As and Pb Presence within the Meoqui-Delicias Aquifer, Chihuahua, Mexico
Abstract
1. Introduction
2. Materials and Methods
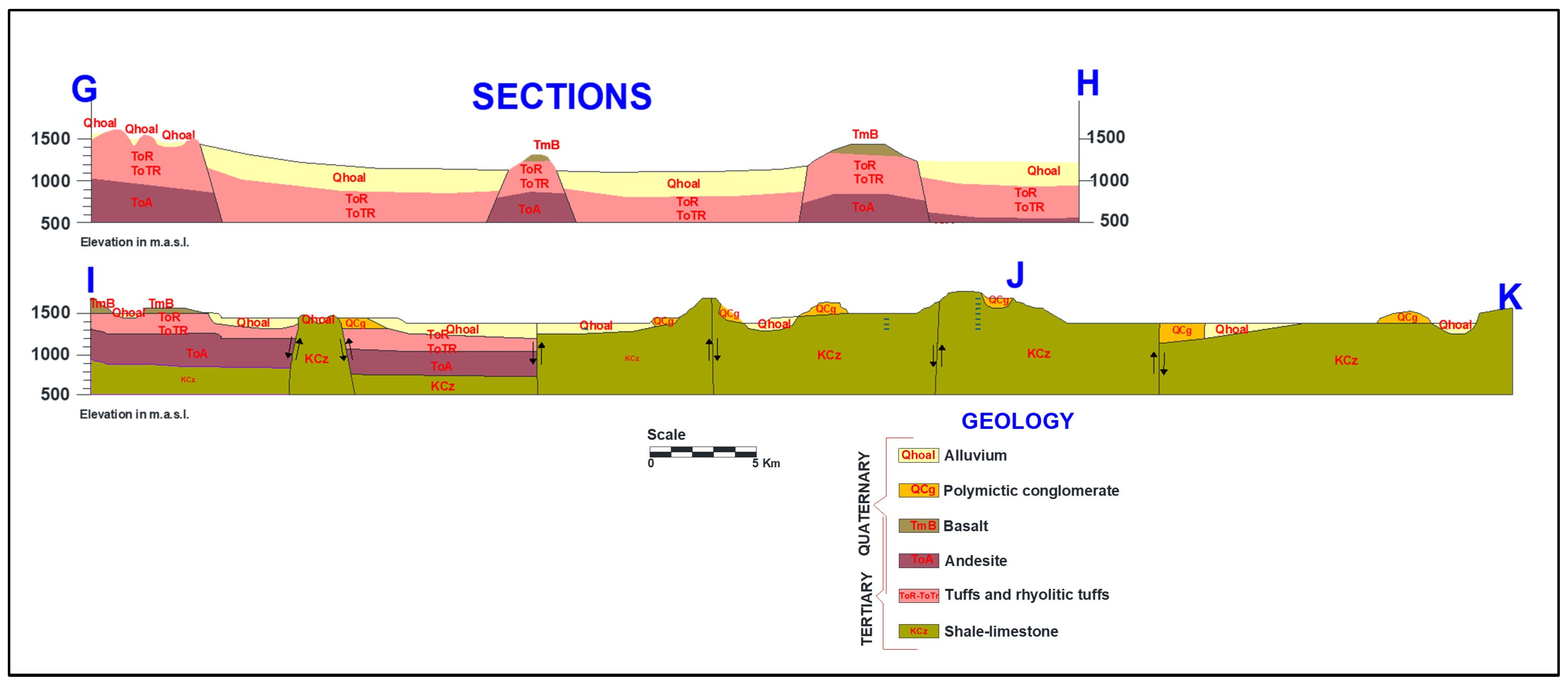
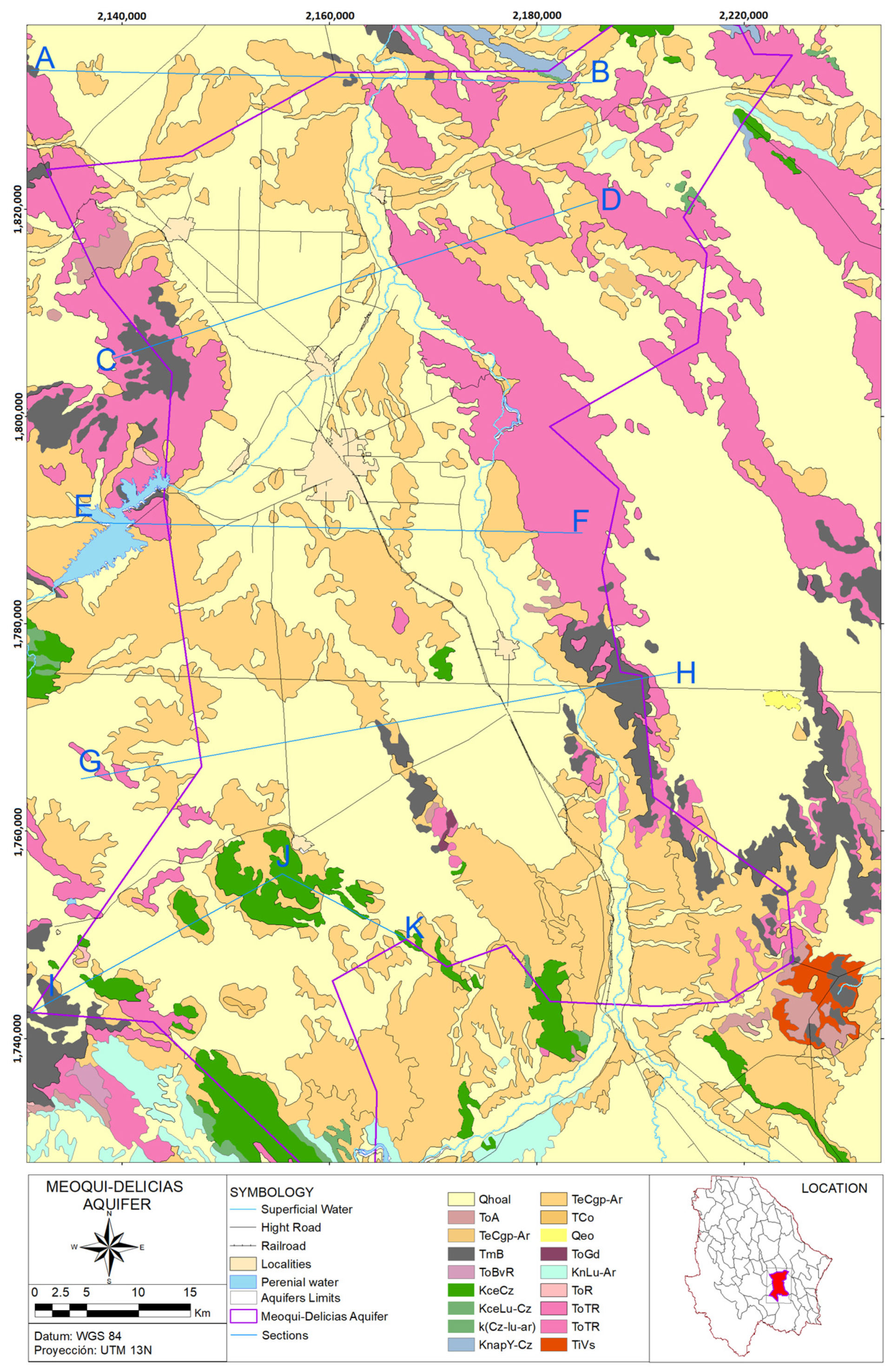
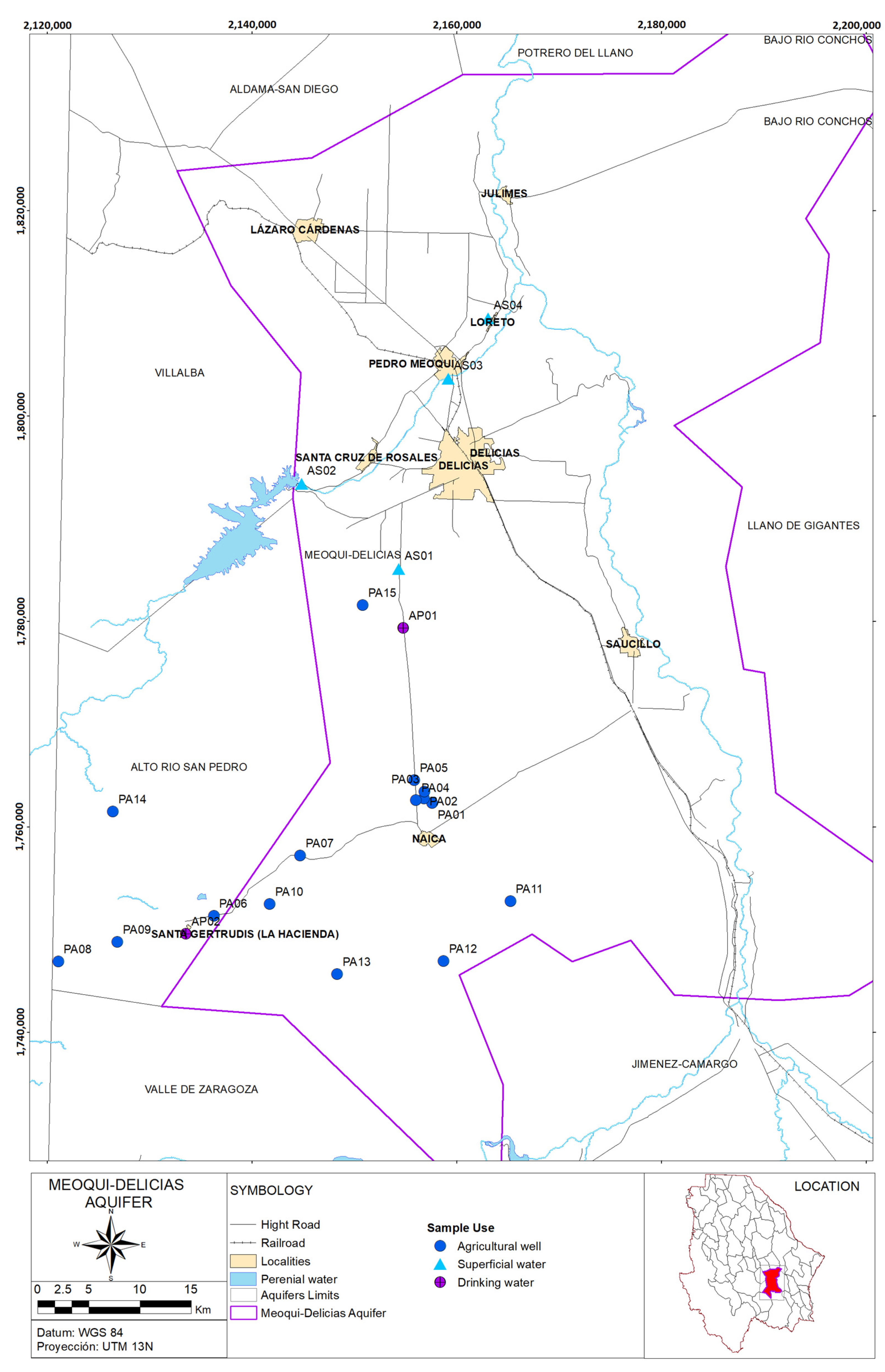
Area of Study


- The drainpipe valve was opened, and the water was allowed to run for about 3 min.
- To avoid interference with the sample, the container was opened near the outlet hole, and the stopper was held down.
- The sample was taken quickly, leaving free space to allow agitation.
- The samples were stored in a cooler and kept at a temperature of about 4 °C.
- Samples were identified with the following data:
- Sample name
- Date and time
- Location coordinates of the sampling point and observations.
- A measures of 100 mL of each sample is taken, which must be at room temperature to avoid variations in volume.
- A measure of 45 mL of sample is taken and placed in a beaker (previously washed with deionized water).
- Add 5 mL of nitric acid (HNO3) and mix.
- The samples are left for 2 h on the electric grill at a temperature of 90 °C. The temperature should not be exceeded because some elements to be analyzed may volatilize.
- Filter the samples into a 100 mL flask.
- After filtering, make up to 100 mL.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| No. | ID | X | Y | Spring | Summer | Autumn | Winter | Use |
|---|---|---|---|---|---|---|---|---|
| 1 | PA01 | 452,097 | 3,084,798 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 10 January 2020 | Agricultural |
| 2 | PA02 | 451,301 | 3,085,203 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 10 January 2020 | Agricultural |
| 3 | PA03 | 451,306 | 3,085,900 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 10 January 2020 | Agricultural |
| 4 | PA04 | 450,511 | 3,085,031 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 10 January 2020 | Agricultural |
| 5 | PA05 | 450,301 | 3,087,001 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 21 January 2020 | Agricultural |
| 6 | AP01 | 448,932 | 3,101,792 | 28 May 2019 | 14 August 2019 | 10 October 2019 | 21 January 2020 | Drinking Water |
| 7 | AP02 | 428,273 | 3,071,545 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 10 January 2020 | Drinking Water |
| 8 | PA06 | 430,944 | 3,073,332 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 10 January 2020 | Agricultural |
| 9 | PA07 | 439,286 | 3,079,426 | 9 May 2019 | 8 August 2019 | 3 October 2019 | 21 January 2020 | Agricultural |
| 10 | PA08 | 415,804 | 3,068,585 | 28 May 2019 | 8 August 2019 | 3 October 2019 | 21 January 2020 | Agricultural |
| 11 | PA09 | 421,566 | 3,070,606 | 28 May 2019 | 8 August 2019 | NA | 21 January 2020 | Agricultural |
| 12 | PA10 | 436,382 | 3,074,631 | 28 May 2019 | 14 August 2019 | 8 October 2019 | 10 January 2020 | Agricultural |
| 13 | PA11 | 459,947 | 3,075,377 | 28 May 2019 | 14 August 2019 | 8 October 2019 | 10 January 2020 | Agricultural |
| 14 | PA12 | 453,510 | 3,069,379 | 28 May 2019 | 14 August 2019 | 8 October 2019 | 10 January 2020 | Agricultural |
| 15 | PA13 | 443,118 | 3,067,889 | 28 May 2019 | 14 August 2019 | 8 October 2019 | 10 January 2020 | Agricultural |
| 16 | PA14 | 420,866 | 3,083,338 | NA | 8 August 2019 | NA | 21 January 2020 | Agricultural |
| 17 | PA15 | 444,909 | 3,103,926 | NA | 8 August 2019 | 8 October 2019 | 21 January 2020 | Agricultural |
| 18 | AS01 | 448,389 | 3,107,565 | NA | 19 August 2019 | NA | NA | Agricultural |
| 19 | AS02 | 438,688 | 3,115,681 | NA | 19 August 2019 | 10 October 2019 | 21 January 2020 | Agricultural |
| 20 | AS03 | 452,841 | 3,126,203 | NA | 19 August 2019 | 10 October 2019 | 21 January 2020 | Agricultural |
| 21 | AS04 | 456,599 | 3,132,179 | NA | 19 August 2019 | NA | 21 January 2020 | Agricultural |
References
- Padilla-Reyes, D.A.; Dueñas-Moreno, J.; Mahlknecht, J.; Mora, A.; Kumar, M.; Ornelas-Soto, N.; Mejía-Avendaño, S.; Navarro-Gómez, C.J.; Bhattacharya, P. Arsenic and fluoride in groundwater triggering a high risk: Probabilistic results using Monte Carlo simulation and species sensitivity distribution. Chemosphere 2024, 359, 142305. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.; Reza AH, M.S.; Hasan, M.I.; Ahsan, M.A.; Moniruzzaman, M.; Hasan, A.B.; Shofi, S.I.; Hridoy, K.M. Evaluating arsenic contamination in northwestern Bangladesh: A GIS-Based assessment of groundwater vulnerability and human health impacts. Heliyon 2024, 10, e27917. [Google Scholar] [CrossRef] [PubMed]
- Dechdacho, P.; Howard, S.; Hershey, R.L.; Parashar, R.; Perez, L.J. Effective removal of arsenic from contaminated groundwater using an iron-based metal-organic framework. Environ. Technol. Innov. 2023, 32, 103406. [Google Scholar] [CrossRef]
- Sumdang, N.; Chotpantarat, S.; Cho, K.H.; Thanh, N.N. The risk assessment of arsenic contamination in the urbanized coastal aquifer of Rayong groundwater basin, Thailand using the machine learning approach. Ecotoxicol. Environ. Saf. 2023, 253, 114665. [Google Scholar] [CrossRef] [PubMed]
- Vega Gleason, S. Riesgos ambientales y salud. Temas Sel. Salud Derecho 2002, 94, 165. [Google Scholar]
- Barbier, E. The Water Paradox: Overcoming the Global Crisis in Water Management; Yale University Press: New Haven, CT, USA, 2019; Volume 76. [Google Scholar]
- Stone, A.; Lanzoni, M.; Smedley, P. Groundwater Resources: Past, Present, and Future; Dadson, S.J., Garrick, D.E., Penning-Rowsell, E.C., Hall, J.W., Hope, R., Hughes, J., Eds.; Water Science, Policy, and Management: A Global Challenge; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019. [Google Scholar]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic contamination of groundwater: A global synopsis with focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Arreguín Cortés, F.I.; Chávez Guillén, R.; Soto Navarro, P.R.; Smedley, P.L. Una revisión de la presencia de arsénico en el agua subterránea en México. Rev. Tlaloc AMH 2010, 45, 1–11. [Google Scholar]
- Liu, F.; Huang, G.; Sun, J.; Jing, J.; Zhang, I. Distribution of arsenic in shallow aquifers of Guangzhou region, China: Natural and anthropogenic impacts. Water Qual. Res. J. Can. 2014, 49, 354–371. [Google Scholar] [CrossRef]
- Kong, H.; Teng, Y.; Song, L.; Wang, J.; Zhang, L. Lead and strontium isotopes as tracers to investigate the potential sources of lead in soil and groundwater: A case study of the Hun River alluvial fan. Appl. Geochem. 2018, 97, 291–300. [Google Scholar] [CrossRef]
- Toyoda, K.; Nakano, S.; Tanaka, S.; Banda, K.; Nyambe, I.A.; Ishikawa, T.; Nakayama, S.; Ishizuka, M. Geochemical identification of particulate lead pollution in shallow groundwater in inhabited areas in Kabwe, Zambia. Appl. Geochem. 2022, 139, 105215. [Google Scholar] [CrossRef]
- Huang, C.; Guo, Z.; Li, T.; Xu, R.; Peng, C.; Gao, Z.; Zhong, L. Source identification and migration fate of metal(loid)s in soil and groundwater from an abandoned Pb/Zn mine. Sci. Total Environ. 2023, 895, 165037. [Google Scholar] [CrossRef]
- García Amaro, E. Modificaciones al Sistema de Clasificación Climática de Köppen, 5th ed.; Instituto de Geografía. de la Universidad Nacional Autónoma de México: Ciudad de México, México, 2004. [Google Scholar]
- CNA. Actualización de la Disponibilidad Media Anual de Agua en el Acuífero Meoqui-Delicias (0831), Estado de Chihuahua; Comisión Nacional del Agua (CNA): Alcaldía Coyoacán, Mexico, 2020.
- Villalobos Gutiérrez, M.N. Evolución Espacial y Temporal de la Presencia de Nitratos en el Acuífero Meoqui-Delicias, Chihuahua. Ph.D. Thesis, Universidad Autonoma de Chihuahua, Chihuahua, Mexico, 2021. [Google Scholar]
- Silva Hidalgo, H. Modelo Matemático Para la Distribución de Agua Superficial en Cuencas Hidrológicas. Ph.D. Thesis, Centro de Investigación en Materiales Avanzados, S.C. (CIMAV), Chihuahua, Mexico, 2010. [Google Scholar]
- CNA. Estudio Hidrogeológico, Hidrogeoquímico y de la Incidencia de Arsénico, Flúor y Hierro en las Zonas Acuíferas de Meoqui-Delicias y Jiménez-Camargo en el estado de Chihuahua, México; Comisión Nacional del Agua (CNA): Alcaldía Coyoacán, Mexico, 1997.
- CNA. Muestreo del Agua de los Acuíferos Jiménez-Camargo, Meoqui-Delicias, para el Estudio Hidrogeoquímico y de Calidad Natural, así como la Localización y Diseño de las Fuentes Alternas; Comisión Nacional del Agua (CNA): Alcaldía Coyoacán, Mexico, 1998.
- Espino Valdés, M.S.; Barrera Prieto, Y.; Herrera Peraza, E. Presencia de arsénico en la sección norte del acuífero Meoqui-Delicias del estado de Chihuahua, México. Tecnociencia Chihuah. 2009, III, 8–18. [Google Scholar]
- SGM (Servicio Geológico Mexicano). Carta Geológica Ciudad Delicias H13-11; SGM (Servicio Geológico Mexicano): Pachuca, Mexico, 2000.
- Instituto Nacional de Estadística y Geografía (INEGI). Carta Geológica Ciudad Delicias H13-11; Instituto Nacional de Estadística y Geografía (INEGI): Aguascalientes, Mexico, 1983.
- Instituto Nacional de Estadística y Geografía (INEGI). Carta Geológica Ciudad Camargo G13-2; Instituto Nacional de Estadística y Geografía (INEGI): Aguascalientes, Mexico, 1990.
- Instituto Nacional de Estadística y Geografía (INEGI). Carta Topográfica Delicias H1311; Instituto Nacional de Estadística y Geografía (INEGI): Aguascalientes, Mexico, 2017.
- Aranda Meléndez, G. Estudio Geológico de los Yacimientos Minerales de Naica, Chihuahua . 1980. Available online: https://repositorioinstitucional.uaslp.mx/xmlui/bitstream/handle/i/3238/IGE1YMN98001.pdf?sequence=4 (accessed on 19 February 2021).
- Franco Rubio, M. Estratigrafía del Albiano-Cenomaniano en la Región de Naica, Chihuahua. UNAM Inst. Geol. 1978, 2, 132–149. [Google Scholar]
- Instituto Nacional de Estadística y Geografía. Carta Topográfica Santa Rosalía de Camargo G1302; Instituto Nacional de Estadística y Geografía (INEGI): Aguascalientes, Mexico, 2017.
- Secretaria de Salud; Norma Oficial Mexicana. NOM-014-SSA1-1993, Procedimientos Sanitarios Para el Muestreo de Agua Para uso y Consumo Humano en Sistemas de Abastecimiento de Agua Públicos y Privados; Norma Oficial Mexicana-Secretaría de Salud: Ciudad de México, Mexico, 1993.
- Golden Software, Inc. Surfer Version 11; Golden Software, Inc.: Golden, CO, USA, 2013. [Google Scholar]
- Secretaria de Salud; NORMA Oficial Mexicana. NOM-127-SSA1-1994, Salud Ambiental, Agua Para Uso y Consumo humano-Límites Permisibles de Calidad y Tratamientos a Que Debe Someterse el Agua Para su Potabilización; Última Reforma Publicada DOF 03-02-1995; Norma Oficial Mexicana-Secretaría de Salud: Ciudad de México, Mexico, 1994; pp. 1–7.
- Secretaria de Salud. MODIFICACIÓN a la Norma Oficial Mexicana. NOM-127-SSA1-1994, Salud Ambiental. Agua Para Uso y Consumo Humano. Límites Permisibles de Calidad y Tratamientos a que debe Someterse el Agua Para su Potabilización; Norma Oficial Mexicana-Secretaría de Salud: Ciudad de México, Mexico, 1998.
- SEMARNAT, Secretaría de Medio Ambiente y Recursos Naturales. NOM-001-SEMARNAT-1996 Límites Máximos Permisibles De Contaminantes en las Descargas de Aguas Residuales en Aguas y Bienes Nacionales; Norma Oficial Mexicana: Ciudad de México, Mexico, 1998; Volume 33.
- CNA. Actualización del Estudio Geohidrológico del Acuífero Meoqui-Delicias, Chihuahua; Comisión Nacional del Agua (CNA): Alcaldía Coyoacán, Mexico, 2005.
- Espinoza, M. Distribución de la Contaminación Natural por Arsénico en las Aguas Subterráneas de la Subcuenca Suroeste del Valle de Sebaco, Matagalpa-Nicaragua. Master’s Thesis, Universidad Nacional Autónoma de Nicaragua, Managu, Nicaragua, 2005; p. 149. Available online: http://repositorio.unan.edu.ni/2378/ (accessed on 7 July 2021).
- Rascon Beltran, B.E. Estudio Hidrogeoquimico y de Vulnerabilidad a la Contmainacion de la Porcion sur del Acuifero Meoqui-Delicias del Estado de Chihuahua. Ph.D. Thesis, Universidad Autónoma de Chihuahua, Chihuahua, Mexico, 2011; p. 150. [Google Scholar]
- Rodriguez Carmona, K.Y. Estudio de la Calidad de Agua Proveniente de Pozos del Acuífero Meoqui—Delicias; Chihuahua, México. 2019. Available online: http://cimav.repositorioinstitucional.mx/jspui/handle/1004/624 (accessed on 19 February 2021).
- Rango, T.; Bianchini, G.; Beccaluva, L.; Tassinari, R. Geochemistry and water quality assessment of central Main Ethiopian Rift natural waters with emphasis on source and occurrence of fluoride and arsenic. J. Afr. Earth Sci. 2010, 57, 479–491. [Google Scholar] [CrossRef]
- Peccerillo, A. A high-pressure experimental study on the evolution of the silicic magmatism of the Main Ethiopian Rift. Lithos 2006, 91, 46–58. [Google Scholar]
- SEMARNAT, Secretaría de Medio Ambiente y Recursos Naturales; NORMA Oficial Mexicana. NOM-001-SEMARNAT-1996, que Establece los Límites Máximos Permisibles de Contaminantes en las Descargas Residuales y Bienes Nacionales; Secretaría de Medio Ambiente y Recursos Naturales: Ciudad de México, Mexico, 1996.
- Consejo de Recursos Minerales. Monografía Geológico-Minera del Estado de Chihuahua; Secretaria De Energia: Ciudad de México, Mexico, 1994.
- SGM (Servicio Geológico Mexicano). Panorama Minero del Estado de Chihuahua; SGM (Servicio Geológico Mexicano): Pachuca, Mexico, 2021.

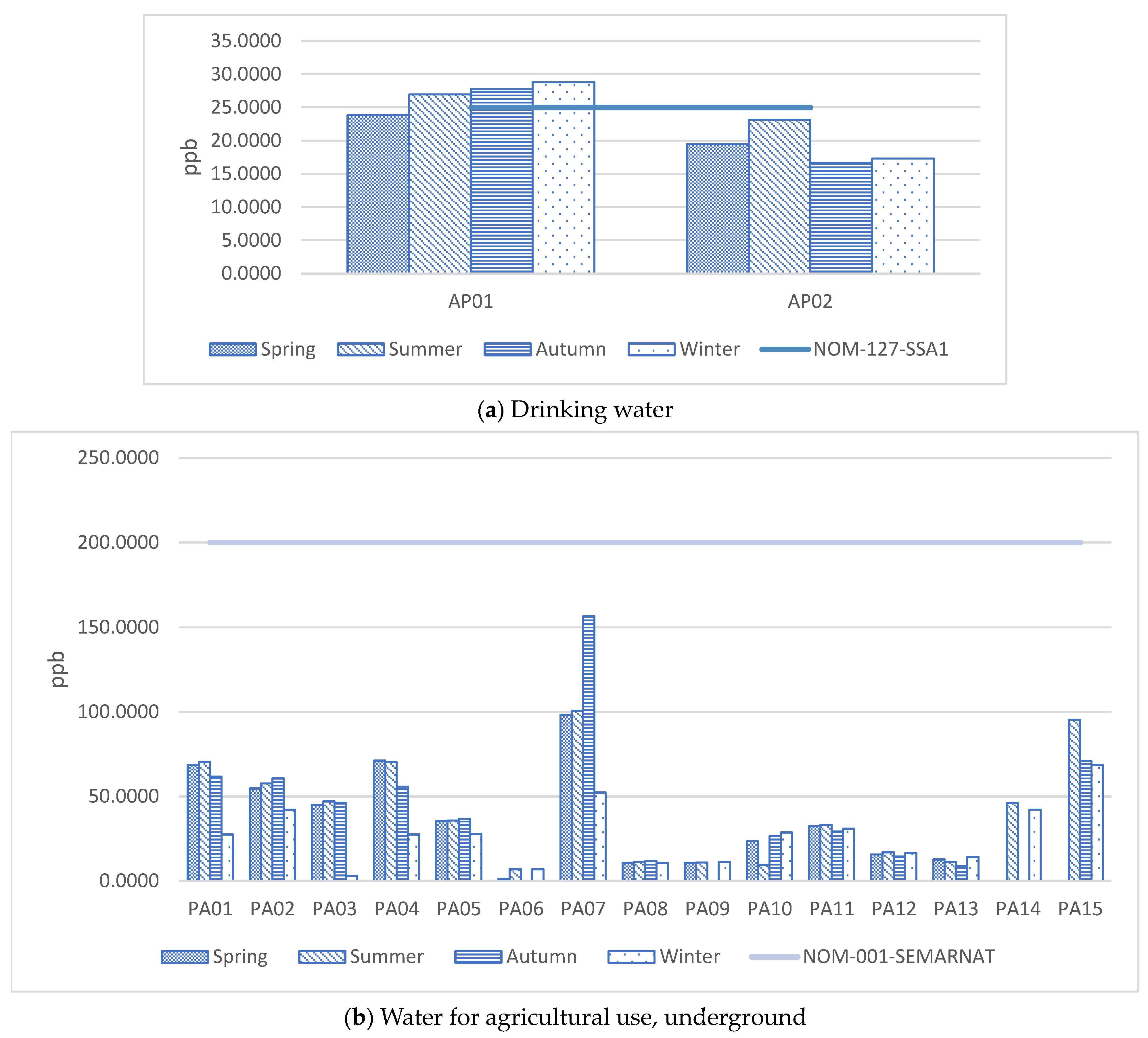
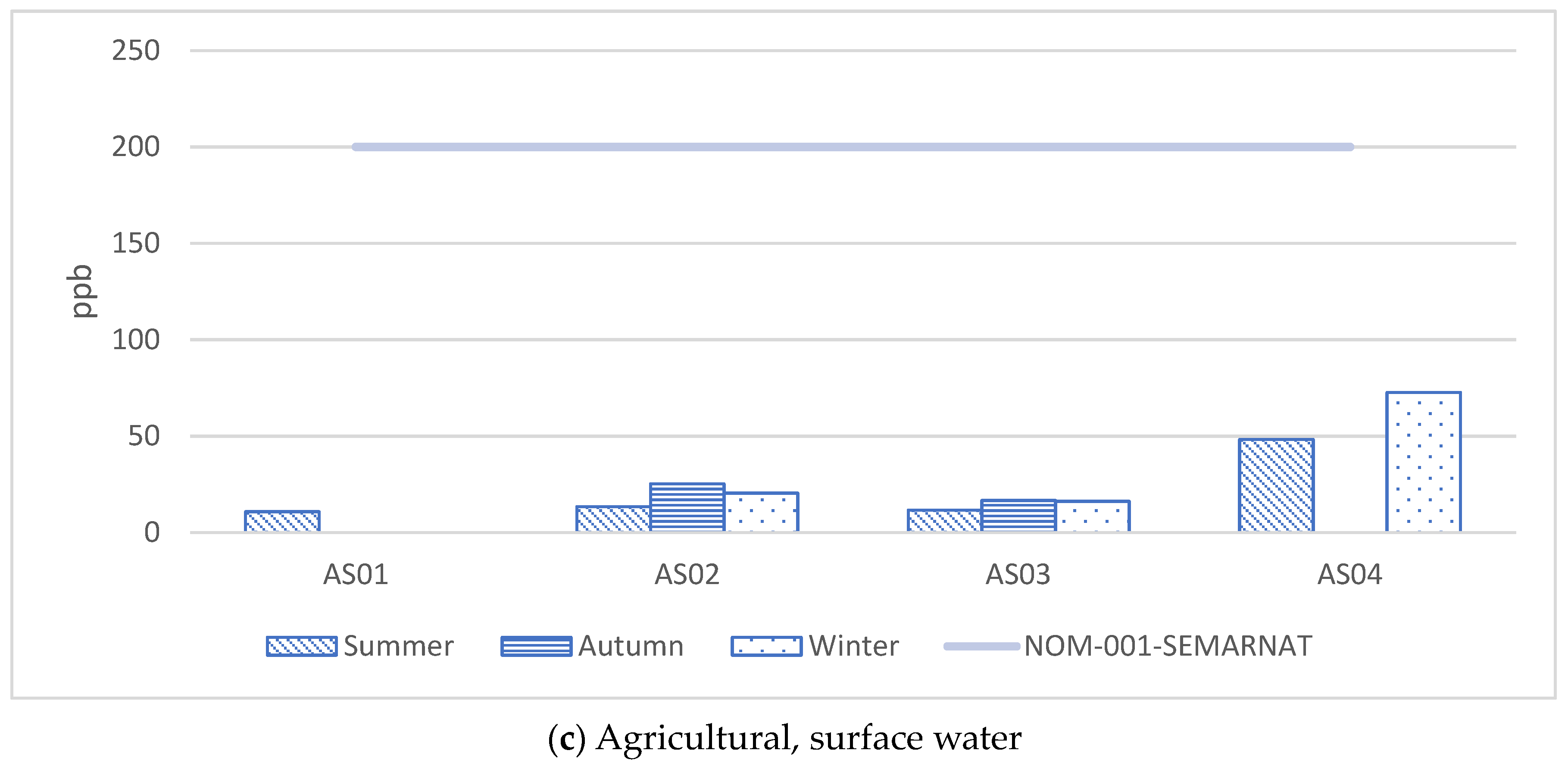
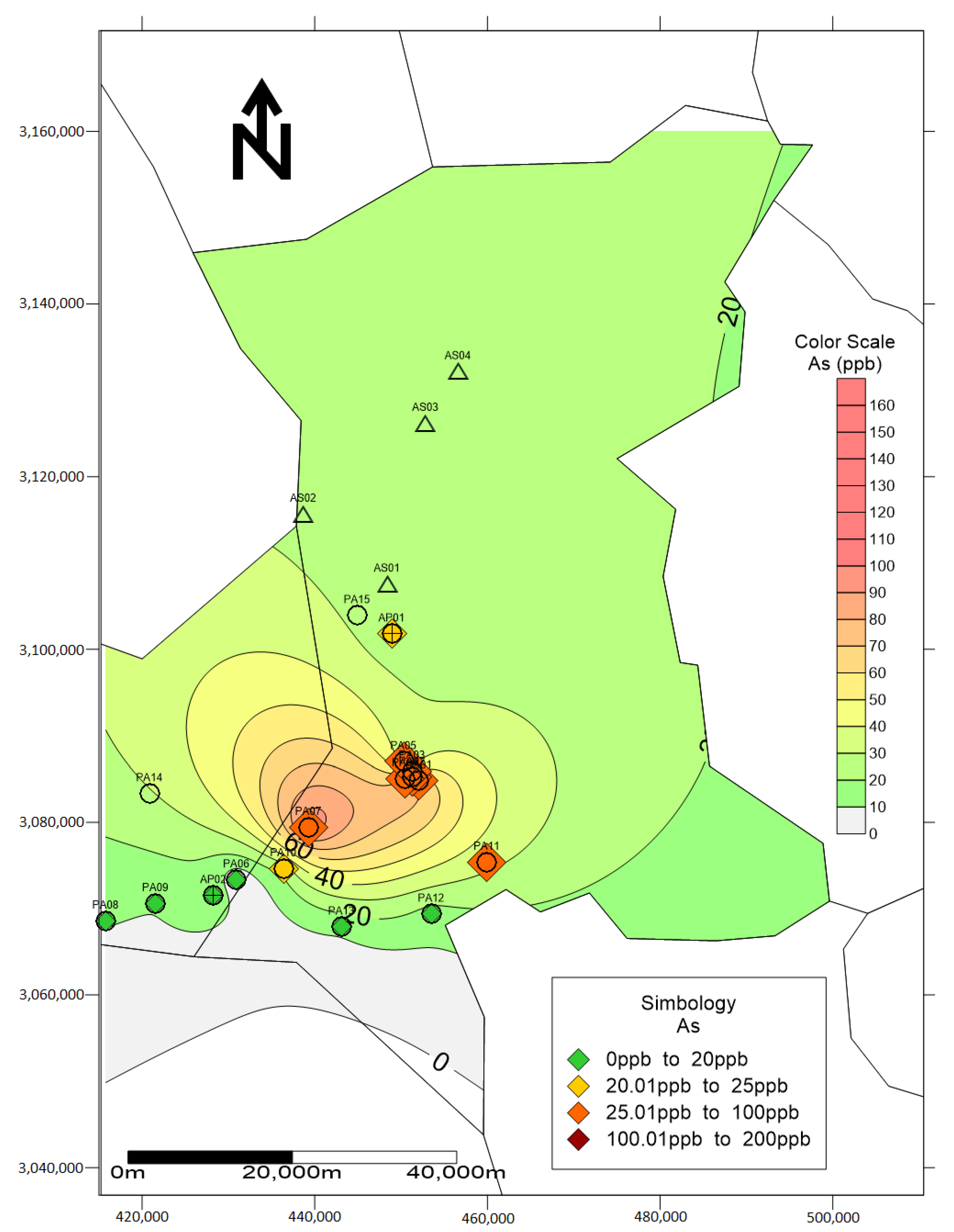
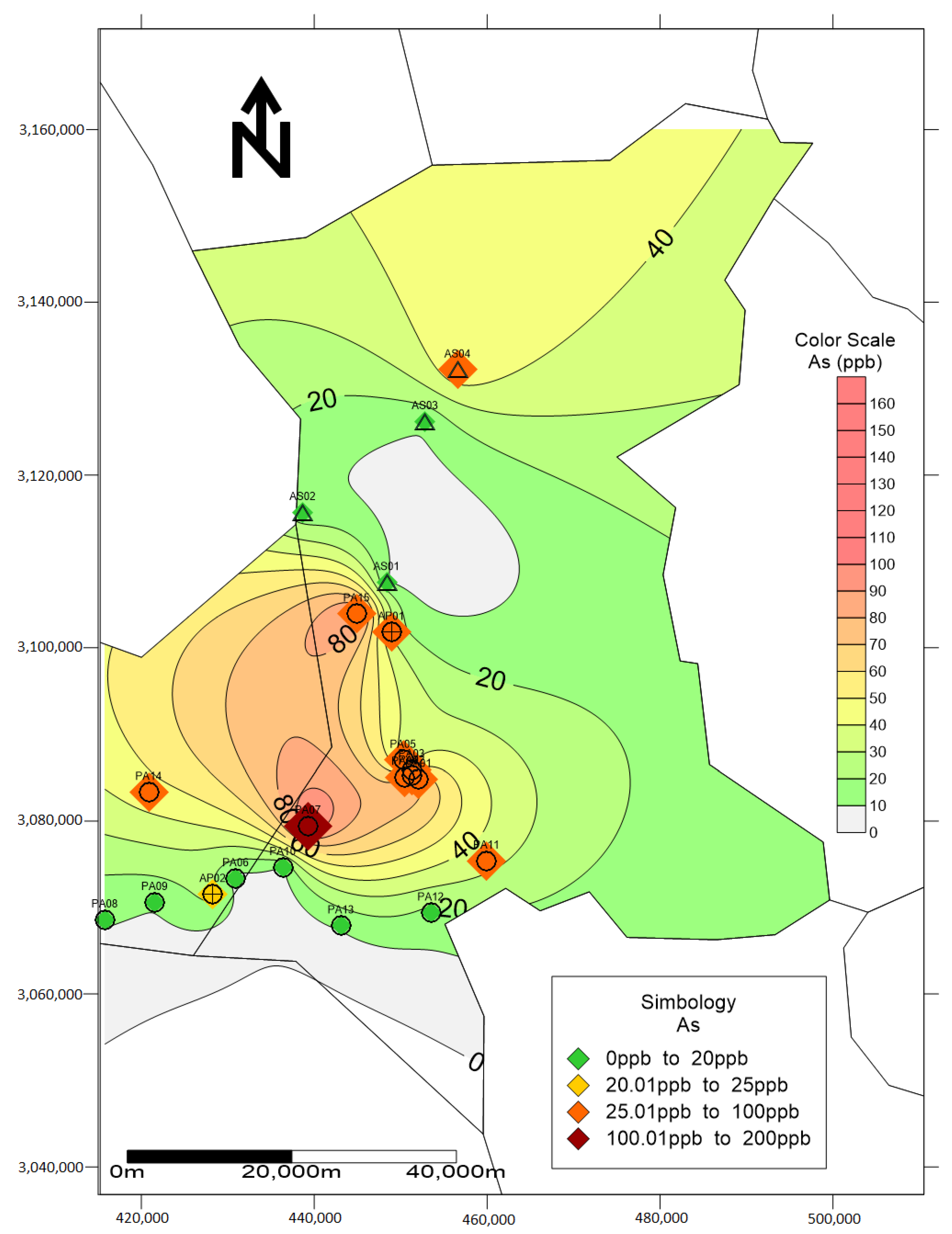
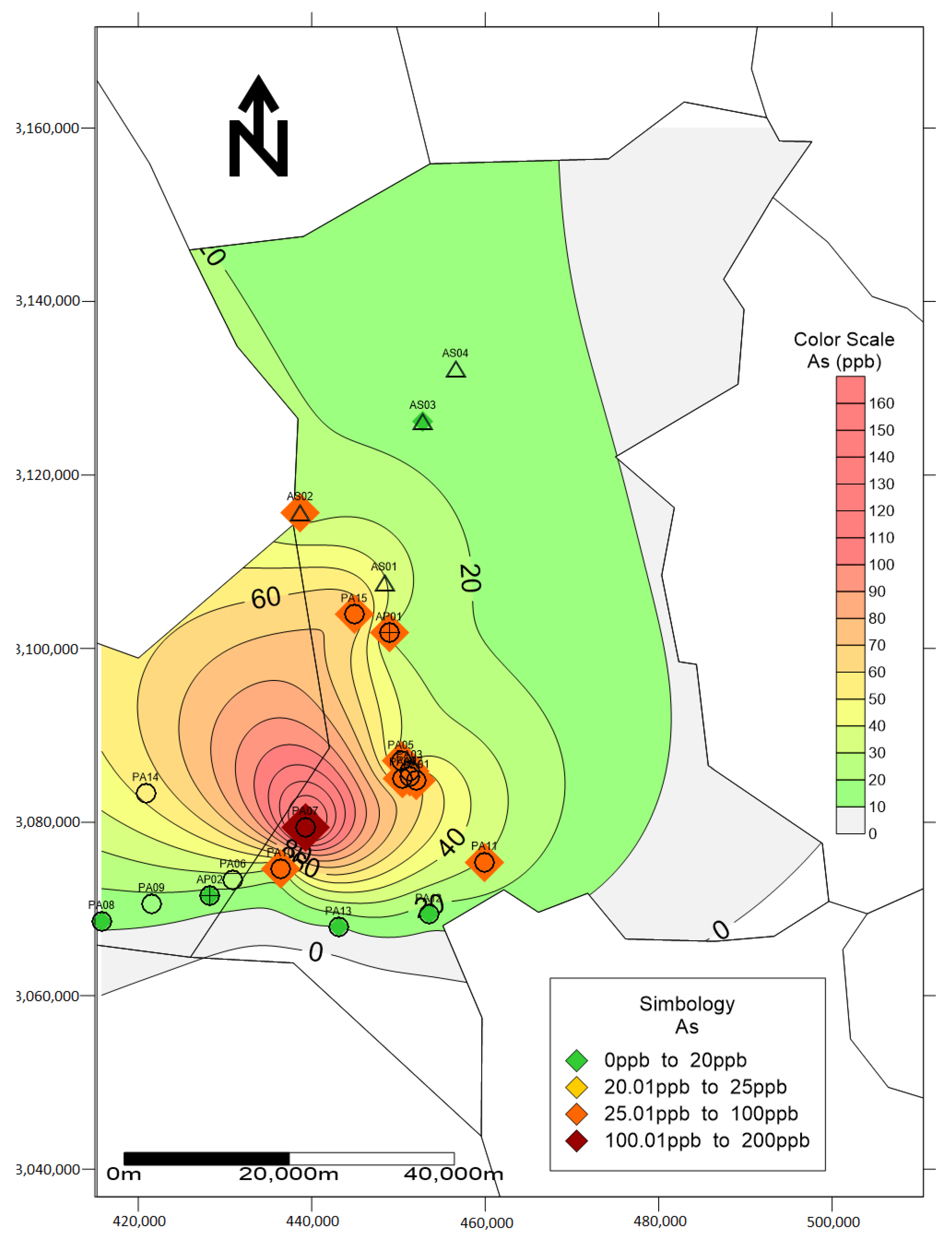
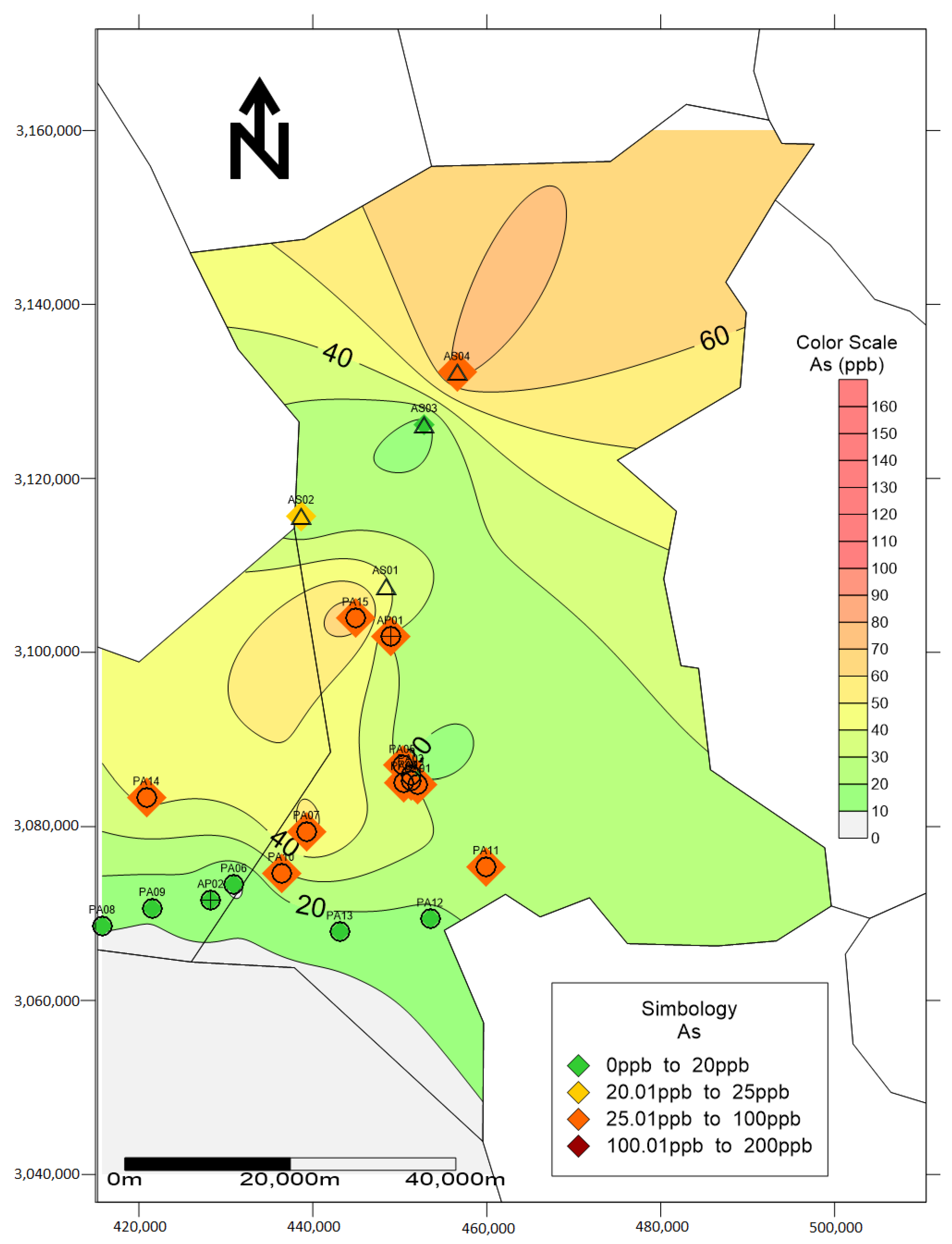

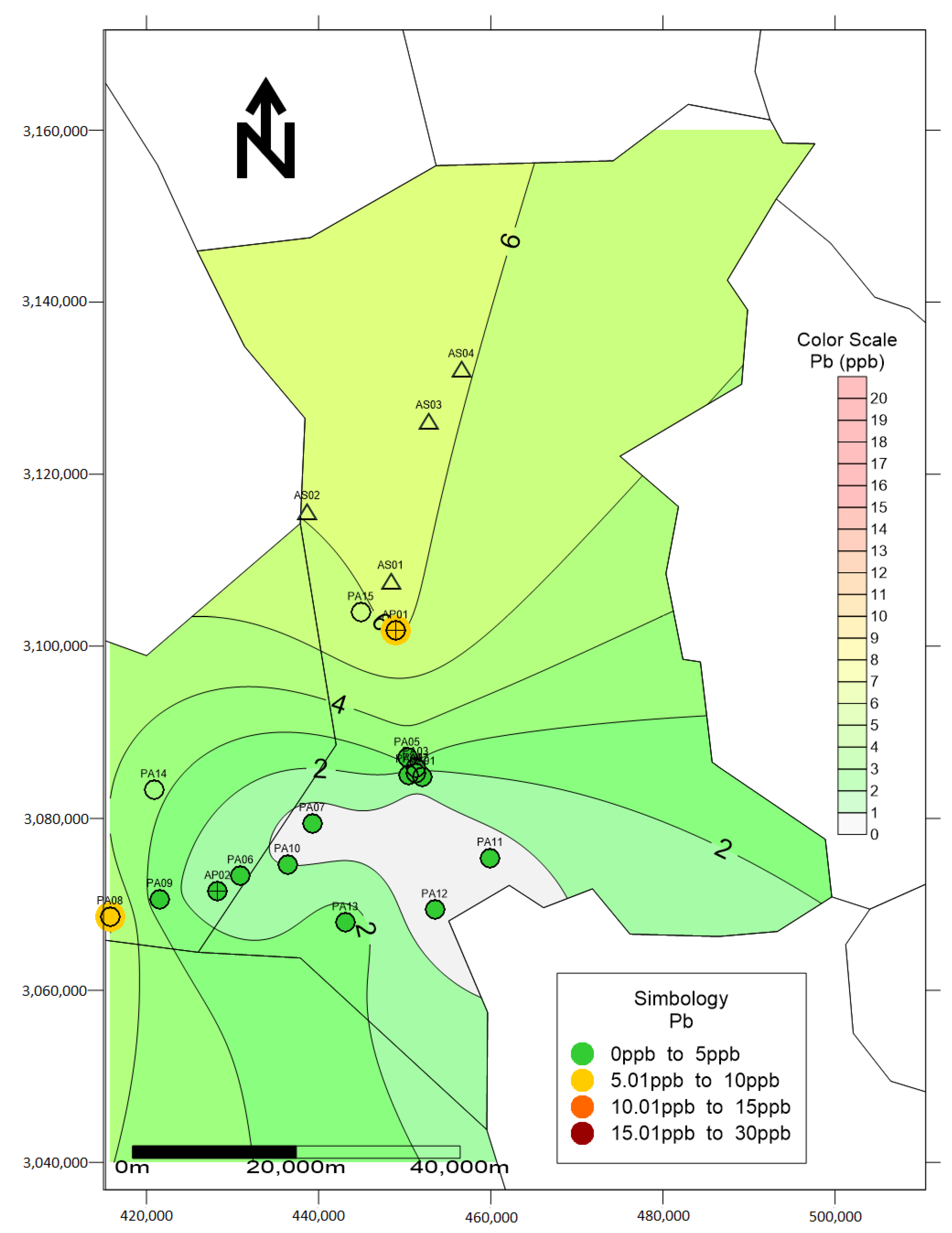



| No. | Element | As | Pb | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NOM-127 | 25 (ppb) | 10 (ppb) | |||||||
| NOM-0001 | 200 (ppb) | 500 (ppb) | |||||||
| Station | Spring | Summer | Autumn | Winter | Spring | Summer | Autumn | Winter | |
| 1 | PA01 | 68.7801 | 70.3980 | 61.7422 | 27.5270 | 1.4247 | 3.9208 | ND | 7.7424 |
| 2 | PA02 | 54.7624 | 57.6879 | 60.6778 | 42.1852 | 2.0608 | 2.2614 | ND | 6.8736 |
| 3 | PA03 | 44.9838 | 47.1107 | 46.2778 | 2.9683 | 2.6138 | 3.8866 | 26.3200 | 6.8144 |
| 4 | PA04 | 71.2084 | 70.3250 | 55.8822 | 27.5643 | 1.4972 | 2.5678 | ND | 7.5011 |
| 5 | PA05 | 35.4676 | 35.8022 | 36.8356 | 27.7268 | 3.4182 | 4.5639 | ND | 8.6339 |
| 6 | AP01 | 23.8593 | 26.9457 | 27.7467 | 28.7897 | 6.1027 | 3.1531 | ND | 4.3952 |
| 7 | AP02 | 19.4893 | 23.1434 | 16.6644 | 17.2978 | 1.4002 | ND | 9.3956 | 6.8941 |
| 8 | PA06 | 1.1999 | 6.9885 | ND | 6.9695 | 1.1903 | 0.9825 | ND | 4.0515 |
| 9 | PA07 | 98.3359 | 100.7361 | 156.5444 | 52.3509 | 0.4633 | 2.3283 | ND | 7.3497 |
| 10 | PA08 | 10.6329 | 11.1670 | 11.7689 | 10.6047 | 5.2683 | 1.4696 | 17.8311 | 8.3421 |
| 11 | PA09 | 10.7063 | 11.0089 | NA | 11.3220 | 2.3719 | 2.7092 | ND | 8.0657 |
| 12 | PA10 | 23.5760 | 9.6176 | 26.5867 | 28.7430 | 0.9794 | 5.3584 | 2.7533 | 6.0670 |
| 13 | PA11 | 32.5038 | 33.2515 | 29.3089 | 30.9716 | 0.6096 | 2.7160 | ND | 22.2817 |
| 14 | PA12 | 15.7230 | 17.0210 | 14.5467 | 16.5281 | 0.2126 | 1.2732 | ND | 5.8739 |
| 15 | PA13 | 12.7527 | 11.5096 | 8.9311 | 14.1539 | 2.6198 | 4.3784 | ND | 7.9070 |
| 16 | PA14 | NA | 46.1733 | NA | 42.2855 | NA | 3.4774 | ND | 4.1821 |
| 17 | PA15 | NA | 95.4749 | 71.0044 | 68.6418 | NA | 5.0414 | ND | 4.6709 |
| 18 | AS01 | NA | 10.8156 | NA | NA | NA | ND | NA | NA |
| 19 | AS02 | NA | 13.4156 | 25.2867 | 20.4510 | NA | ND | ND | 2.7585 |
| 20 | AS03 | NA | 11.5778 | 16.6267 | 16.1863 | NA | ND | ND | 5.6596 |
| 21 | AS04 | NA | 48.2267 | NA | 72.6581 | NA | ND | NA | 7.4740 |
| MIN. | 1.1999 | 6.9885 | 8.9311 | 2.9683 | 0.2126 | 0.9825 | 2.7533 | 2.7585 | |
| MAX. | 98.3359 | 100.7361 | 156.5444 | 72.6581 | 6.1027 | 5.3584 | 26.3200 | 22.2817 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencomo-Calderón, M.; Herrera-Peraza, E.F.; Villalobos-Aragón, A. As and Pb Presence within the Meoqui-Delicias Aquifer, Chihuahua, Mexico. Water 2024, 16, 2538. https://doi.org/10.3390/w16172538
Bencomo-Calderón M, Herrera-Peraza EF, Villalobos-Aragón A. As and Pb Presence within the Meoqui-Delicias Aquifer, Chihuahua, Mexico. Water. 2024; 16(17):2538. https://doi.org/10.3390/w16172538
Chicago/Turabian StyleBencomo-Calderón, Marisol, Eduardo Florencio Herrera-Peraza, and Alejandro Villalobos-Aragón. 2024. "As and Pb Presence within the Meoqui-Delicias Aquifer, Chihuahua, Mexico" Water 16, no. 17: 2538. https://doi.org/10.3390/w16172538
APA StyleBencomo-Calderón, M., Herrera-Peraza, E. F., & Villalobos-Aragón, A. (2024). As and Pb Presence within the Meoqui-Delicias Aquifer, Chihuahua, Mexico. Water, 16(17), 2538. https://doi.org/10.3390/w16172538







