Abstract
Environmental changes are important factors related to shifts in species compositions and abundances of aquatic communities. This study presents the responses of cladoceran communities to realistic scenarios of an increase in temperature and phosphorus concentration. This study was conducted under laboratory conditions, and the outcomes of this study explain the causes of seasonal shifts in both abundance and species composition and allow us to predict their responses to climatic changes in aquatic ecosystems. The results showed that temperature increase was the more important trigger of shifts than phosphorus increase. Moreover, the simultaneous influence of increases in temperature and phosphorus concentration had a significantly higher impact than single factors. Under all the scenarios, the increased contributions of species that were dominant before the changes and the extinction of rare species were observed. Ultimately, cladoceran communities displayed functional overcompensation and loss of species in comparison to prechanged communities.
1. Introduction
Freshwater ecosystems, an important component of the landscape, are strongly impacted by numerous climate- and human-driven factors, including warming, increased nutrient load, and their combined effect [1,2]. Temperature has a strong effect on living organisms at various levels of biological organization, from biochemical processes in cells to the generation time of populations [3]. Temperatures have increased globally, and numerous simulations suggest further warming. According to Christensen et al. [4], climate warming affects freshwater ecosystems more than oceans due to the smaller thermal inertia and lower resources of water available for evaporative cooling on land. According to the Atmosphere–Ocean General Circulation Model, Africa, Europe, the polar regions, Northern and Central Asia, and most of the Americas warm more than the global average [4]. Moreover, diel minimum temperatures increase faster than maximum temperatures, resulting in a global decrease in the diel range of temperatures [5]. Zooplankton populations, including cladocerans, display species-specific (i.e., related to phenology) and habitat-specific (i.e., related to spatial architecture, including quality of refuges) seasonal and diel cycles that are linked to the seasonality and durability of temperature. Under sufficient feeding resources, an increase in temperature within the tolerance range of a species positively influences rates of growth, development, and reproduction. Thus, the size of zooplankton populations displays a strong correlation with seasonal fluctuations in temperature, resulting in an abundance peak that typically occurs earlier with warmer temperatures [6,7]. An increase in temperature might directly result in an increased number of generations and population instability, as well as alterations in the phenology and life histories of zooplankton communities [8]. Shifts in a number of generations seem to be especially important from an ecological perspective since additional generations per unit of time can strongly influence population size. Climate warming can also indirectly influence the population dynamics and life histories of zooplankton through its effect on the seasonality of available feeding resources and other components of the ecosystem, including the span of the growing season [9]. This indirect effect of climate warming might be especially strong in temperate regions where the quantity and quality of phytoplankton display highly seasonal variability [10,11,12].
Phosphorus is a primary component of nucleic acids and some intermediary metabolites. Phosphorus occurs in the pentavalent form (orthophosphates, pyrophosphates, longer-chain polyphosphates, organic phosphate esters and phosphodiesters, and organic phosphonates) in aquatic ecosystems. Lake ecosystems receive most of the phosphorus in surface flows. Phosphorus is delivered to aquatic systems as a mixture of dissolved and particulate inputs, each of which is a complex mixture of these different molecular forms of pentavalent phosphorus. Phosphorus is a very dynamic element, so the particulates may release phosphate and organic soluble phosphates. Moreover, various phosphorus compounds are chemically or enzymatically hydrolyzed to orthophosphate, which is the only form of phosphorus that can be assimilated by bacteria and primary producers. Particulates may be deposited in the bottom sediments, but under appropriate conditions, they can be converted to dissolved orthophosphate. In the northern temperate zone, a warming climate would boost the phosphorus load on lakes due to higher winter rainfall [1]. Erosion processes are major contributors of nutrients, including phosphorus, to water ecosystems; thus, increased surface flow results in increased phosphorus loading in these ecosystems [13]. Apart from natural sources, freshwater ecosystems receive phosphorus from many anthropogenic origins, including the leaching of agricultural soils and industrial and urban sewage. Phosphorus is the main limiting compound in water ecosystems. In water ecosystems, primary producers assimilate inorganic forms of phosphorus during primary production. This production is then utilized by herbivorous animals. Phosphorus limitation impacts the upper trophic levels by reducing the total primary production and thus reducing food availability. However, the quality of food regarded as the algal mineral content of phosphorus also limits the abundance of some herbivorous zooplankton, including cladocerans. This appears when the digestibility of algae or their biochemical profiles are altered under nutrient limitation [14,15]. Under experimental conditions, cladocerans fed phosphorus-limited algae displayed reduced growth and reproduction even when food was provided at high concentrations [16,17,18]. Under natural conditions, seston C: P ratios indicating the phosphorus limitation of herbivores are also often observed [19,20]. On the other hand, the increase in overall lake nutrient levels, including phosphorus, has been identified as an important contributor to worsening water quality in freshwater ecosystems [21].
The order Cladocera is an old group of branchiopod crustaceans known since at least the mid-Mesozoic era [22]. Palaeoecological studies have shown that the diversity and abundance of this group altered in response to the severe climatic and environmental changes that occurred across Earth’s history [23] and resulted in the evolution of modern taxa through extinctions and evolutionary speciation [24]. Cladocerans perform a crucial role in aquatic ecosystems, including active grazing on algae, detritus, and various heterotrophs; taking part in nutrient regeneration; and being food for fish and planktivorous organisms [25]. Cladocerans directly and indirectly support ecosystem services such as clearing water through the top-down control of algae and bacteria and supporting freshwater fauna for human consumption [26]. They also participate in the potential responses of aquatic ecosystems to multiple types of environmental changes and partake in ecosystem stability, i.e., the ability to maintain stable functioning when faced with a varying environment [27]. As with phytoplankton, zooplankton have characteristic seasonal cycles that are strongly linked to the seasonality of temperature (arctic and temperate regions), hydrology (tropical regions), food availability, and predation pressure. Variations in these factors can modify the population fluctuations in these organisms. Indeed, the interplay between seasonal increases in temperature, resource availability, and predation pressure results in the typical unimodal and bimodal patterns in zooplankton seasonality often observed in temperate lakes [28,29], whereas a combination of year-round strong predation pressure and hydrological forcing seems to govern zooplankton seasonality in warmer lakes [30].
This study presents cladoceran responses to increased temperature and phosphorus concentration. Each of these factors might influence cladocerans in different ways. Previous studies have shown that the concentration of phosphorus in lakes correlates with zooplankton abundance [31]. Cladocerans, similar to other ectotherm organisms, can cope with suboptimal temperatures due to perpetuated evolutionary mechanisms and current phenotypic plasticity [32]. Thus, an environmentally realistic increase in temperature might not significantly influence cladocerans due to yearly broad temperature fluctuations in temperate ecosystems, but it might influence the impact of other environmental factors, including phosphorus [33,34].
2. Materials and Methods
2.1. Model System
Water for the laboratory experiment was taken from a small (0.35 ha) and shallow (maximum depth 2.2 m) fishless polyhumic water pool (eastern Poland, 51° N, 23° E). The pool was located in a peat bog formed of Sphagnum angustifolium, Sphagnum cuspidatum, Polytrichum spp., Eriophorum vaginatum, Carex acutiformis sp., and Equisetum limosum. Water, together with all living organisms, was placed in 30 L plastic containers and transported to the laboratory, where the experiment was set up in 30 L glass tanks (0.25 m diameter, 15 cm deep). Moreover, a 4 cm layer of bottom sediment collected from the pool was placed in each tank to simulate the environmental conditions as accurately as possible. In the experiment with the temperature increase, the temperature was increased by 4 °C above the ambient temperature using the TX-30 temperature modification system; thus, the final temperature was 23 °C. In the experiment with the phosphorus increase, the initial concentration of P-PO43− was doubled at the beginning of the experiment to obtain the concentration of 0.01 mg/L. An experiment with a simultaneous increase in temperature and phosphorus (23 °C × 0.01 mg/L of P-PO43−) was also established. Each experimental treatment was conducted in three replications. The experiments were run for seven weeks, and samples were collected on day 1 (before the increase in temperature and phosphorus concentration) and then on days 21, 28, 35, 42, and 49. To determine the abundance of cladocerans in each of the experimental treatments, 100 mL of water was passed through a 70 µm sieve to separate the animals. All individuals were identified at the species level and enumerated using a microscope. In each treatment, the number of species, the density, and the structure of dominance were calculated.
2.2. Response Metrics of Cladoceran Communities
Functional and compositional responses of cladocerans expressed as temporal stability, resistance, resilience, and similarity were calculated for each experimental community. Resistance—i.e., the ability to withstand the change—was calculated as the difference between the community at day 21 (i.e., changed community) and the community at day 1 (i.e., control community). The functional resistance, measured according to the equation ResistD = ln (Dday1/Dday21), ranged between −1 and 1, and ResistD = 0 was the highest resistance. The values of compositional resistance were measured as the initial similarity, ResistC = sim (Cday1/Cday21), where sim is the abundance-based Bray–Curtis index [35] between the 1-day and 21-day communities. The maximum compositional resistance corresponds to a similarity of 1 (100% similarity between day 1 and day 21), while values close to 0 reflect low resistance. Resilience (ResilD, ResilC) was measured for functions as the slope of the log-transformed difference between the community at day 1 and pressed communities over time. Given the log-transformed response variable, the recovery trend was linearized even when growth was exponential, in order to use linear regression. Resil = 0 means the lack of resilience, while Resil > 0 indicates recovery, whereas Resil < 0 means that the community further deviates from the control over time. For the species structure, resilience was reflected corresponding to the slope of similarity between the community at day 1 and the pressed community over time with the same benchmarks. The temporal stability (TstabD, TstabC) was measured as the inverse of the standard deviation of residuals around Resil over time. Larger values of functional and compositional stability mean lower fluctuations around the trend. The similarity of the disturbed communities and those prior to the disturbances (Simil) was measured as the degree of functional (SimilD) or compositional (SimilC) restoration at the end of the experiments, according to the equations SimilD = ln (Dday1/Dday49) and SimilC = sim (Cday1/Cday49). A functional similarity value equal to 0 means the maximum similarity to the status quo, whereas a compositional similarity value equal to 1 means the maximum similarity. Statistical analyses were performed with the use of Statistica® 13.1 software (https://www.statsoft.pl/statistica_13/ (accessed on 15 May 2023)). The normality and homogeneity of variances were determined using the Shapiro–Wilk test and Levene’s test, respectively. Differences in response metrics across treatments were determined by one-way ANOVA followed by pairwise comparisons using Tukey HSD. The level of significance was at least p < 0.05. The results are presented as means ± standard deviation (SD).
3. Results
3.1. Changes in Composition and Abundance of Cladocera during the Experiment
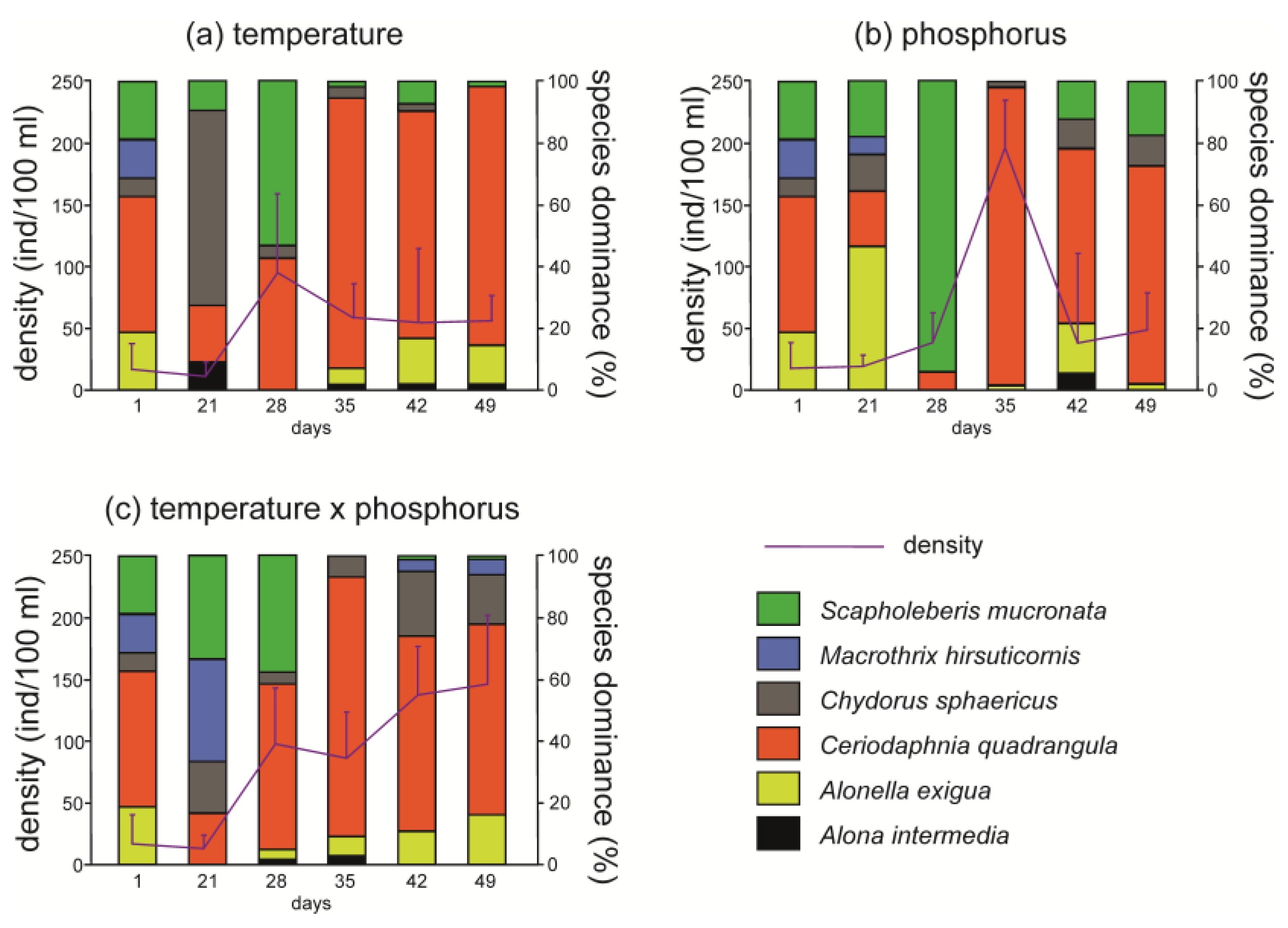
In all the treatments, a maximum of six species was found throughout the experiments, which is a typical number of cladoceran species in these pools [36]. In samples taken at day 1 (i.e., before the changes), five cladoceran species were found, and Ceriodaphnia quadrangula was dominant. The density of cladocerans increased during the experiment in comparison to the situation before the changes. Alterations in species dominance were also observed. In general, at day 21, species dominance displayed the greatest perturbations that would stabilize later. As a result, species dominance at the end of the experiment (day 49) was similar to species dominance at the beginning of the experiment (day 1, Figure 1).
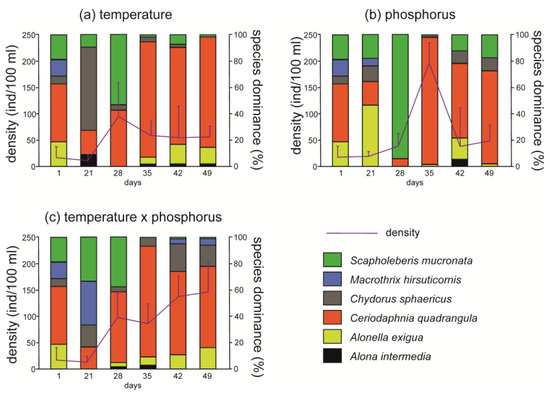
Figure 1.
Structure of dominance and density (mean ± SD, n = 3) of Cladocera in experimental treatments. (a) The treatment with the temperature increase to the final temperature 23 °C. (b) The treatment with the phosphorus increase to the final concentration of 0.01 mg/L. (c) The treatment with a simultaneous increase in temperature and phosphorus (23 °C × 0.01 mg/L of P-PO43−).
3.2. Response Metrics of Cladocera to Temperature and Phosphorus
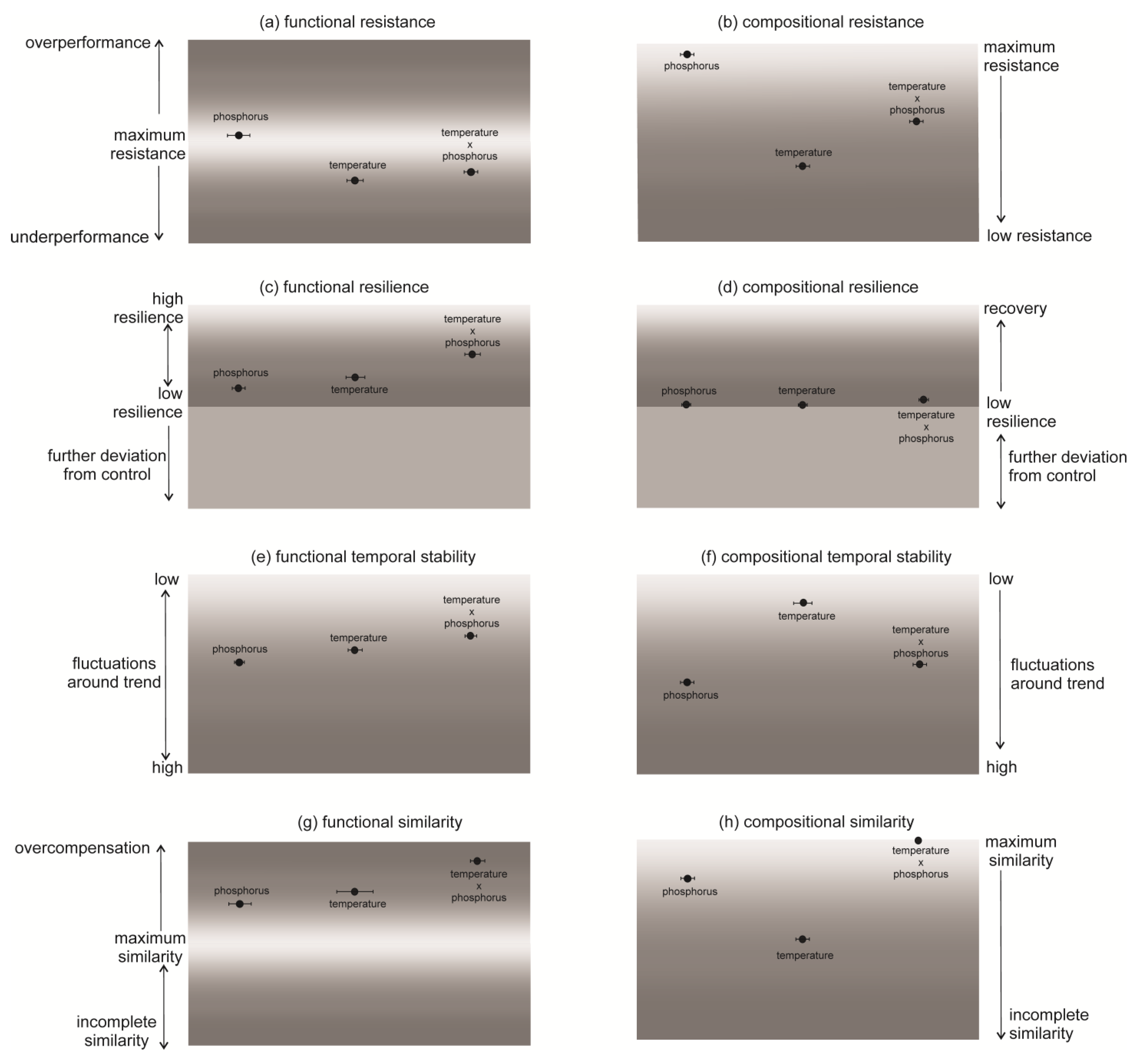
Cladocerans showed the highest functional and compositional resistance to the increase in phosphorus concentration (ResistD = 0.061, ResistC = 0,98). They turned out to be less resistant to the temperature (ResistD = −0.375, ResistC = 0.4) and the combination of the temperature and phosphorus (ResistD = 0.3288, ResistC = 0.64, Figure 2a,b). However, only the differences in functional resistance among treatments were statistically significant (Supplementary Information, Tables S1 and S2). For both function and composition, the populations reared under simultaneously increased temperature and phosphorus (temperature × phosphorus) displayed significantly higher resilience (Supplementary Information, Tables S3 and S4). Functional resilience ranged between ResilD = 0.171 in the treatment with phosphorus and ResilD = 0.507 in the treatment with the temperature x phosphorus increase (Figure 2c). The values of compositional resilience of the communities were much lower and ranged between ResilC = 0.01 for the treatments with temperature and phosphorus and ResilC = 0.07 for the treatment with temperature x phosphorus (Figure 2d). The lowest temporal stability in abundance was found for the phosphorus increase (TstabD = 1.372) and the highest for the temperature x phosphorus increase (TstabD = 2.775, Figure 2e); however, these differences were not statistically significant (Supplementary Information, Tables S5 and S6). Regarding composition, the stabilities of communities differed significantly among treatments (Supplementary Information, Tables S5 and S6). The lowest stability was observed for phosphorus (TstabC = 2.775) and the highest for temperature (TstabC = 5.167, Figure 2f). Finally, all the communities differed from those prior to the changes. Functional overcompensation was observed in all the treatments (SimilD between 0.916 for phosphorus and 2.079 for temperature × phosphorus, Figure 2g). In the case of composition, only the community in the treatment with temperature × phosphorus was similar to that before the change (SimilC = 1), whereas the others undercompensated regarding species composition (SimilC < 1, Figure 2h). Differences in the similarity of the changed communities and prior communities were statistically significant (Supplementary Information, Tables S7 and S8).
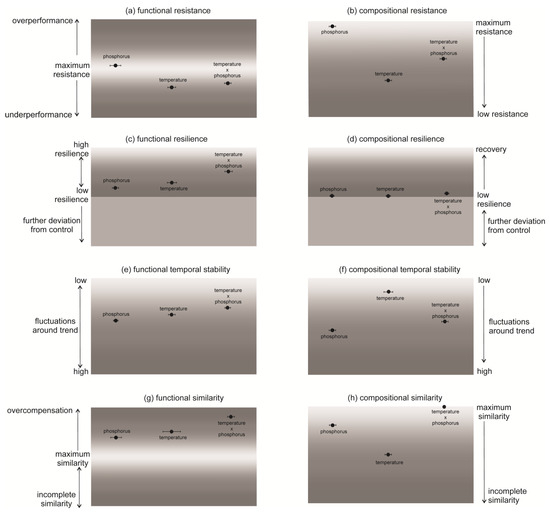
Figure 2.
Functional and compositional responses of Cladocera to increased temperature and phosphorus concentration (mean ± SD, n = 3). (a,b) Resistance—i.e., the ability to withstand the change—was calculated as the difference between the community at day 21 (i.e., changed community) and the community at day 1 (i.e., control community). (c,d) Resilience was measured for functions as the slope of the log-transformed difference between the community at day 1 and pressed communities over time. (e,f) The temporal stability was measured as the inverse of the standard deviation of residuals around Resil over time. (g,h) The similarity of the disturbed communities and these prior disturbances was measured as the degree of functional or compositional restoration at the end of the experiments.
4. Discussion
Ecological stability is the core concept of importance for understanding present-day and predicting future ecosystem dynamics. Ecological stability is supported by functionally important taxa, and changes in the abundance and phenology of these taxa might have repercussions for the functioning of ecosystems [37,38]. Cladocerans fall into the category of functionally important taxa as they are active grazers on algae, detritus, and various heterotrophs taking part in nutrient regeneration, as well as serve as food for fish and planktivorous organisms [25]. In this study, cladocerans were impacted by an environmentally relevant increase in temperature and phosphorus concentration. A lower effect on abundance and species composition was observed in the case of the phosphorus increase. The growth rate hypothesis states that phosphorus content is directly coupled to high demands for phosphorus-rich ribosomal RNA in rapidly growing organisms like cladocerans [39]. According to the above hypothesis, organisms with high demands for phosphorus display the highest growth rates under phosphorus-rich food. Hence, as higher temperatures influence cladocerans through ontogeny and physiology [29], higher phosphorus influences zooplankton through the enhancement of food resources. A moderate phosphorus addition can affect primary productivity and can lead to the bottom-up control of trophic dynamics that, in turn, might result in higher growth rates of populations of grazers [40]. However, the aftereffects of phosphorus addition are more intricate. In this study, some fluctuations in the abundance of cladocerans were observed in the treatment with the phosphorus increase; however, the size of the community at the end of the experiment was similar to that reported before the phosphorus addition. Phosphorus has an indirect effect on the development of Cladocera since it is assimilated together with other inorganic forms of chemical elements (such as carbon and nitrogen) by primary producers; primary production is then consumed by cladocerans and other herbivores, forming, by this process, the supporting base for higher trophic levels. Nutrient limitation might influence higher trophic levels by either reducing the total primary production or altering the biochemical profile (mainly some highly unsaturated fatty acids) [41] and digestibility of primary producers [14] in ways that reduce their quality as food. Thus, under apparent conditions of abundant (but phosphorus-limited) food, cladocerans can experience reduced growth and reproduction [16,19], which directly translates to the sizes of populations. Albeit under conditions of high phosphorus concentration, the efficiency of the trophic transfer between primary and secondary production tremendously varies for a variety of reasons, including imbalances in the chemical composition between consumers and primary producers [42]. Some research reports that Cladocera display a species-specific response to phosphorus concentration. Most studies concerning the effect of phosphorus concentration on the growth and reproduction of Cladocera focus on Daphnia sp. and Bosmina sp., common taxa of Cladocera that are mainly typical of the pelagic zone of deep lakes [43]. There are no data on the response of the cladocerans under consideration in this paper to phosphorus concentration except C. quadrangula, which shows no response to phosphorus concentration, according to the literature [44]. However, C. quadrangula responded to the phosphorus addition by increasing in relative abundance in this experiment. Although phosphorus enrichment had a lower effect on abundance and species composition, the analysis of the response metrics suggests turnovers of the cladoceran community. The functional and compositional resistance of all the communities were measured on day 21 of the experiment. The Cladoceran communities displayed high resistance to phosphorus enrichment; however, a careful analysis of the raw data suggests that phosphorus enrichment has a deferred effect on the development of cladoceran communities. A population decline was observed on day 28 of the experiment, and that decline had a significant influence on the temporal stability of the cladoceran community in the phosphorus-enriched treatment.
Temperature directly impacts all aspects of physiology, including grazing [45], respiration [46,47], the timing of reproduction [48], and the ontogenetic development of communities’ habits, including top-down regulation [49,50] and competitive interactions [51]. The consolidated influence of temperature on various aspects of individual traits results in higher abundance peaks of zooplankton populations under warmer conditions [6,7]. However, climate warming may deploy environmental stress in shallow lakes, ponds, and wetlands since shallow-water ecosystems have large surface areas in relation to their depth. Thus, thermal stratification and temperature refuges are absent. Higher temperatures typically result in higher rates of species-specific traits until an optimum is reached, above which physiological processes rapidly decrease [3]. As a result, some species may respond to warming by changes in population dynamics [52], whereas others can become extinct due to overrunning their absolute thermal niche requirements. Previous studies showed the variable effects of experimental warming on cladoceran communities, including shifts in composition and suppression of the abundance of Cladocera in subalpine freshwaters [53], a lower abundance of cladocerans in the warmer waters of subtropical lakes [54], and no effect of an ambient increase in temperature on cladocerans in temperate lakes [55,56]. In this study, temperature had a positive influence on the abundance of cladoceran communities. Similar effects were observed in the experiments with single populations of Daphnia magna that showed larger sizes at higher temperatures [57]. The observed inconsistencies in the responses of cladoceran communities to warming may result from the various effects of interactions of temperature with other environmental factors in lake ecosystems. The effect of temperature on zooplankton can be modified by the trophic status of ecosystems. Warmer temperatures are sometimes hypothesized to have a negative impact on zooplankton in unproductive ecosystems as a result of strong synergistic interactions between food limitation and thermal stress [58]. During temperature increases, Cladocera populations decreased in size in oligotrophic Lake Annecy but did not respond to warming in meso-eutrophic Lake Geneva [59]. The results presented in this paper show that temperature had a significant influence on the cladoceran community, which displayed low resistance and resilience to a 4 °C increase in temperature; low compositional resistance was especially apparent. Moreover, the community showed high temporal stability (low fluctuations around the trend), suggesting that the newly established species structure was quite stable and resulted from overrunning the thermal niche requirements of cladocerans.
In this experiment, the ecosystems were enriched by externally adding P-PO43− to double the final phosphorus concentration. However, there are some indications that warming also increases nutrient levels. Higher temperatures might diminish the ratio of nutrient storage in sediments, leading to higher nutrient concentrations in the water column. Moreover, the sediment release of phosphorus may be boosted by the reduced oxygen concentrations observed due to the enhanced metabolic rates of the bacteria that take part in the mineralization of organic matter at the water–sediment interface [60]. Finally, warming, in tandem with other symptoms of climate change, may enhance phosphorus loading from catchments to freshwater ecosystems through alterations in precipitation regimes [1]. Some research suggests that interactions between temperature and phosphorus are strong predictors of zooplankton abundance and structure in shallow-water ecosystems [61,62,63]. This study revealed the interactive effects of temperature and phosphorus on cladoceran communities. This observation supports the widely accepted synergistic effect of temperatures and nutrient concentration on the functioning of freshwater communities. However, opposite results were obtained in a fish-free mesocosm experiment that tested the combined effects of warming (4 °C above ambient temperature) and increased nutrient loading on plankton communities and found that warming had a positive influence on zooplankton development, although it was dampened in systems with high nutrient concentrations [64]. This situation could be explained by the effects of increased metabolic costs and the overexploitation of food resources [65] and shows that the impact of temperature and phosphorus load might have an intricate influence on cladoceran communities. Many reports suggest that nutrient enrichment and warming might favor the development of inedible taxa of phytoplankton. Excessive nutrient loading and warmer conditions are often suggested to promote cyanobacteria such that the proportion of cyanobacterial biovolume in phytoplankton communities increases although the overall phytoplankton biomass remains stable. The timing and intensity of inedible algae blooms are important factors of the abundance and taxonomic composition of cladoceran communities. In the shallow eutrophic lakes of warmer climates, a dominance of filamentous and colonial cyanobacteria that periodically or regularly develop intense blooms is often observed [66,67,68]. However, warming might also have a positive impact on the increase in the proportion of other taxa, including edible species of phytoplankton [69], that would enhance the development of cladoceran communities. The response of Cladocera to the simultaneous influence of warming and the increase in phosphorus concentration was not a simple sum of single effects. Although the cladoceran community displayed low functional resistance, it turned out to be more resilient to changes in the environment than the communities influenced by either temperature or phosphorus increase. This response metric indicates that the community recovered functionally to the state observed prior to the changes. Interestingly, the response metrics showed a very low influence of simultaneous warming and phosphorus increase on the taxonomic composition of the cladoceran community.
Overall, changes in species structure under the influence of environmental conditions are the most intriguing issue in terms of the possible extinction of keystone or rare species. In all the treatments, the species dominant before the changes increased at the cost of less abundant species. Moreover, the extinction of the rare species Macrothrix hirsuticornis was observed in the treatments with either the temperature increase or the phosphorus increase; however, this species was still present in the treatment with the temperature x phosphorus increase. Some experimental data suggest that nutrient input has a stronger effect on cladoceran species diversity than warming [55], but this statement was not supported by the present observations. Although some studies suggest that environmental changes might favor some rare taxa [70], rare species are also expected to be affected more negatively by environmental changes [71]. In all the treatments, compositional resilience correlated negatively to functional resilience. Some studies suggest that the increase in functional resilience is a direct consequence of the low compositional resilience reflected by a high species turnover [72,73]. Eventually, regarding similarity to prior-changed communities, the changed communities overcompensated functionally and showed compositional undercompensation due to the loss of species. This is in agreement with common examples of systems recovering functionally, but not in terms of composition, to the prechanged state [74,75].
5. Conclusions
Growing evidence has shown that climate variations significantly affect the seasonal succession of plankton and that the influence of both the warming and the increase in phosphorus concentration considered in this paper eventuate from climate changes. This experiment showed that phosphorus increase had a smaller impact on the abundance and composition of Cladocera, whereas higher temperature, both as a single factor and in tandem with phosphorus increase, indubitably enhanced the functional development of cladoceran communities. Ultimately, cladocerans overcompensated functionally, but regarding composition, a loss of species was observed during the experiment. This study demonstrated that realistic alterations in some environmental factors involve the responses of communities and that the strength of this response is different for various factors. Moreover, comparisons of the presented results with those reviewed in the literature suggest that the ultimate impact of warming and phosphorus load may differ in relation to geographical distribution, morphology, trophic conditions, and the taxonomic structure of trophic levels. Therefore, the impact of changes in environmental factors should be considered as a multifaceted problem to find the general effects of the impact of climate changes on communities of aquatic invertebrates. The outcomes of this study allow for a deeper understanding of the processes of seasonal shifts in cladoceran communities and for predicting their responses to long-lasting climatic changes in aquatic ecosystems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16020249/s1, Table S1: Results of one-way ANOVA for functional and compositional resistance of cladoceran communities, Table S2: Results of Tukey HSD tests showing pairwise comparisons between functional or compositional resistances of cladoceran communities, Table S3: Results of one-way ANOVA for functional and compositional resilience of cladoceran communities, Table S4: Results of Tukey HSD tests showing pairwise comparisons between functional or compositional resistances of cladoceran communities, Table S5: Results of one-way ANOVA for functional and compositional stability of cladoceran communities, Table S6: Results of Tukey HSD tests showing pairwise comparisons between functional or compositional stability of cladoceran communities, Table S7: Results of one-way ANOVA for functional and compositional similarity between disturbed and prior-disturbed cladoceran communities, Table S8: Results of Tukey HSD tests showing pairwise comparisons between functional and compositional similarity of cladoceran communities.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The author declares no conflict of interest.
References
- Jeppesen, E.; Kronvang, B.; Meerhoff, M.; Søndergaard, M.; Hansen, K.M.; Andersen, H.E. Climate change effects on runoff, catchment phosphorus loading and lake ecological state, and potential adaptations. J. Environ. Qual. 2009, 38, 1930–1941. [Google Scholar] [CrossRef] [PubMed]
- Moss, B.; Kosten, S.; Meerhoff, M.; Battarbee, R.; Jeppesen, E.; Mazzeo, N.; Havens, K.; Lacerot, G.; Liu, Z.; de Meester, L.; et al. Allied attack: Climate change and eutrophication. Inland Waters 2011, 1, 101–105. [Google Scholar] [CrossRef]
- Kingsolver, J.G. The well-temperatured biologist. Am. Nat. 2009, 174, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.H.; Hewitson, B.; Busuioc, A.; Chen, A.; Gao, X.; Held, I.; Jones, R.; Kolli, R.K.; Kwon, W.T.; Laprise, R.; et al. Regional climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 847–940. [Google Scholar]
- Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, A.T.; Gregory, J.M. Global climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 747–845. [Google Scholar]
- Gillooly, J.F.; Dodson, S.I. Latitudinal patterns in the size distribution and seasonal dynamics of new world, freshwater cladocerans. Limnol. Oceanogr. 2000, 45, 22–30. [Google Scholar] [CrossRef]
- Straile, D. North Atlantic Oscillation synchronizes food-web interactions in central European lakes. Proc. R. Soc. Lond. B Bio. 2002, 269, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Drake, J.M. Population effects of increased climate variation. Proc. R. Soc. Lond. B Biol. 2005, 272, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Ottersen, G.; Planque, B.; Belgrano, A.; Post, E.; Reid, P.C.; Stenseth, N.C. Ecological effects of the North Atlantic oscillation. Oecologia 2001, 128, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sommer, U.; Gliwicz, Z.M.; Lampert, W.; Duncan, A. The PEG-model of seasonal succession of planktonic events in fresh waters. Arch. Hydrobiol. 1986, 106, 433–471. [Google Scholar] [CrossRef]
- Cushing, D.H. Plankton production and year-class strength in fish populations: An update of the match/mismatch hypothesis. Adv. Mar. Biol. 1990, 26, 249–293. [Google Scholar]
- De Senerpont Domis, L.N.; Mooij, W.M.; Huisman, J. Climate-induced shifts in an experimental phytoplankton community: A mechanistic approach. In Shallow Lakes in a Changing World: Proceedings of the 5th International Symposium on Shallow Lakes, Dalfsen, The Netherlands, 5–9 June 2005; Springer: Amsterdam, The Netherlands, 2005; pp. 403–413. [Google Scholar]
- Praskievicz, S.; Chang, H.J. A review of hydrological modelling of basin-scale climate change and urban development impacts. Prog. Phys. Geogr. 2009, 33, 650–671. [Google Scholar] [CrossRef]
- Van Donk, E.; Hessen, D.O. Reduced digestibility of UV-B stressed and nutrient-limited algae by Daphnia magna. Hydrobiologia 1995, 307, 147–151. [Google Scholar] [CrossRef]
- Ahlgren, G.; Zeipel, K.; Gustafsson, I. Phosphorus limitation effects on the fatty acid content and nutritional quality of a green alga and a diatom. SIL Proc. 1998, 26, 1659–1664. [Google Scholar] [CrossRef]
- DeMott, W.R.; Gulati, R.D.; Siewertsen, S. Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnol. Oceanogr. 1998, 43, 1147–1161. [Google Scholar] [CrossRef]
- Sterner, R.W.; Schulz, K.L. Zooplankton nutrition: Recent progress and reality check. Aquat. Ecol. 1998, 33, 1–19. [Google Scholar]
- Elser, J.J.; Hayakawa, K.; Urabe, J. Nutrient limitation reduces food quality for zooplankton: Daphnia response to seston phosphorus enrichment. Ecology 2001, 82, 898–903. [Google Scholar] [CrossRef]
- Sterner, R.; Hessen, D.O. Algal nutrient limitation and the nutrition of aquatic herbivores. Ann. Rev. Ecol. Syst. 1994, 25, 1–29. [Google Scholar] [CrossRef]
- Sterner, R.W. Modelling interactions of food quality and quantity in homeostatic consumers. Freshw. Biol. 1997, 38, 473–481. [Google Scholar] [CrossRef]
- Genkai-Kato, M.; Carpenter, S.R. Eutrophication due to phosphorus recycling in relation to lake morphometry, temperature, and macrophytes. Ecology 2005, 86, 210–219. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. The Cladocera (Crustacea: Branchiopoda) as a relict group. Zool. J. Linn. Soc. 2006, 147, 109–124. [Google Scholar] [CrossRef][Green Version]
- Smol, J.P. The power of the past: Using sediments to track the effects of multiple stressors on lake ecosystems. Freshw. Biol. 2010, 55, 43–59. [Google Scholar] [CrossRef]
- Martens, K.; Horne, D.J.; Griffiths, H.I. Age and diversity of non-marine ostracods. In Sex and Parthenogenesis, Evolutionary Ecology of Reproductive Modes in Non-Marine Ostracods; Martens, K., Ed.; Backhyus: Leyden, The Netherlands, 1998; pp. 37–55. [Google Scholar]
- Sommer, U.; Stibor, H. Copepoda—Cladocera—Tunicata: The role of three major mesozooplankton groups in pelagic food webs. Ecol. Res. 2002, 17, 161–174. [Google Scholar] [CrossRef]
- Lomartire, S.; Marques, J.C.; Gonçalves, A.M.M. The key role of zooplankton in ecosystem services: A perspective of interaction between zooplankton and fish recruitment. Ecol. Indic. 2021, 129, 107867. [Google Scholar] [CrossRef]
- Pimm, S.L. The complexity and stability of ecosystems. Nature 1984, 307, 321–326. [Google Scholar] [CrossRef]
- Jeppesen, E.; Peder Jensen, J.; Søndergaard, M.; Lauridsen, T.; Junge Pedersen, L.; Jensen, L. Top-down control in freshwater lakes: The role of nutrient state, submerged macrophytes and water depth. Hydrobiologia 1997, 342, 151–164. [Google Scholar] [CrossRef]
- Angilletta Jr, M.J.; Huey, R.B.; Frazier, M.R. Thermodynamic effects on organismal performance: Is hotter better? Physiol. Biochem. Zool. 2010, 83, 197–206. [Google Scholar] [CrossRef]
- Havens, K.E.; Elia, A.C.; Taticchi, M.I.; Fulton, R.S. Zooplankton–phytoplankton relationships in shallow subtropical versus temperate lakes Apopka (Florida, USA) and Trasimeno (Umbria, Italy). Hydrobiologia 2009, 628, 165–175. [Google Scholar] [CrossRef]
- Hunt, R.J.; Matveev, V.F. The effects of nutrients and zooplankton community structure on phytoplankton growth in a subtropical Australian reservoir: An enclosure study. Limnologica 2005, 35, 90–101. [Google Scholar] [CrossRef]
- van Bethem, K.J.; Bruijning, M.; Bonnet, T.; Jongejans, E.; Postma, E.; Ozgul, A. Disentangling evolutionary, plastic and demographic processes underlying trait dynamics: A review of four frameworks. Methods Ecol. Evol. 2017, 8, 75–85. [Google Scholar] [CrossRef]
- Foreman, C.M.; Wolf, C.F.; Priscu, J.C. Impact of episodic warming events. Aquat. Geochem. 2004, 10, 239–268. [Google Scholar] [CrossRef]
- Wilhelm, S.; Adrian, R. Impact of summer warming on the thermal characteristics of a polymictic lake and consequences for oxygen, nutrients and phytoplankton. Freshw. Biol. 2008, 53, 226–237. [Google Scholar] [CrossRef]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Niedźwiecki, M.; Adamczuk, M.; Mieczan, T. Trophic interactions among the heterotrophic components of plankton in man-made peat pools. J. Limnol. 2017, 76, 524–533. [Google Scholar] [CrossRef][Green Version]
- Carpenter, S.R.; Frost, T.M.; Kitchell, J.F.; Kratz, T.K. Species dynamics and global environmental change: A perspective from ecosystem experiments. In Biotic Interactions and Global Change; Kareiva, P.M., Kingsolver, J.G., Huey, R.B., Eds.; Sinaur Associates Inc.: Sunderland, MA, USA, 1993. [Google Scholar]
- Chapin, F.S., III; Zavaleta, E.S.; Eviner, V.T. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.A.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2000, 3, 540–550. [Google Scholar] [CrossRef]
- DeAngelis, D.L. Energy flow, nutrient cycling, and ecosystem resilience. Ecology 1980, 61, 764–771. [Google Scholar] [CrossRef]
- Demott, W.; Müller-Navarra, D. The importance of highly unsaturated fatty acids in zooplankton nutrition: Evidence from experiments with Daphnia, a cyanobacterium and lipid emulsions. Freshw. Biol. 1997, 38, 649–664. [Google Scholar] [CrossRef]
- Cebrian, J.; Shurin, J.B.; Borer, E.T.; Cardinale, B.J.; Smith, M.D. Producer nutritional quality controls ecosystem trophic structure. PLoS ONE 2009, 4, e4929. [Google Scholar] [CrossRef]
- Korovchinsky, N.M. Species richness of pelagic Cladocera of large lakes in the eastern hemisphere. Hydrobiologia 2000, 434, 41–54. [Google Scholar] [CrossRef]
- Thomas, P.K.; Kunze, C.; Van de Waal, D.B.; Hillebrand, H.; Striebel, M. Elemental and biochemical nutrient limitation of zooplankton: A meta-analysis. Ecol. Lett. 2022, 25, 2776–2792. [Google Scholar] [CrossRef]
- Kiørboe, T.; Møhlenberg, F.; Nicolajsen, H. Ingestion rate and gut clearance in the planktonic copepod Centropages hamatus (Lilljeborg) in relation to food concentration and temperature. Ophelia 1982, 21, 181–194. [Google Scholar] [CrossRef]
- Ikeda, T. Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Mar. Biol. 1985, 85, 1–11. [Google Scholar] [CrossRef]
- Thor, P. Specific dynamic action and carbon incorporation in Calanus finmarchicus copepodites and females. J. Exp. Mar. Biol. Ecol. 2002, 272, 159–169. [Google Scholar] [CrossRef]
- Korpelainen, H. The effects of temperature and photoperiod on life history parameters of Daphnia magna (Crustacea: Cladocera). Freshw. Biol. 1986, 16, 615–620. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Davidson, T.A.; Søndergaard, M.; Lauridsen, T.L.; Beklioglu, M.; Brucet, S.; Volta, P.; González-Bergonzoni, I.; et al. Climate change impacts on lakes: An integrated ecological perspective based on a multi-faceted approach, with special focus on shallow lakes. J. Limnol. 2014, 73, 88–111. [Google Scholar] [CrossRef]
- Shurin, J.B.; Clasen, J.L.; Greig, H.S.; Kratina, P.; Thompson, P.L. Warming shifts top–down and bottom–up control of pond food web structure and function. Philos. T. Roy. Soc. B 2012, 367, 3008–3017. [Google Scholar] [CrossRef]
- Fey, S.B.; Cottingham, K.L. Thermal sensitivity predicts the establishment success of nonnative species in a mesocosm warming experiment. Ecology 2012, 93, 2313–2320. [Google Scholar] [CrossRef]
- Schindler, D.W. Widespread effects of climatic warming on freshwater ecosystems in North America. Hydrol. Process. 1997, 11, 1043–1067. [Google Scholar] [CrossRef]
- Strecker, A.L.; Tyler, P.C.; Vinebrooke, R.D. Effects of experimental greenhouse warming on phytoplankton and zooplankton communities in fishless alpine ponds. Limnol. Oceanogr. 2004, 49, 1182–1190. [Google Scholar] [CrossRef]
- Havens, K.E.; Fulton, R.S., III; Beaver, J.R.; Samples, E.E.; Colee, J. Effects of climate variability on cladoceran zooplankton and cyanobacteria in a shallow subtropical lake. J. Plankton Res. 2016, 38, 418–430. [Google Scholar] [CrossRef]
- McKee, D.; Atkinson, D.; Collings, S.E.; Eaton, J.W.; Harvey, I.; Heyes, T.; Hatton, K.; Wilson, D.; Moss, B. Macro-zooplankter responses to simulated climate warming in experimental freshwater microcosms. Freshw. Biol. 2002, 47, 1557–1570. [Google Scholar] [CrossRef]
- Šorf, M.; Davidson, T.A.; Brucet, S.; Menezes, R.F.; Søndergaard, M.; Lauridsen, T.L.; Landkildehus, F.L.; Liboriussen, L.; Jeppesen, E. Zooplankton response to climate warming: A mesocosm experiment at contrasting temperatures and nutrient level. Hydrobiologia 2015, 742, 185–203. [Google Scholar] [CrossRef]
- Adamczuk, M. Population dynamics and life history traits of Daphnia magna across thermal regimes of environments. Sci. Total Environ. 2020, 72, 137963. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.V.; Folt, C.L.; Stemberger, R.S. Consequences of elevated temperatures for zooplankton communities in temperate lakes. Arch. Hydrobiol. 1996, 135, 289–319. [Google Scholar] [CrossRef]
- Anneville, O.; Molinero, J.C.; Souissi, S.; Gerdeaux, D. Seasonal and interannual variability of cladoceran communities in two peri-alpine lakes: Uncoupled response to the 2003 heat wave. J. Plankton Res. 2010, 32, 913–925. [Google Scholar] [CrossRef][Green Version]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Holmgren, K.; González-Bergonzoni, I.; Teixeira-de Mello, F.; Declerck, S.A.; De Meester, L.; Søndergaard, M.; Lauridsen, T.L.; Bjerring, R.; et al. Impacts of climate warming on lake fish community structure and potential effects on ecosystem function. Hydrobiologia 2010, 646, 73–90. [Google Scholar] [CrossRef]
- Kosten, S.; Huszar, V.L.M.; Becares, E.; Costa, L.S.; Donk, E.; Hansson, L.-A.; Jeppesen, E.; Kruk, C.; Lacerot, G.; Mazzeo, N.; et al. Warmer climates boost cyanobacterial dominance in shallow lakes. Glob. Chang. Biol. 2012, 18, 118–126. [Google Scholar] [CrossRef]
- Cremona, F.; Agasild, H.; Haberman, J.; Zingel, P.; Nõges, P.; Nõges, T.; Laas, A. How warming and other stressors affect zooplankton abundance, biomass and community composition in shallow eutrophic lakes. Clim. Chang. 2020, 159, 565–580. [Google Scholar] [CrossRef]
- Feuchtmayr, H.; Moss, B.; Harvey, I.; Moran, R.; Hatton, K.; Connor, L.; Atkinson, D. Differential effects of warming and nutrient loading on the timing and size of the spring zooplankton peak: An experimental approach with hypertrophic freshwater mesocosms. J. Plankton Res. 2010, 32, 1715–1725. [Google Scholar] [CrossRef]
- Beisner, B.E.; Mccauley, E.; Wrona, F.J. The influence of temperature and food chain length on plankton predator-prey dynamics. Can. J. Fish. Aquat. Sci. 1997, 54, 586–595. [Google Scholar]
- Beaver, J.R.; Scotese, K.C.; Manis, E.E.; Juul, S.T.J.; Carroll, J.; Renicker, T.R. Variation in water residence time is the primary determinant of phytoplankton and zooplankton composition in a Pacific Northwest reservoir ecosystem (Lower Snake River, USA). River. Syst. 2015, 21, 261–275. [Google Scholar] [CrossRef]
- Fulton, R.S.; Godwin, W.F.; Schaus, M.H. Water quality changes following nutrient loading reduction and biomanipulation in a large shallow subtropical lake, Lake Griffin, Florida, USA. Hydrobiologia 2015, 753, 243–263. [Google Scholar] [CrossRef]
- Zhu, M.; Paerl, H.W.; Zhu, Z.; Wu, T.; Li, W.; Shi, K.; Zhao, L.; Zhang, Y.; Qin, B.; Caruso, A.M. The role of tropical cyclones in stimulating cyanobacterial (Microcystis spp.) blooms in hypertrophic Lake Taihu, China. Harmful Algae 2014, 39, 310–321. [Google Scholar] [CrossRef]
- Winder, M.; Sommer, U. Phytoplankton response to a changing climate. Hydrobiologia 2012, 698, 5–16. [Google Scholar] [CrossRef]
- Kurm, V.; Geisen, S.; Gera Hol, W.H. A low proportion of rare bacterial taxa responds to abiotic changes compared with dominant taxa. Environ. Microbiol. 2019, 21, 750–758. [Google Scholar] [CrossRef]
- Sykes, L.; Santini, L.; Etard, A.; Newbold, T. Effects of rarity form on species’ responses to land use. Conserv. Biol. 2020, 34, 688–696. [Google Scholar] [CrossRef]
- Tilman, D. Biodiversity: Population versus ecosystem stability. Ecology 1996, 77, 350–363. [Google Scholar] [CrossRef]
- Allan, E.; Weisser, W.; Weigelt, A.; Roscher, C.; Fischer, M.; Hillebrand, H. More diverse plant communities have higher functioning over time due to turnover in complementary dominant species. Proc. Natl. Acad. Sci. USA 2011, 108, 17034–17039. [Google Scholar] [CrossRef]
- Borja, A.; Dauer, D.M.; Elliott, M.; Simenstad, C.A. Medium- and long-term recovery of estuarine and coastal ecosystems: Patterns, rates and restoration effectiveness. Estuar. Coast. 2010, 33, 1249–1260. [Google Scholar] [CrossRef]
- Gaohua, J.; Havens, K.; Beaver, J.R.; East, T.L. Recovery of plankton from hurricane impacts in a large shallow lake. Freshw. Biol. 2018, 63, 366–379. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).