Prey Supply and Predation as Potential Limitations to Feasibility of Anadromous Salmonid Introductions in a Reservoir
Abstract
1. Introduction
2. Methods
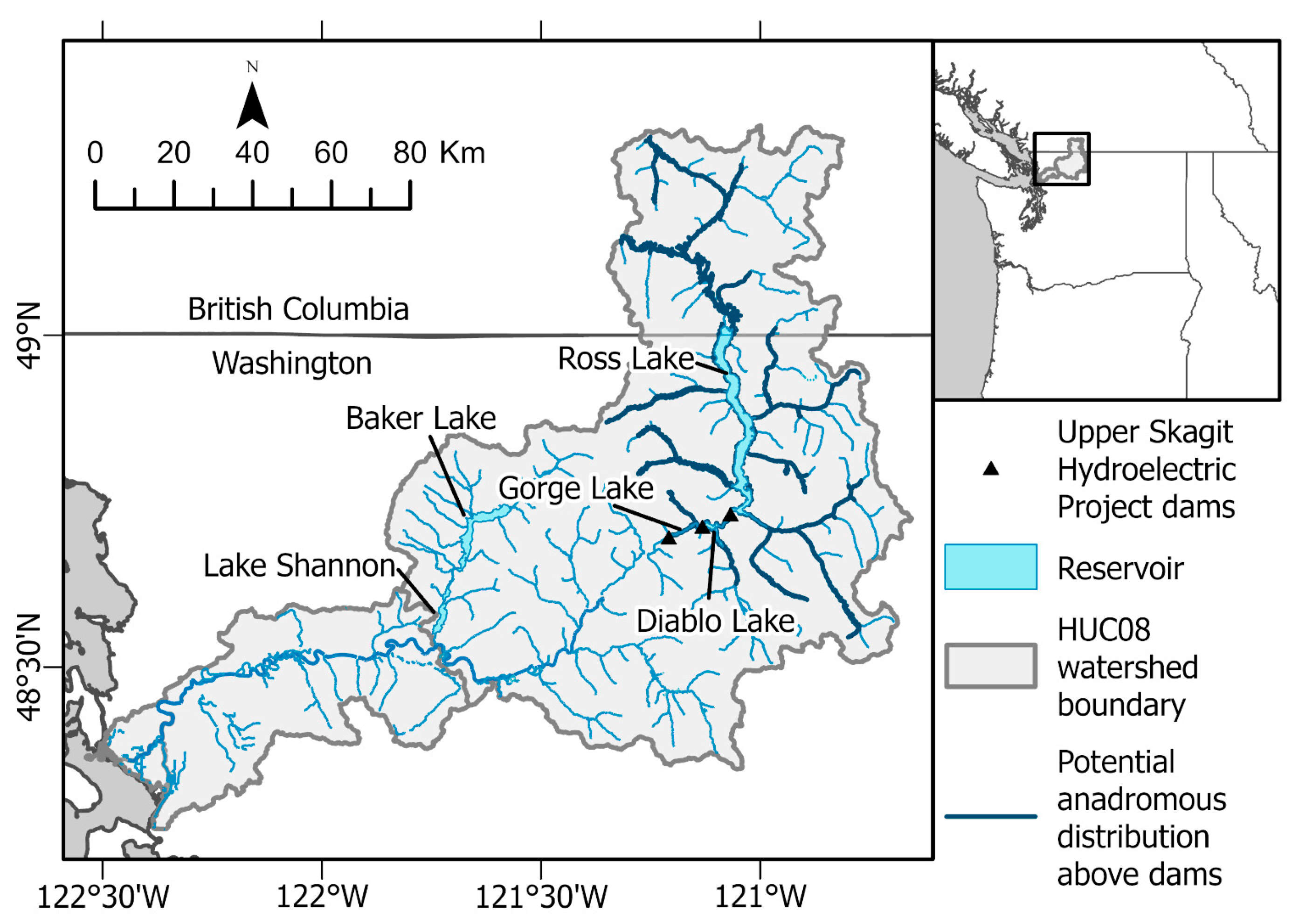
2.1. Study System
2.2. Candidate Anadromous Species and Life Histories
2.3. Bioenergetics Simulations
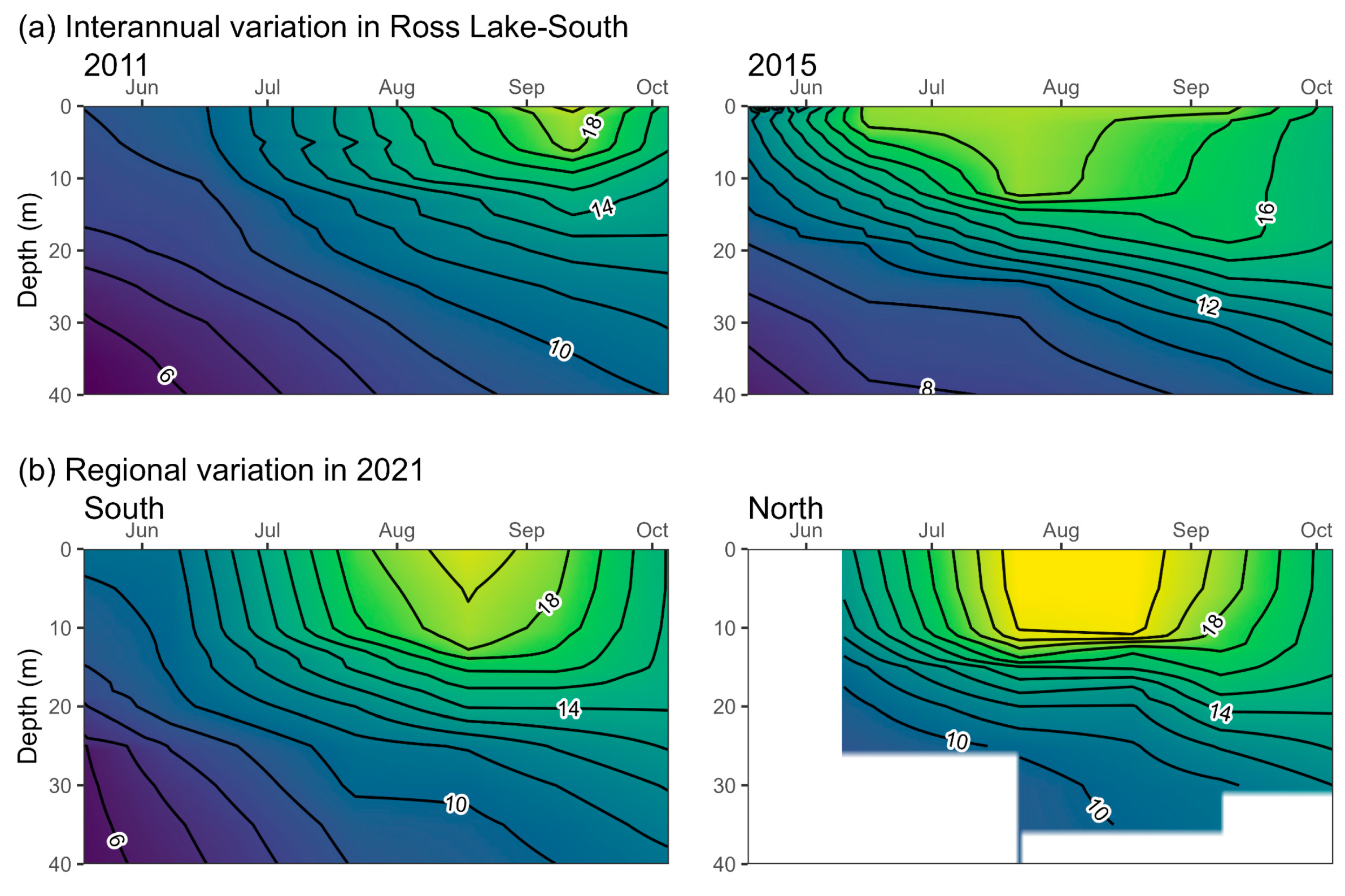
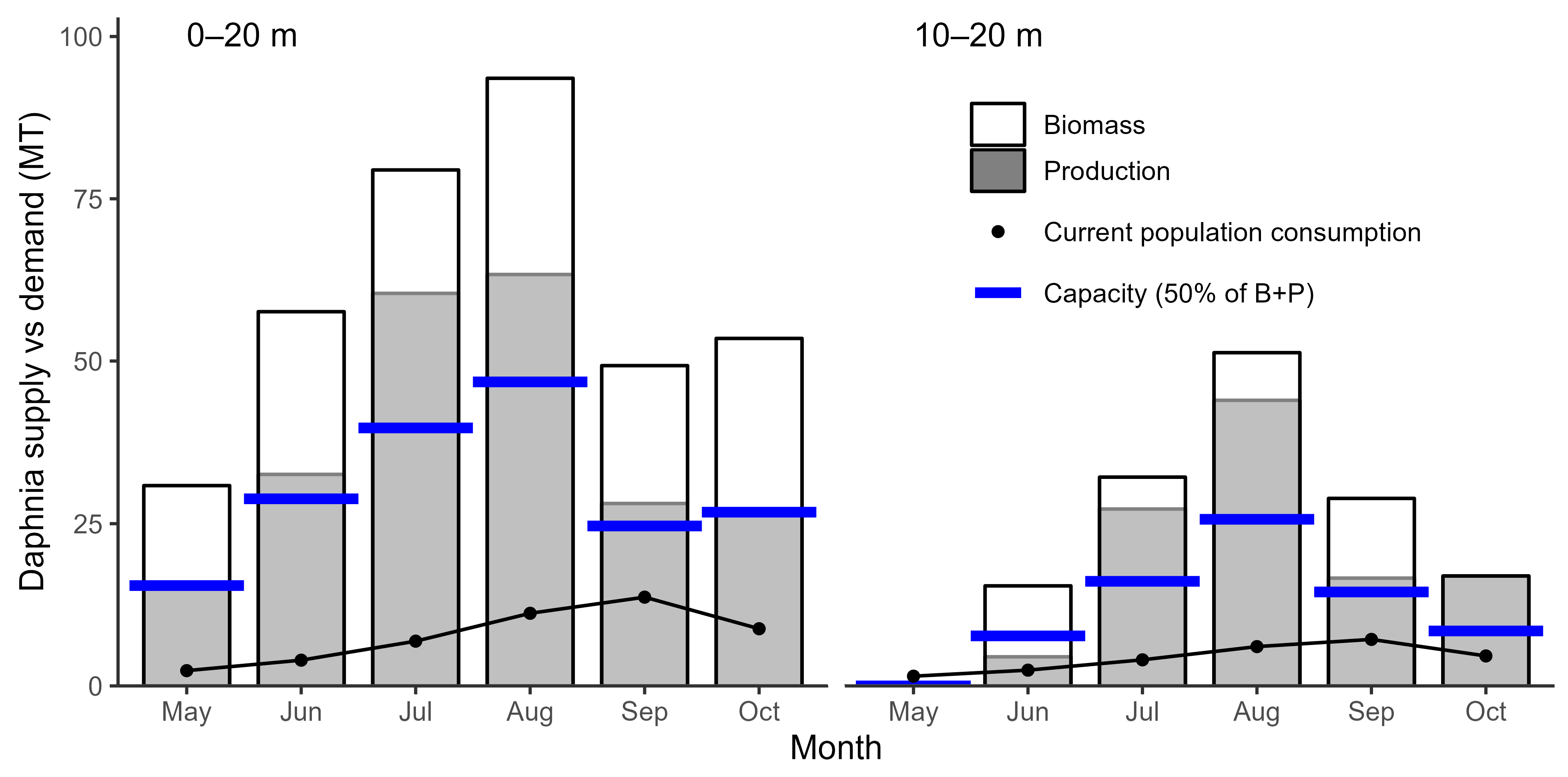
2.4. Daphnia Availability and Sockeye Salmon Capacity in Ross Lake
3. Results
3.1. Daphnia Availability and Sockeye Salmon Capacity
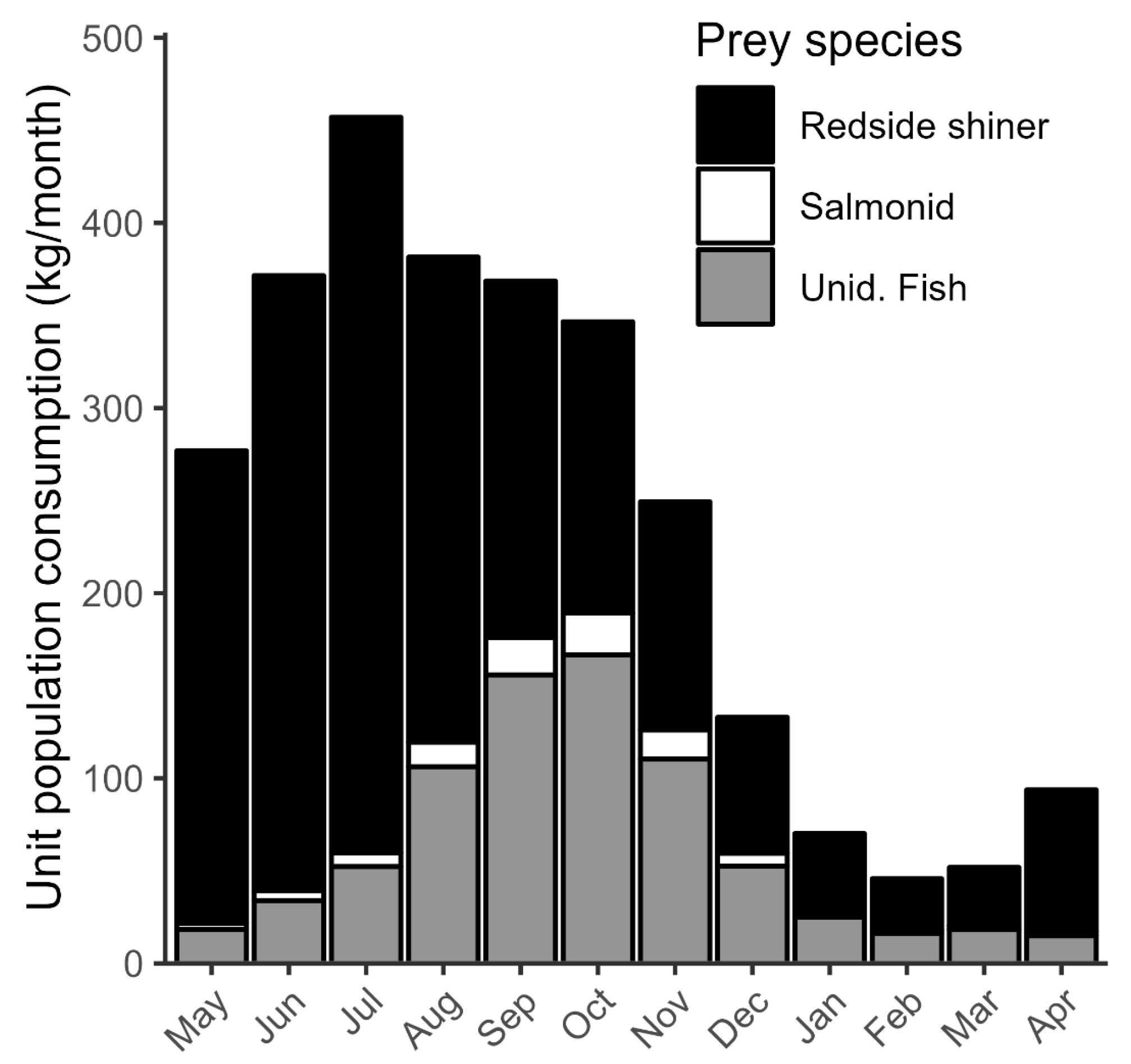
3.2. Predation Mortality
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bubac, C.M.; Johnson, A.C.; Fox, J.A.; Cullingham, C.I. Conservation translocations and post-release monitoring: Identifying trends in failures, biases, and challenges from around the world. Biol. Conserv. 2019, 238, 108239. [Google Scholar] [CrossRef]
- Seddon, P.J.; Armstrong, D.P.; Maloney, R.F. Developing the science of reintroduction biology. Conserv. Biol. 2007, 21, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Swan, K.D.; Lloyd, N.A.; Moehrenschlager, A. Projecting further increases in conservation translocations: A Canadian case study. Biol. Conserv. 2018, 228, 175–182. [Google Scholar] [CrossRef]
- Armstrong, D.P.; Seddon, P.J. Directions in reintroduction biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- IUCN. Guidelines for Reintroductions and Other Conservation Translocations; Version 1.0; IUCN Species Survival Commission: Gland, Switzerland, 2013. [Google Scholar]
- He, F.; Zarfl, C.; Bremerich, V.; David, J.N.W.; Hogan, Z.; Kalinkat, G.; Tockner, K.; Jähnig, S.C. The global decline of freshwater megafauna. Glob. Chang. Biol. 2019, 25, 3883–3892. [Google Scholar] [CrossRef] [PubMed]
- Cochran-Biederman, J.L.; Wyman, K.E.; French, W.E.; Loppnow, G.L. Identifying correlates of success and failure of native freshwater fish reintroductions. Conserv. Biol. 2015, 29, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.H.; Pess, G.R.; Carmichael, R.W.; Ford, M.J.; Cooney, T.D.; Baldwin, C.M.; McClure, M.M. Planning Pacific salmon and steelhead reintroductions aimed at long-term viability and recovery. N. Am. J. Fish. Manag. 2014, 34, 72–93. [Google Scholar] [CrossRef]
- Dunham, J.; Gallo, K.; Shively, D.; Allen, C.; Goehring, B. Assessing the feasibility of native fish reintroductions: A framework applied to threatened bull trout. N. Am. J. Fish. Manag. 2011, 31, 106–115. [Google Scholar] [CrossRef]
- Kock, T.J.; Ferguson, J.W.; Keefer, M.L.; Schreck, C.B. Review of trap-and-haul for managing Pacific salmonids (Oncorhynchus spp.) in impounded river systems. Rev. Fish Biol. Fish. 2021, 31, 53–94. [Google Scholar] [CrossRef]
- Matala, A.P.; Narum, S.R.; Saluskin, B.P.; Johnston, M.V.; Newell, J.E.; Fast, D.E.; Galbreath, P.F. Early observations from monitoring a reintroduction program: Return of sockeye salmon to a nursery lake of historical importance. Trans. Am. Fish. Soc. 2019, 148, 271–288. [Google Scholar] [CrossRef]
- Evans, M.L.; Johnson, M.A.; Jacobson, D.; Wang, J.; Hogansen, M.; O’Malley, K.G. Evaluating a multi-generational reintroduction program for threatened salmon using genetic parentage analysis. Can. J. Fish. Aquat. Sci. 2016, 73, 844–852. [Google Scholar] [CrossRef]
- Sard, N.M.; O’Malley, K.G.; Jacobson, D.P.; Hogansen, M.J.; Johnson, M.A.; Banks, M.A. Factors influencing spawner success in a spring Chinook salmon (Oncorhynchus tshawytscha) reintroduction program. Can. J. Fish. Aquat. Sci. 2015, 72, 1390–1397. [Google Scholar] [CrossRef]
- Kock, T.; Perry, R.; Hansen, G.; Haner, P.; Pope, A.; Plumb, J.; Cogliati, K.; Hansen, A. Evaluation of Chinook Salmon Fry Survival in Lookout Point Reservoir, Western Oregon, 2017; Open-File Report 2019-1011; U.S. Geological Survey: Reston, VA, USA, 2019.
- Flagg, T.A.; Ruehle, T.E. Cle Elum Lake Anadromous Salmon Restoration Feasibility Study: Summary of Research, 2000 Final Report to Bonneville Power Administration; BPA Report DOE/BP-64840-4; Bonneville Power Administration—Environment, Fish and Wildlife Division: Portland, OR, USA, 2000.
- Keefer, M.L.; Taylor, G.A.; Garletts, D.F.; Helms, C.K.; Gauthier, G.A.; Pierce, T.M.; Caudill, C.C. Reservoir entrapment and dam passage mortality of juvenile Chinook salmon in the Middle Fork Willamette River. Ecol. Freshw. Fish 2012, 21, 222–234. [Google Scholar] [CrossRef]
- Naiman, R.J.; Alldredge, J.R.; Beauchamp, D.A.; Bisson, P.A.; Congleton, J.; Henny, C.J.; Huntly, N.; Lamberson, R.; Levings, C.; Merrill, E.N.; et al. Developing a broader scientific foundation for river restoration: Columbia River food webs. Proc. Natl. Acad. Sci. USA 2012, 109, 21201–21207. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.T.J.; Olden, J.D.; Zanden, M.J.V. Dam invaders: Impoundments facilitate biological invasions into freshwaters. Front. Ecol. Environ. 2008, 6, 357–363. [Google Scholar] [CrossRef]
- Hansen, A.G.; Gardner, J.R.; Connelly, K.A.; Polacek, M.; Beauchamp, D.A. Trophic compression of lake food webs under hydrologic disturbance. Ecosphere 2018, 9, e02304. [Google Scholar] [CrossRef]
- Murphy, C.A.; Taylor, G.; Pierce, T.; Arismendi, I.; Johnson, S.L. Short-term reservoir draining to streambed for juvenile salmon passage and non-native fish removal. Ecohydrology 2019, 12, e2096. [Google Scholar] [CrossRef] [PubMed]
- Bourret, S.L.; Kennedy, B.P.; Caudill, C.C.; Chittaro, P.M. Using otolith chemical and structural analysis to investigate reservoir habitat use by juvenile Chinook salmon Oncorhynchus tshawytscha. J. Fish Biol. 2014, 85, 1507–1525. [Google Scholar] [CrossRef] [PubMed]
- Koehler, M.E.; Fresh, K.L.; Beauchamp, D.A.; Cordell, J.R.; Simenstad, C.A.; Seiler, D.E. Diet and bioenergetics of lake-rearing juvenile Chinook salmon in Lake Washington. Trans. Am. Fish. Soc. 2006, 135, 1580–1591. [Google Scholar] [CrossRef]
- Monzyk, F.R.; Emig, R.; Romer, J.D.; Friesen, T.A. Life-History Characteristics of Juvenile Spring Chinook Salmon Rearing in Willamette Valley Reservoirs; Oregon Department of Fish and Wildlife—Upper Willamette Research, Monitoring and Evaluation Program: Corvallis, OR, USA, 2014. [Google Scholar]
- Murphy, C.A.; Lee, C.S.; Johnson, B.; Arismendi, I.; Johnson, S.L. GrowChinook: An optimized multimodel and graphic user interface for predicting juvenile Chinook salmon growth in lentic ecosystems. Can. J. Fish. Aquat. Sci. 2019, 12, 564–575. [Google Scholar] [CrossRef]
- Naughton, G.P.; Keefer, M.L.; Clabough, T.S.; Knoff, M.J.; Blubaugh, T.J.; Sharpe, C.; Caudill, C.C. Reservoir provides cool-water refuge for adult Chinook salmon in a trap-and-haul reintroduction program. Mar. Freshw. Res. 2018, 69, 1995–2007. [Google Scholar] [CrossRef]
- Arostegui, M.C.; Quinn, T.P. Reliance on lakes by salmon, trout and charr (Oncorhynchus, Salmo and Salvelinus): An evaluation of spawning habitats, rearing strategies and trophic polymorphisms. Fish Fish. 2019, 20, 775–794. [Google Scholar] [CrossRef]
- Edmundson, J.A.; Mazumder, A. Linking growth of juvenile sockeye salmon to habitat temperature in Alaskan Lakes. Trans. Am. Fish. Soc. 2001, 130, 644–662. [Google Scholar] [CrossRef]
- Koenings, J.P.; Kyle, G.B. Consequences to juvenile sockeye salmon and the zooplankton community resulting from intense predation. Alsk. Fish. Res. Bull. 1997, 4, 120–135. [Google Scholar]
- Cartwright, M.A.; Beauchamp, D.A.; Bryant, M.D. Quantifying cutthroat trout (Oncorhynchus clarki) predation on sockeye salmon (Oncorhynchus nerka) fry using a bioenergetics approach. Can. J. Fish. Aquat. Sci. 1998, 55, 1285–1295. [Google Scholar] [CrossRef]
- Foerster, R.E.; Ricker, W.E. The effect of reduction of predaceous fish on survival of young sockeye salmon at Cultus Lake. J. Fish. Res. Board Can. 1941, 5, 315–336. [Google Scholar] [CrossRef]
- Sorel, M.H.; Hansen, A.G.; Connelly, K.A.; Wilson, A.C.; Lowery, E.D.; Beauchamp, D.A. Predation by northern pikeminnow and tiger muskellunge on juvenile salmonids in a high-head reservoir: Implications for anadromous fish reintroductions. Trans. Am. Fish. Soc. 2016, 145, 521–536. [Google Scholar] [CrossRef]
- Seattle City Light. Final License Application to the Federal Energy Regulatory Committee. Exhibit E—Appendix G: Skagit Project Upstream and Downstream Fish Passage Program. 2023. Available online: https://www.seattle.gov/light/skagit/Relicensing/default.htm (accessed on 8 November 2023).
- Duda, J.J.; Hardiman, J.M. Applying Intrinsic Potential Models to Evaluate Salmon (Oncorhynchus spp.) Introduction into Main-Stem and Tributary Habitats Upstream from the Skagit River Hydroelectric Project, Northern Washington; Open-File Report 2023-1077; U.S. Geological Survey: Reston, VA, USA, 2023; p. 44.
- Jensen, B.L.; Johnson, R.C.; Duda, J.J.; Ostberg, C.O.; Code, T.J.; Mclean, J.H.; Stenberg, K.D.; Larsen, K.A.; Hoy, M.S.; Beauchamp, D.A. Growth performance of rainbow trout in reservoir tributaries and implications for steelhead growth potential above Skagit River dams. N. Am. J. Fish. Manag. 2023, 43, 1427–1446. [Google Scholar] [CrossRef]
- Johnson, R.C.; Code, T.J.; Stenberg, K.D.; Mclean, J.H.; Jensen, B.L.; Hoy, M.S.; Beauchamp, D.A. Change in growth and prey utilization for a native salmonid following invasion by an omnivorous minnow in an oligotrophic reservoir. Hydrobiologia 2024, in press. [Google Scholar]
- Johnson, R.C.; Hoy, M.S.; Stenberg, K.D.; Mclean, J.H.; Jensen, B.L.; Code, T.J.; Ostberg, C.O.; Beauchamp, D.A. Shift in piscivory following invasion of a minnow in a mid-elevation reservoir. Ecol. Freshw. Fish. 2024, 00, e12778. [Google Scholar] [CrossRef]
- Connor, E.J.; Pflug, D.E. Changes in the distribution and density of pink, chum, and Chinook salmon spawning in the Upper Skagit River in response to flow management measures. N. Am. J. Fish. Manag. 2004, 24, 835–852. [Google Scholar] [CrossRef]
- Smith, M.J. Final Report: Population Structure and Genetic Assignment of Bull Trout (Salvelinus confluentus) in the Skagit River Basin; University of Washington: Seattle, WA, USA, 2010. [Google Scholar]
- WDFW; SRSC. Skagit Chinook Recovery Plan; Washington Department of Fish and Wildlife (WDFW) & Skagit River System Cooperative (SRSC): La Conner, WA, USA, 2005.
- Zimmerman, M.S.; Kinsel, C.; Beamer, E.; Connor, E.J.; Pflug, D.E. Abundance, survival, and life history strategies of juvenile Chinook salmon in the Skagit River, Washington. Trans. Am. Fish. Soc. 2015, 144, 627–641. [Google Scholar] [CrossRef]
- Thompson, J.N.; Beauchamp, D.A. Size-selective mortality of steelhead during freshwater and marine life stages related to freshwater growth in the Skagit River, Washington. Trans. Am. Fish. Soc. 2014, 143, 910–925. [Google Scholar] [CrossRef]
- Lowery, E.D.; Beauchamp, D.A. Baseline Food Web Assessment of the Upper Clackamas River Basin Prior to Reintroduction of Bull Trout; Report # WACFWRU-010-02; US Geological Survey, Washington Cooperative Fish and Wildlife Research Unit: Seattle, WA, USA, 2010.
- Beauchamp, D.A. Riverine predation on sockeye salmon fry migrating to Lake Washington. N. Am. J. Fish. Manag. 1995, 15, 358–365. [Google Scholar] [CrossRef]
- Quinn, T.P.; Losee, J.P. Diverse and changing use of the Salish Sea by Pacific salmon, trout, and char. Can. J. Fish. Aquat. Sci. 2022, 79, 1003–1021. [Google Scholar] [CrossRef]
- Zimmerman, M.S.; Irvine, J.R.; O’Neill, M.; Anderson, J.H.; Greene, C.M.; Weinheimer, J.; Trudel, M.; Rawson, K. Spatial and temporal patterns in smolt survival of wild and hatchery coho salmon in the Salish Sea. Mar. Coast. Fish. 2015, 7, 116–134. [Google Scholar] [CrossRef]
- Deslauriers, D.; Chipps, S.R.; Breck, J.E.; Rice, J.A.; Madenjian, C.P. Fish Bioenergetics 4.0: An R-based modeling application. Fisheries 2017, 42, 586–596. [Google Scholar] [CrossRef]
- Beauchamp, D.A.; Stewart, D.J.; Thomas, G.L. Corroboration of a bioenergetics model for sockeye salmon. Trans. Am. Fish. Soc. 1989, 118, 597–607. [Google Scholar] [CrossRef]
- Johnson, R.C.; Code, T.J.; Hoy, M.S.; Jensen, B.L.; Stenberg, K.D.; Ostberg, C.O.; Duda, J.J. Upper Skagit Reservoir Food Web Data, 2005–2021; U.S. Geological Survey Data Release: Reston, VA, USA, 2024. [CrossRef]
- Hansen, A.G.; Polacek, M.; Connelly, K.A.; Gardner, J.R.; Beauchamp, D.A. Food Web Interactions in Kachess and Keechelus Reservoirs, Washington: Implications for Threatened Adfluvial Bull Trout and Management of Water Storage—Final Report; Washington Cooperative Fish and Wildlife Research Unit: Seattle, WA, USA, 2017. [Google Scholar]
- Scheuerell, J.M.; Schindler, D.E.; Scheuerell, M.D.; Fresh, K.L.; Sibley, T.H.; Litt, A.H.; Shepherd, J.H. Temporal dynamics in foraging behavior of a pelagic predator. Can. J. Fish. Aquat. Sci. 2005, 62, 2494–2501. [Google Scholar] [CrossRef]
- Sorel, M.H.; Hansen, A.G.; Connelly, K.A.; Beauchamp, D.A. Trophic feasibility of reintroducing anadromous salmonids in three reservoirs on the North Fork Lewis River, Washington: Prey supply and consumption demand of resident fishes. Trans. Am. Fish. Soc. 2016, 145, 1331–1347. [Google Scholar] [CrossRef]
- Luecke, C.; Brandt, D. Notes: Estimating the energy density of Daphnid prey for use with rainbow trout bioenergetics models. Trans. Am. Fish. Soc. 1993, 122, 386–389. [Google Scholar] [CrossRef]
- McCarthy, S.G.; Duda, J.J.; Emlen, J.M.; Hodgson, G.R.; Beauchamp, D.A. Linking habitat quality with trophic performance of steelhead along forest gradients in the South Fork Trinity River watershed, California. Trans. Am. Fish. Soc. 2009, 138, 506–521. [Google Scholar] [CrossRef]
- Stockwell, J.D.; Bonfantine, K.L.; Johnson, B.M. Kokanee foraging: A Daphnia in the stomach is worth two in the lake. Trans. Am. Fish. Soc. 1999, 128, 169–174. [Google Scholar] [CrossRef]
- Brett, J.R. Energetic responses of salmon to temperature: A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). Am. Zool. 1971, 11, 99–113. [Google Scholar] [CrossRef]
- Hansen, A.G.; Gardner, J.R.; Beauchamp, D.A.; Paradis, R.; Quinn, T.P. Recovery of sockeye salmon in the Elwha River, Washington, after dam removal: Dependence of smolt production on the resumption of anadromy by landlocked kokanee. Trans. Am. Fish. Soc. 2016, 145, 1303–1317. [Google Scholar] [CrossRef]
- Quinn, T.P. The Behavior and Ecology of Pacific Salmon and Trout, 2nd ed.; University of Washington Press: Seattle, WA, USA, 2018. [Google Scholar]
- Beauchamp, D.A.; Wahl, D.H.; Johnson, B.M. Predator-Prey Interactions In Analysis and Interpretation of Freshwater Fisheries Data; Guy, C.S., Brown, M.R., Eds.; American Fisheries Society: Bethesda, Maryland, 2007; pp. 765–842. [Google Scholar]
- Baldwin, C.M.; Beauchamp, D.A.; Van Tassell, J.J. Bioenergetic assessment of temporal food supply and consumption demand by salmonids in the Strawberry Reservoir food web. Trans. Am. Fish. Soc. 2000, 129, 429–450. [Google Scholar] [CrossRef]
- Beauchamp, D.A.; Van Tassell, J.J. Modeling seasonal trophic interactions of adfluvial bull trout in Lake Billy Chinook, Oregon. Trans. Am. Fish. Soc. 2001, 130, 204–216. [Google Scholar] [CrossRef]
- Juanes, F.; Buckel, J.A.; Scharf, S.F. 2002. Feeding ecology of piscivorous fishes. In Handbook of Fish Biology and Fisheries; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Science Ltd.: Oxford, UK, 2002; pp. 267–283. [Google Scholar]
- Winters, L.K.; Budy, P. Exploring crowded trophic niche space in a novel reservoir fish assemblage: How many is too many? Trans. Am. Fish. Soc. 2015, 144, 1117–1128. [Google Scholar] [CrossRef]
- Thompson, J.N.; Beauchamp, D.A. Growth of juvenile steelhead Oncorhynchus mykiss under size-selective pressure limited by seasonal bioenergetic and environmental constraints. J. Fish Biol. 2016, 89, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.R.; Vidergar, D.T.; Bennett, D.H. Stable isotopes and gut content show diet overlap among native and introduced piscivores in a large oligotrophic lake. Ecol. Freshw. Fish 2005, 14, 267–277. [Google Scholar] [CrossRef]
- Hansen, A.G.; Gardner, J.R.; Connelly, K.A.; Polacek, M.; Beauchamp, D.A. Resource use among top-level piscivores in a temperate reservoir: Implications for a threatened coldwater specialist. Ecol. Freshw. Fish 2022, 31, 469–491. [Google Scholar] [CrossRef]
- Foster, J. Skagit River Snorkel Survey Report; Triton Environmental Consultants Ltd.: Vernon, BC, Canada, 2020. [Google Scholar]
- Nelson, T.C. Final Report: Upper Skagit Watershed Native Char Project 2001–2004; LGL Limited Environmental Research Associates: Sidney, BC, Canada, 2006. [Google Scholar]
- Nelson, T.C.; Mussell, C.E.J.; Rissling, J. Progress Report 2003: Upper Skagit Watershed Native Char Project; LGL Limited Environmental Research Associates: Sidney, BC, Canada, 2004. [Google Scholar]
- Seattle City Light. Biological Evaluation—Supplement: Impacts of entrainment of bull trout. In Skagit River Hydroelectric Project License (FERC no. 553) Amendment: Addition of a Second Power Tunnel at the Gorge Development; Seattle City Light: Seattle, WA, USA, 2012. [Google Scholar]
- Hyatt, K.D.; Stockner, J.G. Responses of sockeye salmon (Oncorhynchus nerka) to fertilization of British Columbia coastal lakes. Can. J. Fish. Aquat. Sci. 1985, 42, 320–331. [Google Scholar] [CrossRef]
- Henderson, M.A.; Cass, A.I. Effect of smolt size on smolt-to-adult survival for Chilko Lake sockeye salmon. Can. J. Fish. Aquat. Sci. 1991, 48, 988–994. [Google Scholar] [CrossRef]
- Koski, M.L.; Johnson, B.M. Functional response of kokanee salmon (Oncorhynchus nerka) to Daphnia at different light levels. Can. J. Fish. Aquat. Sci. 2002, 59, 707–716. [Google Scholar] [CrossRef]
- Murphy, C.A.; Romer, J.D.; Stertz, K.; Arismendi, I.; Emig, R.; Monzyk, F.; Johnson, S.L. Damming salmon fry: Evidence for predation by non-native warmwater fishes in reservoirs. Ecosphere 2021, 12, e03757. [Google Scholar] [CrossRef]
- Eckmann, M.; Dunham, J.; Connor, E.J.; Welch, C.A. Bioenergetic evaluation of diel vertical migration by bull trout (Salvelinus confluentus) in a thermally stratified reservoir. Ecol. Freshw. Fish 2018, 27, 30–43. [Google Scholar] [CrossRef]
- Beauchamp, D.A.; Lariviere, M.G.; Thomas, G.L. Evaluation of competition and predation as limits to juvenile kokanee and sockeye salmon production in Lake Ozette, Washington. N. Am. J. Fish. Manag. 1995, 15, 193–207. [Google Scholar] [CrossRef]
- Furey, N.B.; Hinch, S.G. Bull trout movements match the life history of sockeye salmon: Consumers can exploit seasonally distinct resource pulses. Trans. Am. Fish. Soc. 2017, 146, 450–461. [Google Scholar] [CrossRef]
- Furey, N.B.; Hinch, S.G.; Mesa, M.G.; Beauchamp, D.A. Piscivorous fish exhibit temperature-influenced binge feeding during an annual prey pulse. J. Anim. Ecol. 2016, 85, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Hatton, T.W.; Adams, N.S. Monitoring Fish Abundance and Behavior, Using Multi-Beam Acoustic Imaging Sonar, at a Selective Water Withdrawal Structure in Lake Billy Chinook, Deschutes River, Oregon, 2020; Open-File Report 2022-1038; U.S. Geological Survey: Reston, VA, USA, 2022; pp. 1–31.
- Smith, T.W.; Liermann, B.W.; Eby, L.A. Ecological assessment and evaluation of potential eradication approaches for introduced redside shiners in a montane lake. N. Am. J. Fish. Manag. 2021, 41, 1473–1489. [Google Scholar] [CrossRef]



| Temperature °C | |||
|---|---|---|---|
| Month | Min | Mean | Max |
| May | 7.4 | 8.5 | 9.5 |
| June | 9.6 | 10.6 | 11.8 |
| July | 11.8 | 13.0 | 14.0 |
| August | 13.1 | 13.8 | 14.7 |
| Sepetember | 13.9 | 15.2 | 15.5 |
| October | 12.0 | 13.5 | 14.7 |
| November | 8.8 | 10.4 | 12.1 |
| December | 5.8 | 7.1 | 8.7 |
| January | 4.6 | 5.1 | 5.7 |
| Febuary | 4.1 | 4.2 | 4.6 |
| March | 4.1 | 4.6 | 5.1 |
| April | 5.2 | 6.3 | 7.6 |
| Capacity Definition | Month | No Thermal Restriction (0–20 m) | Thermal Restriction (10–20 m) | ||||
|---|---|---|---|---|---|---|---|
| Capacity | Initial Fry Abundance (S = 8.5%) | Initial Fry Abundance (S = 25%) | Capacity | Initial Fry Abundance (S = 8.5%) | Initial Fry Abundance (S = 25%) | ||
| 50% biomass + production | May | 10,046,618 | 10,046,618 | 10,046,618 | - | - | - |
| June | 10,355,767 | 12,767,573 | 11,649,737 | 2,193,357 | 2,704,179 | 2,467,421 | |
| July | 8,127,822 | 12,271,375 | 10,246,898 | 2,986,596 | 4,509,159 | 3,765,258 | |
| August | 6,170,447 | 11,485,812 | 8,751,223 | 3,399,799 | 6,328,464 | 4,821,757 | |
| September | 1,460,625 | 3,352,045 | 2,330,369 | 968,018 | 2,221,542 | 1,544,434 | |
| October | 2,093,817 | 5,884,409 | 3,743,769 | - | - | - | |
| 50% production | May | 3,940,730 | 3,940,730 | 3,940,730 | - | - | - |
| June | 5,135,605 | 6,331,661 | 5,777,307 | 0 | 0 | 0 | |
| July | 5,780,337 | 8,727,145 | 7,287,380 | 2,380,058 | 3,593,408 | 3,000,584 | |
| August | 3,553,515 | 6,614,595 | 5,039,765 | 2,760,415 | 5,138,300 | 3,914,952 | |
| September | 54,338 | 124,702 | 86,694 | 150,563 | 345,533 | 240,217 | |
| October | 553,376 | 1,555,194 | 989,443 | - | - | - | |
| Adult Returns | |||||
|---|---|---|---|---|---|
| Scenario | Fry-Smolt Survival (%) | Initial Fry Abundance in Lake | Smolt Abundance | SAR: 4.1% | SAR: 6.3% |
| 1 | 3.6 | 2,967,493 | 106,830 | 4380 | 6730 |
| 8.5 | 2,221,542 | 188,831 | 7742 | 11,896 | |
| 25.0 | 1,544,434 | 386,108 | 15,830 | 24,325 | |
| 2 | 3.6 | 4,477,597 | 161,193 | 6609 | 10,155 |
| 8.5 | 3,352,045 | 284,924 | 11,682 | 17,950 | |
| 25.0 | 2,330,369 | 582,592 | 23,886 | 36,703 | |
| Predation Potential (Individuals) | ||||||
|---|---|---|---|---|---|---|
| Species/Life-History | FL (mm) | Weight (g) | Population Estimate | March | May | June |
| Chinook fry-migrant | 39 | 0.72 | 1000 BT | 2745 | - | - |
| 3000 BT | 8237 | - | - | |||
| Chinook parr-migrant | 75 | 4.90 | 1000 BT | - | - | 1062 |
| 3000 BT | - | - | 3185 | |||
| Chinook yearling | 120 | 19.70 | 1000 BT | - | 158 | - |
| 3000 BT | - | 474 | - | |||
| Steelhead age-2 smolt | 130 | 24.90 | 1000 BT | - | 125 | - |
| 3000 BT | - | 375 | - | |||
| Steelhead age-3 smolt | 165 | 50.30 | 1000 BT | - | 62 | - |
| 3000 BT | - | 186 | - | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 This article is a U.S. Government work and is in the public domain in the USA. Submitted for possible open access publication under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, R.C.; Jensen, B.L.; Code, T.J.; Duda, J.J.; Beauchamp, D.A. Prey Supply and Predation as Potential Limitations to Feasibility of Anadromous Salmonid Introductions in a Reservoir. Water 2024, 16, 1157. https://doi.org/10.3390/w16081157
Johnson RC, Jensen BL, Code TJ, Duda JJ, Beauchamp DA. Prey Supply and Predation as Potential Limitations to Feasibility of Anadromous Salmonid Introductions in a Reservoir. Water. 2024; 16(8):1157. https://doi.org/10.3390/w16081157
Chicago/Turabian StyleJohnson, Rachelle C., Benjamin L. Jensen, Tessa J. Code, Jeffrey J. Duda, and David A. Beauchamp. 2024. "Prey Supply and Predation as Potential Limitations to Feasibility of Anadromous Salmonid Introductions in a Reservoir" Water 16, no. 8: 1157. https://doi.org/10.3390/w16081157
APA StyleJohnson, R. C., Jensen, B. L., Code, T. J., Duda, J. J., & Beauchamp, D. A. (2024). Prey Supply and Predation as Potential Limitations to Feasibility of Anadromous Salmonid Introductions in a Reservoir. Water, 16(8), 1157. https://doi.org/10.3390/w16081157






