Geochemical Surveys of Ground and Surface Waters in the Abandoned Hg-Mine of Abbadia San Salvatore (Central Italy): A Preparatory Investigation before Remediation
Abstract
1. Introduction
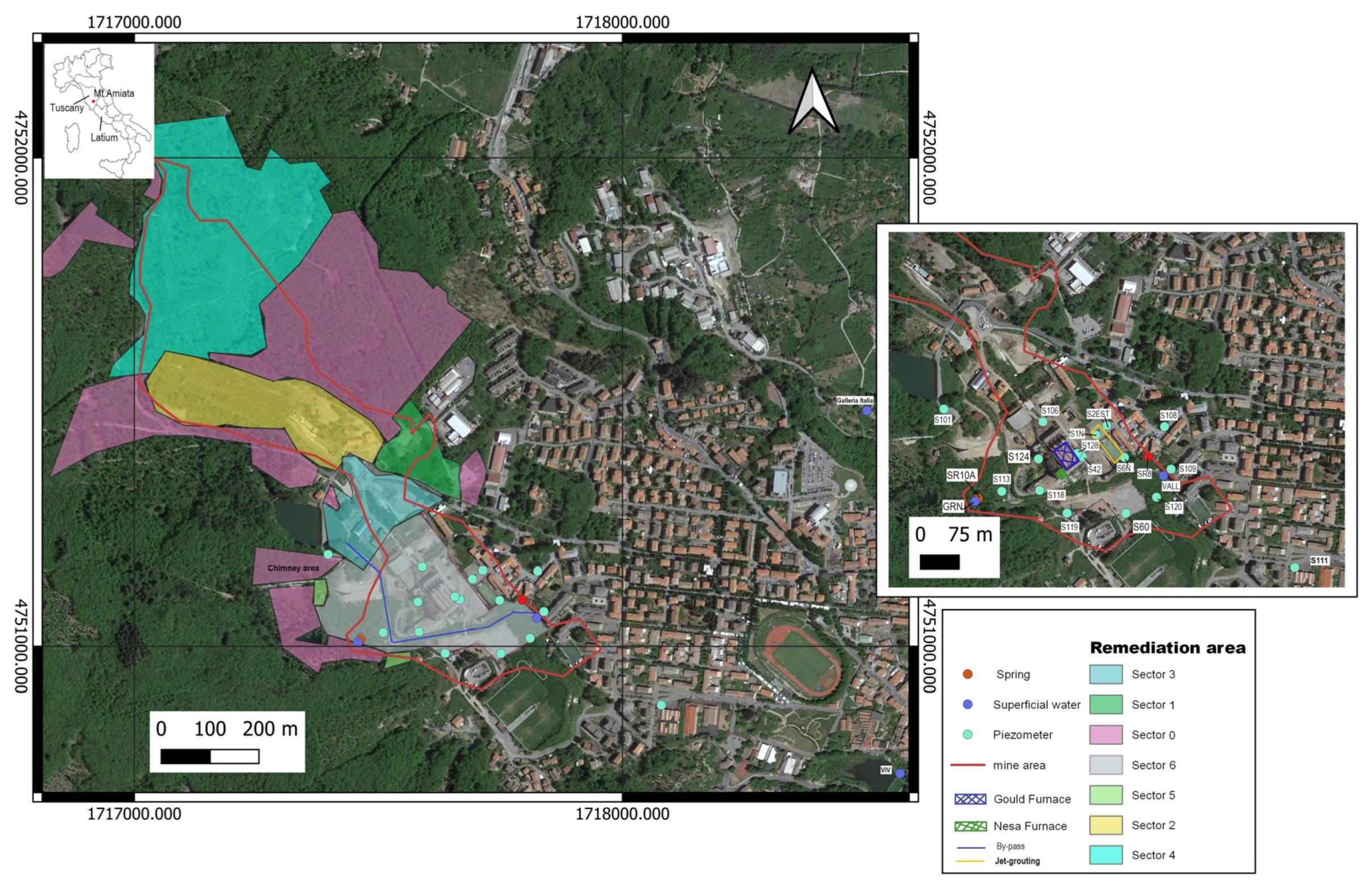
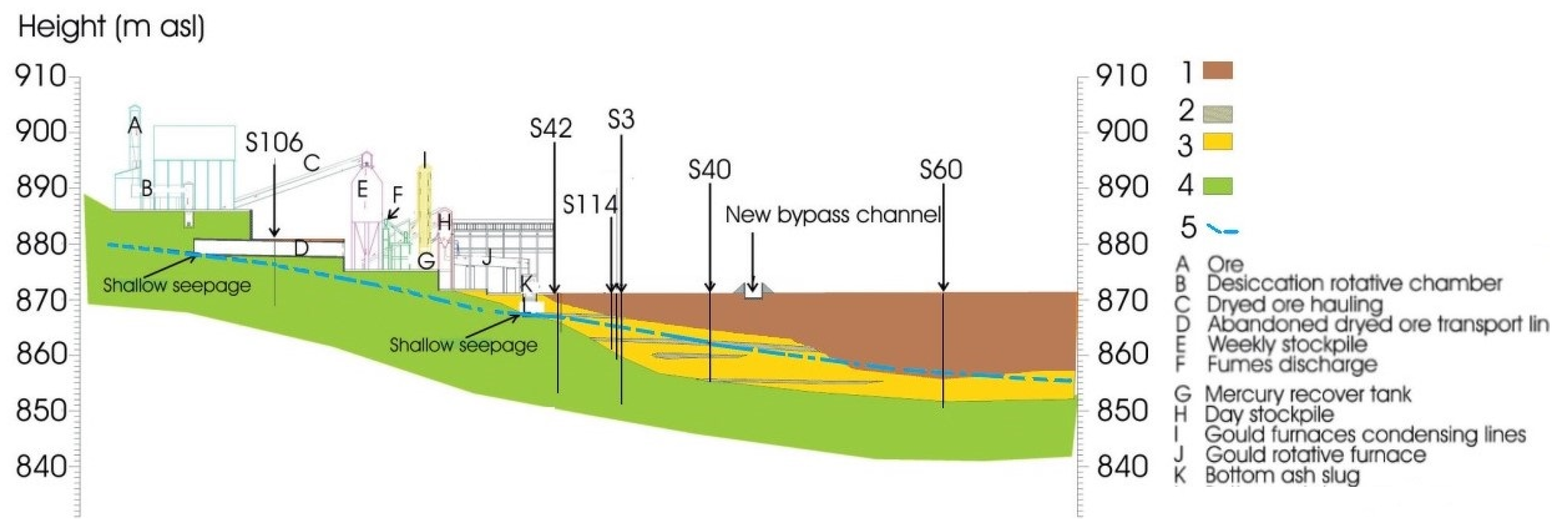
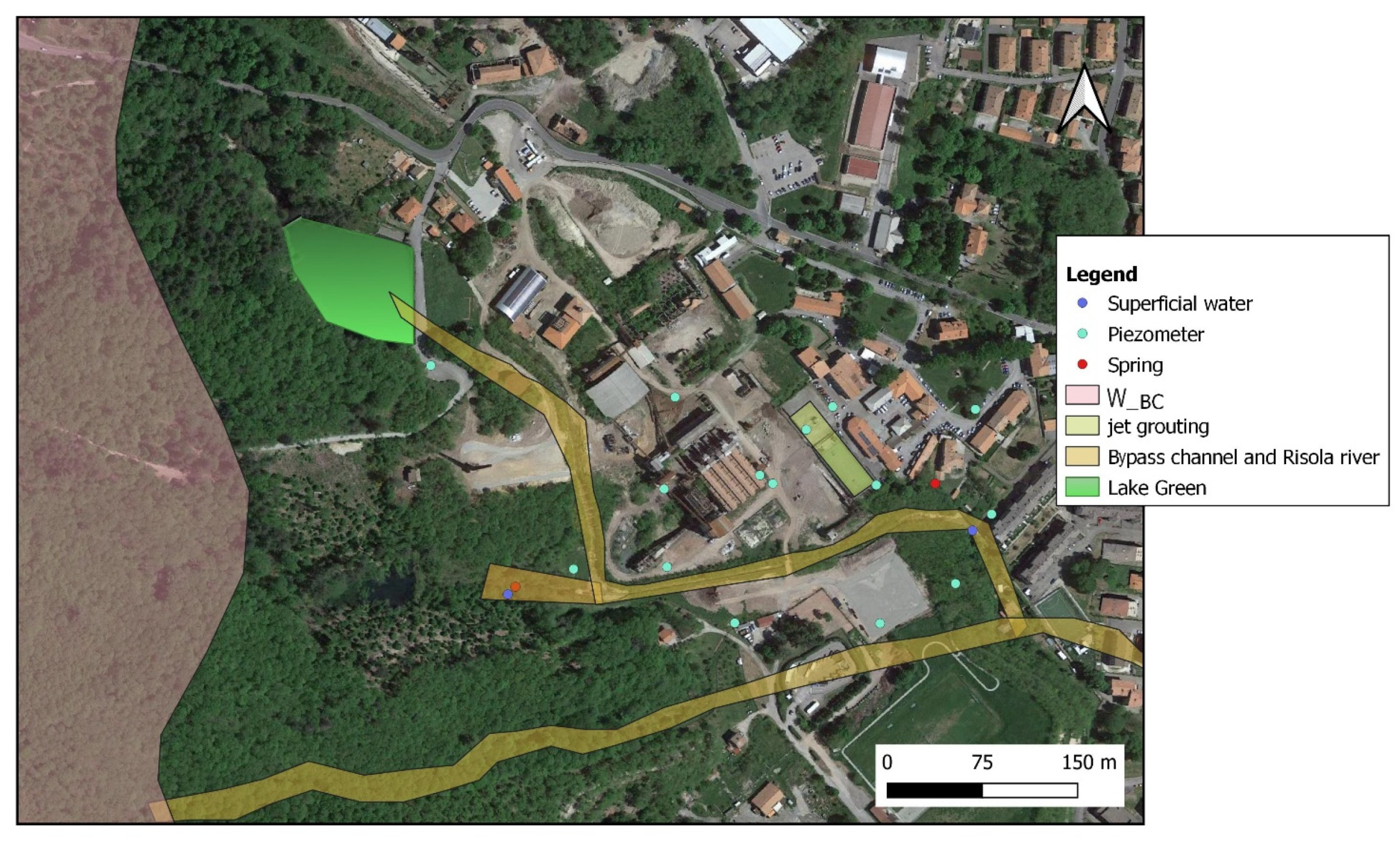
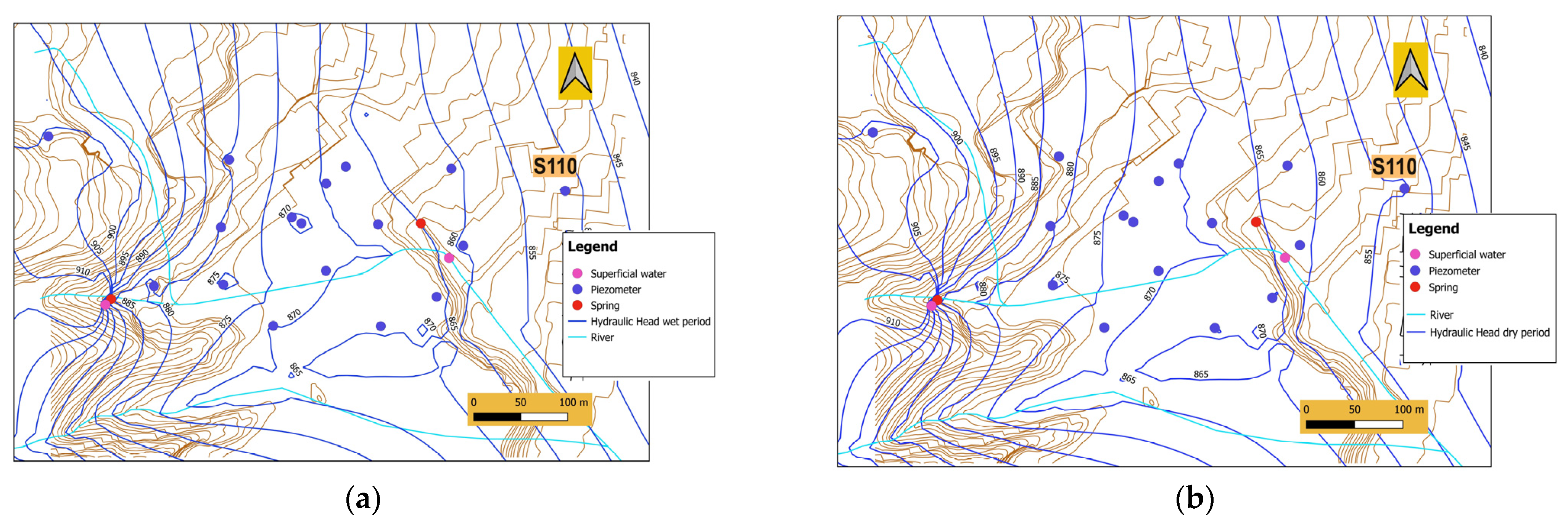
Study Area and Brief Hydrogeological Outlines
2. Sampling and Analytical Methods
2.1. Geochemical Modeling with PHREEQC
2.2. Flow and Hg Transporting Modeling with ModFlow 6.4.2
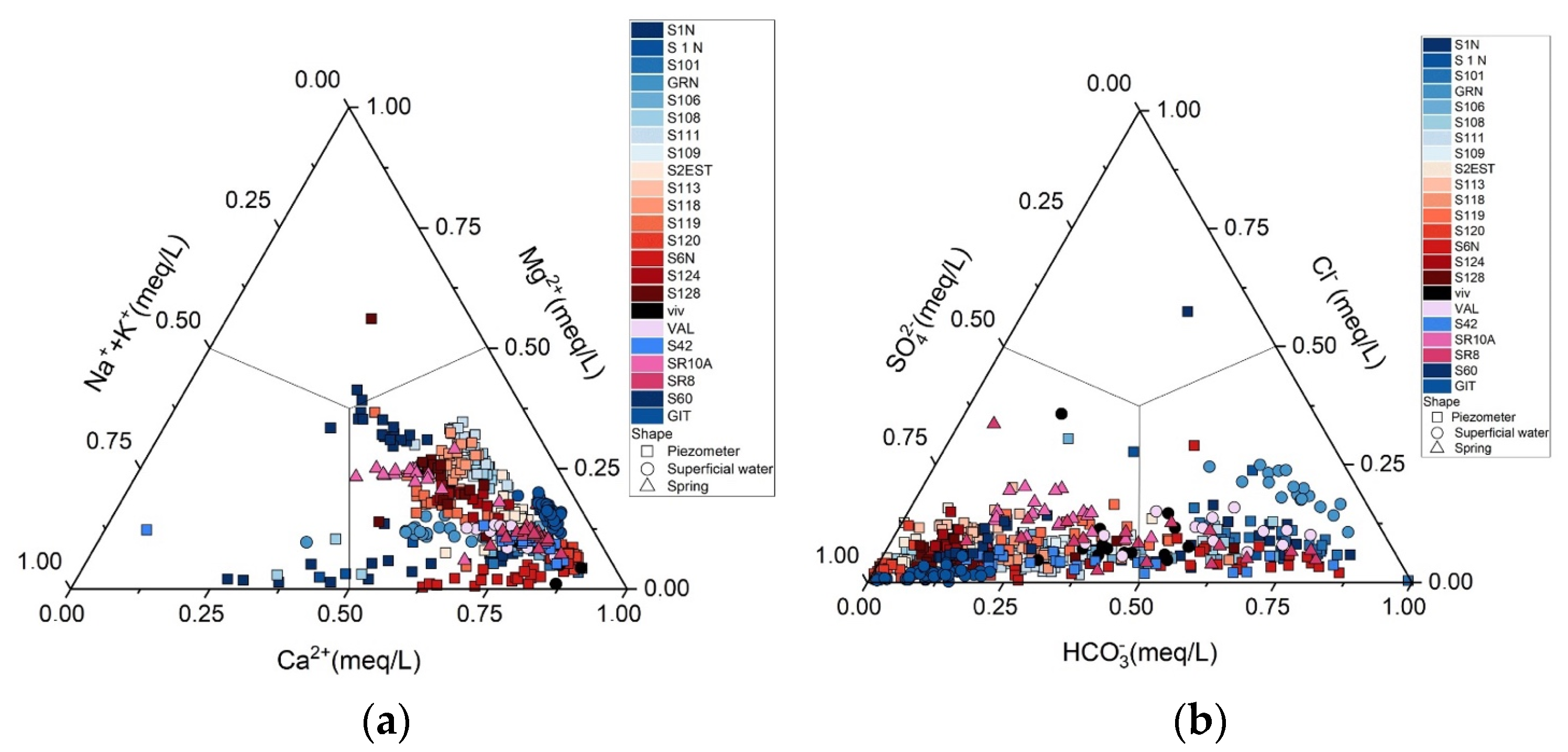
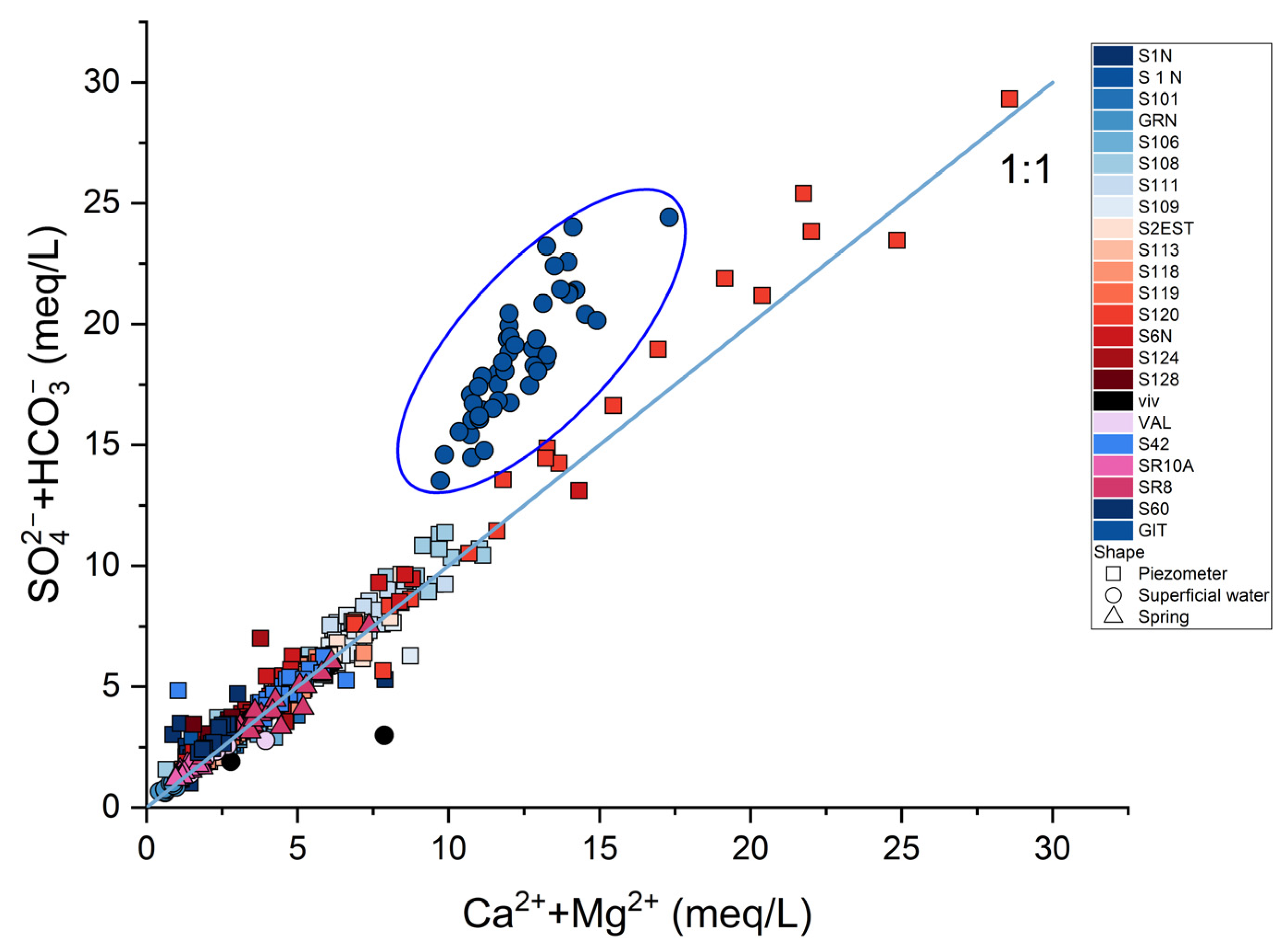
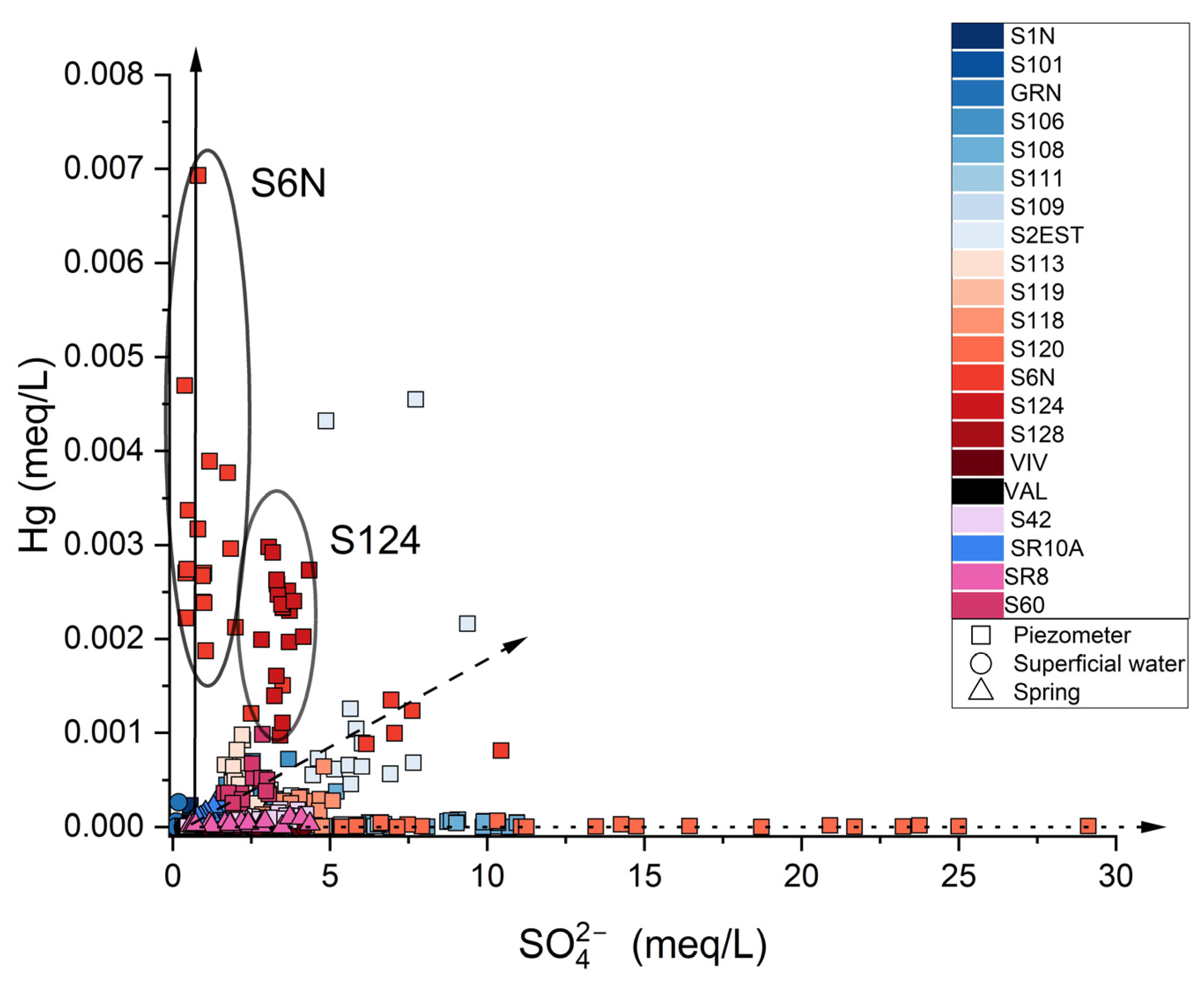
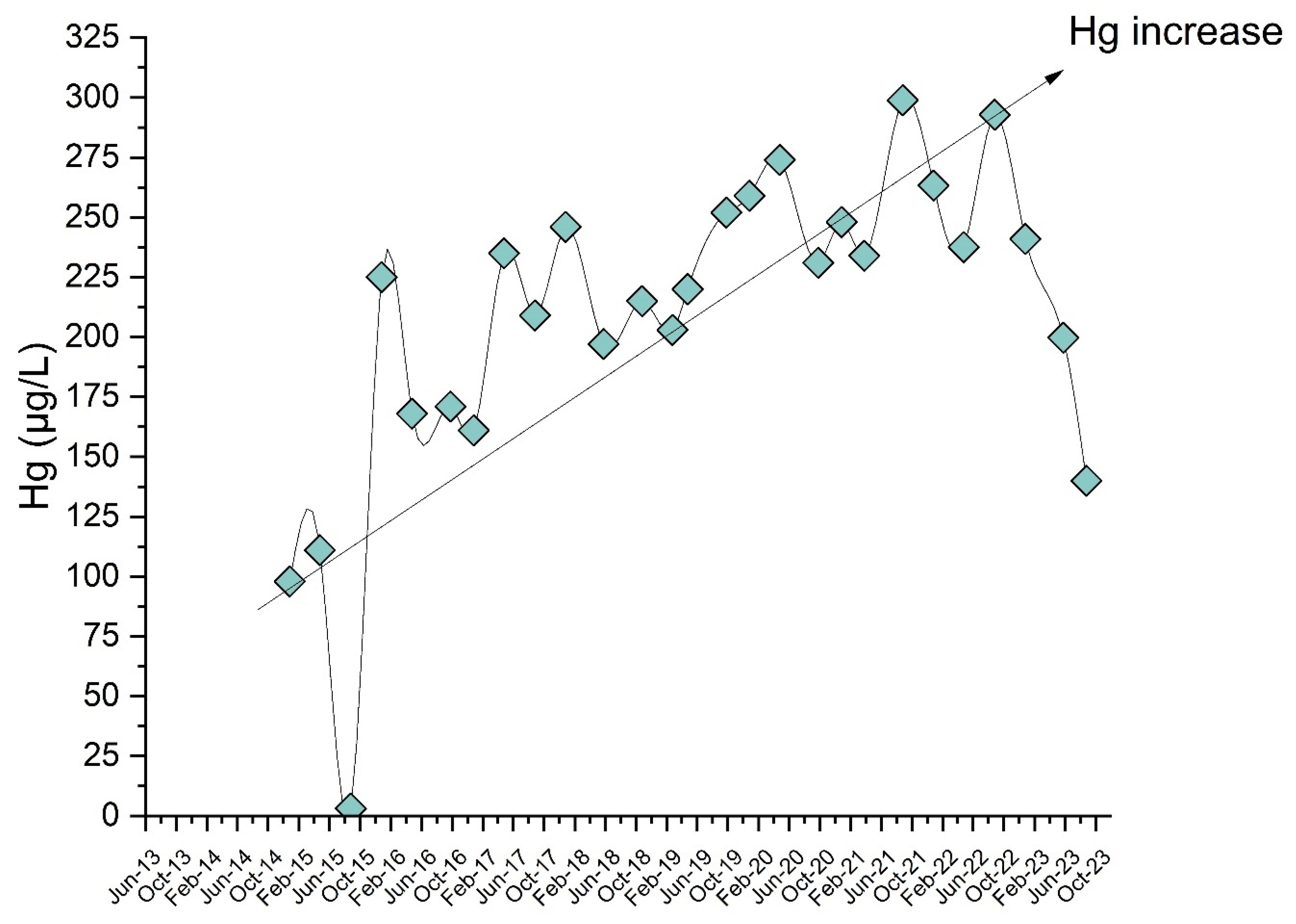
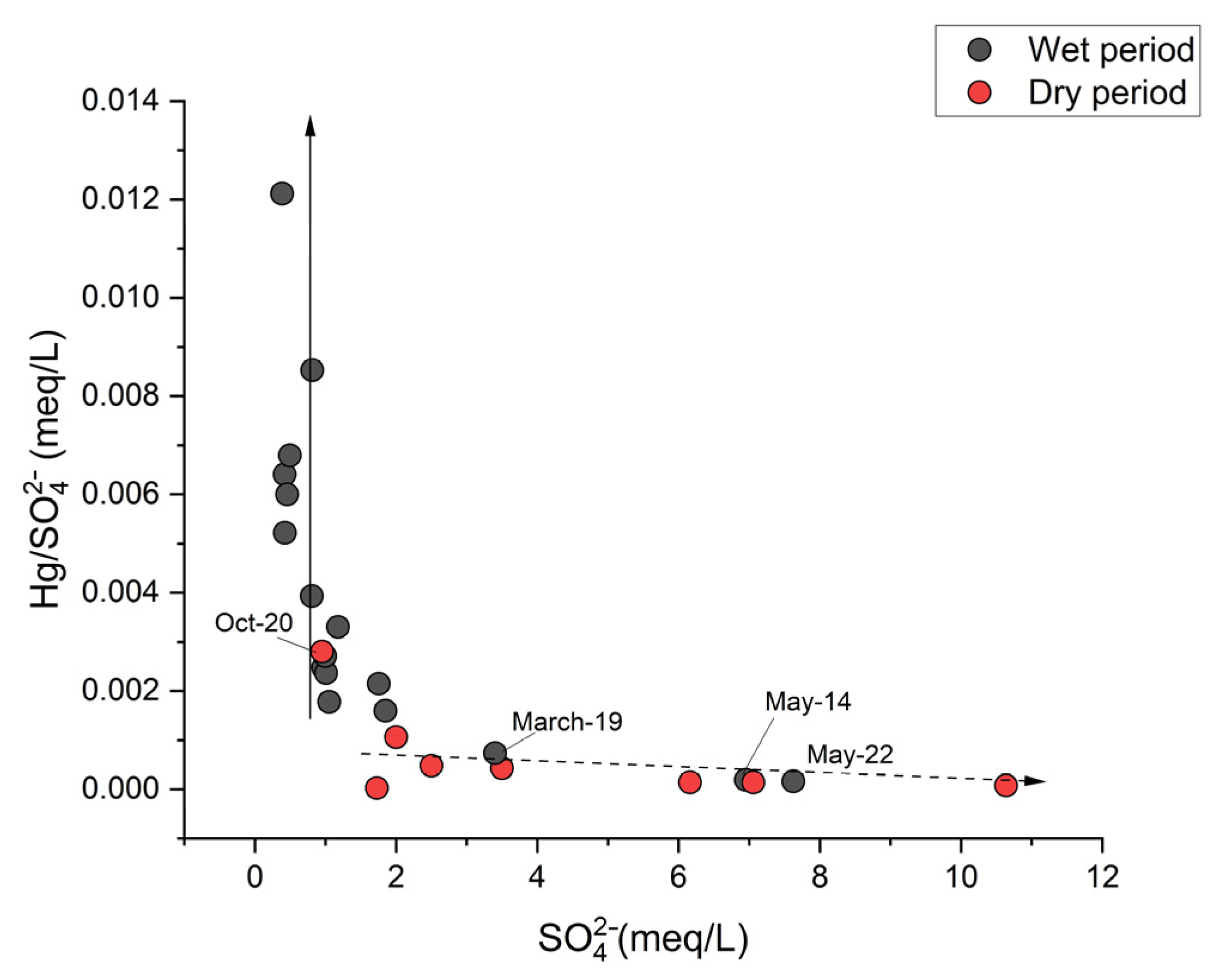
3. Results
4. Discussion
4.1. PHREEQ-C Modeling—Saturation Indices (SI) and Speciation
4.2. Flux and Transport Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bain, J.G.; Mayer, K.U.; Blowes, D.W.; Frind, E.O.; Molson, J.W.H.; Kahnt, R.; Jenk, U. Modelling the closure-related geochemical evolution of groundwater at a former uranium mine. J. Contam. Hydrol. 2001, 52, 109–135. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, Q.; Liu, M.; Guo, J.; Xia, J.; Wang, J.; Qiu, Y.; Zou, J.; He, W.; Jiang, F. Treatment and remediation of metal-contaminated water and groundwater in mining areas by biological sulfidogenic processes: A review. J. Hazard Mater. 2023, 443, 130377. [Google Scholar] [CrossRef] [PubMed]
- Madzivire, G.; Gitari, W.M.; Kumar Vadapalli, V.R.; Ojumu, T.V.; Petrik, L.F. Fate of sulphate removed during the treatment of circumneutral mine water and acid mine drainage with coal fly ash: Modelling and experimental approach. Min. Engin. 2011, 24, 1467–1477. [Google Scholar] [CrossRef]
- Iribar, V.; Izco, F.; Tames, P.; Antiguedad, I.; da Silva, A. Water contamination and remedial measures at the Troya abandoned Pb-Zn mine (The Basque Country, Northern Spain). Environ. Geol. 2000, 39, 800–806. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Hydrogeochemical processes governing the origin, trans-port and fate of major and trace elements from mine wastes and mineralized rock to surface waters. Appl. Geochem. 2011, 26, 1777–1791. [Google Scholar] [CrossRef]
- Nordstrom, D.K. Mine waters: Acidic to circmneutral. Elements 2011, 7, 393–398. [Google Scholar] [CrossRef]
- Galván, L.; Olías, M.; Cerón, J.C.; de Villaran, R.F. Inputs and fate of contaminants in a reservoir with circumneutral water affected by acid mine drainage. Sci. Tot. Environ. 2021, 762, 143614. [Google Scholar] [CrossRef]
- Gomo, M. Conceptual hydrogeochemical characteristics of a calcite and dolomite acid mine drainage neutralised circumneutral groundwater system. Water Sci. 2018, 32, 355–361. [Google Scholar] [CrossRef]
- Bowell, R.J.; Dill, S.; Cowan, J.; Wood, A. A review of sulfate removal options for mine waters. In Mine Water 2004—Proceedings International Mine Water Association Symposium 2; Jarvis, A.P., Dudgeon, B.A., Younger, P.L., Eds.; Newcastle upon Tyne (University of Newcastle): Newcastle, UK, 2004; pp. 75–91. [Google Scholar]
- Johnson, B.D.; Hallberg, K.B. Acid mine drainage remediation options: A review. Sci. Tot. Environ. 2005, 338, 3–14. [Google Scholar] [CrossRef]
- Vaselli, O.; Nisi, B.; Rappuoli, D.; Bianchi, F.; Cabassi, J.; Venturi, S.; Tassi, F.; Raco, B. Geochemical characterization of the ground waters from the former Hg-mining area of Abbadia San Salvatore (Mt. Amiata, central Italy): Criticalities and perspectives for the reclamation process. Ital. J. Geosci. 2015, 134, 304–322. [Google Scholar] [CrossRef]
- Laurenzi, M.A.; Braschi, E.; Casalini, M.; Conticelli, S. New 40Ar-39Ar dating and revision of the geochronology of the Monte Amiata Volcano, Central Italy. Ital. J. Geosci. 2015, 134, 255–265. [Google Scholar] [CrossRef]
- Meloni, F.; Montegrossi, G.; Lazzaroni, M.; Rappuoli, D.; Nisi, B.; Vaselli, O. Total and leached arsenic, mercury and antimony in the mining waste dumping area of Abbadia San Salvatore (Mt. Amiata, Central Italy). Appl. Sci. 2021, 11, 7893. [Google Scholar] [CrossRef]
- Rimondi, V.; Chiaranti, L.; Lattanzi, P.; Benvenuti, M.; Beutel, M.; Colica, A.; Costagliola, P.; Di Bendetto, F.; Gabbani, G.; Gray, J.E.; et al. Metallogeny, explotation and environmental impact of the Mt. Amiata mercury ore district (Southern Tuscany, Italy). Ital. J. Geosci. 2015, 134, 323–336. [Google Scholar] [CrossRef]
- Dini, A. Mines and minerals in the Mining district of Monte Amiata. In Il Vulcano di Monte Amiata; E.S.A. Edizioni Scientifiche e Artistiche: Nola, Italy, 2017; pp. 343–369. [Google Scholar]
- Segreto, L. Monte Amiata. Il mercurio italiano. In Strategie Internazionali e Vincoli Extra-Economici; Angeli, F., Ed.; AbeBooks Inc.: Milano, Italy, 1991. (In Italian) [Google Scholar]
- Gandy, C.J.; Smith, J.W.N.; Jarvis, A.P. Attenuation of mining-derived pollutants in the hyporheic zone: A review. Sci. Tot. Environ. 2017, 373, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Stefania, G.A.; Rotiroti, M.; Fumagalli, L.; Zanotti, C.; Bonomi, T. Numerical modeling of remediation scenarios of a groundwater Cr (VI) plume in an alpine valley aquifer. Geosciences 2018, 8, 209. [Google Scholar] [CrossRef]
- ISPRA. Sviluppo e Valutazione di Modelli di Flusso in Acquiferi Porosi; ISPRA: Roma, Italy, 2021; Volume 193, ISBN 978-88-448-1048-1. (In Italian)
- Parkhurst, D.L.; Appelo, C.A.J. User’s guide to PHREEQC (Version 2)-A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey. Water-Resour. Investig. Rep. 1999, 312, 99–4259. [Google Scholar]
- Vaselli, O.; Nisi, B.; Rappuoli, D.; Cabassi, J.; Tassi, F. Gaseous elemental mercury and total and leached mercury in building materials from the former Hg-mining area of Abbadia San Salvatore (Central Italy). Int. J. Environ. Res. Public Health 2017, 14, 425. [Google Scholar] [CrossRef] [PubMed]
- Vaselli, O.; Rappuoli, D.; Bianchi, F.; Nisi, B.; Niccolini, M.; Esposito, A.; Cabassi, J.; Giannini, L.; Tassi, F. One hundred years of mercury exploitation at the mining area of Abbadia San Salvatore (Mt. Amiata, Central Italy): A methodological approach from a complex reclamation activity before the establishment of a new mining park. In El Patrimonio Geologico y Minero; Identidad y Motor de Desarrollo. Publicaciones del Instituto Geologico y minero de Espana Serie: Cuadernos del Museo Geominero, n. 29; Proceed. XVII Congreso Internacional sobre Patrimonio Geologico’ y Minero; Instituto Geológico y Minero de España: Madrid, Spain, 2019; pp. 1109–1126. ISBN 978-84-9138-081-8. [Google Scholar]
- Meloni, F.; Farieri, A.; Higueras, P.L.; Esbrì, M.J.; Nisi, B.; Cabassi, J.; Rappuoli, D.; Vaselli, D. Mercury distribution in plants and soils from the former mining area of Abbadia San Salvatore (Tuscany, Central Italy). Environ. Geochem. Health 2023, 45, 8523–8538. [Google Scholar] [CrossRef]
- Magi, F.; Doveri, M.; Menichini, M.; Minissale, A.; Vaselli, O. Groundwater response to local climate variability: Hydrogeological and isotopic evidences from the Mt. Amiata volcanic aquifer (Tuscany, central Italy). Rend. Lincei. Sci. Fis. Nat. 2019, 30, 125–136. [Google Scholar] [CrossRef]
- SIR. Available online: http://www.sir.toscana.it (accessed on 28 March 2024).
- Doveri, M.; Menichini, M.; Provenzale, A.; Scozzari, A. Groundwater response to climate changes: Examples of observed and modeled trends on Tuscany aquifers (central Italy). In Atti del Convegno “XVII Giornata Mondiale dell’Acqua-Strategie di Adattamento Alla Domanda e Alla Disponibilità di Risorse Idriche”; Accademia dei Lincei: Roma, Italy, 2017. [Google Scholar]
- Caparrini, F.; Castelli, F.; Ercolani, G. Adattamento ed Implementazione del Modello Idrologico MOBIDIC per il Bilancio dei Bacini Idrografci e Dell’acquifero del Monte Amiata, Relazione Finale per Regione Toscana; Regione Toscana: Florence, Italy, 2011; p. 78. (In Italian) [Google Scholar]
- Bianchi, F.; Corti, F.; Vaselli, O.; Rappuoli, D. Bonifica area mineraria Lotto 6: Relazione conclusiva sulle indagini e studi degli anni 2011 e 2012. In Modalità Operative per la Bonifica; Technical Report, Municipality of Abbadia San Salvatore; Unità di Progetto Bonifica: Abbadia San Salvatore, Italy, 2012; p. 53. [Google Scholar]
- Lazzaroni, M. Investigating Hg Dispersion in the Environment: The Experience from the Abandoned Mining Area of Abbadia San Salvatore (Tuscany, Central Italy) between Research and Remediation Activities of Natural and Anthropic Matrices. Ph.D. Thesis, University of Florence, Florence, Italy, 2022; p. 213. [Google Scholar]
- Delany, J.M.; Lundeen, S.R. The LLNL Thermochemical Database: Revised Data and File Format for the EQ3/6 Package; Lawrence Livermore National Laboratory: Livermore, CA, USA, 1991. [Google Scholar]
- Ball, J.B.; Nordstrom, D.K. User’s Manual for WATEQ4F, with Revised Thermodynamic Data Base and Test Cases for Calculating Speciation of Major, Trace, and Redox Elements in Natural Water; Open-File Report 91-183; U.S. Geological Survey: Reston, VA, USA, 1991.
- Allison, J.D.; Brown, D.S.; Novo-Gradac, K.J. MINTEQA2/PRODEFA2—A Geochemical Assessment Model for Environmental Systems—Version 3.0 User’s Manual; Environmental Research Laboratory, Office of Research and Development U.S. Environmental Protection Agency: Athens, GA, USA, 1990.
- U.S. Environmental Protection Agency. MINTEQA2/PRODEFA2, A Geochemical Assessment Model for Environmental Systems—User Manual Supplement for Version 4.0; National Exposure Research Laboratory, Ecosystems Research Division: Athens, GA, USA, 1998.
- Johnston, S.G.; Bennett, W.W.; Doriean, N.; Hockmann, K.; Karimian, N.; Burton, E.D. Antimony and arsenic speciation, redox-cycling and contrasting mobility in a mining-impacted river system. Sci. Total Environ. 2020, 710, 136354. [Google Scholar] [CrossRef]
- Fournier, R.O.; Potter, I.I. Revised and expanded silica (quartz) geothermometer. Bull. Geotherm. Resour. Counc. 1982, 11, 3–12. [Google Scholar]
- Langevin, C.D.; Hughes, J.D.; Provost, A.M.; Russcher, M.J.; Niswonger, R.G.; Panday; Sorab; Merrick; Damian; Morway, E.D.; et al. MODFLOW 6 Modular Hydrologic Model version 6.4.2: U.S. Geological Survey Software Release; U.S. Geological Survey: Reston, VA, USA, 2023. [CrossRef]
- Franke, O.L.; Reilly, T.E.; Bennett, G.D. Definition of Boundary and Initial Conditions in the Analysis of Saturated Ground-Water Flow Systems: An Introduction. In Techniques of Water-Resources Investigations of the U.S. Geological Survey; USGS: Washington, DC, USA, 1987. [Google Scholar]
- Rappuoli, D. Bonifica del Terreno di Proprietà Comunale Interessato Dall’ex Sito Minerario di Abbadia San Salvatore; Technical Report, Municipality of Abbadia San Salvatore; Unità di Profetto di Bonifica: Abbadia San Salvatore, Italy, 2009; p. 120. [Google Scholar]
- Schofield, S.; Jankowski, J. Hydrochemistry and isotopic composition of Na-HCO3-rich groundwaters from the Ballimore region, central New South Wales, Australia. Chem. Geol. 2004, 211, 111–134. [Google Scholar] [CrossRef]
- Vaselli, O.; Lazzaroni, M.; Nisi, B.; Cabassi, J.; Tassi, F.; Rappuoli, D.; Meloni, F. Discontinuous geochemical monitoring of the galleria Italia circumneutral waters (former Hg-mining area of Abbadia San Salvatore, Tuscany, Central Italy) feeding the Fosso Della Chiusa Creek. Environments 2021, 8, 15. [Google Scholar] [CrossRef]
- Meloni, F.; Nisi, B.; Gozzi, C.; Rimondi, V.; Cabassi, J.; Montegrossi, G.; Rappuoli, D.; Vaselli, O. Background and geochemical baseline values of chalcophile and siderophile elements in soils around the former mining area of Abbadia San Salvatore (Mt. Amiata, southern Tuscany, Italy). J. Geochem. Explor. 2023, 255, 107324. [Google Scholar] [CrossRef]
- Ariya, P.A.; Amyot, M.; Dastoor, A.; Deeds, D.; Feinberg, A.; Kos, G.; Poulain, A.; Ryjkov, A.; Semeniuk, K.; Subir, M.; et al. Mercury Physicochemical and Biogeochemical Transformation in the Atmosphere and at Atmospheric Interfaces: A Review and Future Directions. Chem. Rev. 2015, 115, 3760–3802. [Google Scholar] [CrossRef]
- Barnett, M.O.; Turner, R.R.; Sanger, P.C. Oxidative dissolution of metacinnabar (b-HgS) by dissolved oxygen. Appl. Geochem. 2001, 16, 1499–1512. [Google Scholar] [CrossRef]
- Thanabalasingam, P.; Pickering, W.F. Specific sorption of antimony (III) by the hydrous oxides of Mn, Fe, and Al. Water Air Soil Pollut. 1990, 49, 175–185. [Google Scholar] [CrossRef]
- Masue, Y.; Loeppert, R.H.; Kramer, T.A. Arsenate and arsenite adsorption and desorption behavior on coprecipitated aluminum: Iron hydroxides. Environ. Sci. Technol. 2007, 41, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, Z.; He, M.; Meng, X.; Jin, X.; Qiu, N.; Zhang, J. Adsorption of antimony onto iron oxyhydroxides: Adsorption behavior and surface structure. J. Hazard. Mater. 2014, 276, 339–345. [Google Scholar] [CrossRef]
- Young, S.D. Chemistry of Heavy Metals and Metalloids in Soils. In Book Heavy Metals in Soils. Traces Metals and Metalloids in Soils and Their Bioavailability, 3rd ed.; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2013; Volume 22, pp. 51–95. [Google Scholar]
- Morel, F.M.; Kraepiel, A.M.; Amyot, M. The chemical cycle and bioaccumulation of mercury. Annu. Rev. Ecol. Syst. 1998, 29, 543–566. [Google Scholar] [CrossRef]
- Bagnato, E.; Parello, F.; Valenza, M.; Caliro, S. Mercury content and speciation in the Phlegrean Fields volcanic complex: Evidence from Hydrothermal system and fumaroles. J. Volcanol. Geotherm. Res. 2009, 189, 250–260. [Google Scholar] [CrossRef]
- Park, C.M.; Katz, L.E.; Liljestrand, H.W. Mercury speciation during in situ thermal desorption in soil. J. Hazrd. Mater. 2015, 300, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Seong, H.J.; Chon, C.M.; Park, J.H.; Nam, I.H.; Yoo, K.; Ahn, J.S. Interaction of Sb (III) with iron sulfide under anoxic conditions: Similarities and differences compared to As (III) interactions. Chemosphere 2018, 195, 762–770. [Google Scholar] [CrossRef] [PubMed]


| Sample ID | Year | T | pH | E.C. | Eh | Hydraulic Head | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | |
| S1N | Feb-13/Sep-23 | 5.6 | 15.5 | 12.8 | 13.1 | 6.6 | 11.3 | 8.5 | 7.8 | 122 | 1093 | 327.0 | 250.0 | −226 | 283 | 83.4 | 85.0 | 3.9 | 6.8 | 5.0 | 4.9 |
| S2EST | Feb-13/Sep-23 | 6.3 | 14.0 | 12.8 | 13.0 | 4.9 | 10.0 | 6.0 | 5.7 | 286 | 807 | 563.0 | 603.0 | −15 | 373 | 190.0 | 210.0 | 4.3 | 7.0 | 5.4 | 5.5 |
| S6N | Feb-13/Sep-23 | 4.8 | 14.1 | 12.5 | 12.9 | 6.2 | 9.6 | 7.5 | 7.4 | 220 | 1150 | 494.0 | 399.0 | −86 | 293 | 138.0 | 156.0 | 5.2 | 8.8 | 7.2 | 7.4 |
| S42 | May-13/Sep-23 | 4.9 | 14.5 | 11.6 | 11.5 | 5.7 | 7.6 | 6.7 | 6.7 | 280 | 780 | 526.0 | 540.0 | 18 | 293 | 142.0 | 172.5 | 3.3 | 6.4 | 4.6 | 4.6 |
| S60 | May-13/Sep-21 | 5.5 | 16.0 | 12.2 | 12.0 | 5.7 | 7.3 | 6.5 | 6.6 | 212 | 593 | 372.3 | 357.0 | 40 | 277 | 156.3 | 156.0 | 14.8 | 15.5 | 15.2 | 15.3 |
| S101 | Sep-15/sep-23 | 6.5 | 14.0 | 11.5 | 11.5 | 6.2 | 11.4 | 7.0 | 7.0 | 142 | 735 | 408.0 | 378.0 | −40 | 477 | 186.0 | 171.5 | 7.1 | 13.3 | 9.2 | 8.8 |
| S106 | Feb-13/Sep-23 | 5.1 | 14.0 | 10.9 | 11.0 | 5.4 | 7.6 | 6.7 | 6.6 | 169 | 690 | 389.0 | 370.5 | −18 | 268 | 132.3 | 128.0 | 3.5 | 7.2 | 4.5 | 4.3 |
| S108 | Feb-13/Sep-23 | 6.0 | 15.2 | 13.0 | 13.0 | 5.5 | 10.6 | 7.1 | 6.8 | 220 | 1120 | 756.0 | 839.5 | −140 | 863 | 174.0 | 150.0 | 3.7 | 8.9 | 5.4 | 5.3 |
| S109 | Feb-13/Sep-23 | 6.1 | 16.5 | 12.2 | 12.2 | 5.5 | 7.6 | 6.8 | 6.8 | 169 | 1066 | 692.0 | 701.0 | −2.6 | 302 | 161.0 | 167.0 | 5.9 | 9.6 | 8.1 | 8.2 |
| S111 | Feb-13/Sep-23 | 5.4 | 14.5 | 12.3 | 12.5 | 6.5 | 7.9 | 7.1 | 7.0 | 320 | 1039 | 731.1 | 759.0 | −1.7 | 266 | 142.5 | 149.0 | 4.6 | 6.6 | 5.4 | 5.4 |
| S113 | Feb-13/Sep-23 | 5.1 | 14.8 | 11.4 | 12.4 | 5.4 | 7.7 | 6.2 | 5.0 | 172 | 850 | 372.4 | 340.0 | 2.9 | 340 | 189.0 | 210.0 | 6.6 | 14.8 | 8.0 | 7.0 |
| S118 | Sep-14/Sep-23 | 5.4 | 14.8 | 12.2 | 12.7 | 5.9 | 7.9 | 6.6 | 6.5 | 199 | 738 | 534.3 | 572.0 | 31 | 290 | 154.7 | 151.5 | 5.9 | 6.9 | 6.4 | 6.4 |
| S119 | Sep-14/Sep-23 | 5.2 | 16.0 | 12.9 | 13.2 | 6.0 | 7.8 | 6.7 | 6.6 | 172 | 560 | 280.5 | 245.0 | 26 | 321 | 145.0 | 137.0 | 11.5 | 12.2 | 11.9 | 11.8 |
| S120 | Sep-14/Sep-23 | 4.7 | 15.0 | 12.1 | 12.1 | 4.4 | 7.3 | 5.5 | 5.4 | 220 | 1771 | 1147.5 | 1123.0 | 29 | 368 | 209.9 | 222.0 | 14.6 | 18.2 | 16.2 | 16.3 |
| S124 | Jan-15/Sep-23 | 5.3 | 14.0 | 12.1 | 12.8 | 5.4 | 7.8 | 6.1 | 5.9 | 309 | 2160 | 570.3 | 502.0 | 30 | 324 | 199.6 | 220.5 | 5.9 | 12.0 | 7.1 | 6.8 |
| S128 | Jan-15/Sep-23 | 6.0 | 14.0 | 11.7 | 12.0 | 5.5 | 7.6 | 6.3 | 6.1 | 196 | 540 | 360.8 | 383.0 | −38 | 218 | 76.7 | 44.0 | 2.8 | 6.5 | 4.8 | 5.0 |
| VIV | Sep-17/Sep-23 | 3.0 | 20.0 | 12.4 | 14.1 | 5.9 | 8.3 | 7.4 | 7.4 | 206 | 657 | 402.2 | 385.0 | −21 | 284 | 89.9 | 71.5 | - | - | - | - |
| VAL | Sep-17/Sep-23 | 3.0 | 23.0 | 11.4 | 12.7 | 5.9 | 8.6 | 7.2 | 7.2 | 130 | 520 | 287.6 | 272.0 | 29 | 284 | 128.8 | 119.0 | - | - | - | - |
| SR8 | Sep-14/Sep-23 | 3.7 | 18.8 | 11.9 | 12.3 | 5.8 | 8.4 | 7.3 | 7.2 | 130 | 757 | 458.3 | 402.0 | −2.6 | 263 | 111.4 | 116.0 | - | - | - | - |
| SR10A | Sep-14/Sep-23 | 4.4 | 14.5 | 11.5 | 12.0 | 5.9 | 7.7 | 6.7 | 6.7 | 109 | 530 | 225.9 | 200.0 | 27 | 291 | 151.5 | 164.0 | - | - | - | - |
| GRN | Sep-14/Sep-23 | 3.2 | 16.3 | 11.0 | 12.4 | 5.6 | 8.2 | 7.1 | 7.1 | 69 | 390 | 144.5 | 111.5 | 5.6 | 273 | 124.7 | 125.0 | - | - | - | - |
| GIT | Jan-09/Sep-23 | 7.6 | 19.4 | 14.9 | 15.0 | 5.5 | 7.0 | 6.2 | 6.2 | 736 | 1567 | 930.7 | 916.9 | n.d. | n.d. | n.d. | n.d. | - | - | - | - |
| Sample ID | Year | HCO3− | F− | Cl− | Br− | NO3− | SO42− | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | |
| S1N | Feb-13/Sep-23 | 31 | 152 | 97.6 | 97.7 | <0.1 | 0.5 | 0.3 | 0.3 | 2.4 | 29 | 9.0 | 6.4 | <0.1 | 1.9 | 0.7 | 0.4 | 0.9 | 16 | 3.8 | 2.9 | 6.1 | 160 | 45.0 | 31.0 |
| S2EST | Feb-13/Sep-23 | 1.2 | 87 | 25.8 | 19.5 | <0.1 | 2.0 | 0.6 | 0.4 | 5.7 | 27 | 12.0 | 10.0 | <0.1 | 0.8 | 0.3 | 0.3 | 3.1 | 39 | 12.0 | 10.0 | 44 | 450 | 248.0 | 259.0 |
| S6N | Feb-13/Sep-23 | 80 | 227 | 149.0 | 157.0 | 0.2 | 1.6 | 0.7 | 0.5 | 1.4 | 47 | 8.2 | 6.2 | <0.1 | 1.2 | 0.6 | 0.6 | 0.3 | 7.8 | 2.8 | 2.0 | 19 | 502 | 127.0 | 56.0 |
| S42 | May-13/Sep-23 | 49 | 244 | 114.0 | 8.0 | <0.1 | 0.6 | 0.3 | 0.2 | 2.9 | 14 | 7.8 | 8.1 | <0.1 | 0.9 | 0.6 | 0.6 | 4.0 | 20 | 13.0 | 13.5 | 23 | 234 | 141.0 | 154.0 |
| S60 | May-13/Sep-21 | 21 | 40 | 28.9 | 28.5 | <0.1 | 0.5 | 0.2 | 0.2 | 4.9 | 13 | 7.9 | 7.1 | <0.1 | 0.7 | 0.3 | 0.3 | 2.8 | 6.1 | 4.8 | 4.8 | 78 | 147 | 115.0 | 113.6 |
| S101 | Sep-15/sep-23 | 61 | 217 | 147.0 | 143.0 | <0.1 | 0.5 | 0.2 | 0.2 | 0.5 | 23 | 10.3 | 9.9 | <0.1 | 1.5 | 0.7 | 0.7 | <0.1 | 4.8 | 1.3 | 0.8 | 0.8 | 56 | 31.5 | 33.0 |
| S106 | Feb-13/Sep-23 | 31 | 152 | 76.2 | 59.0 | <0.1 | 0.4 | 0.1 | 0.1 | 4.6 | 47 | 10.9 | 8.9 | <0.1 | 1.3 | 0.6 | 0.5 | 6.3 | 18 | 10.9 | 9.9 | 27 | 177 | 100.0 | 100.0 |
| S108 | Feb-13/Sep-23 | 21 | 226 | 71.2 | 50.0 | <0.1 | 1.7 | 0.3 | 0.2 | 5.6 | 27 | 12.0 | 9.3 | <0.1 | 3.5 | 0.7 | 0.5 | <0.1 | 18 | 7.6 | 8.2 | 16 | 525 | 307.0 | 322.0 |
| S109 | Feb-13/Sep-23 | 26 | 188 | 126.0 | 126.5 | <0.1 | 1.3 | 0.3 | 0.2 | 10.5 | 32 | 16.9 | 16.2 | <0.1 | 1.0 | 0.5 | 0.3 | 1.5 | 18 | 12.0 | 12.5 | 71 | 274 | 203.0 | 213.0 |
| S111 | Feb-13/Sep-23 | 99 | 188 | 142.8 | 146.4 | <0.1 | 1.8 | 0.4 | 0.4 | 4.4 | 40 | 12.7 | 10.6 | <0.1 | 1.8 | 0.7 | 0.6 | <0.1 | 4.7 | 1.8 | 1.6 | 40 | 333 | 238.0 | 255.0 |
| S113 | Feb-13/Sep-23 | 6.0 | 39 | 23.7 | 23.2 | <0.1 | 1.1 | 0.3 | 0.3 | 6.8 | 22 | 12.2 | 11.8 | <0.1 | 0.8 | 0.5 | 0.4 | 0.5 | 15 | 10.5 | 10.8 | 69 | 140 | 104.4 | 103.0 |
| S118 | Sep-14/Sep-23 | 51 | 115 | 72.6 | 68.9 | <0.1 | 0.6 | 0.3 | 0.2 | 3.3 | 19 | 10.8 | 10.6 | <0.1 | 0.7 | 0.6 | 0.7 | 1.7 | 36 | 21.9 | 25.1 | 30 | 244 | 173.2 | 179.3 |
| S119 | Sep-14/Sep-23 | 35 | 73 | 46.5 | 46.0 | <0.1 | 0.6 | 0.2 | 0.2 | 4.7 | 21 | 8.6 | 8.4 | <0.1 | 1.1 | 0.6 | 0.6 | 0.1 | 8.7 | 2.0 | 1.7 | 30 | 134 | 68.8 | 60.2 |
| S120 | Sep-14/Sep-23 | 4.5 | 193 | 27.9 | 12.0 | <0.1 | 1.1 | 0.4 | 0.3 | 2.7 | 56 | 16.0 | 10.8 | <0.1 | 2.2 | 0.7 | 0.4 | 1.8 | 18 | 8.1 | 8.4 | 258 | 1398 | 706.6 | 665.5 |
| S124 | Jan-15/Sep-23 | 22 | 173 | 34.8 | 28.0 | <0.1 | 0.6 | 0.3 | 0.3 | 6.8 | 30 | 11.8 | 10.4 | <0.1 | 1.2 | 0.6 | 0.6 | 17 | 30 | 23.2 | 23.1 | 136 | 209 | 168.3 | 166.3 |
| S128 | Jan-15/Sep-23 | 12 | 117 | 34.7 | 26.8 | 0.1 | 0.8 | 0.4 | 0.3 | 2.3 | 11 | 6.6 | 6.2 | <0.1 | 1.5 | 0.7 | 0.5 | <0.1 | 5.9 | 1.1 | 0.6 | 42 | 162 | 127.2 | 132.4 |
| VIV | Sep-17/Sep-23 | 32 | 123 | 100.8 | 110.0 | <0.1 | 0.5 | 0.2 | 0.2 | 6.1 | 38 | 12.8 | 10.7 | <0.1 | 1.4 | 0.7 | 0.7 | 1.0 | 5.7 | 2.7 | 2.2 | 54 | 201 | 100.5 | 93.7 |
| VAL | Sep-17/Sep-23 | 42 | 188 | 81.3 | 73.0 | <0.1 | 0.8 | 0.3 | 0.2 | 6.0 | 16 | 9.6 | 9.0 | <0.1 | 2.5 | 0.7 | 0.4 | 0.6 | 5.3 | 2.0 | 1.8 | 16 | 161 | 49.8 | 35.7 |
| SR8 | Sep-14/Sep-23 | 24 | 204 | 118.9 | 99.0 | 0.1 | 1.5 | 0.4 | 0.3 | 6.1 | 74 | 15.4 | 11.0 | <0.1 | 0.6 | 0.3 | 0.2 | 0.4 | 13 | 3.8 | 2.7 | 26 | 210 | 104.0 | 97.5 |
| SR10A | Sep-14/Sep-23 | 21 | 51 | 29.8 | 28.6 | <0.1 | 0.4 | 0.2 | 0.2 | 4.2 | 16 | 9.7 | 9.3 | <0.1 | 0.5 | 0.3 | 0.3 | 1.0 | 8.4 | 3.4 | 3.0 | 30 | 75 | 52.7 | 51.0 |
| GRN | Sep-14/Sep-23 | 30 | 77 | 46.6 | 43.9 | <0.1 | 0.9 | 0.2 | 0.1 | 5.5 | 11 | 7.4 | 7.1 | 0.2 | 0.3 | 0.2 | 0.2 | <0.1 | 2.0 | 0.7 | 0.6 | 2.8 | 14 | 6.4 | 5.9 |
| GIT | Jan-13/Sep-23 | 67 | 192 | 128.5 | 120.0 | 0.1 | 2.0 | 0.9 | 0.8 | 1.6 | 34 | 13.7 | 12 | n.d. | n.d. | n.d. | n.d. | 0.1 | 1.6 | 0.3 | 0.2 | 338 | 586 | 473.6 | 475.4 |
| Sample ID | Year | Ca2+ | Mg2+ | Na+ | K+ | NH4+ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | |
| S1N | Feb-13/Sep-23 | 15 | 130.0 | 35.0 | 28.0 | 0.4 | 22 | 3.0 | 1.0 | 3.0 | 46 | 13.9 | 13.3 | 0.1 | 55 | 19.1 | 13.1 | <0.01 | 0.2 | 0.1 | 0.1 |
| S2EST | Feb-13/Sep-23 | 31 | 126 | 88.0 | 95.0 | 2.0 | 27 | 11.0 | 11.0 | 6.7 | 30 | 13.2 | 12.7 | 5.5 | 16 | 10.4 | 9.7 | <0.01 | 1.1 | 0.1 | 0.1 |
| S6N | Feb-13/Sep-23 | 26 | 270 | 84.0 | 65.0 | 0.1 | 10 | 3.0 | 2.0 | 0.2 | 18 | 9.1 | 8.6 | 8.9 | 39 | 20.4 | 20.1 | <0.01 | 0.5 | 0.1 | 0.1 |
| S42 | May-13/Sep-23 | 8.0 | 120 | 79.0 | 79.0 | 3.0 | 8.0 | 6.0 | 6.0 | 3.6 | 91 | 12.0 | 9.0 | 6.9 | 17 | 12.4 | 13.0 | <0.01 | 0.9 | 0.1 | 0.1 |
| S60 | May-13/Sep-21 | 16 | 32 | 25.2 | 26.0 | 9.6 | 16 | 12.8 | 13.0 | 11 | 16 | 13.0 | 12.6 | 3.6 | 14 | 10.6 | 10.9 | <0.01 | 0.4 | 0.1 | 0.1 |
| S101 | Sep-15/sep-23 | 20 | 95 | 56.8 | 53.0 | 0.5 | 4.0 | 3.0 | 3.0 | 1.1 | 10 | 6.0 | 5.6 | 1.6 | 25 | 4.0 | 3.3 | <0.01 | 0.4 | 0.1 | 0.1 |
| S106 | Feb-13/Sep-23 | 44 | 109 | 63.4 | 59.0 | 2.0 | 6.0 | 4.0 | 4.0 | 3.4 | 13 | 7.2 | 6.4 | 1.9 | 10 | 4.9 | 4.2 | <0.01 | 0.5 | 0.1 | 0.1 |
| S108 | Feb-13/Sep-23 | 12 | 170 | 104.5 | 114.0 | 0.6 | 43 | 21.5 | 21.0 | 9.5 | 32 | 16.5 | 17.3 | 10 | 28 | 19.3 | 20.0 | <0.01 | 0.5 | 0.2 | 0.1 |
| S109 | Feb-13/Sep-23 | 29 | 160 | 111.0 | 114.0 | 4.0 | 13 | 8.5 | 9.0 | 3.0 | 22 | 10.4 | 10.0 | 5.9 | 20 | 13.8 | 14.2 | <0.01 | 1.2 | 0.1 | 0.0 |
| S111 | Feb-13/Sep-23 | 30 | 150 | 89.8 | 94.4 | 8.6 | 41 | 27.8 | 30.0 | 8.4 | 42 | 17.0 | 16.9 | 7.1 | 23 | 13.9 | 14.7 | <0.01 | 0.1 | 0.1 | 0.1 |
| S113 | Feb-13/Sep-23 | 27 | 43 | 33.6 | 32.8 | 7.7 | 11 | 8.9 | 8.9 | 8.2 | 11 | 9.9 | 10.0 | 4.8 | 10 | 7.8 | 7.9 | <0.01 | 1.1 | 0.2 | 0.1 |
| S118 | Sep-14/Sep-23 | 37 | 98 | 63.0 | 62.8 | 2.5 | 28 | 17.5 | 18.6 | 1.7 | 15 | 9.5 | 9.6 | 4.7 | 29 | 19.3 | 20.0 | 0.01 | 1.4 | 0.2 | 0.1 |
| S119 | Sep-14/Sep-23 | 17 | 81 | 30.2 | 25.8 | 1.3 | 11 | 5.2 | 4.8 | 8.0 | 13 | 9.5 | 9.0 | 3.9 | 7.6 | 6.2 | 6.3 | <0.01 | 0.3 | 0.1 | 0.0 |
| S120 | Sep-14/Sep-23 | 106 | 534 | 266.9 | 241.6 | 4.1 | 25 | 12.5 | 12.3 | 5.8 | 12 | 8.3 | 8.2 | 9.4 | 59 | 28.1 | 22.2 | <0.01 | 1.0 | 0.1 | 0.1 |
| S124 | Jan-15/Sep-23 | 48 | 76 | 58.5 | 56.5 | 3.0 | 13 | 10.3 | 10.8 | 4.4 | 12 | 10.0 | 10.6 | 3.7 | 21 | 17.5 | 19.0 | 0.04 | 2.5 | 1.7 | 1.8 |
| S128 | Jan-15/Sep-23 | 9.9 | 48 | 36.0 | 36.4 | 4.1 | 13 | 9.4 | 10.2 | 7.8 | 21 | 12.8 | 12.6 | 0.1 | 13 | 6.9 | 6.9 | <0.01 | 1.6 | 0.3 | 0.2 |
| VIV | Sep-17/Sep-23 | 44 | 150 | 72.8 | 65.6 | 0.4 | 8.8 | 5.0 | 5.2 | 5.3 | 8.9 | 7.3 | 7.7 | 4.5 | 10 | 6.9 | 6.8 | 0.02 | 0.5 | 0.2 | 0.1 |
| VAL | Sep-17/Sep-23 | 24 | 73 | 41.0 | 33.5 | 0.5 | 6.4 | 3.5 | 3.5 | 5.2 | 8.5 | 6.7 | 6.6 | 2.6 | 7.0 | 4.5 | 4.1 | <0.01 | 1.0 | 0.1 | 0.1 |
| SR8 | Sep-14/Sep-23 | 33 | 119 | 73.0 | 68.1 | 2.5 | 17 | 6.5 | 5.7 | 6.5 | 13 | 8.8 | 8.4 | 3.5 | 9.3 | 5.5 | 4.8 | 0.01 | 0.6 | 0.2 | 0.1 |
| SR10A | Sep-14/Sep-23 | 13 | 34 | 19.7 | 19.1 | 1.8 | 6.9 | 5.3 | 5.6 | 4.3 | 12 | 8.4 | 8.5 | 1.8 | 11 | 5.5 | 5.3 | <0.01 | 0.1 | 0.0 | 0.0 |
| GRN | Sep-14/Sep-23 | 6.8 | 20 | 13.2 | 13.1 | 1.1 | 3.1 | 1.7 | 1.6 | 4.5 | 9.0 | 5.7 | 5.6 | 1.5 | 4.7 | 3.2 | 3.1 | <0.01 | 0.1 | 0.1 | 0.1 |
| GIT | Jan-13/Sep-23 | 167 | 243 | 193.1 | 191 | 16.5 | 34 | 22.7 | 22.9 | 7.5 | 15 | 11.5 | 11.3 | 5.0 | 13 | 9.4 | 9.5 | 0.02 | 3.5 | 0.8 | 0.2 |
| Sample ID | Year | As | Sb | Hg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | Min | Max | Mean | Median | Min | Max | Mean | Median | Min | Max | Mean | Median | |
| S1N | Feb-13/Sep-23 | 0.3 | 5.8 | 1.8 | 1.3 | 0.3 | 17 | 4.8 | 3.6 | <0.1 | 22 | 3.1 | 1.2 |
| S2EST | Feb-13/Sep-23 | 0.5 | 13 | 1.6 | 1.3 | <0.1 | 1.5 | 0.4 | 0.1 | 3.0 | 456 | 84.1 | 55.4 |
| S6N | Feb-13/Sep-23 | 0.5 | 15 | 5.2 | 4.6 | 8.8 | 37 | 22.3 | 21.1 | 4.6 | 695 | 261.6 | 270.7 |
| S42 | May-13/Sep-23 | 0.1 | 1.6 | 0.7 | 0.7 | <0.1 | 8.9 | 1.2 | 0.5 | <0.1 | 26 | 12.6 | 11.7 |
| S60 | May-13/Sep-21 | 1.0 | 1.8 | 1.3 | 1.3 | <0.1 | 2.7 | 0.4 | 0.2 | 24 | 99 | 42.5 | 37.4 |
| S101 | Sep-15/sep-23 | 0.2 | 2.4 | 1.4 | 1.5 | <0.1 | 2.0 | 0.5 | 0.4 | <0.1 | 2.9 | 0.7 | 0.5 |
| S106 | Feb-13/Sep-23 | <0.1 | 2.7 | 0.8 | 0.4 | <0.1 | 13 | 3.5. | 0.3 | 0.3 | 72 | 29.5 | 29.8 |
| S108 | Feb-13/Sep-23 | 0.6 | 8.6 | 2.1 | 1.9 | <0.1 | 1.2 | 0.3 | 0.2 | <0.1 | 38 | 3.2 | 1.2 |
| S109 | Feb-13/Sep-23 | 0.7 | 1.7 | 1.2 | 1.2 | <0.1 | 2.9 | 0.5 | 0.1 | <0.1 | 2.6 | 0.6 | 0.4 |
| S111 | Feb-13/Sep-23 | 0.5 | 6.7 | 2.2 | 2.2 | <0.1 | 2.5 | 0.5 | 0.3 | <0.1 | 2.6 | 0.6 | 0.4 |
| S113 | Feb-13/Sep-23 | 0.6 | 1.5 | 1.2 | 1.2 | <0.1 | 1.2 | 0.3 | 0.2 | 9.6 | 98 | 45.4 | 49.0 |
| S118 | Sep-14/Sep-23 | 0.9 | 4.0 | 2.1 | 1.9 | <0.1 | 16 | 2.8 | 0.6 | 0.5 | 64 | 23.3 | 25.2 |
| S119 | Sep-14/Sep-23 | 5.5 | 8.6 | 6.6 | 6.5 | <0.1 | 0.9 | 0.2 | 0.2 | <0.1 | 3.3 | 1.0 | 0.6 |
| S120 | Sep-14/Sep-23 | <0.1 | 3.9 | 1.3 | 1.0 | <0.1 | 2.2 | 0.5 | 0.2 | <0.1 | 9.1 | 1.8 | 0.9 |
| S124 | Jan-15/Sep-23 | 0.6 | 2.1 | 1.7 | 1.7 | <0.1 | 18 | 1.6 | 0.1 | 3.1 | 299 | 208.6 | 225.0 |
| S128 | Jan-15/Sep-23 | <0.1 | 2.1 | 1.1 | 1.1 | <0.1 | 1.5 | 0.3 | 0.1 | <0.1 | 6.7 | 1.5 | 0.9 |
| VIV | Sep-17/Sep-23 | 1.8 | 5.2 | 3.2 | 3.0 | 0.3 | 1.2 | 0.7 | 0.6 | <0.1 | 2.5 | 0.5 | 0.3 |
| VAL | Sep-17/Sep-23 | 1.2 | 8.1 | 3.5 | 2.7 | <0.1 | 1.4 | 0.7 | 0.6 | <0.1 | 2.5 | 1.1 | 0.9 |
| SR8 | Sep-14/Sep-23 | 1.5 | 6.8 | 4.0 | 4.1 | <0.1 | 1.8 | 0.4 | 0.3 | 0.1 | 10 | 2.8 | 1.7 |
| SR10A | Sep-14/Sep-23 | 1.5 | 2.9 | 2.1 | 2.1 | <0.1 | 0.9 | 0.2 | 0.1 | 3.6 | 29 | 12.9 | 11.7 |
| GRN | Sep-14/Sep-23 | 0.9 | 3.8 | 1.7 | 1.7 | 0.2 | 1.2 | 0.4 | 0.4 | <0.1 | 26 | 1.4 | 0.2 |
| GIT | Jan-13/Sep-23 | 7.0 | 12 | 9.4 | 9.4 | <0.1 | 0.6 | 0.4 | 0.4 | <0.1 | 2.2 | 0.4 | 0.2 |
| Sample ID | Year | Al | Fe | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Range | Min | Max | Mean | Median | Min | Max | Mean | Median | |
| S1N | Sep-21/Sep-23 | 40 | 3877 | 1390.5 | 196.0 | 3.4 | 85 | 22.7 | 9.9 |
| S2EST | Sep-21/Sep-23 | 86 | 577 | 266.6 | 249.9 | 4.0 | 15 | 8.5 | 8.4 |
| S6N | Sep-21/Sep-23 | 13 | 49 | 22.0 | 16.4 | 3.5 | 472 | 87.2 | 29.7 |
| S42 | Sep-21/Sep-23 | 2.5 | 86 | 27.1 | 6.0 | 1.0 | 64 | 13.7 | 5.0 |
| S101 | Sep-21/Sep-23 | 0.1 | 74 | 16.4 | 7.2 | 0.9 | 21 | 5.5 | 2.0 |
| S106 | Sep-21/Sep-23 | 21 | 23 | 22.1 | 22.1 | 3.9 | 6.0 | 5.0 | 5.0 |
| S108 | Sep-21/Sep-23 | 7.8 | 48 | 19.3 | 12.1 | 3.2 | 63 | 18.9 | 6.5 |
| S109 | Sep-21/Sep-23 | 4.6 | 69 | 17.8 | 9.4 | 1.3 | 20 | 7.4 | 3.0 |
| S111 | Sep-21/Sep-23 | 6.4 | 27 | 11.4 | 8.0 | 2.7 | 604 | 96.6 | 7.0 |
| S113 | Sep-21/Sep-23 | 28 | 40 | 33.7 | 35.2 | 1.9 | 13 | 5.3 | 4.0 |
| S118 | Sep-21/Sep-23 | 6.7 | 107 | 30.6 | 23.2 | 6.0 | 51 | 22.8 | 14.3 |
| S119 | Sep-21/Sep-23 | 3.5 | 12 | 6.7 | 5.5 | 1.6 | 6.7 | 3.3 | 2.6 |
| S120 | Sep-21/Sep-23 | 1021 | 1434 | 1224.3 | 1207.0 | 7.1 | 112 | 26.2 | 14.0 |
| S124 | Sep-21/Sep-23 | 0.1 | 19 | 11.4 | 12.5 | 0.4 | 8.0 | 5.6 | 6.1 |
| S128 | Sep-21/Sep-23 | 7.0 | 38 | 14.0 | 9.0 | 16 | 11,832 | 8136.9 | 10,839.4 |
| VIV | Sep-21/Sep-23 | 13 | 96 | 36.8 | 22.5 | 22 | 113 | 65.1 | 56.7 |
| VAL | Sep-21/Sep-23 | 13 | 65 | 32.6 | 33.2 | 5.4 | 102 | 48.4 | 39.4 |
| SR8 | Sep-21/Sep-23 | 16 | 64 | 36.3 | 33.1 | 4.1 | 138 | 42.6 | 15.0 |
| SR10A | Sep-21/Sep-23 | 12 | 49 | 23.7 | 18.6 | 1.6 | 13 | 4.9 | 3.1 |
| GRN | Sep-21/Sep-23 | 6.6 | 30 | 15.0 | 14.3 | 2.7 | 212 | 83.7 | 79.0 |
| GIT | Sep-21/Sep-23 | 149 | 6596 | 701.8 | 346.0 | 5876 | 32,100 | 17,928.3 | 17,642.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, F.; Montegrossi, G.; Cabassi, J.; Bianchi, F.; Nisi, B.; Rappuoli, D.; Vaselli, O. Geochemical Surveys of Ground and Surface Waters in the Abandoned Hg-Mine of Abbadia San Salvatore (Central Italy): A Preparatory Investigation before Remediation. Water 2024, 16, 1210. https://doi.org/10.3390/w16091210
Meloni F, Montegrossi G, Cabassi J, Bianchi F, Nisi B, Rappuoli D, Vaselli O. Geochemical Surveys of Ground and Surface Waters in the Abandoned Hg-Mine of Abbadia San Salvatore (Central Italy): A Preparatory Investigation before Remediation. Water. 2024; 16(9):1210. https://doi.org/10.3390/w16091210
Chicago/Turabian StyleMeloni, Federica, Giordano Montegrossi, Jacopo Cabassi, Francesco Bianchi, Barbara Nisi, Daniele Rappuoli, and Orlando Vaselli. 2024. "Geochemical Surveys of Ground and Surface Waters in the Abandoned Hg-Mine of Abbadia San Salvatore (Central Italy): A Preparatory Investigation before Remediation" Water 16, no. 9: 1210. https://doi.org/10.3390/w16091210
APA StyleMeloni, F., Montegrossi, G., Cabassi, J., Bianchi, F., Nisi, B., Rappuoli, D., & Vaselli, O. (2024). Geochemical Surveys of Ground and Surface Waters in the Abandoned Hg-Mine of Abbadia San Salvatore (Central Italy): A Preparatory Investigation before Remediation. Water, 16(9), 1210. https://doi.org/10.3390/w16091210









