Development and Field Application of a Diffusive Gradients in Thin-Films Passive Sampler for Monitoring Three Polycyclic Aromatic Hydrocarbon Derivatives and One Polycyclic Aromatic Hydrocarbon in Waters
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Analysis
2.2. DGT Preparation
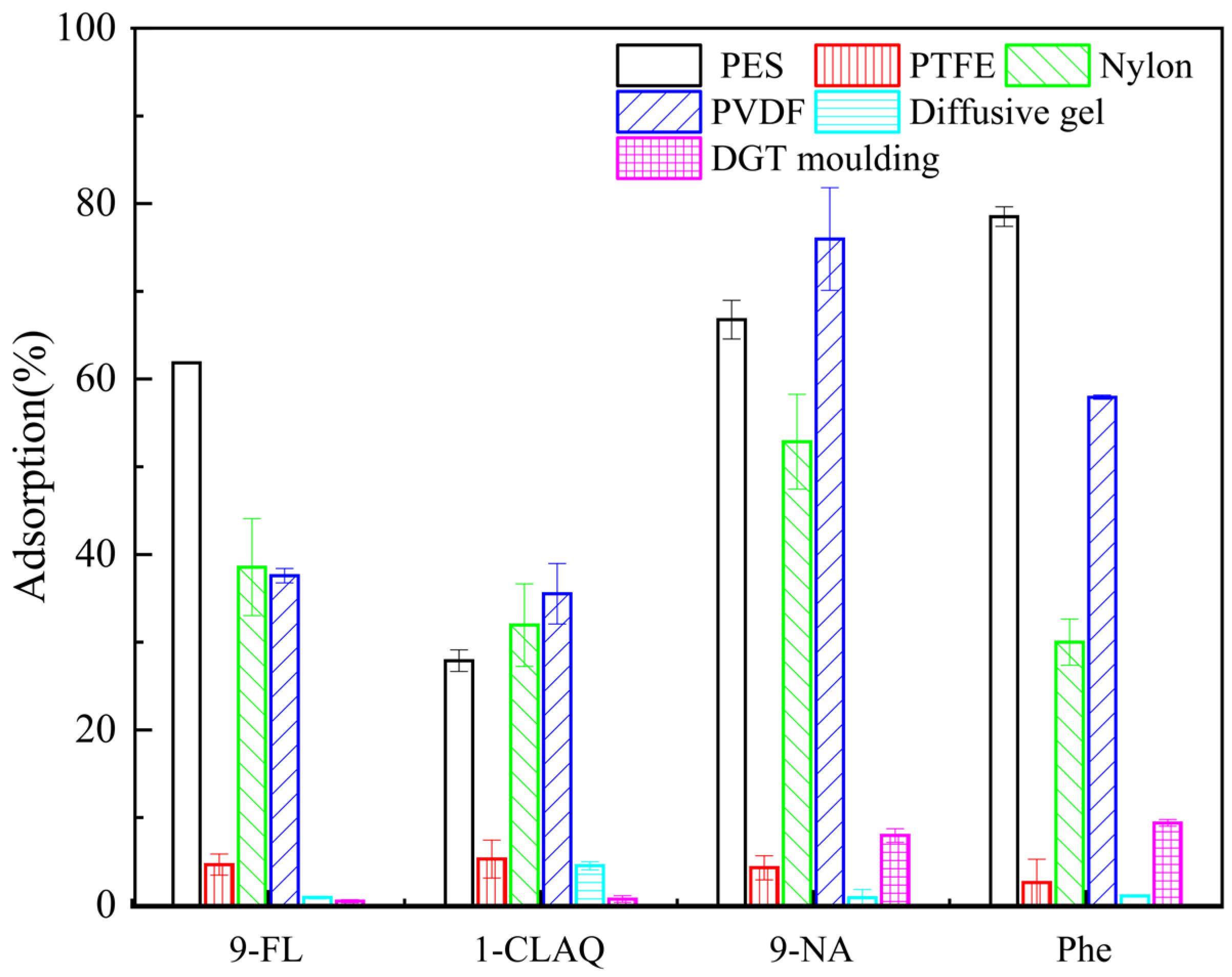
2.3. Characteristics of the Binding Gel
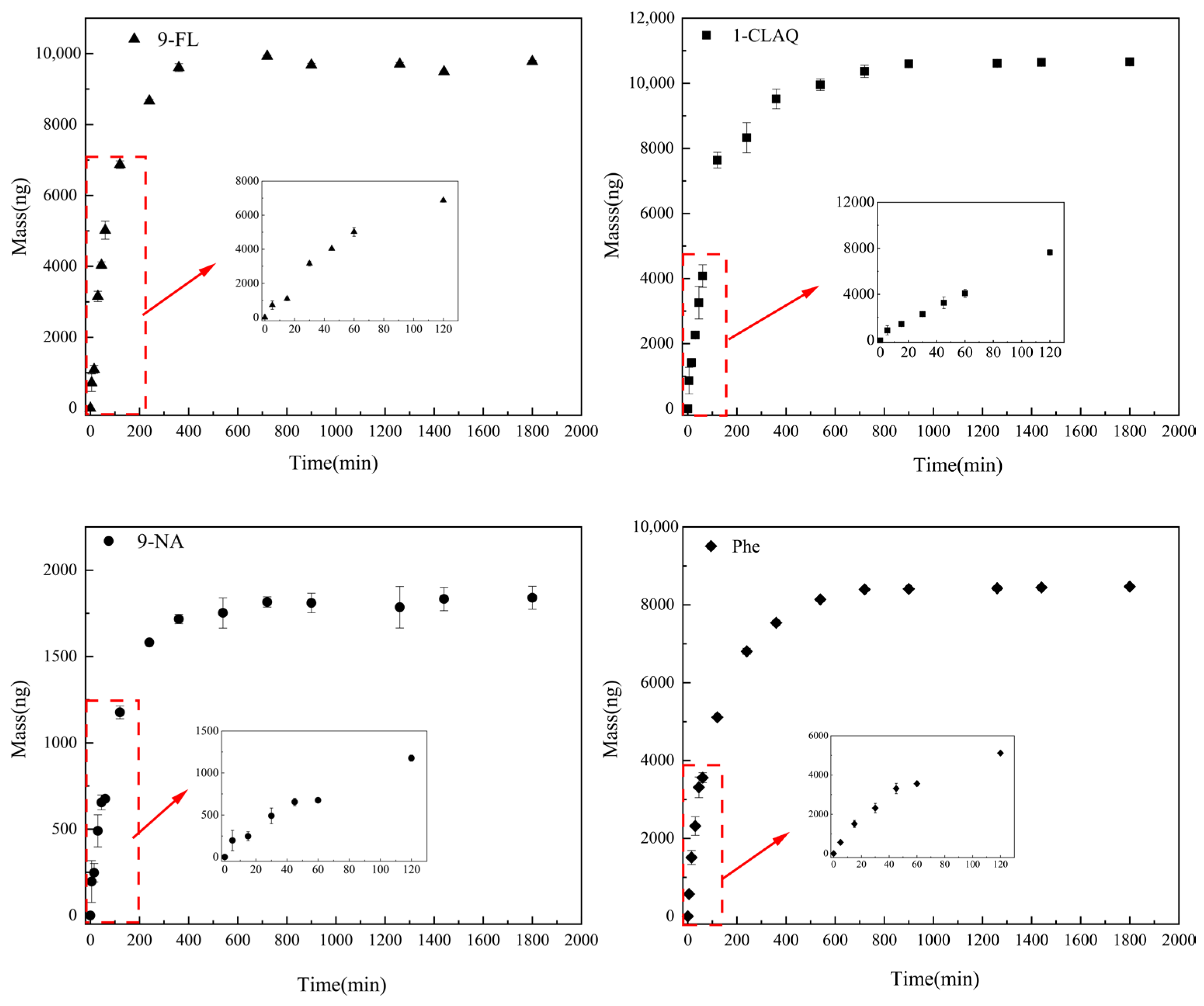
2.3.1. Adsorption Kinetics
2.3.2. Elution Efficiency
2.3.3. Adsorption Isotherm
2.4. Diffusion Coefficient Determination
2.5. DGT Performance Tests under Different Conditions
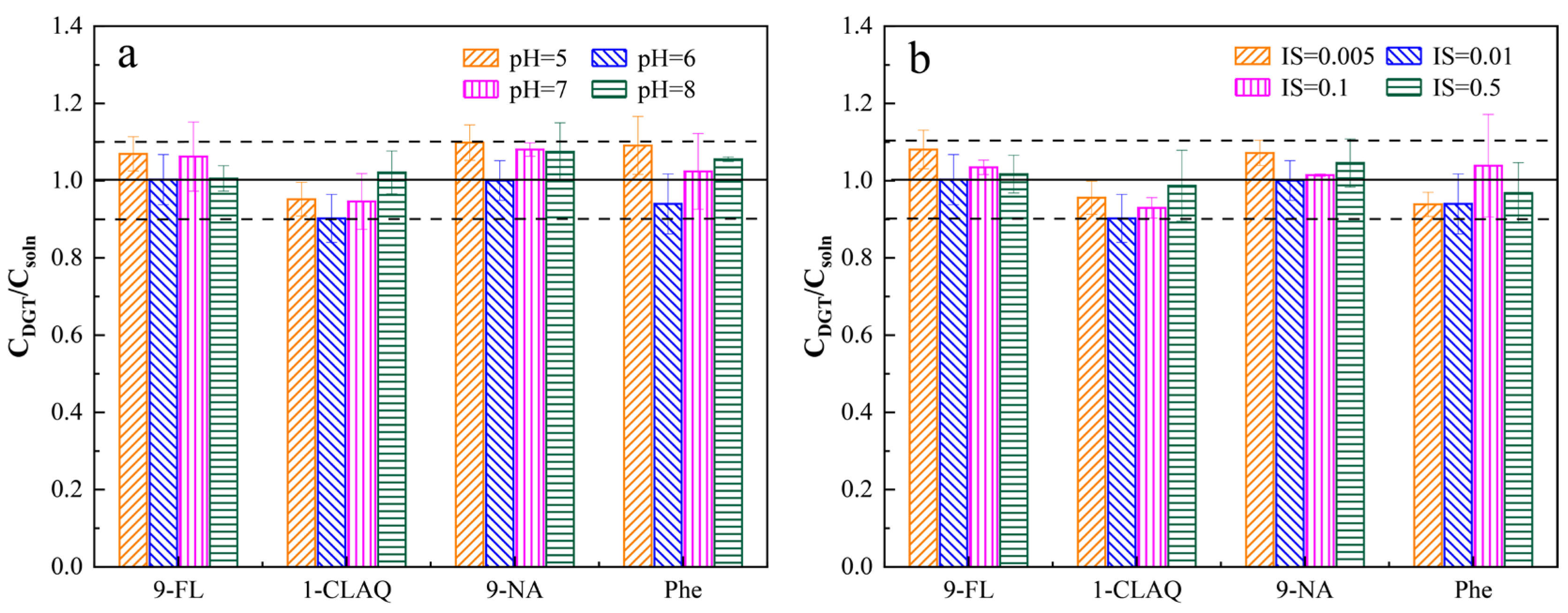
2.5.1. Effects of pH and IS
2.5.2. Influences of Diffusion Film Thickness and Deployment Time
2.5.3. Competition Effects and Aging Effects
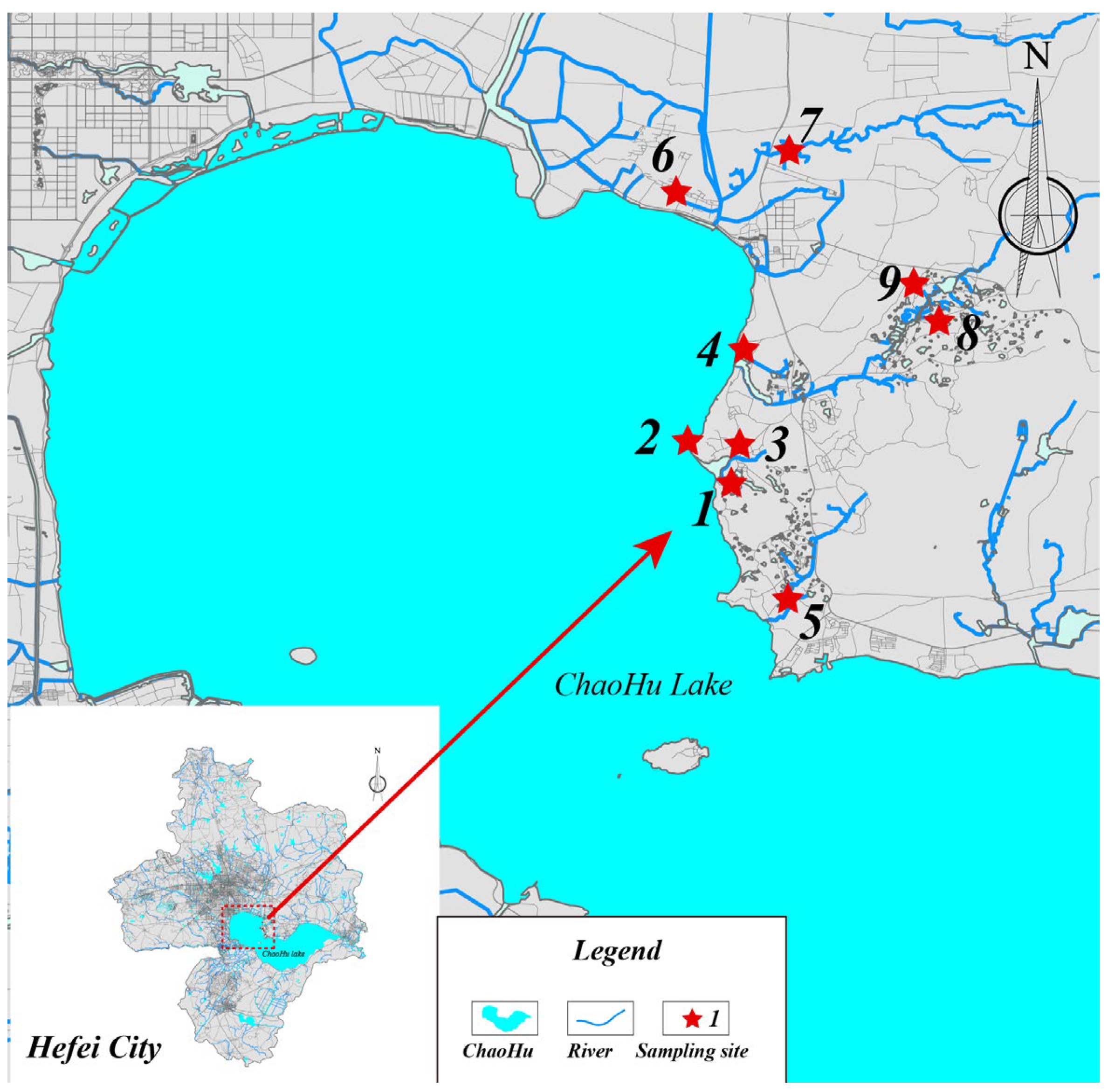
2.6. Field Trials
3. Results and Discussion
3.1. DGT Device Preparation
3.2. Characteristics of the Binding Gel
3.2.1. Adsorption Kinetics
3.2.2. Elution Efficiencies
3.2.3. Adsorption Isotherm
3.3. Diffusion Coefficient
3.4. Factors Affecting DGT Performance
3.4.1. Effects of pH and IS
3.4.2. Effects of Diffusion Film Thickness and Deployment Time
3.4.3. Competition of Compounds and Aging Effect of XAD18 Binding Gels
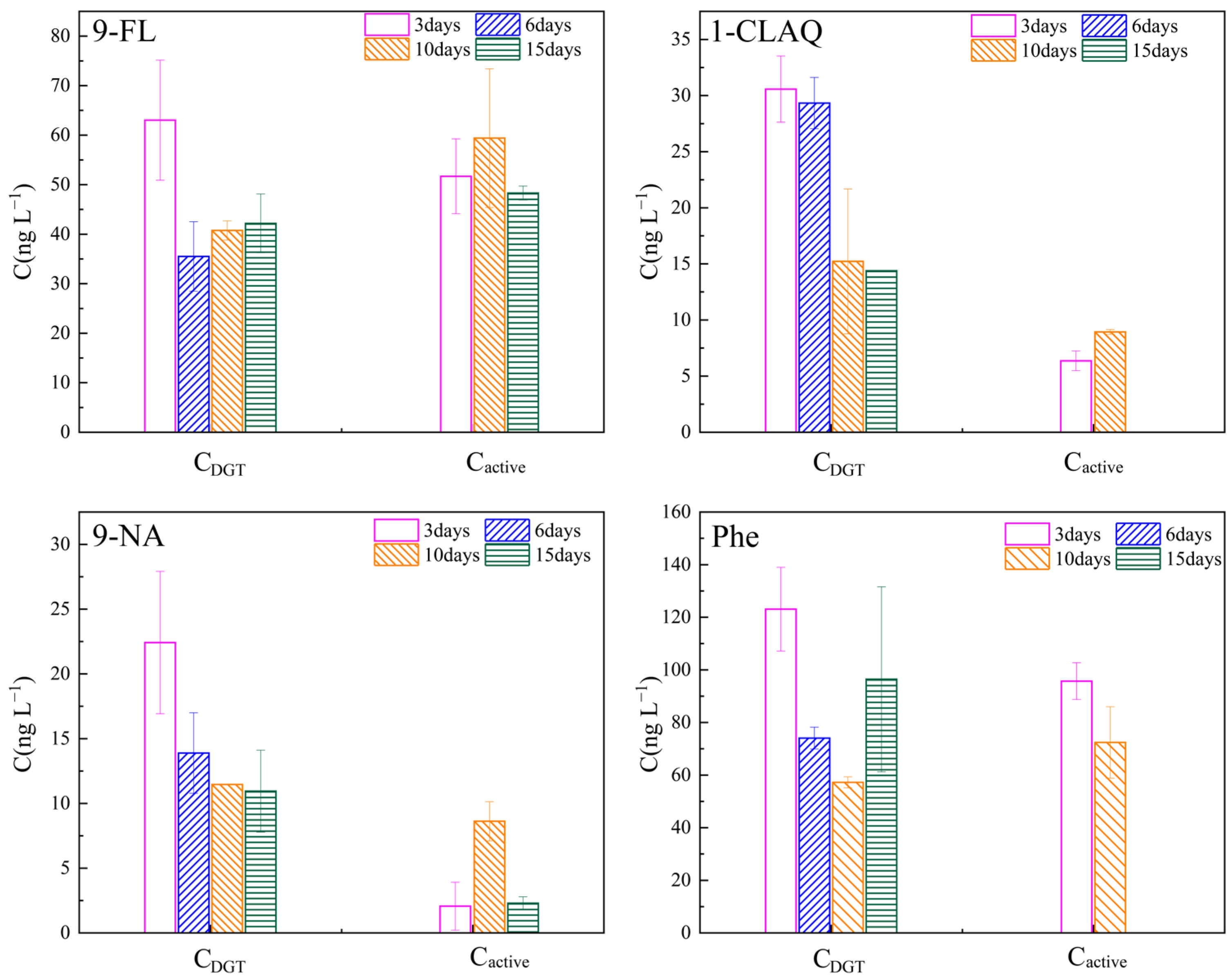
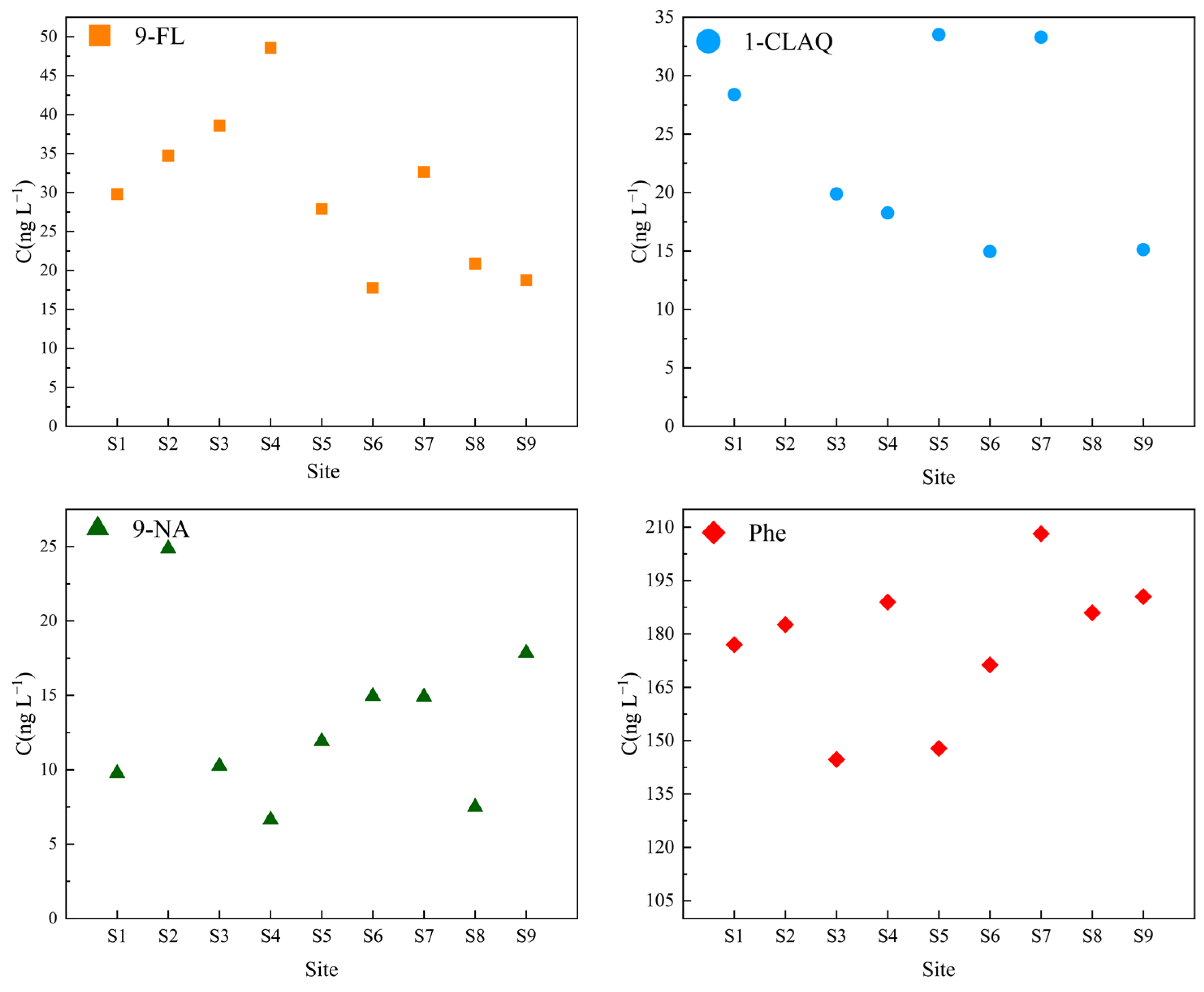
3.5. Field Evaluation and Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ross, J.A.; Nesnow, S. Polycyclic aromatic hydrocarbons: Correlations between DNA adducts and ras oncogene mutations. Mutat. Res. 1999, 424, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Saber, A.N.; Zhang, H.; Islam, A.; Yang, M. Occurrence, fates, and carcinogenic risks of substituted polycyclic aromatic hydrocarbons in two coking wastewater treatment systems. Sci. Total Environ. 2021, 789, 147808. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Dong, Q.; Li, F.; Wang, T.; Qiu, X.; Zhu, T. A Systematic Review of Polycyclic Aromatic Hydrocarbon Derivatives: Occurrences, Levels, Biotransformation, Exposure Biomarkers, and Toxicity. Environ. Sci. Technol. 2023, 57, 15314–15335. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Qi, W.; Liu, H.; Qu, J. Oxygenated, nitrated, methyl and parent polycyclic aromatic hydrocarbons in rivers of Haihe River System, China: Occurrence, possible formation, and source and fate in a water-shortage area. Sci. Total Environ. 2014, 481, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Qiao, M.; Fu, L.; Li, Z.; Liu, D.; Bai, Y.; Zhao, X. Distribution and ecological risk of substituted and parent polycyclic aromatic hydrocarbons in surface waters of the Bai, Chao, and Chaobai rivers in northern China. Environ. Pollut. 2020, 257, 113600. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, S.; White, P.A.; Lemieux, C.L.; Lynes, K.D.; Lambert, L.B.; Oberg, L.; Haglund, P.; Tysklind, M. Sources, fate, and toxic hazards of oxygenated polycyclic aromatic hydrocarbons (PAHs) at PAH-contaminated sites. Ambio 2007, 36, 475–485. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.L.; Busby, W.F., Jr.; Lafleur, A.L.; Penman, B.W.; Crespi, C.L. Human cell mutagenicity of oxygenated, nitrated and unsubstituted polycyclic aromatic hydrocarbons associated with urban aerosols. Mutat. Res. 1996, 371, 123–157. [Google Scholar] [CrossRef]
- Bandowe, B.A.M.; Meusel, H. Nitrated polycyclic aromatic hydrocarbons (nitro-PAHs) in the environment—A review. Sci. Total Environ. 2017, 581, 237–257. [Google Scholar] [CrossRef]
- Zhao, K.; Peng, G.; Wang, K.; Li, F. Distribution, sources, and health risk of polycyclic aromatic hydrocarbons and their derivatives in the watershed: The case of Yitong River, China. Environ. Sci. Pollut. Res. 2023, 30, 68536–68547. [Google Scholar] [CrossRef]
- Umbuzeiro, G.A.; Franco, A.; Martins, M.H.; Kummrow, F.; Carvalho, L.; Schmeiser, H.H.; Leykauf, J.; Stiborova, M.; Claxton, L.D. Mutagenicity and DNA adduct formation of PAH, nitro-PAH, and oxy-PAH fractions of atmospheric particulate matter from Sao Paulo, Brazil. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008, 652, 72–80. [Google Scholar] [CrossRef]
- Ohura, T.; Suhara, T.; Kamiya, Y.; Ikemori, F.; Kageyama, S.; Nakajima, D. Distributions and multiple sources of chlorinated polycyclic aromatic hydrocarbons in the air over Japan. Sci. Total Environ. 2019, 649, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Ohura, T.; Morita, M.; Makino, M.; Amagai, T.; Shimoi, K. Aryl hydrocarbon receptor-mediated effects of chlorinated polycyclic aromatic hydrocarbons. Chem. Res. Toxicol. 2007, 20, 1237–1241. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Khim, J.S.; Higley, E.B.; Giesy, J.P.; Ohura, T.; Kannan, K. Relative Potencies of Individual Chlorinated and Brominated Polycyclic Aromatic Hydrocarbons for Induction of Aryl Hydrocarbon Receptor-Mediated Responses. Environ. Sci. Technol. 2009, 43, 2159–2165. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Li, K.; Li, Y.; Zhang, H.; Jones, K.C.; Liu, X.; Liu, S.; Ma, L.Q.; Luo, J. Development and Application of the Diffusive Gradients in Thin-Films Technique for Measuring Psychiatric Pharmaceuticals in Natural Waters. Environ. Sci. Technol. 2019, 53, 11223–11231. [Google Scholar] [CrossRef] [PubMed]
- Amdany, R.; Chimuka, L.; Cukrowska, E. Determination of naproxen, ibuprofen and triclosan in wastewater using the polar organic chemical integrative sampler (POCIS): A laboratory calibration and field application. Water SA 2014, 40, 407–414. [Google Scholar] [CrossRef]
- Vrana, B.; Mills, G.A.; Allan, I.J.; Dominiak, E.; Svensson, K.; Knutsson, J.; Morrison, G.; Greenwood, R. Passive sampling techniques for monitoring pollutants in water. Trac-Trends Anal. Chem. 2005, 24, 845–868. [Google Scholar] [CrossRef]
- Zou, Y.-T.; Fang, Z.; Li, Y.; Wang, R.; Zhang, H.; Jones, K.C.; Cui, X.-Y.; Shi, X.-Y.; Yin, D.; Li, C.; et al. Novel Method for in Situ Monitoring of Organophosphorus Flame Retardants in Waters. Anal. Chem. 2018, 90, 10016–10023. [Google Scholar] [CrossRef]
- Zabiegala, B.; Kot-Wasik, A.; Urbanowicz, M.; Namiesnik, J. Passive sampling as a tool for obtaining reliable analytical information in environmental quality monitoring. Anal. Bioanal. Chem. 2010, 396, 273–296. [Google Scholar] [CrossRef]
- Namiesnik, J.; Zabiegala, B.; Kot-Wasik, A.; Partyka, M.; Wasik, A. Passive sampling and/or extraction techniques in environmental analysis: A review. Anal. Bioanal. Chem. 2005, 381, 279–301. [Google Scholar] [CrossRef]
- Lombard, N.J.; Bokare, M.; Harrison, R.; Yonkos, L.; Pinkney, A.; Murali, D.; Ghosh, U. Codeployment of Passive Samplers and Mussels Reveals Major Source of Ongoing PCB Inputs to the Anacostia River in Washington, DC. Environ. Sci. Technol. 2023, 57, 1320–1331. [Google Scholar] [CrossRef]
- Yao, Y.; Meng, X.-Z.; Wu, C.-C.; Bao, L.-J.; Wang, F.; Wu, F.-C.; Zeng, E.Y. Tracking human footprints in Antarctica through passive sampling of polycyclic aromatic hydrocarbons in inland lakes. Environ. Pollut. 2016, 213, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Vrana, B.; Klucarova, V.; Benicka, E.; Abou-Mrad, N.; Amdany, R.; Horakova, S.; Draxler, A.; Humer, F.; Gans, O. Passive sampling: An effective method for monitoring seasonal and spatial variability of dissolved hydrophobic organic contaminants and metals in the Danube river. Environ. Pollut. 2014, 184, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Booij, K.; van Bommel, R.; van Aken, H.M.; van Haren, H.; Brummer, G.-J.A.; Ridderinkhof, H. Passive sampling of nonpolar contaminants at three deep-ocean sites. Environ. Pollut. 2014, 195, 101–108. [Google Scholar] [CrossRef]
- Lohmann, R.; Muir, D. Global Aquatic Passive Sampling (AQUA-GAPS): Using Passive Samplers to Monitor POPs in the Waters of the World. Environ. Sci. Technol. 2010, 44, 860–864. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.-J.; Xu, S.-P.; Liang, Y.; Zeng, E.Y. Development of a low-density polyethylene-containing passive sampler for measuring dissolved hydrophobic organic compounds in open waters. Environ. Toxicol. Chem. 2012, 31, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davison, W. Performance Characteristics of Diffusion Gradients in Thin Films for the In Situ Measurement of Trace Metals in Aqueous Solution. Environ. Sci. 1995, 67, 3391–3400. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H. In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 1994, 367, 546–548. [Google Scholar] [CrossRef]
- Challis, J.K.; Stroski, K.M.; Luong, K.H.; Hanson, M.L.; Wong, C.S. Field Evaluation and in Situ Stress Testing of the Organic-Diffusive Gradients in Thin-Films Passive Sampler. Environ. Sci. Technol. 2018, 52, 12573–12582. [Google Scholar] [CrossRef]
- D’Angelo, E. Development and evaluation of a sensitive, Diffusive Gradients in Thin-Films (DGT) method for determining microcystin-LR concentrations in freshwater and seawater. Harmful Algae 2019, 89, 101668. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Guan, D.-X.; Luo, J.; Zhang, H.; Davison, W.; Cui, X.-Y.; Wang, L.-H.; Ma, L.Q. Activated Charcoal Based Diffusive Gradients in Thin Films for in Situ Monitoring of Bisphenols in Waters. Anal. Chem. 2015, 87, 801–807. [Google Scholar] [CrossRef]
- Davison, W.; Zhang, H. Progress in understanding the use of diffusive gradients in thin films (DGT)-back to basics. Environ. Chem. 2012, 9, 1–13. [Google Scholar] [CrossRef]
- Chen, W.; Li, Y.Y.; Chen, C.E.; Sweetman, A.J.; Zhang, H.; Jones, K.C. DGT Passive Sampling for Quantitative in Situ Measurements of Compounds from Household and Personal Care Products in Waters. Environ. Sci. Technol. 2017, 51, 13274–13281. [Google Scholar] [CrossRef]
- Qin, N.; He, W.; Liu, W.; Kong, X.; Xu, F.; Giesy, J.P. Tissue distribution, bioaccumulation, and carcinogenic risk of polycyclic aromatic hydrocarbons in aquatic organisms from Lake Chaohu, China. Sci. Total Environ. 2020, 749, 141577. [Google Scholar] [CrossRef]
- Qiao, M.; Qi, W.; Liu, H.; Qu, J. Simultaneous determination of typical substituted and parent polycyclic aromatic hydrocarbons in water and solid matrix by gas chromatography-mass spectrometry. J. Chromatogr. A 2013, 1291, 129–136. [Google Scholar] [CrossRef]
- Zeng, C.Y.; Xu, H.; Xu, Y.; You, S.H.; Wang, X.; Hu, Y.; Huang, S.E.; Bian, R.; Qi, W.X.; Lan, H.C.; et al. Distribution and health risk of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in surface water of the Yangtze River. Acta Sci. Circumstantiae 2021, 41, 4932–4941. [Google Scholar] [CrossRef]
| Compound | Mass of the Compound Loaded on the Binding Gels | ||||
|---|---|---|---|---|---|
| 450 ng | 900 ng | 4500 ng | Mean | S.D. | |
| 9-FL | 94.88 ± 3.08 | 103.21 ± 7.66 | 96.32 ± 1.60 | 98.14 | 4.45 |
| 1-CLAQ | 92.81 ± 4.59 | 90.54 ± 3.31 | 86.16 ± 1.67 | 89.84 | 3.38 |
| 9-NA | 93.08 ± 1.35 | 84.33 ± 4.12 | 83.81 ± 1.29 | 87.07 | 5.21 |
| Phe | 95.18 ± 6.85 | 99.04 ± 0.19 | 96.99 ± 1.35 | 97.07 | 1.93 |
| T (°C) | Compound | ||||
|---|---|---|---|---|---|
| 9-FL | 1-CLAQ | 9-NA | Phe | ||
| 25 °C | DDGT (cm2 s−1) | 5.30 × 10−6 | 4.51 × 10−6 | 4.03 × 10 | 3.34 × 10−6 |
| 20 °C | DDGT (cm2 s−1) | 4.47 × 10−6 | 3.72 × 10−6 | 3.36 × 10−6 | 2.67 × 10−6 |
| Deq (cm2 s−1) | 4.61 × 10−6 | 3.93 × 10−6 | 3.51 × 10−6 | 2.91 × 10−6 | |
| DDGT/Deq | 0.97 | 0.95 | 0.96 | 0.92 | |
| 28 °C | DDGT (cm2 s−1) | 5.68 × 10−6 | 4.98 × 10−6 | 4.13 × 10−6 | 3.74 × 10−6 |
| Deq (cm2 s−1) | 5.74 × 10−6 | 4.88 × 10−6 | 4.36 × 10−6 | 3.62 × 10−6 | |
| DDGT/Deq | 0.99 | 1.02 | 0.95 | 1.03 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, S.; Li, L.; Li, Y.; Wu, J.; Dou, Y. Development and Field Application of a Diffusive Gradients in Thin-Films Passive Sampler for Monitoring Three Polycyclic Aromatic Hydrocarbon Derivatives and One Polycyclic Aromatic Hydrocarbon in Waters. Water 2024, 16, 684. https://doi.org/10.3390/w16050684
Ren S, Li L, Li Y, Wu J, Dou Y. Development and Field Application of a Diffusive Gradients in Thin-Films Passive Sampler for Monitoring Three Polycyclic Aromatic Hydrocarbon Derivatives and One Polycyclic Aromatic Hydrocarbon in Waters. Water. 2024; 16(5):684. https://doi.org/10.3390/w16050684
Chicago/Turabian StyleRen, Shiyu, Liangshen Li, Yucheng Li, Juan Wu, and Yueqin Dou. 2024. "Development and Field Application of a Diffusive Gradients in Thin-Films Passive Sampler for Monitoring Three Polycyclic Aromatic Hydrocarbon Derivatives and One Polycyclic Aromatic Hydrocarbon in Waters" Water 16, no. 5: 684. https://doi.org/10.3390/w16050684
APA StyleRen, S., Li, L., Li, Y., Wu, J., & Dou, Y. (2024). Development and Field Application of a Diffusive Gradients in Thin-Films Passive Sampler for Monitoring Three Polycyclic Aromatic Hydrocarbon Derivatives and One Polycyclic Aromatic Hydrocarbon in Waters. Water, 16(5), 684. https://doi.org/10.3390/w16050684





