by metagenomic Next-Generation Sequencing: A retrospective study. medRxiv 2024. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Prosser, T.; Stevens, M. Opportunistic pathogens in drinking water distribution systems—A review. Microorganisms 2024, 12, 916. [Google Scholar] [CrossRef]
- Barbosa, A.; Azevedo, N.F.; Goeres, D.M.; Cerqueira, L. Ecology of Legionella pneumophila biofilms: The link between transcriptional activity and the biphasic cycle. Biofilm 2024, 7, 100196. [Google Scholar] [CrossRef]
- Ariyadasa, S.; van Hamelsveld, S.; Taylor, W.; Lin, S.; Sitthirit, P.; Pang, L.; Billington, C.; Weaver, L. Diversity of Free-Living Amoebae in New Zealand Groundwater and Their Ability to Feed on Legionella pneumophila. Pathogens 2024, 13, 665. [Google Scholar] [CrossRef]
- Margot, C.; Rhoads, W.; Gabrielli, M.; Olive, M.; Hammes, F. Dynamics of drinking water biofilm formation associated with Legionella spp. colonization. NPJ Biofilms Microbiomes 2024, 10, 101. [Google Scholar] [CrossRef]
- Moeletsi, M.E. Socio-economic barriers to adoption of electric vehicles in South Africa: Case study of the gauteng province. World Electr. Veh. J. 2021, 12, 167. [Google Scholar] [CrossRef]
- Muchemwa, M.; Batisai, K. Social networks and residential choice: Zimbabwean migrants’ experiences in Hillbrow, Johannesburg. Soc. Sci. Humanit. Open 2024, 10, 101090. [Google Scholar] [CrossRef]
- Bastaraud, A.; Cecchi, P.; Handschumacher, P.; Altmann, M.; Jambou, R. Urbanization and waterborne pathogen emergence in low-income countries: Where and how to conduct surveys? Int. J. Environ. Res. Public Health 2020, 17, 480. [Google Scholar] [CrossRef] [PubMed]
- Potgieter, N.; van der Loo, C.; Barnard, T.G. Co-existence of free-living amoebae and potential human pathogenic bacteria isolated from rural household water storage containers. Biology 2021, 10, 1228. [Google Scholar] [CrossRef] [PubMed]
- Slavik, I.; Oliveira, K.R.; Cheung, P.B.; Uhl, W. Water quality aspects related to domestic drinking water storage tanks and consideration in current standards and guidelines throughout the world—A review. J. Water Health 2020, 18, 439–463. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.; Silva, A.R.; Melo, L.F. Legionella and biofilms—Integrated surveillance to bridge science and real-field demands. Microorganisms 2021, 9, 1212. [Google Scholar] [CrossRef]
- The National Institute for Communicable Diseases (NICD), Division of Public Health, Surveillance and Response. Notifiable Medical Conditions Surveillance System. Available online: https://www.health.gov.au/our-work/nndss (accessed on 20 March 2025).
- Muchesa, P.; Leifels, M.; Jurzik, L.; Barnard, T.; Bartie, C. Detection of amoeba-associated Legionella pneumophila in hospital water networks of Johannesburg. South. Afr. J. Infect. Dis. 2018, 33, 72–75. [Google Scholar] [CrossRef]
- Couturier, J.; Ginevra, C.; Nesa, D.; Adam, M.; Gouot, C.; Descours, G.; Campèse, C.; Battipaglia, G.; Brissot, E.; Beraud, L. Transmission of Legionnaires’ disease through toilet flushing. Emerg. Infect. Dis. 2020, 26, 1526. [Google Scholar] [CrossRef]
- Bartie, C.; Muchesa, P.; Barnard, T.G. An Investigation into the Presence of Free-Living Amoebae and Amoeba Resistant Bacteria in Drinking Water Distribution Systems of Health Care Institutions in Johannesburg, South Africa: Report to the Water Research Commission; Water Research Commission: Pretoria, South Africa, 2016. [Google Scholar]
- Omar, K.; Barnard, T. Detection of diarrhoeagenic Escherichia coli in clinical and environmental water sources in South Africa using single-step 11-gene m-PCR. World J. Microbiol. Biotechnol. 2014, 30, 2663–2671. [Google Scholar] [CrossRef]
- Rafiee, M.; Jahangiri-Rad, M.; Hajjaran, H.; Mesdaghinia, A.; Hajaghazadeh, M. Detection and identification of Legionella species in hospital water supplies through Polymerase Chain Reaction (16S rRNA). J. Environ. Health Sci. Eng. 2014, 12, 1–6. [Google Scholar] [CrossRef]
- Schwake, D.O.; Alum, A.; Abbaszadegan, M. Impact of environmental factors on Legionella populations in drinking water. Pathogens 2015, 4, 269–282. [Google Scholar] [CrossRef]
- Tambi, A.; Brighu, U.; Gupta, A. Methods for detection and enumeration of coliforms in drinking water: A review. Water Supply 2023, 23, 4047–4058. [Google Scholar] [CrossRef]
- Yao, X.H.; Shen, F.; Hao, J.; Huang, L.; Keng, B. A review of Legionella transmission risk in built environments: Sources, regulations, sampling, and detection. Front. Public Health 2024, 12, 1415157. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.R.; Rhoads, W.J.; Keane, T.; Salehi, M.; Hamilton, K.; Pieper, K.J.; Cwiertny, D.M.; Prévost, M.; Whelton, A.J. Considerations for large building water quality after extended stagnation. AWWA Water Sci. 2020, 2, e1186. [Google Scholar] [CrossRef] [PubMed]
- Shangase, S.I.; Ndlovu, T.S.; Pillay, P. The Evaluation of the Quality of Sanitation and Stored Water for Domestic Use in the Umlazi P Section Informal Settlement in South Africa. Stud. J. Health Res. Afr. 2024, 5, 1198. [Google Scholar] [CrossRef]
- Arberas-Jiménez, I.; Sifaoui, I.; Reyes-Batlle, M.; Rizo-Liendo, A.; Sancho, L.; Urruticoechea, A.; Piñero, J.E.; Lorenzo-Morales, J. Ultraviolet–Chlorine combined treatment efficiency to eliminate Naegleria fowleri in artificial surf lagoons. Heliyon 2022, 8, e11625. [Google Scholar] [CrossRef]
- van der Kooij, D.; Veenendaal, H.R.; Italiaander, R. Corroding copper and steel exposed to intermittently flowing tap water promote biofilm formation and growth of Legionella pneumophila. Water Res. 2020, 183, 115951. [Google Scholar] [CrossRef]
- Szwetkowski, K.J.; Falkinham, J.O., III. Methylobacterium spp. as emerging opportunistic premise plumbing pathogens. Pathogens 2020, 9, 149. [Google Scholar] [CrossRef]
- Clark, G.G.; Zhang, G.; Zhao, S.; Nguyen, T.H. Assessment of Legionella pneumophila in Rural Homes Supplied by Private Well Water. ACS ES&T Water 2025, 5, 1041–1054. [Google Scholar]
- Girolamini, L.; Salaris, S.; Pascale, M.R.; Mazzotta, M.; Cristino, S. Dynamics of Legionella community interactions in response to temperature and disinfection treatment: 7 years of investigation. Microb. Ecol. 2022, 83, 353–362. [Google Scholar] [CrossRef]
- Martin, R.L.; Harrison, K.; Proctor, C.R.; Martin, A.; Williams, K.; Pruden, A.; Edwards, M.A. Chlorine disinfection of Legionella spp.; L. pneumophila, and acanthamoeba under warm water premise plumbing conditions. Microorganisms 2020, 8, 1452. [Google Scholar] [CrossRef]
- Pinel, I.; Hankinson, P.; Moed, D.; Wyseure, L.; Vrouwenvelder, J.S.; van Loosdrecht, M.C. Efficient cooling tower operation at alkaline pH for the control of Legionella pneumophila and other pathogenic genera. Water Res. 2021, 197, 117047. [Google Scholar] [CrossRef]
- McFarland, M.L.; Provin, T.L.; Boellstorff, D.E. Drinking Water Problems: Corrosion; E-616; Texas AgriLife Extension Service; The Texas A&M System: College Station, TX, USA, 2024. [Google Scholar]
- Atkinson, A.J.; Morrison, C.M.; Frehner, W.; Gerrity, D.; Wert, E.C. Design and operational considerations in response to Legionella occurrence in Las Vegas Valley groundwater. Water Res. 2022, 220, 118615. [Google Scholar] [CrossRef] [PubMed]
- Mraz, A.L.; Weir, M.H. Knowledge to predict pathogens: Legionella pneumophila lifecycle systematic review part II growth within and egress from a host cell. Microorganisms 2022, 10, 141. [Google Scholar] [CrossRef] [PubMed]
- Assaidi, A.; Ellouali, M.; Latrache, H.; Zahir, H.; Karoumi, A.; Mliji, E.M. Chlorine disinfection against Legionella pneumophila biofilms. J. Water Sanit. Hyg. Dev. 2020, 10, 885–893. [Google Scholar] [CrossRef]
- Nkwenkwezi, M.G. Applications of Statistical Techniques on the Assessment of Water Quality Parameters. Master’s Thesis, University of Johannesburg, Johannesburg, South Africa, 2021. [Google Scholar]
- Pijnacker, R.; Brandsema, P.; Euser, S.; Vahidnia, A.; Kuiter, A.; Limaheluw, J.; Schout, C.; Mohammad, G.H.; Raven, S. An outbreak of Legionnaires’ disease linked to a municipal and industrial wastewater treatment plant, The Netherlands, September–October 2022. Eurosurveillance 2024, 29, 2300506. [Google Scholar] [CrossRef]
- Quon, H.; Jiang, S. Quantitative Microbial Risk Assessment of Antibiotic-Resistant E. coli, Legionella pneumophila, and Mycobacteria in Nonpotable Wastewater Reuse Applications. Environ. Sci. Technol. 2024, 58, 12888–12898. [Google Scholar] [CrossRef]
- Monteiro, S.N.; Robalo, A.M.; Santos, R.J. Evaluation of Legiolert™ for the detection of Legionella pneumophila and comparison with spread-plate culture and qPCR methods. Curr. Microbiol. 2021, 78, 1792–1797. [Google Scholar] [CrossRef]
- Karia, K.; Yui, S.; Muzslay, M.; Ali, S. Concordance between IDEXX LegiolertTM (liquid culture assay) and plate culture (ISO 11731: 2017) for the detection and quantification of Legionella pneumophila in water samples. J. Hosp. Infect. 2024, 150, 163–168. [Google Scholar] [CrossRef]
- Barzegar, L.; Ghanizadeh, G.; Esmaeili, D. Study of Gorge Fisher Plumbing System Effects on Growth Inhibition and Amplification of Legionella pneumophila by Culture and PCR Based on 16sRNA and mip Genes. Middle East J. Rehabil. Health Stud. 2022, 9, e122683. [Google Scholar] [CrossRef]
- Yi, Q.; Zhang, G.; Wang, T.; Li, J.; Kang, W.; Zhang, J.; Liu, Y.; Xu, Y. Comparative Analysis of Metagenomic Next-Generation Sequencing, Sanger Sequencing, and Conventional Culture for Detecting Common Pathogens Causing Lower Respiratory Tract Infections in Clinical Samples. Microorganisms 2025, 13, 682. [Google Scholar] [CrossRef]



| Primer | Sequence 5′-3′ | Size (bp) | Reference |
|---|---|---|---|
| Leg 225 (F) | AAG ATT AGC CTG CGT CCG AT | 654 | [33] |
| Leg 858 (R) | GTC AAC TTA TCG CGT TTG CT | ||
| L. pneumophila (F) | CCG ATG CCA CAT CAT TAG C | 150 | [34] |
| L. pneumophila (R) | CCA ATT GAG CGC CAC TCA TAG |
| Strain | Primer | Size (bp) | Sequence | Reference |
|---|---|---|---|---|
| L. pneu | LpneuF | 150 | 5′-CCGATGCCACATCATTAGC-3′ | [29] |
| LpneuR | 5′-CCAATTGAGCGCCACTCATAG-3′ | |||
| LpneuP (Probe) | 5′-6-carboxyfluorescein [FAM]-TGCCTTTAGCCATTGCTTCCG-BHQ1–3′ |
| Parameter | Category | n = 129 | Hillbrow | Zandspruit | Melusi | Atteridgeville |
|---|---|---|---|---|---|---|
| Water source | Tap | 90 | 27 | 25 | 16 | 22 |
| Storage container | 39 | 0 | 5 | 20 | 14 | |
| Stored water refill frequency | Once a day | 25 | 0 | 5 | 9 | 11 |
| More than once a day | 6 | 0 | 0 | 3 | 3 | |
| Every two day | 6 | 0 | 0 | 6 | 0 | |
| Every three days | 1 | 0 | 0 | 1 | 0 | |
| Once a week | 1 | 0 | 0 | 1 | 0 | |
| Water treatment | Treated before use | 20 | 3 | 0 | 0 | 17 |
| Not treated | 109 | 24 | 30 | 36 | 19 | |
| Water quality perception | Odourless, no taste/discoloration | 127 | 27 | 30 | 34 | 36 |
| Discoloured, Sour/metallic taste | 2 | 0 | 0 | 2 | 0 | |
| Plumbing material used | Not specified | 110 | 24 | 30 | 36 | 20 |
| Iron | 6 | 3 | 0 | 0 | 3 | |
| PVC | 6 | 0 | 0 | 0 | 6 | |
| Copper | 6 | 0 | 0 | 0 | 6 | |
| PEX | 1 | 0 | 0 | 0 | 1 | |
| Plumbing maintenance records | No maintenance record | 99 | 0 | 30 | 36 | 33 |
| Annually | 24 | 21 | 0 | 0 | 3 | |
| Only when needed | 6 | 6 | 0 | 0 | 0 | |
| Plumbing renovation records | No renovation recorded | 121 | 21 | 30 | 36 | 34 |
| Recently renovated | 8 | 6 | 0 | 0 | 2 | |
| Toilet facilities | With flushing | 93 | 27 | 30 | 0 | 36 |
| With pit toilet | 36 | 0 | 0 | 36 | 0 | |
| With toilet lids | 52 | 27 | 4 | 0 | 21 | |
| Point of aerosolization observed | yes | 41 | 0 | 26 | 0 | 15 |
| Study Site | Total Samples | Samples Positive for Total Coliform | Samples Positive for E. coli |
|---|---|---|---|
| Hillbrow | 27 | 8 | 0 |
| Zandspruit | 31 | 3 | 0 |
| Melusi | 36 | 14 | 0 |
| Atteridgeville | 40 | 1 | 0 |
| Site | Slope (β₁) | Intercept (β₀) | R2 | Interpretation |
|---|---|---|---|---|
| Hillbrow | 15.38 | −375.59 | 0.13 | Moderate association; MPN increases with temperature |
| Zandspruit | 3.28 | −36.18 | 0.03 | Weak relationship; minimal predictive power |
| Melusi | 3.05 | −18.96 | 0.16 | Moderate association; MPN partly temperature-dependent |
| Site | n | Legionella spp. | L. pneumophila |
|---|---|---|---|
| Hillbrow | 27 | 21 | 20 |
| % | 78 | 74 | |
| Zandspruit | 31 | 2 | 25 |
| % | 6 | 81 | |
| Melusi | 36 | 16 | 29 |
| % | 44 | 81 | |
| Atteridgeville | 40 | 16 | 34 |
| % | 40 | 85 | |
| Total | 134 | 55 | 108 |
| % | 41 | 81 |
| Sample Name | Copies/µL |
|---|---|
| Z1 | 1.027 × 103 |
| Z2 | 47.32 × 107 |
| Z3 | 9.576 × 101 |
| Z4 | 1.3278 |
| Z5 | 8.617 × 101 |
| Z8 | 3.9095 × 104 |
| Z9 | 2.35 × 103 |
| Z10 | 1.627 × 101 |
| Z13 | 6.59 × 102 |
| Z14 | 1.724 |
| Z15 | 1.7588 × 101 |
| Z16 | 4.835 × 101 |
| Z17 | 3.032 × 101 |
| Z18 | 1.882 × 103 |
| Z19 | 4.718 × 102 |
| Z21 | 4.288 |
| Z22 | 4.057 |
| Z23 | 1.7524 × 105 |
| Z24 | 4.115 |
| Z26 | 6.106 × 10 |
| Z29 | 1.387 |
| Z30 | 7.416 |
| A28 | 1.9482 |
| A30 | 1.5054 × 101 |
| A31 | 3.719 × 104 |
| A32 | 4.6035 × 103 |
| A33 | 6.425 × 103 |
| A34 | 1.0573 × 10 |
| A36 | 1.4018 × 103 |
| Sample | Description | Query Cover | E-Value | Accession Number |
|---|---|---|---|---|
| H3 | Legionella pneumophila strain LEG1117 chromosome | 98% | 3.00 × 10−51 | LS483410.1 |
| H11 | L. pneumophila isolate L1860 macrophage infectivity potentiator surface protein (mip) gene, partial cds | 98% | 3.00 × 10−51 | KC410215.1 |
| Z1 | Legionella pneumophila strain Edelstein isolate SI7 macrophage infectivity potentiator (mip) gene, partial cds | 98% | 3.00 × 10−51 | MW524769.1 |
| Z2 | Legionella pneumophila strain MIP1 macrophage infectivity potentiator (mip) gene, partial cds | 98% | 3.00 × 10−51 | KJ160890.1 |
| Z4 | Legionella pneumophila strain H3 chromosome, complete genome | 98% | 3.00 × 10−51 | CP114576.1 |
| Z5 | Legionella pneumophila partial mip gene for macrophage infectivity potentiator, | 98% | 3.00 × 10−51 | AJ810195.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mnisi, Z.F.; Delair, Z.; Singh, A. Legionella in Urban and Rural Water, a Tale of Two Environments. Water 2025, 17, 1491. https://doi.org/10.3390/w17101491
Mnisi ZF, Delair Z, Singh A. Legionella in Urban and Rural Water, a Tale of Two Environments. Water. 2025; 17(10):1491. https://doi.org/10.3390/w17101491
Chicago/Turabian StyleMnisi, Zandice Faith, Zaakirah Delair, and Atheesha Singh. 2025. "Legionella in Urban and Rural Water, a Tale of Two Environments" Water 17, no. 10: 1491. https://doi.org/10.3390/w17101491
APA StyleMnisi, Z. F., Delair, Z., & Singh, A. (2025). Legionella in Urban and Rural Water, a Tale of Two Environments. Water, 17(10), 1491. https://doi.org/10.3390/w17101491






