Comparative Analysis of Livestock Wastewater Reuse Under Summer and Winter Conditions at a Scale-Down Microalgae Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Cultivation Conditions
2.2. Analytics and Measurement
2.3. Disinfection Analysis
2.4. Light Response Tests
3. Results and Discussion
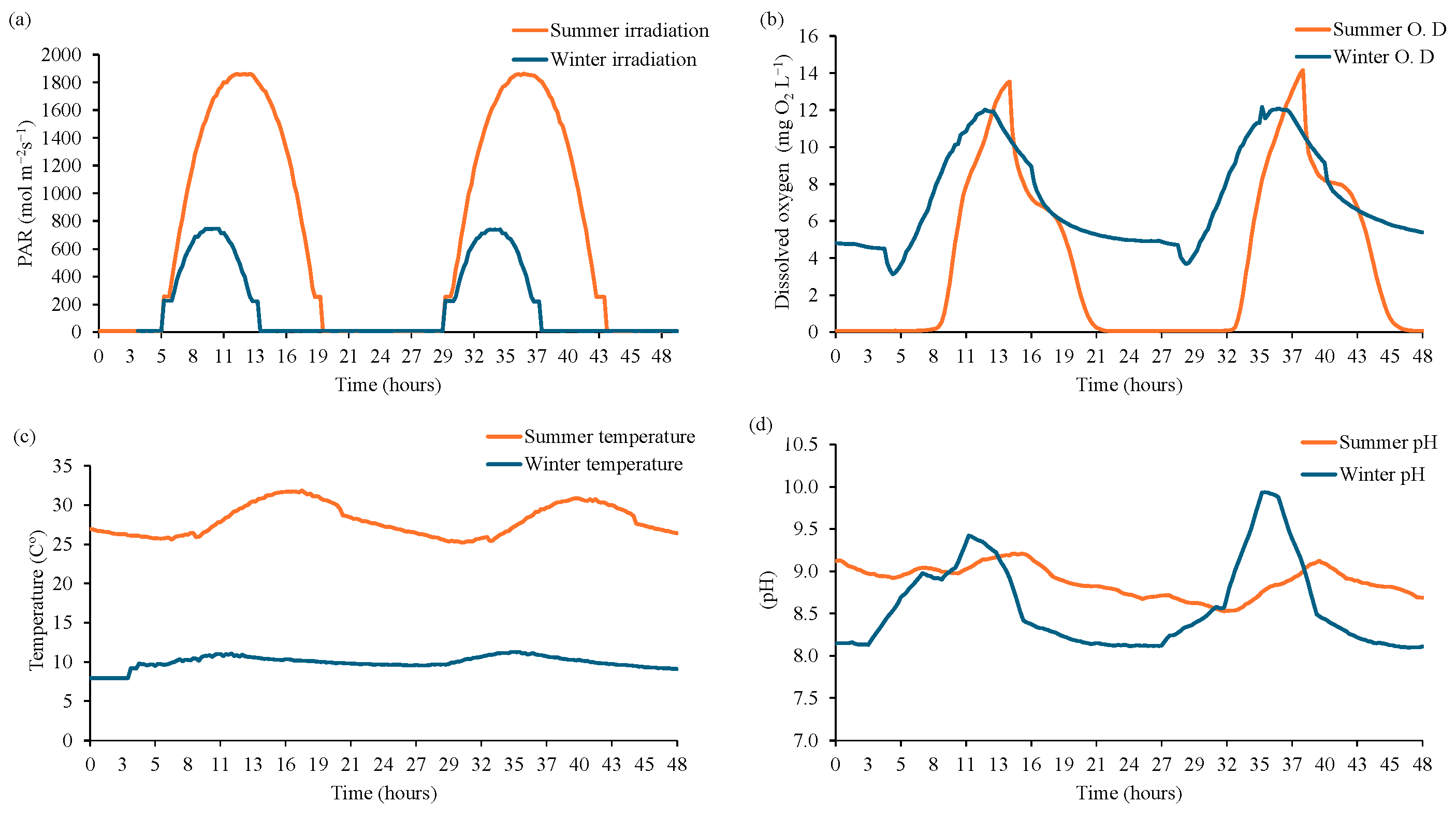
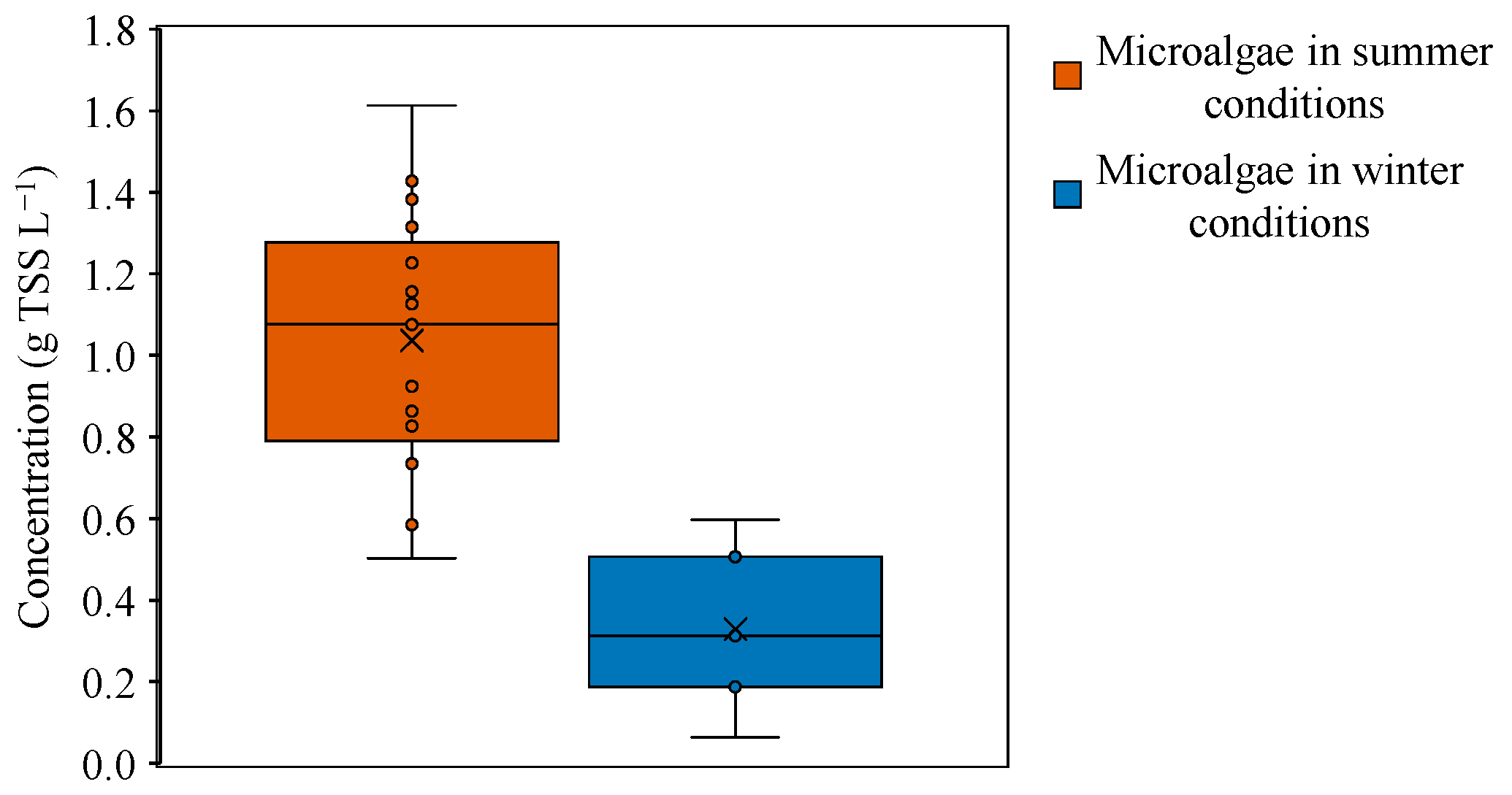
3.1. Microalgae Culture Monitorization
3.2. Chemical Pollution Removal
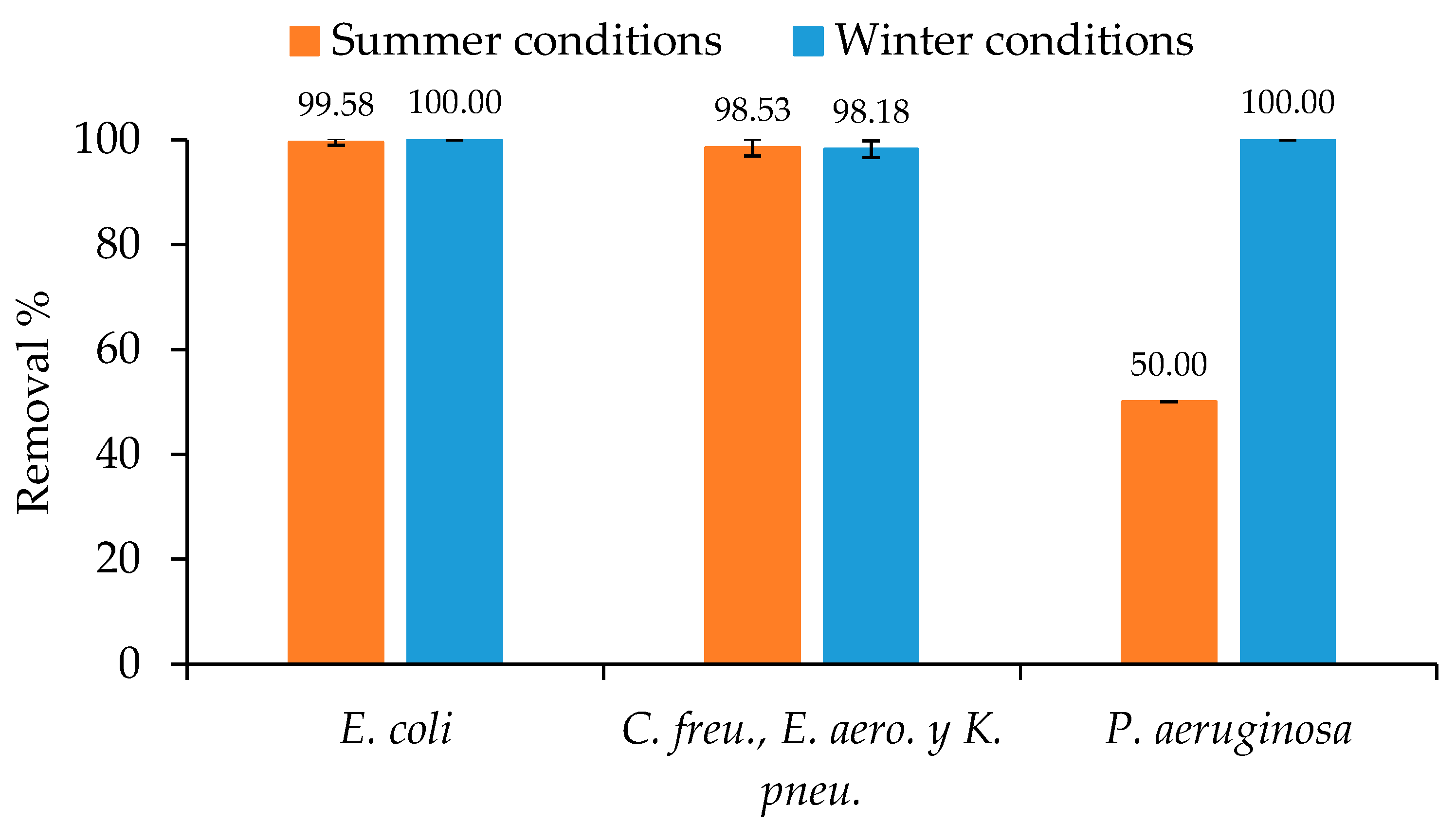
3.3. Bacterial Pathogen Removal
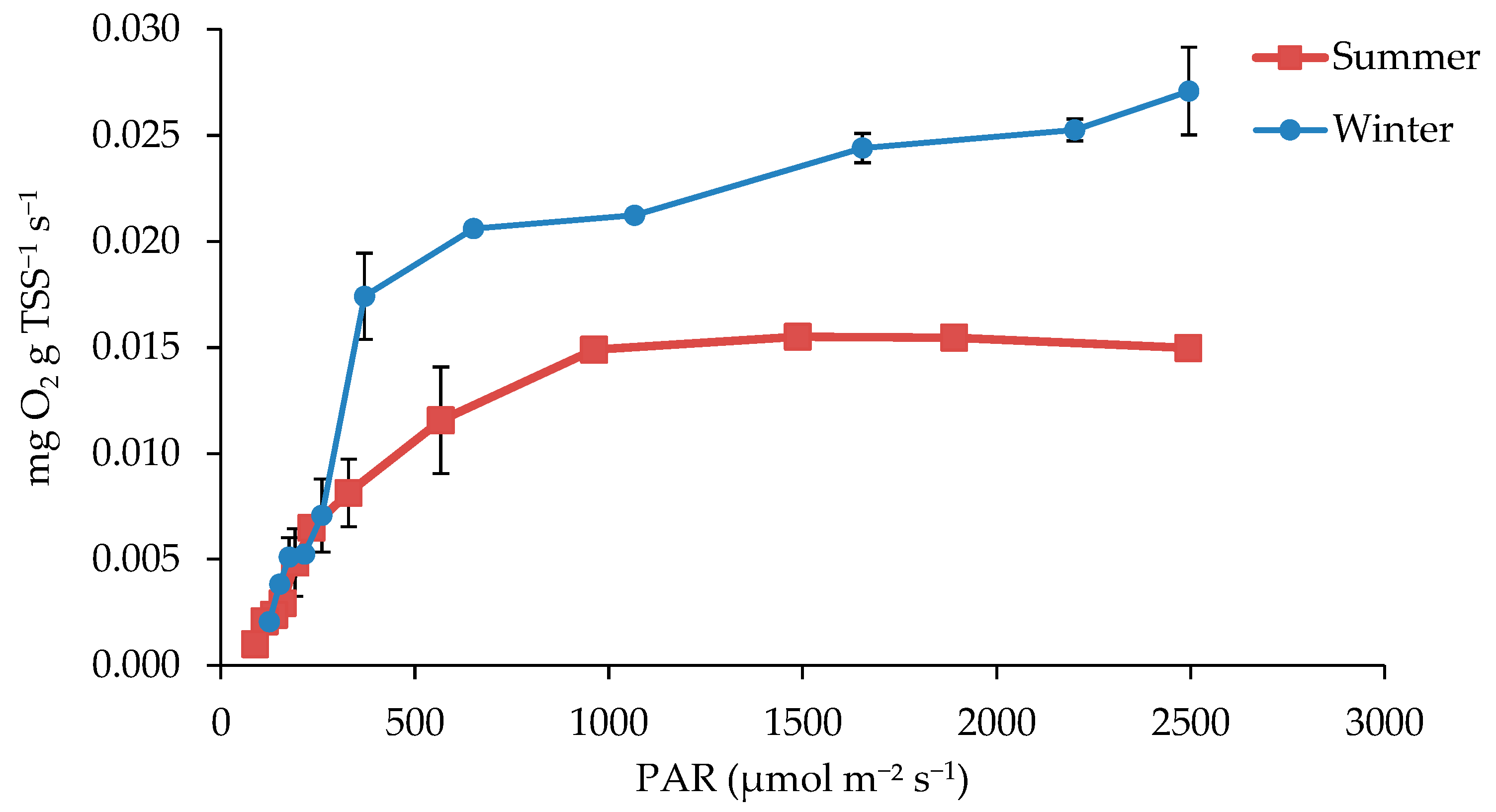
3.4. Light Response Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, X.M.; Ma, J.R.; Ying, J.; Cai, M.; Kong, Y.Q. Inferring future warming in the Arctic from the observed global warming trend and CMIP6 simulations. Adv. Clim. Change Res. 2021, 12, 499–507. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, P.; Gojenko, B.; Yu, J.; Wei, L.; Luo, D.; Xiao, T. A review of water pollution arising from agriculture and mining activities in Central Asia: Facts, causes and effects. Environ. Pollut. 2021, 291, 118209. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Xin, X.; Li, J.; Jia, H.; Wen, L.; Yin, W. Water level–driven agricultural nonpoint source pollution dominated the ammonia variation in China’s second largest reservoir. Environ. Res. 2022, 215, 114367. [Google Scholar] [CrossRef] [PubMed]
- Food Safety. Available online: https://www.who.int/es/news-room/fact-sheets/detail/food-safety (accessed on 9 April 2025).
- Bari, M.L.; Yeasmin, S. Foodborne Diseases and Responsible Agents. In Food Safety and Preservation; Academic Press: Cambridge, MA, USA, 2018; pp. 195–229. [Google Scholar] [CrossRef]
- FAO. The Future of Food and Agriculture; FAO: Rome, Italy, 2017; pp. 1–47. [Google Scholar]
- Chong, C.C.; Cheng, Y.W.; Ishak, S.; Lam, M.K.; Lim, J.W.; Tan, I.S.; Show, P.L.; Lee, K.T. Anaerobic digestate as a low-cost nutrient source for sustainable microalgae cultivation: A way forward through waste valorization approach. Sci. Total Environ. 2022, 803, 150070. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Díaz, V.; Leyva-Díaz, J.C.; Almécija, M.C.; Poyatos, J.M.; del Mar Muñío, M.; Martín-Pascual, J. Microalgae bioreactor for nutrient removal and resource recovery from wastewater in the paradigm of circular economy. Bioresour. Technol. 2022, 363, 127968. [Google Scholar] [CrossRef]
- Saranya, D.; Shanthakumar, S. An integrated approach for tannery effluent treatment with ozonation and phycoremediation: A feasibility study. Environ. Res. 2020, 183, 109163. [Google Scholar] [CrossRef]
- Fthenakis, V.M.; Kim, H.C. Environmental Impacts of Photovoltaic Life Cycles. Compr. Renew. Energy 2012, 1, 143–159. [Google Scholar] [CrossRef]
- Do, C.V.T.; Pham, M.H.T.; Pham, T.Y.T.; Dinh, C.T.; Bui, T.U.T.; Tran, T.D.; Nguyen, V.T. Microalgae and bioremediation of domestic wastewater. Curr. Opin. Green. Sustain. Chem. 2022, 34, 100595. [Google Scholar] [CrossRef]
- Quan, X.; Hu, R.; Chang, H.; Tang, X.; Huang, X.; Cheng, C.; Zhong, N.; Yang, L. Enhancing microalgae growth and landfill leachate treatment through ozonization. J. Clean. Prod. 2020, 248, 119182. [Google Scholar] [CrossRef]
- de Godos, I.; Arbib, Z.; Lara, E.; Cano, R. Wastewater treatment in algal systems. In Innovative Wastewater Treatment & Resource Recovery Technologies: Impacts on Energy, Economy and Environment; IWA Publishing: London, UK, 2017. [Google Scholar]
- Alcántara, C.; De Godos, I.; Muñoz, R. Wastewater treatment and biomass generation with algae. In Wastewater Treatment Residues as Resources for Biorefinery Products and Biofuels; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Salama, E.-S.; Kurade, M.B.; Abou-Shanab, R.A.; El-Dalatony, M.M.; Yang, I.-S.; Min, B.; Jeon, B.-H. Recent progress in microalgal biomass production coupled with wastewater treatment for biofuel generation. Renew. Sustain. Energy Rev. 2017, 79, 1189–1211. [Google Scholar] [CrossRef]
- Ibrahim, F.G.G.; Gómez, V.A.; Torre, R.M.; de Godos Crespo, I. Scale-down of high-rate algae ponds systems for urban wastewater reuse. J. Water Process Eng. 2023, 56, 104342. [Google Scholar] [CrossRef]
- García, D.; de Godos, I.; Domínguez, C.; Turiel, S.; Bolado, S.; Muñoz, R. A systematic comparison of the potential of microalgae-bacteria and purple phototrophic bacteria consortia for the treatment of piggery wastewater. Bioresour. Technol. 2019, 276, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Palomar, C.R.; Álvaro, A.G.; Hermosilla, D.; Gascó, A.; Muñoz, R.; de Godos, I. Ozonation as Pretreatment of Digested Swine Manure Prior to Microalgae Culture. Water 2024, 16, 1740. [Google Scholar] [CrossRef]
- American Water Works Association & Water Pollution Control Federation; American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1985. [Google Scholar]
- Diario Oficial de la Unión Europea. Reglamento (UE) 2020/741 del Parlamento Europeo y del Consejo Relativo a los Requisitos Mínimos para la Reutilización del Agua; European Union: Brussels, Belgium, 2020. [Google Scholar]
- Chromocult® Coliform Agar—Evaluación del Grado de Contaminación Microbiana Para Alimentos Sólidos y Líquidos. Available online: https://www.merckmillipore.com/ES/es/product/Chromocult-Coliform-Agar,MM_NF-C164546 (accessed on 29 April 2025).
- Barreiro-vescovo, S.; González-fernández, C. Activity determination of an algal-bacterial consortium developed during wastewater treatment based on oxygen evolution. J. Water Process Eng. 2020, 36, 101278. [Google Scholar] [CrossRef]
- Costache, T.A.; Fernández, F.G.A. Comprehensive model of microalgae photosynthesis rate as a function of culture conditions in photobioreactors. Appl. Microbiol. Biotechnol. 2013, 97, 7627–7637. [Google Scholar] [CrossRef]
- de Godos, I.; Mendoza, J.; Acién, F.; Molina, E.; Banks, C.; Heaven, S.; Rogalla, F. Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour. Technol. 2014, 153, 307–314. [Google Scholar] [CrossRef]
- Muñoz, R.; Guieysse, B. Algal—Bacterial processes for the treatment of hazardous contaminants: A review. Water Res. 2006, 40, 2799–2815. [Google Scholar] [CrossRef]
- Mendoza, J.; Granados, M.; de Godos, I.; Acién, F.; Molina, E.; Banks, C.; Heaven, S. Fluid-dynamic characterization of real-scale raceway reactors for microalgae production. Biomass Bioenergy 2013, 54, 267–275. [Google Scholar] [CrossRef]
- De Godos, I.; Arbib, Z.; Lara, E.; Rogalla, F. Bioresource Technology Evaluation of High Rate Algae Ponds for treatment of anaerobically digested wastewater: Effect of CO2 addition and modification of dilution rate. Bioresour. Technol. 2016, 220, 253–261. [Google Scholar] [CrossRef]
- Arbib, Z.; De Godos, I.; Corona, E.L. Understanding the biological activity of high rate algae ponds through the calculation of oxygen balances. Appl. Microbiol. Biotechnol. 2017, 101, 5189–5198. [Google Scholar] [CrossRef] [PubMed]
- de Godos, I.; Blanco, S.; García-Encina, P.A.; Becares, E.; Muñoz, R. Influence of flue gas sparging on the performance of high rate algae ponds treating agro-industrial wastewaters. J. Hazard. Mater. 2010, 179, 1049–1054. [Google Scholar] [CrossRef] [PubMed]
- Metcalf; Eddy. Wastewater Engineering: Treatment and Reuse, 4th ed.; Tchobanoglus, G., Burton, F., Stensel, H.D., Eds.; McGraw-Hill: Boston, MA, USA, 2003. [Google Scholar]
- Cho, S.; Lee, N.; Park, S.; Yu, J.; Luong, T.T.; Oh, Y.-K.; Lee, T. Bioresource Technology Microalgae cultivation for bioenergy production using wastewaters from a municipal WWTP as nutritional sources. Bioresour. Technol. 2013, 131, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Arun, J.; Varshini, P.; Prithvinath, P.K.; Priyadarshini, V.; Gopinath, K. Enrichment of bio-oil after hydrothermal liquefaction (HTL) of microalgae C. vulgaris grown in wastewater: Bio-char and post HTL wastewater utilization studies. Bioresour. Technol. 2018, 261, 182–187. [Google Scholar] [CrossRef]
- Ling, Y.; Sun, L.P.; Wang, S.Y.; Lin, C.S.K.; Sun, Z.; Zhou, Z.G. Cultivation of oleaginous microalga Scenedesmus obliquus coupled with wastewater treatment for enhanced biomass and lipid production. Biochem. Eng. J. 2019, 148, 162–169. [Google Scholar] [CrossRef]
- Yun, J.H.; Smith, V.H.; Pate, R.C. Managing nutrients and system operations for biofuel production from freshwater macroalgae. Algal Res. 2015, 11, 13–21. [Google Scholar] [CrossRef]
- Razzak, S.A. Effect of temperature and CO2 concentration on biological nutrient removal from tertiary municipal wastewater using microalgae Chlorella prototheocoides. Biotechnol. Notes 2025, 6, 32–43. [Google Scholar] [CrossRef]
- de Godos, I.; Vargas, V.; Guzmán, H.; Soto, R.; García, B.; García, P.; Muñoz, R. Assessing carbon and nitrogen removal in a novel anoxic-aerobic cyanobacterial-bacterial photobioreactor configuration with enhanced biomass sedimentation. Water Res. 2014, 61, 77–85. [Google Scholar] [CrossRef]
- Fallowfield, H.J.; Young, P.; Taylor, M.J.; Buchanan, N.; Cromar, N.; Keegan, A.; Monis, P. Independent validation and regulatory agency approval for high rate algal ponds to treat wastewater from rural communities. Environ. Sci. Water Res. Technol. 2018, 4, 195–205. [Google Scholar] [CrossRef]
- Marchello, A.E.; Lombardi, A.T.; Dellamano-Oliveira, M.J.; de Souza, C.W.O. Microalgae population dynamics in photobioreactors with secondary sewage effluent as culture medium. Braz. J. Microbiol. 2015, 46, 75–84. [Google Scholar] [CrossRef]
- Chambonniere, P.; Bronlund, J.; Guieysse, B. Pathogen removal in high-rate algae pond: State of the art and opportunities. J. Appl. Phycol. 2021, 33, 1501–1511. [Google Scholar] [CrossRef]
- Amaro, H.M.; Salgado, E.M.; Nunes, O.C.; Pires, J.C.M.; Esteves, A.F. Microalgae systems—Environmental agents for wastewater treatment and further potential biomass valorisation. J. Environ. Manag. 2023, 337, 117678. [Google Scholar] [CrossRef]


| Parameter | Initial Concentration in Summer | Summer Removal | Initial Concentration in Winter | Winter Removal |
|---|---|---|---|---|
| TSS | 0.71 (g L−1) | 48.94 ± 78.20 | 0.32 (g L−1) | 96.20 ± 2.01 |
| NH3 | 110.18 (mg L−1) | 78.26 ± 20.77 | 22.81 (mg L−1) | 16.82 ± 54.11 |
| PO43− | 21.72 (mg L−1) | 69.83 ± 48.28 | 12.29 (mg L−1) | 34.83 ± 42.50 |
| DQO | 1.38 (g L−1) | 65.34 ± 25.04 | 0.54 (g L−1) | 91.08 ± 4.57 |
| E. coli | 6000 (UFC mL−1) | 99.6 | 210 (UFC mL−1) | 100.0 |
| C. freu., E. aero., K. pneu. | 1700 (UFC mL−1) | 98.5 | 55 (UFC mL−1) | 98.2 |
| P. aeruginosa | 20,000 (UFC mL−1) | 50.0 | 35 (UFC mL−1) | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz Palomar, C.; García Álvaro, A.; Hermosilla, D.; Gonzalo Ibrahím, F.G.; Muñoz, R.; de Godos, I. Comparative Analysis of Livestock Wastewater Reuse Under Summer and Winter Conditions at a Scale-Down Microalgae Culture. Water 2025, 17, 1483. https://doi.org/10.3390/w17101483
Ruiz Palomar C, García Álvaro A, Hermosilla D, Gonzalo Ibrahím FG, Muñoz R, de Godos I. Comparative Analysis of Livestock Wastewater Reuse Under Summer and Winter Conditions at a Scale-Down Microalgae Culture. Water. 2025; 17(10):1483. https://doi.org/10.3390/w17101483
Chicago/Turabian StyleRuiz Palomar, César, Alfonso García Álvaro, Daphne Hermosilla, Félix Gaspar Gonzalo Ibrahím, Raúl Muñoz, and Ignacio de Godos. 2025. "Comparative Analysis of Livestock Wastewater Reuse Under Summer and Winter Conditions at a Scale-Down Microalgae Culture" Water 17, no. 10: 1483. https://doi.org/10.3390/w17101483
APA StyleRuiz Palomar, C., García Álvaro, A., Hermosilla, D., Gonzalo Ibrahím, F. G., Muñoz, R., & de Godos, I. (2025). Comparative Analysis of Livestock Wastewater Reuse Under Summer and Winter Conditions at a Scale-Down Microalgae Culture. Water, 17(10), 1483. https://doi.org/10.3390/w17101483








