Dual Effect of Microplastics and Cadmium on Stream Litter Decomposition and Invertebrate Feeding Behavior
Abstract
1. Introduction
- (1)
- (2)
- (3)
- The impact of combined pollution on the feeding behavior of aquatic invertebrates: Investigating whether the combined pollution of MPs and Cd alters the nature of litter, thereby causing changes in invertebrate feeding behavior. We speculated on the ecological consequences of this behavior change and its feedback effects on nutrient and pollutant dynamics within the ecosystem [43,44].
2. Materials and Methods
2.1. Collection and Pre-Treatment of Litter
2.2. Preparation of Micropollutant Solutions
2.3. Microcosm Experiment Setup
2.4. Determination of Remaining Mass and Nutrient Content of Litter
2.5. Determination of Lignin and Cellulose Content
2.6. Microbial Metabolic Activity Assessment
2.7. Fungal Biomass Measurement
2.8. Invertebrate Feeding Experiment
2.9. Fungal Community Structure Analysis
2.10. Data Analysis
3. Results
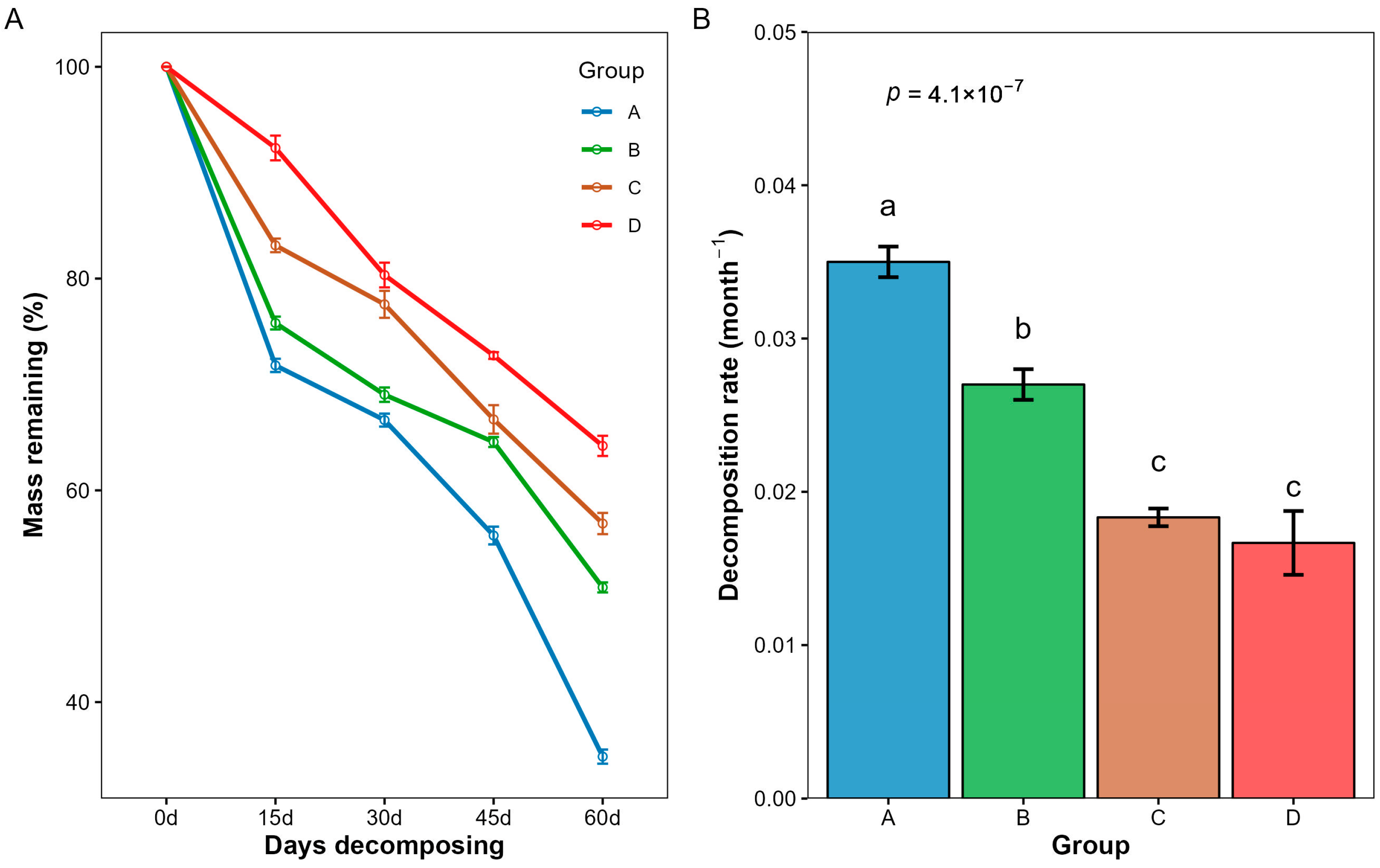
3.1. Changes in Mass Remaining and Decomposition Rate of Litter
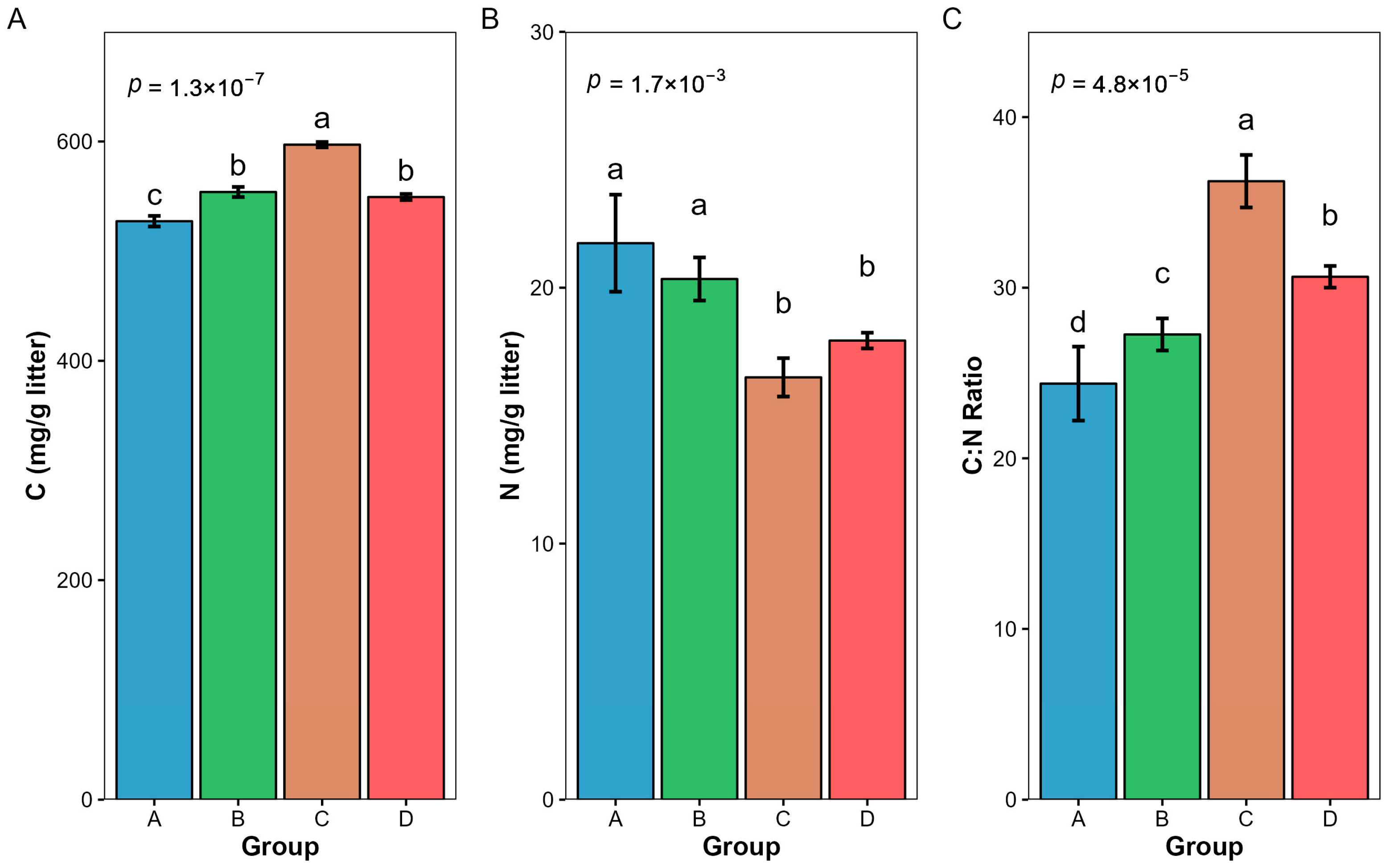
3.2. Changes in Nutrient Content and Carbon–Nitrogen Ratio of Litter
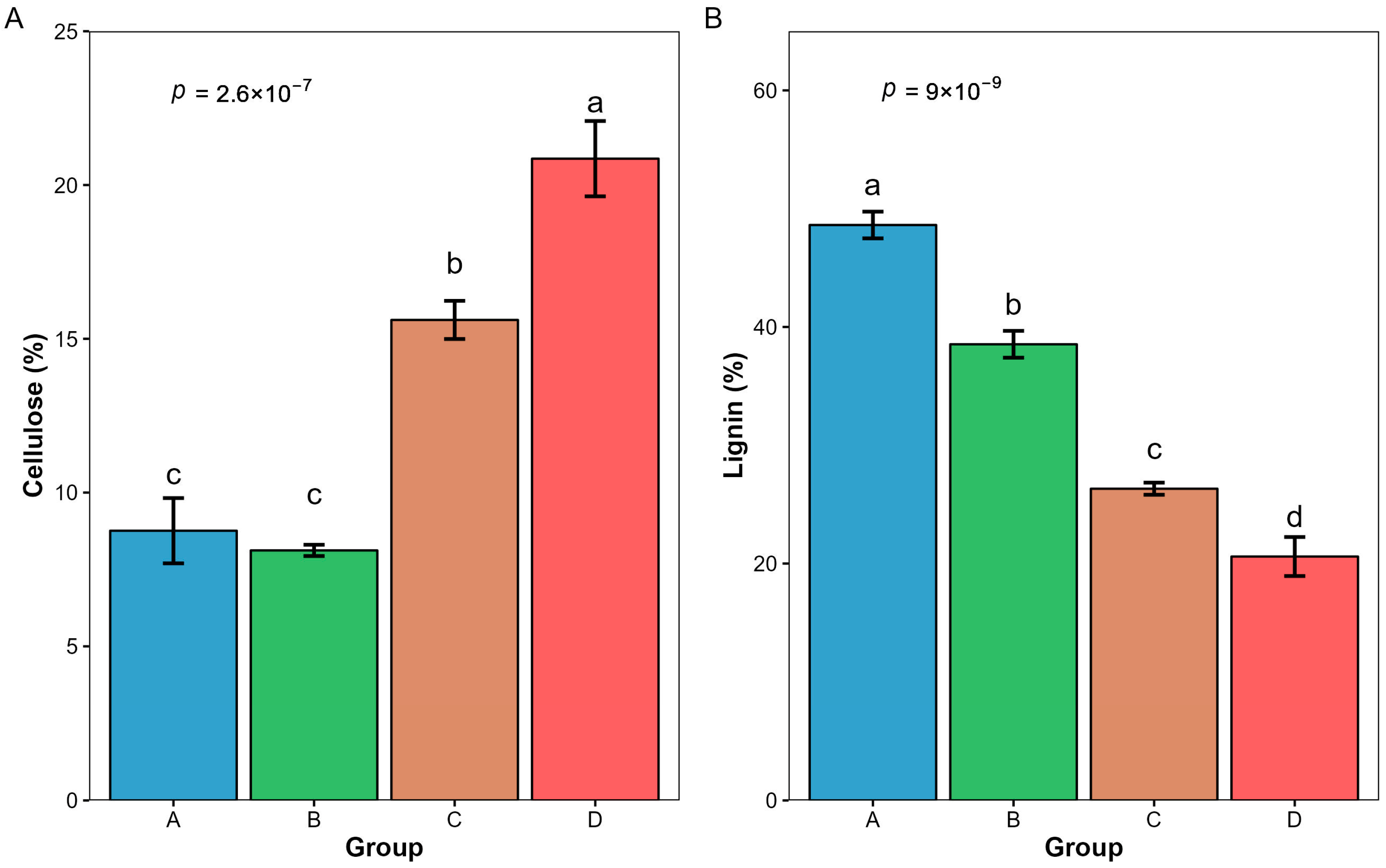
3.3. Changes in Cellulose and Lignin Content of Litter
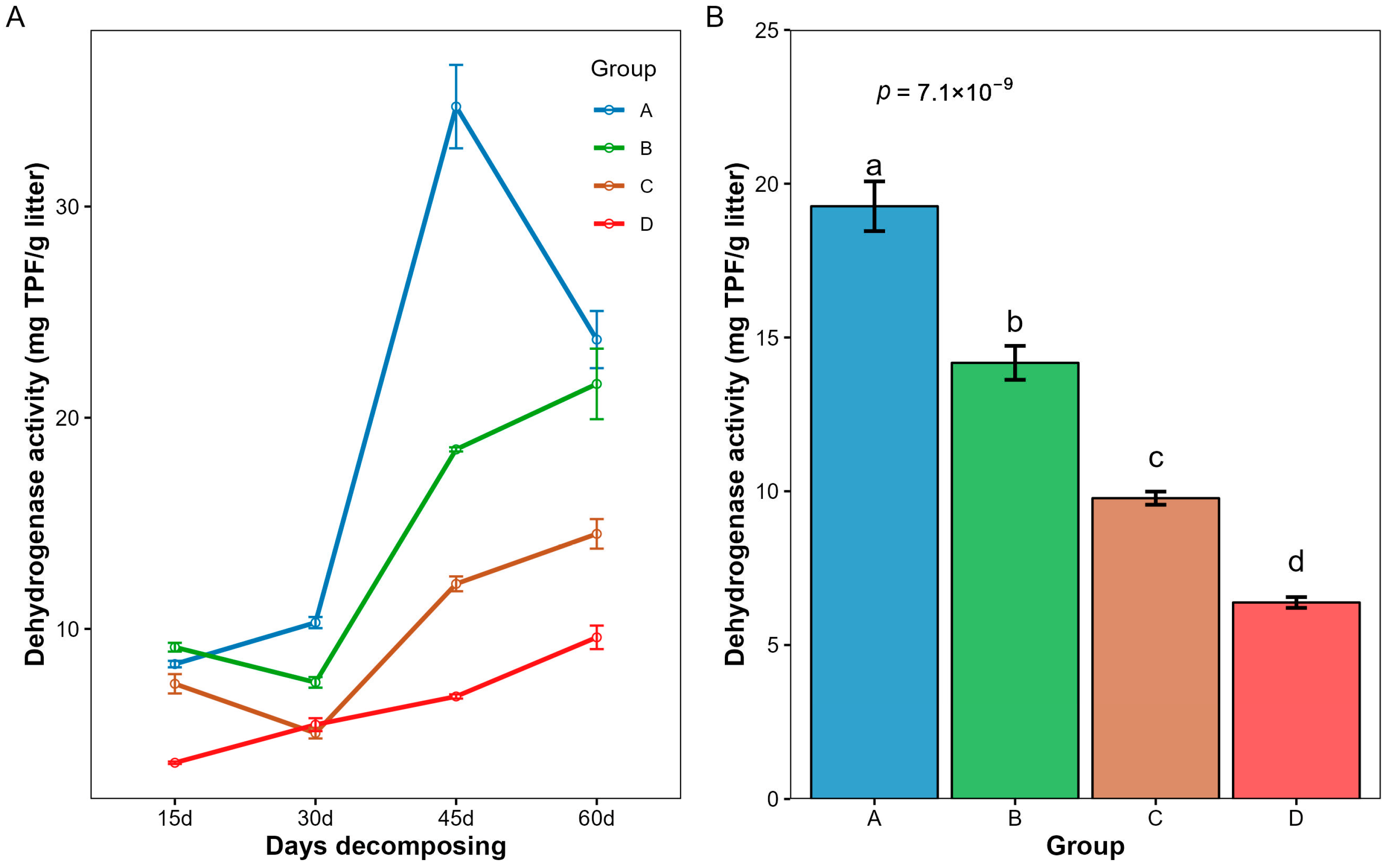
3.4. Changes in Microbial Activity
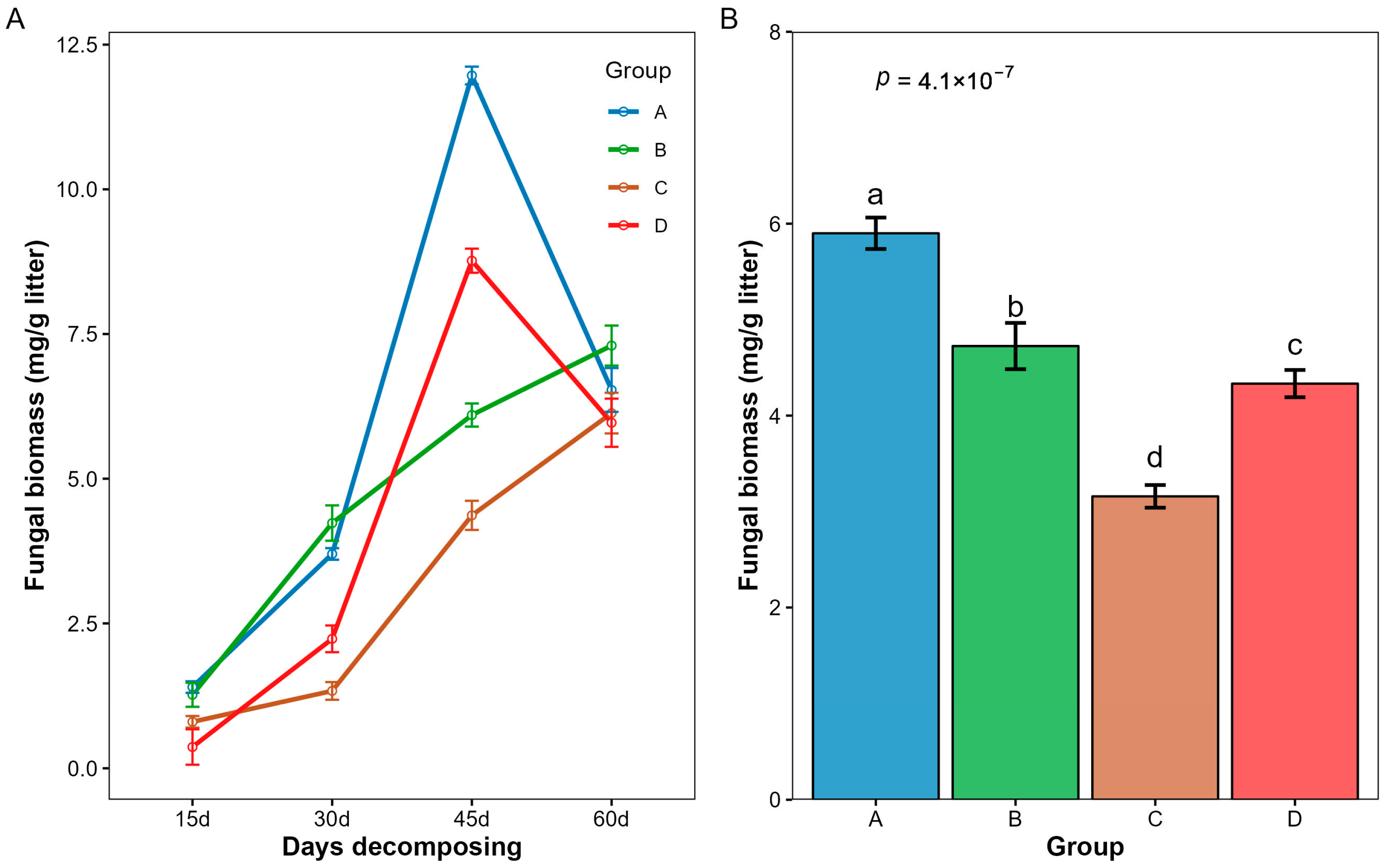
3.5. Changes in Fungal Biomass
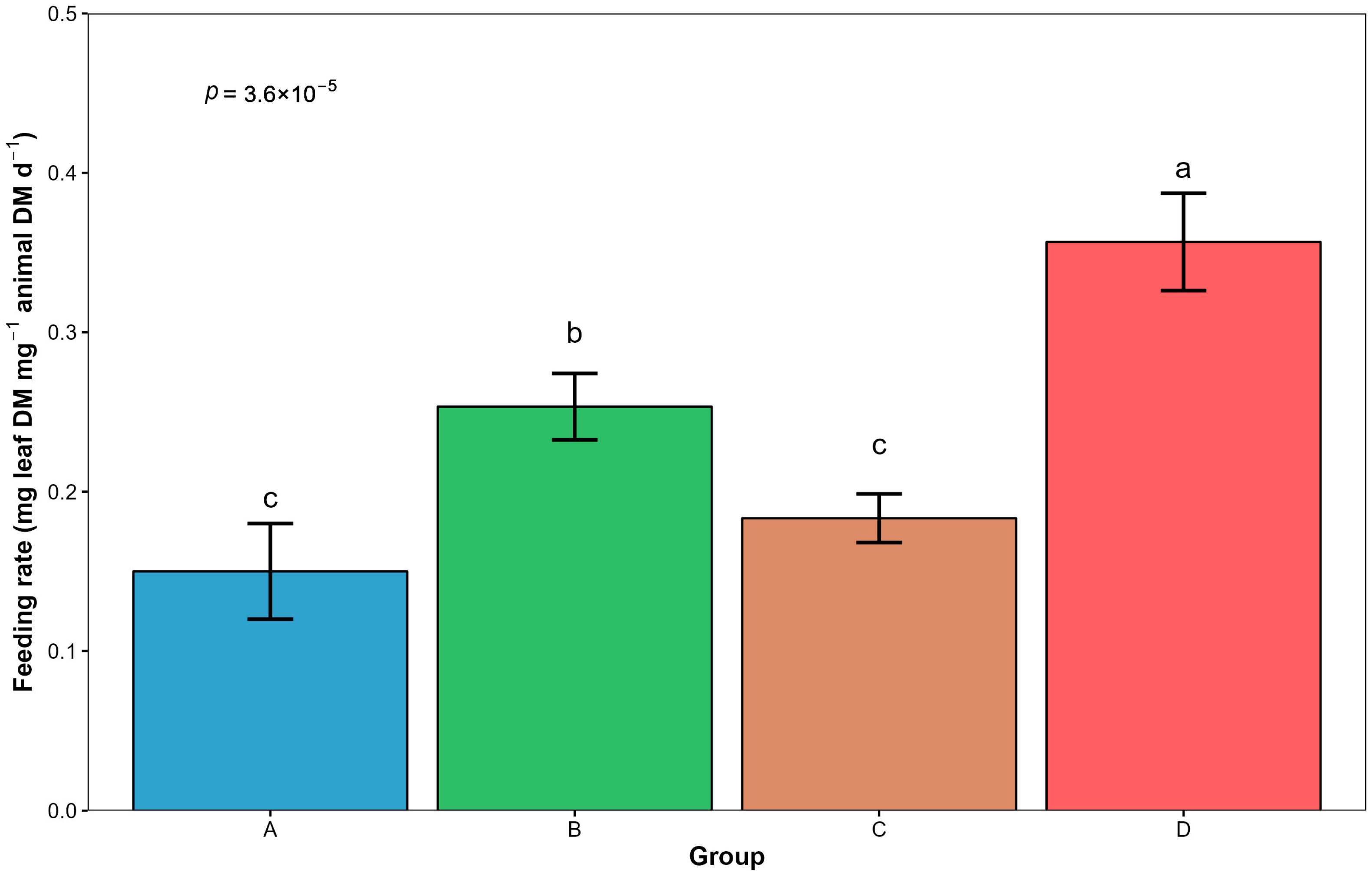
3.6. Changes in Invertebrate Feeding Rate
3.7. Relationships between Litter Decomposition Rate, Invertebrate Feeding Rate, and Environmental Factors
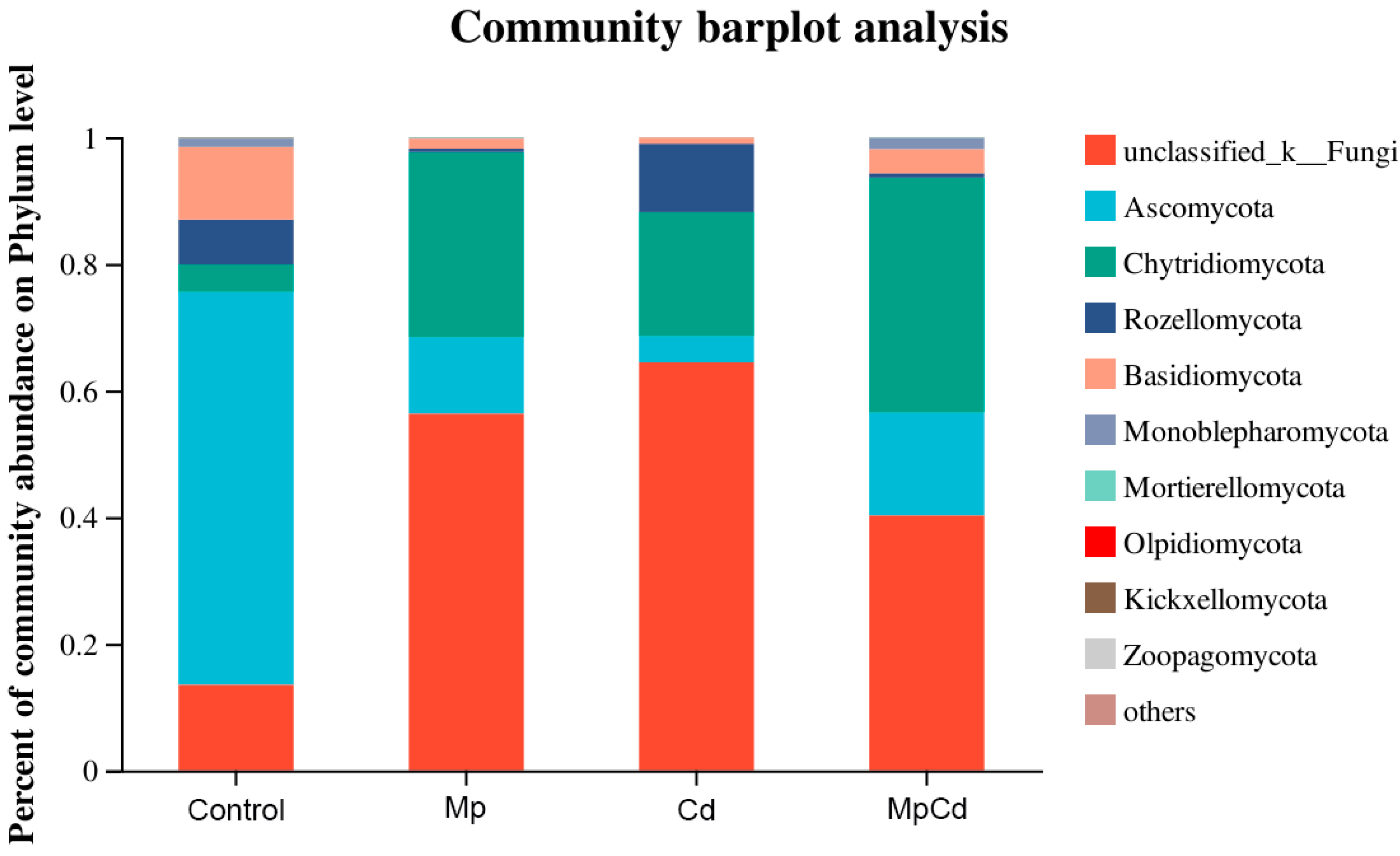
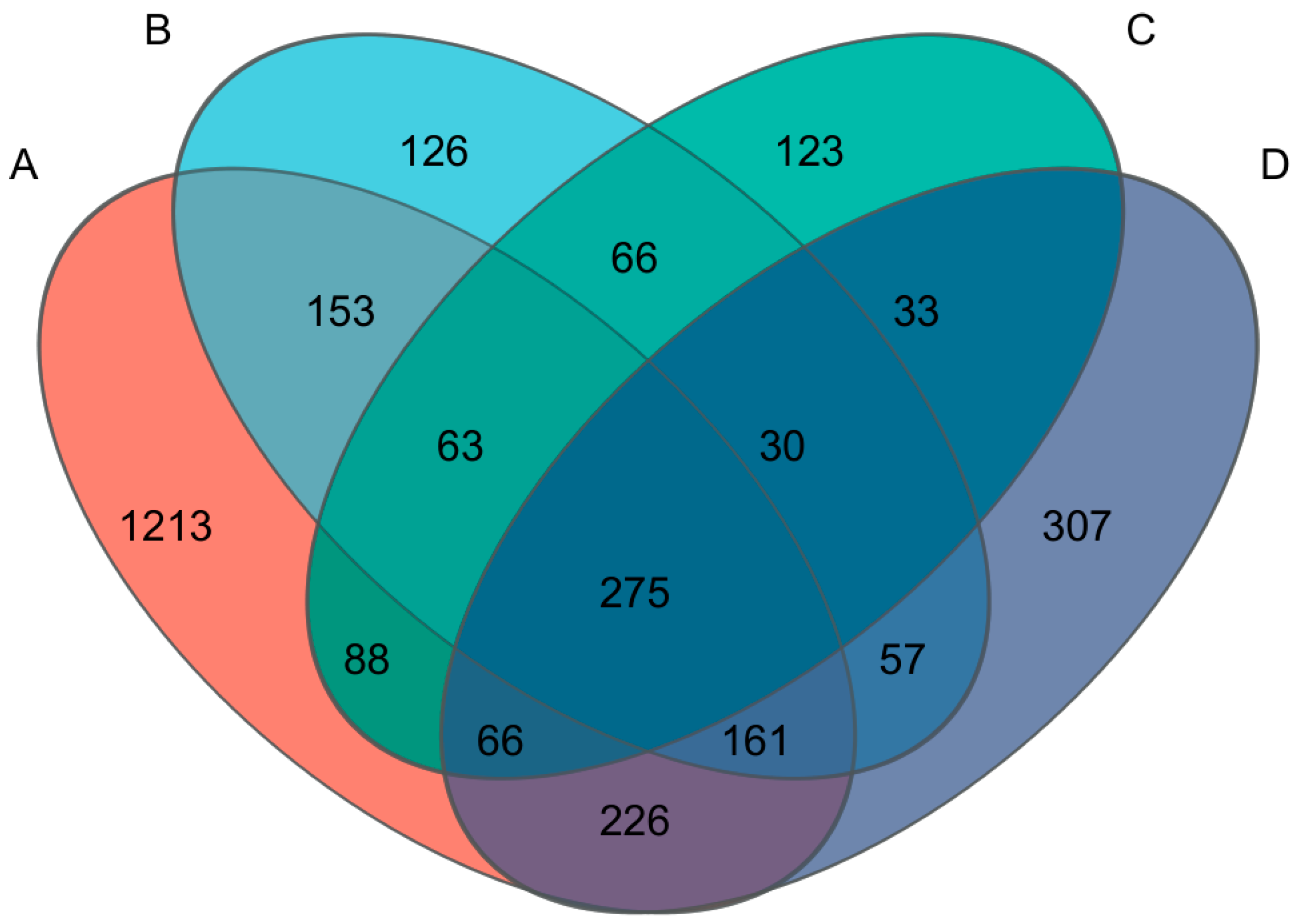
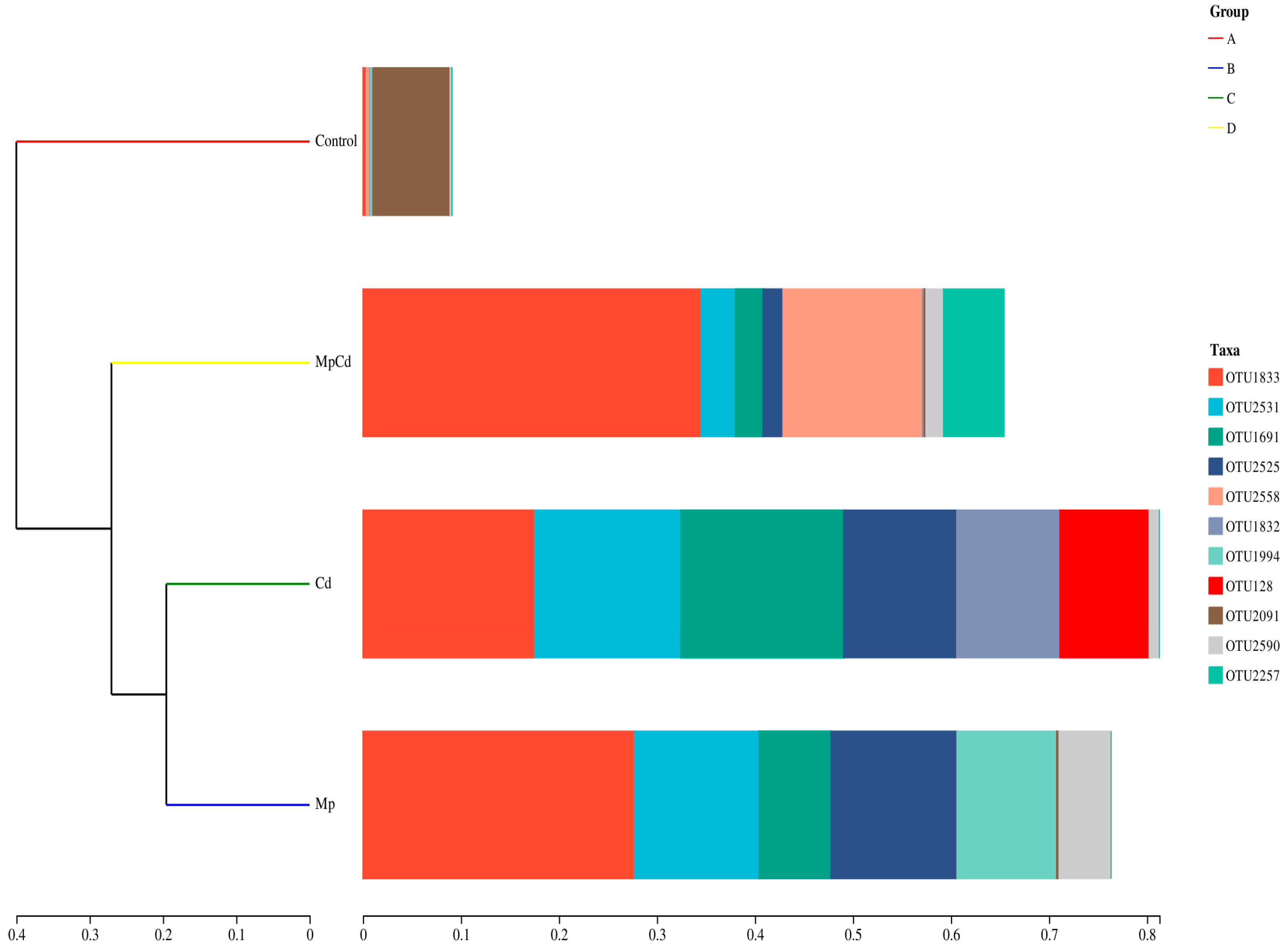
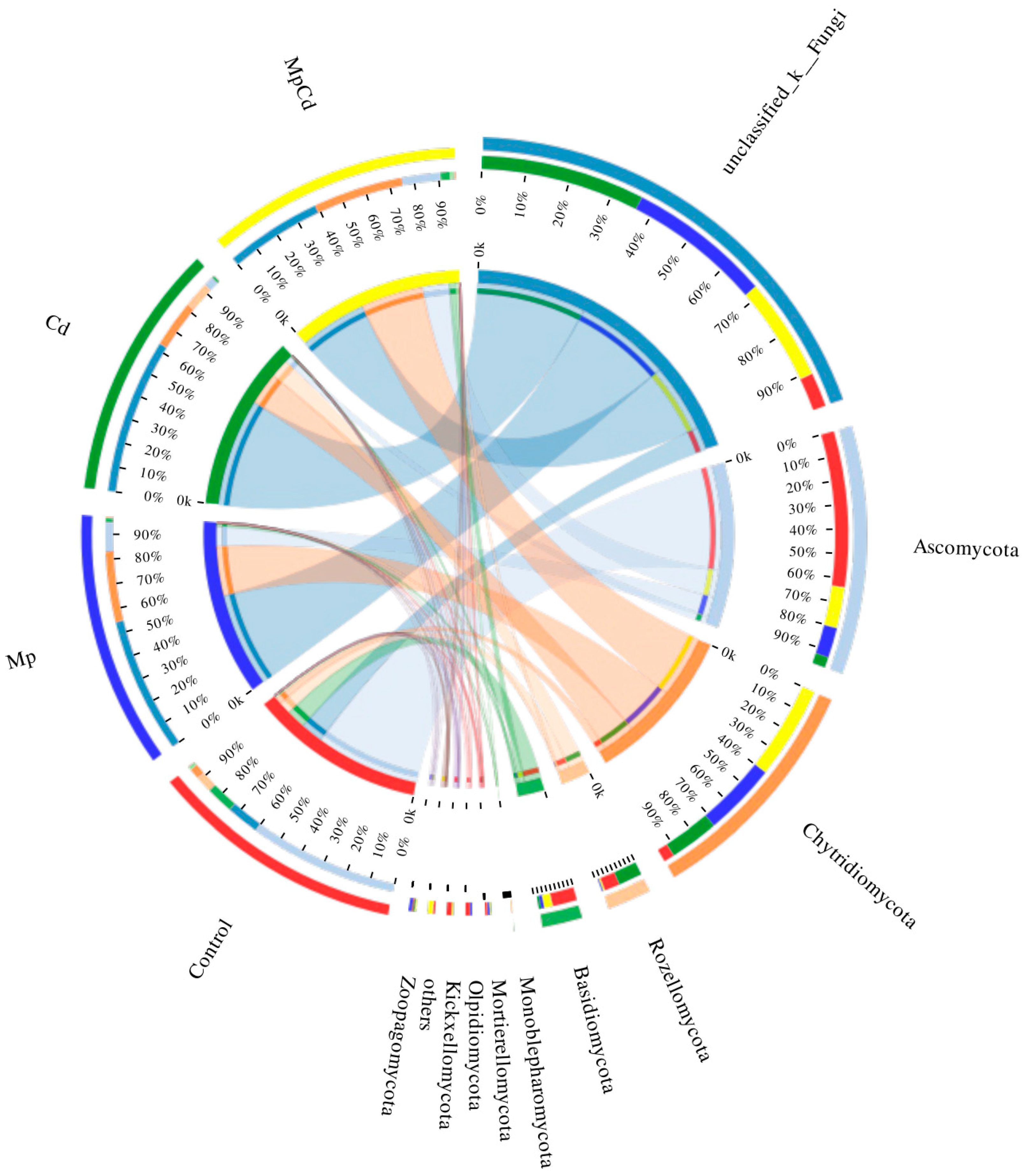
3.8. Changes in Fungal Community Structure
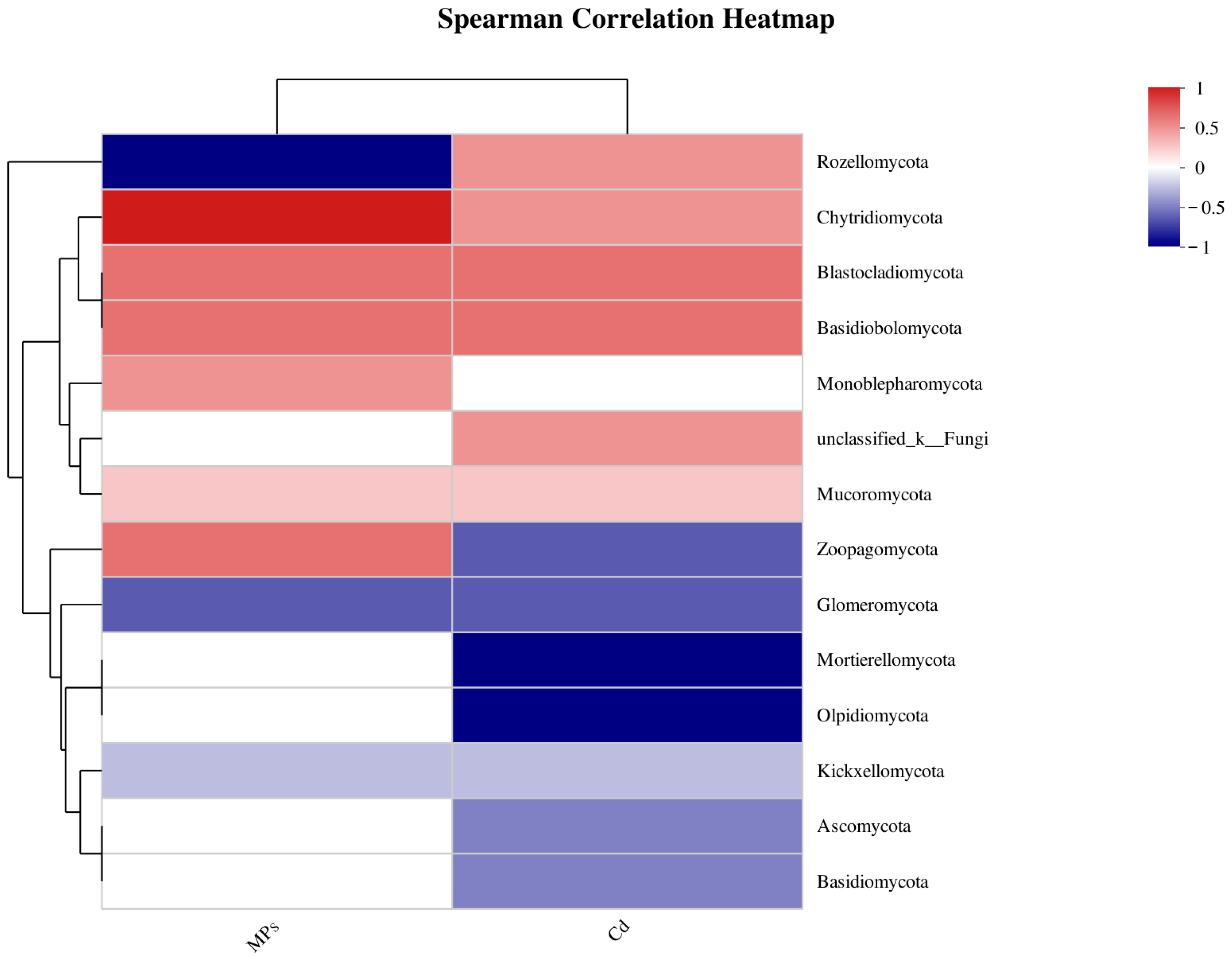
3.9. Relationship between Fungal Diversity and Environmental Factors
4. Discussion
4.1. Inhibitory Effects of Micropollutants on Litter Decomposition
4.2. Regulatory Effects of Micropollutants on Aquatic Fungal Community Structure
4.3. Promoting Effects of Micropollutants on Feeding by Aquatic Invertebrates
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Thompson, R.C.; Olsen, Y.; Mitchell, R.P.; Davis, A.; Rowland, S.J.; John, A.W.G.; McGonigle, D.; Russell, A.E. Lost at sea—Where is all the plastic. Science 2004, 304, 838. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dong, H.; Wang, Y.; Ren, R.; Qin, X.; Wang, S. Effects of microplastics and their adsorption of cadmium as vectors on the cladoceran Daday: Implications for plastic-ingesting organisms. J. Hazard. Mater. 2020, 400, 123239. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.F.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption mechanism of cadmium on microplastics and their desorption behavior in sediment and gut environments: The roles of water pH, lead ions, natural organic matter and phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef] [PubMed]
- Rachman, C.M. Microplastics research—From sink to source. Science 2018, 360, 28–29. [Google Scholar] [CrossRef] [PubMed]
- Eerkes-Medrano, D.; Thompson, R.C.; Aldridge, D.C. Microplastics in freshwater systems: A review of the emerging threats, identification of knowledge gaps and prioritisation of research needs. Water Res. 2015, 75, 63–82. [Google Scholar] [CrossRef]
- Khalid, N.; Aqeel, M.; Noman, A.; Khan, S.M.; Akhter, N. Interactions and effects of microplastics with heavy metals in aquatic and terrestrial environments. Environ. Pollut. 2021, 290, 118104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Huang, J.; Zhang, W.; Shi, L.; Yi, K.; Yu, H.; Zhang, C.; Li, S.; Li, J. Microplastics as a vehicle of heavy metals in aquatic environments: A review of adsorption factors, mechanisms, and biological effects. J. Environ. Manag. 2022, 302, 113995. [Google Scholar] [CrossRef] [PubMed]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- De Falco, F.; Di Pace, E.; Cocca, M.; Avella, M. The contribution of washing processes of synthetic clothes to microplastic pollution. Sci. Rep. 2019, 9, 6633. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Liang, J.; Feng, C.; Zeng, G.; Zhong, M.; Gao, X.; Li, X.; He, X.; Li, X.; Fang, Y.; Mo, D. Atmospheric deposition of mercury and cadmium impacts on topsoil in a typical coal mine city, Lianyuan, China. Chemosphere 2017, 189, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.W.; Luo, T.; Liu, X.; Hua, H.; Zhuang, Y.; Zhang, X.; Zhang, L.; Zhang, Y.; Xu, W.; Ren, J. Tracing anthropogenic cadmium emissions: From sources to pollution. Sci. Total Environ. 2019, 676, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.; Chapman, D.; Carvalho, L.; Spears, B.M.; Andersen, H.E.; Argillier, C.; Auer, S.; Baattrup-Pedersen, A.; Banin, L.; Beklioğlu, M.; et al. Impacts of multiple stressors on freshwater biota across spatial scales and ecosystems. Nat. Ecol. Evol. 2020, 4, 1060–1068. [Google Scholar] [CrossRef] [PubMed]
- Svara, V.; Krauss, M.; Michalski, S.G.; Altenburger, R.; Brack, W.; Luckenbach, T. Chemical Pollution Levels in a River Explain Site-Specific Sensitivities to Micropollutants within a Genetically Homogeneous Population of Freshwater Amphipods. Environ. Sci. Technol. 2021, 55, 6087–6096. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.M.; Mo, W.Y.; Luukkonen, T. Adsorption behaviour and interaction of organic micropollutants with nano and microplastics—A review. Sci. Total Environ. 2021, 797, 149140. [Google Scholar] [CrossRef]
- Adyari, B.; Shen, D.; Li, S.; Zhang, L.; Rashid, A.; Sun, Q.; Hu, A.; Chen, N.; Yu, C.-P. Strong impact of micropollutants on prokaryotic communities at the horizontal but not vertical scales in a subtropical reservoir, China. Sci. Total Environ. 2020, 721, 137767. [Google Scholar] [CrossRef]
- Meyer, A.; Alric, B.; Dézerald, O.; Billoir, E.; Coulaud, R.; Larras, F.; Mondy, C.P.; Usseglio-Polatera, P. Linking Micropollutants to Trait Syndromes across Freshwater Diatom, Macroinvertebrate, and Fish Assemblages. Water 2022, 14, 1184. [Google Scholar] [CrossRef]
- Franzellitti, S.; Canesi, L.; Auguste, M.; Wathsala, R.H.G.R.; Fabbri, E. Microplastic exposure and effects in aquatic organisms: A physiological perspective. Environ. Toxicol. Pharmacol. 2019, 68, 37–51. [Google Scholar] [CrossRef] [PubMed]
- López-Rojo, N.; Pérez, J.; Alonso, A.; Correa-Araneda, F.; Boyero, L. Microplastics have lethal and sublethal effects on stream invertebrates and affect stream ecosystem functioning. Environ. Pollut. 2020, 259, 113898. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as vector for heavy metal contamination from the marine environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Liu, G.X.; Dave, P.H.; Kwong, R.W.M.; Wu, M.; Zhong, H. Influence of Microplastics on the Mobility, Bioavailability, and Toxicity of Heavy Metals: A Review. Bull. Environ. Contam. Toxicol. 2021, 107, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.K.; Kumar, R. Microplastic effects in aquatic ecosystems with special reference to fungi–zooplankton interaction: Identification of knowledge gaps and prioritization of research needs. Front. Ecol. Evol. 2023, 11, 1279589. [Google Scholar] [CrossRef]
- Quainoo, S.; Seena, S.; Graça, M.A.S. Copper tolerant ecotypes of Heliscus lugdunensis differ in their ecological function and growth. Sci. Total Environ. 2016, 544, 168–174. [Google Scholar] [CrossRef]
- Cao, Y.X.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A critical review on the interactions of microplastics with heavy metals: Mechanism and their combined effect on organisms and humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef]
- Naqash, N.; Prakash, S.; Kapoor, D.; Singh, R. Interaction of freshwater microplastics with biota and heavy metals: A review. Environ. Chem. Lett. 2020, 18, 1813–1824. [Google Scholar] [CrossRef]
- Foley, C.J.; Feiner, Z.S.; Malinich, T.D.; Höök, T.O. A meta-analysis of the effects of exposure to microplastics on fish and aquatic invertebrates. Sci. Total Environ. 2018, 631–632, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Martín-Folgar, R.; Martínez-Guitarte, J.L. Cadmium alters the expression of small heat shock protein genes in the aquatic midge. Chemosphere 2017, 169, 485–492. [Google Scholar] [CrossRef]
- Wang, F.L.; Wang, X.X.; Song, N.N. Polyethylene microplastics increase cadmium uptake in lettuce (Lactuca sativa L.) by altering the soil microenvironment. Sci. Total Environ. 2021, 784, 147133. [Google Scholar] [CrossRef]
- Hess, S.; Hof, D.; Oetken, M.; Sundermann, A. Effects of multiple stressors on benthic invertebrates using Water Framework Directive monitoring data. Sci. Total Environ. 2023, 878, 162952. [Google Scholar] [CrossRef]
- Tiegs, S.D.; Costello, D.M.; Isken, M.W.; Woodward, G.; McIntyre, P.B.; Gessner, M.O.; Chauvet, E.; Griffiths, N.A.; Flecker, A.S.; Acuña, V.; et al. Global patterns and drivers of ecosystem functioning in rivers and riparian zones. Sci. Adv. 2019, 5, eaav0486. [Google Scholar] [CrossRef]
- Kuehn, K.A. Lentic and lotic habitats as templets for fungal communities: Traits, adaptations, and their significance to litter decomposition within freshwater ecosystems. Fungal Ecol. 2016, 19, 135–154. [Google Scholar] [CrossRef]
- Mille-Lindblom, C.; Tranvik, L.J. Antagonism between bacteria and fungi on decomposing aquatic plant litter. Microb. Ecol. 2003, 45, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, A.; Pérez, J.; López-Rojo, N.; García, G.; Pérez, E.; Guerra, A.; Nieto, C.; Boyero, L. Litter decomposition can be reduced by pesticide effects on detritivores and decomposers: Implications for tropical stream. Environ. Pollut. 2021, 285, 117243. [Google Scholar] [CrossRef]
- Pérez, J.; Ferreira, V.; Graça, M.A.S.; Boyero, L. Litter Quality Is a Stronger Driver than Temperature of Early Microbial Decomposition in Oligotrophic Streams: A Microcosm Study. Microb. Ecol. 2021, 82, 897–908. [Google Scholar] [CrossRef]
- Wallace, J.B.; Webster, J.R. The role of macroinvertebrates in stream ecosystem function. Annu. Rev. Entomol. 1996, 41, 115–139. [Google Scholar] [CrossRef]
- Rugenski, A.T.; Múrria, C.; Whiles, M.R. Tadpoles enhance microbial activity and leaf decomposition in a neotropical headwater stream. Freshw. Biol. 2012, 57, 1904–1913. [Google Scholar] [CrossRef]
- Rasmussen, J.J.; Friberg, N.; Larsen, S.E. Impact of lambda-cyhalothrin on a macroinvertebrate assemblage in outdoor experimental channels: Implications for ecosystem functioning. Aquat. Toxicol. 2008, 90, 228–234. [Google Scholar] [CrossRef]
- Pereira, A.; Ferreira, V. Invasion of Native Riparian Forests by Species Affects In-Stream Litter Decomposition and Associated Microbial Decomposers. Microb. Ecol. 2021, 81, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Yang, W.; Tan, B.; Peng, Y.; Huang, C.; Xu, Z.; Ni, X.; Yang, Y.; Zhou, W.; Zhang, L.; et al. Immobilization of heavy metals during aquatic and terrestrial litter decomposition in an alpine forest. Chemosphere 2019, 216, 419–427. [Google Scholar] [CrossRef]
- Zhao, D.; Li, J.; Lv, L.; Zhang, M.; Liu, Z.; An, S. Effect of cadmium contamination on the eutrophic secondary pollution of aquatic macrophytes by litter decomposition. J. Environ. Manag. 2019, 231, 1100–1105. [Google Scholar] [CrossRef]
- He, B.B.; Duodu, G.O.; Rintoul, L.; Ayoko, G.A.; Goonetilleke, A. Influence of microplastics on nutrients and metal concentrations in river sediments. Environ. Pollut. 2020, 263, 114490. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Li, G.; Wang, H.-T.; Duan, G.-L. Effects of nano- or microplastic exposure combined with arsenic on soil bacterial, fungal, and protistan communities. Chemosphere 2021, 281, 130998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Wang, M.; Chen, X.; Yang, C.; Wu, L. Combined toxicity of microplastics and cadmium on the zebrafish embryos (Danio rerio). Sci. Total Environ. 2020, 743, 140638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, J.; Geng, X.; Jiang, Y. Does microplastic ingestion dramatically decrease the biomass of protozoa grazers? A case study on the marine ciliate. Chemosphere 2021, 267, 129308. [Google Scholar] [CrossRef]
- Du, J.; Qv, W.; Niu, Y.; Qv, M.; Jin, K.; Xie, J.; Li, Z. Nanoplastic pollution inhibits stream leaf decomposition through modulating microbial metabolic activity and fungal community structure. J. Hazard. Mater. 2022, 424, 127392. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Cui, M.; Yang, J.; Lin, Z.; Zhang, H. Evidence for negative effects of ZnO nanoparticles on leaf litter decomposition in freshwater ecosystems. Environ. Sci. Nano 2017, 4, 2377–2387. [Google Scholar] [CrossRef]
- Du, J.; Zhang, Y.; Guo, R.; Meng, F.; Gao, Y.; Ma, C.; Zhang, H. Harmful effect of nanoparticles on the functions of freshwater ecosystems: Insight into nanoZnO-polluted stream. Chemosphere 2019, 214, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Assmann, C.; Rinke, K.; Nechwatal, J.; von Elert, E. Consequences of the colonisation of leaves by fungi and oomycetes for leaf consumption by a gammarid shredder. Freshw. Biol. 2011, 56, 839–852. [Google Scholar] [CrossRef]
- Gigault, J.; ter Halle, A.; Baudrimont, M.; Pascal, P.-Y.; Gauffre, F.; Phi, T.-L.; El Hadri, H.; Grassl, B.; Reynaud, S. Current opinion: What is a nanoplastic? Environ. Pollut. 2018, 235, 1030–1034. [Google Scholar] [CrossRef]
- Du, J.; Qv, M.; Qv, W.; Liu, L.; Zhang, Y.; Cui, M.; Zhang, H. Potential threats of zeolitic imidazolate framework-8 nanoparticles to aquatic fungi associated with leaf decomposition. J. Hazard. Mater. 2021, 401, 123273. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic waste inputs from land into the ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S. Effects of microplastics on European flat oysters, Ostrea edulis and their associated benthic communities. Environ. Pollut. 2016, 216, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Batista, D.; Pascoal, C.; Cássio, F. Impacts of warming on aquatic decomposers along a gradient of cadmium stress. Environ. Pollut. 2012, 169, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Zhang, Y.; Guo, W.; Li, N.; Gao, C.; Cui, M.; Lin, Z.; Wei, M.; Zhang, H. Chronic impacts of TiO2 nanoparticles on Populus nigra L. leaf decomposition in freshwater ecosystem. J. Hazard. Mater. 2018, 350, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.S. Energy-Storage and Balance of Producers and Decomposers in Ecological-Systems. Ecology 1963, 44, 322–331. [Google Scholar] [CrossRef]
- Valenzuela-Solano, C.; Crohn, D.M. Are decomposition and N release from organic mulches determined mainly by their chemical composition? Soil Biol. Biochem. 2006, 38, 377–384. [Google Scholar] [CrossRef]
- Tryfona, T.; Pankratova, Y.; Petrik, D.; Moran, D.R.; Wightman, R.; Yu, X.; Echevarría-Poza, A.; Deralia, P.K.; Vilaplana, F.; Anderson, C.T.; et al. Altering the substitution and cross-linking of glucuronoarabinoxylans affects cell wall architecture in. New Phytol. 2024, 242, 524–543. [Google Scholar] [CrossRef] [PubMed]
- Yanni, S.F.; Whalen, J.K.; Simpson, M.J.; Janzen, H.H. Plant lignin and nitrogen contents control carbon dioxide production and nitrogen mineralization in soils incubated with Bt and non-Bt corn residues. Soil Biol. Biochem. 2011, 43, 63–69. [Google Scholar] [CrossRef]
- Aziz, M.A.; Khan, K.S.; Khalid, R.; Shabaan, M.; Alghamdi, A.G.; Alasmary, Z.; Majrashi, M.A. Integrated application of biochar and chemical fertilizers improves wheat (Triticum aestivum) productivity by enhancing soil microbial activities. Plant Soil 2024. [Google Scholar] [CrossRef]
- Alekseyeva, K.S.; Mähnert, B.; Berthiller, F.; Breyer, E.; Herndl, G.J.; Baltar, F. Adapting an Ergosterol Extraction Method with Marine Yeasts for the Quantification of Oceanic Fungal Biomass. J. Fungi 2021, 7, 690. [Google Scholar] [CrossRef]
- Mansoldo, F.R.P.; Firpo, R.; da Silva Cardoso, V.; Queiroz, G.N.; Lage Cedrola, S.M.; de Godoy, M.G.; Vermelho, A.B. New method for rapid identification and quantification of fungal biomass using ergosterol autofluorescence. Talanta 2020, 219, 121238. [Google Scholar] [CrossRef] [PubMed]
- Kingsbury, S.E.; McAlpine, D.F.; Cheng, Y.; Parker, E.; Campbell, L.M. A review of the non-indigenous Chinese mystery snail, Cipangopaludina chinensis (Viviparidae), in North America, with emphasis on occurrence in Canada and the potential impact on indigenous aquatic species. Environ. Rev. 2021, 29, 182–200. [Google Scholar] [CrossRef]
- Cui, J.G.; Shan, B.Q.; Tang, W.Z. Effect of periphyton community structure on heavy metal accumulation in mystery snail (Cipangopaludina chinensis): A case study of the Bai River, China. J. Environ. Sci. 2012, 24, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.Q.; Qin, J.; Pang, H.; Chen, Z.; Huang, Y.; Li, W.; Du, X.; Wen, L.; Pan, X.; Lin, Y. Comparison of the composition and function of gut microbes between adult and juvenile Cipangopaludina chinensis in the rice snail system. PeerJ 2022, 10, e13042. [Google Scholar] [CrossRef] [PubMed]
- Graça, M.A.S.; Ferreira, V.; Canhoto, C.; Encalada, A.C.; Guerrero-Bolaño, F.; Wantzen, K.M.; Boyero, L. A conceptual model of litter breakdown in low order streams. Int. Rev. Hydrobiol. 2015, 100, 1–12. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Chauvet, E.; Barlocher, F.; Graca, M.A.S.; Canhoto, C. Top-down and bottom-up control of litter decomposers in streams. Freshw. Biol. 2014, 59, 2172–2182. [Google Scholar] [CrossRef]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between microplastics and soil fauna: A critical review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Silva, J.M.C.; Machado, A.L.; Campos, D.; Rodrigues, A.C.M.; Silva, A.L.P.; Soares, A.M.V.M.; Pestana, J.L.T. Microplastics in freshwater sediments: Effects on benthic invertebrate communities and ecosystem functioning assessed in artificial streams. Sci. Total Environ. 2022, 804, 150118. [Google Scholar] [CrossRef]
- Miao, L.Z.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef]
- Rummel, C.D.; Jahnke, A.; Gorokhova, E.; Kühnel, D.; Schmitt-Jansen, M. Impacts of Biofilm Formation on the Fate and Potential Effects of Microplastic in the Aquatic Environment. Environ. Sci. Technol. Lett. 2017, 4, 258–267. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wei, M.; Wang, S.; Wu, B.; Du, D. Cadmium influences the litter decomposition of Solidago canadensis L. and soil N-fixing bacterial communities. Chemosphere 2020, 246, 125717. [Google Scholar] [CrossRef]
- Pinto-Poblete, A.; Retamal-Salgado, J.; López, M.D.; Zapata, N.; Sierra-Almeida, A.; Schoebitz, M. Combined Effect of Microplastics and Cd Alters the Enzymatic Activity of Soil and the Productivity of Strawberry Plants. Plants 2022, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.Y.; Zhang, X.; Zhang, S.; Zhang, S.; Sun, Y. Interactions of microplastics and cadmium on plant growth and arbuscular mycorrhizal fungal communities in an agricultural soil. Chemosphere 2020, 254, 126791. [Google Scholar] [CrossRef]
- Liu, P.; Chen, S.; Cui, Y.; Tan, W. Insights into the inhibition effects of Cd on soil enzyme activities: From spatial microscale to macroscale. J. Hazard. Mater. 2021, 418, 126274. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, Y.; Wang, J.; Zhang, M.; Jia, W.; Qin, X. LDPE microplastic films alter microbial community composition and enzymatic activities in soil. Environ. Pollut. 2019, 254, 112983. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.P.; Wang, Z.; Lu, G.; He, W.; Wei, G.; Huang, F.; Xu, X.; Shen, W. Kinetics of soil dehydrogenase in response to exogenous Cd toxicity. J. Hazard. Mater. 2017, 329, 299–309. [Google Scholar] [CrossRef]
- Schilling, J.S.; Ayres, A.; Kaffenberger, J.T.; Powers, J.S. Initial white rot type dominance of wood decomposition and its functional consequences in a regenerating tropical dry forest. Soil Biol. Biochem. 2015, 88, 58–68. [Google Scholar] [CrossRef]
- Spohn, M. Microbial respiration per unit microbial biomass depends on litter layer carbon-to-nitrogen ratio. Biogeosciences 2015, 12, 817–823. [Google Scholar] [CrossRef]
- Stagg, C.L.; Baustian, M.M.; Perry, C.L.; Carruthers, T.J.B.; Hall, C.T. Direct and indirect controls on organic matter decomposition in four coastal wetland communities along a landscape salinity gradient. J. Ecol. 2018, 106, 655–670. [Google Scholar] [CrossRef]
- Messinetti, S.; Mercurio, S.; Parolini, M.; Sugni, M.; Pennati, R. Effects of polystyrene microplastics on early stages of two marine invertebrates with different feeding strategies. Environ. Pollut. 2018, 237, 1080–1087. [Google Scholar] [CrossRef]
- Santonja, M.; Pellan, L.; Piscart, C. Macroinvertebrate identity mediates the effects of litter quality and microbial conditioning on leaf litter recycling in temperate streams. Ecol. Evol. 2018, 8, 2542–2553. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.P.; Reich, P.B.; Hobbie, S.E.; Stefanski, A.; Rich, R.; Rice, K.E.; Eddy, W.C.; Eisenhauer, N. Reduced feeding activity of soil detritivores under warmer and drier conditions. Nat. Clim. Chang. 2018, 8, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Kettner, M.T.; Rojas-Jimenez, K.; Oberbeckmann, S.; Labrenz, M.; Grossart, H. Microplastics alter composition of fungal communities in aquatic ecosystems. Environ. Microbiol. 2017, 19, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Kuehn, K.A.; Phipps, S.W. Fungal contributions to carbon flow and nutrient cycling during decomposition of standing Typha domingensis leaves in a subtropical freshwater marsh. Freshw. Biol. 2015, 60, 2100–2112. [Google Scholar] [CrossRef]
- Danger, M.; Cornut, J.; Chauvet, E.; Chavez, P.; Elger, A.; Lecerf, A. Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: A case of aquatic priming effect? Ecology 2013, 94, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Truchy, A.; Sarremejane, R.; Muotka, T.; Mykrä, H.; Angeler, D.G.; Lehosmaa, K.; Huusko, A.; Johnson, R.K.; Sponseller, R.A.; McKie, B.G. Habitat patchiness, ecological connectivity and the uneven recovery of boreal stream ecosystems from an experimental drought. Glob. Change Biol. 2020, 26, 3455–3472. [Google Scholar] [CrossRef]
- Azevedo, M.M.; Carvalho, A.; Pascoal, C.; Rodrigues, F.; Cássio, F. Responses of antioxidant defenses to Cu and Zn stress in two aquatic fungi. Sci. Total Environ. 2007, 377, 233–243. [Google Scholar] [CrossRef]
- Zhou, Y.T.; Sun, Y.; Liu, J.; Ren, X.; Zhang, Z.; Wang, Q. Effects of microplastics on humification and fungal community during cow manure composting. Sci. Total Environ. 2022, 803, 150029. [Google Scholar] [CrossRef]
- Lin, L.T.; Chen, Y.; Qu, L.; Zhang, Y.; Ma, K. Cd heavy metal and plants, rather than soil nutrient conditions, affect soil arbuscular mycorrhizal fungal diversity in green spaces during urbanization. Sci. Total Environ. 2020, 726, 138594. [Google Scholar] [CrossRef]
- Luo, G.W.; Jin, T.; Zhang, H.; Peng, J.; Zuo, N.; Huang, Y.; Han, Y.; Tian, C.; Yang, Y.; Peng, K.; et al. Deciphering the diversity and functions of plastisphere bacterial communities in plastic-mulching croplands of subtropical China. J. Hazard. Mater. 2022, 422, 126865. [Google Scholar] [CrossRef]
- Koh, J.; Bairoliya, S.; Salta, M.; Cho, Z.T.; Fong, J.; Neo, M.L.; Cragg, S.; Cao, B. Sediment-driven plastisphere community assembly on plastic debris in tropical coastal and marine environments. Environ. Int. 2023, 179, 108153. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.Y.; Li, T.; Li, M.; Yang, S.; Shen, M.; Liu, H. Research advances on the toxicity of biodegradable plastics derived micro/nanoplastics in the environment: A review. Sci. Total Environ. 2024, 916, 170299. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wolosker, M.B.; Zhao, Y.; Ren, H.; Lemos, B. Exposure to microplastics cause gut damage, locomotor dysfunction, epigenetic silencing, and aggravate cadmium (Cd) toxicity in. Sci. Total Environ. 2020, 744, 140979. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P. Genetic variation in compensatory feeding for dietary dilution in a generalist caterpillar. Sci. Rep. 2017, 7, 7461. [Google Scholar] [CrossRef] [PubMed]
- Alian, R.S.; Dziewięcka, M.; Kędziorski, A.; Majchrzycki, L.; Augustyniak, M. Do nanoparticles cause hormesis? Early physiological compensatory response in house crickets to a dietary admixture of GO, Ag, and GOAg composite. Sci. Total Environ. 2021, 788, 147801. [Google Scholar] [CrossRef] [PubMed]
- Seena, S.; Gutiérrez, I.B.; Barros, J.; Nunes, C.; Marques, J.C.; Kumar, S.; Gonçalves, A.M. Impacts of low concentrations of nanoplastics on leaf litter decomposition and food quality for detritivores in streams. J. Hazard. Mater. 2022, 429, 128320. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Godbold, J.A.; Lewis, C.N.; Savage, G.; Solan, M.; Galloway, T.S. Microplastic burden in marine benthic invertebrates depends on species traits and feeding ecology within biogeographical provinces. Nat. Commun. 2023, 14, 8023. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Pan, Y.; Fang, Y.; Pang, L.; Shen, J.; Tian, X. Effects of heavy metal-mediated intraspecific variation in leaf litter on the feeding preferences of stream detritivores. Sci. Total Environ. 2021, 763, 144591. [Google Scholar] [CrossRef]
- Hobbelen, P.H.F.; Koolhaas, J.E.; van Gestel, C.A.M. Risk assessment of heavy metal pollution for detritivores in floodplain soils in the Biesbosch, the Netherlands, taking bioavailability into account. Environ. Pollut. 2004, 129, 409–419. [Google Scholar] [CrossRef]
- Peng, X.Z.; Fan, Y.; Jin, J.; Xiong, S.; Liu, J.; Tang, C. Bioaccumulation and biomagnification of ultraviolet absorbents in marine wildlife of the Pearl River Estuarine, South China Sea. Environ. Pollut. 2017, 225, 55–65. [Google Scholar] [CrossRef]
- Quiblier, C.; Susanna, W.; Isidora, E.-S.; Mark, H.; Aurelie, V.; Jean-Francois, H. A review of current knowledge on toxic benthic freshwater cyanobacteria—Ecology, toxin production and risk management. Water Res. 2013, 47, 5464–5479. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, H.; Cai, S.; Chen, S.; Li, Q.; Luo, Y.; Zeng, X.; Ye, R.; Wan, P.; Tian, X. Dual Effect of Microplastics and Cadmium on Stream Litter Decomposition and Invertebrate Feeding Behavior. Water 2024, 16, 1295. https://doi.org/10.3390/w16091295
He H, Cai S, Chen S, Li Q, Luo Y, Zeng X, Ye R, Wan P, Tian X. Dual Effect of Microplastics and Cadmium on Stream Litter Decomposition and Invertebrate Feeding Behavior. Water. 2024; 16(9):1295. https://doi.org/10.3390/w16091295
Chicago/Turabian StyleHe, Hualong, Sulin Cai, Siyuan Chen, Qiang Li, Yunchao Luo, Xiaoyi Zeng, Rumeng Ye, Pengwei Wan, and Xingjun Tian. 2024. "Dual Effect of Microplastics and Cadmium on Stream Litter Decomposition and Invertebrate Feeding Behavior" Water 16, no. 9: 1295. https://doi.org/10.3390/w16091295
APA StyleHe, H., Cai, S., Chen, S., Li, Q., Luo, Y., Zeng, X., Ye, R., Wan, P., & Tian, X. (2024). Dual Effect of Microplastics and Cadmium on Stream Litter Decomposition and Invertebrate Feeding Behavior. Water, 16(9), 1295. https://doi.org/10.3390/w16091295






