Abstract
The present work aims to assess the effectiveness and efficiency of orange peels as a low-cost biosorbent for removing Cr(VI) from an aqueous solution by the biosorbent process. The orange peels as adsorbent was characterized using different methods, such as FTIR, pHpzc, equilibrium pH, TGA, XRD, SEM, and (BET). The tests were conducted in the batch mode, and the effects of different parameters, such as the pH, dosage of the bioadsorbent, influent Cr(VI), and time, on the biosorption of Cr(VI) were investigated. The adsorption kinetics proved that a contact time of 90 min resulted in the highest (approximately 97.8%) Cr(VI) removal, with an adsorption capacity of 4.96 mg/g. Moreover, the increase in the biosorbent dosage (from 1 to 10 g/L) resulted in the enhancement in the Cr(VI) removal effectiveness. Moreover, the pH of the solution also affected significantly the effectiveness of the removal. The tests were conducted under acidic pH solution conditions, and the prediction of the pH value at a zero charge (pH pzc) was confirmed experimentally. Furthermore, the results from the batch-mode assays were successfully tested by an experimental design (full factorial design). The biosorption of Cr(VI) on orange peels occurred mostly according to the pseudo-second-order kinetic model and the uptake of Cr(VI) was satisfactorily described by the Langmuir model.
1. Introduction
Chromium (Cr) is one of the most widely used chemicals in the industry thanks to its valuable effects in processing skin for tannery [1], textiles [2], wood and manufacturing dye [3] as well as in paint and paper [4] and in oil refining [5]. Cr(III) and Cr(VI) are two stable oxidation states of Cr that persist in the environment [6]. Cr (III) is essential for human nutrition, especially in glucose metabolism, while Cr(VI) is toxic for humans, animals, and bacteria. Cr(VI) compounds are known to be carcinogenic [7,8]. According to the World Health Organization (WHO) guidelines, the concentration thresholds of Cr(VI) are 0.1 mg/L and 0.05 mg/L in wastewater discharged into inland surface waters and drinking water, respectively [9].
Various methods are used for removing Cr(VI) substances from wastewater, industrial waters, surface water, and drinking water were reported in the literature [10,11,12,13]; for instance, conventional treatment methods, such as chemical precipitation, re commonly used for the removal of chromium from industrial effluents, such as tannery wastewater [14,15,16,17,18,19,20,21,22]. Using this method, other various substances can be separated in addition to chromium. Ion exchange [23,24,25], electro-coagulation [26], membrane separation [27], coupled coagulation/precipitation [28], and integrated reduced/oxidation [29] have been used. However, they are costly to use to safely remove residual sludges and the chemicals necessary for removing Cr(VI) are expensive. They also suffer from unsatisfactory Cr(VI) removal, whereas adsorption is considered to be a promising method, as it requires low operating costs and, when it is combined with a suitable desorption phase, the concern for the residual sludge disposal is negligible [30].
A number of low-cost adsorbents have been already tested successfully for the removal of toxic pollutants from wastewater [31,32,33,34,35,36]. In recent years, several natural adsorbents, like wool, olive cake, sawdust, pine needles, almond shells, cactus leaves, soot, hazelnut shell, coconut shell, banana peel, orange peel, seaweed, dead fungal biomass, cyano-bacteria, and green algae, have been used to remove Cr from water [37,38,39,40]. However, many of these adsorbents present a low Cr adsorption yield and slow process kinetics. Thus, it is of interest to find other low-cost materials with enhanced performance. In such a context, the aim of this study is to test the efficiency of orange peels in removing Cr(IV) from water. We validate the results using the Minitab software 21.1.0 (Minitab, LLC, State College, PA, USA) and determine the optimal conditions corresponding to the best chromium removal efficiency and effectiveness. Compared to other publications, the novelties of this study include: (1) Creating a mathematical model to promote the adsorption process of water with similar chromium properties, potentially omitting the laboratory optimization step, i.e., only mathematical models are used; (2) Kinetic studies and adsorption isotherms; (3) Chemical structure considerations of orange peel and their reaction with chromium.
2. Materials and Methods
2.1. Biosorbent Preparation
With the aim of developing an inexpensive biomaterial for the removal of Cr(VI) from wastewater, orange (Citrus sinensis) peels were chosen as a natural source for the preparation of the biosorbent. It was obtained according to the following sequence of operation:
- The orange peels were washed with tap water and then with distilled water to remove residual impurities and soluble parts. The washing process was repeated several times until clear washing water was obtained.
- The orange peels were exposed to the sun for several days until completely dry.
- Once dried, the peels were crushed using an electric household grinder.
- The ground peels were sifted using a sieve with a mesh size of 0.315 mm.
- After sieving, the biosorbent was stored for the experimental period in dry bottles.
- Before each use, the orange peels in powder form were dried in an oven at a temperature of 105 °C to constant weight.
2.2. Biosorbent Characterization
For the physical and chemical characterization of the bioadsorbent (orange peels in powder size), several techniques were used. The Fourier-transfer infrared (FTIR) spectrum was used to examine the nature of the chemical bonds with a JASCO FT/IR -4600 type device (JASCO, Tokyo, Japan). A thermogravimetric analysis (TGA) was performed to study the thermal stability with the TGAQ50 module from the Thermal Analyzes device. The crystalline structure of the orange peels was evaluated by X-ray diffractometer using Cu with wavelength K-Alpha (1.54) at 40 mA and 40 kV with a scan analysis. The Scanning electron microscopic (SEM) analysis of the bioadsorbent was recorded with a scanning electron microscope Quanta 650 (FEI Company, Hillsboro, OR, USA). The Brunauer–Emmett–Teller (BET) method was employed to measure the surface area of the orange peel powder. Moreover, the identification of the points of zero net charge, iodine number, and contact-free pH was determined.
2.2.1. pH Value at the Point of Zero Charge
The pH at the point of zero charge (pHpzc) of materials is a good indicator of the chemical and electronic properties of functional groups on their surface. The pHpzc of the biosorbent was calculated as follows: Solutions of 50 mL of NaCl (0.01 M) were placed in flasks. The pH in each flask was adjusted by adding drops of solutions of NaOH or HCl (0.1 M) to achieve initial pH (pHi) values ranging between 2 and 12. In each flask, 0.15 g of the biosorbent (Orange peel) was added. The flasks were kept closed at room temperature for 48 h, and then the final pH (pHf) was determined. On the graph of pHf = f(pHi), the intersection of the curve with the quadrant bisector represents the iso-electric point (pHpzc) [41,42].
2.2.2. Contact-Free pH
The contact-free pH was determined by introducing 1 g of the biosorbent in 100 mL of distilled water, and the sample was stirred at 300 rpm for 24 h. The sample was centrifuged and, finally, the pH of the filtrate was measured using a pH meter (Jenway model 3540, Camlab, Cambridge, UK).
2.2.3. Iodine Number
The iodine number (Id) was calculated according to the following procedure involving two tests. The first test was the blank test conducted with 10 mL of a 0.1 N iodine solution placed in a beaker and dosed with a 0.1 N solution of sodium thiosulfate (Na2SO3) until the color disappeared with the adding of a few drops of a starch solution (indicator). The second test consisted of adding 0.2 g of the biosorbent into a beaker that contained 15 mL of a 0.1 N iodine solution and mixing it for 4 min with a stirring equipment. After that time, a centrifugation process was carried out and 10 mL of the filtrate (which contained iodine) was dosed with a 0.1 N solution of sodium thiosulfate (Na2SO3) until the color disappeared with the adding of a few drops of a starch solution (indicator) [43].
Id is calculated according to the following Equation (1).
where
- (VB − VS): difference between the titration volumes calculated using the blank test and the biosorbent with VB = 12.9 mL and VS = 9 mL of 0.1 N solution of thiosulfate.
- N: normality of the sodium thiosulfate solution (0.1 N).
- 126.9: the atomic mass of iodine (g/mol).
- m: mass of the biosorbent (0.2 g).
2.3. Adsorption Tests
2.3.1. Chromium Solutions
A Cr(VI) stock solution of 1 g/L was prepared by dissolving 2.828 g of potassium dichromate (K2Cr2O7) salt in 1000 mL of distilled water. Seven solutions at different Cr(VI) concentrations (i.e., 10, 20, 30, 50, 60, 80, and 100 mg/L) were obtained from the stock solution by the appropriate dilution rates. The pH was adjusted by adding 0.1 N hydrochloric acid (HCl) and 0.1 N caustic soda (NaOH) solutions, and measured by using a pH meter (type 3505, JENWAY).
2.3.2. Operating Procedure
The adsorption tests were conducted according to the following procedure:
- (1)
- Preparation of a suspension (bio-sorbent/contaminated solution) for a ratio r (S/L) = 1.5 and 10 g/L as the biosorbent dosage;
- (2)
- The initial Cr(VI) was set at the desired concentration, as reported in Section 2.3.1;
- (3)
- The biosorbent suspension was stirred at 300 rpm for 24 h at room temperature (22 ± 1 °C);
- (4)
- Volumes of 5 mL were collected with a syringe from a suspension at different reaction times during the first period (350 min) of experimentation in order to determine the equilibrium time;
- (5)
- The pH was adjusted by adding a HCl or NaOH solution in the range of 2–10;
- (6)
- The samples collected from the suspension were filtrated with a Millipore filter (0.45 µm);
- (7)
- The filtrated fraction was analyzed by a UV–visible spectrometer SHIMADZU UV-160A model.
The biosorbent efficiency expressed as the Crremoval (%) was assessed by calculating the amount of Cr(VI) removed according to the following Equation (2):
whereas the quantity of the adsorbed pollutant (q) expressed in mg of pollutant per g of adsorbent (mg/g) was calculated according to the following Equation (3):
where
- C0 = the initial concentration of Cr(VI) in mg/L.
- Ce and Ct = the residual concentrations of Cr(VI) at the equilibrium and “t” times, respectively, in mg/L.
- m = mass of the biosorbent (g).
- V = volume of the Cr solution (mL).
2.4. Effect of the Parameters
The effects of the following parameters were investigated: solid–liquid ratio (r), operating pH, and the initial concentration of Cr(VI).
The examination of the influence of the solid–liquid ratio (r) on the adsorption capacity of Cr(VI) on the orange peels was conducted by varying the biosorbent dosage among 0.1, 0.5, and 1 g in 100 mL working solution.
To optimize the pH, a study of the Cr(VI) adsorption as a function of the pH varying withing the range from 2 to 10 was carried out with an initial Cr(VI) concentration of 50 mg/L and a dosage of the adsorbent of 10 g/L.
Finally, in order to study the effect of the initial concentration of Cr(VI), the following values were considered: 10, 20, 30, 50, 60, 80, and 100 mg/L.
2.5. Study of the Effects and Interactions between the Parameters Affecting the Cr(IV) Adsorption Process
To understand in depth the effect of the operating parameters and their interactions on the Cr(VI) removal efficiency, a full factorial design study was conducted and is described in the following subsections.
Full Factorial Design
In order to obtain the optimal conditions for adsorption, a full nk factorial design was performed, where n = the number of levels and k = the number of factors being evaluated. For the present study, n = 2 and k = 4, as detailed in Table 1. Thus, the total number of required experiments was 24. If Y is the response variable, the four parameters’ regression equation and their interactions are evaluated through the following Equation (4):
where b0, b1, b2, b3, and b4 are the linear coefficients, and b12, b13, b14, b23, b24, and b34 are the second-order interaction terms. X1, X2, X3, and X4 are the coded dimension factors of the following studied parameters: the biosorbent dosage (m), pH, the initial concentration (C) of Cr(VI), and contact time (t), respectively. Table 1 reports the low and high levels for the studied parameters. The minimum and maximum values of the variables in Table 1 were selected based on several conditions for the preliminary experimentations, for example, the contact time was chosen when the equilibrium was reached. For the initial concentration, we chose to ascribe to the synthetic solutions a character like that of wastewater effluents, which nearly reach these concentrations. For the pH, it was better to use a large interval in order to optimize the available values.
Y = b0 + b1X1 + b2X2 + b3X3 + b4X4 + b12X1X2 +b13X1X3 + b14X1X4 + b23X2X3 + b24X2X4 + b34X3X4

Table 1.
Minimum and maximum levels of the studied parameters.
2.6. Kinetic Study
The kinetic studies of the adsorption process on the biosorbent were carried out at 22 °C (room temperature) and different initial Cr(VI) concentrations (from 10 to 100 mg/L). The efficiency of the adsorption process was assessed at regular time intervals.
The mathematical expressions of the pseudo-first- [44] and pseudo-second-order [45] models as well as the intra-particle kinetic model are reported in the following Equations (5)–(7):
where
- qe (mg/g): adsorbed amount at equilibrium.
- qt, (mg/g): adsorbed amount at time t (min).
- K1 (min−1): equilibrium constant for the adsorption rate of the pseudo-first-order equation.
- K2 (g/mg·min): equilibrium constant for the adsorption rate of the pseudo-second-order equation.
- Kin (mg/g·min1/2): equilibrium constant for the adsorption rate of the intra-particle model equation.
2.6.1. Adsorption Isotherms
Different adsorption isotherm models are available in the literature to process the adsorption process experimental data [46,47]. Among them, in this work, the Langmuir, Freundlich, and Elovich isotherms were used. In what follows, we report the linear relationships of the 3 models: Equations (8), (9), and (10), respectively.
where
- Ce (mg/L): equilibrium concentration.
- qe (mg/g): adsorbed amount at equilibrium.
- qmax (mg/g): adsorbed maximum amount.
- KL, KF, and KE are, respectively, the Langmuir constant (L/mg), Freundlich constant (mg(1−n)·Ln/g), and Elovich constant (L/mg).
- n: constant relating to energy.
3. Results
3.1. Biosorbent Characterization
3.1.1. FTIR Analysis
FTIR measurements were conducted to understand the possible functional groups existing for interactions between metallic ions and the orange peel adsorbent. Figure 1 shows that the spectrum is characterized by the presence of a characteristic peak at 3311.28 cm−1 attributed to the elongation of the N–H [48,49] or most likely representing O–H group stretching vibrations (of carboxyl, phenols, or alcohols) [50,51]. This is quite logical if one considers that the main constituents of orange peels are cellulose, lignin, and hemicelluloses, all constituents that are rich in different kinds of –OH groups, as well as vicinal phenolic –OHs and –OCH3 groups belonging to flavonoids, such as hesperidin glucoside, known to be abundant in orange peels. The band at 2926.58 cm−1 generally characterizes aliphatic C-H stretching vibrations [52,53,54]. The band appearing in the orange peel powder spectrum at 1611 cm−1 is attributed to the C=O groups of carboxylic acids, mainly both the acetate groups (COO−) of hemicelluloses and of the esters of citric acid, present in all citrus peels, as well as the carbonyl groups of ketones, like the one on the C4 site of hesperidin, as well as traces of aldehydes or lactones [55]. The presence of a peak at 1358 cm−1 attributed to C–O stretching supports the existence of numerous and varied carboxyl/alcohol/ether/ester functional groups in the absorbent. The probability of the presence of aliphatic fluorinated compounds (C-F), which are characterized by a peak at 1011.5 cm−1 in the FTIR spectrum of the orange peel [56,57], exists but is unlikely, as more likely, it is a confirmation of the existence of aromatic nuclei in the mixture of the material constituents.
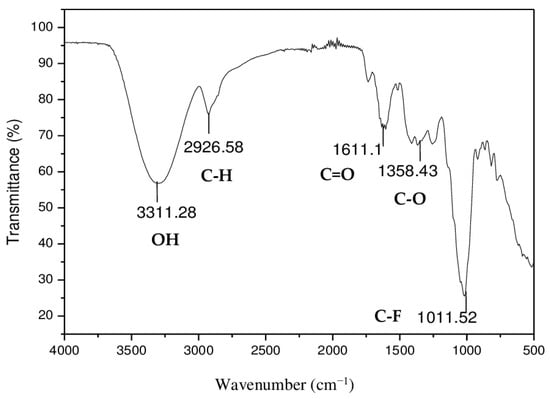
Figure 1.
Orange peel powder infrared spectrum.
3.1.2. pH Value at the Point of Zero Charge
Figure 2 reports the graphical calculation of pHpzc; at pH = 2.63, the surface charge is zero. For pH values higher than pHpzc, the biosorbent surface charge is negative [58,59].
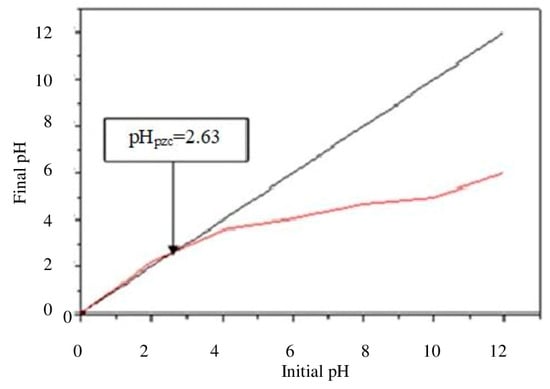
Figure 2.
pH value at the point of zero charge (pHpzc) calculated for the orange peel bio-sorbent.
Therefore, on the basis of the operating pH, three different conditions can occur:
- (1)
- At pH < pHpzc, the orange peel surface is positively charged;
- (2)
- At pH = pHpzc, the orange peel surface is not charged (neutral);
- (3)
- At pH > pHpzc, the orange peel surface is negatively charged.
3.1.3. Contact-Free pH
The value of the contact-free pH obtained was 4.3 (Figure 3). Therefore, the biosorbent is slightly acid.
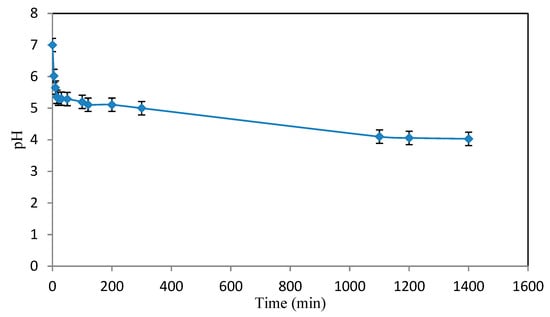
Figure 3.
Contact-free pH.
3.1.4. Iodine Number
The resulting value of Id was 133.11 mg/g. This value proves that the biosorbent material is characterized by meso-pores and shows a moderately high iodine retention capacity.
3.1.5. Thermogravimetric Analysis
A thermogravimetry analysis (TGA) was carried out to evaluate the biosorbent composition in terms of water, cellulose, hemicellulose, and lignin. From Figure 4, it can be noticed that three steps of weight loss occurred: The first step between 25 °C and 180 °C is due to water evaporation (moisture loss). The second step in the range of 180–380 °C is a consequence of hemicellulose and cellulose degradation. The last step, when temperature is over 380 °C, is attributed to the decomposition of residual lignin. Generally, lignin accounts for 15% of the total dry weight [60,61].
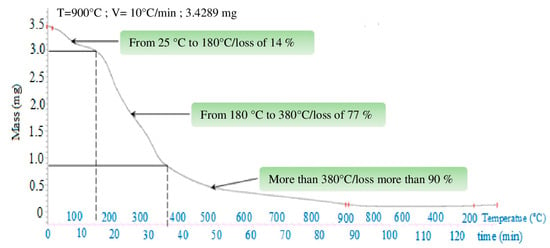
Figure 4.
Orange peel thermogravimetric analysis (TGA).
3.1.6. XRD Analysis
Figure 5 shows the XRD spectra of the orange peel powder in its natural form. The graph shows that the constituent mixture is amorphous. This can be explained by the rupture of multiple C-C bonds (the aromatic rings) and the formations of groups and functions on the surface of the biomaterial (powder or orange peels). The spectrum shows two peaks, the first at 2θ = 21° and a large one at 2θ = [30°–45°]; this second one reveals that the adsorbent has a largely amorphous structure. A notable property of well-defined adsorbents is the absence of sharp peaks, which indicates that it has a largely amorphous structure [62,63,64,65].
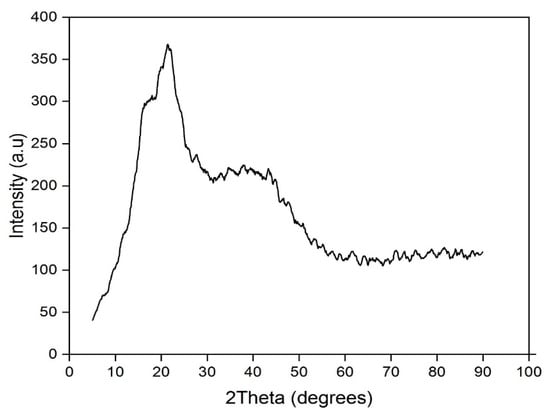
Figure 5.
X-ray analysis of the orange peel powder.
3.1.7. SEM Analysis
In Figure 6, the surface morphology of the raw orange peels in powder form with the different magnifications of (a: ×1500), (b: ×800), and (c: ×400) confirms the heterogeneous nature of this adsorbent with large pore size. This structure facilitates the process of adsorption. It indicates that the porous structure of the material ensures a high surface area, facilitating adsorption. However, the chemical complexation of Cr(VI) by carbohydrate polymers and oligomers is well documented [66] as well as the ortho-diphenol metallic complexes by flavonoids (such as hesperidin) [67].
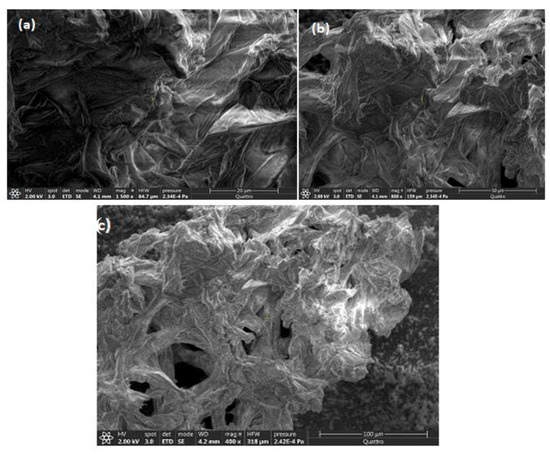
Figure 6.
SEM of the orange peels prior to adsorption with different magnifications ((a) ×1500), ((b) ×800), and ((c) ×400).
3.1.8. BET Surface Area Analysis
A BET analysis was used to measure the surface area, the pore volume, and the pore diameter of the orange peels in powder form. This analysis showed that the pore diameter (pd), the pore volume (Vp), and the specific surface area (SBET) were 152.238 A°, 32.270 m3/g, and 1511.278 m2/g, respectively. The obtained results confirm that the orange peel powder has a large surface area, which implies a high number of vacant sites that vaporize the adsorption of the pollutant.
3.2. Equilibrium Study
As shown in Figure 7, the chemical equilibrium for the adsorption process is reached in almost 90 min, with a satisfactory removal efficiency of approximately 99%. Such a result was obtained with pH = 5.1 and a biosorbent particle size (d) ≤ 0.315 mm. It is worthy of note that the removal efficiency value of 99% was obtained in the preliminary study conducted to assess the time needed to reach the chemical equilibrium condition. The results show that 90 min is a sufficient time to reach the chemical equilibrium. This time was, therefore, used to conduct further tests designed to study the effects of the remaining operating parameters, such as the dosage of the biosorbent, contact time, pH, and the initial concentration of Cr(VI).
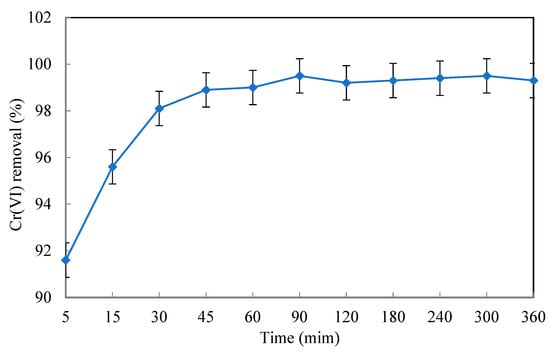
Figure 7.
Cr(VI) removal efficiency. Operating conditions: C0 = 50 mg/L, T = 22 ± 2 °C, r = 10 g/L, d = 0.315 mm, and pH = 5.1.
3.3. Adsorption Process Performance
Adsorption is affected by several parameters, such as the biosorbent dosage, contact time, pH, and initial Cr(VI) concentration. The effects of each previously mentioned parameter are discussed in the following dedicated subsections.
3.3.1. Effect of the Biosorbent Dosage
According to Figure 8, the adsorption capacity (q) defined by Equation (3) is inversely proportional to the solid–liquid ratio (r), whereas the efficiency of the removal process increases by increasing the dosage of the biosorbent, thus increasing the working adsorbent surface and consequently the number of active sites. The obtained result is confirmed by other researchers [68,69,70].
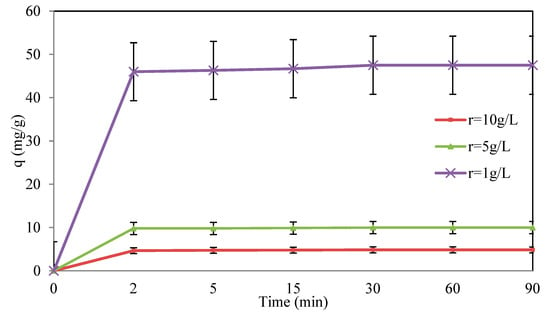
Figure 8.
Effect of the solid–liquid ratio on the Cr(VI) removal efficiency. Operating conditions: C0 = 50 mg/L, T = 22 ± 2 °C, pH = 5.1, and d = 0.315 mm.
3.3.2. Effect of the Contact Time
Figure 9 shows the trend of Cr(VI) adsorption. In this figure, two different phases of the process can be clearly noticed: a first stage lasting 15 min, where the process occurs very fast, and a second stage with a slower adsorption and even no adsorption. This trend is actually the result of a progressive saturation of the vacant sites on the surface of the biosorbent.
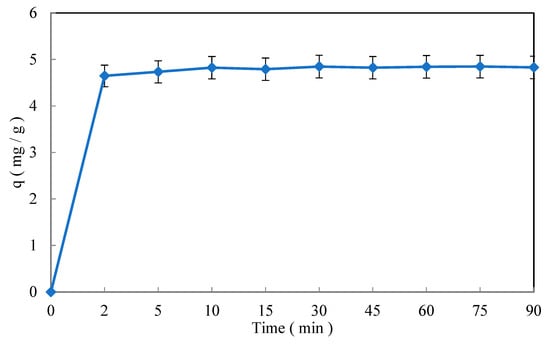
Figure 9.
The effect of the contact time on the Cr(VI) removal efficiency. Operating conditions: C0 = 50 mg/L, T = 22 ± 2 °C, pH = 5.1, and d = 0.315 mm.
3.3.3. Effect of the pH
Figure 10 clearly shows that the Cr(VI) removal capacity at the chemical equilibrium condition decreases by raising the initial pH from 2 to 10. Comparing the curves in Figure 10, it can be stated that, for a pH ranging between 2 and 4, the adsorption capacity is at its maximum (4.96 and 4.91 mg/g, respectively), whereas, at the opposite extreme, i.e., at pH = 10, this capacity decreases to 4.88 mg/g.
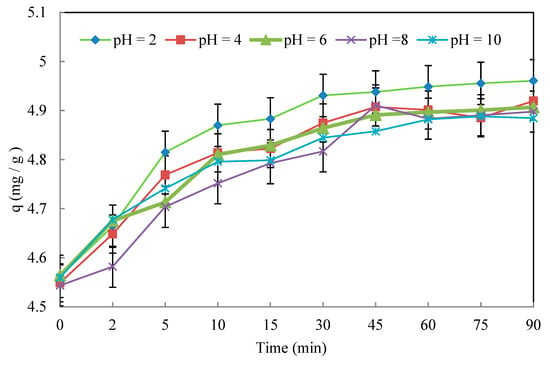
Figure 10.
The effect of the pH on the Cr(VI) removal efficiency. Operating conditions: C0 = 50 mg/L, T = 22 ± 2 °C, d = 0.315 mm, and r = 10 g/L.
Figure 11 reports the removal efficiency of Cr (IV) as a function of the pH: Increasing the initial pH from 2 to 10, the removal efficiency of Cr(VI) increases up to 99.21% at pH = 2. The minimum removal rate (i.e., 97.68%) was obtained at pH = 10. Anyway, this value is high and still satisfying, but at a pH higher than 6, another process occurs, which is precipitation, as mentioned in another work [71].
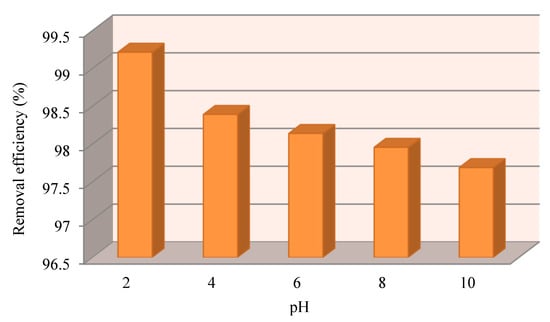
Figure 11.
Cr(VI) removal efficiency as a function of the pH.
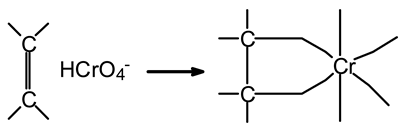
The highest removal rate of Cr(VI) at pH = 2 is a consequence of the protonation of the biosorbent as well as Cr(VI) speciation. Indeed, at a low pH, the proton concentration is high, and the negative charges on the pore surface of the biosorbent are, thus, new adsorption sites that are consequently available with positive charges, which is confirmed the pHpzc result [72]. Conversely, Cr(VI) in a solution can coexist as chromate (CrO4−2), dichromate (Cr2O7−2), hydrogen chromate (HCrO4−), or chromic acid (H2CrO4), according to the reaction scheme reported in Figure 12. At a low pH, ions of HCrO4− are the most abundant in a solution compared to those of Cr2O7−2, and ions of HCrO4− are smaller in size than those of Cr2O7−2 [73]. Therefore, monovalent ions of HCrO4− diffuse smoothly and are adsorbed more easily and in greater amounts by the pore surface than ions of Cr2O7−2. At a pH below 1, H2CrO4 predominates in the solution as a polycyclic anhydride species that is difficult to be adsorbed by the pores [74].
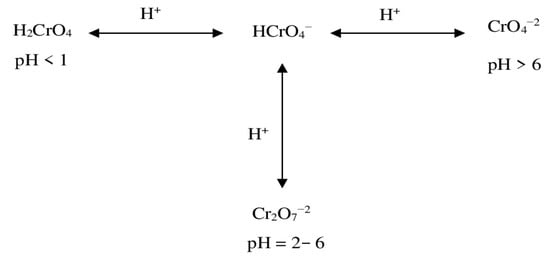
Figure 12.
Cr(VI) chemical reaction scheme.
3.3.4. Effect of the Initial Concentration of Cr(VI)
Figure 13 shows that the highest removal yield of 98% is achieved after 30 min, with a Cr(VI) concentration of 50 mg/L at pH = 5.1. With higher Cr(VI) concentrations, the removal efficiency decreases. This result occurs because, for a specific dosage of the biosorbent, when the concentration of Cr(VI) is the lowest, the ratio between the active sites of the surface and the molecules of the metals in the solution is the highest, and thus a higher amount of molecules is retained by the biosorbent and removed from the solution. Conversely, at higher Cr(VI) concentrations, the amount of metal ions adsorbed per unit mass of the biosorbent is greater, thus causing a saturation of the biosorbent surface and, therefore, a relevant amount of metal ions remains dissolved in the solution [75].
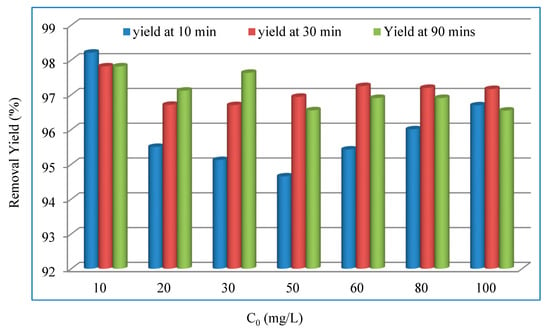
Figure 13.
Variation in the Cr(VI) removal efficiency. Operating conditions: T = 22 ± 2 °C, pH = 5.1, d = 0.315 mm, and r = 10 g/L.
3.4. Results of the Full Factorial Design
According to the factorial plan for Cr(VI) removal by adsorption on the orange peels, 16 experimental tests (24) were conducted, as reported in Table 2. In the last column of Table 2, the Cr(VI) removal efficiency is indicated.

Table 2.
Matrix of the experimental plan.
The mathematical expression to calculate the Cr(VI) removal efficiency (YCr) is as follows:
where
YCr (%) = 98.02 + 0.64 m − 0.69 pH + 0.22 t + 0.13 C0 + 0,54 m × pH − 0.012 m × t − 0.43 m × C0 + 0.051 pH × t + 0.66 pH × C0 − 0.008125 t × C0 + ζ
- m: mass of the biosorbent (mg);
- C0: the initial concentration of Cr(VI) (mg/L);
- t: contact time (min);
- ζ: residual error.
The analysis of variance (ANOVA table) resulted in a determination factor R2(adjusted) = 58% and R2 = 85.89%.
The “p”-value (probability of risk) was lower than 0.05 (5%) for only the mass of the biosorbent, the pH, and the (pH/Cr(VI) concentration) interaction; so, these set factors have the largest effect on the adsorption process of Cr(VI) on the orange peels.
Figure 14 shows that both parameters, the pH and biosorbent dosage, had a relevant effect on the removal efficiency of Cr(VI). The pH value was inversely correlated to the removal efficiency. Actually, an increase in the pH resulted in a decrease in the removal efficiency; therefore, Cr(VI) removal was more efficient in acidic media at pH ≥ 2. This finding can be explained by the predominant presence of HCrO4− ions when the pH is equal to 2 as well as by the surface of the biosorbent being charged positively because the pHpzc is higher than the pH value of the solution. The biosorbent dosage also has a significant effect on the removal of Cr(VI), because the amount of active sites capable of adsorbing metal ions depends on it.
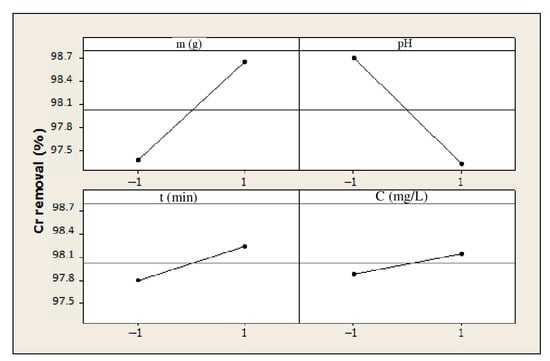
Figure 14.
Effect of each single parameter on Cr removal (%).
Figure 15 reports the effects of each parameter and their mutual interactions. The red and black lines indicate a different level (i.e., +1 and −1) for each parameter. Regarding the biosorbent dosage, the results display that the amount of 2000 mg is more effective than that of 500 mg for all the mutual interactions. Conversely, for the initial concentration of Cr(VI), it can be seen that the lower the concentration, the more efficient the removal of Cr(VI). Finally, concerning the pH, as already mentioned, at a low value (an acidic medium), the removal of Cr(VI) is more pronounced with a contact time of 90 min. Regarding interaction factors, in Figure 14, it can be noticed that any interaction with the contact time does not have a significant effect on the adsorption process. The interactions that significantly affect the removal rate are as follows: the biosorbent dosage with the pH, the pH with the initial Cr(VI)concentration, and the biosorbent dosage with the initial Cr(VI)concentration. Consequently, the parameters and mutual interactions that showed negligible effects on the Cr(VI) removal efficiency were deleted from Equation (11), thus resulting in a simplified expression as follows:
YCr (%) = 98.02 + 0.64 m − 0.69 pH + 0.22 t + 0.13 C0 + 0.54 m × pH − 0.43 m × C0 + 0.66 pH × C0 + ζ
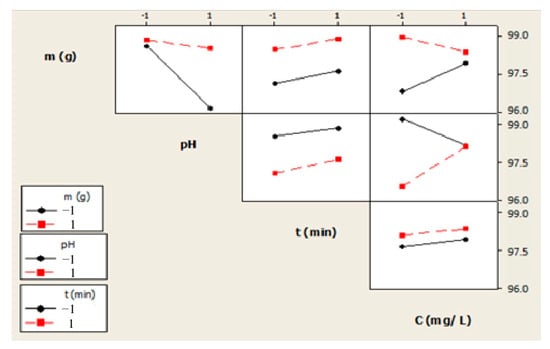
Figure 15.
Effect of the interactions among the parameters on the Cr(VI) removal efficiency.
According to the ANOVA study:
R2 = 85.89%; R2 (adjusted) = 73.55%.
Also, the “p”-value was lower than 5% for all the parameters investigated and their mutual interactions, except for the contact time and the initial Cr(VI) concentration.
3.5. Kinetic Study
The values of qe, K1, K2, and Kin were determined from the intercept and slope of the line representing the linear relationship for each kinetic model (Figure 16, Figure 17 and Figure 18).
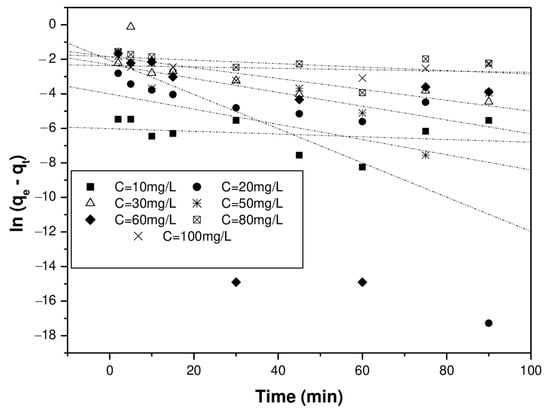
Figure 16.
Kinetics of the pseudo-first-order model.
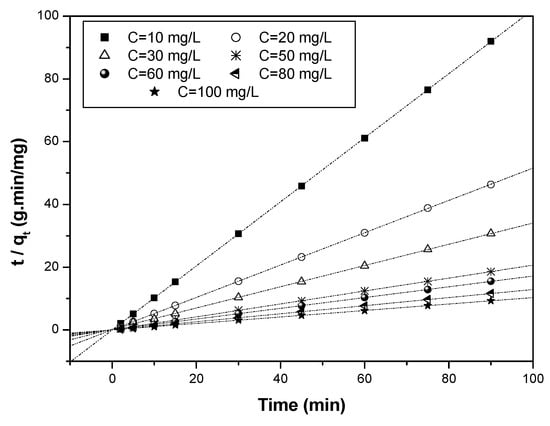
Figure 17.
Kinetics of the pseudo-second-order model.
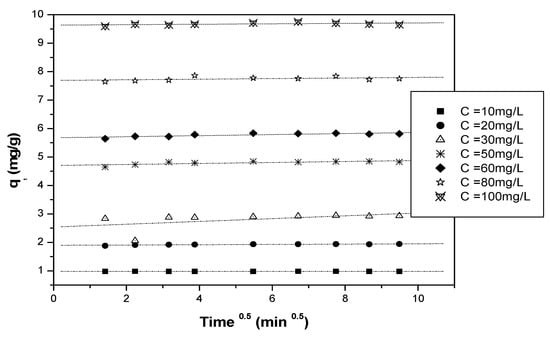
Figure 18.
Kinetics of the intra-particle model.
The results are reported in Table 3.

Table 3.
Kinetic constants of the first-order, second-order, and intra-particle scattering models for the different initial Cr(VI) concentrations.
From the linear regressions of the three previously mentioned kinetics (pseudo-first-order, pseudo-second-order, and intra-particle models), it can be noticed that the adsorption of Cr(VI) on the biosorbent surface is satisfactorily modeled by a second-order kinetics for all the initial Cr(VI) concentrations examined. The kinetic constants of each model are reported in Table 3.
3.6. Adsorption Isotherm Modeling
The adsorption capacity is often represented by isothermal curves, which can also provide information on the adsorption mechanism.
Figure 19 shows that the shape of the curve is almost similar to a type (II) isothermal curve. This type indicates a single-layer adsorption at a low concentration, followed by multilayer adsorption at a higher concentration. The lower the concentration at which multilayer adsorption begins, the stronger the interaction occurring between the adsorbed substance and the adsorbent.
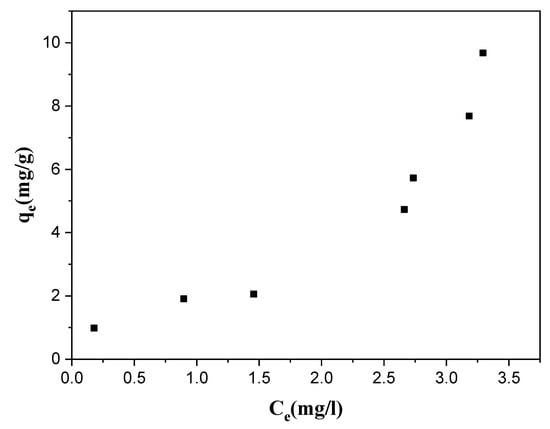
Figure 19.
Isotherm study of Cr(VI) adsorption on the orange peel powder at laboratory temperature.
This type of isotherm is characteristic of an exclusively physical adsorption on non-porous solids or solids presenting mainly macro-pores [76].
The equilibrium isotherms for the adsorption of Cr(VI) on the orange peel powder samples over a wide range of concentrations (10–100 mg/L) were modeled.
To examine the relationship between the sorbed (qe) and aqueous concentrations (Ce) at equilibrium, sorption isotherm models are widely employed for fitting the data, of which the Langmuir, Freundlich, and Elovich equations are the ones most widely used.
To ensure equilibrium conditions, the linear form of the three models’ equations cited above (Section 2.6.1) was applied to the experimental data.
The equilibrium parameters obtained with the adsorption model of the Langmuir, Freundlich, and Elovich isotherms are reported in Table 4. The values of the correlation coefficient (R2) are higher for the Langmuir and Freundlich isotherms than the Elovich isotherm model. Therefore, the Langmuir model assumes that the uptake of adsorbate molecules occurs on a homogenous surface by monolayer adsorption without any interaction between the adsorbed molecules. The Freundlich model is suitable for non-ideal adsorption on heterogeneous surfaces [77]. This result may indicate the formation of single-layer adsorption on the active sites of the surface at a low concentration in the first time, followed by multilayer adsorption at a higher concentration.

Table 4.
Isotherm constants for the adsorption of Cr(VI) on the biosorbent.
The graphical maximum adsorption capacity of the Langmuir isotherm (qmax) was 5.46 mg/g.
The balancing parameter, RL, of the Langmuir model was calculated using the following equation:
where RL (dimensionless) indicates the tendency of the adsorption process to take place when the following conditions occur:
- (a)
- 0 < RL < 1, the adsorption is favorable;
- (b)
- RL > 1, the adsorption is unfavorable;
- (c)
- RL = 0, the adsorption is irreversible;
- (d)
- RL = 1, the isothermal representation is linear [78].
The RL values ranging from 0.205 to 0.826 for the different initial Cr(VI) concentrations studied indicate that the process of Cr(VI) adsorption by the biosorbent is favorable.
3.7. Chemical Structure Considerations
It must be considered that, when one talks of the absorption of Cr(VI) on a porous surface, it is not the generic interaction of a metal ion with the unspecified sites of the surface of the porous medium, but that there are clear interactions by secondary forces and perhaps the coordination complexing of some kind with particular structures of the orange peel constituents, i.e., specific adhesion. First of all, carbohydrate oligomers and polymers are well known to easily complex chromium (VI) and Cr (III) with structures of the types that follow: Cr(VI) being reduced to Cr (III) by the carbohydrates and the carbohydrates being simultaneously oxidized as follows:
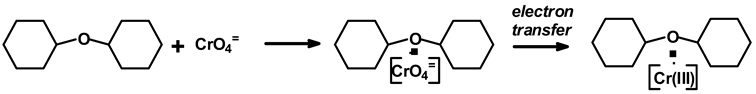
This is a simplified form of the specific adhesion mechanism by carbohydrates, with the series of mechanisms involved being considerably more complex but well determined [66].
Equally, coordination complexes can occur with hesperidin glucosides, where complexation, and hence the absorption of Cr(VI), can occur with both the ortho –OH and –OCH3 groups of the B-ring of its flavonoid as well as with the carbohydrate chain linked to it. Thus, the structure of hesperidin is:
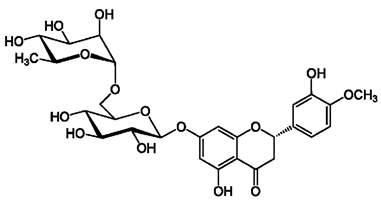
Not only coordination and absorption can occur in carbohydrates, but also in the classical coordination of ortho-oxygens of the B-ring for complexes of this kind [67].
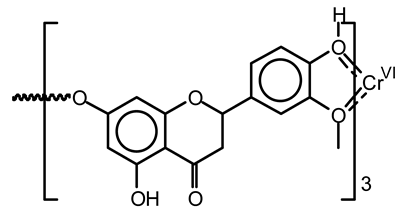
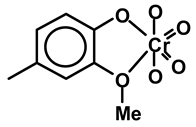
Moreover, different types of coordination complexes can form in lignin [79]. These are:
1. Covalently bonded CrO4= chromate esters, in which some CrO4= oxygens are covalently bonded to the aromatic nuclei of phenylpropopane units [80,81].
2. CrO4− coordination complexes with hydroxyl and methoxy groups of the aromatic nuclei of lignin phenylpropane [80,82]; hence, the same type of coordination complexes, as shown above for hesperidin, can occur. Such complexes can also occur with Cr (III), especially the Cr(III) produced by reduction from Cr(VI) by the oxidation of carbohydrates.
3. Ferrocene-type complexes, presenting structures of the type as shown, with the aromatic rings of lignin or any other aromatic rings present, may also occur [79].
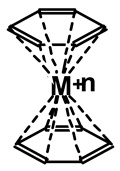
In the above, M is either CrO4=, hence Cr(VI), or Cr(III).
All these types of complexes have already been found and characterized for the adsorption of chromium on lignocellulosic material.
One can think that the citric acid present in orange peels can also react to form esters, but it definitely esterifies both carbohydrates and lignin during the drying of the orange peel, as already determined in the case of other applications of citric acid to lignocellulosic materials [83].
4. Conclusions
A preliminary study of the influence of some parameters on the biosorption process was conducted. The results of this study show that orange peel is efficient as a biosorbent and the adsorption process is fast, since the chemical equilibrium is achieved in a relatively short first time, i.e., from 5 to 15 min. The highest removal efficiency was 99.2%, and it was achieved in an acidic solution (pH = 2), thus confirming the validity of the theory of pHpzc and contact-free pH.
Moreover, an analytic study on the effects and mutual interactions of the operating parameters, such as the pH, the biosorbent dosage, the initial concentration of Cr(VI), and the contact time, was conducted through a full factorial experimental design. This study confirmed that the adsorption process mainly depended on the biosorbent dosage and the pH, whose optimum value was 2. Furthermore, the study of the effect of the biosorbent dosage was extremely useful to set the optimum value of the solid/liquid ratio (r) at 10 g/L as the dosage of the biosorbent. Under such conditions, the Cr(VI) removal efficiency was around 97% when the initial concentration of Cr(VI) was set at 50 mg/L. In addition, a kinetics study was also performed and the adsorption process was satisfactorily modeled by pseudo-second-order kinetics.
Finally, the experimental results were compared to the Freundlich, Langmuir, and Elovich isotherm models. The results demonstrate that the adsorption of Cr(VI) on the biosorbent are well described by the isotherm models of Langmuir and Freundlich. According to Langmuir’s dimensionless factor (RL), it can be concluded that the Cr(VI) adsorption process on the orange peels in their natural state is favorable (0 < RL < 1).
Author Contributions
Conceptualization, A.K., A.B., K.D., A.P. (Antonio Panico) and A.P. (Antonio Pizzi); methodology, A.B., A.K., A.P. (Antonio Panico), Z.S., A.H. and K.D.; formal analysis, A.P. (Antonio Panico); investigation, A.B. and A.P. (Antonio Panico); data curation, A.K., A.B., K.D. and A.P. (Antonio Pizzi); writing—original draft preparation, A.K., A.B., A.P. (Antonio Panico), Z.S., A.H., K.D. and A.P. (Antonio Pizzi); writing—review and editing, K.D., A.K., A.B. and A.P. (Antonio Pizzi); supervision, A.K., K.D. and A.P. (Antonio Pizzi); project administration, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data that support the findings of this study are available upon request from the corresponding author.
Acknowledgments
We warmly thank the University of Constantine 3 and the National Polytechnic of Constantine for their support in conducting the experimental part as well as the physicochemical analyses and the characterization of the bioadsorbent.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Achouri, O.; Bencheikh-Lehocine, M.; Panico, A.; Derbal, K.; Pirozzi, F. Effect of Chemical Coagulation Pretreatment on Anaerobic Digestion of Tannery Wastewater. J. Environ. Eng. 2019, 9, 1–5. [Google Scholar] [CrossRef]
- Priyadharshini, M.R.; Soundhirajan, K. Removal of Chromium (VI) from Textile Waste Water by Using Activated Carbon Prepared from Neem Leaves and Garlic Husk. Middle East J. Appl. Sci. Technol. 2021, 4, 19–28. [Google Scholar] [CrossRef]
- Phan, T.; Ullah, A.; Khatri, M.; Ngoc, N. A Review on the Fabrication of Several Carbohydrate Polymers into Nanofibrous Structures Using Electrospinning for Removal of Metal Ions and Dyes. Carbohydr. Polym. 2021, 252, 117175. [Google Scholar] [CrossRef]
- Selatnia, A.A.A.; Khodja, H.E.S.M.; Daoud, S.M.N. Biosorption of Hexavalent Chromium and Congo Red Dye onto Pleurotus Mutilus Biomass in Aqueous Solutions. Int. J. Environ. Sci. Technol. 2021, 19, 2477–2492. [Google Scholar] [CrossRef]
- Ghaedi, M.; Hossainian, H.; Montazerozohori, M.; Shokrollahi, A.; Shojaipour, F.; Soylak, M.; Purkait, M.K. A Novel Acorn Based Adsorbent for the Removal of Brilliant Green. Desalination 2011, 281, 226–233. [Google Scholar] [CrossRef]
- Nomanbhay, S.M. Removal of Heavy Metal from Industrial Wastewater Using Chitosan Coated Oil Palm Shell Charcoal Removal of Heavy Metal from Industrial Wastewater Using Chitosan Coated Oil Palm Shell Charcoal. Electron. J. Biotechnol. 2005, 8, 43–53. [Google Scholar] [CrossRef]
- Raji, C.; Anirudhan, T.S. Batch Cr(VI) Removal by Polyacrylamide-Grafted Sawdust: Kinetics and Thermodynamics. Water Res. 1998, 32, 3772–3780. [Google Scholar] [CrossRef]
- Richard, F.C.; Bourg, A.C.M. Aqueous Geochemistry of Chromium: A Review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- WHO. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; ISBN 978-92-4-154815-1. [Google Scholar]
- Jung, C.; Heo, J.; Han, J.; Her, N.; Lee, S.J.; Oh, J.; Ryu, J.; Yoon, Y. Hexavalent Chromium Removal by Various Adsorbents: Powdered Activated Carbon, Chitosan, and Single/Multi-Walled Carbon Nanotubes. Sep. Purif. Technol. 2013, 106, 63–71. [Google Scholar] [CrossRef]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Saha, R.; Nandi, R.; Saha, B. Sources and Toxicity of Hexavalent Chromium. J. Coord. Chem. 2011, 64, 1782–1806. [Google Scholar] [CrossRef]
- Oze, C.; Bird, D.K.; Fendorf, S. Genesis of Hexavalent Chromium from Natural Sources in Soil and Groundwater. Proc. Natl. Acad. Sci. USA 2007, 104, 6544–6549. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.R.; Zhang, G.; Fang, J.; Dou, X. Enhanced Chromium Recovery from Tanning Wastewater. J. Clean. Prod. 2006, 14, 75–79. [Google Scholar] [CrossRef]
- Li, M.; Jia, X.; Wang, J.; Wang, Y.; Chen, Y.; Wu, J.; Wang, Y.; Shen, M.; Xue, H. Quantitative Analysis of the Research Development Status and Trends of Tannery Wastewater Treatment Technology. Catalysts 2022, 12, 1317. [Google Scholar] [CrossRef]
- Hintermeyer, B.H.; Lacour, N.A.; Perez Padilla, A.; Tavani, E.L. Separation of the Chromium(III) Present in a Tanning Wastewater by Means of Precipitation, Reverse Osmosis and Adsorption. Lat. Am. Appl. Res. 2008, 38, 63–71. [Google Scholar]
- Wang, D.; He, S.; Shan, C.; Ye, Y.; Ma, H.; Zhang, X.; Zhang, W.; Pan, B. Chromium Speciation in Tannery Effluent after Alkaline Precipitation: Isolation and Characterization. J. Hazard. Mater. 2016, 316, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.; Abdulla, H.M.; Mohamed, A.H.; El-Bassuony, A.D. Remediation and Recycling of Chromium from Tannery Wastewater Using Combined Chemical–Biological Treatment System. Process Saf. Environ. Prot. 2016, 104, 1–10. [Google Scholar] [CrossRef]
- Abdulla, H.M.; Kamal, E.M.; Mohamed, A.H.; El-bassuony, A.D. Chromium Removal From Tannery Wastewater Using Chemical and Biological Techniques Aiming Zero Discharge of Pollution. In Proceeding of Fifth Scientific Environmental Conference, Zagazig, Egypt, 21–23 December 2010; pp. 171–183. [Google Scholar]
- Ifthikar, J.; Shahib, I.I.; Jiang, W.; Senthilnithy, R.; Elkhlifi, Z.; Wang, J.; Chen, Z. Review on Technologies for the Development of Effective and Practical Chromate Removal from Wastewaters. J. Environ. Chem. Eng. 2023, 11, 11. [Google Scholar] [CrossRef]
- Younas, F.; Younas, S.; Bibi, I.; Farooqi, Z.U.R.; Hameed, M.A.; Mohy-Ud-Din, W.; Shehzad, M.T.; Hussain, M.M.; Shakil, Q.; Shahid, M.; et al. A Critical Review on the Separation of Heavy Metal(Loid)s from the Contaminated Water Using Various Agricultural Wastes. Int. J. Phytoremediation 2023, 26, 349–368. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Rejeb, Z.B.; Zaoui, A.; Park, C.B. Sustainable and Efficient Technologies for Removal and Recovery of Toxic and Valuable Metals from Wastewater: Recent Progress, Challenges, and Future Perspectives. Chemosphere 2022, 292, 292. [Google Scholar] [CrossRef]
- Cavaco, S.A.; Fernandes, S.; Quina, M.M.; Ferreira, L.M. Removal of Chromium from Electroplating Industry Effluents by Ion Exchange Resins. J. Hazard. Mater. 2007, 144, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Kusku, O.; Rivas, B.L.; Urbano, B.F.; Arda, M.; Kabay, N.; Bryjak, M. A Comparative Study of Removal of Cr(VI) by Ion Exchange Resins Bearing Quaternary Ammonium Groups. J. Chem. Technol. Biotechnol. 2014, 89, 851–857. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Wang, C.; Ji, M. Cr(VI) Removal by a New Type of Anion Exchange Resin DEX-Cr: Adsorption Affecting Factors, Isotherms, Kinetics, and Desorption Regeneration. Environ. Prog. Sustain. Energy 2014, 33, 676–680. [Google Scholar] [CrossRef]
- Akoulih, M.; Tigani, S.; Saadane, R.; Tazi, A. Electrocoagulation Based Chromium Removal Efficiency Classification Using Logistic Regression. Appl. Sci. 2020, 10, 5179. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Othman, M.H.D.; Adam, M.R.; Liang, X.; Goh, H.; Anouzla, A.; Sillanpää, M.; Mohyuddin, A.; Chew, K.W. Chromium Removal from Aqueous Solution Using Natural Clinoptilolite. Water 2023, 15, 1667. [Google Scholar] [CrossRef]
- Golbaz, S.; Jafari, A.J.; Rafiee, M.; Kalantary, R.R. Separate and Simultaneous Removal of Phenol, Chromium, and Cyanide from Aqueous Solution by Coagulation/Precipitation: Mechanisms and Theory. Chem. Eng. J. 2014, 253, 251–257. [Google Scholar] [CrossRef]
- Dittert, I.M.; de Lima Brandão, H.; Pina, F.; da Silva, E.A.B.; De Souza, S.M.A.G.U.; de Souza, A.Ô.A.U.; Botelho, C.M.S.; Boaventura, R.A.R.; Vilar, V.J.P. Integrated Reduction/Oxidation Reactions and Sorption Processes for Cr(VI) Removal from Aqueous Solutions Using Laminaria Digitata Macro-Algae. Chem. Eng. J. 2014, 237, 443–454. [Google Scholar] [CrossRef]
- Arora, R. ScienceDirect Adsorption of Heavy Metals—A Review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Y.; Li, T. Adsorption and Desorption of Heavy Metals from Water Using Aminoethyl Reduced Graphene Oxide. Environ. Eng. Sci. 2018, 35, 978–987. [Google Scholar] [CrossRef]
- Gallouze, H.; Akretche, D.; Daniel, C.; Coelhoso, I. Removal of Synthetic Estrogen from Water by Adsorption on Modified Bentonites. Environ. Eng. Sci. 2021, 38, 4–14. [Google Scholar] [CrossRef]
- Bailey, S.E.; Trudy, J.O.; Bricka, R.M.; Adrian, D.D. A Review of Potentially Low-Cost Sorbents for Heavy Metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Mittal, A.; Kurup, L.; Gupta, V.K. Use of Waste Materials—Bottom Ash and De-Oiled Soya, as Potential Adsorbents for the Removal of Amaranth from Aqueous Solutions. J. Hazard. Mater. 2005, 117, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.K.; Rastogi, A.; Dwivedi, M.K. Process Development for the Removal of Zinc and Cadmium from Wastewater Using Slag—A Blast Furnace Waste Material. Sep. Sci. Technol. 1997, 32, 2883–2912. [Google Scholar] [CrossRef]
- Hadi, M.; Sanaei, D.; Ali, I.; Bhatnagar, A. Removal of Chromium (VI) from Aqueous Solution Using Treated Waste Newspaper as a Low-Cost Adsorbent: Kinetic Modeling and Isotherm Studies. J. Mol. Liq. 2016, 215, 671–679. [Google Scholar] [CrossRef]
- Al-Homaidan, A.A.; Al-Qahtani, H.S.; Al-Ghanayem, A.A.; Ameen, F.; Ibraheem, I.B.M. Potential Use of Green Algae as a Biosorbent for Hexavalent Chromium Removal from Aqueous Solutions. Saudi J. Biol. Sci. 2018, 25, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Amel, K.; Hassena, M.A.; Kerroum, D. Isotherm and Kinetics Study of Biosorption of Cationic Dye onto Banana Peel. Energy Procedia 2012, 19, 286–2952012. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Meniai, A.H. Application of Chemically Modified Orange Peels for Removal of Copper(II) from Aqueous Solutions. Theor. Found. Chem. Eng. 2012, 46, 732–739. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Bendjamaa, I.; Bensid, T.; Meniai, A.H.; Derbal, K. Effect of Calcination on Orange Peels Characteristics: Application of an Industrial Dye Adsorption. Chem. Eng. Trans. 2014, 38, 361–366. [Google Scholar] [CrossRef]
- Wibowo, N.; Setyadhi, L.; Wibowo, D.; Setiawan, J.; Ismadji, S. Adsorption of Benzene and Toluene from Aqueous Solutions onto Activated Carbon and Its Acid and Heat Treated Forms: Influence of Surface Chemistry on Adsorption. J. Hazard. Mater. 2007, 19, 237–242. [Google Scholar] [CrossRef]
- Laggoun, Z.; Khalfaoui, A.; Benalia, A.; Ghomrani, A.F.; Bouchareb, R.; Mahfouf, A.; Pizzi, A.; Panico, A.; Derbal, K. Applied Sciences Application of Response Surface Design for Optimization of Direct Red Dye Biosorption onto Cockleshells. Appl. Sci. 2023, 13, 12333. [Google Scholar] [CrossRef]
- Bencheikh, I.; Azoulay, K.; Mabrouki, J.; El Hajjaji, S.; Dahchour, A.; Moufti, A.; Dhiba, D. The Adsorptive Removal of MB Using Chemically Treated Artichoke Leaves: Parametric, Kinetic, Isotherm and Thermodynamic Study. Sci. Afr. 2020, 9, e00509. [Google Scholar] [CrossRef]
- Kolasniski, K.W. Zur Theorie Der Sogenannten Adsorption Geloster Stofe”, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Blanchard, G.; Maunaye, M.; Martin, G. Removal of Heavy Metals from Waters by Means of Natural Zeolites. Water Res. 1984, 18, 1501–1507. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surface of Glass”, Mica and Olatinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.E.; Saad, E.A.; El-khatib, A.M.; Soliman, M.A.; Allam, E.A. Green Solid Synthesis of Polyaniline-Silver Oxide Nanocomposite for the Adsorptive Removal of Ionic Divalent Species of Zn/Co and Their Radioactive Isotopes 65Zn/60Co. Environ. Sci. Pollut. Res. 2018, 25, 22120–22135. [Google Scholar] [CrossRef] [PubMed]
- Benalia, A. Extraction et Valorisation Des Produits Actifs Des Plantes Naturelles En Tant Que Bio Coagulants Utiles Dans l’amelioration de Qualite Des Eaux. Ph.D. Thesis, University of Constantine 3, El Khroub, Algeria, 2023. [Google Scholar]
- Shakoor, S.; Nasar, A. Utilization of Punica Granatum Peel as an Eco-Friendly Biosorbent for the Removal of Methylene Blue Dye from Aqueous Solution. J. Appl. Biotechnol. Bioeng. 2018, 5, 242–249. [Google Scholar] [CrossRef]
- Bouchareb, R.; Derbal, K.; Benalia, A. Optimization of Active Coagulant Agent Extraction Method from Moringa Oleifera Seeds for Municipal Wastewater Treatment. Water Sci. Technol. 2021, 84, 393–403. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Pizzi, A.; Medjahdi, G. The Use of Aloe Vera as Natural Coagulant in Algerian Drinking Water Treatment Plant. J. Renew. Mater. 2022, 10, 625–637. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K.; Khalfaoui, A.; Bouchareb, R.; Panico, A.; Gisonni, C.; Crispino, G.; Pirozzi, F.; Pizzi, A. Use of Aloe Vera as an Organic Coagulant for Improving Drinking Water Quality. Water 2021, 13, 2024–2039. [Google Scholar] [CrossRef]
- Benalia, A.; Derbal, K. Etude Expérimentale et Modélisation Du Processus de La Coagulation Floculation: Application Aux Eaux Destinée a La Consommation. Master’s Thesis, University of Constantine 3, El Khroub, Algeria, 2015. [Google Scholar]
- Khalfaoui, B.; Meniai, A.H.; Borja, R. Removal of Copper from Industrial Wastewater by Raw Charcoal Obtained from Reeds. J. Chem. Technol. Biotechnol. 1995, 64, 153–156. [Google Scholar] [CrossRef]
- Khalfaoui, A.; Khelifi, M.N.; Khelfaoui, A.; Benalia, A.; Derbal, K. The Adsorptive Removal of Bengal Rose by Artichoke Leaves: Optimization by Full Factorials Design. Water 2022, 14, 2251. [Google Scholar] [CrossRef]
- Mashkoor, F.; Nasar, A.; Inamuddin; Asiri, A.M. Exploring the Reusability of Synthetically Contaminated Wastewater Containing Crystal Violet Dye Using Tectona Grandis Sawdust as a Very Low-Cost Adsorbent. Sci. Rep. 2018, 8, 8314. [Google Scholar] [CrossRef]
- Saka, C. BET, TG-DTG, FT-IR, SEM, Iodine Number Analysis and Preparation of Activated Carbon from Acorn Shell by Chemical Activation with ZnCl2. J. Anal. Appl. Pyrolysis 2012, 95, 21–24. [Google Scholar] [CrossRef]
- Baatache, O.; Derbal, K.; Benalia, A.; Aberkane, I.; Guizah, Q.E.; Khalfaoui, A.; Pizzi, A. Valorization of Pine Cones (Pinus nigras) for Industrial Wastewater Treatment and Crystal Violet Removal: A Sustainable Approach Based on Bio-Coagulants and a Bio-Adsorbent. Water 2024, 16, 260. [Google Scholar] [CrossRef]
- Ali, A.; Saeed, K.; Mabood, F. Removal of Chromium (VI) from Aqueous Medium Using Chemically Modified Banana Peels as Efficient Low-Cost Adsorbent. Alex. Eng. J. 2016, 55, 2933–2942. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Saad, S.R.; Mohamed, R.H.A. Promoted Removal of Metformin Hydrochloride Anti-Diabetic Drug from Water by Fabricated and Modified Nanobiochar from Artichoke Leaves. Sustain. Chem. Pharm. 2020, 18, 100336. [Google Scholar] [CrossRef]
- Kennedy, L.J.; Vijaya, J.J.; Kayalvizhi, K.; Sekaran, G. Adsorption of Phenol from Aqueous Solutions Using Mesoporous Carbon Prepared by Two-Stage Process. Chem. Eng. J. 2007, 132, 279–287. [Google Scholar] [CrossRef]
- Aini Zakaria, H.; Wan Mansor, W.S.; Shahrin, N. Development of Water Treatment Sachets From the Seeds of Moringa Oleifera and Activated Carbon. MATTER Int. J. Sci. Technol. 2018, 3, 240–252. [Google Scholar] [CrossRef]
- Benalia, A.; Chaibraa, W.; Djeghar, S.; Derbal, K.; Khalfaoui, A.; Mahfouf, A.; Bouchareb, R.; Panico, A.; Pizzi, A. Use of Extracted Proteins from Oak Leaves as Bio-Coagulant for Water and Wastewater Treatment: Optimization by a Fractional Factorial Design. Water 2023, 15, 1984. [Google Scholar] [CrossRef]
- Benalia, A.; Baatache, O.; Derbal, K.; Khalfaoui, A. The Use of Central Composite Design (CCD) to Optimize and Model the Coagulation-Flocculation Process Using a Natural Coagulant: Application in Jar Test and Semi-Industrial Scale. J. Water Process Eng. 2024, 57, 104704. [Google Scholar] [CrossRef]
- Pizzi, A. Chromium Interactions in CCA/CCB Wood Preservatives Part I. Interactions with Wood Carbohydrates. Holzforschung 1990, 44, 373–380. [Google Scholar] [CrossRef]
- Braghiroli, F.L.; Amaral-Labat, G.; Boss, A.F.N.; Lacoste, C.; Pizzi, A. Tannin Gels and Their Carbon Derivatives: A Review. Biomolecules 2019, 9, 587. [Google Scholar] [CrossRef]
- Morales-Barrera, L.; Cristiani-Urbina, E. Hexavalent Chromium Removal by a Trichoderma Inhamatum Fungal Strain Isolated from Tannery Effluent. Water. Air. Soil Pollut. 2008, 187, 327–336. [Google Scholar] [CrossRef]
- Hossain, M.A.; Kumita, M.; Michigami, Y.; Mori, S. Optimization of Parameters for Cr(VI) Adsorption on Used Black Tea Leaves. Adsorption 2005, 11, 561–568. [Google Scholar] [CrossRef]
- Memon, J.R.; Memon, S.Q.; Bhanger, M.I.; Khuhawar, M.Y. Banana Peel: A Green and Economical Sorbent for Cr(III) Removal. Adsorption 2008, 11, 20–25. [Google Scholar]
- Ramakrishnaiah, C.R.; Prathima, B. Hexavalent Chromium Removal From Industrial Watsewater by Chemical Precipitation Method. Int. J. Eng. Res. Appl. 2012, 2, 599–603. [Google Scholar]
- Tran, H.N.; You, S.; Chao, H. Effect of Pyrolysis Temperatures and Times on the Adsorption of Cadmium onto Orange Peel Derived Biochar. Waste Manag. Res. 2016, 34, 129–138. [Google Scholar] [CrossRef]
- Sa, M. Adsorbent—Adsorbate Interactions in the Adsorption of Cd (II) and Hg (II) on Ozonized Activated Carbons. Environ. Sci. Technol. 2002, 36, 3850–3854. [Google Scholar] [CrossRef]
- Brito, F.; Ascanio, J.; Mateo, S.; Hernfindez, C.; Araujo, L.; Gili, P.; Martín-Zarza, P.; Domínguez, S.; Mederos, A. Equilibrium of Cr(VI) Species in Acid Medium and Adsorption Studies of These Species. Polyhedron 1997, 16, 3835–3846. [Google Scholar] [CrossRef]
- Gupta, B.V.B.S. Adsorption of Cr(VI) Using Activated Neem Leaves: Kinetic Studies. Adsorption 2008, 14, 85–92. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting Physisorption Data for Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Ali, R.; Naushad, M. Hexavalent Chromium Removal from Aqueous Medium by Activated Carbon Prepared from Peanut Shell: Adsorption Kinetics, Equilibrium and Thermodynamic Studies. Chem. Eng. J. 2012, 184, 238–247. [Google Scholar] [CrossRef]
- Reddad, Z. Methods of Removing Metal Ions by Adsorption on a Natural Polysaccharide-Experimental Study and Modeling. Ph.D. Thesis, National School of Industrial Techniques and Mines of Nantes, Nantes, France, 2002. [Google Scholar]
- Pizzi, A. Chromium Interactions in CCA/CCB Wood Preservatives. Part II. Interactions with Lignin. Holzforschung 1990, 44, 419–424. [Google Scholar] [CrossRef]
- Pizzi, A. Wood Waterproofing and Lignin Crosslinking by Means of Chromium Trioxide/Guajacyl Units Complexes. J. Appl. Polym. Sci. 1980, 25, 2547–2553. [Google Scholar] [CrossRef]
- Ostmeyer, J.G.; Elder, T.J.; Littrell, D.M.; Tatarchuk, B.J.; Winandy, J.E. Spectroscopic Analysis of Southern Pine Treated with Chromated Copper Arsenate. I. X-Ray Photoelectron Spectroscopy (Xps)-17. J. Wood Chem. Technol. 1988, 8, 413–439. [Google Scholar] [CrossRef]
- Pizzi, A. A New Approach to the Formulation and Application of CCA Preservatives. Wood Sci. Technol. 1983, 17, 303–319. [Google Scholar] [CrossRef]
- Del Menezzi, C.; Amirou, S.; Pizzi, A.; Xi, X.; Delmotte, L. Reactions with Wood Carbohydrates and Lignin of Citric Acid as a Bond Promoter of Wood Veneer Panels. Polymers 2018, 10, 833. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).