Zooplankton Temporal, Longitudinal, and Vertical Diversity Patterns in the Floodplains of the Western Amazon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Protocols
2.3. Statistical Analyses
3. Results
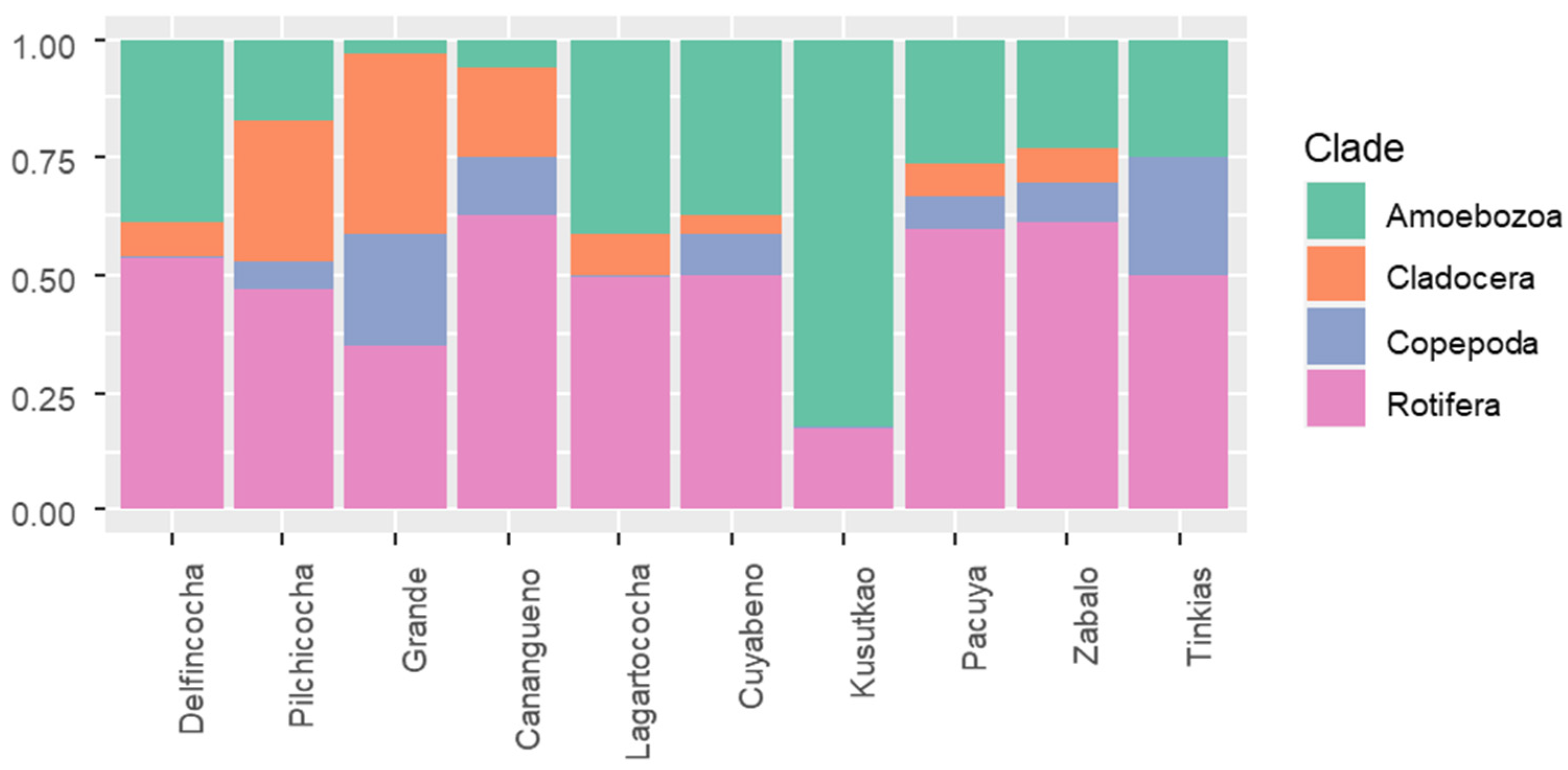
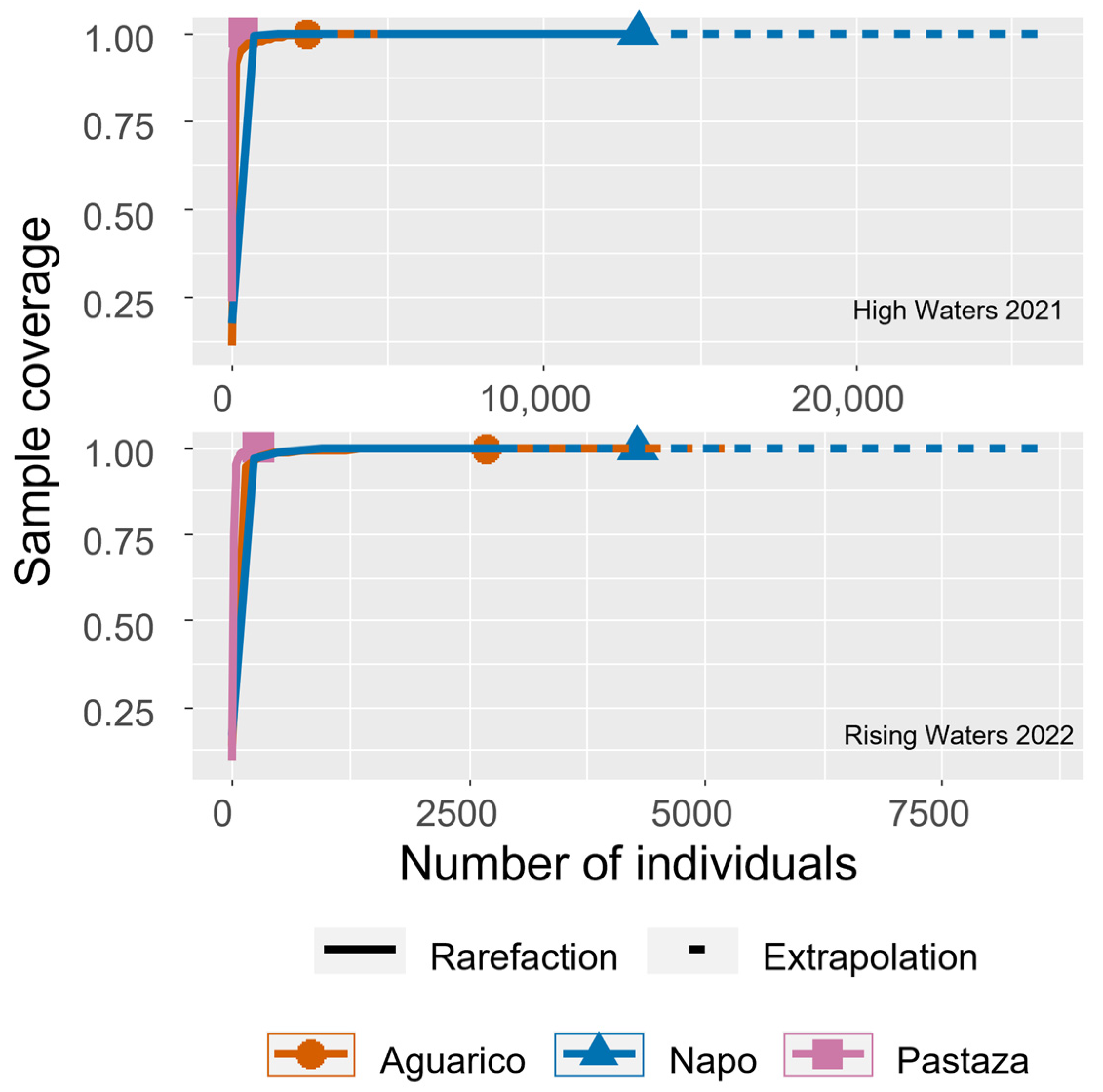
3.1. Zooplankton Diversity Patterns in the Floodplains of the Napo and Pastaza Watersheds
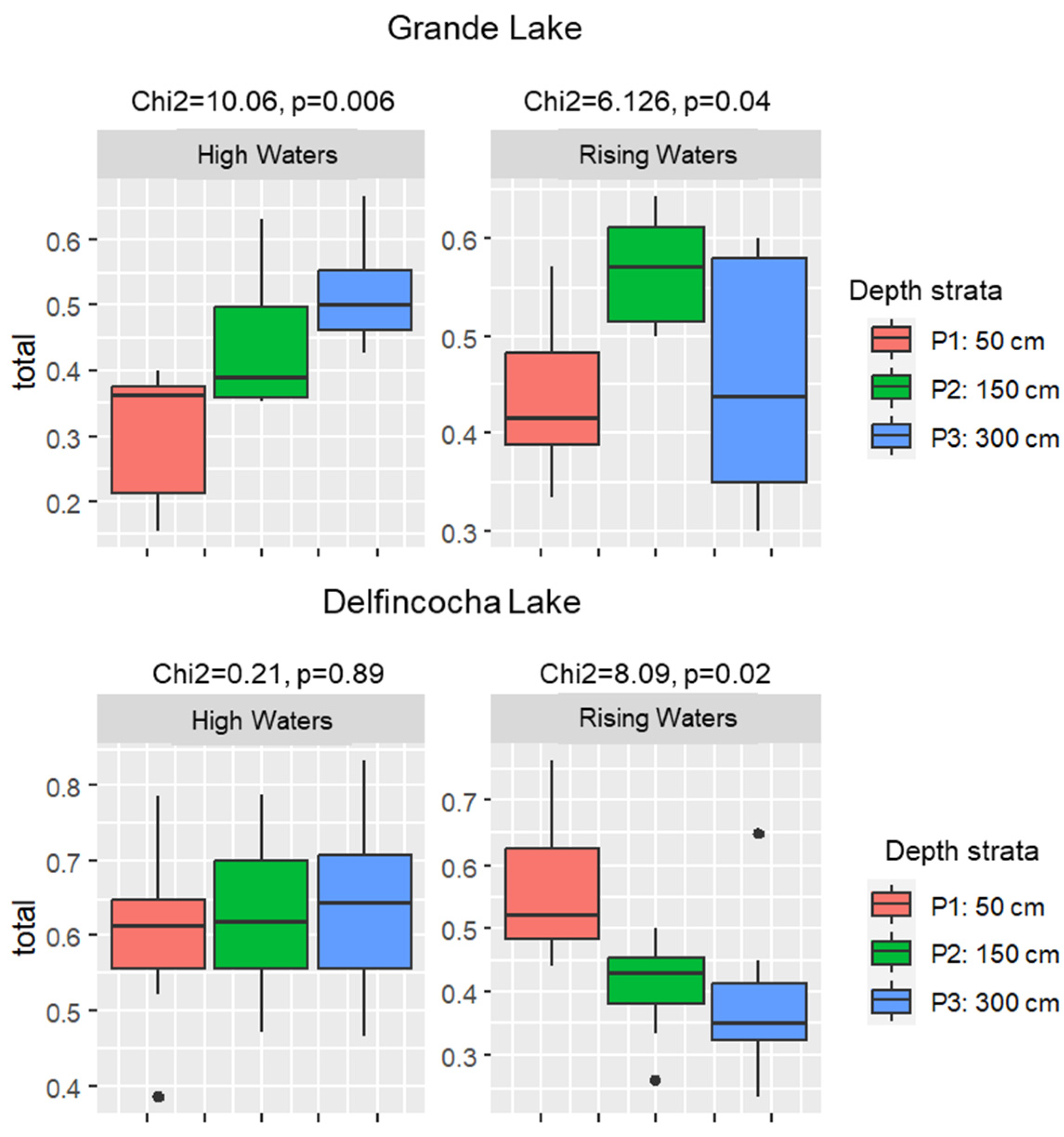
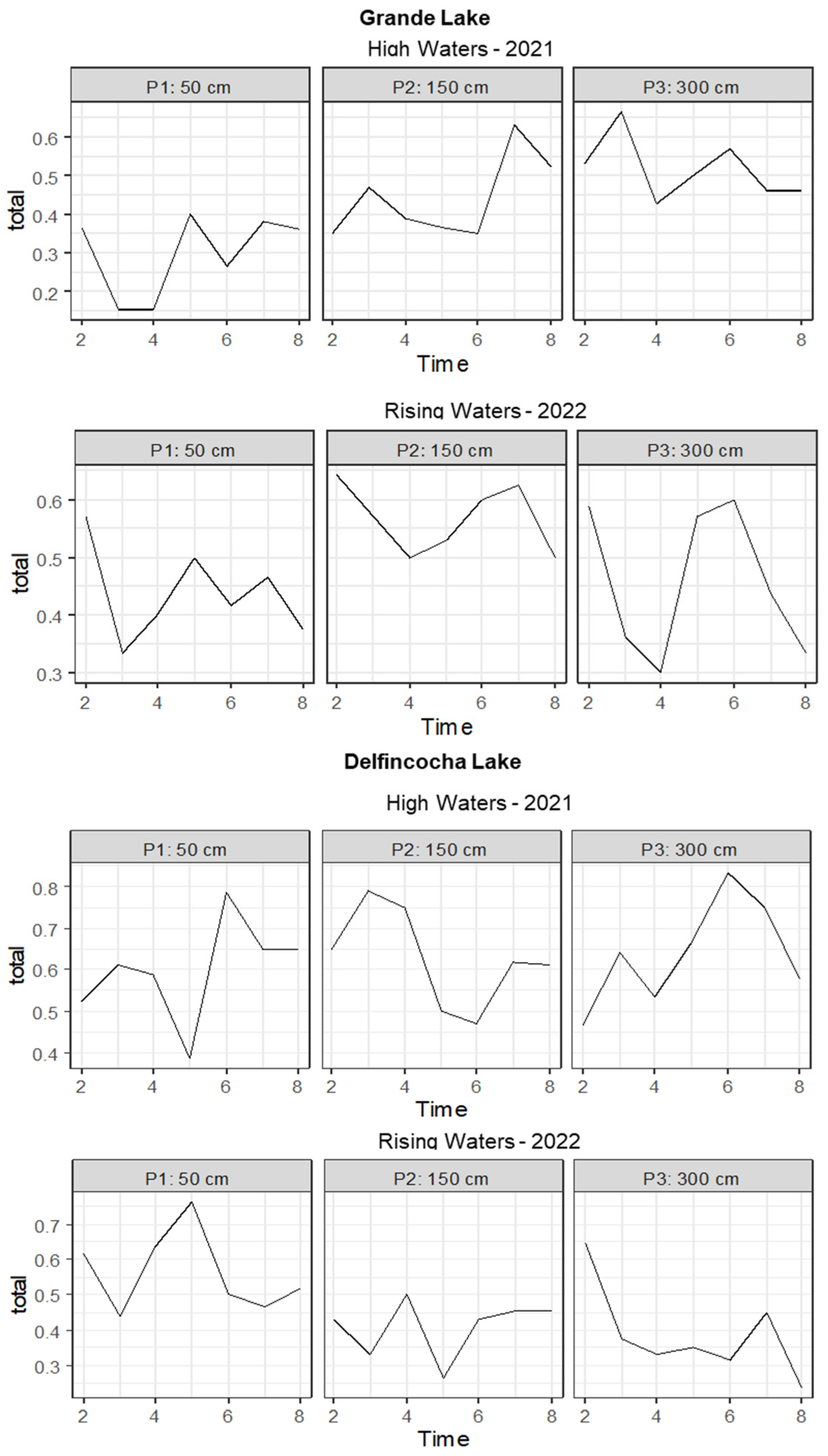
3.2. Temporal and Vertical Zooplankton Diversity Patterns within Delfincocha and Grande Lakes
4. Discussion
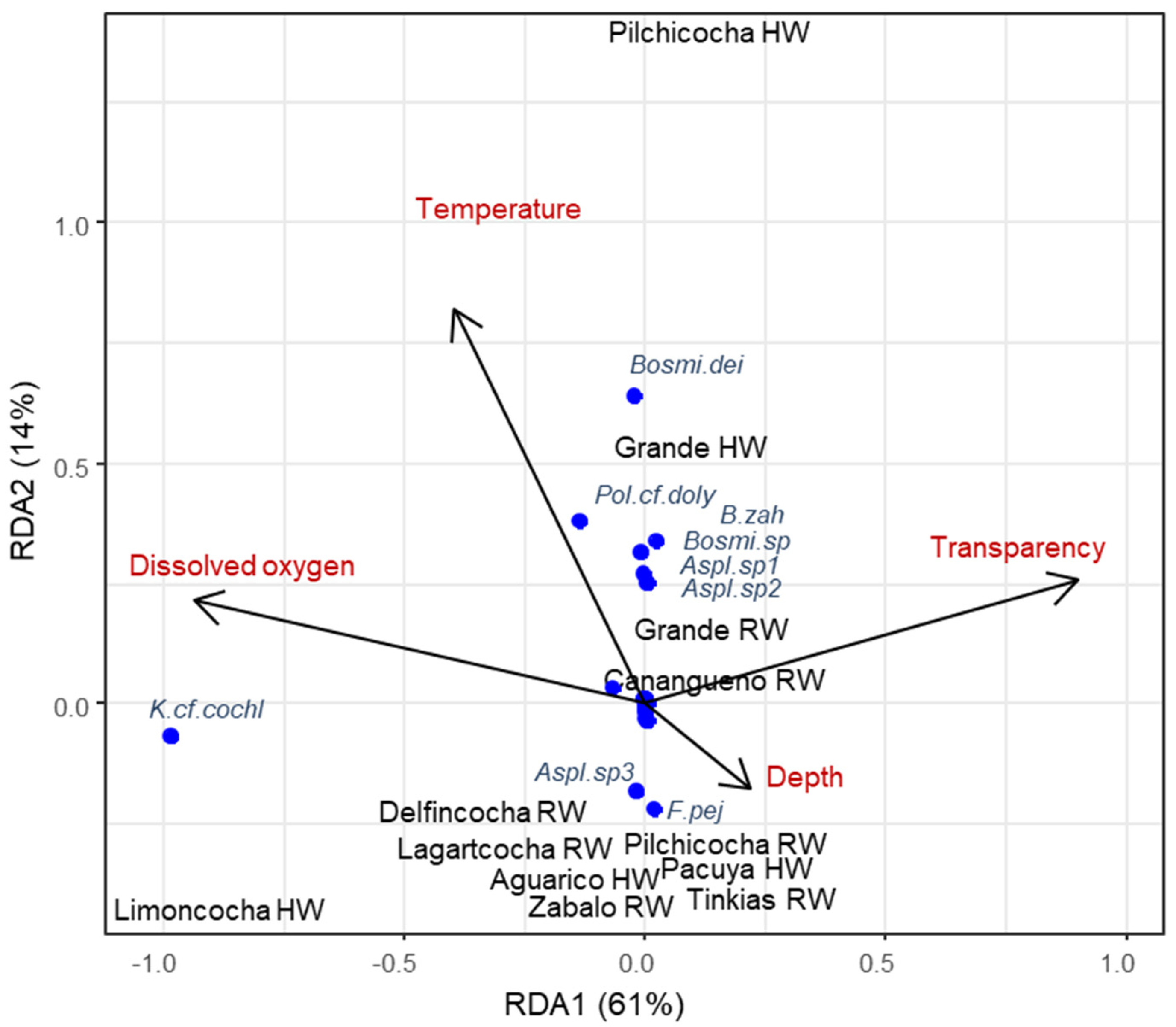
4.1. Zooplankton Diversity Patterns in the Floodplains of the Napo and Pastaza Watersheds
4.2. Vertical and Temporal Zooplankton Diversity Patterns
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poff, N.L. Landscape filters and species traits: Towards mechanistic understanding and prediction in stream ecology. J. N. Am. Benthol. Soc. 1997, 16, 391–409. [Google Scholar] [CrossRef]
- Thorp, J.; Thoms, M.; Delong, M. The Riverine Ecosystem Synthesis; Elsevier: London, UK, 2008. [Google Scholar]
- Aranguren-Riaño, N.; Guisande, C.; Ospina, R. Factors controlling crustacean zooplankton species richness in Neotropical lakes. J. Plankton Res. 2011, 33, 1295–1303. [Google Scholar] [CrossRef]
- Simões, N.R.; Dias, J.D.; Leal, C.M.; de Souza Magalhães Braghin, L.; Lansac-Tôha, F.A.; Bonecker, C.C. Floods control the influence of environmental gradients on the diversity of zooplankton communities in a neotropical floodplain. Aquat. Sci. 2013, 75, 607–617. [Google Scholar] [CrossRef]
- Thomaz, S.M.; Bini, L.M.; Bozelli, R.L. Floods increase similarity among aquatic habitats in river-floodplain systems. Hydrobiologia 2007, 579, 1–13. [Google Scholar] [CrossRef]
- Junk, W.; Bayley, P.; Sparks, E. The Flood Pulse Concept in River—Floodplain Systems. Can. J. Fish. Aquat. Sci. 1989, 106, 110–127. [Google Scholar]
- Blackburn-Desbiens, P.; Grosbois, G.; Power, M.; Culp, J.; Rautio, M. Integrating hydrological connectivity and zooplankton composition in Arctic ponds and lakes. Freshw. Biol. 2023, 68, 2131–2150. [Google Scholar] [CrossRef]
- Napiórkowski, P.; Bąkowska, M.; Mrozińska, N.; Szymańska, M.; Kolarova, N.; Obolewski, K. The effect of hydrological connectivity on the zooplankton structure in floodplain lakes of a regulated large river (the Lower Vistula, Poland). Water 2019, 11, 1924. [Google Scholar] [CrossRef]
- Jenkins, C.N.; Pimm, S.L.; Joppa, L.N. Global patterns of terrestrial vertebrate diversity and conservation. Proc. Natl. Acad. Sci. USA 2013, 110, E2602–E2610. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Reines, J.M.; de Roa, E.Z. New additions to the cladoceran fauna of Ciénaga Grande de Santa Marta and Colombia. Check List 2013, 9, 9–24. [Google Scholar] [CrossRef][Green Version]
- López, C.; Mosquera, P.V.; Hampel, H.; Neretina, A.N.; Alonso, M.; Van, D.K.; Kotov, A.A. An annotated checklist of the freshwater cladocerans (Crustacea: Branchiopoda: Cladocera) of Ecuador and the Galápagos Islands. Invertebr. Zool. 2018, 15, 277–291. [Google Scholar] [CrossRef]
- López, C.; Soto, L.M.; Lafuente, W.; Stamou, G.; Michaloudi, E.; Papakostas, S.; Fontaneto, D. Rotifers from inland water bodies of continental Ecuador and Galápagos Islands: An updated checklist. Zootaxa 2020, 4768, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Flores, J.M.; Garate-Quispe, J.; Molinos, J.G.; Pillaca-Ortiz, J.M.; Caballero-Espejo, J.; Ascorra, C.; Silman, M.; Fernandez, L.E. Seasonality and aquatic metacommunity assemblage in three abandoned gold mining ponds in the southwestern Amazon, Madre de Dios (Peru). Ecol. Indic. 2021, 125, 107455. [Google Scholar] [CrossRef]
- Andrade-Sossa, C.; Alvarez-Silva, J.P.; Aranguren-Riaño, N.; Cupitra-Gómez, O.S.; Villabona-González, S.L.; Torres-Bejarano, A.M.; López, C. Zooplankton studies in Colombian fresh and brackish water ecosystems: A review and future perspectives. Limnologica 2023, 100, 126081. [Google Scholar] [CrossRef]
- Castello, L.; McGrath, D.G.; Hess, L.L.; Coe, M.T.; Lefebvre, P.A.; Petry, P.; Macedo, M.N.; Renó, V.F.; Arantes, C.C. The vulnerability of Amazon freshwater ecosystems. Conserv. Lett. 2013, 6, 217–229. [Google Scholar] [CrossRef]
- Finer, M.; Jenkins, C.N.; Pimm, S.L.; Keane, B.; Ross, C. Oil and Gas Projects in the Western Amazon: Threats to Wilderness, Biodiversity, and Indigenous Peoples. PLoS ONE 2008, 3, e2932. [Google Scholar] [CrossRef] [PubMed]
- Mestanza-Ramón, C.; Cuenca-Cumbicus, J.; D’orio, G.; Flores-Toala, J.; Segovia-Cáceres, S.; Bonilla-Bonilla, A.; Straface, S. Gold Mining in the Amazon Region of Ecuador: History and a Review of Its Socio-Environmental Impacts. Land 2022, 11, 221. [Google Scholar] [CrossRef]
- Comité ERFEN-Ecuador. BOLETÍN ERFEN Nro. 012-2021. 2021. Available online: www.inocar.mil.ec (accessed on 1 March 2022).
- Wetzel, R.G.; Likens, G. Limnological Analyses; Springer Science & Business Media: New York, USA, 2000. [Google Scholar]
- Sousa, F.D.R.; Elmoor-Loureiro, L.M.A. Identification key for the Brazilian genera and species of Aloninae (Crustacea, Branchiopoda, Anomopoda, Chydoridae). Pap Avulsos Zool. 2019, 59, e20195924. [Google Scholar] [CrossRef]
- Elmoor-Loureiro, L.M.A. Manual de Identificação de Cladóceros Límnicos do Brasil; Universa Brasília: Brasilia, Brazil, 1997; Volume 1. [Google Scholar]
- Kotov, A.A.; Stifter, P. Notes on the genus Ilyocryptus Sars, 1862 (Cladocera: Anomopoda: Ilyocryptidae). 1. Ilyocryptus plumosus sp. n., a primitive Neotropical member of the I. spinifer-group. Arthropoda Selecta. Русский Артрoпoдoлoгический журнал 2004, 13, 193–198. [Google Scholar]
- Fuentes-Reinés, J.M.; Elmoor-Loureiro, L.M.A. Annotated checklist and new records of Cladocera from the Ciénaga El Convento, Atlántico-Colombia. Panam. J. Aquat. Sci. 2015, 10, 189–202. [Google Scholar]
- Andrade-Sossa, C.; Buitron-Caicedo, L.; Elías-Gutiérrez, M. A new species of Scapholeberis Schoedler, 1858 (Anomopoda: Daphniidae: Scapholeberinae) from the Colombian Amazon basin highlighted by DNA barcodes and morphology. PeerJ 2020, 8, e9989. [Google Scholar] [CrossRef] [PubMed]
- Witty, L.M. Practical Guide to Identifying Freshwater Crustacean Zooplankton, 2nd ed.; Cooperative Freshwater Ecology Unit: Sudbury, ON, USA, 2004. [Google Scholar]
- Siemensma, F.J. Microworld, Mundo de los Organismos Ameboides. Publicación Electrónica Mundial. Available online: https://arcella.nl/ (accessed on 1 May 2022).
- De Caceres, M.; Jansen, F.; De Caceres, M.M. Relationship between Species and Groups of Sites. Package ‘Indicspecies’. 2016, Version 1.6. Available online: https://emf-creaf.github.io/indicspecies/ (accessed on 20 February 2024).
- Li, D. hillR: Taxonomic, functional, and phylogenetic diversity and similarity through Hill Numbers. J. Open Source Softw. 2018, 3, 1041. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNEXT. Interpolation and Extrapolation for Species Diversity.” cran.r-project.org, p. 18. 2016. Available online: http://chao.stat.nthu.edu.tw/blog/software-download/ (accessed on 1 July 2022).
- Gotelli, N.J.; Chao, A. Measuring and Estimating Species Richness, Species Diversity, and Biotic Similarity from Sampling Data. In Encyclopedia of Biodiversity, 2nd ed.; Academic Press: Waltham, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5, pp. 195–211. [Google Scholar] [CrossRef]
- Alberdi, A. Integral Analysis of Diversity Based on Hill Numbers.” cran.r-project.org, p. 34. 2022. Available online: https://github.com/anttonalberdi/hilldiv (accessed on 1 July 2022).
- Legendre, P.; Legendre, L. Numerical Ecology, 2nd ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Hallett, L.M.; Jones, S.K.; MacDonald, A.A.M.; Jones, M.B.; Flynn, D.F.B.; Ripplinger, J.; Slaughter, P.; Gries, C.; Collins, S.L. codyn: An r package of community dynamics metrics. Methods Ecol. Evol. 2016, 7, 1146–1151. [Google Scholar] [CrossRef]
- Lessmann, J.; Fajardo, J.; Muñoz, J.; Bonaccorso, E. Large expansion of oil industry in the Ecuadorian Amazon: Biodiversity vulnerability and conservation alternatives. Ecol. Evol. 2016, 6, 4997–5012. [Google Scholar] [CrossRef] [PubMed]
- Wernersson, A. Aquatic ecotoxicity due to oil pollution in the Ecuadorian Amazon Aquatic ecotoxicity due to oil pollution in the Ecuadorian Amazon. Aquat. Ecosyst. Health Manag. 2004, 7, 127–136. [Google Scholar] [CrossRef]
- Bozelli, R.L.; Thomaz, S.M.; Padial, A.A.; Lopes, P.M.; Bini, L.M. Floods decrease zooplankton beta diversity and environmental heterogeneity in an Amazonian floodplain system. Hydrobiologia 2015, 753, 233–241. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, X.; Qiu, X.; Liang, T.; Chen, J.; Zhao, S.; Ouyang, S.; Jin, B.; Wu, X. Changes and drivers of zooplankton diversity patterns in the middle reach of Yangtze River floodplain lakes, China. Ecol. Evol. 2021, 11, 17885–17900. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.K.; Sharma, S. Zooplankton diversity of Loktak Lake, Manipur, India. J. Threat. Taxa 2011, 3, 1745–1755. [Google Scholar] [CrossRef]
- Bagatini, I.L.; Spínola, A.L.G.; Peres, B.d.M.; Mansano, A.d.S.; Rodrigues, M.A.A.; Batalha, M.A.P.L.; de Lucca, J.V.; Godinho, M.J.L.; Tundisi, T.M.; Seleghim, M.H.R. Protozooplankton and its relationship with environmental conditions in 13 water bodies of the Mogi-Guaçu basin-SP, Brazil. Biota Neotrop. 2013, 13, 152–163. [Google Scholar] [CrossRef]
- Picapedra, P.H.D.S.; Fernandes, C.; Baumgartner, G.; Sanches, P.V. Drivers of zooplankton spatial dynamics in a small neotropical river. Acta Limnol. Bras. 2022, 34, e13. [Google Scholar] [CrossRef]
- Gabaldón, C.; Devetter, M.; Hejzlar, J.; Šimek, K.; Znachor, P.; Nedoma, J.; Seda, J. Repeated flood disturbance enhances rotifer dominance and diversity in a zooplankton community of a small dammed mountain pond. J. Limnol. 2017, 76. [Google Scholar] [CrossRef]
- Allan, J.D. Life History Patterns in Zooplankton. Am. Nat. 1976, 110, 165–180. [Google Scholar] [CrossRef]
- Pinel-Alloul, B.; Chemli, A.; Taranu, Z.E.; Bertolo, A. Using the diversity, taxonomic and functional attributes of a zooplankton community to determine lake environmental typology in the natural southern boreal lakes (Québec, Canada). Water 2022, 14, 578. [Google Scholar] [CrossRef]
- Lima, A.F.; Lansac-Tôha, F.A.; Velho, L.F.M.; Bini, L.M.; Takeda, A.M. Composition and abundance of Cladocera (Crustacea) assemblages associated with Eichhornia azurea (Swartz) Kunth stands in the Upper Paraná River floodplain. Acta Sci. Biol. Sci. 2003, 25, 41–48. [Google Scholar]
- Lima, A.F.; Lima, F.A.L.-T.; Velho, L.F.M.; Bini, L.M. Environmental Influence on Planktonic Cladocerans and Copepods in the Floodplain of the Upper River Paraná, Brazil. Stud. Neotrop Fauna Environ. 1998, 33, 188–196. [Google Scholar] [CrossRef]
- Tockner, K.; Malard, F.; Ward, J.V. An extension of the flood pulse concept. Hydrol. Process 2000, 14, 2861–2883. [Google Scholar] [CrossRef]
- Zhang, K.; Jiang, F.; Chen, H.; Dibar, D.T.; Wu, Q.; Zhou, Z. Temporal and spatial variations in zooplankton communities in relation to environmental factors in four floodplain lakes located in the middle reach of the Yangtze River, China. Environ. Pollut. 2019, 251, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Bonecker, C.C.; Simões, N.R.; Minte-Vera, C.V.; Lansac-Tôha, F.A.; Velho, L.F.M.; Agostinho, Â.A. Temporal changes in zooplankton species diversity in response to environmental changes in an alluvial valley. Limnologica 2013, 43, 114–121. [Google Scholar] [CrossRef]
- Pereira, A.L.A.; de Carvalho, P.; Granzotti, R.V.; Vieira, L.C.G.; Bini, L.M. Temporal beta diversity increases with environmental variability in zooplankton floodplain communities. Limnology 2024, 25, 1–10. [Google Scholar] [CrossRef]
- Diniz, L.P.; Petsch, D.K.; Bonecker, C.C. Zooplankton β diversity dynamics and metacommunity structure depend on spatial and temporal scales in a Neotropical floodplain. Freshw. Biol. 2021, 66, 1328–1342. [Google Scholar] [CrossRef]
- Ramos, E.A.; de Morais-Junior, C.S.; Rodrigues-Filho, C.A.S.; Sánchez-Botero, J.I.; Júnior, M.M.; Novaes, J.L.C. Influence of spatial and environmental factors on the structure of a zooplankton metacommunity in an intermittent river. Aquat. Ecol. 2022, 56, 239–249. [Google Scholar] [CrossRef]
- Gabriel, W.; Thomas, B. Vertical migration of zooplankton as an evolutionarily stable strategy. Am. Nat. 1988, 132, 199–216. [Google Scholar] [CrossRef]
- Bandara, K.; Varpe, Ø.; Wijewardene, L.; Tverberg, V.; Eiane, K. Two hundred years of zooplankton vertical migration research. Biol. Rev. 2021, 96, 1547–1589. [Google Scholar] [CrossRef] [PubMed]
- Lampert, W. The adaptive significance of diel vertical migration of zooplankton. Funct. Ecol. 1989, 3, 21–27. [Google Scholar] [CrossRef]
- Dodson, S. Predicting diel vertical migration of zooplankton. Limnol. Oceanogr. 1990, 35, 1195–1200. [Google Scholar] [CrossRef]
- Rejas, D.; De Meester, L.; Ferrufino, L.; Maldonado, M.; Ollevier, F. Diel vertical migration of zooplankton in an Amazonian várzea lake (Laguna Bufeos, Bolivia). Stud. Neotrop. Fauna Environ. 2007, 42, 71–81. [Google Scholar] [CrossRef]
- Antón-Pardo, M.; Muška, M.; Jůza, T.; Vejříková, I.; Vejřík, L.; Blabolil, P.; Čech, M.; Draštík, V.; Frouzová, J.; Holubová, M.; et al. Diel changes in vertical and horizontal distribution of cladocerans in two deep lakes during early and late summer. Sci. Total Environ. 2021, 751, 141601. [Google Scholar] [CrossRef] [PubMed]
- Echevarría, G.; Rodríguez, J.P.; Machado-Allison, A. Seasonal fluctuations in taxonomic and functional diversity in assemblages of catfishes in the Venezuelan Arauca River Floodplain. Stud. Neotrop. Fauna Environ. 2018, 53, 38–53. [Google Scholar] [CrossRef]
- Coelho, P.N.; Henry, R. Functional groups of microcrustaceans along a horizontal gradient in a Neotropical lake colonized by macrophytes. Aquat. Sci. 2020, 83, 3. [Google Scholar] [CrossRef]
- Stansfield, J.H.; Perrow, M.R.; Tench, L.D.; Jowitt, A.J.D.; Taylor, A.A.L. Submerged macrophytes as refuges for grazing Cladocera against fish predation: Observations on seasonal changes in relation to macrophyte cover and predation pressure. In Shallow Lakes ’95: Trophic Cascades in Shallow Freshwater and Brackish Lakes; Kufel, L., Prejs, A., Rybak, J.I., Eds.; Springer: Dordrecht, The Netherlands, 1997; pp. 229–240. [Google Scholar] [CrossRef]
- Corgosinho, P.H.C.; Holynska, M.; Marrone, F.; Geraldes-Primeiro, L.J.d.O.; dos Santos-Silva, E.N.; Perbiche-Neves, G.; Lopez, C. An annotated checklist of freshwater Copepoda (Crustacea, Hexanauplia) from continental Ecuador and the Galapagos Archipelago. Zookeys 2019, 871, 55. [Google Scholar] [CrossRef] [PubMed]

| Hill Number | Diversity | ||
|---|---|---|---|
| High Waters—2021 | |||
| q | Gamma | Alpha | Beta |
| 0 | 81.00 | 19.40 | 4.17 |
| 1 | 22.9 | 6.54 | 3.50 |
| 2 | 15.64 | 3.62 | 4.28 |
| Rising Waters—2022 | |||
| q | Gamma | Alpha | Beta |
| 0 | 48 | 14.27 | 3.36 |
| 1 | 21.5 | 6.65 | 3.23 |
| 2 | 14.95 | 4.02 | 3.72 |
| Indicator Species | A | B | Stat | p Value |
|---|---|---|---|---|
| High Waters—2021: Group 1 + 3: Limoncocha and Canangueno Lakes | ||||
| Keratella cf. cochlearis | 0.9942 | 1 | 0.997 | 0.049 |
| Hexarthra intermedia braziliensis | 0.9764 | 1 | 0.988 | 0.049 |
| Copepoda Calanoida.sp3 | 0.9446 | 1 | 0.972 | 0.026 |
| Rising Waters—2022: Group 2 + 3: Zancudococha and Delfincocha Lakes | ||||
| Hexarthra intermedia braziliensis | 0.9985 | 1 | 0.999 | 0.033 |
| Watershed | Water Body | High Waters—2021 | Rising Waters—2022 | ||||
|---|---|---|---|---|---|---|---|
| q0 | q1 | q2 | q0 | q1 | q2 | ||
| Aguarico | Lagartococha River | 22 | 12.34 | 8.23 | 21.00 | 10.90 | 6.49 |
| Delfincocha Lake | 40 | 19.30 | 11.20 | 28.00 | 8.06 | 3.75 | |
| Canangueno Lake | 16 | 2.92 | 1.83 | 14.00 | 8.19 | 7.05 | |
| Grande Lake | 33 | 9.26 | 5.68 | 16.00 | 7.48 | 5.13 | |
| Cuyabeno River | 22 | 14.64 | 11.05 | 17.00 | 13.20 | 11.08 | |
| Zancudococha Lake | NA | NA | NA | 6.00 | 3.05 | 2.32 | |
| Aguarico River | 4 | 2.92 | 2.47 | NA | NA | NA | |
| Zabalo River | NA | NA | NA | 11.00 | 4.92 | 2.91 | |
| Napo | Pilchicocha Lake | 16 | 5.64 | 4.37 | 15.00 | 5.63 | 3.03 |
| Pacuya River | NA | NA | NA | 13.00 | 6.71 | 4.06 | |
| Limoncocha Lake | 16 | 3.91 | 2.30 | NA | NA | NA | |
| Pañacocha Lake | 16 | 4.43 | 2.35 | NA | NA | NA | |
| Pastaza | Kusutkao River | 9 | 5.37 | 4.13 | 13.00 | 10.84 | 10.04 |
| Nueva Tinkias Lake | NA | NA | NA | 3.00 | 2.59 | 2.27 | |
| Delfincocha Lake | |||
| Estimate | t value | p value | |
| Temperature | −0.171 | −3.190 | 0.003 |
| Dissolved oxygen | 0.034 | 0.257 | 0.799 |
| Grande Lake | |||
| Estimate | t value | p value | |
| Temperature | 0.068 | 2.064 | 0.046 |
| Dissolved oxygen | −0.077 | −3.302 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez, F.; Cuesta, F.; Echevarría, G. Zooplankton Temporal, Longitudinal, and Vertical Diversity Patterns in the Floodplains of the Western Amazon. Water 2024, 16, 1166. https://doi.org/10.3390/w16081166
Sánchez F, Cuesta F, Echevarría G. Zooplankton Temporal, Longitudinal, and Vertical Diversity Patterns in the Floodplains of the Western Amazon. Water. 2024; 16(8):1166. https://doi.org/10.3390/w16081166
Chicago/Turabian StyleSánchez, Fernando, Francisco Cuesta, and Gabriela Echevarría. 2024. "Zooplankton Temporal, Longitudinal, and Vertical Diversity Patterns in the Floodplains of the Western Amazon" Water 16, no. 8: 1166. https://doi.org/10.3390/w16081166
APA StyleSánchez, F., Cuesta, F., & Echevarría, G. (2024). Zooplankton Temporal, Longitudinal, and Vertical Diversity Patterns in the Floodplains of the Western Amazon. Water, 16(8), 1166. https://doi.org/10.3390/w16081166





