Abstract
The traditional taxonomy of freshwater invertebrates is a labor-intensive process requiring extensive knowledge and experience. In addition, this science is largely subjective, which makes its digitalization difficult. However, accurate species attribution is becoming increasingly important for environmental surveys and conservation efforts. In the 21st century, molecular biology methods have proven to be a successful tool for objectively determining biodiversity. Achieving objectivity in identifying the taxa of non-parasitic leeches of the family Erpobdellidae was the main goal of this study. To this end, various bioinformatic approaches to DNA sequence analysis have been tested. As a result, the phylogenetic diversity amounted to 47 species versus 29 morphospecies defined traditionally. The use of molecular species delimitation methods made it possible to identify seven complexes of morphologically hidden (cryptic) species and some morphological misidentifications, as well as to discover a new species from Eastern Siberia (Erpobdella sibirica) with a unique molecular diagnosis (DNA barcode). A pioneering attempt to apply the same approach to higher taxa showed that Erpobdellidae probably consists of seven genera, including the unambiguous elimination of the genus Motobdella. Obtaining quantitative and, therefore, objective data is an advantage of the molecular approach, which has proven to be effective in recognizing species and genera, at least in leeches.
1. Introduction
Modern environmental surveys currently require a fast and accurate determination of the biodiversity level, bypassing a lengthy procedure of traditional morphological analysis. DNA identification tools, as a part of the molecular ecology, have been developed to solve this problem. Sequence-based methods have proven to be very successful tools for solving the disputable moments in classical taxonomy, for which it is important to represent and understand biodiversity in general and to identify the most vulnerable species in particular [1,2,3]. To date, DNA barcoding has become a standardized technique for creating a database from the information about the 648 bp region of the mitochondrial cytochrome-c oxidase I (COI-5′) for different metazoans [4,5,6]. Some studies have cast doubt on the concept of a barcoding gap, given that both false-positive and false-negative errors could occur during its search (e.g., [7,8,9]). Authors and supporters of DNA barcoding acknowledge its limitations and inability to work with evolutionarily young species [10,11]. Nevertheless, DNA barcoding is actively being introduced, and although the technique is not universal, its usefulness remains undeniable. Previously, this tool was usefully applied to taxonomic issues in leeches [12,13,14,15,16,17,18,19,20,21,22]. Here, its utility in the taxonomically difficult but ecologically important freshwater genus Erpobdella De Blainville, 1818, was investigated. Despite erpobdellids having been studied phylogenetically more than any other group of leeches in the early period of molecular methods development (e.g., [23,24,25,26,27]), the level of differences among its taxa was not definitely resolved and still remains open. The identification of morphological species within this genus is often difficult, owing to poor knowledge of the fauna and indistinct descriptions of some species due to the morphological inertness within the genus [28]. At the same time, the lack of external distinctive features and a very large intraspecific variability cause contradiction and hamper the reliable taxonomic definition of the most common species, including E. octoculata (Linnaeus, 1758) and E. vilnensis (Liskiewicz, 1925) [29,30]. Therefore, morphologically indistinguishable complexes of cryptic species are common among freshwater non-parasitic leeches [13,23,31,32,33]. The difficulties in taxonomic determination have led to the expansion of species diagnoses and, consequently, an underestimation of biodiversity. Moreover, there is still no consensus on the genera within Erpobdellidae. The idea of synonymizing most genera (Croatobranchus, Dina, Nephelopsis, Mooreobdella, Motobdella, and Trocheta) meets with more and more supporters from North America [14,26,31,33,34], and at the same time, opponents of the abolition of at least Eurasian Dina and Trocheta [25,28,32,35,36,37].
The present study aims to test and refine the phylogenetic diversity and generic status of species putatively assigned to Erpobdella using modern bioinformatics approaches to species delimitation based on molecular data.
2. Materials and Methods
2.1. New Sample Collection and Morphological Analysis
Biological material was collected from 2011 to 2018 from the basins of the Eastern Siberian rivers (Angara, Lena, and Selenga), the warming-up coves of two major bays of Lake Baikal (the Maloye More Strait and Chivyrkuy Bay), and Lake Izumrudnoe (Table 1). In addition, two samples of predatory leeches from the Lopan River (Eastern Ukraine) were included in the study. Leeches were caught manually or by hydrobiological tools in the coastal zone of the water bodies at a depth of 0.5–1.5 m. The collected animals were pre-anesthetized and then fixed with 80% ethanol. Specimens are kept in the collection of the Limnological Institute, Irkutsk.
Morphological analysis of the erpobdellid leeches was conducted using an MSP-2 var.2 (LOMO, St. Petersburg, Russia) stereomicroscope. Currently, existing taxonomic keys [29,30], original descriptions of some species [38,39], and later interpretations [40] were used in attempts to determine the specimens up to the species level. Novel leech species were additionally examined through scanning electronic microscopy (FEI Company, Hillsboro, OR, USA). Photos of the whole individuals were taken with a Nikon D7000 (Tokyo, Japan) camera.
2.2. DNA Extraction, Amplification, and Sequencing
A small portion of muscle tissue from the posterior leech sucker of approximately 0.1–0.2 mm3 was used for DNA extraction with the QIAamp DNA Mini Kit (QIAGEN, Venlo, The Netherlands) according to the manufacturer’s protocol. This tissue was chosen to take into account the possible collection significance and maximum availability of specimens for further research, as well as in order to minimize contamination with the host’s blood. Double-stranded templates of the cytochrome C oxidase subunit I (cox1) gene fragment that were suitable for sequencing were prepared through PCR amplification with the primers universal to most invertebrates, namely LCO1490 and HCO2198 [41] and Taq DNA polymerase (“Lytech” Co. Ltd., Moscow, Russia) under the following conditions: preheating for 1 min at 94 °C; 25 cycles, including denaturation (15 s at 95 °C), annealing (5 s at 45 °C), and elongation (15 s at 95 °C); then, final elongation (7 min at 72 °C). Sanger sequencing was performed at the «Syntol» Company (Moscow, Russia). All new cox1 sequences were checked manually for the absence of insertions, deletions, and nonsense- or stop codons to avoid nuclear pseudogene amplification; then, consensus sequences were created from the complementary strands using MEGA11 [42]. Final DNA sequences were deposited in GenBank under the accession numbers MN245518–MN245562 and KM220015–KM220020 (Table 1).

Table 1.
Information on newly sequenced Erpobdella specimens from Eastern Siberia. Specimens are deposited in the collection of the Limnological Institute (Irkutsk) under corresponding voucher numbers.
Table 1.
Information on newly sequenced Erpobdella specimens from Eastern Siberia. Specimens are deposited in the collection of the Limnological Institute (Irkutsk) under corresponding voucher numbers.
| Collection Site | Coordinates | Voucher | Accession Number |
|---|---|---|---|
| Erpobdella octoculata | |||
| LB: Lake Izumrudnoe | 51°23′39″N 104°38′33″E | E275, E276 | MN245559, MN245560 |
| DRb: Lopan River* | 49°59′19″N, 36°13′38″E | KI37, KI38 | MN245561, MN245562 |
| Erpobdella sibirica sp. nov. | |||
| ARb: Angara River | 53°04′31″N, 104°19′05″E | KI34 | MN245518 |
| ARb: Angara River | 52°14′59″N, 104°17′04″E | KI36 | MN245519 |
| ARb: Kudarejka River | 52°51′53″N, 104°27′05″E | KI10 | MN245532 |
| ARb: B Edogon River | 54°12′03″N, 100°05′25″E | E183, E185, E186 | MN245520-MN245522 |
| ARb: Oyok pond | 52°36′20″N, 104°29′02″E | E248, E251 | MN245548, MN245549 |
| LRb: Lake Dalnee | 56°05′28″N, 108°16′07″E | E180, E181 | MN245527, MN245528 |
| LRb: Tukolon River | 55°24′44″N, 107°03′68″E | E176 | MN245554 |
| LB: Shida Bay | 53°03′55″N, 106°47′33″E | KI19, E19 | MN245542, KM220015 |
| LB: Ulirba Bay | 53°04′08″N, 106°48′25″E | KI1, E1, E7, E8 | MN245543, KM220016-KM220018 |
| LB: Khuzhir-Nugo Bay | 53°04′04″N, 106°48′52″E | KI9, E9 | MN245544, KM220019 |
| LB: Kurkut Bay | 53°01′12″N, 106°53′49″E | F1, E101, E17 | MN245539, MN245541, KM220020 |
| LB: Tulkhane Bay | 53°02′48″N, 106°46′36″E | E102 | MN245540 |
| LB: Lake Kurma | 53°11′13″N, 106°58′58″E | E112, E114 | MN245533, MN245534 |
| LB: Lake Kurma | 53°10′57″N, 106°58′41″E | E116 | MN245535 |
| LB: Lake Surhaitornur | 53°16′53″N, 107°09′19″E | E137 | MN245553 |
| LB: Chivyrkuy Bay | 53°46′10″N, 109°00′28″E | KI17 | MN245526 |
| LB: Chivyrkuy Bay | 53°46′05″N, 109°01′22″E | KI2, KI3 | MN245525, MN245523 |
| LB: Chivyrkuy Bay | 53°45′58″N, 109°02′13″E | KI33 | MN245524 |
| LRb: Lena River | 58°07′13″N, 108°46′05″E | KI4, KI5 | MN245536, MN245537 |
| LRb: Lena River | 57°46′26″N, 108°07′43″E | KI6 | MN245538 |
| LRb: Lake Severnoe | 54°19′22″N, 108°22′43″E | 925 | MN245550 |
| LRb: Lake Severnoe | 54°19′15″N, 108°22′40″E | 926 | MN245551 |
| LRb: Lake Severnoe | 54°19′18″N, 108°22′45″E | 927 | MN245552 |
| SRb: Lake Gusinoe | 51°15′57″N, 106°23′54″E | G60 | MN245529 |
| SRb: Lake Gusinoe | 51°17′31″N, 106°28′42″E | G81 | MN245530 |
| SRb: Lake Gusinoe | 51°17′28″N, 106°28′47″E | G82 | MN245531 |
| SRb: Lake Ulaagchny | 48°22′28″N, 96°10′40″E | 915, 916, 917 | MN245545-MN245547 |
| SRb: Lake Ulaagchny | 48°22′37″N, 96°08′56″E | 918,919, 922,923 | MN245555- MN245558 |
Note(s): ARb, Angara River basin; LB, Lake Baikal; LRb, Lena River basin; SRb, Selenga River basin; DRb, non-Siberian locality.
2.3. Bioinformatic Analysis of Molecular Data
A comparison of the nucleotide sequences with the sequence databases, an estimation of the statistical significance of the matches, and a search for the regions of local similarity among the homologous DNA fragments were performed using BLAST+ ver. 2.1.5.0 [43]. Sequences downloaded from GenBank and used as a comparison group or outgroup are described in the Supplementary Materials (Table S1).
Phylogenetic relationships, as well as intra- and interspecific genetic distances, were estimated using MEGA 11.0.13 [42], except for Bayesian inference, which was constructed in BEAST2 v.2.7.6 [44]. Maximum likelihood and Bayesian probability trees were inferred based on a best-fit model of molecular evolution selected with jModelTest v 2.1.10 [45].
To overcome the difficulties associated with assessing species, modern molecular-based algorithms, i.e., ASAP, GMYC, and PTP [46,47,48], were integrated with classical DNA barcoding [4,10]. In this context, DNA barcoding, as a formalized method for including genetic attributes in a taxonomic analysis, elucidates the intellectual content of taxonomy from a molecular perspective. The method hypothesizes the existence of a barcode gap or a threshold value, visualizing a difference in the frequency distribution of intra- and interspecific variations in the nucleotide composition in DNA marker sequences.
The procedure of assembling species by automatic partitioning (ASAP [46]) was applied to the differentiation of the aligned sequences into hypothetical species (or MOTUs) by detecting the optimal barcoding gap for this dataset.
To detect the threshold value of intraspecific genetic distance, the generalized mixed Yule coalescent (GMYC) was implemented. This method utilizes a predicted difference in the rates of evolution for speciation (interspecific relation) and coalescent (intraspecific relation) processes [47].
Alternatively, the Bayesian implementation of the Poisson tree processes (PTP) model for species delimitation (bPTP) was applied to infer putative species boundaries on a high-resolution-input Bayesian ultrametric tree [48].
3. Results
The performed molecular analysis made it possible to decipher the nucleotide sequences of the cox1 gene fragment of 652–682 bp in length for 51 specimens, including 49 Siberian and 2 European erpobdellid leeches (Table 1). This part of the genome was selected since it was recognized as a versatile tool for the DNA barcoding of Metazoa [4,5,6] and successfully adopted in several recent studies discovering obscured leech species (e.g., [18,21,22,49]).
The BLAST analysis revealed the nucleotide sequences most similar to the Siberian samples throughout the worldwide genetic data available at the moment. The resulting set of cox1 sequences consisted of 100 sequences belonging to different Erpobdellidae species (with an identity of 88% and higher), whose genetic identity ranged from 88.26 to 93.76%. The cox1 sequences obtained from the BLAST list belonged to the genera Dina and Erpobdella. At the top of the list, there were 10 Erpobdella sequences with an identity ratio ranging from 88.68 to 93.76%, of which E. vilnensis had the highest values (Table 2).

Table 2.
Top 10 Erpobdellidae homologous sequences selected by BLAST, with the statistical significance of the matches.
To form a molecular dataset, a search for the cox1 sequences available at the NCBI database was performed with the following command line: (“Erpobdella”[Organism] OR Erpobdella[All Fields]) AND cytochrome[All Fields]. As a result, 527 sequences were found, of which 29 were not Erpobdellidae (search engine error); of the rest, only 372 had nominal names and were significantly longer than 600 bp. Because some of the scientific community tends to synonymize the genera Dina, Trocheta, Mooreobdella, and Motobdella with Erpobdella, 43 sequences of identified species belonging to these genera that meet the length requirements were retrieved from public sources and included in the ingroup (species names with GenBank accession numbers are in Table S1). Eight homologous sequences of the genera Orobdella (Erpobdellidae) and Haemopis (Haemopidae) were selected as the outgroup: AB67966 O. esulcata, AB938009 O. masaakikuroiwai, AB698862 and AB698864 O. mononoke, OK447864 H. grandis, KM611858 H. marmorata, and AF462021 H. sanguisuga. The final dataset matrix consisted of 474 terminals (350 Nearctic and 116 Palaearctic, including 51 cox1 sequences assembled in this work) and 648 aligned nucleotide characters. Based on the resulting alignment, the phylogenetic relationships of the Erpobdella leeches were hypothesized using different approaches to phylogeny reconstruction, which were based on distance (neighbor-joining) or probabilistic (maximum likelihood and Bayesian probability) criteria. The nearest neighbor-joining (NJ) tree was inferred under the Kimura two-parameter algorithm (K2P). The selection relied on recommendations for performing standardized DNA barcoding [4,50]. Maximum likelihood (ML) and Bayesian (BI) trees were built with GTR+I+G as an optimal evolutionary model according to jModelTest (Figure 1). The NJ and ML phylogenies are presented in the Supplementary Materials (Figures S1 and S2).
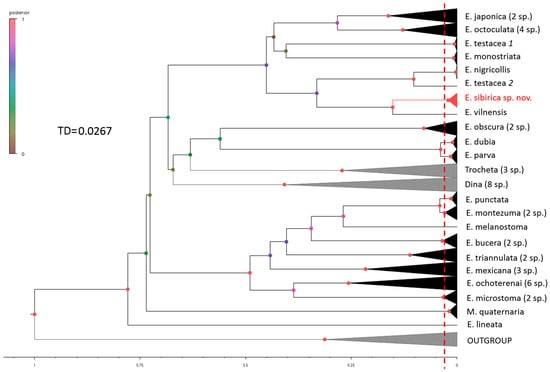
Figure 1.
Bayesian tree based on the barcoding genome fragment alignment of 474 sequences with intraspecific variability threshold (TD, dotted red line) within the family Erpobdellidae according to GMYC analysis. The tip labels of the 47 cox1 sequences of new Siberian species are colored in red.
To molecularly delimit species in the original dataset, the results from several modern MOTU-picking methods were compared. The concept of DNA barcoding is based on the recognition of 2–3% nucleotide substitutions in a 648 bp fragment of the cox1 gene as a threshold value for intraspecific genetic polymorphism; all values exceeding this figure correspond to groups of independent species status [4,50,51]. Standing by this hypothesis, 48 species were found in the dataset consisting of 474 sequences assigned to 29 morphological species of Erpobdellidae. The genetic distances matrix is presented in Table S3. The average interspecific genetic distance in Erpobdellidae was 14.33%; in the genera Trocheta, Dina, Erpobdella, and Mooreobdella, it was 9.93, 11.69, 13.85, and 15.72%, respectively, wherein the maximum intraspecific genetic variability was equal to 2.47 ± 0.43%.
The ASAP method, which automatically assembles species based only on pairwise genetic distances, was used to test the hypothesis that a gap in the barcode exists. As a result, the intended molecular operational taxonomic units (MOTUs) of the species rank were identified, and the gap between grades of 2.62–10.5% was interpreted as a barcode gap (Figure 2). The recursive application of the calculated value of the barcode interval allowed us to decompose the initial dataset and merge the sequences, the differences between which were below this threshold value, into a single cluster. Consequently, the set of 474 cox1 sequences was divided into 48 groups of potential species of Erpobdellidae (Table S2) with a maximum threshold distance of p = 0.0262.
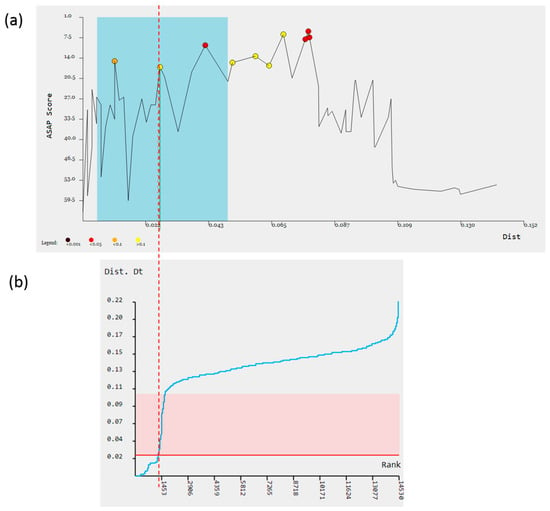
Figure 2.
Distribution of pairwise distances between sequences of Erpobdellidae samples obtained by ASAP program. The red dotted line indicates the threshold of intraspecific polymorphism in the studied group. (a) Boxed species graph with the most relevant partitions marked in blue rectangle and estimated threshold distance (red dotted line); (b) ranked distances with indication of TD (red line), the best rank (dotted red line), and relative barcode gap width (pink range).
Using the generalized mixed Yule coalescence (GMYC) analysis as the de novo species delimitation based on the ultrametric input tree (Figure 1), the threshold of -0.02665188 for the studied erpobdellid taxa was identified; it coincides with the peak value of maximum likelihood and limits the 50 terminal branches within the dataset of 474 sequences as species-level groups (with confidence ranging between 48 and 51).
The Poisson tree processes (PTP) model was alternatively applied to infer putative species boundaries on a given BI tree (Figure 1) using the evolutionary placement algorithm (EPA-PTP) to count the number of species in phylogenetic placements. The estimated number of putative species for the same dataset was 49 at a threshold distance of 0.0308. The bPTP contradicts the GMYC in terms of delimiting the species E. dubia (Moore et Meyer, 1951) and E. parva (Moore, 1912), supporting the hypothesis of their merging under the ASAP while denying the homogeneity of E. microstoma (Moore, 1901) and E. montezuma (Davies et al., 1985).
Thus, both the distant (p-distance, ASAP) and coalescent (GMYC, bPTP)) approaches to molecular delimitation discovered 47–50 species of Erpobdellidae within the 29 nominal taxa identified morphologically (Table 3). This implies the presence of 7–10 cryptic groups consisting of 2–6 species (Figure 1, Table 4), as well as the detection of misidentifications when the same name species diverge along different phylogenetic lineages (D. prokletijaca, E. nigricollis, E. testacea, and E. punctata) and vice versa when a phylogenetic lineage includes representatives of two species (E. dubia and E. parva). However, all methods confirm the existence of E. sibirica sp. nov. (Appendix A) by unambiguously grouping together its 47 cox1 sequences.

Table 3.
Summary data on the delimitation of Erpobdellidae species.
Despite the inference algorithm and rotation of some branches, the NJ, ML, and BI phylogenies are consistent in splitting the Erpobdellidae dataset into eight major clades, apparently corresponding to the genus level (Figure 1, Figures S1 and S2). Dina, Trocheta, and Mooreobdella (concerning only M. quaternaria) are consistently clustered apart from the others, thereby confirming their generic status. For the first time, the question of dividing Palaearctic and Nearctic Erpobdella into separate genera is being raised since, in all phylogenetic schemes, they consistently cluster distantly (Figure 1, Figures S1 and S2). In addition, the lineages of E. obscura, E. parva (together with E. dubia as a junior synonym), and E. lineata from Korea probably also represent independent genera. The intra- and intergeneric distances average 8.5 and 15% of the nucleotide substitutions in the barcoding genome fragment, respectively.
4. Discussion
Molecular systematics, a rapidly developing branch of biosciences, shows that nominal species can include dozens of morphologically identical but genetically distinct species [49,52,53,54,55,56]. The phenomenon is widespread among animals and contributes significantly to global species richness [57,58], and yet cryptic species rarely receive official status, remaining undescribed and therefore inaccessible to conservation practice [59]. This situation still persists in most aquatic invertebrates, including annelids, as recent studies illustrate [13,18,21,22,35]. To increase the efficiency of taxonomic identifications, methods that are universal for all organisms are in demand. The best solution today is the molecular delimitation of taxa, which does not require an in-depth study of morphology and allows rare specimens of the collection to be preserved intact. By reducing the time of analysis, this approach expands its capabilities and makes the study of biodiversity more accessible to a wide range of specialists. The current development of molecular research methods and their bioinformatic analyses provides a previously unattainable array of data on the phylogenetic diversity of life forms and significantly increases the reliability of taxonomic studies.
Twenty years ago, DNA barcoding was one of the first approaches used for the molecular delimitation of taxa; its concept is still based on the existence of a gap between intra- and interspecific genetic polymorphisms. For most multicellular organisms, a 648 bp fragment of the mitochondrial cox1 gene turned out to be a universal tool, with intraspecific variability typically limited to 2–3% due to nucleotide substitutions [4,10,50]. All subsequent methods are based on the original concept but use different algorithms to automatically search for a barcoding gap.
By comparing the results of ASAP, GMYC, bPTP, and classical DNA barcoding, a threshold of 2.47% was determined to be sufficient to distinguish 47 species-level groups among 29 erpobdellid morphospecies (Table 3). As a consequence, at least seven genetically heterogeneous groups were discovered, two of them being among Palaearctic and five being among Nearctic fauna (Table 4). Despite the ambiguous bioinformatic assessment of the morphospecies D. lineata, E. microstoma, and M. montezuma, low values of intraspecific genetic distances (1.16, 2.06, and 1.33%, respectively) indicate their homogeneity and, therefore, the absence of cryptic species within them. In the case of E. mexicana and E. obscura, the two species are indeed cryptic because the genetic distances between the lineages within them are sufficient to recognize each as a valid species (Table 4 and Table S3). As for E. dubia and E. parva, the validity of these species is more complicated. In the resulting phylogenies (NJ, ML, and BI: Figure 1, Figures S1 and S2), these species clearly form two branches, but the E. dubia clade always includes several E. parva specimens (MN612835, MN612836, MN612857, MN612858, and MN613025). Is this an erroneous attribution of these specimens to E. parva or the impossibility of morphological distinction between E. dubia and E. parva species in principle? I am inclined to the second, like ASAP, bPTP (Table 3), especially since the same is indicated by an intraspecific distance below 2% and an interspecific distance above 3% (Table 4), which is consistent with the basic requirements of DNA barcoding.

Table 4.
Number of cryptic species depending on genetic variability (Pi) within morphospecies (MS) and between cryptic species (CS).
Table 4.
Number of cryptic species depending on genetic variability (Pi) within morphospecies (MS) and between cryptic species (CS).
| Region | Morphospecies | Pi within MS | Number of CS | Pi between CS (Average) |
|---|---|---|---|---|
| PA | Dina lineata * | 0.0167 | 1(2) | 0.0196 |
| Erpobdella japonica | 0.0678 | 2 | 0.0973 | |
| Erpobdella octoculata | 0.0555 | 4 | 0.0559–0.0890 (avg. 0.0808) | |
| NA | Erpobdella bucera | 0.0331 | 2 | 0.0331 |
| Erpobdella mexicana | 0.0214 | 3 | 0.0431–0.1149 (avg. 0.0872) | |
| Erpobdella obscura | 0.0188 | 2 | 0.0554 | |
| Erpobdella ochoterenai | 0.0626 | 6 | 0.0612–0.1204 (avg. 0.0972) | |
| Erpobdella triannulata | 0.0492 | 2 | 0.0658 | |
| Erpobdella microstoma * | 0.0206 | 1(2) | 0.0315 | |
| Motobdella Montezuma * | 0.0133 | 1(2) | 0.0265 | |
| Erpobdella dubia/parva ** | 0.0199 | 1(2) | 0.0308 |
Note(s): * species suggested to be cryptic by bioinformatic inferences; ** species synonymized by most approaches.
The molecular evidence supports the phylogenetic relationship of E. puncta and M. montezuma, suggesting synonymy of the genera Erpobdella and Motobdella. Moreover, these species appear so morphologically similar that they are difficult to distinguish since 28 of the 102 E. punctata specimens are genetically related to M. montezuma (Table S2).
Thus, the combination of different approaches implies the presence of seven cryptic species groups consisting of two or more genetically distant species, as well as the detection of misidentifications when the same name species diverge along different phylogenetic lineages (D. prokletijaca, E. nigricollis, E. testacea, and E. punctata) and vice versa when a phylogenetic lineage includes representatives of two species (E. dubia and E. parva). This conclusion confirms the recent findings of [14,33,49,60] and raises a requirement for an in-depth morphological revision of these species groups.
Until recently, only two representatives of Erpobdellidae have been considered to inhabit Siberian fresh waters: Erpobdella octoculata was indicated as widespread throughout Siberia, whereas Erpobdella nigricollis was found only in Western Siberia [29]. A series of further studies allowed for hypothesizing the presence of the Erpobdella species in Eastern Siberia with an uncertain attribution [49,61,62,63]. This study shows that 47 Siberian Erpobdella sequences form a single distinct lineage within the Palaearctic species clade (with a probability of 97–100%, depending on the tree). This division suggests a new species from Eastern Siberia (Figure A1 in Appendix A). Moreover, the DNA barcode of the new species contains 48 unique nucleotides that distinguish E. sibirica sp. nov. from the closest sister species, E. vilnensis (Figure A2 in Appendix A). The existence of E. sibirica sp. nov. is unambiguously confirmed by all methods used in this study.
As follows from the above, species issues are more or less easily resolved using modern DNA barcoding methods, while attempts to unify the approaches to molecular delimitation at the genus level are still rare and shaky (e.g., [64]). However, disagreements regarding the assignment of species to a genus within the Erpobdellidae still occur. Although Siddall [26] synonymized all Erpobdellidae genera (except Motobdella) into Erpobdella, the European scientific community did not accept this decision in part of the Palaearctic genera Dina and Trocheta. The Dina species complex, previously relegated to a subgenus within Erpobdella [29], was later returned to genus status based on its morphology [30], which was subsequently reconfirmed by detailed molecular data [32]. The formal disappearance of the Palaearctic genus Trocheta was also not accepted [27,35,37,65,66]. In this study, three different phylogenetic reconstruction algorithms (NJ, ML, and BI) supported the independent positions of Dina and Trocheta, as well as the separately clustered Palearctic and Nearctic representatives of Erpobdella (Figure 1, Figures S1 and S2). In addition, the resulting phylogenies were consistent with the findings of M. Siddall regarding the transfer of most North American species into the genus Erpobdella [26], further suggesting that Motobdella is also a synonym of Erpobdella. However, three lineages of E. dubia/parva, E. obscura, and M. quaternaria, despite their unstable position in the phylogenetic trees, have a level equal to the genus. The position of E. lineata from South Korea was always far distant from the group of European Dina lineata, although these names are synonymous. The Mooreobdella melanostoma clade had a stable location among the Nearctic Erpobdella, close to E. punctata and E. montezuma, indicating its doubtless belonging to this genus in contrast to M. quaternaria, confirming the existence of the genus Mooreobdella. Jueg and Grosser [67] proposed to synonymize this genus with the recently described Erpobdellopsis Grosser, 2017. However, according to the International Code of Zoological Nomenclature, the name Mooreobdella Pawlowski, 1955, has priority as a previously described taxon [68].
Genetic distances of 12.6–16.7% between genus-level groups (Table S4) support the idea of at least seven genera within Erpobdellidae instead of the four currently recognized. Thus, in addition to the long-established Palaearctic genera Dina, Erpobdella, and Trocheta, four Nearctic groups can receive the status of a separate genus (“Nearctic Erpobdella”, “obscura”, “dubia/parva”, and de novo Mooreobdella), while Motobdella should be synonymized with “Nearctic Erpobdella”. The taxonomic status of the sample from Korea with DNA barcode accession number KF966549 requires clarification since it may not be assigned to any of the genera within Erpobdellidae.
In this regard, a long-felt need for additional study of molecular delimitation of genera has reached a crisis point. Unquestionably, a reliable distinction between higher taxa requires further study involving additional morphological and multilocus molecular data as well as different approaches to their analysis.
5. Conclusions
This study was focused on the partition of a molecular dataset into potential taxonomic groups, mainly species, as the ultimate unit of classification of organisms and, therefore, the basic unit of evolution and biodiversity. Freshwater leeches were chosen as an experimental group, which, like Mendelian peas for genetics, turned out to be an ideal group for DNA barcoding (e.g., [12]). Previously, the method was successfully tested on flat and fish leeches [18,22]. This time, non-parasitic leeches were selected for an innovative study with an integrative approach that combines different bioinformatic algorithms to partition molecular data into subsets of species. As a result, the phylogenetic diversity of the available Erpobdellidae amounted to 47 species versus 29 morphospecies defined traditionally. In addition, numerous taxonomic misattributions within the data deposited with GenBank (incl. seven cryptic species and dozens of misidentifications) were revealed, and a new species from Siberia was discovered and described (Appendix A). Moreover, a pioneering attempt to apply a species-level approach to distinguish higher-level groups was successful in detecting the molecular signatures of genera. It was shown for the first time that Erpobdellidae probably consists of seven genera, given the unequivocal abolition of the genus Motobdella.
The results obtained are important not only for molecular systematics in itself. Erroneous taxonomic identification can distort the meaning of biodiversity and, therefore, prevent a clear understanding of the ecological and evolutionary signals and patterns.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16071030/s1, Figure S1: NJ tree of Erpobdellidae based on the barcoding genome fragment; Figure S2: ML tree of Erpobdellidae based on the barcoding genome fragment; Table S1: Collection of genetic sequences found to be a proper comparison group and outgroup; Table S2: Species grouping according to ASAP analysis; Table S3: Estimates of evolutionary divergence over sequence pairs between species groups; Table S4: Estimates of average evolutionary divergence over sequence pairs between potential genera.
Funding
This research was carried out in the frame of State Task No. 0279-2021-0011 and received no external funding. The APC was funded by Irina Kaygorodova.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original molecular genetic data of this study are open access and available at https://www.ncbi.nlm.nih.gov/ (accessed on 5 March 2024), reference numbers MN245518–MN245562 and KM220015–KM220020. A new species of the Erpobdella leeches was registered in ZooBank (lsid: zoobank.org:pub:5B2E54F1-D940-403B-8FC7-AACACB952B81).
Acknowledgments
The author is grateful to Natalia Sorokovikova (Limnological Institute, Irkutsk) for her technical help in sampling and sequencing, as well as to Ekaterina Matveenko (Limnological Institute, Irkutsk), Elena Dzuba (Limnological Institute, Irkutsk), Lyudmila Fedorova (Surgut State University, Surgut), Natalia Shaburova (Baikal-Lena Nature Reserve, Irkutsk), and Chananbaatar Ayushsuren (Institute of Biology, Ulaanbaatar) for their contribution to the sample collection.
Conflicts of Interest
The author declares no conflicts of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Appendix A. Molecular Description of Erpobdella sibirica sp. nov. (Figure A1)
Holotype. Russia, Lake Severnoe (54°19′22″N, 108°22′43″E), depth of 0.5 m, voucher 925 (Table 1), leg. N. Shaburova (14-Aug-2012); DNA barcode accession number MN245550. The holotype is deposited in the collection at the Limnological Institute, Irkutsk, under voucher number 925.

Figure A1.
Erpobdella sibirica sp. nov.: (A): live leech with its cocoon; (B,C): fixed specimens, dorsal and ventral views, respectively.
Paratypes are deposited in the collection at the Limnological Institute, Irkutsk (see Table 1 for the voucher numbers of 46 specimens). Their DNA barcodes have the following accession numbers: MN245518–MN245549, MN245551-MN245558, and KM220015–KM220020.
Etymology. The specific name indicates the wide range of the species and its geographical distribution in the water bodies of Eastern Siberia.
Species diagnosis. The worms are of medium size, and the body length of sexually mature individuals reaches 28–35 mm (Figure A1). The molecular genetic identifier of the new species Erpobdella sibirica sp. nov. was formed on the basis of 47 nucleotide sequences (Table 1, Figure A2).

Figure A2.
Molecular identifier (DNA barcode) of the new species Erpobdella sibirica sp. nov., reconstructed from 47 cox1 sequences of geographically distant sample localities in Eastern Siberia.
The molecular structure of the cox1 nucleotide sequences of 648 bp long contains 48 sites that distinguish E. sibirica sp. nov. from its closest relative E. vilnensis, and of them, eight unique diagnostic features in positions 19, 145, 238, and 412 are guanines (G); in 188, 232, and 583—cytosines (C); and in 613—thymine (T).
References
- Valentini, A.; Pompanon, F.; Taberlet, P. DNA barcoding for ecologists. Trends Ecol. Evol. 2009, 24, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mora, C.; Tittensor, D.; Adl, S.; Simpson, A.; Worm, B. How many species are there on Earth and in the ocean? PLoS Biol. 2011, 9, e1001127. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.; Barnosky, A.; García, A.; Pringle, R.; Todd, P. Accelerated modern human-induced species losses: Entering the sixth mass extinction. Sci. Adv. 2015, 1, e1400253. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.; Cywinska, A.; Ball, S.; de Waard, J.R. Biological identifications through DNA barcodes. Proc. Royal Soc. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.; Stoeckle, M.; Zemlak, T.; Francis, C. Identification of Birds through DNA Barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.; Hollingsworth, P.; Hajibabaei, M. From writing to reading the encyclopedia of life. Phil. Trans. R. Soc. B 2016, 371, 20150321. [Google Scholar] [CrossRef] [PubMed]
- Wiemers, M.; Fiedler, K. Does the DNA barcoding gap exist?—A case study in blue butterflies (Lepidoptera: Lycaenidae). Front. Zool. 2007, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Freudenstein, J.; Broe, M.; Folk, R.; Sinn, B. Biodiversity and the Species Concept—Lineages are not Enough. Syst. Biol. 2017, 66, 644–656. [Google Scholar] [CrossRef]
- Estensmo, E.; Maurice, S.; Morgado, L.; Martin-Sanchez, P.; Skrede, I.; Kauserud, H. The influence of intraspecific sequence variation during DNA metabarcoding: A case study of eleven fungal species. Mol. Ecol. Resour. 2021, 21, 1141–1148. [Google Scholar] [CrossRef]
- Hebert, P.; Gregory, T. The promise of DNA Barcoding for taxonomy. Syst. Biol. 2005, 54, 852–859. [Google Scholar] [CrossRef]
- Burns, J.; Janzen, D.; Hajibabaei, M.; Hallwachs, W.; Hebert, P. DNA barcodes and cryptic species of skipper butterflies in the genus Perichares in Area de Conservación Guanacaste, Costa Rica. Proc. Natl. Acad. Sci. USA 2008, 105, 6350–6355. [Google Scholar] [CrossRef] [PubMed]
- Bely, A.; Weisblat, D. Lessons from leeches: A call for DNA barcoding in the lab. Evol. Dev. 2006, 8, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Koperski, P.; Milanowski, R.; Krzyk, A. Searching for cryptic species in Erpobdella ocloculata (L.) (Hirudinea: Clitellata): Discordance between the results of genetic analysis and cross-breeding experiments. Contrib. Zool. 2011, 80, 85–94. [Google Scholar] [CrossRef]
- Oceguera-Figueroa, A.; Phillips, A.; Pacheco-Chaves, B.; Reeves, W.; Siddall, M. Phylogeny of macrophagous leeches (Hirudinea, Clitellata) based on molecular data and evaluation of the barcoding locus. Zool. Scr. 2011, 40, 194–203. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Mandzyak, N. Molecular phylogeny of Siberian Glossiphoniidae (Hirudinea). Mol. Biol. 2014, 48, 452–455. [Google Scholar] [CrossRef]
- Utevsky, S.; Dubov, P.; Prokin, A. First Russian record of Erpobdella monostriata: DNA barcoding and geographical distribution. Spixiana 2015, 38, 161–168. [Google Scholar]
- de Waard, J.; Ratnasingham, S.; Zakharov, E.; Borisenko, A.; Steinke, D.; Tefler, A.; Perez, A.; Sones, J.; Young, M.; Levesque-Beaudin, V.; et al. A reference library for Canadian invertebrates with 1.5 million barcodes, voucher specimens, and DNA samples. Sci. Data 2019, 6, 308. [Google Scholar] [CrossRef] [PubMed]
- Kaygorodova, I.; Bolbat, N.; Bolbat, A. Species delimitation through DNA barcoding of freshwater leeches of the Glossiphonia genus (Hirudinea: Glossiphoniidae) from Eastern Siberia, Russia. J. Zool. Syst. Evol. 2020, 58, 1437–1446. [Google Scholar] [CrossRef]
- Jovanović, M.; Haring, E.; Sattmann, H.; Grosser, C.; Pesic, V. DNA barcoding for species delimitation of the freshwater leech genus Glossiphonia from the Western Balkan (Hirudinea, Glossiphoniidae). Biodivers. Data J. 2021, 9, e66347. [Google Scholar] [CrossRef]
- Kondratov, I.; Sitnikova, T.; Kaygorodova, I.; Denikina, N.; Annenkov, V.; Khanaev, I.; Kirilchik, S.; Nebesnykh, I.; Dzyuba, E. Amazing discoveries of benthic fauna from the abyssal zone of Lake Baikal. Biology 2021, 10, 972. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Matveenko, E.; Dzyuba, E. Unexpected discovery of an ectoparasitic invasion first detected in the Baikal coregonid fish population. Fishes 2022, 7, 298. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Matveenko, E. Diversity of the Piscicola species (Hirudinea, Piscicolidae) in the Eastern Palaearctic with a description of three new species and notes on their biogeography. Diversity 2023, 15, 98. [Google Scholar] [CrossRef]
- Trontelj, P.; Sket, B.; Dovč, P.; Steinbrück, G. Phylogenetic relationships in European erpobdellid leeches (Hirudinea: Erpobdellidae) inferred from restriction-site data of the 18S ribosomal gene and ITS2 region. J. Zool. Syst. Evol. Res. 1996, 34, 85–93. [Google Scholar] [CrossRef]
- Govedich, F.; Blinn, D.; Keim, P.; Davies, R. Phylogenetic relationships of three genera of Erpobdellidae (Hirudinoidea), with description of a new genus, Motobdella, and species Motobdella sedonensis. Can. J. Zool. 1998, 76, 2164–2171. [Google Scholar] [CrossRef]
- Trontelj, P.; Sket, B. Molecular re-assessment of some phylogenetic, taxonomic and biogeographic relationships between the leech genera Dina and Trocheta (Hirudinea: Erpobdellidae). Hydrobiologia 2000, 438, 227–235. [Google Scholar] [CrossRef]
- Siddall, M. Phylogeny of the leech family Erpobdellidae (Hirudinida: Oligochaeta). Invertebr. Syst. 2002, 16, 1–6. [Google Scholar] [CrossRef]
- Pffeiffer, I.; Brenig, B.; Kutschera, U. Molecular phylogeny of selected predaceous leeches with reference to the evolution of body size and terrestrialism. Theory Biosci. 2005, 124, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Sket, B. Intralacustrine speciation in the genus Dina (Hirudinea, Erpobdellidae) in Lake Ohrid (Yugoslavia). Hydrobiologia 1989, 182, 49–59. [Google Scholar] [CrossRef]
- Lukin, E. Leeches of Fresh and Saline Waters; Nauka: Leningrad, Russia, 1976; 484p. [Google Scholar]
- Nesemann, H.; Neubert, E. Annelida, Clitellata: Branchiobdellida, Acanthobdellea, Hirudinea, Susswasserfauna von Mitteleuropa; Spectrum Akademischer Verlag: Heidelberg, Germany, 1999; 178p. [Google Scholar]
- Hovingh, P. Erpobdella (Dina) parva complex (Annelida: Hirudinea: Arhynchobdellida: Erpobdellidae): Additional description of Erpobdella parva, E. dubia, and E. lahontana and taxonomic revision. Hydrobiologia 2004, 517, 89–105. [Google Scholar] [CrossRef]
- Trajanovski, S.; Albrecht, C.; Schreiber, K.; Schultheiß, R.; Stadler, T.; Benke, M.; Wilke, T. Testing the spatial and temporal framework of speciation in an ancient lake species flock: The leech genus Dina (Hirudinea: Erpobdellidae) in Lake Ohrid. Biogeosciences 2010, 7, 3387–3402. [Google Scholar] [CrossRef]
- Anderson, K.; Braoudakis, G.; Kvist, S. Genetic variation, pseudocryptic diversity, and phylogeny of Erpobdella (Annelida: Hirudinida: Erpobdelliformes), with emphasis on Canadian species. Mol. Phylogenet. Evol. 2020, 143, 106688. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.; Siddall, M. Poly-paraphyly of Hirudinidae: Many lineages of medicinal leeches. BMC Evol. Biol. 2009, 9, 246. [Google Scholar] [CrossRef] [PubMed]
- Khomenko, A.; Utevsky, S.; Utevsky, A.; Trontelj, P. Unrecognized diversity of Trocheta species (Hirudinea: Erpobdellidae): Resolving a century-old taxonomic problem in Crimean leeches. Syst. Biodivers. 2020, 18, 129–141. [Google Scholar] [CrossRef]
- Pešić, V.; Grosser, C. Dina serbica, a new species of leeches (Annelida: Hirudinea: Erpobdellidae) from Serbia, based on morphological and molecular evidence. Ecol. Montenegrina 2022, 51, 1–14. [Google Scholar] [CrossRef]
- Grosser, C.; Barjadze, S.; Maghradze, E. Trocheta ariescornuta n. sp. (Annelida, Hirudinida: Erpobdellidae)—A new cavernicolous leech from Motena Cave in Georgia. Ecol. Montenegrina 2021, 44, 32–43. [Google Scholar] [CrossRef]
- Cichocka, J.; Bielecki, A.; Kur, J.; Pikuła, D.; Kilikowska, A.; Biernacka, B. A new leech species (Hirudinida: Erpobdellidae: Erpobdella) from a cave in the West Azerbaijan province of Iran. Zootaxa 2015, 4013, 413–427. [Google Scholar] [CrossRef] [PubMed]
- Liskiewicz, S. Leeches (Hirudinea) of the Kazan province (Russia, Eastern). Proc. Soc. Friends Sci. Vilnius 1925, 2, 2–8. [Google Scholar]
- Utevsky, S.; Son, M.; Dyadichko, V.; Kaygorodova, I. New information on the geographical distribution of Erpobdella vilnensis (Liskiewicz, 1925) (Hirudinida, Erpobdellidae) in Ukraine. Lauterbornia 2012, 75, 75–78. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Camacho, C.; Boratyn, G.; Joukov, V.; Vera Alvarez, R.; Madden, T. ElasticBLAST: Accelerating sequence search via cloud computing. BMC Bioinform. 2023, 24, 117. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, S.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Brouillet, S.; Achaz, G. ASAP: Assemble species by automatic partitioning. Mol. Ecol. Resour. 2021, 21, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, T.; Barraclough, T. Delimiting species using single-locus data and the Generalized Mixed Yule Coalescent approach: A revised method and evaluation on simulated data sets. Syst Biol. 2013, 62, 707–724. [Google Scholar] [CrossRef]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Bolbat, A.; Kaygorodova, I.; Bukin, Y.; Fedorova, L.; Sorokovikova, N. Application of bioinformational methods for species delimitation in the genus Erpobdella (Erpobdellidae, Hirudinea). Bull. Irkutsk State Univ. Ser. Biol. Ecol. 2017, 20, 3–13. [Google Scholar]
- Hebert, P.; Ratnasingham, S.; de Waard, J. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. B 2003, 270, S96–S99. [Google Scholar] [CrossRef]
- Kekkonen, M.; Hebert, P. DNA barcode-based delineation of putative species: Efficient start for taxonomic workflows. Mol. Ecol. Resour. 2014, 14, 706–715. [Google Scholar] [CrossRef]
- Bugarski-Stanojević, V.; Stamenković, G.; Jojić, V.; Ćosić, N.; Ćirović, D.; Stojković, O.; Veličković, J.; Savić, I. Cryptic diversity of the european blind mole rat Nannospalax leucodon species complex: Implications for Conservation. Animals 2022, 12, 1097. [Google Scholar] [CrossRef]
- Cerca, J.; Meyer, C.; Purschke, G.; Struck, T. Delimitation of cryptic species drastically reduces the geographical ranges of marine interstitial ghost-worms (Stygocapitella; Annelida, Sedentaria). Mol. Phylogenet. Evol. 2020, 143, 106663. [Google Scholar] [CrossRef] [PubMed]
- Jörger, K.; Norenburg, J.; Wilson, N.; Schrödl, M. Barcoding against a paradox? Combined molecular species delineations reveal multiple cryptic lineages in elusive meiofaunal sea slugs. BMC Evol. Biol. 2012, 12, 245. [Google Scholar] [CrossRef] [PubMed]
- Novo, M.; Almodovar, A.; Fernandez, R.; Cosin, D. Cryptic speciation of hormogastrid earthworms revealed by mitochondrial and nuclear data. Mol. Phylogenet. Evol. 2010, 56, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Kon, T.; Yoshino, T.; Mukai, T.; Nishida, M. DNA sequences identify numerous cryptic species of the vertebrate: A lesson from the gobioid fish Schindleria. Mol. Phylogen. Evol. 2007, 44, 53–62. [Google Scholar] [CrossRef]
- Trontelj, P.; Fišer, C. Perspectives: Cryptic species diversity should not be trivialized. Syst. Biodivers. 2009, 7, 1–3. [Google Scholar] [CrossRef]
- Poulin, R.; Pérez-Ponce de Leόn, G. Global analysis reveals that cryptic diversity is linked with habitat but not mode of life. J. Evol. Biol. 2017, 30, 641–649. [Google Scholar] [CrossRef]
- Pante, E.; Puillandre, N.; Viricel, A.; Arnaud-Haond, S.; Aurelle, D.; Castelin, M.; Chenuil, A.; Destombe, C.; Forcioli, D.; Valero, M.; et al. Species are hypotheses: Avoid connectivity assessments based on pillars of sand. Mol. Ecol. 2015, 24, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Tessler, M.; Siddall, M.; Oceguera-Figueroa, A. Leeches from Chiapas, Mexico with a new species of Erpobdella (Hirudinida: Erpobdellidae). Am. Mus. Novit. 2018, 3895, 129–134. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Pronin, N. New records of Lake Baikal Leech Fauna: Species Diversity and Spatial Distribution in Chivyrkuy Gulf. Sci. World J. 2013, 2013, 206590. [Google Scholar] [CrossRef]
- Kaygorodova, I.; Mandzyak, N.; Petryaeva, E.; Pronin, N. Genetic diversity of leeches in Lake Gusinoe (Eastern Siberia, Russia). Sci. World J. 2014, 2014, 619127. [Google Scholar] [CrossRef]
- Kaygorodova, I. Annotated checklist of the leech species diversity in the Maloe More Strait of Lake Baikal, Russia. ZooKeys 2015, 545, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Bolbat, A.; Bukin, Y.; Kaygorodova, I. Genome-based taxa delimitation (GBTD): A new approach. Diversity 2022, 14, 948. [Google Scholar] [CrossRef]
- Grosser, C.; Kutschera, U. Feeding behaviour and reproductive biology of the semiaquatic leech Trocheta haskonis (Hirudinea: Erpobdellidae). Lauterbornia 2004, 52, 163–169. [Google Scholar]
- Kutschera, U.; Pfeiffer, I.; Ebermann, E. The European land leech: Biology and DNA-based taxonomy of a rare species that is threatened by climate warming. Naturwissenschaften 2007, 94, 967–974. [Google Scholar] [CrossRef]
- Jueg, U.; Grosser, C. Erpobdellopsis graacki n. gen., n. sp. —A peculiar leech from Spain (Annelida, Hirudinida: Erpobdellidae). Lauterbornia 2017, 84, 69–87. [Google Scholar]
- ICZN. International Code of Zoological Nomenclature, 4th ed.; The International Trust for Zoological Nomenclature: London, UK, 1999; Available online: https://www.iczn.org/the-code/the-code-online/ (accessed on 20 March 2024).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).