Abstract
The use of marine invertebrates in ecotoxicology is important for an integrated approach which takes into consideration physiological responses and chemical levels in environmental matrices. Standard protocols have been developed and organisms belonging to different trophic levels are needed as model organisms to evaluate toxicant bioavailability and assess their impact on marine biota. The calanoid copepod Acartia tonsa is commonly used in ecotoxicology due to its widespread distribution and well-studied biology. However, different strains coming from various geographical areas are available, and possible variations in physiological characteristics raise concerns about the comparability of ecotoxicological results. This study compares the life cycle assessment and sensitivity of Adriatic and Baltic strains of A. tonsa exposed to nickel (Ni2+) in standardized acute and semi-chronic tests. Life cycle assessments revealed differences in egg production, egg-hatching success, and naupliar viability between the strains. The acute toxicity test demonstrated the significantly higher sensitivity of Adriatic strain nauplii to Ni2+ compared to the Baltic strain, whereas the semi-chronic test showed no significant difference in sensitivity between the strains. These findings suggest that while strain-specific differences exist in different geographical populations, responses to toxicants are not significantly different. Particularly, the semi-chronic assessments with both A. tonsa strains emphasized the robustness of this species as a model organism in ecotoxicology.
1. Introduction
The use of marine invertebrates as model organisms in ecotoxicology and the application of an integrated approach, which takes into consideration both physiological responses and chemical levels in the environment, have become routinary activities to monitor marine environment. These approaches are considered valid methods to better evaluate the bioavailability of toxicants and their potential threats to marine organisms [1] and are included in the Italian legislation [2] to regulate the management of polluted matrices in order to protect marine environments. Moreover, an integrated approach can also be a valuable tool for the management of protected marine sites to characterize the overall ecological status of these areas and improve conservation strategies in highly anthropized environmental contexts. The choice of the better organism to be used in ecotoxicology depends mainly on the relevance of the trophic level to be studied, the matrices to be assessed (sediment or water), and also the availability of organisms [3]. Most of the species used as models can be reared, purchased, or collected directly from the environment. Finding a good model organism in ecotoxicology and a common method for assessing environmental risk are mandatory to compare results in different geographical areas and at different times, so in recent years, standard protocols have been proposed for species belonging to various trophic levels (e.g., bacteria [4], microalgae [5,6], mollusks [7,8]; rotifers [9]; echinoderms [10]). All of these protocols consider many different physiological end points, depending on the species and duration of the exposure. Among marine crustaceans, the marine planktonic calanoid copepod Acartia tonsa (Dana, 1849) is a cosmopolitan, eurythermal, and euryhaline marine zooplanktonic organism common in subtropical and temperate latitudes and abundant in coastal and estuarine waters [11,12]. This is a well-known model species, with its biology and ecology having been studied since the 1980s [13,14]. Moreover, this planktonic organism is distributed worldwide in marine and estuarine environments, being the most abundant species in the Atlantic and Pacific American coasts [15,16]. In the Mediterranean and Baltic seas, A. tonsa was introduced by ship ballast waters in the 1980s [17,18] and has adapted well, since then, to euryhaline conditions, surviving sudden changes in salinities [19].
For these reasons, A. tonsa is widely used in ecotoxicology; bioassay protocols consider different end points, such as mortality, development, or fecundity in different life stages (eggs, nauplii, adults) after 48 h short-term exposure (acute test) [20,21] after 5–6 days in a larval development assay [22], after 7 days in a semi-chronic test [23], and after 4 days in a long-term incubation test [24]. Therefore, several studies in which A. tonsa has been used for the assessment of the quality of marine–brackish sediments [24,25] and for the toxicity of emerging contaminants [26,27,28] have been published.
Commonly, Acartia tonsa copepods are collected directly in the natural environment, reared in laboratory conditions, or purchased. However, different places of origin of A. tonsa (i.e., Adriatic, Baltic or Atlantic strains) may result in different physiological characteristics which can compromise the interpretation of the ecotoxicological results. For example, it has been demonstrated that Adriatic and Baltic strains of Acartia spp. showed different egg survival rates after storage at low temperatures [14]. The hypothesis that the geographical origin of the strain/population selected for ecotoxicological tests may influence the results is realistic, with the risk of under- or over-estimating the ecological risk assessment.
The aim of this study was to compare the ecotoxicological responses of two strains of Acartia tonsa, Adriatic and Baltic, exposed to the same reference toxicant. Both strains, widely used in standardized acute and semi-chronic bioassays, were exposed to the same reference toxicant, nickel chloride (NiCl2), following the standard procedures reported in the UNICHIM [21,23] protocol. The end point of naupliar immobilization or mortality, recorded after 48 h and 7 days exposure, in acute and semi-chronic tests, respectively, was considered. Moreover, both Adriatic and Baltic A. tonsa life traits, reared with the same laboratory protocols, were followed and compared. Life cycle, daily adult survival, egg production (fecundity), percentage of egg-hatching success, and naupliar viability were recorded. The final aim was to ascertain the comparability of the ecotoxicological responses of A. tonsa strains coming from different geographical areas.
2. Materials and Methods
2.1. Chemicals
Nickel chloride hexahydrate NiCl2 × 6H2O (Carlo Erba Reagents, Milan, Italy) was used as a reference toxicant for the ecotoxicological tests. A concentration of 1000 mg of Ni L−1 was diluted in distilled water (DW) to obtain an effective dissolved Ni2+ concentration of 10.0 ± 0.9 mg L−1, analyzed with ICP OES 720 (Agilent Technologies, Santa Clara, CA, USA) [29].
2.2. Copepod Culture
The Mediterranean strain of the calanoid copepod Acartia tonsa was originally collected in the northern Adriatic Sea (referred to as the Adriatic strain) and reared in a laboratory (ISPRA, Italy) for several generations. The Baltic strain was purchased from Guernsey Sea Farms (Guernsey, UK) and reared in a laboratory for several generations. Copepod cultures were reared as reported by Zhang et al. [30], in 20 L propylene tanks containing 0.22 µm of filtered seawater (FSW) (Millipore 90 mm holder YY3009000 Merk Life Science Srl, Milan, Italy) at 30 Practical Salinity Units (PSU). Natural seawater collected from an unpolluted site along the Tyrrhenian coast was filtered with a 0.22 μm mesh net filter, and the salinity was adjusted with distilled MilliQ water (BdW) at 30 PSU.
Copepods were fed twice a week with a mixed algal diet of Isochrys galbana, Rhodomonas baltica, and Rhinomonas reticulata in the exponential-growth phase and cultured in f/2 medium without silicates at the final concentration of 1500 μg C L−1. Both A. tonsa and the monoalgal cultures were maintained in a thermostatic chamber at 20 ± 1 °C and a 14:10 h L:D photoperiod.
2.3. Life Cycle Assessment
To analyze the life cycle, adults of the A. tonsa Adriatic and Baltic strains were collected from the main cultures and incubated in 800 mL beakers containing FSW and 3000 µg carbon L−1 of I. galbana and R. reticulata at the density of maximum 1 ind. mL−1 [30]. After 24 h, almost 2000 eggs were collected from the bottom of the beaker, counted under a stereomicroscope (Leica Microsystems S9i, Milan, Italy), and transferred in new 800 mL beakers containing 500 mL of FSW. Early naupliar stages (F1) were supplied with 750 µg carbon L−1 of I. galbana and 750 µg carbon L−1 of R. reticulata every 48 h. In the copepodite stages (CI), a monoalgal diet of R. reticulata was supplied at 1500 µg carbon L−1 every 48 h. When F1 reached the adult stage and few eggs were counted on the bottom of the beaker, 4 males and 4 females were sorted and isolated pairwise in 4 crystallizers filled with 50 mL of FSW and R. reticulata at 1500 µg carbon L−1 for the first 15 days, which was doubled after 16 days up to the end of the experiment. Couples were transferred to a new crystallizer with FSW and R. reticulata every 24 h and observed under a stereomicroscope to control viability. Females and males that died in the first 20 days of the experiment were replaced with adults coming from the main F1 culture from which they had been isolated. In this case, the replacement adults were the same age as the dead ones (Figure 1).
The main F1 culture was maintained in an 800 mL FSW beaker in the same condition as the isolated couples, and every 48 h, the eggs were removed to avoid the mixing of different generations.
For each couple of A. tonsa, the following aspects were evaluated:
Daily egg production per female (EPR) (eggs female−1 day−1);
Percentage of egg-hatching success (EHS) calculated 48 h after egg laying, as follows:
Egg-hatching success = [(N° hatched eggs)/(N° hatched eggs + N° unhatched eggs)] × 100
Percentage of naupliar viability (NV) calculated as follows:
Naupliar viability = 1 − [(N° death nauplii)/(N° hatched eggs)] × 100
The following flow-chart in Figure 1 synthetizes the method used:
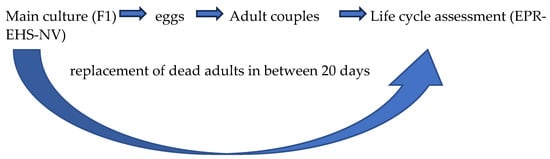

Figure 1.
Acartia tonsa culture method. Flow chart synthetized the method used to collect eggs and adults for the analysis of egg production rate (EPR), egg hatching success (EHS) and naupliar viability (NV) during the life cycle assessment of both A. tonsa Adriatic and Baltic strains. Females and males that died in the first 20 days of the experiment in the crystallizers were replaced with adults coming from the main F1 culture from which they had been isolated.
2.4. Ecotoxicological Tests
For ecotoxicological tests, mature A. tonsa adult copepods of both Adriatic and Baltic strains were sorted from the main culture by filtration through a 300 µm mesh net filter, incubated at a density ≤ 40 ind. L−1 in 800 mL beakers and fed daily with a mixture of exponentially growing algae R. reticulata and R. baltica, at a concentration > 500 µg carbon L−1. After 18–24 h, eggs at the bottom of the beaker were collected with a 50 µm mesh net filter, gently rinsed with FSW, and manually sorted using a Leica stereomicroscope (Milan, Italy). For the ecotoxicological test, the eggs were collected during the first 15 days since the adult stages appeared and stored at 4 °C for a maximum of one month before being used [29].
The acute test was performed according to the methods reported by Gorbi et al. [31], with some modifications. Briefly, a total of 10 eggs were sorted from the main culture and singularly incubated in 2.5 mL well plates containing FSW (negative control) or NiCl2 solutions (positive controls) at the final concentrations of 0.4, 0.2, 0.1, and 0.05 mg Ni2+ L−1. The plates, performed in triplicate, were maintained for 48 h in a thermostatic chamber at 20 ± 1 °C and a 14:10 L:D photoperiod. The end point considered was the immobilization or mortality of the nauplii after 48 h of incubation. Egg-hatching success and naupliar immobilization or mortality were evaluated after 48 h of exposure under an inverted microscope (Nikon TMS). Nauplii were considered immobilized or dead if, after 15 s of observation and physical stimulation, they did not actively swim.
The percentage of naupliar immobilization/mortality (NI) was calculated as follows:
where IN represents the number of immobilized or dead nauplii and HE represents the number of hatched eggs.
NI (%) = [IN/(HE)] × 100
The test was considered acceptable if the negative control had egg-hatching success ≥80% and naupliar immobilization or mortality ≤ 20%, and if EC50 in the positive control was 0.24 ± 0.12 mg Ni2+ L−1 after 48 h of exposure [21,31]. EC50 was calculated using PROBIT Analysis version 1.5 [21].
A semi-chronic 7-day test with A. tonsa was performed according to the method reported by Gorbi et al. [31]. Briefly, groups of three eggs were transferred in each camera test consisting of a 100 mL glass beaker equipped with a 20 µm mesh filter tube and filled with 25 mL of test solution. Three replicates for each experimental group, consisting of three camera tests, were performed and placed in a climate-controlled room at 20 ± 1 °C. The control group in FSW and dilutions of the reference toxicant (Ni2+) included aliquots of algal cultures in the exponential-growth phase at the final density of 5 × 104 cells mL−1 of I. galbana and 0.365 × 104 cells mL−1 of R. baltica. Final concentrations of 0.1, 0.063, 0.040, and 0.0025 mg Ni2+ L−1 were tested. The medium was renewed after 48 h and 5 days by transferring the 20 µm mesh net filter into a new camera containing fresh medium prepared as indicated above. At each renewal and after 7 days, the number of alive, dead, and immobilized nauplii in each camera test was recorded. Naupliar immobilization or death (NI) is expressed as follows:
where NI = naupliar immobilization, and TN = total nauplii.
NI (%) = [NI/(TN)] × 100
The test was considered valid if egg-hatching success in the control was ≥80% and naupliar immobilization was ≤30%.
2.5. Statistical Analyses
Control charts were prepared using EC50 values obtained by the relative test (acute or semi-chronic) with the reference toxicant (Ni2+) by different laboratories with Adriatic and Baltic strains. EC50 was calculated using Mosaic free loaded software (https://mosaic.univ-lyon1.fr/ (accessed on 13 July 2023)). All results were plotted, and upper and lower chart limits were calculated considering 2-fold standard deviation (2SD) ± mean EC50; means were than compared with Student’s t-test and considered statistically different for p < 0.05.
3. Results
3.1. Life Cycle Assessment
The life cycle of both A. tonsa strains is schematized in Figure 2; in our laboratory conditions, the eggs hatched within 48 h and naupliar stages lasted almost 7 days.
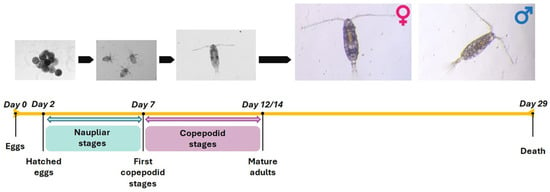
Figure 2.
Acartia tonsa life cycle timing. Schematic representation of the life cycle of A. tonsa Adriatic and Baltic strains.
Mature males and females were observed after 12 ± 2 days from hatching when viable eggs started to be produced. The lifespan of adults, daily egg production (fecundity), egg-hatching success, and naupliar viability were followed until the death of the females (Figure 3). The total lifespan of adults was variable, depending on the sex and the strain; specifically, for the Adriatic strain, males had a higher mortality rate than females within the first 26 days. They were replaced eight times, with alive males coming from the same F1 generation (Table 1); thereafter, males died after 29 and 27 days in couples 3 and 4 and after 32 days in couples 1 and 2 and were not replaced (Table 2). Five A. tonsa Adriatic strain females were replaced in couples 3 and 4 during the first 18 days and were replaced with the same F1 generation; thereafter, they died after 32 and 33 days, respectively, whereas females 1 and 2 died after 35 days (Table 1).
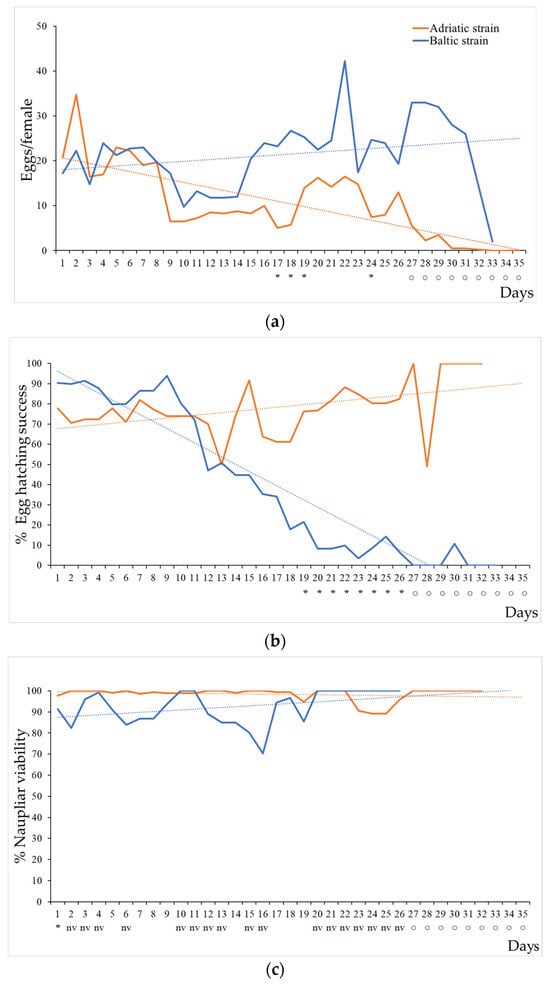
Figure 3.
Fecundity, percentage of egg-hatching success, and naupliar viability of Acartia tonsa Adriatic (orange line) and Baltic strains (blue line) fed a monoalgal diet of Rhinomanas reticulata. (a) Mean egg production per female, (b) percentage of egg-hatching success after 48 h, and (c) percentage of naupliar viability calculated on the number of hatched eggs. Dotted lines are linear trends (Adriatic, orange line; Baltic, blue line). Asterisks indicate significant differences (p < 0.05), and empty circles and nv indicate lack of data (0 egg production) or data with zero variance (nv).

Table 1.
Acartia tonsa Adriatic and Baltic strains’ daily survival of males (M) and females (F) fed with a monoalgal diet of Rhinomanas reticulata. Blue and red empty circles indicate dead males and females replaced with live copepods coming from the same culture; blue and red full circles indicate dead males and females, respectively, not replaced with live individuals.

Table 2.
Acartia tonsa Adriatic and Baltic strains. Mean and standard deviation (sd) of egg number per female per day, percentage of egg-hatching success, and naupliar viability calculated over the whole period of lifespan. Different letters indicate statistically significant differences (p < 0.05). Adult survival refers to the longer survival time of females (F) and males (M).
Regarding the Baltic strain, four males and two females died within 22 days in two couples and were replaced, whereas those who died after 23 days were not replaced considering the end of their life cycle (Table 1). The maximum survival recorded was 29 and 33 days for males and females, respectively (Table 2).
Daily fecundity (number of eggs per female), percentage of egg-hatching success, and naupliar viability are shown in Figure 2.
During the first week, egg production was similar in both A. tonsa Adriatic and Baltic strains, with a mean of 21.63 ± 12.7 and 20.63 ± 10.14 eggs per female per day, respectively. Thereafter, the production rapidly declined for the Adriatic strain (mean of 7.09 ± 6.32) compared to the Baltic strain (21.53 ± 9.8), with a statistically significant difference after 17–19 and 24 days. Females did not produce eggs after 32 days up to their death (Figure 3a). The egg production of the Baltic strain remained stable and increased after the second week, declining after 32 days shortly before their death (Figure 3a). On average, the Baltic strain produced twice as many eggs as the Adriatic strain, with an average of 21.3 eggs per female per day (p < 0.05; n = 68) (Table 2).
The percentage of egg-hatching success was variable during the life cycle for both strains; for the Adriatic one, the percentage ranged from 50 to 100%, with a mean of 77.9 ± 13.1% (Figure 3b, Table 2). In contrast, the percentage of egg-hatching success for the A. tonsa Baltic strain declined with the age of the females soon after 10 days. After 19 days, the mean percentage of egg-hatching success was statistically lower than that of the Adriatic strain and an average of 39.5% was calculated for the whole period, which was also significantly different from the Adriatic strain (p < 0.05; n = 65) (Figure 3b, Table 2).
3.2. Ecotoxicological Tests
3.2.1. Acute Test
Figure 4 shows Shewart-like control charts of EC50 depicting acute toxicity tests (naupliar immobilization or mortality) with A. tonsa Adriatic (a) and Baltic (b) strains exposed to the reference toxicant.
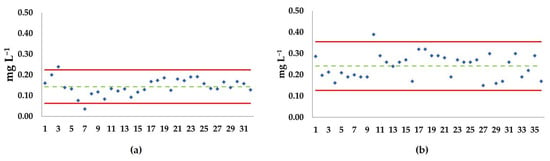
Figure 4.
Shewart-like control chart of EC50 of naupliar immobilization or mortality with NiCl2 in acute test (48 h) with Acartia tonsa eggs. (a) Adriatic strain (modified by [32]); (b) Baltic strain. Green dotted line: mean EC50 value; red lines: upper and lower limits (2 × standard deviation ± mean); blue diamonds: single EC50 values. The x-axis represents different, independent tests.
Thirty-two bioassays were conducted with the Adriatic strain; data were partially derived by Rotolo et al. [32], except for the first two (Figure 4a). Single EC50 values and the mean EC50 of 0.144 mg Ni2+ L−1 exhibited homogeneous dispersion within the two limits (2SD = ± 0.081 mg Ni2+ L−1), with two outliers (0.24 and 0.04 mg Ni2+ L−1). Regarding the Baltic strain (Figure 4b), results from 36 independent tests are plotted, revealing a mean EC50 of 0.241 mg Ni2+ L−1. The collected data were homogeneously dispersed within the two limits (2SD = ± 0.114 mg Ni2+ L−1), with only one outlier (0.39 mg Ni2+ L−1) (Figure 4b). The statistical comparison between EC50s in acute test exposure indicated significantly higher sensitivity to the reference toxicant for the Adriatic strain compared to the Baltic strain (Figure 5).
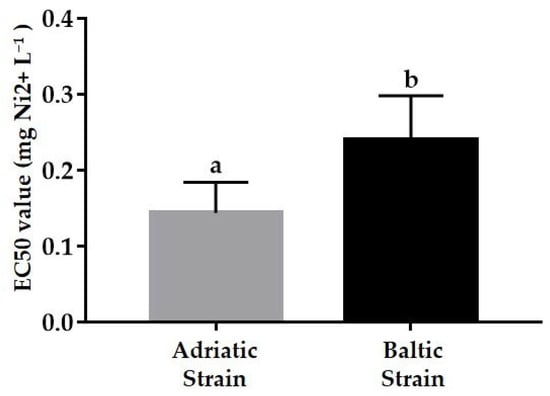
Figure 5.
Effective concentration inducing 50% naupliar immobilization or mortality (EC50) in Acartia tonsa eggs exposed in acute test (48 h) (mean ± sd); Baltic (n = 36) and Adriatic (n = 32) strains. Different letters indicate statistically significant difference (Student’s t-test, p < 0.05).
3.2.2. Semi-Chronic Test
Figure 6 shows Shewart-like control charts of EC50s calculated for semi-chronic exposure tests of the A. tonsa Baltic and Adriatic strains to Ni2+ (a and b, respectively).
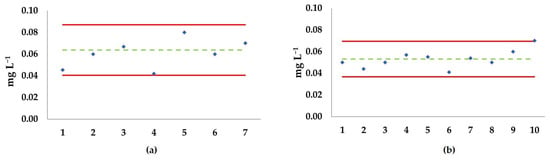
Figure 6.
Shewart-like control chart of EC50 of naupliar immobilization or mortality with NiCl2 in semi-chronic test (7 days) with Acartia tonsa eggs. (a) Adriatic strain; (b) Baltic strain. Green dotted line: mean EC50 value; red lines: upper and lower limits (2 × standard deviation ± mean); diamonds: single EC50 values. The x-axis represents different, independent tests.
For both strains, the single tests exhibited a well-dispersed pattern around their relative mean EC50, devoid of outliers, and no significant differences between the means of the Baltic (0.053 mg Ni2+ L−1) and Adriatic (0.061 mg Ni2+ L−1) strains were detected (Figure 7).
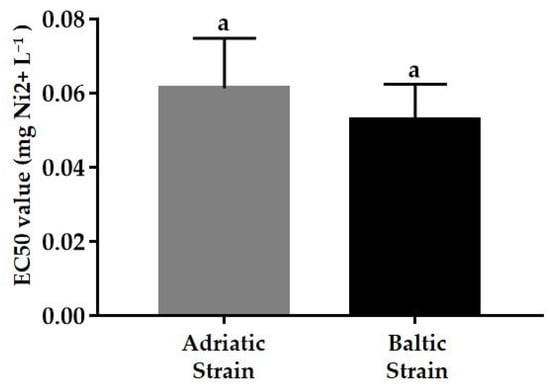
Figure 7.
Effective concentration inducing 50% naupliar immobilization or mortality (EC50) in Acartia tonsa eggs exposed in semi-chronic test (7 days) (mean ± sd); Baltic (n = 10) and Adriatic (n = 20) strains. Similar letters indicate a non-statistically significant difference (Student’s t-test, p < 0.05).
4. Discussion
Acartia tonsa copepods from the Mediterranean and Baltic areas were cultured and exposed to the same reference toxicant to test whether they responded differently to the same culture conditions in terms of reproduction and productivity, survival, and life cycle, and had different levels of sensitivity during ecotoxicological assays.
Differences in larval developmental time within one nominal copepod species have been observed in natural populations, pointing out possible geographical origin effects on life history traits [33]. Moreover, genetically distinct clades have been reported within the nominal, cosmopolitan species Acartia tonsa [34,35,36]. Investigations of genetic differentiation suggested that European populations of this species may have resulted from single or multiple invasions of American strains [37]. Comparative studies on different strains of A. tonsa have also been conducted in order to select the most suitable ones for aquaculture purposes, investigating both genetic traits and physiological characteristics, such as the biochemical composition of various life stages from four different strains. These studies demonstrated genetic differentiation between strains, as indicated by differences in two mitochondrial gene loci between three of the four strains and differences in mortality, egg production, hatching success, and the biochemical composition of eggs and adults [37,38].
We did not analyze genetic differentiation between the strains, and our results showed that life cycle and adult survival were comparable, despite the higher mortality rate observed in the Adriatic strain compared to the Baltic one. In both cases, adults showed a maximum survivability longer than 30 days, in accordance with the results obtained from the Kattegat Øresund (Denmark) population by Kiørboe et al. [39] (35 ± 1.3 and 31 ± 1.2 day of life span for females and males, respectively).
The productivity, indicated by the number of eggs produced, showed differences between the two strains starting from the second week of the culture. The low productivity of the Adriatic strain derived mainly from the low egg production of couple 2 during the entire monitoring period. However, the mean values calculated considering all couples were comparable with those obtained with the same Mediterranean strain by Zhang et al. [30]. On average, the daily egg production of the Baltic strain increased soon after the specimens reached the adult stage. In general, the maximum daily egg production in the first week was between 24 and 40 eggs. It is interesting to note that an increase in egg production was observed for all couples in the Baltic strain when the food supplied was doubled (after day 16). In this second phase of the trial, daily egg production reached a maximum of 66 eggs. The effect due to the increase in food supply was more evident in the Adriatic strain after 2 days, but the productivity recorded during the first week was not able to be restored. This suggests that the decline in egg production was only partially due to the amount of food supplied and was most likely caused by a physiological state, probably more influenced by the age of the adults.
Despite the higher number of eggs produced by the Baltic strain compared to the Adriatic strain, its quality in terms of percentage of hatching success was lower than that of the Adriatic strain. This difference was much more evident after 10 days of culture. Before that time, the percentage of successfully hatched eggs was comparable in both strains and in the range of 79.83–93.96%, which was considered suitable for ecotoxicological assay performances and in line or even higher than the values reported in the literature [38,40,41]. This result may have mainly depended on the physiological state of the females. Acartia tonsa females tend to remove undesirable substances, such as oxidative stress products, through eggs, with the consequence of a high content of oxidative products in the eggs released by older females [40,42], which may have been reflected in the lower egg-hatching success at the end of their life cycle.
It is interesting to note different strategies evolved between the strains; the Adriatic strain seemed to respond to aging by reducing the energy involved in the production of eggs, which in turn were of better quality (fewer eggs with a high hatching rate), while the Baltic strain spared no energy to devote to laying eggs, which, however, were of poor quality in terms of hatching success. Probably, in nature, the final effect considered as the recruitment rate of individuals to the population is the same, but in ecotoxicology, the egg-hatching success is a parameter that must be taken into consideration for the validation of the results. In fact, for the validation of the bioassays with A. tonsa, the egg-hatching success in the controls should be ≥80% [21,31]. Considering that the differences between the strains were observed in egg-hatching success rate, we suggest taking the physiological characteristics of the strain used for culture maintenance and for setting up bioassays into consideration.
Regarding the results of ecotoxicological tests, standardized tests with A. tonsa have been included in the methods used to determine the quality and management of dredged marine sediments [2]. The selection of species and bioassays to set up a battery of marine model organisms follows specific criteria in order to represent different trophic levels, end points, and matrices. Within these criteria, it is possible to choose alternative, different model species for ecotoxicological bioassays. In fact, despite some differences in end points, authors demonstrated that different species can be used interchangeably for an integrated sediment quality assessment [43,44,45].
Our results of the acute exposure test with both A. tonsa strains showed EC50s in the control chart close to the mean value, especially for those tests conducted in the second half of the graph, indicating the quality and the robustness of the data during the time. The preliminary results regarding EC50 for naupliar immobilization or mortality in the semi-chronic test were also close to the mean value, suggesting the accuracy and reproducibility of the data (Figure 4 and Figure 6). However, the overall mean of EC50 in the acute test for the Adriatic strain of A. tonsa was statistically lower by about half, compared to the Baltic strain, indicating its high sensitivity. It is known that populations coming from different zoogeographical regions can show physiological differences, for example, related to tolerance to stressful conditions, such as low temperatures [13].
However, several studies indicated that different strains of aquatic species, such as bacteria [46], microalgae [47], and invertebrates [48], can show different levels of sensitivity to contaminants or stressors.
Among freshwater crustaceans, a greater number of comparative studies are available for Daphnia magna, a widely used organism in ecotoxicology which is prescribed as a model by international regulations [49,50]. Barata et al. [51] recorded variations in sensitivity when different laboratory clones or a wild population of D. magna were exposed to toxic compounds.
Population-specific responses are also frequently reported in marine copepods exposed to the same stressors [52,53]. Although environmental factors such as diet and culture conditions remain the major cause of inter-laboratory variations [54], various genotypes can respond differently to the same substances [55]. Picado et al. [56] suggested performing an analysis on gene expression and genetic stability in order to obtain well-characterized and stable standard clones to use on standard tests, reducing confounding factors among laboratories. Gene expression analyses have been conducted on co-generic Acartia exposed to the same toxicant [24], but, to our knowledge, a comparison between toxicological responses of the same species coming from different geographical areas has never been reported until now.
Overall, our data regarding the sensitivity of both A. tonsa strains to Ni exposure in acute toxicity tests agree with those reported by Gorbi et al. [31], suggesting the interchangeability of both strains.
Similarly, our results regarding semi-chronic bioassays indicated a mean EC50 in alignment with that reported by Gorbi et al. [31]. The number of semi-chronic assays performed was limited to a few tests compared to the acute tests; however, the preliminary results suggested that the pre-selection of different A. tonsa strains is not necessary for this test.
5. Conclusions
What emerged from this study was a difference in productivity and egg viability between A. tonsa Adriatic and Baltic strains reared in the same laboratory conditions. It has clarified the importance of detailed knowledge of the biology and strain specificity of model organisms, in this case, the marine calanoid copepod, to prevent potential confounding factors during ecotoxicological bioassays, which may invalidate tests or over-/under-estimate the toxicity of chemicals and natural matrices. Shewart-like control charts have proven to be a useful tool to evaluate the EC50 variability in long-term plotted data and should be considered for all model species and strains to define the level of acceptability and sensitivity in ecotoxicological tests according to the standard protocols.
Author Contributions
Conceptualization, V.V. and I.B.; methodology, V.V., I.B., M.O., M.R., A.C., G.F., S.A. and T.B.; software, V.V., M.O. and S.A.; validation, V.V., M.O., M.R., A.C., S.A., G.F., D.P., C.P. and I.B.; investigation, V.V., M.O., M.R., A.C., G.F., S.A., T.B., I.M. and V.S.; resources, D.P. and C.P.; writing—original draft preparation, V.V., M.O., A.C., I.M. and I.B.; writing—review and editing, V.V., M.O., M.R., A.C., I.M. and I.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by: PORTUGUESE SCIENCE FOUNDATION (FUNDAÇÃO PARA A CIÊNCIA E A TECNOLOGIA, FCT), grant number PD/BD/150609/2020 to A.C.; the authors acknowledge financial support to CESAM by FCT/MCTES (UIDP/50017/2020+UIDB/50017/2020+LA/P/0094/2020).
Data Availability Statement
The raw data supporting the conclusions of this article have been made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Piva, F.; Ciaprini, F.; Onorati, F.; Benedetti, M.; Fattorini, D.; Ausili, A.; Regoli, F. Assessing sediment hazard through a Weight of Evidence approach with bioindicator organisms: A practical model to elaborate data from sediment chemistry, bioavailability, biomarkers and ecotoxicological bioassays. Chemosphere 2011, 83, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Ministerial Decree 173. Regolamento Recante Modalità e Criteri per L’autorizzazione All’immersione in Mare Dei Materiali di Escavo di Fondali Marini—Technical Attachment. Decreto del Ministero dell’Ambiente e della Tutela del Territorio e del Mare. 2016. Available online: https://www.gazzettaufficiale.it/eli/id/2016/09/06/16G00184/sg (accessed on 13 July 2023).
- Pandard, P.; Devillers, J.; Charissou, A.-M.; Poulsen, V.; Jourdain, M.-J.; Férard, J.-F.; Grand, C.; Bispo, A. Selecting a battery of bioassays for ecotoxicological characterization of wastes. Sci. Total Environ. 2006, 363, 114–125. [Google Scholar] [CrossRef] [PubMed]
- ISO 11348-3; Water Quality—Determination of the Inhibitory Effect of Water Samples on the Light Emission of Vibrio fischeri (Luminescent bacteria test). Part 3: Method Using Freeze-Dried Bacteria. International Organization for Standardization: Genève, Switzerland, 2007.
- ISO 10253; Water Quality—Marine Algal Growth Inhibition Test with Skeletonema sp. and Phaeodactylum tricornutum. International Organization for Standardization: Genève, Switzerland, 2016.
- ASTM E1218; Standard Guide for Conducting Static Toxicity Tests with Microalgae. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ISO 17244; Water Quality—Determination of the Toxicity of Water Samples on the Embryo-Larval Development of Japanese Oyster (Crassostrea gigas) and Mussel (Mytilus edulis or Mytilus galloprovincialis). International Organization for Standardization: Genève, Switzerland, 2015.
- ASTM E724; Standard Guide for Conducting Static Short-Term Chronic Toxicity Tests Starting with Embryos of Four Species of Saltwater Bivalve Molluscs. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ISO 19820; Water Quality—Determination of the Acute Toxicity to the Marine Rotifer Brachionus plicatilis. International Organization for Standardization: Genève, Switzerland, 2016.
- USEPA 600-4-90-027F; Methods of Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms. United States Environmental Protection Agency: Washington, DC, USA, 1993.
- Razouls, C.; Desreumaux, N.; Kouwenberg, J.; de Bovée, F. Biodiversity of Marine Planktonic Copepods (Morphology, Geographical Distribution and Biological Data). Sorbonne University, CNRS Centre National de la Recherche Scientifique: Paris, France, 2005–2024. Available online: http://copepodes.obs-banyuls.fr/en (accessed on 25 March 2024).
- Sarkisian, B.L.; Lemus, J.T.; Apeitos, A.; Blaylock, R.B.; Saillant, E.A. An intensive, large-scale batch culture system to produce the calanoid copepod, Acartia tonsa. Aquaculture 2019, 501, 272–278. [Google Scholar] [CrossRef]
- Parrish, K.K.; Wilson, D.F. Fecundity studies on Acartia tonsa (Copepoda: Calanoida) in standardized culture. Mar. Biol. 1978, 46, 65–81. [Google Scholar] [CrossRef]
- Hansen, B.W.; Buttino, I.; Cunha, M.E.; Drillet, G. Embryonic cold storage capability from seven strains of Acartia spp. isolated in different geographical areas. Aquaculture 2016, 457, 131–139. [Google Scholar] [CrossRef]
- Hoffmeyer, M.S. Decadal change in zooplankton seasonal succession in the Bahía Blanca Estuary, Argentina, following introduction of two zooplankton species. J. Plankton Res. 1994, 26, 181–189. [Google Scholar] [CrossRef]
- Durbin, E.G.; Durbin, A.G.; Smayda, T.J.; Verity, P.G. Food limitation of production by adult Acartia tonsa in Narragansett Bay, Rhode Island. Limnol. Oceanogr. 1983, 28, 1199–1213. [Google Scholar] [CrossRef]
- Gruszka, P. The River Odra estuary as a gateway for alien species immigration to the Baltic Sea basin. Acta Hydrochim. Hydrobiol. 1999, 27, 374–382. [Google Scholar] [CrossRef]
- Brylinski, J.M. Report on the presence of Acartia tonsa Dana (Copepoda) in the harbour of Dunkirk (France) and its geo-graphical distribution in Europe. J. Plankton Res. 1981, 3, 255–260. [Google Scholar] [CrossRef]
- Calliari, D.; Andersen Borg, M.C.; Thor, P.; Gorokhova, E.; Tiselius, P. Instantaneous salinity reductions affect the survival and feeding rates of the cooccurring copepods Acartia tonsa Dana and A. clausi Giesbrecht differently. J. Exp. Mar. Biol. Ecol. 2008, 362, 18–25. [Google Scholar] [CrossRef]
- ISO 14669; Water Quality—Determination of Acute Lethal Toxicity to Marine Copepods (Copepoda, Crustacea). International Organization for Standardization: Genève, Switzerland, 1999.
- UNICHIM M.U. 2365; Qualità dell’acqua: Determinazione Dell’inibizione Della Mobilità di Naupli di Acartia tonsa Dana (Crustacea: Copepoda) dopo 24 h e 48 h di Esposizione. Associazione per l‘Unificazione del Settore dell’Industria Chimica: Milan, Italy, 2012; p. 22.
- ISO 16778; Water Quality—Calanoid Copepod Early-Life Stage Test with Acartia tonsa. International Organization for Stand-ardization: Genève, Switzerland, 2015.
- UNICHIM M.U. 2366; Qualità Dell’acqua: Determinazione Dell’inibizione Della Mobilità di Naupli di Acartia tonsa Dana (Crustacea: Copepoda) Dopo 7 Giorni di Esposizione. Associazione per l‘Unificazione del Settore dell’Industria Chimica: Milan, Italy, 2012; p. 24.
- Carotenuto, Y.; Vitiello, V.; Gallo, A.; Libralato, G.; Trifuoggi, M.; Toscanesi, M.; Lofrano, G.; Esposito, F.; Buttino, I. Assessment of the relative sensitivity of the copepods Acartia tonsa and Acartia clausi exposed to sediment-derived elutriates from the Bagnoli-Coroglio industrial area. Mar. Environ. Res. 2020, 155, 104878. [Google Scholar] [CrossRef] [PubMed]
- Picone, M.; Bergamin, M.; Delaney, E.; Volpi Ghirardini, A.; Kusk, K.O. Testing lagoonal sediments with early life stages of the copepod Acartia tonsa (Dana): An approach to assess sediment toxicity in the Venice Lagoon. Ecotox. Environ. Safe 2018, 147, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Picone, M.; Distefano, G.G.; Marchetto, D.; Russo, M.; Vecchiato, M.; Gambaro, A.; Barbante, C.; Volpi Ghirardini, A. Fragrance materials (FMs) affect the larval development of the copepod Acartia tonsa: An emerging issue for marine ecosystems. Ecotox. Environ. Safe 2021, 215, 112146. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, F. Acartia spp. (Copepoda: Calanoida) as Model Organisms to Evaluate the Toxicity of Emerging Contaminants: An Ecotoxicogenomic Approach. Ph.D. Thesis, The Open University, Milton Keynes, UK, 2023. [Google Scholar] [CrossRef]
- Sørensen, L.; Størseth, T.R.; Altin, D.; Nordtug, T.; Faksness, L.-G.; Hansen, B.H. A simple protocol for estimating the acute toxicity of unresolved polar compounds from field weathered oils. Toxicol. Mech. Method. 2024, 34, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, V.; Zhou, C.; Scuderi, A.; Pellegrini, D.; Buttino, I. Cold storage of Acartia tonsa eggs: A practical use in ecotoxicological studies. Ecotoxicology 2016, 25, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ianora, A.; Wu, C.; Pellegrini, D.; Esposito, F.; Buttino, I. How to increase productivity of the copepod Acartia tonsa (Dana): Effects of population density and food concentration. Aquac. Res. 2015, 46, 2982–2990. [Google Scholar] [CrossRef]
- Gorbi, G.; Invidia, M.; Savorelli, F.; Faraponova, O.; Giacco, E.; Cigar, M.; Buttino, I.; Leoni, T.; Prato, E.; Lacchetti, I.; et al. Standardized methods for acute and semichronic toxicity tests with the copepod Acartia tonsa. Environ. Toxicol. Chem. 2012, 31, 2023–2028. [Google Scholar] [CrossRef] [PubMed]
- Rotolo, F.; Vitiello, V.; Pellegrini, D.; Carotenuto, Y.; Buttino, I. Historical control data in ecotoxicology: Eight years of tests with the copepod Acartia tonsa. Environ. Pollut. 2021, 284, 117468. [Google Scholar] [CrossRef] [PubMed]
- Leandro, S.M.; Queiroga, H.; Rodriguez-Grana, L.; Tiselius, P. Temperature dependent development and somatic growth in two allopatric populations of Acartia clausi (Copepoda: Calanoida). Mar. Ecol. Prog. Ser. 2006, 322, 189–197. [Google Scholar] [CrossRef]
- Caudill, C.C.; Bucklin, A. Molecular phylogeography and evolutionary history of the estuarine copepod, Acartia tonsa, on the Northwest Atlantic coast. Hydrobiologia 2004, 511, 91–102. [Google Scholar] [CrossRef]
- Hill, R.S. Genetic Diversity and Structure of Calanoid Copepods: Molecular Evolutionary Patterns in Coastal Estuaries (Acartia tonsa) and the Open Ocean (Calanus spp.). Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2004. Available online: https://scholars.unh.edu/dissertation/246 (accessed on 13 July 2023).
- Chen, F.; Marcus, N.H. Subitaneous, diapause, and delayed-hatching eggs of planktonic copepods from the northern Gulf of Mexico: Morphology and hatching success. Mar. Biol. 1997, 127, 587–597. [Google Scholar] [CrossRef]
- Drillet, G.; Goetze, E.; Jepsen, P.M.; Højgaard, J.K.; Hansen, B.W. Strain-specific vital rates in four Acartia tonsa cultures, I: Strain origin, genetic differentiation and egg survivorship. Aquaculture 2008, 280, 109–116. [Google Scholar] [CrossRef]
- Drillet, G.; Jepsen, P.M.; Højgaard, J.K.; Jørgensen, N.O.G.; Hansen, B.W. Strainspecific vital rates in four Acartia tonsa cultures, II: Life history traits and biochemical contents of eggs and adults. Aquaculture 2008, 279, 47–54. [Google Scholar] [CrossRef]
- Kiørboe, T.; Ceballos, S.; Thygesen, U.H. Interrelations between senescence, life-history traits, and behavior in planktonic copepods. Ecology 2015, 96, 2225–2235. [Google Scholar] [CrossRef] [PubMed]
- Holste, L.; Peck, M.A. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): A laboratory investigation. Mar. Biol. 2006, 148, 1061–1070. [Google Scholar] [CrossRef]
- Jepsen, P.M.; Andersen, N.; Holm, T.; Jørgensen, A.T.; Højgaard, J.K.; Hansen, B.W. Effects of adult stocking density on egg production and viability in cultures of the calanoid copepod Acartia tonsa (Dana). Aquac. Res. 2007, 38, 764–772. [Google Scholar] [CrossRef]
- Rodríguez-Graña, L.; Calliari, D.; Tiselius, P.; Hansen, B.W.; Sköld, H.N. Gender-specific ageing and non-Mendelian inher-itance of oxidative damage in marine copepods. Mar. Ecol. Prog. Ser. 2010, 401, 1–13. [Google Scholar] [CrossRef]
- Khosrovyan, A.; Rodríguez-Romero, A.; Salamanca, M.J.; Del Valls, T.A.; Riba, I.; Serrano, F. Comparative performances of eggs and embryos of sea urchin (Paracentrotus lividus) in toxicity bioassays used for assessment of marine sediment quality. Mar. Pollut. Bull. 2013, 70, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Romero, A.; Khosrovyan, A.; Del Valls, T.A.; Obispo, R.; Serrano, F.; Conradi, M.; Riba, I. Several benthic species can be used interchangeably in integrated sediment quality assessment. Ecotox. Environ. Safe 2013, 92, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, A.; Morroni, L.; Valentini, A.; Vitiello, V.; Renzi, M.; Nuccio, C.; Pellegrini, D. Comparison of different ecotoxicological batteries with WOE approach for the environmental quality evaluation of harbour sediments. Aquat. Toxicol. 2021, 237, 105905. [Google Scholar] [CrossRef] [PubMed]
- Voloshko, L.N.; Gavrilova, O.V.; Moustakas, M.B. Response of cyanobacteria strains to stress induced by heavy metal ions. Nova Hedwig. Beih. 2001, 123, 487–498. [Google Scholar]
- Reis, M.; Kraberg, A.C.; Erler, K.; Luckas, B. Ecotoxicology of different strains of Lingulodinium polyedrum from the Portugese coast. In Proceedings of the 12th International Conference on Harmful Algae, Copenhagen, Denmark, 4–8 September 2006; pp. 323–325. [Google Scholar]
- Soucek, D.J.; Mount, D.R.; Dickinson, A.; Hockett, R.; McEwen, A.R. Contrasting effects of chloride on growth, reproduction, and toxicant sensitivity in two genetically distinct strains of Hyalella azteca. Environ. Toxicol. Chem. 2015, 34, 2354–2362. [Google Scholar] [CrossRef] [PubMed]
- EC Regulation N. 1907 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/ EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EC, 93/67/EEC, 93/105/EC and 2001/21/EC, The European Parliament and the Council of the European Union. 2006. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1907-20140410 (accessed on 2 April 2023).
- EC Regulation N. 1272 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of Substances and Mixtures, Amending and Repealing Directives 67/548/EEC and 1999/45/EEC, and Amending Regulation 1907/2006. European Commission, ed. 2008. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:353:0001:1355:EN:PDF (accessed on 13 July 2023).
- Barata, C.; Baird, D.J.; Mitchell, S.E.; Soares, A.M.V.M. Among- and within-population variability in tolerance to cadmium stress in natural populations of Daphnia magna: Implications for ecological risk assessment. Environ. Toxicol. Chem. 2002, 21, 1058–1064. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.H.; Altin, D.; Rørvik, S.F.; Øverjordet, I.B.; Olsen, A.J.; Nordtug, T. Comparative study on acute effects of water accommodated fractions of an artificially weathered crude oil on Calanus finmarchicus and Calanus glacialis (Crustacea: Copepoda). Sci. Total Environ. 2011, 409, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Carotenuto, Y.; Miralto, A.; Procaccini, G.; Ianora, A. Copepod population-specific response to a toxic diatom diet. PLoS ONE 2012, 7, e47262. [Google Scholar] [CrossRef] [PubMed]
- Baird, D.J.; Barata, C. Genetic variation in the response of Daphnia to toxic substances: Implications for risk assessment. In Genetic and Ecotoxicology; Forbes, V.E., Ed.; Taylor & Francis: Philadelphia, PA, USA, 1998; pp. 207–220. [Google Scholar] [CrossRef]
- Lovett Doust, L.; Lovett Doust, J.; Schmidt, M. In praise of plants as biomonitors send in the clones. Funct. Ecol. 1993, 7, 754–758. [Google Scholar]
- Picado, A.; Chankova, S.; Fernandes, A.; Simões, F.; Leverett, D.; Johnson, I.; Hernan, R.; Pires, A.M.; Matos, J. Genetic varia-bility in Daphnia magna and ecotoxicological evaluation. Ecotoxicol. Environ. Safe 2007, 67, 406–410. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).