Effects of Phosphate and Arsenate on As Metabolism in Microcystis aeruginosa at Different Growth Phases
Abstract
1. Introduction
2. Materials and Methods
2.1. Algae Culture and Pretreatment
2.2. Experimental Design
2.3. Speciation and Content Analysis of Extracellular and Intracellular As
2.4. The Principles and Computation Methods for Various Factors
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effects of P and iAsV Additions on Extracellular As Species and Content at Different Growth Phases
3.2. Effects of P and iAsV Additions on Intracellular As Species and Content at Different Growth Phases
3.3. Effects of P and iAsV Additions on As Metabolism at Different Growth Phases
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rakhmania; Kamyab, H.; Yuzir, M.A.; Al-Qaim, F.F.; Purba, L.D.A.; Riyadi, F.A. Application of Box-Behnken design to mineralization and color removal of palm oil mill effluent by electrocoagulation process. Environ. Sci. Pollut. Res. 2023, 30, 71741–71753. [Google Scholar] [CrossRef]
- Filella, M.; Wey, S.; Matoušek, T.; Coster, M.; Rodríguez-Murillo, J.; Loizeau, J. Arsenic in Lake Geneva (Switzerland, France): Long term monitoring, and redox and methylation speciation in an As unpolluted, oligo-mesotrophic lake. Environ. Sci. Process. Impacts 2023, 25, 850–869. [Google Scholar] [CrossRef]
- Gao, Z.; Jiang, C.; Lyu, R.; Yang, Z.; Zhang, T. Optimization of the preparation of fungal-algal pellets for use in the remediation of arsenic-contaminated water. Environ. Sci. Pollut. Res. 2020, 27, 36789–36798. [Google Scholar] [CrossRef]
- Malsawmdawngzela, R.; Lalhmunsiama; Tiwari, D.; Lee, S. Synthesis of novel clay-based nanocomposite materials and its application in the remediation of arsenic contaminated water. Int. J. Environ. Sci. Technol. 2022, 20, 10285–10296. [Google Scholar] [CrossRef]
- Mahdavi, H.; Ulrich, A.C.; Liu, Y. Metal removal from oil sands tailings pond water by indigenous micro-alga. Chemosphere 2012, 89, 350–354. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Xu, P.; Liu, C.; Liu, M.; Wang, Y.; Wang, C.; Zhang, C.; Ge, Y. Review of arsenic speciation, toxicity and metabolism in microalgae. Rev. Environ. Sci. Biotechnol. 2015, 14, 427–451. [Google Scholar] [CrossRef]
- Pal, A.; Paknikar, K.M. Microorganisms in Environmental Management; Springer: Dordrecht, The Netherlands, 2012; pp. 477–523. [Google Scholar]
- Wang, Z.; Gui, H.; Luo, Z.; Zhen, Z.; Yan, C.; Xing, B. Dissolved organic phosphorus enhances arsenate bioaccumulation and biotransformation in Microcystis aeruginosa. Environ. Pollut. 2019, 252, 1755–1763. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Z.; Yan, C.; Xing, B. Impacts of environmental factors on arsenate biotransformation and release in Microcystis aeruginosa using the Taguchi experimental design approach. Water Res. 2017, 118, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Markley, C.T.; Herbert, B.E. Modeling phosphate pnfluence on arsenate reduction kinetics by a freshwater Cyanobacterium. Environ. Model. Assess. 2010, 15, 361–368. [Google Scholar] [CrossRef]
- Ashfaq, M.H.; Shahid, S.; Javed, M.; Iqbal, S.; Hakami, O.; Aljazzar, S.O.; Fatima, U.; Elkaeed, E.B.; Pashameah, R.A.; Alzahrani, E.; et al. Controlled growth of TiO2/Zeolite nanocomposites for simultaneous removal of ammonium and phosphate ions to prevent eutrophication. Front. Mater. 2022, 9, 1007485. [Google Scholar] [CrossRef]
- Qin, C.; Liu, H.; Liu, L.; Smith, S.; Sedlak, D.L.; Gu, A.Z. Bioavailability and characterization of dissolved organic nitrogen and dissolved organic phosphorus in wastewater effluents. Sci. Total Environ. 2015, 511, 47–53. [Google Scholar] [CrossRef]
- Davis, T.W.; Bullerjahn, G.S.; Tuttle, T.; McKay, R.M.; Watson, S.B. Effects of increasing nitrogen and phosphorus concentrations on phytoplankton community growth and toxicity during Planktothrix blooms in Sandusky Bay, Lake Erie. Environ. Sci. Technol. 2015, 49, 7197–7207. [Google Scholar] [CrossRef]
- Elias, M.; Wellner, A.; Goldin-Azulay, K.; Chabriere, E.; Vorholt, J.A.; Erb, T.J.; Tawfik, D.S. The molecular basis of phosphate discrimination in arsenate-rich environments. Nature 2012, 491, 134–137. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.H.; Lin, M.M.; Ge, Y. A symbiotic bacterium differentially influences arsenate absorption and transformation in Dunaliella salina under different phosphate regimes. J. Hazard. Mater. 2016, 318, 443–451. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, Z.; Yan, C.; Che, F.; Yan, Y. Arsenic uptake and depuration kinetics in Microcystis aeruginosa under different phosphate regimes. J. Hazard. Mater. 2014, 276, 393–399. [Google Scholar] [CrossRef]
- Schoffelen, N.J.; Mohr, W.; Ferdelman, T.G.; Littmann, S.; Duerschlag, J.; Zubkov, M.V.; Ploug, H.; Kuypers, M.M.M. Single-cell imaging of phosphorus uptake shows that key harmful algae rely on different phosphorus sources for growth. Sci. Rep. 2018, 8, 17182. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Wang, Z.; Liu, A.; Yan, Y.; Wu, Y.; Zhang, X. New insights into toxic effects of arsenate on four Microcystis species under different phosphorus regimes. Environ. Sci. Pollut. Res. 2020, 27, 44460–44469. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, S.; Yang, F.; Wei, C. Effects of arsenate on the growth and arsenic uptake in Microcystis aeruginosa at different growth phases and phosphorus levels. Acta Sci. Circumstantiae 2017, 37, 2061–2068. (In Chinese) [Google Scholar]
- Xie, S.; Liu, J.; Yang, F.; Feng, H.; Wei, C.; Wu, F. Arsenic uptake, transformation, and release by three freshwater algae under conditions with and without growth stress. Environ. Sci. Pollut. Res. 2018, 25, 19413–19422. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Liu, C.; Xu, P.; Li, H.; Lin, Q.; Zhang, C.; Ge, Y. Arsenate toxicity and metabolism in the halotolerant microalga Dunaliella salina under various phosphate regimes. Environ. Sci. Process. Impacts 2016, 18, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ning, Z.; Wang, Y.; Zheng, Y.; Zhang, C.; Figeys, D. Quantitative proteomic analysis of Dunaliella salina upon acute arsenate exposure. Chemosphere 2016, 145, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Salvador, D.; Churro, C.; Valério, E. Evaluating the influence of light intensity in mcyA gene expression and microcystin production in toxic strains of Planktothrix agardhii and Microcystis aeruginosa. J. Microbiol. Methods 2016, 123, 4–12. [Google Scholar] [CrossRef]
- Baker, J.; Wallschläger, D. The role of phosphorus in the metabolism of arsenate by a freshwater green alga, Chlorella vulgaris. J. Environ. Sci. 2016, 49, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wang, Z.; Chen, Y.; Luo, Y.; Luo, Z. Enhancing arsenate metabolism in Microcystis aeruginosa and relieving risks of arsenite and microcystins by nano-Fe2O3 under dissolved organic phosphorus conditions. Environ. Pollut. 2023, 330, 121801. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Jagadevan, S. Genetic identification of arsenate reductase and arsenite oxidase in redox transformations carried out by arsenic metabolising prokaryotes—A comprehensive review. Chemosphere 2016, 163, 400–412. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Wang, Y.; Yang, M.; Shi, R.; Ma, T.; Cui, G.; Li, X. Distribution and speciation of arsenic in seasonally stratified reservoirs: Implications for biotransformation mechanisms governing interannual variability. Sci. Total Environ. 2022, 806, 150925. [Google Scholar] [CrossRef]
- Che, F.; Du, M.; Yan, C. Arsenate biotransformation by Microcystis aeruginosa under different nitrogen and phosphorus levels. J. Environ. Sci. 2018, 66, 41–49. [Google Scholar] [CrossRef]
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A review of high arsenic groundwater in Mainland and Taiwan, China: Distribution, characteristics and geochemical processes. Appl. Geochem. 2014, 41, 196–217. [Google Scholar] [CrossRef]
- Wang, N.; Li, Y.; Deng, X.; Miao, A.; Ji, R.; Yang, L. Toxicity and bioaccumulation kinetics of arsenate in two freshwater green algae under different phosphate regimes. Water Res. 2013, 47, 2497–2506. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hogan, B.; Duncan, E.; Doyle, C.; Krassoi, R.; Rahman, M.M.; Naidu, R.; Lim, R.P.; Maher, W.; Hassler, C. Toxicity of arsenic species to three freshwater organisms and biotransformation of inorganic arsenic by freshwater phytoplankton (Chlorella sp. CE-35). Ecotoxicol. Environ. Saf. 2014, 106, 126–135. [Google Scholar] [CrossRef]
- Amirnia, S.; Asaeda, T.; Takeuchi, C.; Kaneko, Y. Manganese-mediated immobilization of arsenic by calcifying macro-algae, Chara braunii. Sci. Total Environ. 2019, 646, 661–669. [Google Scholar] [CrossRef]
- Wang, N.; Huang, B.; Xu, S.; Wei, Z.; Miao, A.; Ji, R.; Yang, L. Effects of nitrogen and phosphorus on arsenite accumulation, oxidation, and toxicity in Chlamydomonas reinhardtii. Aquat. Toxicol. 2014, 157, 167–174. [Google Scholar] [CrossRef]
- Karadjova, I.B.; Slaveykova, V.I.; Tsalev, D.L. The biouptake and toxicity of arsenic species on the green microalga Chlorella salina in seawater. Aquat. Toxicol. 2008, 87, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Castro, M.C.; Urrea, G.; Guasch, H. Influence of the interaction between phosphate and arsenate on periphyton’s growth and its nutrient uptake capacity. Sci. Total Environ. 2014, 503–504, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Han, F.; Xu, H.; Mu, J.; Chen, D.; Feng, B.; Zeng, H. Potential lipid accumulation and growth characteristic of the green alga Chlorella with combination cultivation mode of nitrogen (N) and phosphorus (P). Bioresour. Technol. 2014, 174, 24–32. [Google Scholar] [CrossRef] [PubMed]
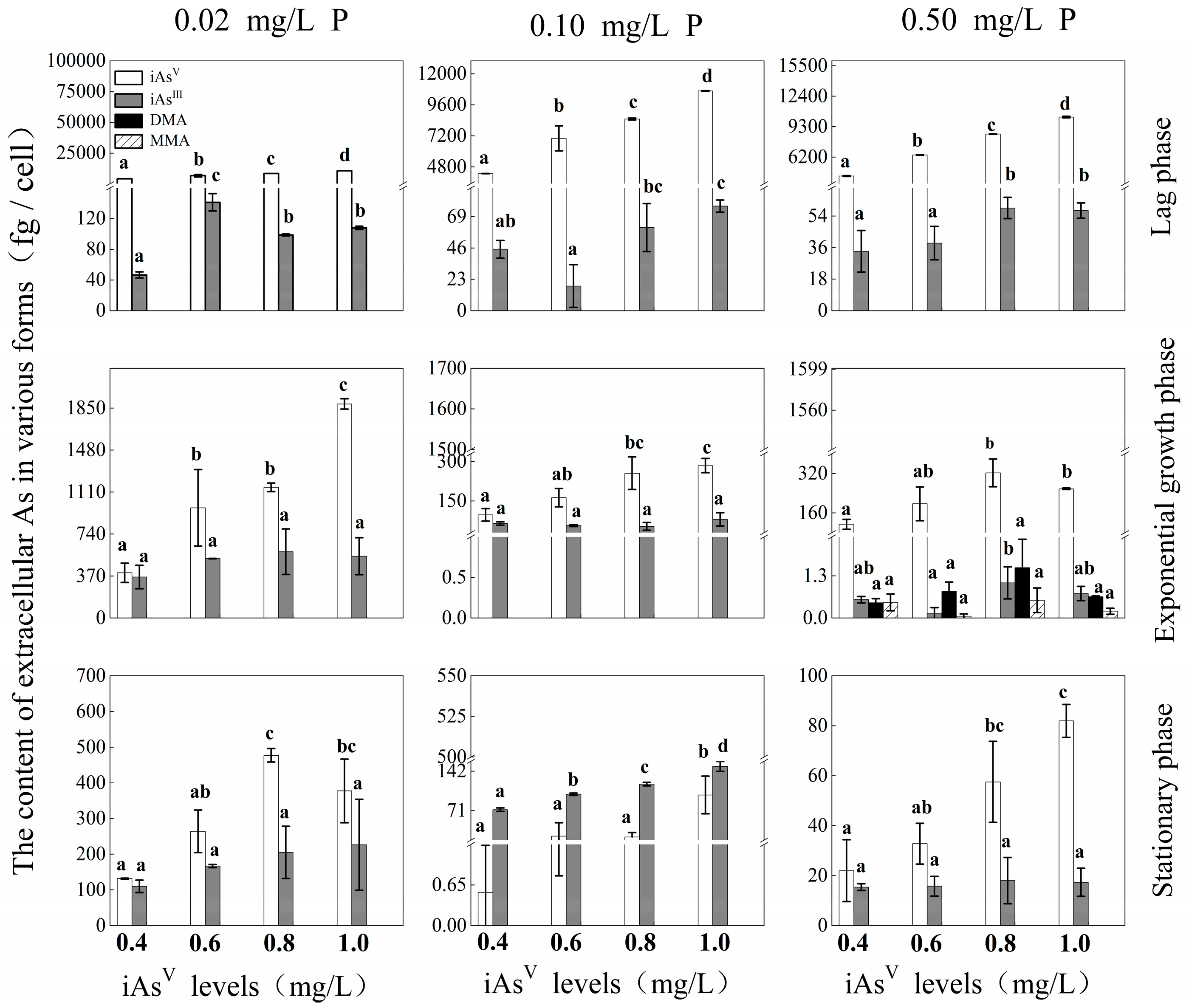
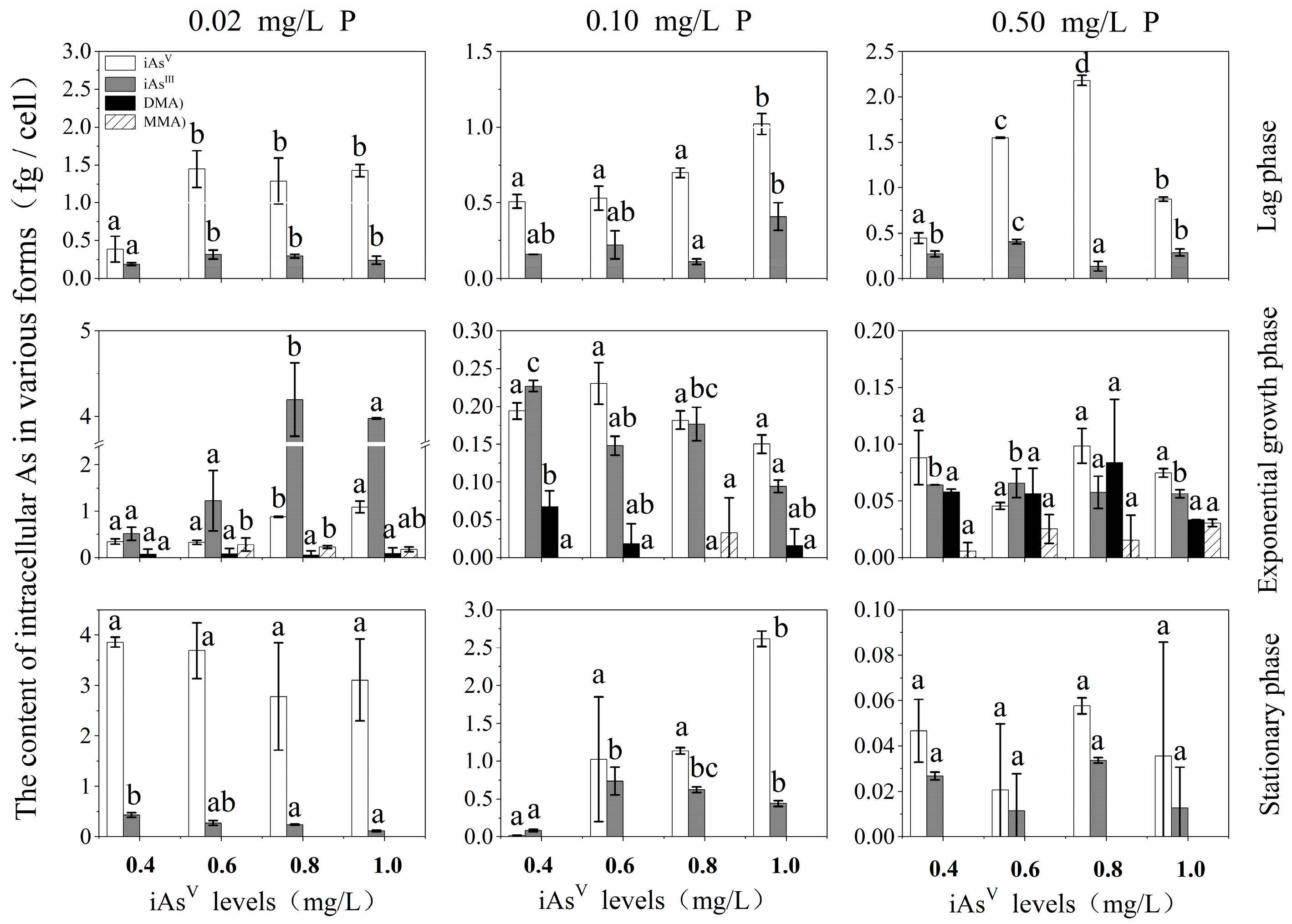
| Serial Number | iAsV Levels (mg/L) | P Levels (mg/L) |
|---|---|---|
| 1 | 0.4 | 0.02 |
| 2 | 0.1 | |
| 3 | 0.5 | |
| 4 | 0.6 | 0.02 |
| 5 | 0.1 | |
| 6 | 0.5 | |
| 7 | 0.8 | 0.02 |
| 8 | 0.1 | |
| 9 | 0.5 | |
| 10 | 1 | 0.02 |
| 11 | 0.1 | |
| 12 | 0.5 |
| P Levels (mg/L) | iAsV Add Time Points | ||
|---|---|---|---|
| Lag Phase | Exponential Growth Phase | Stationary Phase | |
| 0.02 | Day 0 | Day 8 | Day 16 |
| 0.1 | Day 0 | Day 11 | Day 22 |
| 0.5 | Day 0 | Day 12 | Day 24 |
| As Addition (mg/L) | P Levels (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lag Growth Phase | Exponential Growth Phase | Stationary Growth Phase | |||||||
| 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | |
| 0.4 | 46.98 ± 3.11 Ab | 49.33 ± 0.84 Aab | 34.46 ± 11.83 Aa | 362.07 ± 103.30 Ba | 65.47 ± 6.33 Aa | 1.70 ± 0.07 Aa | 113.95 ± 17.87 Ca | 72.96 ± 2.99 Ba | 15.43 ± 1.31 Aa |
| 0.6 | 143.23 ± 11.76 Bc | 18.64 ± 15.48 Aa | 40.28 ± 9.55 Aa | 525.42 ± 1.83 Ca | 57.06 ± 3.58 Ba | 1.15 ± 0.08 Aa | 170.54 ± 5.24 Ca | 102.26 ± 3.43 Bb | 15.82 ± 3.91 Aa |
| 0.8 | 100.12 ± 1.70 Bb | 64.43 ± 23.88 Ab | 60.64 ± 6.03 Ab | 589.50 ± 201.76 Ba | 54.63 ± 14.77 Aa | 3.36 ± 1.86 Aa | 207.91 ± 72.10 Ba | 120.52 ± 3.15 ABc | 18.10 ± 9.30 Aa |
| 1 | 109.43 ± 2.25 Bb | 70.34 ± 13.50 Ab | 57.94 ± 4.32 Ab | 549.20 ± 162.99 Ba | 80.82 ± 25.13 Aa | 1.76 ± 0.15 Aa | 229.32 ± 126.03 Aa | 153.61 ± 8.88 Ad | 17.43 ± 5.55 Aa |
| As Addition (mg/L) | P Levels (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lag Growth Phase | Exponential Growth Phase | Stationary Growth Phase | |||||||
| 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | |
| 0.4 | 0.01 ± 0.01 Aa | 0.01 Ab | 0.01 Ab | 0.09 ± 0.02 Aa | 0.26 ± 0.04 Bc | 0.17 Ab | 1.68 ± 0.07 Bb | 0.13 Aa | 0.17 ± 0.01 Ab |
| 0.6 | 0.03 Bb | 0.01 Aa | 0.03 Bd | 0.10 ± 0.04 Aa | 0.14 ± 0.04 Ab | 0.07 ± 0.01 Aa | 0.90 ± 0.35 Aa | 1.29 ± 0.73 Ab | 0.10 Aa |
| 0.8 | 0.02 ± 0.01 ABa | 0.01 Aa | 0.02 Bc | 0.29 ± 0.01 Cc | 0.10 Bab | 0.05 ± 0.02 Aa | 0.41 ± 0.23 Aa | 1.21 ± 0.13 Bab | 0.10 ± 0.04 Aa |
| 1 | 0.01 Ba | 0.01 Bb | 0.01 Aa | 0.21 ± 0.01 Bb | 0.05 ± 0.02 Aa | 0.05 Aa | 0.49 ± 0.22 Aa | 1.22 ± 0.18 Ab | 0.08 Aa |
| As Addition (mg/L) | P Levels (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lag Growth Phase | Exponential Growth Phase | Stationary Growth Phase | |||||||
| 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | |
| 0.4 | 99.43 ± 0.50 Aa | 99.14 ± 0.12 Aa | 98.83 ± 0.29 Ab | 99.94 ± 0.07 Aa | 99.77 Ab | 95.21 ± 2.80 Aa | 96.75 ± 0.43 Aa | 99.98 ± 0.03 Bb | 99.72 ± 0.15 Ba |
| 0.6 | 98.86 ± 0.28 Aa | 74.58 ± 42.08 Aa | 96.30 ± 1.11 Aa | 99.97 ± 0.01 Aa | 99.69 ± 0.04 Aa | 98.40 ± 0.37 Aa | 97.93 ± 0.46 Aa | 99.06 ± 0.99 Aab | 99.72 Aa |
| 0.8 | 98.84 ± 0.60 Ba | 98.97 ± 0.27 Ba | 96.64 ± 0.29 Aa | 99.87 ± 0.06 Ba | 99.76 ± 0.01 Bb | 98.16 ± 0.37 Aa | 98.55 ± 1.14 Aa | 99.08 ± 0.09 Aab | 99.71 ± 0.10 Aa |
| 1 | 98.81 ± 0.27 Aa | 98.62 ± 0.29 Aa | 98.82 ± 0.05 Ab | 99.82 ± 0.11 Ba | 00.87 ± 0.03 Bc | 97.59 ± 0.42 Aa | 98.37 ± 1.41 Aa | 98.30 ± 0.04 Aa | 99.55 Aa |
| As Addition (mg/L) | P levels (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Lag Growth Phase | Exponential Growth Phase | Stationary Growth Phase | |||||||
| 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | 0.02 | 0.1 | 0.5 | |
| 0.4 | 99.11 ± 0.54 Aa | 98.81 ± 0.14 Aa | 98.13 ± 0.55 Ab | 99.81 ± 0.01 Bb | 99.37 ± 0.04 Ba | 88.76 ± 1.74 Aa | 96.41 ± 0.42 Aa | 99.87 Bb | 99.58 ± 0.18 Ba |
| 0.6 | 98.67 ± 0.26 Aa | 65.58 ± 56.79 Aa | 95.50 ± 1.22 Aa | 99.70 ± 0.19 Ba | 99.46 ± 0.10 Bab | 88.22 ± 4.14 Aa | 97.80 ± 0.52 Aa | 98.35 ± 1.18 Aab | 99.61 Aa |
| 0.8 | 98.57 ± 0.53 Ba | 98.84 ± 0.45 Ba | 96.58 ± 0.39 Aa | 99.10 ± 0.21 Ba | 99.40 ± 0.25 Bab | 94.73 ± 0.13 Aa | 98.47 ± 1.18 Aa | 98.58 ± 0.08 Aab | 99.58 ± 0.09 Aa |
| 1 | 98.66 ± 0.26 Aa | 98.03 ± 0.69 Aa | 98.54 ± 0.10 Ab | 99.04 ± 0.32 Ba | 99.78 ± 0.01 Bb | 92.11 ± 0.90 Aa | 98.34 ± 1.41 Aa | 98.04 ± 0.06 Aa | 99.42 Aa |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, J.; Yang, F.; Xie, S.; Wei, C. Effects of Phosphate and Arsenate on As Metabolism in Microcystis aeruginosa at Different Growth Phases. Water 2024, 16, 940. https://doi.org/10.3390/w16070940
Zhang P, Liu J, Yang F, Xie S, Wei C. Effects of Phosphate and Arsenate on As Metabolism in Microcystis aeruginosa at Different Growth Phases. Water. 2024; 16(7):940. https://doi.org/10.3390/w16070940
Chicago/Turabian StyleZhang, Ping, Jinxin Liu, Fen Yang, Shaowen Xie, and Chaoyang Wei. 2024. "Effects of Phosphate and Arsenate on As Metabolism in Microcystis aeruginosa at Different Growth Phases" Water 16, no. 7: 940. https://doi.org/10.3390/w16070940
APA StyleZhang, P., Liu, J., Yang, F., Xie, S., & Wei, C. (2024). Effects of Phosphate and Arsenate on As Metabolism in Microcystis aeruginosa at Different Growth Phases. Water, 16(7), 940. https://doi.org/10.3390/w16070940







