Abstract
Due to urbanization and population growth, freshwater resources have become a long-term concern for most developing countries. With the growth of population, the demand for fresh water is increasing and the requirement for sewage treatment is also increasing. In recent years, the demand for sewage recycling has increased sharply. Constructed wetlands (CWs) are an effective sewage treatment system with low energy consumption, minimal maintenance requirements, and a low operation cost, which will meet the current demand for the removal of nutrients and pathogens. The application of CWs in sewage treatment has attracted more and more attention because it is also a nature-based solution (NbS). These systems are capable of removing not only nitrogen (N) and phosphorus (P), but also pathogen indicators, such as fecal coliform and Escherichia coli. The presence of these indicators also suggests the influx of other pathogens into aquatic systems, thereby threatening aquatic ecological health. However, research on the removal of pathogens in CWs is relatively scare and their removal mechanisms are not fully understood. Despite their widespread application, the role of plants in CWs, particularly in the specific mechanism of pathogens and nitrogen removal, remains largely unknown. This article will help us to better understand this technology and provide help for our further research. In this paper, the coupled denitrification mechanism between microorganisms and plants in the process of nitrogen transformation was discussed. Plants affect nitrogen transformation microorganisms by releasing oxygen and secretions from their roots and provide substrates for bioremediation. The removal effects of different types of CWs on pathogen and nitrogen species were also summarized. Overall, the removal effect of subsurface flow wetlands outperforms that of surface flow wetlands, with multi-stage wetland systems being the most effective. The main factors affecting the removal of pathogens and nitrogen species in CWs include plants, substrates, operating parameters, UV radiation, temperature, water composition, and pH. Finally, the research frontiers on the removal of pathogens in CWs were prospected.
1. Introduction
Due to population growth and economic development, the over exploitation and pollution of water resources has led to a significant decrease in available water per capita [1]. The rapid urbanization and population growth in the 21st century require fresh water supplies to maintain livelihoods [2], thus calling for the development of appropriate sewage treatment technologies and their subsequent reuse. Although various sewage treatment technologies have been developed, the overall treatment capacity in developing countries remains relatively low due to economic constraints. Due to the lack of centralized wastewater collection and treatment systems in China, it has brought great challenges to the management of domestic and agricultural wastewater in China [3]. Official statistics show that the sewage treatment rate in rural areas is 11.4%, far lower than the reported 91.9% in urban areas [4]. The low treatment rate of rural sewage in China will lead to the direct discharge of domestic sewage to nearby water bodies, which will lead to serious eutrophication and potential biological risks of diffusion [5]. A recent survey shows that about half of the CWs have been built in eastern China, of which 48.41% are located in rural areas of Zhejiang Province. These are mainly responsible for the treatment of typical domestic sewage components, including chemical oxygen demand, total nitrogen, ammonia nitrogen, and total phosphorus [6]. As an emerging eco-friendly sewage treatment approach and NbS, CWs have been increasingly implemented and applied on a large scale in China. This method has been successfully applied to the removal of pollutants from domestic wastewater [7], industrial wastewater [8], and agricultural wastewater [9]. Treated wastewater from CWs, once meeting the relevant sanitary standards, can be used for landscape supplementation, green space irrigation, and agricultural irrigation, allowing for nutrient circulation. This not only effectively reduces the pollution load on receiving water bodies, but also offers certain landscape and economic benefits [10].
CWs demonstrate high efficiency in the removal of nitrogenous pollutants [11], attracting widespread concern from researchers. In the purification process of constructed wetlands, microorganisms play a primary role in the transformation and degradation of nitrogenous pollutants. Their involvement in nitrification and denitrification reactions effectively removes nitrogenous pollutants from the constructed wetland systems. Plants can absorb nitrogen and provide substrates for bioremediation, while also influencing nitrifying and denitrifying bacteria through the secretion of oxygen and exudates from their roots [12]. Plants play an important role in the nitrification and denitrification processes within CWs, and plant species are the key factors affecting these processes [13].
In addition, the standard limits for wastewater quality control mainly target organic compounds (such as COD and BOD), nitrogen, and phosphorus. So far, the aquatic ecological health impacts of pathogens in sewage have not received much attention. The helminths parasites most commonly identified in wastewater include Ascaris lumbricoides, Ancylostoma duodenale, and Trichuris trichura [14]. The utilization of this contaminated wastewater for agricultural irrigation poses significant health risks, due to the high viability of insect eggs in the environment. The soil or crops provide favorable conditions for insect eggs to develop into toxic larvae within a few days. Consequently, helminths are transmitted via eggs through a human–water–soil–crop–human pathway [15]. However, pathogens remaining in treatment water should not pose risks to aquatic health. Many studies have shown that pathogens in CWs can be removed individually or in combination through physical, chemical, and biological factors, such as sedimentation, filtration, adsorption, predation, natural die-off, etc. [16,17,18]. However, due to the complexity of the influencing factors, the understanding of the fate and removal of these pathogens in CWs is still insufficient, especially regarding the role of plants in these systems and their specific mechanism of pathogen removal. This paper mainly discusses the various factors affecting the removal of pathogens.
2. The Role of Plants in CWs
2.1. The Coupling Role of Plants with Nitrifying and Denitrifying Microorganisms
Nitrogen removal in CWs is the result of the combined action of multiple factors. Plant roots can directly absorb inorganic nitrogen such as ammonium and nitrate from soil or water, converting it into organic nitrogen within the plant body to meet the needs of its growth and development. Other microorganisms in the wetland also participate in the absorption and conversion process of nitrogen. Ammonification is also an important step in nitrogen removal. In this process, organic nitrogen is decomposed into ammonia nitrogen under the action of microorganisms, providing a foundation for subsequent nitrification. The first stage is nitrosification, where ammonia nitrogen is oxidized to nitrite by nitrosifying bacteria. The second stage is nitrification, where nitrite is further oxidized to nitrate by nitrifying bacteria. Under anaerobic or hypoxic conditions, denitrifying bacteria reduce nitrate or nitrite to gaseous nitrogen (such as nitrogen gas), thereby removing it from the system.
Plants are an important component of CWs, not only providing a vast surface area for the development of microorganisms, but also offering a suitable environment for their growth. The contribution of plant uptake to the total nitrogen removal is relatively small. In pilot-scale CWs, the contribution of plants to total nitrogen removal is less than 10% [19]. Microbial denitrification is the most important pathway for nitrogen removal in CWs. These functional microorganisms do not transform nitrogen in isolation, but rather in cooperation with plants, substrates, and other microorganisms. Plants can absorb nitrogen and provide substrates for microbial attachment, while also influencing nitrification and denitrification bacteria through the secretion of oxygen and exudates from their roots [12]. Plants play an important role in nitrification and denitrification within CWs, with plant species being the key factors influencing these processes [13].
Plant characteristics, such as root exudates, oxygen transport, and root structure, will affect microbial diversity and rhizosphere enzyme activity. The main components of root exudates are small molecular organic acids, phenolics, aromatic proteins, etc., varying with plant species, predominantly in the form of organic carbon. Rhizosphere microorganisms are strongly affected by root exudates [20]. Root exudates can determine the distribution patterns of microorganisms in micro-polluted soil [21] and the microbial activity in rhizosphere soil is often higher than that in non-rhizosphere soil [22]. Oxygen is essential for the activity and growth of nitrifying and denitrifying bacteria. Albuquerque et al. [23] demonstrated, through experiments, the ability of plants to provide oxygen to the aquatic environment. Extensive plant aeration tissues transport excess oxygen to the roots, which then diffuses to the surrounding environment, thus forming an oxidative–reductive rhizosphere micro ecosystem in CWs [24].
Plants are the key factors directly or indirectly affecting nitrification and denitrification in wetland rhizospheres [25]. They support the nitrification process by releasing dissolved oxygen and nutrients from root exudates, promoting the growth of nitrifying bacteria, and enhancing the denitrification process by providing carbon and energy for denitrifying bacteria in root exudates [21]. Compared to non-planted treatments, the potential nitrate reduction and nitrification activities [26], as well as the nitrate reductase activity [27], were notably higher for planted treatments. In addition, compared to single-species planting, CWs with mixed planting demonstrated a higher percentage of nitrogen metabolism-related bacteria, as well as a higher microbial diversity and abundance [28]. Plant species have an important impact on nitrifying bacteria and denitrifying bacteria. Mixed planting holds considerable potential for improving total nitrogen removal efficiency in septic tanks. However, the difference in plant–microbe denitrification coupling mechanisms under various vegetation combination conditions is not yet clear and requires more detailed research to optimize denitrification efficiency.
2.2. The Effect of Plants on the Removal of Pathogens
The specific mechanisms by which plants influence the removal of pathogens from CWs are currently unclear. It could be through adsorption and filtration by the plant roots, oxygen permeation, or the release of root exudates from plant species with antimicrobial activity, as well as secretions affecting the environment of the surrounding water body and, thus, rendering it unsuitable for the survival of pathogens [29]. This section discusses how different types of plants affect the removal efficiency of pathogens and the importance of plant selection in constructed wetland design.
Avelar et al. [30] conducted a comparative study to compare the effectiveness of horizontal subsurface flow constructed wetlands either with or without Mentha aquatica planting on the removal of coliforms from effluent at different hydraulic retention times (HRTs) (HRTs of 1.5 to 6.0 d). They observed that total coliform (TC) and Escherichia coli (EC) removal efficiencies were improved in horizontal subsurface flow constructed wetlands planted with Mentha aquatica. Seidel [31] studied the removal of EC by different species of herbs (Mentha aquatica, Alisma plantago, and Juncus effusus) and showed that Juncus effusus was more effective in removing EC (99%) compared to the other two plants. The report attributed the higher removal of pathogens by Juncus effusus to differences in the nature and density of the root system. Although the high density and large surface area of the root system contribute to the removal of pathogens, various other processes such as biofilm formation, oxygen permeation of the root system, and secretion of phytoconstituents are still unknown and require further in-depth studies. Kipasika et al. [32] compared the ability of the following four types of wetland vegetation: Typha latifolia, Cyperus papyrus, Cyperus alternifolius, and Phragmites mauritianus to remove wetland pathogens, and the results of the study showed that although there were differences in the removal ability between the different plants, the plants were effective in reducing pathogens in the wastewater, with the removal rates of salmonellae and Escherichia coli being above 98%. All these prove the positive role of plants in removing pathogens from wastewater and we have to choose different plants for different situations to achieve the desired removal effect. Table 1 lists the effectiveness of planted CWs in removing pathogens.

Table 1.
Effectiveness of planted CWs in removing pathogens.
However, some studies have different opinions on the effectiveness of plants in removing pathogens. For surface flow constructed wetlands, the role of plants in pathogen removal differs from that in other subsurface flow constructed wetlands [29]. In a study conducted by Lekeufack et al. [36], the surface flow constructed wetlands planted with E. pyramidalis showed a decreased efficiency in removing fecal streptococci when compared to a plant-free control group. This may be due to the inactivating effect of UV radiation on pathogens in unvegetated CWs exposed to prolonged sunlight, or the shade provided by vegetation that reduces UV exposure and may reduce microbial removal from free water surface constructed wetlands. Moreover, the efficiency of removing fecal bacteria was higher in the first year than in the second year, probably when the high plant density in the second year prevented the effect of UV radiation on wetland bacteria [36]. Manios et al. [37] reported no significant difference in the performance of planted and unplanted reed beds for the removal of EC and fecal coliforms. There are different explanations for the removal mechanism of pathogens removed by plants from CWs, which need to be studied in depth.
These studies are also very challenging with many variables to control. Although the influence of plants may seem negligible at times, their impact on biofilm formation and the overall framework of the wetland system cannot be denied.
3. The Removal Efficiency of Different Constructed Wetland Types on Pathogens and Nitrogen Species
CWs can be classified in various ways, based on the relative position of water flow and substrates. They are divided into free water surface constructed wetlands (FWSs) and subsurface flow constructed wetlands (SSFs). Among them, the subsurface flow constructed wetlands can be divided into horizontal subsurface flow constructed wetlands (HSFs) and vertical subsurface flow constructed wetlands (VSFs) according to the direction of water flow. The advantages of FWSs are simple design and low operating costs, but due to their low load and limited decontamination capacity, they are now less commonly used. SSFs are less affected by climate, have a strong load-bearing capacity, and are currently widely used. Table 2 shows a comparison of the advantages and disadvantages of the various types of CWs. Table 3 summarizes the removal effectiveness of different types of CWs for pollutants.

Table 2.
Comparison of advantages and disadvantages of various types of CWs.

Table 3.
The removal effectiveness of different types of CWs for pollutants.
Constructed wetlands are a wastewater treatment technology which is coordinated by plants, microorganisms, and substrates. The matrix can provide a growth medium for plants and microorganisms and the functions of adsorption, interception, filtration, and precipitation of pollutants. Plants can provide a suitable living environment for microorganisms and can also remove some pollutants in water by absorption and interception. Their roots can also provide oxygen and alleviate matrix blockage. Meanwhile, decaying plant tissues can provide a carbon source for denitrification processes. The ammonifying bacteria, nitrifying bacteria, and denitrifying bacteria in microorganisms can make the wetland have a good nitrogen removal effect. Table 4 lists the main mechanisms of pollutant removal by CWs. Figure 1 shows the removal mechanism of pathogens and nitrogen.

Table 4.
Main mechanisms for pollutant removal in CWs.
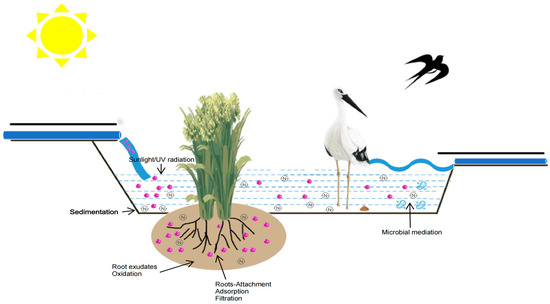
Figure 1.
The removal mechanism of pathogens and nitrogen.
Numerous studies and literature reviews (Rita P. Shingare [41], Shubiao Wu [29]) indicate that both types of CWs are effective in removing pathogens, in which the SSFs have better removal effect than the FWSs. The results showed that under the condition of high concentration of pathogens, although the removal effect of SSFs is still better than that of FWSs, the effluent from single-stage CWs may be at a safety risk due to the effluent pathogens concentration still being high. Therefore, multi-stage CWs may be considered to enhance their removal. Combinations of different types of CWs are also now widely used. Based on the data collected by Shubiao Wu et al. [29] from 91 CWs (including 20 FWSs, 53 HSFs, and 18 different types of multi-stage CWs), the mean values of the reduction in these fecal indicator organisms after wastewater flowed through the three types of CWs were calculated, and the HSFs were found to be more effective in removing EC (+1.10 log10 CFU/100 mL), FC (+0.20 log10 CFU/100 mL), FS (+0.90 log10 CFU/100 mL), Clostridium perfringens (+0.60 log10 CFU/100 mL), and Staphylococcus spp. (+0.80 log10 CFU/100 mL), than the FWSs, except for TC (−0.90 log10 CFU/100 mL). The combined multistage CWs improved the removal of EC, TC, and FC by 1.50, 1.20, and 0.30 log10 CFU/100 mL, respectively, compared to the HSFs. This conclusion was also supported by a study by Rita P. Shingare et al. [41] that investigated the effectiveness of more than 90 different types of CWs for the removal of pathogens and found that the average log reduction rates for multi-stage CWs were 3.14 log CFU/100 mL for EC, 3.80 log CFU/100 mL for FC, 3.24 log CFU/100 mL for FS and TC was 3.39 log CFU/100 mL. While the HSFs showed mean log reduction rates of 3.04, 2.88, 3.16, and 3.27 log CFU/100 mL for EC, FC, FS, and TC. Table 5 lists the pathogen removal effects of different types of CWs.

Table 5.
The removal efficiency of different types of CWs.
Although a combination of different types of CWs can significantly reduce the concentration of pathogens in wastewater, they do not always meet regulatory standards for wastewater reuse, or there is no more economically available space to increase the size of wetlands. As such, further improvements in wetland technology or combining wetlands with different chemical or physical disinfection methods, such as wetland bed aeration, chlorination, ultraviolet disinfection, or advanced oxidation, will be required [29,41].
4. Factors Affecting the Removal of Pathogens and Nitrogen Species
CWs treat wastewater through the physical, chemical, and biological action of various components, with numerous influencing factors. These different factors affect different pathogens in different ways. Factors affecting the removal of pathogens from CWs include plants, substrates, operational operating parameters, UV radiation, temperature, water composition, and pH. The role of plants has already been described in Section 2.
4.1. Substrates
The substrate is an important component of constructed wetland systems, providing a growth medium for plants and microorganisms to adsorb, retain, filter, and precipitate pollutants. There are two main ways for pollutants to adsorb on the substrate, as follows: physical adsorption and chemical adsorption, with physical adsorption being the main focus.
Redder et al. [46] conducted comparative experiments to study the effect of two substrates on Giardia lamblia and Cryptosporidium. In their experiments, seven pilot-scale SSFs, both vertical and horizontal flow types, were filled with washed sand (particle size 0–2 mm), while the other seven wetlands were filled with mixtures of expanded clay and sand with particle sizes of 2–4 mm and 0–2 mm, respectively. Their results suggest that wastewater filtration is the main effective mechanism for reducing protozoan parasites and that small particles (0–2 mm sand grains) may have a more favorable protozoan reduction. Tanner et al. [47] found that VSFs with fine sand media (median 3.20 log10 CFU/100 mL removal rate) had significantly better removal rates compared to coarse gravel media (median 1.90 log10 CFU/100 mL removal rate). The size of the particles is clearly an important factor in the removal of pathogens. Similar results were found by Shingare et al. [35], who studied different substrates for the removal of enteric pathogens and showed a higher reduction in enteric pathogens when sand (<2 mm) was used as a substrate compared to the effect of marble chips (10–15 mm). Ushijima et al. [48] reported that by using fine soil as a substrate in the HSF, the log units of Escherichia coli and MS2 phage were reduced by five and three, respectively; whereas, coarse soil failed to remove these microorganisms. This may be due to the grain size of the filtration substrate being one of the determining factors of the removal efficiency, as smaller grain sizes provide a larger specific surface area for various interactions.
Depending on the nature of the effluent and different discharge standards, matching substrates or combinations of substrates can be selected. Current studies mainly focus on the nitrogen and phosphorus removal effects of the substrates, while relatively little research has been carried out on the removal effect of pathogens. Therefore, on the basis of satisfying the removal of nitrogen and phosphorus pollutants, the substrate needs to be further investigated on the adsorption and removal effect of pathogens.
4.2. Hydraulic Retention Time (HRT)
In practical applications, it is generally believed that once the type of CWs has been determined, the type of substrate and plant species are also determined. The operational parameters will affect the action time between pathogens and the wetland biofilm and, thus, the removal efficiency of the wetland for pathogens. Specific reasons may include that longer hydraulic retention times increase the time spent in removing bacteria such as sedimentation, adsorption to organic matter, predation, the effects of microbial or phytotoxins, and ultraviolet radiation [49].
Sawaittayothin and Polprasert [50] reported a positive correlation between the removal of fecal indicator bacteria and HRT in HSFs, which showed that when the HRT was 1, 3, 5, and 8 d, the removal of total coliforms was 72.5%, 90.1%, 94.3%, and 99.7%, and the removal of fecal coliforms was 63.5%, 88.5%, 94.5%, and 99.2%. However, Tunçsiper et al. [51] pointed out that the relationship between HRT and average removal rate is a polynomial relationship, which means that HRT does not follow the idea of the longer the better, and there exists a “saturation value” in the removal of indicator bacteria in CWs, i.e., when it reaches the “saturation value”, then increasing HRT will not increase the removal rate of indicator bacteria. There is a “saturation value” in the removal of indicator bacteria in artificial wetlands, i.e., when the “saturation value” is reached, increasing the HRT will not increase the removal rate of the indicator bacteria. Various factors affecting the hydraulic retention time include the structure and porosity of the substrate medium, vegetation, water depth, and flow velocity [51,52].
4.3. Water Composition, Oxygen, and pH
The quality of wastewater can affect the treatment process in CWs and also the elimination of pathogens from the CWs. Diaz et al. [49] reported a positive correlation between the number of indicator bacteria and the concentration of fixed nitrogen compounds in wastewater, suggesting that these microorganisms may survive longer or self-propagate faster in the presence of available nitrogen. The reduced removal of indicator bacteria was observed under conditions of water rich in organic matter and surfactants. These compounds will reduce bacterial adsorption in porous media by competing for adsorption sites [53] and by decreasing the affinity of the bacterial surface for adsorption [54]. This suggests that organic matter and nutrients in the water play multiple roles for indicator microorganisms, both stimulating the growth of indicator bacteria and inhibiting it by competing for adsorption sites and altering factors such as light exposure.
Vymazal [55] reported that the presence of oxygen also creates unfavorable conditions for enteric pathogens, as they are either parthenogenetic or specialized anaerobes in nature. Elevated dissolved oxygen levels are directly related to pathogen reduction. Headley et al. [56] conducted an in-depth comparison of EC removal in different types of CWs, all of which used the same influent, water, and showed that the aeration system had a significant performance in removing pathogens.
Generally, these microorganisms’ effluents survive better at pH values between 5.50 and 7.50, with their survival rate rapidly decreasing outside this range. Most pH values in wetland wastewater are within this optimal range [29]. However, in some CWs, the nitrification rate of ammonia is high, leading to higher concentrations of bacteria and natural organic acids (e.g., humic and tannic acids); there is also the presence of effluents with relatively low pH, which are also thought to have antiviral properties [57]. In addition to being due to the composition of the water in the wetland itself, such changes in pH are sometimes caused by the type of wetland and how it is supplied with water. García et al. [44] found that the change in pH in the effluent compared to HSFs was due to the system arrangement of VSFs. Zhao et al. [58] found similar results with intermittent water supply in a composite vertical subsurface flow system. This is because intermittent water supply leads to the passive aeration of the system [10], whereas horizontal flow-based treatment systems mainly rely on air diffusion and oxygen release from plant roots.
4.4. UV Radiation
In FWSs, UV radiation has been one of the important influences on the removal of pathogens, due to the fact that the effluent is advancing in a push-flow pattern over the wetland surface, leaving the pathogens fully exposed to sunlight, leading to DNA damage. In contrast, in horizontal submerged and vertical submerged wetlands, the influencing factors of UV radiation are negligible. Lekeufack et al. [36] conducted a study on the removal of pathogens through FWSs and found that fecal bacteria were removed more efficiently in the first year than in the second year. High plant densities in the second year may have reduced the effects of UV radiation on wetland bacteria. Some studies have also shown that UV radiation can also cause the inactivation of viruses, but probably to a lesser extent than bacteria. Silverman et al. [59] discussed the inactivation of viruses by UV radiation and the effects of different parts of the solar spectrum on different virus species. Wigginton and Kohn [60] enumerated the mechanisms of the UV inactivation of viruses to include the disruption of viral genomes and proteins, disruption of phosphodiester bonds, and others. A proper understanding of the sunlight-induced deactivation processes and their role in pathogen reduction is needed to optimize the design of treatments and to enhance the understanding of mechanisms for pathogen removal in FWSs.
UV radiation not only has a certain bactericidal effect, but also plays a crucial role in the AOP process. Specifically, ultraviolet radiation is mainly used to activate oxidants, such as ozone or hydrogen peroxide, to produce strong oxidizing effects. When ultraviolet radiation combines with these oxidants, they can generate free radicals with extremely high oxidation ability, such as hydroxyl radicals. These free radicals are very active and can react rapidly with organics and micro pollutants in water, as well as decomposing them into low toxic or non-toxic small molecular substances and, even, eventually converting them into carbon dioxide and water. This strong oxidation makes the AOP process able to efficiently remove refractory organics, drug residues, and other micro pollutants in water.
4.5. Temperature/Seasonal Fluctuation
The removal of pathogens by temperature is also controversial. Jokerst et al. [61] seasonally evaluated the effluent treatment efficiency of constructed wetland systems with FWSs and HSFs in a one-year study. Results showed that the wetland removed significantly more EC in the fall, spring, and summer (1.70 log10 CFU/100 mL) than in the winter (1.00 log10 CFU/100 mL) [61]. Elfanssi et al. [62] observed that the maximum removal efficiencies of the bacterial indicators for TC, FC, and FS were 4.80, 4.67, and 4.07 log reductions in summer in a hybrid constructed wetland system, while the lowest performance was observed in winter, with log unit reductions of 3.91, 3.88, and 3.46 for TC, FC, and FS, respectively. Sartori et al. [63] studied SSF systems, which treated domestic wastewater generated by a small village of 150 inhabitants and noted that the removal of EC ranged from 98% during the stationary phase to >99% during the growth phase; the results of the study also showed that the inactivation of fecal bacteria was not affected by the season, but only by the operational artefactual parameters. The results of Thurston et al. [64] showed that the concentration of fecal coliforms increased with temperature in SSF systems. However, the increase in TC and FC in summer compared to winter may be due to animal activity, seasonal changes in plant growth, or colonization of coliforms in wetlands [64].
5. Challenges and Outlook
One significant challenge of CW research is the issue of scale. Predominantly conducted in laboratory settings, the extrapolation of these findings to full-scale CWs remains contentious, raising questions about their applicability. Another major challenge lies in assessing the long-term performance of CWs. Most current research is based on short-term studies, typically not exceeding a year. Like other treatment systems, CWs are designed to provide services for decades rather than a year. In order to improve the technical level of CWs, it is necessary to carry out more research on large-scale CWs for sewage treatment. CWs possess a certain degree of long-term sustainability in removing pollutants, but are also affected by many factors, such as seasonal factors, plant growth status, matrix blockage, and so on. Therefore, it needs regular maintenance and management to deal with various potential risks and challenges and to ensure its long-term stable operation and efficient purification.
There are numerous strategies for nitrogen removal in wastewater treatment processes. For urban wastewater collection and treatment systems, biological treatment methods are commonly employed, including traditional activated sludge process, oxidation ditch process, SBR process, and A2/O process. In conventional secondary and tertiary wastewater treatment processes aimed at improving water quality, ozone oxidation, chlorination, and ultraviolet disinfection are the most successful techniques used for pathogen inactivation in tertiary treatment [65]. While chlorination is widely used as a disinfection method, the presence of natural organic residues in wastewater can lead to the formation of carcinogenic trihalomethanes and other organochlorine compounds [65]. Other methods of pathogen inactivation, such as ultraviolet light and ozone oxidation, are effective but come with higher costs and maintenance requirements. In contrast, CWs offer not only excellent removal efficiency but also environmental friendliness, low operational costs, strong ecological functions, and high sustainability compared to traditional methods. They have the potential to address the issue of low wastewater treatment rates in rural areas and thus possess broad application prospects. However, further research is still needed on the technology of CWs.
- (1)
- In most CWs, planting systems have a higher removal efficiency of pathogens and nitrogen than no-planting systems. We should delve deeper into understanding the role of plants in CWs.
- (2)
- As aquatic ecological health issues related to pathogen pollution in wastewater become increasingly severe, water quality standards may become higher. Therefore, it may be necessary to conduct a detailed investigation of a wider range of indicator bacteria and to explore more efficient detection methods.
- (3)
- As concerns the combination of CWs and other processes, although CWs can significantly reduce the concentration of pathogens and nitrogen in wastewater, if combined with other removal processes (different chemical and physical disinfection methods), this will develop and improve more efficient and economical wetland treatment technologies.
- (4)
- As concerns the model simulation and optimization research of hydraulic, conventional pollutant (such as N and P) removal, as well as pathogen removal in CWs, providing a theoretical basis and technical support for the promotion and application of CWs is required.
6. Conclusions
Nitrogen removal through microorganisms is complicated. Different microbial coupling mechanisms occur under different conditions in different CWs. In this paper, the mechanism of microbial coupling denitrification was introduced from the coupling of microorganisms and plants. The importance of removing different types of nitrogen is well known and people should better understand non-conventional denitrification mechanisms [66] and improve the nitrogen removal rate in wastewater through multiple pathways. An in-depth study on the removal mechanism and influencing factors of pathogens in CWs is required.
Author Contributions
Conceptualization, X.Y.; methodology, Y.Z.; writing—original draft preparation, Z.Y.; writing—review and editing, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Department of Science and Technology of Jilin Province, grant number [20230508089RC] and the National Natural Science Foundation of China grant number [42171107, 42271129].
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- WHO. Guidelines for the Safe Use of Wastewater, Excreta and Greywater; Volume 2: Wastewater use in agriculture; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Hansen, K. Meeting the challenge of water scarcity in the Western United States. In Competition for Water Resources; Elsevier: Amsterdam, The Netherlands, 2017; pp. 2–18. [Google Scholar]
- Tong, Y.; Bu, X.; Chen, C.; Yang, X.; Lu, X.; Liang, H.; Liu, M.; Lin, H.; Zhang, H.; Lin, Y.; et al. Impacts of sanitation improvement on reduction of nitrogen discharges entering the environment from human excreta in China. Sci. Total Environ. 2017, 593, 439–448. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, W.; Wang, W.; Lin, W.; Liu, H.; Ma, X.; Zhou, Y.; Lei, P.; Wei, D.; Zhang, L.; et al. A review on China’s constructed wetlands in recent three decades: Application and practice. J. Environ. Sci. 2021, 104, 53–68. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, S.; Zhang, Y.; Lv, M.; Kinhoun, J.J.R.; Qian, T.; Fan, B. Constructed wetland treatment of source separated washing wastewater in rural areas of southern China. Sep. Purif. Technol. 2021, 272, 118725. [Google Scholar] [CrossRef]
- Calderón-Franco, D.; Sarelse, R.; Christou, S.; Pronk, M.; van Loosdrecht, M.C.; Abeel, T.; Weissbrodt, D.G. Metagenomic profiling and transfer dynamics of antibiotic resistance determinants in a full-scale granular sludge wastewater treatment plant. Water Res. 2022, 219, 118571. [Google Scholar] [CrossRef] [PubMed]
- Vymazal, J. The use of sub-surface constructed wetlands for wastewater treatment in the Czech Republic: 10 years experience. Ecol. Eng. 2002, 18, 633–646. [Google Scholar] [CrossRef]
- Calheiros, C.S.C.; Teixeira, A.; Pires, C.; Franco, A.R.; Duque, A.F.; Crispim, L.F.C.; Moula, S.C.; Castro, P.M.L. Bacterial community dynamics in horizontal flow constructed wetlands with different plants for high salinity industrial wastewater polishing. Water Res. 2010, 44, 5032–5038. [Google Scholar] [CrossRef] [PubMed]
- Trand, J.A.; Weisner, S.E.B. Effects of wetland construction on nitrogen transport and species richness in the agricultural landscape—Experiences from Sweden. Ecol. Eng. 2013, 56, 14–25. [Google Scholar] [CrossRef]
- Stephanakis, A.; Tsihrintzis, V. Effect of loading, resting period porous media, vegetation and aeration on performance of two pilot scale vertical flow constructed wetlands. Chem. Eng. J. 2012, 181, 182. [Google Scholar]
- Li, J.; Yang, X.; Wang, Z.; Shan, Y.; Zheng, Z. Comparison of four aquatic plant treatment systems for nutrient removal from eutrophied water. Bioresour. Technol. 2015, 179, 1–7. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Zhou, Q.; Vymazal, J. Effects of plant biomass on denitrifying genes in subsurface-flow constructed wetlands. Bioresour. Technol. 2014, 157, 341–345. [Google Scholar] [CrossRef]
- Aranychianakis, N.V.; Tsiknia, M.; Kalogerakis, N. Pathways regulating the removal of nitrogen in planted and unplanted subsurface flow constructed wetlands. Water Res. 2016, 102, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Levy, G.; Fine, P.; Bar-Tal, A. (Eds.) Treated Wastewater in Agriculture: Use and Iimpacts on the Soil Environment and Crops; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Jiménez, B.; Maya, C.; Barrios, J.A.; Navarro, I. Helminths and their role in environmental engineering. In Human Helminthiasis; IntechOpen: London, UK, 2017. [Google Scholar]
- Garcia, M.; Becares, E. Bacterial removal in three pilot-scale wastewater treatment systems for rural areas. Water Sci. Technol. 1997, 35, 197–200. [Google Scholar] [CrossRef][Green Version]
- Ottová, V.; Balcarová, J.; Vymazal, J. Microbial characteristics of constructed wetlands. Water Sci. Technol. 1997, 35, 117–123. [Google Scholar] [CrossRef]
- Boutilier, L.; Jamieson, R.; Gordon, R.; Lake, C.; Hart, W. Adsorption, sedimentation, and inactivation of E. coli within wastewater treatment wetlands. Water Res. 2009, 43, 4370–4380. [Google Scholar] [CrossRef]
- Cui, H.; Yang, Y.; Ding, Y.; Li, D.; Zhen, G.; Lu, X.; Huang, M.; Huang, X. A novel pilot-scale tubular bioreactor-enhanced floating treatment wetland for efficient in situ nitrogen removal from urban landscape water: Long-term performance and microbial mechanisms. Water Environ. Res. 2019, 91, 1498–1508. [Google Scholar] [CrossRef]
- Bever, J.D.; Dickie, I.A.; Facelli, E.; Facelli, J.M.; Klironomos, J.; Moora, M.; Rillig, M.C.; Stock, W.D.; Tibbett, M.; Zobel, M. Rooting theories of plant community ecology in microbial interactions. Trends Ecol. Evol. 2010, 25, 468–478. [Google Scholar] [CrossRef]
- Wu, H.; Wang, X.; He, X.; Zhang, S.; Liang, R.; Shen, J. Effects of root exudates on denitrifier gene abundance, community structure and activity in a micro-polluted constructed wetland. Sci. Total Environ. 2017, 598, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xu, Y.; Jin, J.; Liu, J.; Zhang, Q.; Liu, X. Effect of soil type and soybean genotype on fungal community in soybean rhizosphere during reproductive growth stages. Plant Soil 2009, 317, 135–144. [Google Scholar] [CrossRef]
- Albuquerque, A.; Randerson, P.; Białowiec, A. Oxygen transfer capacity as a measure of water aeration by floating reed plants: Initial laboratory studies. Processes 2020, 8, 1270. [Google Scholar] [CrossRef]
- Mei, X.Q.; Yang, Y.; Tam, N.F.Y.; Wang, Y.W.; Li, L. Roles of root porosity, radial oxygen loss, Fe plaque formation on nutrient removal and tolerance of wetland plants to domestic wastewater. Water Res. 2014, 50, 147–159. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, D.H.; Oh, S.; Moon, H.S. Effects of water level and vegetation on nitrate dynamics at varying sediment depths in laboratory-scale wetland mesocosms. Sci. Total Environ. 2020, 703, 134741. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Rueda, O.; Hallin, S.; Baneras, L. Structure and function of denitrifying and nitrifying bacterial communities in relation to the plant species in a constructed wetland. FEMS Microbiol. Ecol. 2009, 67, 308–319. [Google Scholar] [CrossRef]
- Zhang, C.B.; Wang, J.; Liu, W.L.; Zhu, S.X.; Liu, D.; Chang, S.X.; Chang, J.; Ge, Y. Effects of plant diversity on nutrient retention and enzyme activities in a full-scale constructed wetland. Bioresour. Technol. 2010, 101, 1686–1692. [Google Scholar] [CrossRef]
- Huang, X.F.; Ye, G.Y.; Yi, N.K.; Lu, L.J.; Zhang, L.; Yang, L.Y.; Xiao, L.; Liu, J. Effect of plant physiological characteristics on the removal of conventional and emerging pollutants from aquaculture wastewater by constructed wetlands. Ecol. Eng. 2019, 135, 45–53. [Google Scholar] [CrossRef]
- Wu, S.; Carvalho, P.N.; Müller, J.A.; Manoj, V.R.; Dong, R. Sanitation in constructed wetlands: A review on the removal of human pathogens and fecal indicators. Sci. Total Environ. 2016, 541, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Avelar, F.F.; de Matos, A.T.; de Matos, M.P.; Borges, A.C. Coliform bacteria removal from sewage in constructed wetlands planted with Mentha aquatica. Environ. Technol. 2014, 35, 2095–2103. [Google Scholar] [CrossRef]
- Seidel, K. Macrophytes as functional elements in the environment of man. Hidrobiol. Bucur. 1971, 12, 121–130. [Google Scholar]
- Kipasika, H.J.; Buza, J.; Smith, W.A.; Njau, K. Removal capacity of faecal pathogens from wastewater by four wetland vegetation: Typha latifolia, Cyperus papyrus, Cyperus alternifolius and Phragmites australis. Afr. J. Microbiol. Res. 2016, 10, 654–661. [Google Scholar]
- Chang, N.B.; Xuan, Z.; Daranpob, A.; Wanielista, M. A subsurface upflow wetland system for removal of nutrients and pathogens in on-site sewage treatment and disposal systems. Environ. Eng. Sci. 2011, 28, 11–24. [Google Scholar] [CrossRef]
- García, M.; Soto, F.; González, J.M.; Bécares, E. A comparison of bacterial removal efficiencies in constructed wetlands and algae-based systems. Ecol. Eng. 2008, 32, 238–243. [Google Scholar] [CrossRef]
- Shingare, R.P.; Nanekar, S.V.; Thawale, P.R.; Karthik, R.; Juwarkar, A.A. Comparative study on removal of enteric pathogens from domestic wastewater using Typha latifolia and Cyperus rotundus along with different substrates. Int. J. Phytoremediat. 2017, 19, 899–908. [Google Scholar] [CrossRef]
- Lekeufack, M.; Fonkou, T.; Pamo, T.E.; Amougou, A. Removal of faecal bacteria and nutrients from domestic wastewater in a horizontal surface flow wetland vegetated with Echinochloa pyramidalis. Afr. J. Environ. Sci. Technol. 2012, 6, 337–345. [Google Scholar]
- Manios, T.; Stentiford, E.I.; Millner, P.A. The removal of indicator microorganisms from primary treated wastewater in subsurface reed beds using different substrates. Environ. Technol. 2002, 23, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Afrin, R.; Al Muyeed, A.; Sun, G. Treatment of tannery wastewater in a pilot-scale hybrid constructed wetland system in Bangladesh. Chemosphere 2012, 88, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Saeed, T.; Muntaha, S.; Rashid, M.; Sun, G.; Hasnat, A. Industrial wastewater treatment in constructed wetlands packed with construction materials and agricultural by-products. J. Clean. Prod. 2018, 189, 442–453. [Google Scholar] [CrossRef]
- Saeed, T.; Al-Muyeed, A.; Afrin, R.; Rahman, H.; Sun, G. Pollutant removal from municipal wastewater employing baffled subsurface flow and integrated surface flow-floating treatment wetlands. J. Environ. Sci. 2014, 26, 726–736. [Google Scholar] [CrossRef]
- Shingare, R.P.; Thawale, P.R.; Raghunathan, K.; Mishra, A.; Kumar, S. Constructed wetland for wastewater reuse: Role and efficiency in removing enteric pathogens. J. Environ. Manag. 2019, 246, 444–461. [Google Scholar] [CrossRef]
- Kadlec, R.H.; Cuvellier, C.; Stober, T. Performance of the Columbia, Missouri, treatment wetland. Ecol. Eng. 2010, 36, 672–684. [Google Scholar] [CrossRef]
- Galvão, A.; Matos, J.; Silva, M.; Ferreira, F. Constructed wetland performance and potential for microbial removal. Desalin. Water Treat. 2009, 4, 76–84. [Google Scholar] [CrossRef]
- García, J.A.; Paredes, D.; Cubillos, J.A. Effect of plants and the combination of wetland treatment type systems on pathogen removal in tropical climate conditions. Ecol. Eng. 2013, 58, 57–62. [Google Scholar] [CrossRef]
- Ayaz, S.C. Post-treatment and reuse of tertiary treated wastewater by constructed wetlands. Desalination 2008, 226, 249–255. [Google Scholar] [CrossRef]
- Redder, A.; Dürr, M.; Daeschlein, G.; Baeder-Bederski, O.; Koch, C.; Müller, R.; Exner, M.; Borneff-Lipp, M. Constructed wetlands–Are they safe in reducing protozoan parasites? Int. J. Hyg. Environ. Health 2010, 213, 72–77. [Google Scholar] [CrossRef]
- Tanner, C.C.; Sukias, J.P.; Headley, T.R.; Yates, C.R.; Stott, R. Constructed wetlands and denitrifying bioreactors for on-site and decentralised wastewater treatment: Comparison of five alternative configurations. Ecol. Eng. 2012, 42, 112–123. [Google Scholar] [CrossRef]
- Ushijima, K.; Ito, K.; Ito, R.; Funamizu, N. Greywater treatment by slanted soil system. Ecol. Eng. 2013, 50, 62–68. [Google Scholar] [CrossRef]
- Díaz, F.J.; O’Geen, A.T.; Dahlgren, R.A. Efficacy of constructed wetlands for removal of bacterial contamination from agricultural return flows. Agric. Water Manag. 2010, 97, 1813–1821. [Google Scholar] [CrossRef]
- Sawaittayothin, V.; Polprasert, C. Nitrogen mass balance and microbial analysis of constructed wetlands treating municipal landfill leachate. Bioresour. Technol. 2007, 98, 565–570. [Google Scholar] [CrossRef]
- Tunçsiper, B.; Ayaz, S.Ç.; Akça, L. Coliform bacteria removal from septic wastewater in a pilot-scale combined constructed wetland system. Environ. Eng. Manag. J. EEMJ 2012, 11, 1873–1879. [Google Scholar] [CrossRef]
- Kansiime, F.; Van Bruggen, J.J.A. Distribution and retention of faecal coliforms in the Nakivubo wetland in Kampala, Uganda. Water Sci. Technol. 2001, 44, 199–206. [Google Scholar] [CrossRef]
- Stevik, T.K.; Aa, K.; Ausland, G.; Hanssen, J.F. Retention and removal of pathogenic bacteria in wastewater percolating through porous media: A review. Water Res. 2004, 38, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.G.; Jaffé, P.R. Effects of nonionic surfactants on bacterial transport through porous media. Environ. Sci. Technol. 2001, 35, 3877–3883. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of enteric bacteria in constructed treatment wetlands with emergent macrophytes: A review. J. Environ. Sci. Health 2005, 40, 1355–1367. [Google Scholar] [CrossRef] [PubMed]
- Headley, T.; Nivala, J.; Kassa, K.; Olsson, L.; Wallace, S.; Brix, H.; Afferden, M.v.; Müller, R. Escherichia coli removal and internal dynamics in subsurface flow ecotechnologies: Effects of design and plants. Ecol. Eng. 2013, 61, 564–574. [Google Scholar] [CrossRef]
- Olson, M.R.; Axler, R.P.; Hicks, R.E. Effects of freezing and storage temperature on MS2 viability. J. Virol. Methods 2004, 122, 147–152. [Google Scholar] [CrossRef]
- Zhao, Y.J.; Hui, Z.; Chao, X.; Nie, E.; Li, H.J.; He, J.; Zheng, Z. Efficiency of two-stage combinations of subsurface vertical down-flow and up-flow constructed wetland systems for treating variation in influent C/N ratios of domestic wastewater. Ecol. Eng. 2011, 37, 1546–1554. [Google Scholar] [CrossRef]
- Silverman, A.I.; Nguyen, M.T.; Schilling, I.E.; Wenk, J.; Nelson, K.L. Sunlight inactivation of viruses in open-water unit process treatment wetlands: Modeling endogenous and exogenous inactivation rates. Environ. Sci. Technol. 2015, 49, 2757–2766. [Google Scholar] [CrossRef]
- Wigginton, K.R.; Kohn, T. Virus disinfection mechanisms: The role of virus composition, structure, and function. Curr. Opin. Virol. 2012, 2, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Jokerst, A.; Sharvelle, S.E.; Hollowed, M.E.; Roesner, L.A. Seasonal performance of an outdoor constructed wetland for graywater treatment in a temperate climate. Water Environ. Res. 2011, 83, 2187–2198. [Google Scholar] [CrossRef] [PubMed]
- Elfanssi, S.; Ouazzani, N.; Latrach, L.; Hejjaj, A.; Mandi, L. Phytoremediation of domestic wastewater using a hybrid constructed wetland in mountainous rural area. Int. J. Phytoremediat. 2018, 20, 75–87. [Google Scholar] [CrossRef]
- Sartori, L.; Canobbio, S.; Fornaroli, R.; Cabrini, R.; Marazzi, F.; Mezzanotte, V. COD, nutrient removal and disinfection efficiency of a combined subsurface and surface flow constructed wetland: A case study. Int. J. Phytoremediat. 2016, 18, 416–422. [Google Scholar] [CrossRef]
- Thurston, J.A.; Foster, K.E.; Karpiscak, M.M.; Gerba, C.P. Fate of indicator microorganisms, Giardia and Cryptosporidium in subsurface flow constructed wetlands. Water Res. 2001, 35, 1547–1551. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Benigna, I.; Sorlini, S.; Torretta, V. Overview of the main disinfection processes for wastewater and drinking water treatment plants. Sustainability 2017, 10, 86. [Google Scholar] [CrossRef]
- Albuquerque, A.; Oliveira, J.; Semitela, S.; Amaral, L. Influence of bed media characteristics on ammonia and nitrate removal in shallow horizontal subsurface flow constructed wetlands. Bioresour. Technol. 2009, 100, 6269–6277. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).