Abstract
The presence of antibiotics and antibiotic resistance genes (ARGs) in natural habitats has recently sparked increased concern. Vertical-flow constructed wetlands (VFCWs) represent a novel approach to reducing these new contaminants. In the current work, four laboratory-scale VFCW models with various substrates were built to decrease oxytetracycline (OTC) and ARGs. The findings showed that the combination of zeolite and activated carbon exhibited high OTC removal efficiency (up to 97%), with lesser accumulation than in other experimental groups. Furthermore, the combination of zeolite and activated carbon had the lowest absolute and relative abundance of ARGs. This was ascribed to the synergistic benefits of zeolite and activated carbon in CW-D, which exceeded other VFCWs in terms of ARGs removal efficiency. The treatment groups had a considerable but not absolute inhibitory impact on ARGs proliferation; this was attributable to the fact that many dominant bacteria in the community under antibiotic stress were antibiotic-resistant, allowing ARGs to propagate more easily. Network analysis and correlation analysis emphasized the importance of horizontal gene transfer (HGT) in ARGs dissemination, and antibiotic pressure is unlikely to have a substantial influence on ARGs propagation in the medium-term future. Furthermore, it was found that hydrophilic phages and Legionella species might serve as possible hosts for ARGs.
1. Introduction
The emergence of antibiotics has transformed the management of bacterial infections, offering a direct and efficient approach to treating illnesses and significantly enhancing the health and safety of both people and animals [1]. Nonetheless, previous studies have reported that only a small proportion of the antibiotics ingested by people or animals is metabolized, and the remainder is excreted into the environment in derivative and primitive forms [2]. The excessive use of antibiotics leads to their persistent discharge into the environment via human activities such as medical, veterinary, agricultural, and aquacultural practices, hence hastening the development of antibiotic resistance. The introduction of antimicrobial drugs into the environment has raised widespread concern. This directly facilitates the acquisition of antibiotic resistance genes (ARGs) by microbes, even at low doses. Although certain documents indicate that solar photolysis, hydrolysis, ionizing radiation, contact electro-catalysis (CEC), or advanced oxidation processes (AOPs) can degrade specific antibiotics, the ARGs linked to these substances endure in natural environments due to the growth and proliferation of microorganisms. The spread of antimicrobial resistance genes undermines the therapeutic effectiveness of antibiotics against pathogenic microorganisms, endangering both ecosystems and human health [3]. Therefore, the possible risks associated with ARGs must be recognized and handled promptly. This emphasizes the need for a holistic strategy in regulating antibiotic use and alleviating the ecological consequences of these powerful agents.
Antimicrobial resistance genes and antibiotics mostly exist in aquatic environments [4,5]. Various types of water may include ARGs, e.g., drinking water [6,7], groundwater [8,9,10], freshwater [11,12], and saltwater [13,14]. Research indicates that the main sources of these contaminants are the pharmaceutical, healthcare, and animal sectors [15,16,17,18]. Antibiotic residues in wastewater impose continuous selection pressure on microbial populations, leading to an increase in ARGs and HGT across various bacteria [19,20]. For instance, ARGs may last for extended durations even after the removal of antibiotics, demonstrating the robust correlation between antibiotics and ARGs [21,22]. Although wastewater treatment plants (WWTPs) are designed to manage these pollutants, studies have demonstrated that typical WWTPs are inadequate [23,24]. Because these contaminants are persistent, new methods for the degradation and control of pollution must be developed. Examples include a novel membrane filtering approach [25], aerobic granular sludge (AGS) technology [26], and the UV/PAA combination disinfection process [27]. However, the practical use of these sophisticated treatment technologies is significantly impeded by the fact that they often need significant energy inputs, as well as incurring high installation, operating, and maintenance costs.
Constructed wetlands (CWs) are widely used in wastewater treatment and are renowned for their low energy costs, environmental advantages, and low installation and maintenance requirements. Furthermore, CWs have been shown to efficiently reduce antibiotics and their ARGs [28]. CWs effectively remediate wastewater via a synergistic interplay among vegetation, substrates, and microbiological processes [28]. The substrate is an essential part of these systems because it facilitates multiple physical and chemical interactions in wetlands while also providing a nurturing environment for bacteria and plants [29]. Substrates in CWs usually lower antibiotics and ARGs by adsorption, biodegradation, chemical oxidation, and filtration [30,31]. Huang et al. [31] used vertical-flow constructed wetlands (VFCWs) to remove these pollutants from swine wastewater, investigating the effects of various substrate types and hydraulic conditions. The findings demonstrated that the substrate selection may have a significant influence on antibiotic clearance in artificial wetlands. In a similar vein, Ma et al. [29] demonstrated that VFCWs supplemented with plant material and matrix materials such as zeolites and brick balls were very successful in eliminating ARGs and antibiotics. However, Song et al. [32] recognized a possible issue: VFCWs are controlled by a complex collection of processes, and additional study is required to understand how they function in different contexts.
The current work aimed to analyze the ability of various substrates in CWs to remove ARGs and antibiotics. Four laboratory-scale VFCWs have been designed and built, each with a unique combination of substrates for the removal of ARGs and antibiotics. Meanwhile, the microbiota alterations of these VFCWs were assessed. This work is the first to thoroughly examine the dynamic development and response mechanisms of microbial communities in the composite substrate system of VFCWs during the treatment of antibiotic-contaminated wastewater. It offers a novel approach to exposing microbial roles in complex environmental engineering systems and successfully bridges the theoretical gap in understanding the operational processes of VFCWs at the microbial scale.
2. Materials and Methods
2.1. Constructed Wetland Design and Operation
The experiment was conducted in four lab-scale VFCWs that were 15 cm in diameter and 75 cm in height. Four types of VFCW systems were constructed: CW-A—gravel; CW-B—gravel + activated carbon (V:V = 1:2); CW-C—gravel + zeolite (V:V = 1:2); CW-D—activated carbon + zeolite (V:V = 1:2). As depicted in Figure 1, each VFCW was designed with two layers. For instance, CW-C was composed of a 20 cm bottom layer of gravel and a 50 cm top layer of gravel and zeolite in a 1:2 ratio. Common reed (Calamus) was the selected plant species for the wetlands, with a density of 225 plants/m2 (Figure 1).
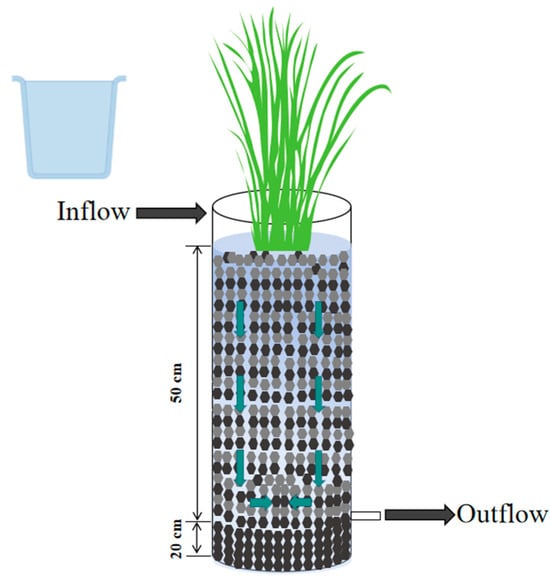
Figure 1.
Schematic diagram of VFCWs.
The synthetic domestic wastewater contained glucose (128 mg/L), protein soup (118 mg/L), CH3COONa (30 mg/L), KH2PO4 (17.5 mg/L), NH4Cl (19 mg/L), and a trace element solution consisting of MgCl4 (6 mg/L), CaCl2 (2 mg/L), CuSO4·5H2O (0.025 mg/L), H3BO3 (6.2 mg/L), MnSO4·H2O (6.37 mg/L), ZnSO4·7H2O (10.75 mg/L), CoCl (0.025 mg/L), and Na2Mo4·2H2O (0.25 mg/L). Experimental data were obtained after 20 days of stable operation, with a hydraulic retention time (HRT) of 2 days for the reactors. The sludge used to inoculate the VFCWs was sourced from the anaerobic tank of a sewage treatment plant in Zhengzhou.
The domestication time was 20 d, and the hydraulic retention time for all devices was set to 2 d. The following experiment was divided into three phases: the first phase operated for 8 days, the second phase for 12 days, and the third phase for 4 days. Oxytetracycline (OTC) was added to the reactors at varying concentrations at different stages (0.01 mg/L, 0.05 mg/L, 0.1 mg/L, 0.2 mg/L, 0.5 mg/L, 0.8 mg/L, 1 mg/L). The device operating temperature of the artificial wetland was 25–30 °C.
2.2. Sample Collection
Water samples were collected at a frequency of once every two days from the inflow and outflow sections of the VFCWs. After collection, the samples were filtered through a 0.22 µm glass fiber membrane and transferred into 100 mL amber glass bottles, which were then promptly transported to the laboratory. Upon arrival, the samples were stored at 4 °C to ensure their stability. At the conclusion of the first and third experimental phases, three random locations on the surface of each VFCWs were selected, and 10 g substrate samples were collected from a depth of 10 cm below the surface. The substrate samples from each VFCWs were subsequently pooled to maximize the reduction of random errors in the experimental results. All collected substrate samples were stored at −20 °C for subsequent analysis and research. The actual operating condition of the VFCWs is shown in Figure 2.

Figure 2.
Photographs of the actual operation of VFCWs.
2.3. Detection of Antibiotics
All samples were tested for antibiotics within 24 h. The solid-phase extraction process of OTC in water was as follows. A 1 L water sample was filtered with a 0.45 μm filter membrane. The pH of the filtered water sample was adjusted to 3.0 by adding 6 mol/L HCl; then, 0.2 g EDTA was added to eliminate the interference of heavy metals. An HLB solid-phase extraction column (Waters Corporation, Milford, MA, USA) was used to enrich the OTC in the water. Each HLB column was sequentially preconditioned with 10 mL methanol and 10 mL ultrapure water. Then, the water samples were passed through the column at a flow rate of about 10.0 mL/min. After this, the HLB column was cleaned with 10 mL ultrapure water and the water was removed by a vacuum pump. The antibiotics in the HLB column were eluted with 6.0 mL pure methanol, and the eluent was collected in a brown test tube. The eluent was dried with nitrogen for 20 min to near dryness and then re-dissolved in 20% methanol water solution to a volume of 1.0 mL. Finally, it was filtered with a 0.22 μm filter membrane. The filtered sample was subjected to further analysis.
The substrate samples were all freeze-dried. Next, 5.0 g of each solid sample was weighed, and 0.5 g of Na2EDTA and 50 mL of a mixed solution containing phosphate buffer (pH = 3.0) and acetonitrile (1:1, v/v) were added. The tubes were shaken at a speed of 300 rpm for 20 min, sonicated for 10 min, and then centrifuged at 10,000 rpm for 10 min to collect the supernatant. The extraction process was performed 3 times for each sample. The obtained supernatants were combined and then diluted to 500 mL with ultrapure water. The following process was performed according to the exaction method of the water samples.
The concentrations of OTC in each sample were measured by a triple quadrupole liquid chromatography–mass spectrometry (TQ-LC-MS) system (Triple Quad™ 3500, SCIEX, Framingham, MA, USA) in multiple reaction monitoring (MRM) mode, which was in accordance with previous studies [33,34].
2.4. DNA Extraction and Quantification and PCR
Intracellular DNA pre-processing is primarily aimed at extracting and analyzing intracellular DNA (iDNA) to detect ARGs. This pre-processing involves methods such as cell lysis to release the DNA, followed by purification and extraction. The substrate samples were subjected to sonication and shaking, followed by filtration through a 0.22 μm mixed cellulose ester membrane to extract and retain cellular components for intracellular DNA pre-processing, and the weight of the substrate sample used for extraction was recorded [32]. After cutting with clean, sterile scissors, the filter membranes were transferred to a sterile collection tube. Microbial DNA was extracted by the Ezup Column Soil DNA Purification Kit (Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China), according to the manufacturer’s instructions. The concentration of DNA was measured using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
Tetracycline ARGs (tetA, tetX), mobile genetic elements (MGEs) (intI1, intI2, ISCR1), and the 16S rRNA gene were analyzed by real-time fluorescence quantitative PCR (qPCR) with a StepOnePlus™ qPCR (Thermo Fisher Scientific, Waltham, MA, USA). The primer sequences for the respective target genes were used as documented in our previous research [34]; the gene fragment was synthesized by Sangon Biotech (Shanghai) Co., Ltd., Shanghai, China. A total of 40 cycles were applied to improve the likelihood of product formation from a low initial template concentration. The PCR volume was 20 μL, which contained 10 µL of AceQ SYBR® Green Master Mix, 0.2 mM of each primer, and 2 µL of DNA template. The complete methodology, including the qPCR parameters, amplification efficacy, and reference curves, was derived from our previous research [35].
2.5. Microbial Community
The extracted DNA obtained above was paired-end sequenced (2 × 250) on an Illumina MiSeqPE2500 high-throughput sequencing platform to build small fragment libraries for sequencing. The primers for 16S rRNA V3-V4 were 338F (5-ACTCCTACGGGAGGCAGCA-3) and 806R (5-GGACTACHVGGGTWTCTAAT-3). The data were filtered, spliced, and removed via the QIIME dada2 process. Sequences with scores less than 20, base ambiguity, primer mismatch, or sequencing lengths less than 150 bp were removed. According to the barcodes, the sequence information of each processing group was classified in terms of operational taxonomic units (OTUs) for species classification, and the OTU similarity was set to 97%. By comparison with the Silva database, the species classification information of each OTU was obtained.
2.6. Data Analysis
T-tests, multifactorial analysis of variance, and Pearson correlation analyses were conducted using the IBM SPSS 19.0 statistical software, and p < 0.05 was used to identify significant differences. The relationships between the ARGs, dominant bacterial genera, and OTC were visualized using a Pearson correlation heatmap, which was generated with the Origin 2021 software. Community differences were assessed through LEfSe analysis performed on the real-time interactive online data analysis platform WekaMine Bioincloud (https://www.bioincloud.tech). Co-occurrence networks were constructed using the Gephi 0.9 software.
3. Results and Discussion
3.1. Accumulation of OTC in Constructed Wetlands
As demonstrated in Figure 3, all VFCWs had excellent OTC reduction capabilities. CW-A, CW-B, CW-C, and CW-D removed more than 97% of OTC from wastewater on average throughout the operating period. The OTC removal capabilities of the VFCWs were as follows: CW-D was followed by CW-C, CW-B, and, finally, CW-A. CW-D had the greatest removal rate, but there was no significant difference across the other VFCWs (p > 0.05). The findings showed that activated carbon and zeolite had better adsorption abilities for OTC than gravel, and the adsorption capacity of each substrate was diminished with the operation of the VFCWs. This was attributable to the adsorption effect, which is the key factor determining OTC elimination [33].
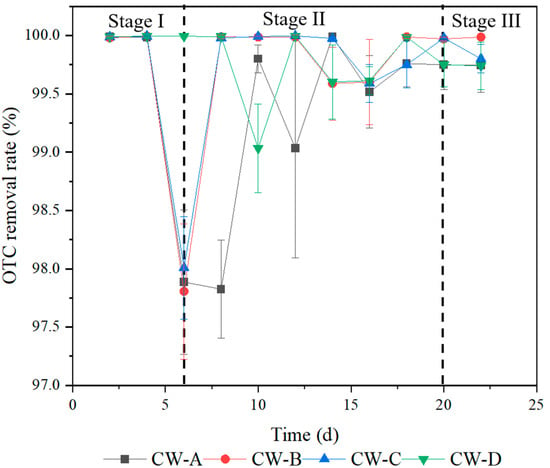
Figure 3.
The removal rates of OTC in wastewater by VFCWs.
This research investigated the buildup of OTC in several VFCWs during stages I and III. As seen in Figure 4, it was found that OTC accumulated in all VFCWs in diverse ways over time. At the conclusion of stage I, the average OTC concentration in CW-C was 42.16 μg/L. The OTC concentrations in CW-A, CW-B, and CW-D varied from 17.11 to 18.48 μg/L. During stage III, CW-B had the highest OTC concentration (197.69 μg/L), followed by CW-C (124.49 μg/L), CW-D (117.41 μg/L), and CW-A (95.85 μg/L). Adsorption was the predominant mode of clearance for OTC [36]; hence, antibiotic buildup in various substrates was not unusual. The accumulation of OTC in all experimental groups (CW-B, CW-C, and CW-D) was significantly higher than in the control group (CW-A), demonstrating that zeolite and activated carbon were the primary substrates driving OTC accumulation in the created wetlands. The CW-B system had the largest total amount of OTC. VFCWs using activated carbon as the substrate had greater accumulation levels than other VFCWs, most likely due to the activated carbon substrates’ stronger adsorption capabilities as compared to gravel and zeolite. Activated carbon is a carbon-dense material with a high specific surface area, a large pore volume, and a variety of functional groups (including carbonyl, carboxyl, hydroxyl, and phenolic hydroxyl groups), resulting in high adsorption capabilities. Furthermore, a previous study also showed that activated carbon may substantially decrease tetracyclines by adsorption [25].
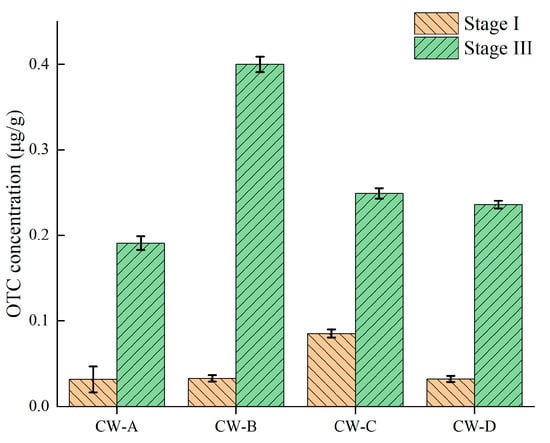
Figure 4.
Accumulation of OTC in VFCWs.
At the conclusion of stage I, the OTC accumulation level of CW-C surpassed those of the other groups. This indicates that zeolite has significant adsorption capabilities during the early operational phase. Previous publications have demonstrated that zeolite may function as a highly selective adsorbent for antibiotics [37]. Zeolite has a strong affinity for antibiotics, aligning with the findings of the current investigation. By the conclusion of the third phase, the total quantity of CW-D was inferior to that of CW-B. This may be ascribed to the distinctive Si-OH groups of zeolite and its catalytic influence on diverse chemical reactions, which, alongside biodegradation via adsorption (predominantly chemical adsorption and electrostatic interactions), also mitigated pollution in wastewater containing various antibiotics and ARGs (e.g., extensive surface area for microbial adhesion) [31].
3.2. Abundance of ARGs in Constructed Wetlands
This study used qPCR to quantitatively analyze the absolute abundance of two ARGs (tetA, tetX) and three MGEs (intI1, intI2, ISCR1) in the VFCWs, as shown in Figure 5. The copy numbers of the 16S rRNA gene across all groups ranged from 1.4 × 105 copies/L to 1.98 × 106 copies/L. Notably, the copy numbers of the 16S rRNA gene were higher in stages I and II compared to stage III. This divergence may be ascribed to the decreased antibiotic concentrations throughout the first and secondary phases, which presumably presented a lesser risk to microbial viability. The decrease in biomass during stage III may be attributed to the increased concentration of antibiotics, which exerted inhibitory effects on the normal survival of microorganisms. Likewise, prior research has shown that elevated antibiotic concentrations, to a certain degree, impede the normal metabolism of bacteria, thereby hindering the growth of microbial biomass [38].
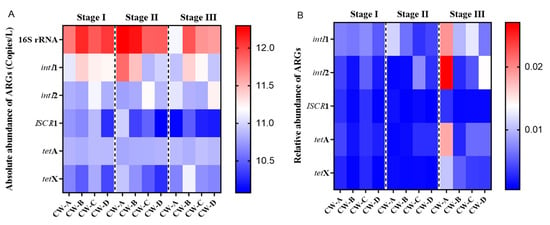
Figure 5.
Absolute and relative abundance of ARGs in VFCWs. (A) The absolute abundance of ARGs; (B) the relative abundance of ARGs.
Figure 5 shows that intI1 has higher absolute abundance than intI2 and ISCR1, which might be attributed to its significant environmental accumulation. Given the strong relationship between intI1 and the spread of OTC-related ARGs, it is possible that OTC-related ARGs may undergo horizontal gene transfer inside VFCWs. Following VFCW operation, both the absolute and relative abundance of tetA and tetX increased in the majority of cases, showing the enrichment of ARGs inside the VFCW. The aforementioned data indicate that, although VFCWs may successfully remove antibiotics, they may also contribute to ARGs enrichment. This occurrence indicates that the presence of antibiotics may drastically alter the quantity and diversity of microbiota, as well as exerting selection pressure on the enrichment of possible antibiotic-resistant bacteria in this system. CW-B showed the highest absolute abundance of ARGs, but the lowest relative abundance. The relative abundance of ARGs in CW-A was much higher than in the other groups. This suggests that activated carbon and zeolite might diminish the occurrence of ARGs [39,40]. Previous research similarly proposed that activated carbon might boost microbial biomass by increasing carbon availability inside the system [41]. The increased absolute abundance, as well as the reduced relative abundance of ARGs in CW-B, may have been due to the higher microbial biomass in the activated carbon-enriched group compared to the other groups. This increased microbial diversity results in a greater absolute number of ARGs. ARGs had the lowest absolute and relative abundance across all categories in CW-D. This was due to the synergistic advantages of the combined use of zeolite and activated carbon in CW-D for the removal of ARGs. Such synergism is not only reflected in the enhanced adsorption capacity but also encompasses the optimization of microbial growth environments, promotion of biofilm formation, facilitation of biodegradation processes, and improvements in cost-effectiveness. These multifaceted benefits contribute to the improved removal efficiency of ARGs.
3.3. Analysis of Microbial Community Structure
The abundance of bacterial communities in each VFCW is shown in Figure 6. The findings showed that the number of operational taxonomic units (OTUs) in all experimental groups was considerably greater than in the control group, CW-A. This shows that the microbial populations in the treatment groups were rather abundant. MiSeq sequencing of 16SrRNA-amplified fragments was performed on all samples, resulting in 247,847 valid sequences. The bacterial community’s OTUs were classified into 41 phyla and 775 genera. The microbiological diversity of the samples is shown in Table 1. The CW-A diversity index was considerably lower than that of the treatment groups, showing that the treatment may increase the microbial abundance and variety. This discovery demonstrates that changing substrates may lead to alterations in the microbiome [42,43].
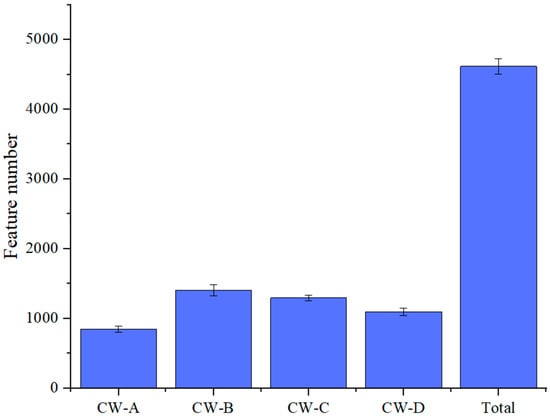
Figure 6.
OTU number distribution diagram.

Table 1.
Bacterial diversity indices of substrates.
Figure 7 shows an analysis of the microbial community structure at the phylum and gene levels. The most prevalent bacterial phyla were Proteobacteria (43.06–53.32%), Bacteroidota (10.62–26.75%), and Patescibacteria (9.93–18.34%). The PCoA analysis (Figure 8) shows variability in the microbial communities across various substrate types and compositions in the VFCWs, suggesting that the substrate type and composition may influence the community structure [44]. This study employed LEfSe analysis to detect variability in bacteria at different levels inside the VFCWs (Figure 9). The primary phyla in the control group were Proteobacteria, Firmicutes, and Synergistota, while the dominating phyla in the experimental group were Actinobacteriota, Planctomycetota, Verrucomicrobiota, and Proteobacteria. Notably, Bacteroidetes and Patescibacteria, the most common bacterial phyla in aquaculture, were anticipated to be key repositories for a diverse spectrum of ARGs and MGEs [45]. Furthermore, Bacteroidota and Firmicutes have been identified as the most likely ARGs hosts in sludge [46]. Proteobacteria, Actinobacteria, and Bacteroidota were obviously the most common bacteria in the ARGs-contaminated environment [47,48]. The present investigation discovered that the control cohort had a much larger abundance of Bacteroidota and Proteobacteria than the experimental group. This research demonstrates that ARGs accumulation occurs often in various VFCWs, which explains why the control group had greater ARGs accumulation levels.
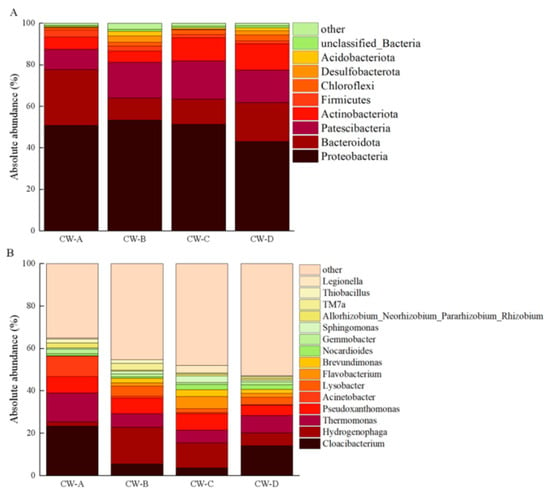
Figure 7.
Microbial community structure in VFCWs. (A) Species with bacterial abundance in the top 9 at the phylum level; (B) species with bacterial abundance at the gene level in the top 15.
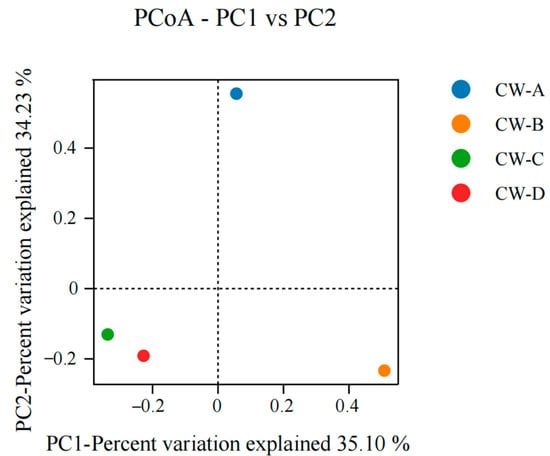
Figure 8.
PCoA analysis of microbial communities across various substrate types and compositions in VFCWs.
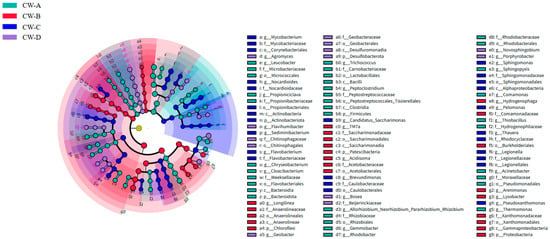
Figure 9.
The different compositions of bacterial communities based on LEfSe analysis in VFCWs.
The four most common genera found in the VFCWs were Cloacibacterium (3.61–23.23%), Hydrogenophaga (6.07–17.35%), Thermomonas (6.06–13.63%), and Pseudoxanthomonas (7.30–7.84%). Cloacibacterium is found throughout nature, particularly in soil and water [49], and may have contributed significantly to the generation and replication of ARGs [50]. Hydrogenophaga mites also have a considerable impact on tetracycline degradation [51]. Thermomonas was a major genus in the control group, whereas Pseudoxanthomonas was more common in the experimental groups. Thermomonas is a crucial component of microbial communities, responsible for their structure and functioning, and has been linked to antibiotic resistance and disintegration [52]. Furthermore, Thermomonas has been identified as a possible ARGs reservoir [53]. Conversely, Pseudoxanthomonas has been associated with antibiotic degradation [54]; however, it may help in the spread of ARGs [55]. Our findings show that the common microbiota in the VFCW environment has the capacity to either boost ARGs transmission or reduce the antibiotic concentrations in response to OTC-induced selection pressure.
3.4. Correlation Analysis Among ARGs, MGEs, and Microbial Community
3.4.1. Correlation Analysis of MGEs, Antibiotics, and ARGs
The associations between ARGs and MGEs were examined using Spearman correlation analysis (Figure 10). MGEs are known to facilitate the spread of ARGs in various media [56]. Figure 10 shows that intI1, intI2, and ISCR1 have a favorable correlation with tetracycline-related ARGs (p < 0.01). In the VFCWs, intI1 had the greatest impact on the gene tetX [57], while intI2 had a substantial effect on tetA. These data indicate MGEs’ important involvement in the spread of tetracycline ARGs. Under the selective pressure of OTC, VFCWs may aid in the spread of ARGs via HGT. This is consistent with earlier research, which has shown that HGT contributes to the propagation of ARGs [10,58]. Interestingly, a substantial association was found between tetA and tetX, suggesting that ARGs are not only closely tied to MGEs but also impacted by other ARGs, perhaps owing to the common host range of tetA and tetX. Furthermore, this research found that antibiotics had no significant effect on ARGs in the short term, indicating that antibiotics may not directly alter ARGs but rather exercise their effects indirectly [59].
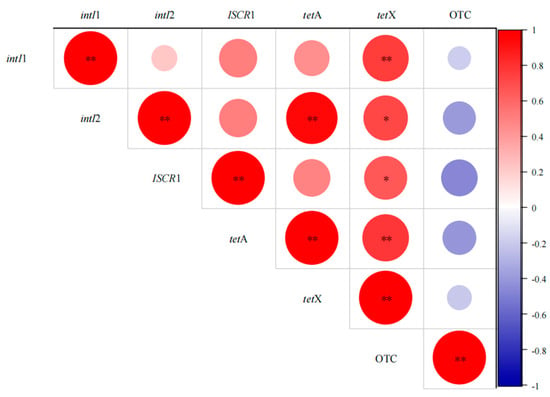
Figure 10.
Heatmap based on the relationship between ARGs, MGEs, and antibiotics. * p ≤ 0.05, ** p ≤ 0.01.
3.4.2. Co-Occurrence Among ARGs, MGEs, and Potential Hosts
The relationship between ARGs and microbial communities was investigated by examining the co-occurrence patterns of two ARGs, three MGEs, and 25 bacterial taxa (Figure 11). The network study consisted of 19 nodes and 42 edges, with a modular index of 10, indicating a modular structure inside the network. The network structure analysis map showed the interactions and strengths between ARGs, MGEs, and microorganisms. The study found that intI1 and ISCR1 exhibited a negative association with Allorhizobium, Neorhizobium, Pararhizobium, Rhizobium, and Rhodobacter (p < 0.01), but a positive correlation with Hydrogenophaga. TetX showed a positive connection with intI1 and ISCR1 in ARGs. TetX had a negative association with Allorhizobium_Neorhizobium_Pararhizobium_Rhizobium (p < 0.01) but a significant positive correlation with Hydrogenophaga (p < 0.01). There is a significant negative association between intI1 and Rhodobacter, as well as Rhodobacter and tetX (p < 0.01). These findings suggest that the effects of some bacteria on tetX may be enhanced by their interactions with MGEs. These findings also show a significant relationship between ARGs, MGEs, and host bacteria, with HGT supporting the spread of antibiotic resistance [60]. Michaelis et al. [61] showed that ARGs may be rapidly propagated by HGT, particularly inside biofilms. The results show that the VFCWs primarily facilitated the transmission and amplification of ARGs across populations via HGT. As a result, future studies may focus on limiting the spread of ARGs by blocking horizontal gene transfer in built wetlands [61].
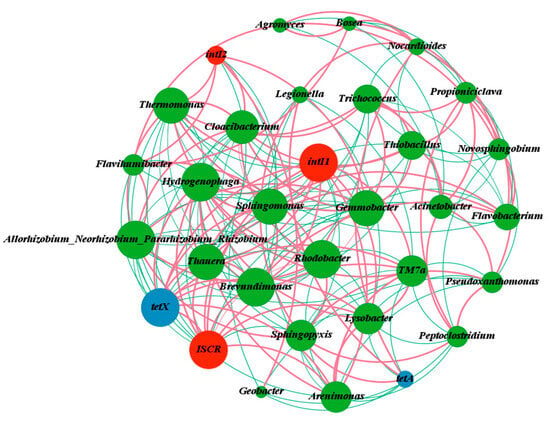
Figure 11.
Co-occurrence network of ARGs and potential bacterial hosts. Nodes represent ARGs, MGEs, or bacterial genera; positive correlation is shown by red line; negative correlation is shown by green line.
In this research, tetX and tetA had a significant positive correlation with Hydrogenophaga and Legionella. The data suggest that these bacterial species might be major potential hosts for tet-associated ARGs. Hydrogenophaga, an aerobic denitrifying bacterium, may reduce nitrification activity and inhibit nitrifying bacteria [62]. In addition, Hydrogenophaga has been used to increase the performance of biological treatment methods in the treatment of antibiotic-contaminated wastewater. Hydrogenophaga has also been demonstrated to aid in the breakdown of tetracyclines [51]. Zhou et al. [63] also identified Hydrogenophaga as a core genus that is strongly related to ARGs. Additionally, Hydrogenophaga has been shown to be resistant to chloramphenicol stress via an efflux pump mechanism. Legionella is a common opportunistic pathogen found in nature. It may cause fever and respiratory symptoms, known as Legionnaires’ disease. The most common and serious clinical condition is lung infection [64]. First-line and other antibiotics, such as azithromycin, fluoroquinolones, and amoxicillin/clavulanate, which are often used to treat Legionnaires’ disease patients, have been shown to diminish the susceptibility of Legionella pneumophila strains from Portugal [65]. Dowling et al. [66] found that β-lactams and aminoglycosides were less efficient against Legionella. These findings imply that Hydrogenophaga and Legionella, as ARGs hosts, play an important role in the spread of ARGs in VFCWs. The LEfSe study indicated that Hydrogenophaga and Legionella were significantly different species in CW-B and CW-C. These data indicate that these two wetlands were more prone to ARGs contamination.
The links between other bacterial genera and ARGs were not significant, most likely owing to the smaller numbers of microorganisms in the VFCWs and the wide heterogeneity in their distribution. The network analysis revealed substantial co-occurrences between bacterial taxa and ARGs subtypes, shedding light on possible ARGs hosts in VFCW settings. These results not only give useful data for the identification of major microbial participants in ARGs propagation but also provide new insights toward assessing and perhaps mitigating antibiotic resistance in human-engineered wetland systems.
4. Conclusions
This research found that the combination of zeolite and activated carbon was the most effective treatment for OTC-contaminated wastewater. While VFCWs show potential in antibiotic removal, antibiotic selection pressure may promote ARGs spread and reduce non-resistant microbial biomass. Notably, the combination of activated carbon and zeolite showed the lowest ARGs buildup and the best antibiotic elimination efficiency, indicating its potential as a composite matrix. Different substrate types in VFCWs support diverse microbial communities, with the dominant antibiotic-resistant bacteria leading to increased ARGs abundance. The LEfSe analysis revealed that the treatment group had a considerable, but not absolute, suppressive effect on ARG growth, suggesting that the possibility of ARGs contamination is not trivial. The relevant research shows that there is no direct relationship between antibiotics and ARGs, although drugs may enhance ARGs amplification via influencing MGEs. The network analysis demonstrated that ARGs had the same associations as MGEs and host bacteria, emphasizing the importance of HGT in ARGs propagation. Furthermore, it has been proven that Hydrophilicphages and Legionella are the principal possible hosts for tet-related ARGs.
Author Contributions
Conceptualization, W.Y. and P.D.; methodology, Y.L. and Y.S.; software, M.B. and L.L.; validation, L.L. and X.L.; formal analysis, Y.L. and Y.S.; investigation, L.L. and X.L.; resources, Y.G., W.Y. and J.L.; data curation, X.L. and L.L. writing—original draft preparation, P.D., J.L. and W.Y.; writing—review and editing, L.R. and W.Y.; visualization, X.L. and P.D.; supervision, Y.G. and W.Y.; funding acquisition, P.D. and Y.G. All authors have read and agreed to the published version of the manuscript.
Funding
1. Key Scientific and Technological Project of Henan Province (NO. 232102321110). 2. The Key R&D projects in Henan Province (No.241111320200). 3. Henan Provincial Fiscal Forestry and Grassland Projects: Investigation of birds and habitat background resources in the Yellow River wetlands in Henan Province.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yuan, W.; Zeng, X.; Cao, Y.; Yang, Q.; Riaz, L.; Wang, Q. Distribution of antibiotic resistance genes from human and animal origins to their receiving environments: A regional scale survey of urban settings. Environ. Pollut. 2022, 293, 118512. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.E.; Weatherly, L.; Shane, H.L. Contribution of antimicrobials to the development of allergic disease. Curr. Opin. Immunol. 2019, 60, 91–95. [Google Scholar] [CrossRef]
- Hu, Y.; Jin, L.; Zhao, Y.; Jiang, L.; Yao, S.; Zhou, W.; Lin, K.; Cui, C. Annual trends and health risks of antibiotics and antibiotic resistance genes in a drinking water source in East China. Sci. Total Environ. 2021, 791, 148152. [Google Scholar] [CrossRef]
- de la Fuente-Nunez, C.; Cesaro, A.; Hancock, R.E. Antibiotic failure: Beyond antimicrobial resistance. Drug Resist. Updat. 2023, 71, 101012. [Google Scholar] [CrossRef] [PubMed]
- Cacace, D.; Fatta-Kassinos, D.; Manaia, C.M.; Cytryn, E.; Kreuzinger, N.; Rizzo, L.; Karaolia, P.; Schwartz, T.; Alexander, J.; Merlin, C. Antibiotic resistance genes in treated wastewater and in the receiving water bodies: A pan-European survey of urban settings. Water Res. 2019, 162, 320–330. [Google Scholar] [CrossRef]
- Sabatino, R.; Cabello-Yeves, P.J.; Eckert, E.M.; Corno, G.; Callieri, C.; Brambilla, D.; Dzhembekova, N.; Moncheva, S.; Di Cesare, A. Antibiotic resistance genes correlate with metal resistances and accumulate in the deep water layers of the Black Sea. Environ. Pollut. 2022, 312, 120033. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Campos, L.C.; Canales, M.; Ciric, L. Drinking water biofiltration: Behaviour of antibiotic resistance genes and the association with bacterial community. Water Res. 2020, 182, 115954. [Google Scholar] [CrossRef]
- Guo, Z.-F.; Boeing, W.J.; Xu, Y.-Y.; Borgomeo, E.; Liu, D.; Zhu, Y.-G. Data-driven discoveries on widespread contamination of freshwater reservoirs by dominant antibiotic resistance genes. Water Res. 2023, 229, 119466. [Google Scholar] [CrossRef]
- Wu, D.-L.; Zhang, M.; He, L.-X.; Zou, H.-Y.; Liu, Y.-S.; Li, B.-B.; Yang, Y.-Y.; Liu, C.; He, L.-Y.; Ying, G.-G. Contamination profile of antibiotic resistance genes in ground water in comparison with surface water. Sci. Total Environ. 2020, 715, 136975. [Google Scholar] [CrossRef]
- Szekeres, E.; Chiriac, C.M.; Baricz, A.; Szőke-Nagy, T.; Lung, I.; Soran, M.-L.; Rudi, K.; Dragos, N.; Coman, C. Investigating antibiotics, antibiotic resistance genes, and microbial contaminants in groundwater in relation to the proximity of urban areas. Environ. Pollut. 2018, 236, 734–744. [Google Scholar] [CrossRef]
- Singh, A.K.; Kaur, R.; Verma, S.; Singh, S. Antimicrobials and antibiotic resistance genes in water bodies: Pollution, risk, and control. Front. Environ. Sci. 2022, 10, 830861. [Google Scholar] [CrossRef]
- Nnadozie, C.F.; Odume, O.N. Freshwater environments as reservoirs of antibiotic resistant bacteria and their role in the dissemination of antibiotic resistance genes. Environ. Pollut. 2019, 254, 113067. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.-Z.; Zou, H.-Y.; Wu, D.-L.; Chen, S.; He, L.-Y.; Zhang, M.; Bai, H.; Ying, G.-G. Swine farming elevated the proliferation of Acinetobacter with the prevalence of antibiotic resistance genes in the groundwater. Environ. Intern. 2020, 136, 105484. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Yang, Z.; Wei, Y.; Miao, J.; Yang, D.; Yin, J.; Li, H.; Chen, T.; Zhou, S.; Li, J. Spatial and temporal analysis of the seasonal dynamics of antibiotic resistance gene occurrence in recreational marine water. Sci. Total Environ. 2023, 893, 164816. [Google Scholar] [CrossRef]
- Kazanjian, P.H. Efforts to regulate antibiotic misuse in hospitals: A history. Infect. Control Hosp. Epidemiol. 2022, 43, 1119–1122. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, L.; Zhang, T.; Jin, L.; Han, Q.; Zhang, D.; Lin, K.; Cui, C. Occurrence and removal of sulfonamide antibiotics and antibiotic resistance genes in conventional and advanced drinking water treatment processes. J. Hazard. Mater. 2018, 360, 364–372. [Google Scholar] [CrossRef]
- Obayiuwana, A.; Ogunjobi, A.; Ibekwe, A. Prevalence of antibiotic resistance genes in pharmaceutical wastewaters. Water 2021, 13, 1731. [Google Scholar] [CrossRef]
- Lien, L.T.Q.; Lan, P.T.; Chuc, N.T.K.; Hoa, N.Q.; Nhung, P.H.; Thoa, N.T.M.; Diwan, V.; Tamhankar, A.J.; Stålsby Lundborg, C. Antibiotic resistance and antibiotic resistance genes in Escherichia coli isolates from hospital wastewater in Vietnam. Int. J. Environ. Res. Public Health 2017, 14, 699. [Google Scholar] [CrossRef]
- Hassoun-Kheir, N.; Stabholz, Y.; Kreft, J.-U.; De La Cruz, R.; Romalde, J.L.; Nesme, J.; Sørensen, S.J.; Smets, B.F.; Graham, D.; Paul, M. Comparison of antibiotic-resistant bacteria and antibiotic resistance genes abundance in hospital and community wastewater: A systematic review. Sci. Total Environ. 2020, 743, 140804. [Google Scholar] [CrossRef]
- Zhao, R.; Yu, K.; Zhang, J.; Zhang, G.; Huang, J.; Ma, L.; Deng, C.; Li, X.; Li, B. Deciphering the mobility and bacterial hosts of antibiotic resistance genes under antibiotic selection pressure by metagenomic assembly and binning approaches. Water Res. 2020, 186, 116318. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, M.; Zhang, B.; He, Y.; Zhong, Y. Changes of antibiotic resistance genes and bacterial communities in the advanced biological wastewater treatment system under low selective pressure of tetracycline. Water Res. 2021, 207, 117834. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.-S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2023, 311, 136977. [Google Scholar] [CrossRef]
- Liu, L.; Xin, Y.; Huang, X.; Liu, C. Response of antibiotic resistance genes in constructed wetlands during treatment of livestock wastewater with different exogenous inducers: Antibiotic and antibiotic-resistant bacteria. Bioresour. Technol. 2020, 314, 123779. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chu, L.; Wojnárovits, L.; Takács, E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: An overview. Sci. Total Environ. 2020, 744, 140997. [Google Scholar] [CrossRef]
- Genç, N.; Dogan, E.C. Adsorption kinetics of the antibiotic ciprofloxacin on bentonite, activated carbon, zeolite, and pumice. Desalin. Water Treat. 2015, 53, 785–793. [Google Scholar] [CrossRef]
- Ping, Q.; Yan, T.; Wang, L.; Li, Y.; Lin, Y. Insight into using a novel ultraviolet/peracetic acid combination disinfection process to simultaneously remove antibiotics and antibiotic resistance genes in wastewater: Mechanism and comparison with conventional processes. Water Res. 2022, 210, 118019. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, L.; Gu, J.-D.; Deng, S.; Huang, C.; Luo, L. The factors controlling antibiotic resistance genes in different treatment processes of mainstream full-scale wastewater treatment plants. Sci. Total Environ. 2023, 900, 165815. [Google Scholar] [CrossRef]
- Chen, J.; Wei, X.D.; Liu, Y.S.; Ying, G.G.; Liu, S.S.; He, L.Y.; Su, H.C.; Hu, L.X.; Chen, F.R.; Yang, Y.Q. Removal of antibiotics and antibiotic resistance genes from domestic sewage by constructed wetlands: Optimization of wetland substrates and hydraulic loading. Sci. Total Environ. 2016, 565, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Cui, Y.; Li, A.; Zou, X.; Ma, C.; Chen, Z. Antibiotics and antibiotic resistance genes from wastewater treated in constructed wetlands. Ecol. Eng. 2022, 177, 106548. [Google Scholar] [CrossRef]
- Parde, D.; Patwa, A.; Shukla, A.; Vijay, R.; Killedar, D.J.; Kumar, R. A review of constructed wetland on type, treatment and technology of wastewater. Environ. Technol. Innov. 2021, 21, 101261. [Google Scholar] [CrossRef]
- Huang, X.; Zheng, J.; Liu, C.; Liu, L.; Liu, Y.; Fan, H. Removal of antibiotics and resistance genes from swine wastewater using vertical flow constructed wetlands: Effect of hydraulic flow direction and substrate type. Chem. Eng. J. 2017, 308, 692–699. [Google Scholar] [CrossRef]
- Song, H.-L.; Zhang, S.; Guo, J.; Yang, Y.-L.; Zhang, L.-M.; Li, H.; Yang, X.-L.; Liu, X. Vertical up-flow constructed wetlands exhibited efficient antibiotic removal but induced antibiotic resistance genes in effluent. Chemosphere 2018, 203, 434–441. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Y.; Riaz, L.; Yang, Q.; Du, B.; Wang, R. Multiple antibiotic resistance and DNA methylation in Enterobacteriaceae isolates from different environments. J. Hazard. Mater. 2021, 402, 123822. [Google Scholar] [CrossRef]
- Riaz, L.; Wang, Q.; Yang, Q.; Li, X.; Yuan, W. Potential of industrial composting and anaerobic digestion for the removal of antibiotics, antibiotic resistance genes and heavy metals from chicken manure. Sci. Total Environ. 2020, 718, 137414. [Google Scholar] [CrossRef]
- Yuan, W.; Liu, Y.; Liu, R.; Li, L.; Deng, P.; Fu, S.; Riaz, L.; Lu, J.; Li, G.; Yang, Z. Unveiling the overlooked threat: Antibiotic resistance in groundwater near an abandoned sulfuric acid plant in Xingyang, China. Environ. Geochem. Health 2024, 46, 309. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Xiang, Y.; Wang, P.; Zhang, J.; Zhang, F.; Wei, J.; Luo, L.; Lei, M.; Tang, L. Modification of biochar derived from sawdust and its application in removal of tetracycline and copper from aqueous solution: Adsorption mechanism and modelling. Bioresour. Technol. 2017, 245, 266–273. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Ngo, H.H.; Wen, H.; Li, N.; Wu, W. Performance evaluation of powdered activated carbon for removing 28 types of antibiotics from water. Environ. Manag. 2016, 172, 193–200. [Google Scholar] [CrossRef]
- Belviso, C.; Guerra, G.; Abdolrahimi, M.; Peddis, D.; Maraschi, F.; Cavalcante, F.; Ferretti, M.; Martucci, A.; Sturini, M. Efficiency in Ofloxacin antibiotic water remediation by magnetic zeolites formed combining pure sources and wastes. Processes 2021, 9, 2137. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P. Antibiotic-resistance genes in waste water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhao, Y.; Wang, C.; Zhang, H.; Chen, Q.; Zhang, X.; Zhang, L.; Wu, J.; Wu, Z.; Zhou, Q. Removal performance of antibiotics and antibiotic resistance genes in swine wastewater by integrated vertical-flow constructed wetlands with zeolite substrate. Sci. Total Environ. 2020, 721, 137765. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Canales, M.; Zhou, Q.; Karu, K.; Zhou, X.; Su, J.; Campos, L.C.; Ciric, L. Antibiotic resistance genes and the association with bacterial community in biofilms occurring during the drinking water granular activated carbon (GAC) sandwich biofiltration. J. Hazard. Mater. 2023, 460, 132511. [Google Scholar] [CrossRef]
- Mason-Jones, K.; Breidenbach, A.; Dyckmans, J.; Banfield, C.C.; Dippold, M.A. Intracellular carbon storage by microorganisms is an overlooked pathway of biomass growth. Nat. Commun. 2023, 14, 2240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Wu, X.; Gao, Y.; Fan, S.; Zhou, H.; Zhang, X. Diversity shifts in the root microbiome of cucumber under different plant cultivation substrates. Front. Microbiol. 2022, 13, 878409. [Google Scholar] [CrossRef]
- Arroyo, P.; Ansola, G.; de Miera, L.E.S. Effects of substrate, vegetation and flow on arsenic and zinc removal efficiency and microbial diversity in constructed wetlands. Ecol. Eng. 2013, 51, 95–103. [Google Scholar] [CrossRef]
- Abou-Kandil, A.; Shibli, A.; Azaizeh, H.; Wolff, D.; Wick, A.; Jadoun, J. Fate and removal of bacteria and antibiotic resistance genes in horizontal subsurface constructed wetlands: Effect of mixed vegetation and substrate type. Sci. Total Environ. 2021, 759, 144193. [Google Scholar] [CrossRef]
- Suyamud, B.; Lohwacharin, J.; Yang, Y.; Sharma, V.K. Antibiotic resistant bacteria and genes in shrimp aquaculture water: Identification and removal by ferrate (VI). J. Hazard. Mater. 2021, 420, 126572. [Google Scholar] [CrossRef]
- Mortezaei, Y.; Demirer, G.N.; Williams, M.R. Fate of intracellular and extracellular antibiotic resistance genes in sewage sludge by full-scale anaerobic digestion. Sci. Total Environ. 2024, 951, 175760. [Google Scholar] [CrossRef]
- Wang, Y.; Chu, L.; Ma, J.; Chi, G.; Lu, C.; Chen, X. Effects of multiple antibiotics residues in broiler manure on composting process. Sci. Total Environ. 2022, 817, 152808. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Xu, H.; Wang, L.; Liu, R.; Jia, X. Fate of antibiotic resistance genes and bacteria in a coupled water-processing system with wastewater treatment plants and constructed wetlands in coastal eco-industrial parks. Ecotoxicol. Environ. Safet. 2023, 252, 114606. [Google Scholar] [CrossRef] [PubMed]
- De Mandal, S.; Mathipi, V.; Muthukumaran, R.B.; Gurusubramanian, G.; Lalnunmawii, E.; Kumar, N.S. Amplicon sequencing and imputed metagenomic analysis of waste soil and sediment microbiome reveals unique bacterial communities and their functional attributes. Environ. Monit. Assess. 2019, 191, 778. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Wu, X. Microbial degradation of tetracycline in the aquatic environment: A review. Crit. Rev. Biotechnol. 2020, 40, 1010–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lu, Z.; Wu, B.; Xie, H.; Liu, G. Antibiotics and antibiotic resistance genes removal in biological aerated filter. Bioresour. Technol. 2024, 395, 130392. [Google Scholar] [CrossRef]
- Guo, N.; Wang, T.; Jin, Y.; Wu, D.; Chen, F.; Cheng, X.; Wang, J.; Feng, L.; Song, H.; Wang, L. Enhanced antibiotic removal in a nitrifying sludge system by ammonia-oxidizing bacteria and heterotrophs. J. Environ. Chem. Eng. 2022, 10, 108585. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, T.; Liu, X.; Zhan, M.; Song, X.; Xu, Y.; Yu, R. Sludge biolysis pretreatment to reduce antibiotic resistance genes (ARGs): Insight into the relationship between potential ARGs hosts and BALOs’ preferred prey. Water Res. 2024, 260, 121949. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Liu, H.; Wang, Y.; Wang, Q.; Zhang, L.; An, F.; Chen, Y. Impacts of cefalexin on nitrite accumulation, antibiotic degradation, and microbial community structure in nitrification systems. J. Hazard. Mater. 2024, 478, 135430. [Google Scholar] [CrossRef]
- Du, W.; Wang, T.; Wang, F.; Li, Z.; Huang, W.; Tai, J.; Fang, S.; Cheng, X.; Cao, J.; Su, Y. Para-chloro-meta-xylenol reshaped the fates of antibiotic resistance genes during sludge fermentation: Insights of cell membrane permeability, bacterial structure and biological pathways. Sci. Total Environ. 2022, 850, 158083. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, Z.; Sun, L.; Dong, C.; Jin, Y.; Hu, B.; Cheng, D. Mobile genetic elements mediate the cross-media transmission of antibiotic resistance genes from pig farms and their risks. Sci. Total Environ. 2024, 926, 172115. [Google Scholar] [CrossRef]
- Jang, H.M.; Lee, J.; Choi, S.; Shin, J.; Kan, E.; Kim, Y.M. Response of antibiotic and heavy metal resistance genes to two different temperature sequences in anaerobic digestion of waste activated sludge. Bioresour. Technol. 2018, 267, 303–310. [Google Scholar] [CrossRef]
- Jiang, L.; Zhai, W.; Wang, J.; Li, G.; Zhou, Z.; Li, B.; Zhuo, H. Antibiotics and antibiotic resistance genes in the water sources of the Wuhan stretch of the Yangtze River: Occurrence, distribution, and ecological risks. Environ. Res. 2023, 239, 117295. [Google Scholar] [CrossRef]
- Newman, M.E. Modularity and community structure in networks. Proc. Natl. Acad. Sci. USA 2006, 103, 8577–8582. [Google Scholar] [CrossRef]
- Michaelis, C.; Grohmann, E. Horizontal gene transfer of antibiotic resistance genes in biofilms. Antibiotics 2023, 12, 328. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Zhou, G.; Wang, H. Nanoscale zero-valent iron inhibits the horizontal gene transfer of antibiotic resistance genes in chicken manure compost. J. Hazard. Mater. 2022, 422, 126883. [Google Scholar] [CrossRef]
- Zhang, Z.-X.; Fan, X.-Y.; Li, X.; Gao, Y.-X.; Zhao, J.-R. Effects of combined antibiotics on nitrification, bacteria and antibiotic resistance genes in activated sludge: Insights from legacy effect of antibiotics. J. Environ. Sci. 2023, 131, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Y.; Li, W.-X.; Qu, J.-H.; Chen, T.; Wang, Y.-P.; Geng, N.-Y. Deciphering the microbial community tolerance mechanism and alteration of antibiotic resistance genes during chloramphenicol wastewater treatment. Int. Biodeterior. Biodegrad. 2023, 178, 105546. [Google Scholar] [CrossRef]
- Stallworth, C.; Steed, L.; Fisher, M.A.; Nolte, F.S. Legionnaires’ disease caused by Legionella londiniensis. J. Clin. Microbiol. 2012, 50, 4178–4179. [Google Scholar] [CrossRef]
- Dowling, J.; Pasculle, A. Bactericidal activity of antibiotics against Legionella micdadei (Pittsburgh pneumonia agent). Antimicrob. Agents Chemother. 1982, 22, 272–276. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).