Comparative Adsorption of Cu(II), Zn(II), Cd(II), and Mn(II) from Aquatic Solution and Neutral Mine Drainage Using Paper Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Atomic Absorption Spectrometry (AAS)
2.2. Determination of pH
2.3. Adsorption of Metal Ions
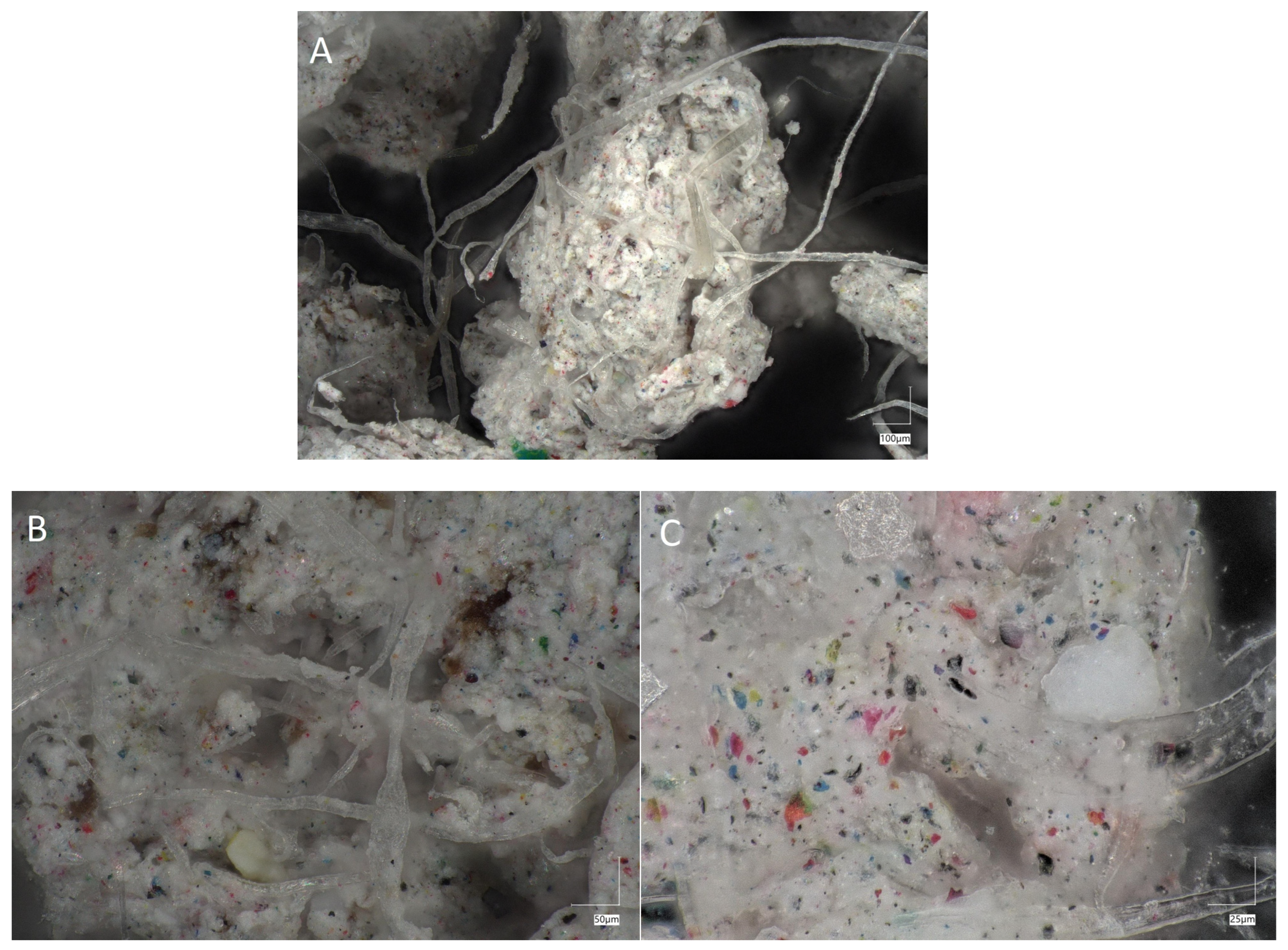
2.4. Microscopic Images of Crystal Size
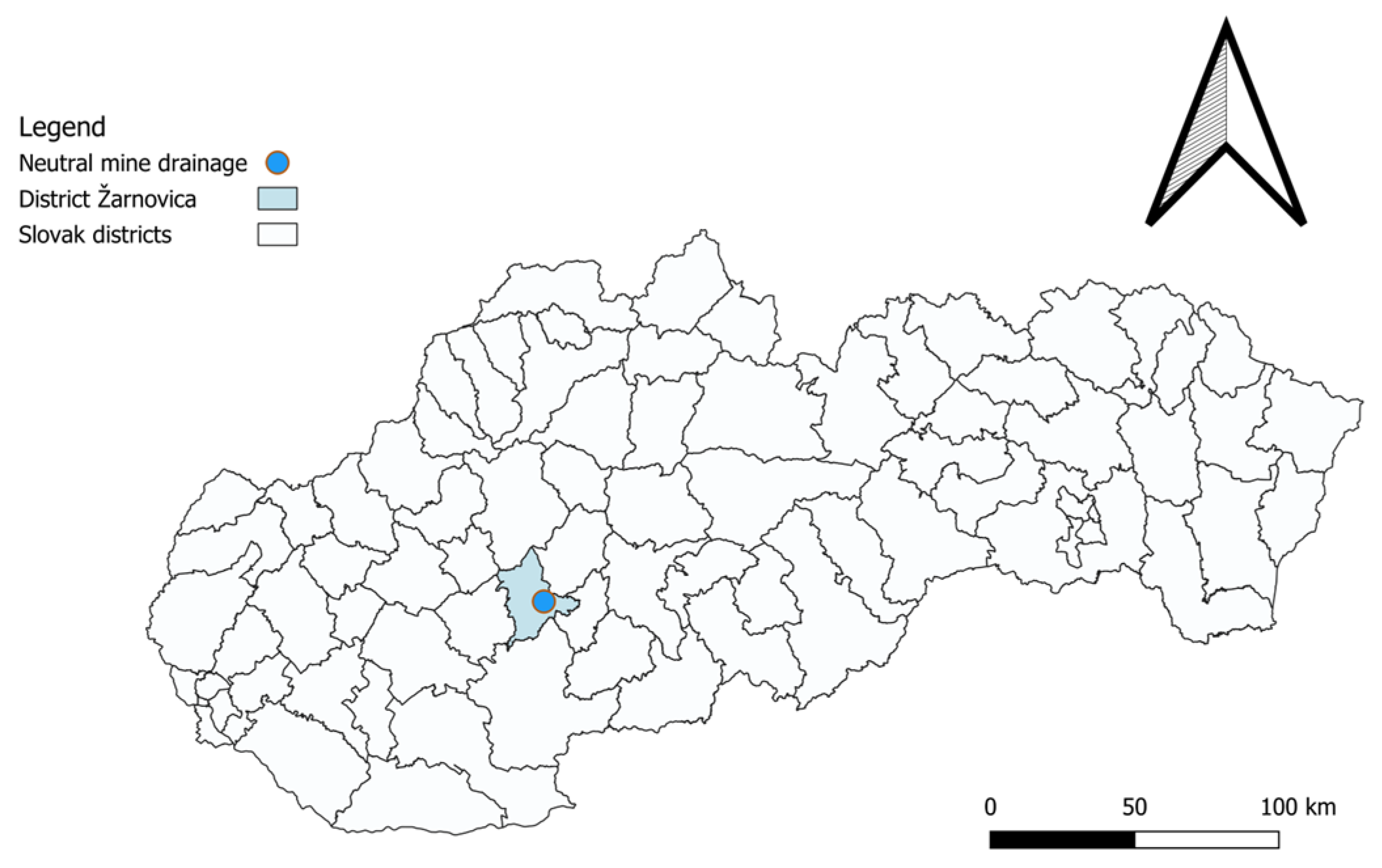
2.5. Sampling Sites of Neutral Mine Drainage
2.6. Characteristics of Neutral Mine Drainage
2.7. Characteristics of Paper Sludge
2.8. Pretreatment of Paper Mill Sludge (Physicochemical Steps)
2.9. Calculations
2.9.1. Adsorption Capacity
2.9.2. Freundlich and Langmuir Adsorption Isotherms
2.9.3. Freundlich Adsorption Isotherm
2.9.4. Langmuir Adsorption Isotherm
2.9.5. Separation Factor
3. Results and Discussion
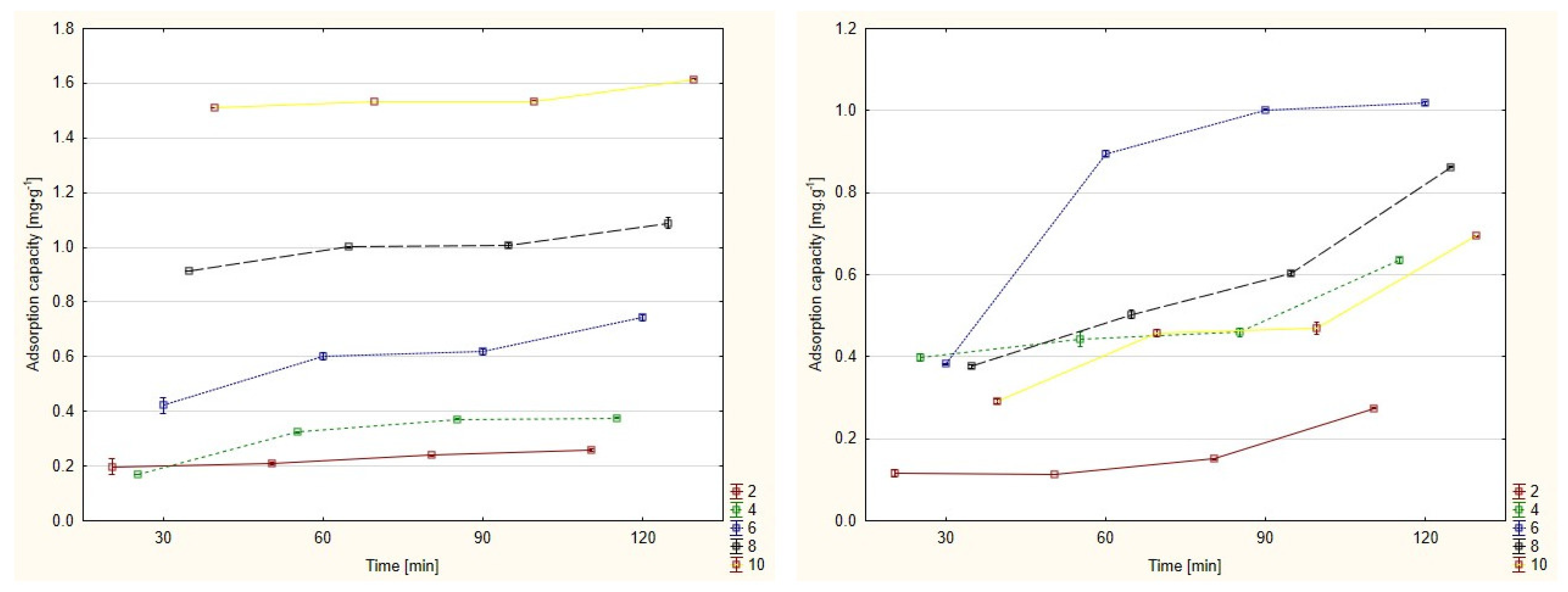
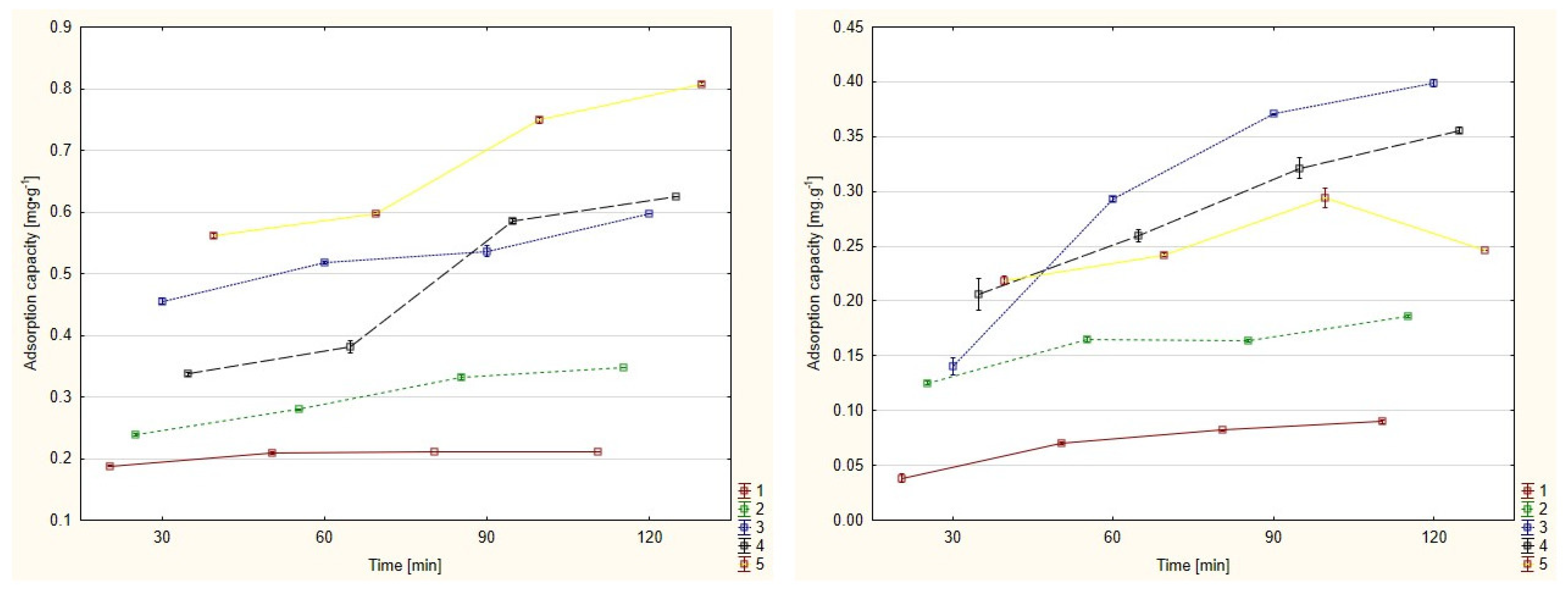
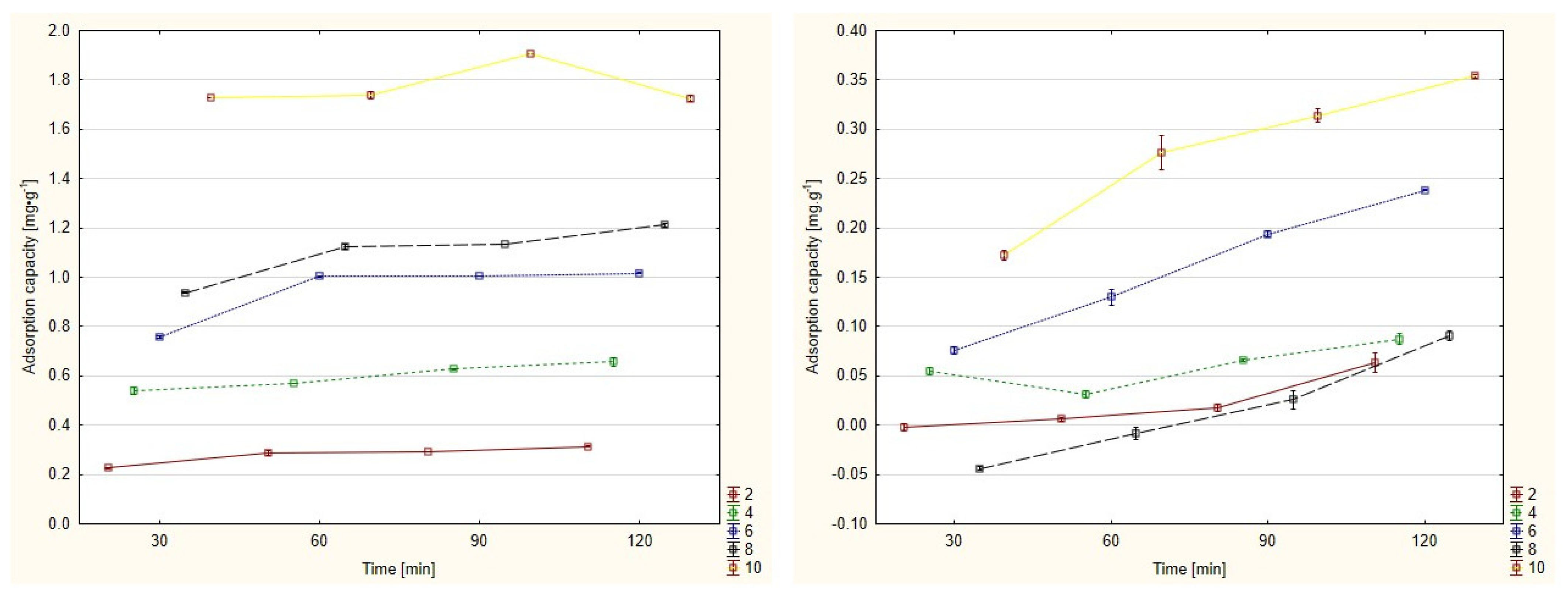
3.1. Adsorption Capacity
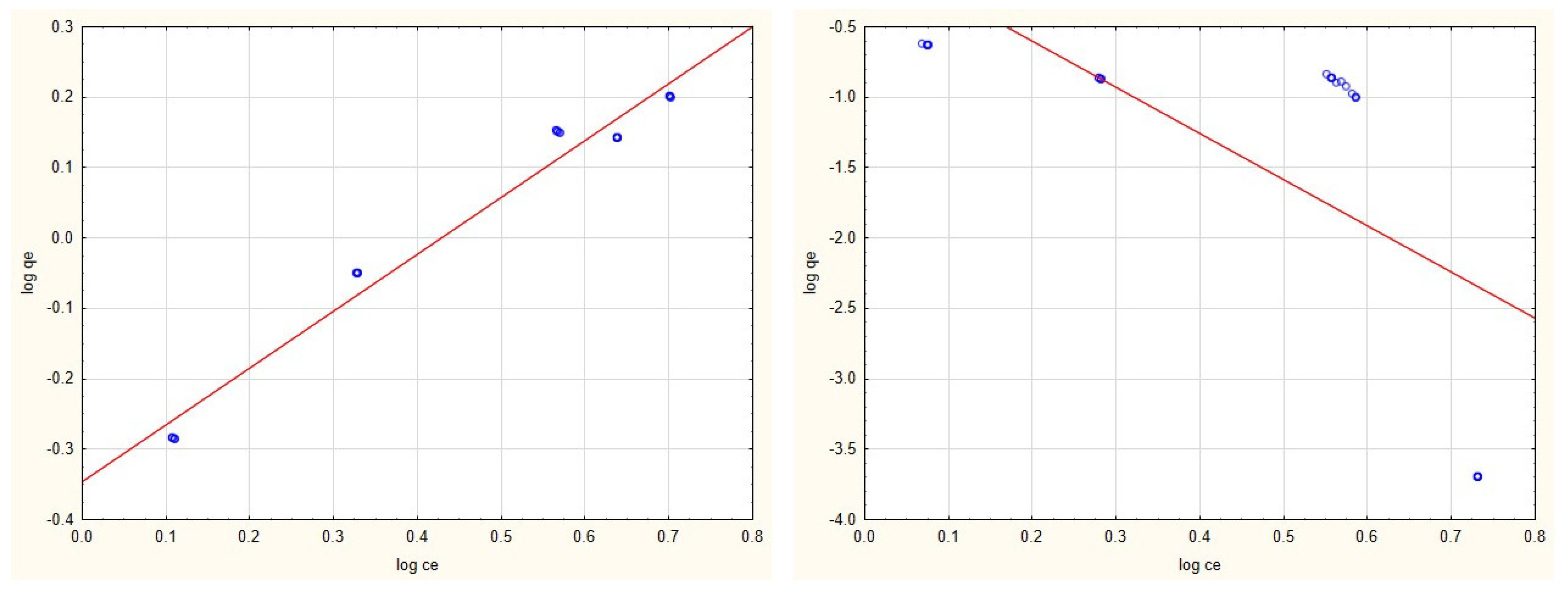
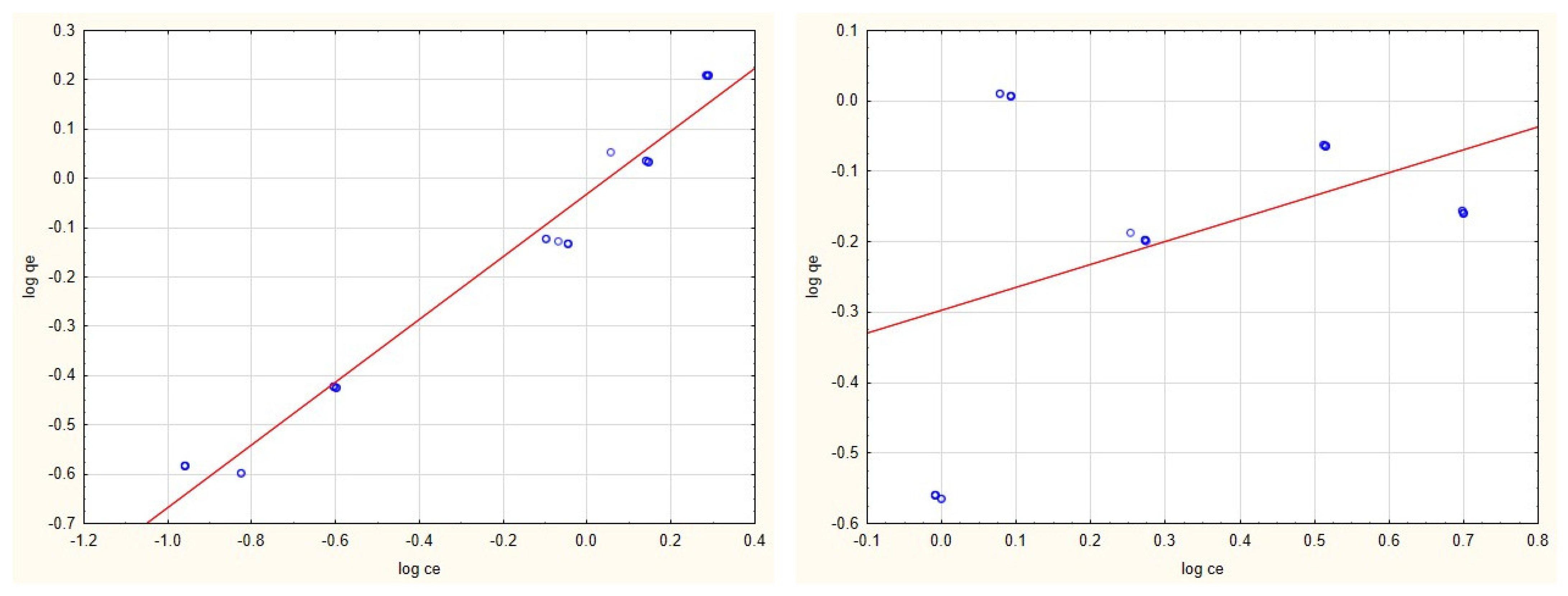
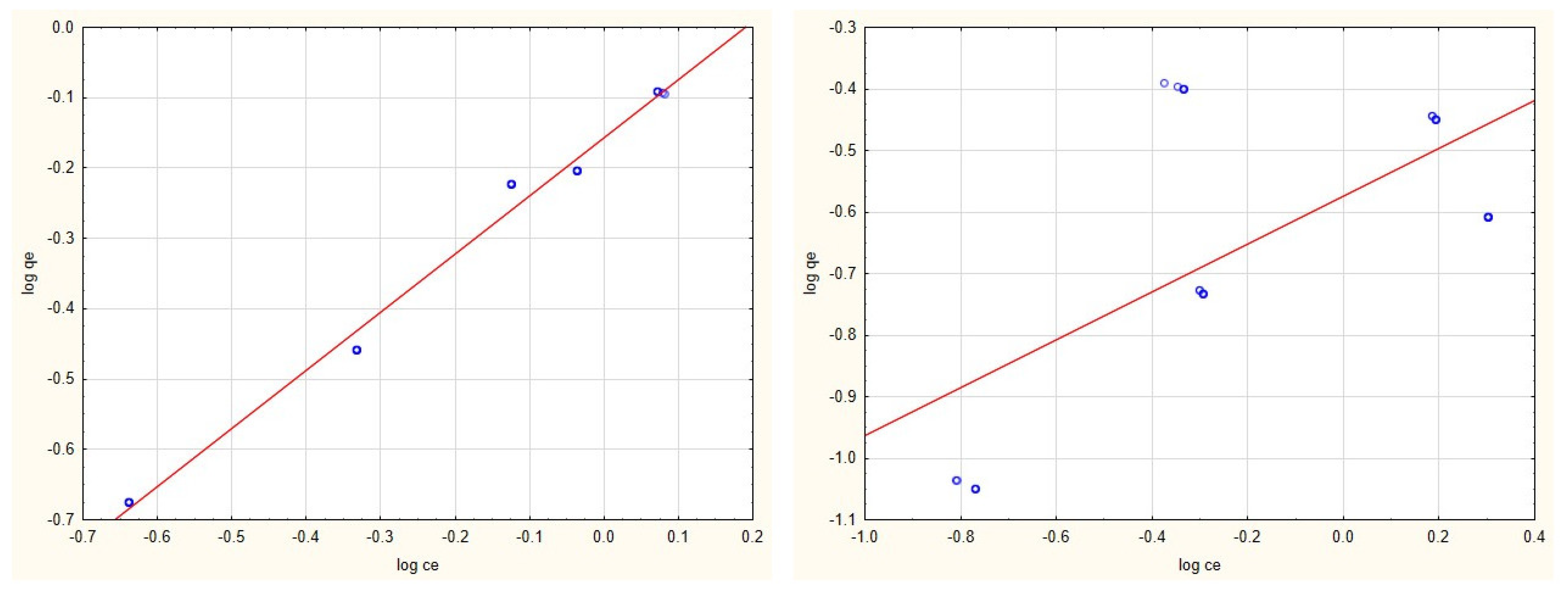
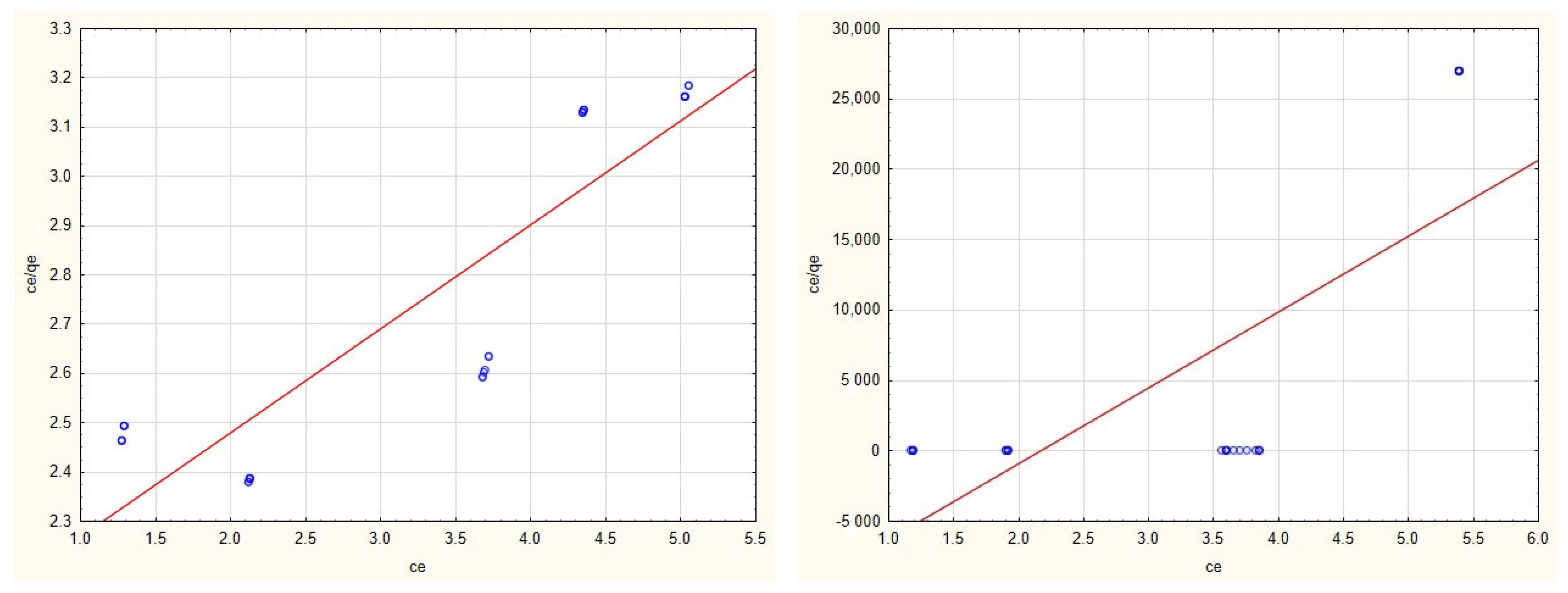
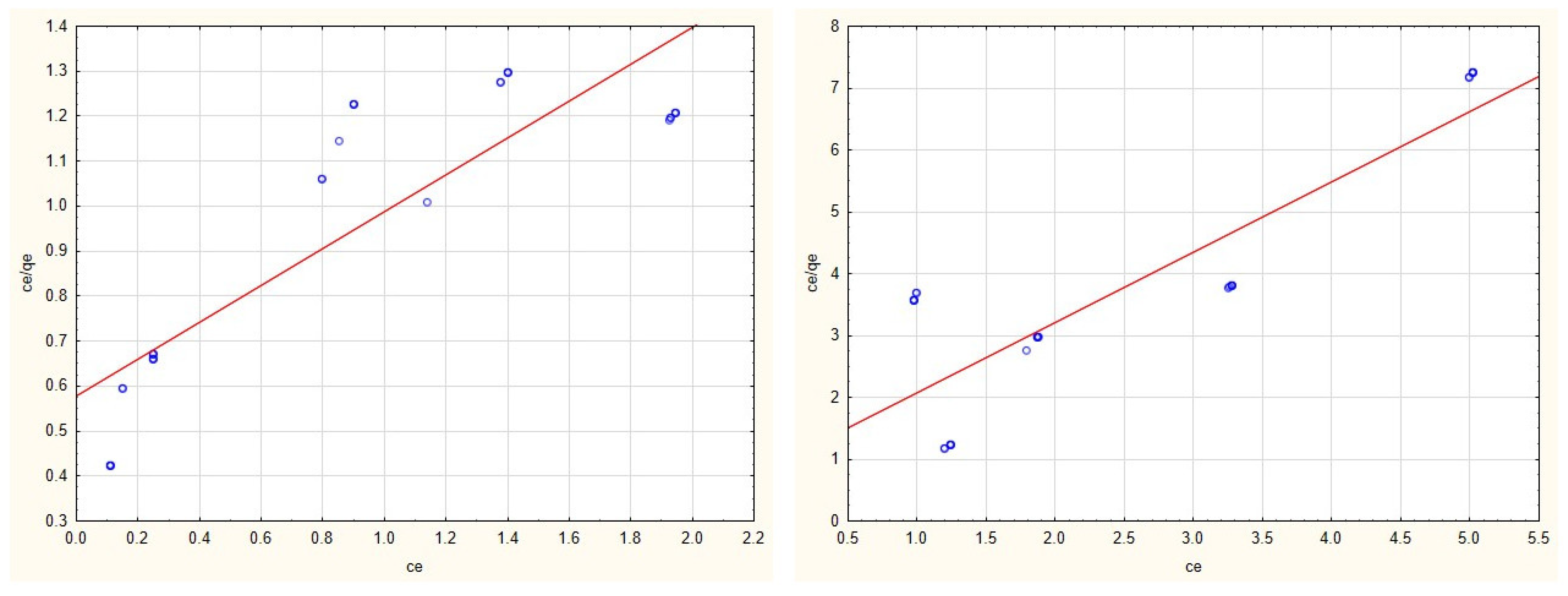
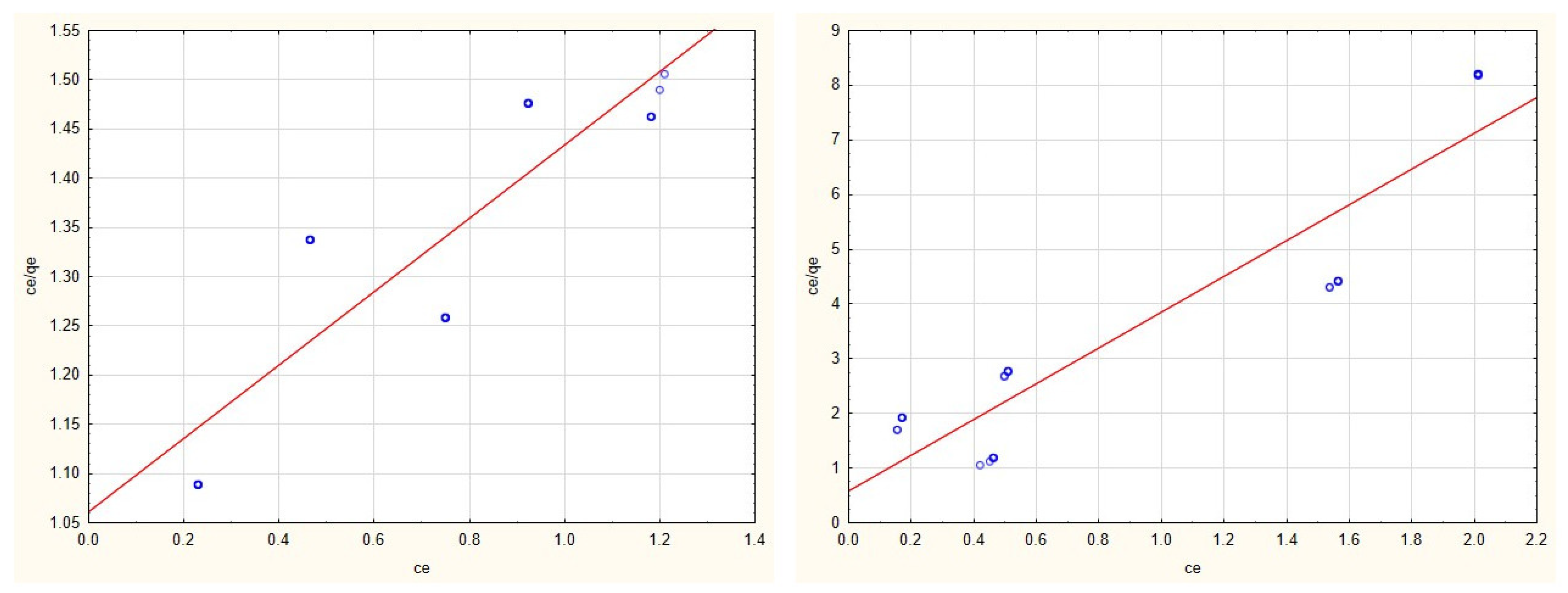
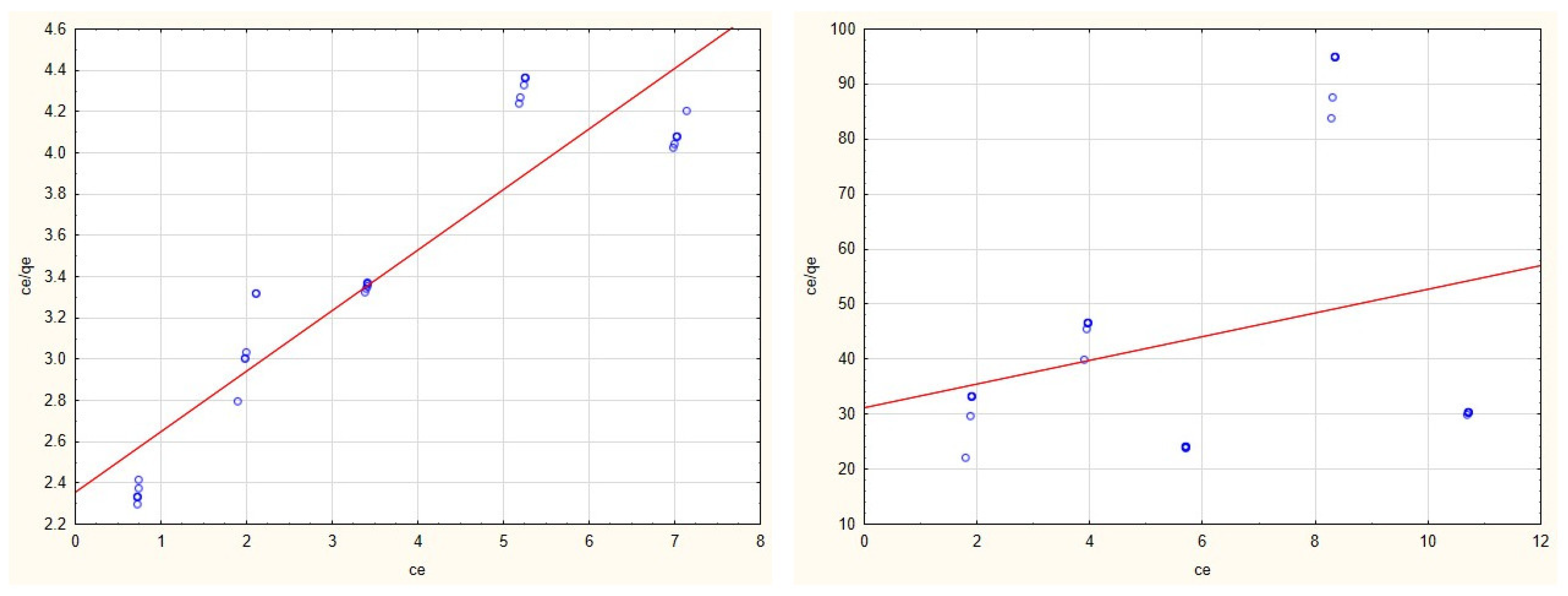
3.2. Adsorption Isotherms
3.3. Evaluation of Adsorption Capacity
3.4. Evaluation of Adsorption Isotherms
3.5. Evaluation of the Separation Factor
3.6. Recyclability and Stability of the Adsorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and Paper Mill Sludge Management Practices: What Are the Challenges to Assess the Impacts on Greenhouse Gas Emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef]
- de Azevedo, A.R.G.; Alexandre, J.; Xavier, G.d.C.; Pedroti, L.G. Recycling Paper Industry Effluent Sludge for Use in Mortars: A Sustainability Perspective. J. Clean. Prod. 2018, 192, 335–346. [Google Scholar] [CrossRef]
- Cusidó, J.A.; Cremades, L.V.; Soriano, C.; Devant, M. Incorporation of Paper Sludge in Clay Brick Formulation: Ten Years of Industrial Experience. Appl. Clay Sci. 2015, 108, 191–198. [Google Scholar] [CrossRef]
- Kizinievič, O.; Kizinievič, V.; Malaiškienė, J. Analysis of the Effect of Paper Sludge on the Properties, Microstructure and Frost Resistance of Clay Bricks. Constr. Build. Mater. 2018, 169, 689–696. [Google Scholar] [CrossRef]
- Jaria, G.; Silva, C.P.; Ferreira, C.I.A.; Otero, M.; Calisto, V. Sludge from Paper Mill Effluent Treatment as Raw Material to Produce Carbon Adsorbents: An Alternative Waste Management Strategy. J. Environ. Manag. 2017, 188, 203–211. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, Y.; Lin, Y.; Yang, D.; Zheng, H. Adsorption Behaviors of Paper Mill Sludge Biochar to Remove Cu, Zn and As in Wastewater. Environ. Technol. Innov. 2021, 23, 101616. [Google Scholar] [CrossRef]
- Gorzin, F.; Bahri Rasht Abadi, M. Adsorption of Cr(VI) from Aqueous Solution by Adsorbent Prepared from Paper Mill Sludge: Kinetics and Thermodynamics Studies. Adsorpt. Sci. Technol. 2018, 36, 149–169. [Google Scholar] [CrossRef]
- Xu, K.; Li, L.; Huang, Z.; Tian, Z.; Li, H. Efficient Adsorption of Heavy Metals from Wastewater on Nanocomposite Beads Prepared by Chitosan and Paper Sludge. Sci. Total Environ. 2022, 846, 157399. [Google Scholar] [CrossRef]
- Wang, Z.; Miao, R.; Ning, P.; He, L.; Guan, Q. From Wastes to Functions: A Paper Mill Sludge-Based Calcium-Containing Porous Biochar Adsorbent for Phosphorus Removal. J. Colloid Interface Sci. 2021, 593, 434–446. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Van, H.T.; Nguyen, Q.T.; Nguyen, T.H.; Nguyen, T.B.L.; Nguyen, V.Q.; Bui, T.U.; Le Sy, H. Paper Waste Sludge Derived-Hydrochar Modified by Iron (III) Chloride for Effective Removal of Cr(VI) from Aqueous Solution: Kinetic and Isotherm Studies. J. Water Process Eng. 2021, 39, 101877. [Google Scholar] [CrossRef]
- Li, E.; Zeng, X.; Fan, Y. Removal of Chromium Ion (III) from Aqueous Solution by Manganese Oxide and Microemulsion Modified Diatomite. Desalination 2009, 238, 158–165. [Google Scholar] [CrossRef]
- Fernández-Nava, Y.; Ulmanu, M.; Anger, I.; Marañón, E.; Castrillón, L. Use of Granular Bentonite in the Removal of Mercury (II), Cadmium (II) and Lead (II) from Aqueous Solutions. Water Air Soil Pollut. 2011, 215, 239–249. [Google Scholar] [CrossRef]
- Šuránek, M.; Melichová, Z.; Kureková, V.; Kljajević, L.; Nenadović, S. Removal of Nickel from Aqueous Solutions by Natural Bentonites from Slovakia. Materials 2021, 14, 282. [Google Scholar] [CrossRef]
- Badii, K.; Shabani, K.S.; Ardejani, F.D.; Olya, M.E. Acid mine drainage treatment by perlite nanomineral, batch and continuous systems. Arch. Min. Sci. 2014, 59, 89–104. [Google Scholar] [CrossRef]
- Zhang, T.; Tu, Z.; Lu, G.; Duan, X.; Yi, X.; Guo, C.; Dang, Z. Removal of Heavy Metals from Acid Mine Drainage Using Chicken Eggshells in Column Mode. J. Environ. Manag. 2017, 188, 1–8. [Google Scholar] [CrossRef]
- Ramutshatsha-Makhwedzha, D.; Mbaya, T.; Mavhungu, A.; Mavhunga, M.L.; Mbaya, R. Adsorptive Removal of Cd2+, Pb2+, and Fe2+ from Acid Mine Drainage Using a Mixture of Waste Orange and Lemon Activated Carbon (WOLAC): Equilibrium Study. J. Iran. Chem. Soc. 2023, 20, 1119–1133. [Google Scholar] [CrossRef]
- Calace, N.; Nardi, E.; Petronio, B.M.; Pietroletti, M.; Tosti, G. Metal Ion Removal from Water by Sorption on Paper Mill Sludge. Chemosphere 2003, 51, 797–803. [Google Scholar] [CrossRef]
- Yoon, K.; Cho, D.-W.; Tsang, D.C.W.; Bolan, N.; Rinklebe, J.; Song, H. Fabrication of Engineered Biochar from Paper Mill Sludge and Its Application into Removal of Arsenic and Cadmium in Acidic Water. Bioresour. Technol. 2017, 246, 69–75. [Google Scholar] [CrossRef]
- Olegario-Sanchez, E.; Pelicano, C.M. Characterization of Philippine Natural Zeolite and Its Application for Heavy Metal Removal from Acid Mine Drainage (AMD). Key Eng. Mater. 2017, 737, 407–411. [Google Scholar] [CrossRef]
- Masindi, V.; Gitari, M.W.; Tutu, H.; DeBeer, M. Synthesis of Cryptocrystalline Magnesite–Bentonite Clay Composite and Its Application for Neutralization and Attenuation of Inorganic Contaminants in Acidic and Metalliferous Mine Drainage. J. Water Process Eng. 2017, 15, 2–17. [Google Scholar] [CrossRef]
- Ryu, S.; Naidu, G.; Hasan Johir, M.A.; Choi, Y.; Jeong, S.; Vigneswaran, S. Acid Mine Drainage Treatment by Integrated Submerged Membrane Distillation–Sorption System. Chemosphere 2019, 218, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Ma, J.; Zhang, X.; Zhang, Q.; Xiao, Y.; Ma, Q.; Wang, S. Magnetic Natural Composite Fe3O4-Chitosan@bentonite for Removal of Heavy Metals from Acid Mine Drainage. J. Colloid Interface Sci. 2019, 538, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Wulandari, E.; Hidayat, A.E.; Moersidik, S.S. Comparison of Copper Adsorption Effectivity in Acid Mine Drainage Using Natural Zeolite and Synthesized Zeolite. IOP Conf. Ser. Earth Environ. Sci. 2020, 473, 012143. [Google Scholar] [CrossRef]
- Gumede, S.; Musonge, P. Characterisation of Mg-Al Hydrotalcite and Surfactant-Modified Bentonite Nano Clays for the Treatment of Acid Mine Drainage. Sustainability 2022, 14, 9501. [Google Scholar] [CrossRef]
- Majzlan, J.; Števko, M.; Chovan, M.; Luptáková, J.; Milovská, S.; Milovský, R.; Jeleň, S.; Sýkorová, M.; Pollok, K.; Göttlicher, J.; et al. Mineralogy and Geochemistry of the Copper-Dominated Neutral Mine Drainage at the Cu Deposit Ľubietová-Podlipa (Slovakia). Appl. Geochem. 2018, 92, 59–70. [Google Scholar] [CrossRef]
- Kisková, J.; Perháčová, Z.; Vlčko, L.; Sedláková, J.; Kvasnová, S.; Pristaš, P. The Bacterial Population of Neutral Mine Drainage Water of Elizabeth’s Shaft (Slovinky, Slovakia). Curr. Microbiol. 2018, 75, 988–996. [Google Scholar] [CrossRef]
- Sracek, O.; Filip, J.; Mihaljevič, M.; Kříbek, B.; Majer, V.; Veselovský, F. Attenuation of Dissolved Metals in Neutral Mine Drainage in the Zambian Copperbelt. Environ. Monit. Assess. 2011, 172, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Trumm, D.; Pope, J. Passive Treatment of Neutral Mine Drainage at a Metal Mine in New Zealand Using an Oxidizing System and Slag Leaching Bed. Mine Water Environ. 2015, 34, 430–441. [Google Scholar] [CrossRef]
- Jarvis, A.P.; Gandy, C.J.; Webb, J.A. Controls on the Generation and Geochemistry of Neutral Mine Drainage: Evidence from Force Crag Mine, Cumbria, UK. Minerals 2023, 13, 592. [Google Scholar] [CrossRef]
- STN EN ISO 10390 (838445); Zemina, Upravené Bioodpady a Kaly. Stanovenie pH. Slovenská technická norma: Bratislava, Slovakia, 1 June 2022. Available online: https://eshop.normservis.sk/norma/stneniso-10390-1.6.2022.html (accessed on 10 December 2024).
- Balintova, M.; Holub, M.; Stevulova, N.; Cigasova, J.; Tesarcikova, N. Sorption in Acidic Environment—Biosorbents in Comparison with Commercial Adsorbents. Chem. Eng. Trans. 2014, 39, 625–630. [Google Scholar] [CrossRef]
- Melichová, Z.; Ďuricová, A.; Samešová, D.; Nagyová, I. Hodnotenie Rizík Vybraných Kovových Prvkov vo Vodách; Univerzita Mateja Bela, Fakulta prírodných vied: Banská Bystrica, Slovakia, 2017; ISBN 978-80-557-1372-4. [Google Scholar]
- Melichová, Z.; Ľuptáková, A. Removing Lead from Aqueous Solutions Using Different Low-Cost Abundant Adsorbents. Desalination Water Treat. 2016, 57, 5025–5034. [Google Scholar] [CrossRef]
- Dada, A.O.; Olalekan, A.; Olatunya, A.; Dada, O. Langmuir, Freundlich, Temkin and Dubinin–Radushkevich Isotherms Studies of Equilibrium Sorption of Zn2+ Unto Phosphoric Acid Modified Rice Husk. J. Appl. Chem. 2012, 3, 38–45. [Google Scholar] [CrossRef]
- Alatalo, S.-M.; Repo, E.; Mäkilä, E.; Salonen, J.; Vakkilainen, E.; Sillanpää, M. Adsorption Behavior of Hydrothermally Treated Municipal Sludge & Pulp and Paper Industry Sludge. Bioresour. Technol. 2013, 147, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Chaukura, N.; Gwenzi, W.; Mupatsi, N.; Ruziwa, D.T.; Chimuka, C. Comparative Adsorption of Zn2+ from Aqueous Solution Using Hydroxylated and Sulphonated Biochars Derived from Pulp and Paper Sludge. Water Air Soil Pollut. 2016, 228, 7. [Google Scholar] [CrossRef]
- Baek, J. Assessment of the Adsorption Characteristics of Cadmium and Arsenic on Modified Paper Mill Sludge. Master’s Thesis, Graduate School, Seoul National University, Seoul, Republic of Korea, 2014. [Google Scholar]
- Du, X.; Cui, S.; Fang, X.; Wang, Q.; Liu, G. Adsorption of Cd(II), Cu(II), and Zn(II) by Granules Prepared Using Sludge from a Drinking Water Purification Plant. J. Environ. Chem. Eng. 2020, 8, 104530. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, F.; Xue, J.; Chen, S.; Wang, J.; Yang, Y. Enhanced Removal of Heavy Metal Ions from Aqueous Solution Using Manganese Dioxide-Loaded Biochar: Behavior and Mechanism. Sci. Rep. 2020, 10, 6067. [Google Scholar] [CrossRef]
- Chen, S.; Zhong, M.; Wang, H.; Zhou, S.; Li, W.; Wang, T.; Li, J. Study on Adsorption of Cu2+, Pb2+, Cd2+, and Zn2+ by the KMnO4 Modified Biochar Derived from Walnut Shell. Int. J. Environ. Sci. Technol. 2023, 20, 1551–1568. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from Aqueous Single Metal Solutions by Guanyl-Modified Cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef]
- Zhou, G.; Luo, J.; Liu, C.; Chu, L.; Crittenden, J. Efficient Heavy Metal Removal from Industrial Melting Effluent Using Fixed-Bed Process Based on Porous Hydrogel Adsorbents. Water Res. 2018, 131, 246–254. [Google Scholar] [CrossRef]
- Merah, M.; Boudoukha, C.; Avalos Ramirez, A.; Haroun, M.F.; Maane, S. High Biosorption of Cationic Dye onto a Novel Material Based on Paper Mill Sludge. Sci. Rep. 2023, 13, 15926. [Google Scholar] [CrossRef]
- Staszak, K.; Regel-Rosocka, M. Removing Heavy Metals: Cutting-Edge Strategies and Advancements in Biosorption Technology. Materials 2024, 17, 1155. [Google Scholar] [CrossRef] [PubMed]
- Ifthikar, J.; Jiao, X.; Ngambia, A.; Wang, T.; Khan, A.; Jawad, A.; Xue, Q.; Liu, L.; Chen, Z. Facile One-Pot Synthesis of Sustainable Carboxymethyl Chitosan—Sewage Sludge Biochar for Effective Heavy Metal Chelation and Regeneration. Bioresour. Technol. 2018, 262, 22–31. [Google Scholar] [CrossRef]
- Rosazlin, A.; Fauziah, C.I.; Rasidah, W.W.; Rosenani, A.B. Leaching of Heavy Metals (Cu, Mn, Zn, Ni, Pb and As) after Six Months Application of Raw and Composted Recycled Paper Mill Sludge. In Proceedings of the 2010 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; Available online: https://old.iuss.org/19th%20WCSS/Symposium/pdf/0659.pdf (accessed on 1 January 2025).
- Salameh, Y.; Albadarin, A.; Allen, S.; Walker, G.; Ahmad, M. Arsenic(III,V) Adsorption onto Charred Dolomite: Charring Optimization and Batch Studies. Chem. Eng. J. 2015, 259, 663–671. [Google Scholar] [CrossRef]
- Chakravarty, S.; Bhattacharjee, S.; Gupta, K.K.; Singh, M.; Chaturvedi, H.T.; Maity, S. Adsorption of Zinc from Aqueous Solution Using Chemically Treated Newspaper Pulp. Bioresour. Technol. 2007, 98, 3136–3141. [Google Scholar] [CrossRef] [PubMed]
- Calace, N.; Di Muro, A.; Nardi, E.; Petronio, B.M.; Pietroletti, M. Adsorption Isotherms for Describing Heavy-Metal Retention in Paper Mill Sludges. Ind. Eng. Chem. Res. 2002, 41, 5491–5497. [Google Scholar] [CrossRef]
- Calvo, B.; Canoira, L.; Morante, F.; Martínez-Bedia, J.M.; Vinagre, C.; García-González, J.-E.; Elsen, J.; Alcantara, R. Continuous Elimination of Pb2+, Cu2+, Zn2+, H+ and NH4+ from Acidic Waters by Ionic Exchange on Natural Zeolites. J. Hazard. Mater. 2009, 166, 619–627. [Google Scholar] [CrossRef]
- Hasan, H.A.; Abdullah, S.R.S.; Kofli, N.T.; Kamarudin, S.K. Isotherm Equilibria of Mn2+ Biosorption in Drinking Water Treatment by Locally Isolated Bacillus Species and Sewage Activated Sludge. J. Environ. Manag. 2012, 111, 34–43. [Google Scholar] [CrossRef]
- Goldberg, S. Equations and Models Describing Adsorption Processes in Soils. In Chemical Processes in Soils; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 489–517. ISBN 978-0-89118-892-6. [Google Scholar]
- Abdullah, R.; Kadir, W.R.; Ishak, C.; Rosenani, A.; Ahmad, R. Growth and Residual Nutrients in Soil of Intercropped Stand of Khaya Senegalensis and Orthosiphon Stamineus Treated with Paper Mill Biosludge. J. Trop. For. Sci. 2015, 27, 255–266. [Google Scholar]


| PTEs [μg/L] | Cu * [μg/L] | Zn * [μg/L] | Cd * [μg/L] | Mn * [μg/L] | Ni [μg/L] | Pb [μg/L] | Al [μg/L] | Fe [μg/L] | pH [–] |
|---|---|---|---|---|---|---|---|---|---|
| LV | Cu 1.1 μg/L for 1st and 2nd hardness classes and 8.8 μg/L for 4th and 5th hardness classes | 7.8 μg/L for 1st and 2nd hardness classes and up to 52 μg/L for 4th and 5th hardness classes | 0.08 μg/L for 1st hardness class and up to 0.25 μg/L for 5th hardness class | Total Mn 300 μg/L | 20 μg/L | 7.2 μg/L | 200 μg/L | Total Fe 2 mg/ L | – |
| MV | 18.2 | 5356.7 | 20.55 | 3520.7 | 8.3 | 1.8 | 152 | 12.3 | 7.6 |
| Property | Sludge | Unit |
|---|---|---|
| Dry matter | 95.30 | % |
| pH | 6.83 | - |
| Cadmium | 0.48 | mg/kg |
| Lead | 7.93 | mg/kg |
| Nickel | 3.7 | mg/kg |
| Magnesium | 432 | mg/kg |
| Calcium | 29,231 | mg/kg |
| Potassium | 142 | mg/kg |
| Phosphorus | 33.7 | mg/kg |
| Manganese | 11.5 | mg/kg |
| Copper | 1.02 | mg/kg |
| Zinc | 25.8 | mg/kg |
| Iron | 111 | mg/kg |
| Sodium | 45.6 | mg/kg |
| CaO | 26.84 | % |
| Al2O3 | 4789 | % |
| Carbonates | 53.2 | % |
| Corg | 19.7 | % |
| Nitrogen | 4380 | g/kg |
| Time | Initial Concentration [mg/L] | Mine Drainage | Water Solution | ||
|---|---|---|---|---|---|
| Mean [mg/g] | Std.Dev. [mg/g] | Mean [mg/g] | Std.Dev. [mg/g] | ||
| 30 | 4 | 0.240 | 0.002 | 0.422 | 0.001 |
| 30 | 8 | 0.103 | 0.011 | 0.823 | 0.005 |
| 30 | 12 | 0.402 | 0.006 | 1.271 | 0.004 |
| 30 | 16 | 0.284 | 0.005 | 1.261 | 0.052 |
| 30 | 20 | 0.627 | 0.001 | 1.430 | 0.002 |
| 60 | 4 | 0.265 | 0.005 | 0.514 | 0.004 |
| 60 | 8 | 0.194 | 0.009 | 0.832 | 0.005 |
| 60 | 12 | 0.493 | 0.005 | 1.271 | 0.004 |
| 60 | 16 | 0.488 | 0.001 | 1.250 | 0.011 |
| 60 | 20 | 0.609 | 0.023 | 1.441 | 0.011 |
| 90 | 4 | 0.255 | 0.001 | 0.516 | 0.002 |
| 90 | 8 | 0.133 | 0.004 | 0.885 | 0.008 |
| 90 | 12 | 0.296 | 0.013 | 1.343 | 0.014 |
| 90 | 16 | 0.465 | 0.004 | 1.305 | 0.012 |
| 90 | 20 | 0.367 | 0.011 | 1.442 | 0.011 |
| 120 | 4 | 0.235 | 0.002 | 0.518 | 0.001 |
| 120 | 8 | 0.135 | 0.002 | 0.892 | 0.000 |
| 120 | 12 | 0.138 | 0.006 | 1.416 | 0.004 |
| 120 | 16 | 0.108 | 0.013 | 1.390 | 0.000 |
| 120 | 20 | 0.000 | 0.000 | 1.589 | 0.002 |
| Time | Initial Concentration [mg/L] | Mine Drainage | Water Solution | ||
|---|---|---|---|---|---|
| Mean [mg/g] | Std.Dev. [mg/g] | Mean [mg/g] | Std.Dev. [mg/g] | ||
| 30 | 2 | 0.116 | 0.008 | 0.198 | 0.026 |
| 30 | 4 | 0.399 | 0.008 | 0.170 | 0.001 |
| 30 | 6 | 0.382 | 0.002 | 0.422 | 0.027 |
| 30 | 8 | 0.376 | 0.005 | 0.914 | 0.001 |
| 30 | 10 | 0.291 | 0.006 | 1.512 | 0.001 |
| 60 | 2 | 0.113 | 0.001 | 0.209 | 0.004 |
| 60 | 4 | 0.443 | 0.017 | 0.323 | 0.001 |
| 60 | 6 | 0.895 | 0.007 | 0.602 | 0.012 |
| 60 | 8 | 0.503 | 0.010 | 1.002 | 0.002 |
| 60 | 10 | 0.458 | 0.009 | 1.533 | 0.001 |
| 90 | 2 | 0.151 | 0.001 | 0.239 | 0.001 |
| 90 | 4 | 0.460 | 0.010 | 0.372 | 0.001 |
| 90 | 6 | 1.002 | 0.001 | 0.618 | 0.010 |
| 90 | 8 | 0.603 | 0.005 | 1.008 | 0.008 |
| 90 | 10 | 0.470 | 0.014 | 1.534 | 0.002 |
| 120 | 2 | 0.274 | 0.002 | 0.258 | 0.004 |
| 120 | 4 | 0.636 | 0.007 | 0.377 | 0.001 |
| 120 | 6 | 1.018 | 0.004 | 0.743 | 0.010 |
| 120 | 8 | 0.862 | 0.002 | 1.089 | 0.021 |
| 120 | 10 | 0.694 | 0.002 | 1.614 | 0.002 |
| Time | Initial Concentration [mg/L] | Mine Drainage | Water Solution | ||
|---|---|---|---|---|---|
| Mean [mg/g] | Std.Dev. [mg/g] | Mean [mg/g] | Std.Dev. [mg/g] | ||
| 30 | 1 | 0.038 | 0.004 | 0.188 | 0.001 |
| 30 | 2 | 0.125 | 0.002 | 0.239 | 0.001 |
| 30 | 3 | 0.141 | 0.007 | 0.455 | 0.005 |
| 30 | 4 | 0.206 | 0.014 | 0.337 | 0.004 |
| 30 | 5 | 0.219 | 0.004 | 0.562 | 0.004 |
| 60 | 1 | 0.071 | 0.001 | 0.208 | 0.002 |
| 60 | 2 | 0.165 | 0.002 | 0.280 | 0.002 |
| 60 | 3 | 0.293 | 0.002 | 0.518 | 0.001 |
| 60 | 4 | 0.260 | 0.006 | 0.381 | 0.010 |
| 60 | 5 | 0.242 | 0.002 | 0.597 | 0.002 |
| 90 | 1 | 0.082 | 0.001 | 0.209 | 0.005 |
| 90 | 2 | 0.163 | 0.001 | 0.331 | 0.004 |
| 90 | 3 | 0.370 | 0.001 | 0.536 | 0.008 |
| 90 | 4 | 0.321 | 0.009 | 0.586 | 0.004 |
| 90 | 5 | 0.294 | 0.008 | 0.749 | 0.005 |
| 120 | 1 | 0.090 | 0.001 | 0.211 | 0.006 |
| 120 | 2 | 0.186 | 0.001 | 0.347 | 0.003 |
| 120 | 3 | 0.399 | 0.003 | 0.592 | 0.008 |
| 120 | 4 | 0.356 | 0.003 | 0.625 | 0.002 |
| 120 | 5 | 0.246 | 0.000 | 0.808 | 0.002 |
| Time | Initial Concentration [mg/L] | Mine Drainage | Water Solution | ||
|---|---|---|---|---|---|
| Mean [mg/g] | Std.Dev. [mg/g] | Mean [mg/g] | Std.Dev. [mg/g] | ||
| 30 | 2 | 0.001 | 0.001 | 0.226 | 0.001 |
| 30 | 4 | 0.055 | 0.004 | 0.539 | 0.014 |
| 30 | 6 | 0.076 | 0.004 | 0.758 | 0.006 |
| 30 | 8 | 0.002 | 0.001 | 0.938 | 0.003 |
| 30 | 10 | 0.172 | 0.005 | 1.730 | 0.001 |
| 60 | 2 | 0.005 | 0.003 | 0.288 | 0.008 |
| 60 | 4 | 0.032 | 0.004 | 0.569 | 0.001 |
| 60 | 6 | 0.130 | 0.007 | 1.003 | 0.002 |
| 60 | 8 | 0.001 | 0.001 | 1.124 | 0.010 |
| 60 | 10 | 0.277 | 0.017 | 1.740 | 0.014 |
| 90 | 2 | 0.017 | 0.004 | 0.291 | 0.004 |
| 90 | 4 | 0.066 | 0.001 | 0.626 | 0.004 |
| 90 | 6 | 0.194 | 0.003 | 1.005 | 0.001 |
| 90 | 8 | 0.026 | 0.009 | 1.134 | 0.001 |
| 90 | 10 | 0.314 | 0.007 | 1.905 | 0.001 |
| 120 | 2 | 0.063 | 0.010 | 0.314 | 0.002 |
| 120 | 4 | 0.087 | 0.005 | 0.656 | 0.017 |
| 120 | 6 | 0.238 | 0.001 | 1.017 | 0.002 |
| 120 | 8 | 0.091 | 0.005 | 1.212 | 0.007 |
| 120 | 10 | 0.354 | 0.002 | 1.724 | 0.012 |
| Adsorbate Ion | Isotherm Constants | |||||||
| Aquatic Solution | Mine Drainage | |||||||
| Langmuir Constants | ||||||||
| qm [mg/g] | b [L/mg] | Equation | R2 | qm [mg/g] | b [L/mg] | Equation | R2 | |
| Cu(II) | 4.742 | 0.102 | y = 2.0586 + 0.211 × x | 0.789 | 0.0002 | −0.4593 | y = −11,733.6892 + 5390.6591 × x | 0.554 |
| Zn(II) | 2.472 | 0.694 | y = 0.579 + 0.4089 × x | 0.767 | 0.880 | 1.207 | y = 0.9412 + 1.1361 × x | 0.754 |
| Cd(II) | 2.685 | 0.351 | y = 1.0613 + 0.3727 × x | 0.744 | 0.307 | 5.537 | y = 0.5897 + 3.2613 × x | 0.863 |
| Mn(II) | 3.413 | 0.1244 | y = 2.354 + 0.2934 × x | 0.856 | 0.455 | 0.072 | y = 31.183 + 2.1601 × x | 0.077 |
| Freundlich Constants | ||||||||
| Kf [mg/g] | n | Equation | R2 | Kf [mg/g] | n [-] | Equation | R2 | |
| Cu(II) | 0.450 | 1.238 | y = −0.3467 + 0.8074 × x | 0.973 | 1.1429 | −0.3039 | y = 0.0583−3.291 × x | 0.453 |
| Zn(II) | 0.932 | 1.564 | y = −0.0314 + 0.6351 × x | 0.984 | 0.505 | 3.072 | y = −0.2967 + 0.3252 × x | 0.188 |
| Cd(II) | 1.435 | 1.212 | y = −0.1569 + 0.8251 × x | 0.989 | 0.266 | 2.567 | y = −0.575 + 0.3891 × x | 0.439 |
| Mn(II) | 2.539 | 1.364 | y = −0.4044 + 0.7324 × x | 0.993 | 0.036 | 1.267 | y = −1.4398 + 0.7875 × x | 0.526 |
| Adsorbate Ion | Adsorbate | Initial Concentration [mg/L] | ||||
|---|---|---|---|---|---|---|
| KR | ||||||
| 4 | 8 | 12 | 16 | 20 | ||
| Cu(II) | aquatic solution | 0.709 | 0.550 | 0.449 | 0.379 | 0.328 |
| neutral mine drainage | −1.194 | −0.374 | −0.222 | −0.157 | −0.122 | |
| 2 | 4 | 6 | 8 | 10 | ||
| Zn(II) | aquatic solution | 0.418 | 0.265 | 0.193 | 0.152 | 0.126 |
| neutral mine drainage | 0.293 | 0.172 | 0.121 | 0.094 | 0.077 | |
| 1 | 2 | 3 | 4 | 5 | ||
| Cd(II) | aquatic solution | 0.740 | 0.588 | 0.487 | 0.416 | 0.363 |
| neutral mine drainage | 0.153 | 0.083 | 0.057 | 0.043 | 0.035 | |
| 2 | 4 | 6 | 8 | 10 | ||
| Mn(II) | aquatic solution | 0.801 | 0.668 | 0.573 | 0.501 | 0.446 |
| neutral mine drainage | 0.875 | 0.778 | 0.700 | 0.636 | 0.583 | |
| References | Adsorbent | qmax Cu (mg/g) | qmax Zn (mg/g) | qmax Cd (mg/g) | qmax Mn (mg/g) | Temperature (°C) | pH/Key Conditions |
|---|---|---|---|---|---|---|---|
| Our results | Untreated paper sludge | 4.742 | 2.472 | 2.685 | 3.413 | 25 | pH ≈ 7; 5 g/L adsorbent |
| [38] | Sludge–clay granules (GSC) | 2.76 | 1.23 | 1.53 | 25 | pH 5.0 | |
| [39] | Water–hyacinth biochar + nano-MnO2 | 103.9 | 68.4 | 151.4 | 25 | pH > 6 (not specified) | |
| [40] | KMnO4-modified walnut shell biochar (MWSC) | 30.18 | 58.96 | 44.94 | 25 | pH 5.0 | |
| [41] | Guanidyl-modified cellulose (Gu-MC) | 82 | 77 | 69 | 25 | pH 6.0 | |
| [42] | Porous Jute/PAA hydrogel | 401.7 | 20 | pH 6.0 (Cd only) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samešová, D.; Pochyba, A.; Ďuricová, A.; Poništ, J.; Prepilková, V.Š.; Schwarz, M.; Veverková, D.; Salva, J.; Schmidtová, J. Comparative Adsorption of Cu(II), Zn(II), Cd(II), and Mn(II) from Aquatic Solution and Neutral Mine Drainage Using Paper Sludge. Water 2025, 17, 1471. https://doi.org/10.3390/w17101471
Samešová D, Pochyba A, Ďuricová A, Poništ J, Prepilková VŠ, Schwarz M, Veverková D, Salva J, Schmidtová J. Comparative Adsorption of Cu(II), Zn(II), Cd(II), and Mn(II) from Aquatic Solution and Neutral Mine Drainage Using Paper Sludge. Water. 2025; 17(10):1471. https://doi.org/10.3390/w17101471
Chicago/Turabian StyleSamešová, Dagmar, Adam Pochyba, Anna Ďuricová, Juraj Poništ, Veronika Štefanka Prepilková, Marián Schwarz, Darina Veverková, Jozef Salva, and Jarmila Schmidtová. 2025. "Comparative Adsorption of Cu(II), Zn(II), Cd(II), and Mn(II) from Aquatic Solution and Neutral Mine Drainage Using Paper Sludge" Water 17, no. 10: 1471. https://doi.org/10.3390/w17101471
APA StyleSamešová, D., Pochyba, A., Ďuricová, A., Poništ, J., Prepilková, V. Š., Schwarz, M., Veverková, D., Salva, J., & Schmidtová, J. (2025). Comparative Adsorption of Cu(II), Zn(II), Cd(II), and Mn(II) from Aquatic Solution and Neutral Mine Drainage Using Paper Sludge. Water, 17(10), 1471. https://doi.org/10.3390/w17101471






