Aquifers and Groundwater: Challenges and Opportunities in Water Resource Management in Colombia
Abstract
1. Introduction
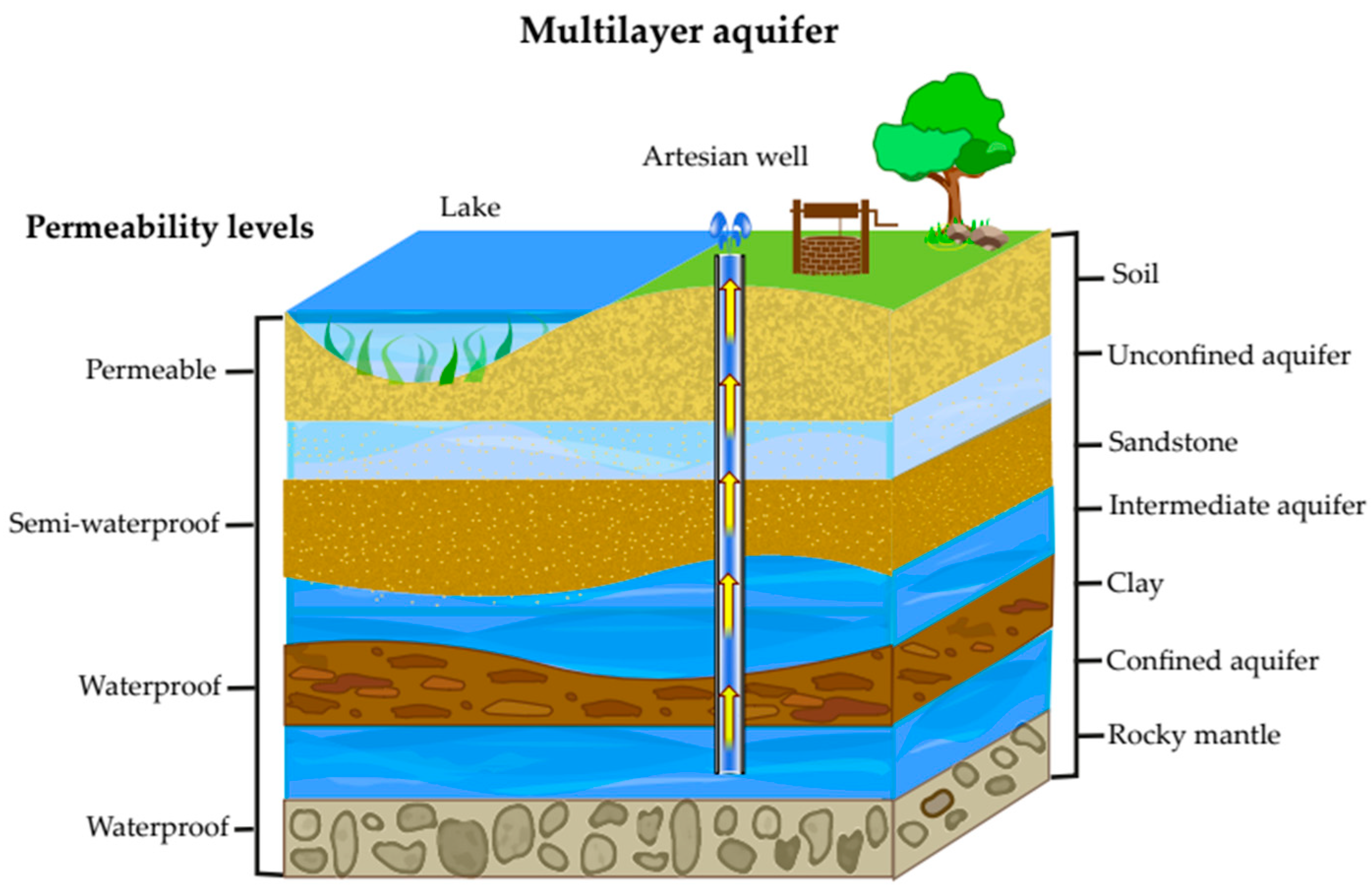
2. Aquifers
2.1. Types of Aquifers
2.1.1. Unconfined or Shallow Aquifers
2.1.2. Confined Aquifers
2.1.3. Intermediate, Semi-Confined, or Leaking Aquifers
3. Groundwater
3.1. Colombian Grounwater
3.2. Biological Propierties of Groundwater
3.3. Physicochemical Properties of Groundwater
3.4. Groundwater Contamination
4. Mineral–Medicinal Groundwater
5. Classification of Mineral Waters
5.1. Waters with More than One Gram per Liter of Mineralizing Substances
- Sulfated: sulfate anion predominates, and its therapeutic properties are strongly influenced by other ions such as sodium, magnesium, bicarbonate, and chloride [119].
- Chlorinated: the chloride ion is usually accompanied by sodium in a similar proportion. The composition of this type of water reflects a deep origin and the presence of past seas. The occurrence of faults and cracks facilitates its rise to the surface. They are subdivided into sources: (>50 g/L), medium (between 10 and 50 g/L) and weak (<10 g/L).
- Bicarbonated: the bicarbonate ion is accompanied by calcium, magnesium, sodium, chloride, and others. When these waters have a large amount of free acids (CO2, >250 mg/L), they are also called carbonated or carbogaseous [119].
5.2. Waters with Less Than One Gram per Liter of Mineralizing Substances
5.3. Waters with Special Components Recognized for Their Biological Activity in Certain Proportions
6. Uses Associated with the Consumption of Mineral Waters
7. Risks Associated with the Consumption of Mineral Waters
7.1. Chemical Contaminants
7.2. Microplastics
7.3. Pathogenic Biological Agents
8. Groundwater Exploration and Analysis
8.1. Extraction
8.2. Groundwater and Aquifer Legislation and Control
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Frenkel-Pinter, M.; Rajaei, V.; Glass, J.B.; Hud, N.V.; Williams, L.D. Water and Life: The Medium Is the Message. J. Mol. Evol. 2021, 89, 2–11. [Google Scholar] [CrossRef]
- Sawka, M.N.; Cheuvront, S.N.; Carter, R. Human Water Needs. Nutr. Rev. 2005, 63, S30–S39. [Google Scholar] [CrossRef] [PubMed]
- Margat, J.; van der Gun, J. Groundwater around the World: A Geographic Synopsis; Routledge: London, UK, 2013. [Google Scholar]
- Vélez, M.V.; Pimienta, C.O.; Vargas, M.C. Las Aguas Subterraneas Un Enfoque Practico; Ingeominas, Universidad Nacional de Colombia: Bogotá, Colombia, 2011. [Google Scholar]
- Senthil Kumar, P.; Yaashikaa, P.R. Introduction—Water. In Water in Textiles and Fashion: Consumption, Footprint, and Life Cycle Assessment; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Falkenmark, M.; Wang-Erlandsson, L.; Rockström, J. Understanding of Water Resilience in the Anthropocene. J. Hydrol. X 2019, 2, 100009. [Google Scholar] [CrossRef]
- Chamizo-Checa, S.; Otazo-Sánchez, E.; Gordillo-Martínez, A.; Suárez-Sánchez, J.; González-Ramírez, C.; Muñoz-Nava, H. Megacitywastewater Poured into a Nearby Basin: Looking for Sustainable Scenarios in a Case Study. Water 2020, 12, 824. [Google Scholar] [CrossRef]
- Macdonald, A.M.; Bell, R.A.; Kebede, S.; Azagegn, T.; Yehualaeshet, T.; Pichon, F.; Young, M.; McKenzie, A.A.; Lapworth, D.J.; Black, E.; et al. Groundwater and Resilience to Drought in the Ethiopian Highlands. Environ. Res. Lett. 2019, 14, 095003. [Google Scholar] [CrossRef]
- Lapworth, D.J.; Boving, T.B.; Kreamer, D.K.; Kebede, S.; Smedley, P.L. Groundwater Quality: Global Threats, Opportunities and Realising the Potential of Groundwater. Sci. Total Environ. 2022, 811, 152471. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Cadena, J.; Pavón, N.P.; Balvanera, P.; Sánchez-Rojas, G.; Razo-Zarate, R. Water Availability–Demand Balance under Climate Change Scenarios in an Overpopulated Region of Mexico. Int. J. Environ. Res. Public Health 2021, 18, 1846. [Google Scholar] [CrossRef] [PubMed]
- Guellouz, L.; Khayat, F. A Data Completion Method for Identifying Pollution Intrusion in Aquifers. Sci. Rep. 2022, 12, 16200. [Google Scholar] [CrossRef]
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- Viaroli, S.; Lancia, M.; Re, V. Microplastics Contamination of Groundwater: Current Evidence and Future Perspectives. A Review. Sci. Total Environ. 2022, 824, 153851. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, S.; Shi, J.; Ma, T.; Xie, X.; Deng, Y.; Du, Y.; Gan, Y.; Guo, Z.; Dong, Y.; et al. Groundwater Quality and Health: Making the Invisible Visible. Environ. Sci. Technol. 2023, 57, 5125–5136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, Y.; Kanyerere, T. A Review of the Managed Aquifer Recharge: Historical Development, Current Situation and Perspectives. Phys. Chem. Earth Parts A/B/C 2020, 118–119, 102887. [Google Scholar] [CrossRef]
- Singh, A. Groundwater Resources Management through the Applications of Simulation Modeling: A Review. Sci. Total Environ. 2014, 499, 414–423. [Google Scholar] [CrossRef]
- IDEAM. Estudio Nacional Del Agua 2018; Instituto de Hidrología, Meteorología y Estudios Ambientales: Bogotá, Colombia, 2019; ISBN 9789585489127.
- IDEAM; IGAC; IAvH; Invemar; Sinchi; IIAP. Ecosistemas Continentales, Costeros y Marinos de Colombia; Instituto Geográfico Agustín Codazzi: Bogotá, Colombia, 2007.
- IDEAM. Zonificación y Codificación de Uniades Hidrográficas e Hidrogeológicas de Colombia; Publicación aprobada por el Comité de Comunicaciones y Publicaciones del IDEAM; IDEAM: Bogotá, Colombia, 2013.
- Rodriquez, C.O.; Vargas, N.O.; Jaramillo, O.; Piñeros, A.; Cañas, H. Oferta y Uso de Agua Subterránea En Colombia. In Estudio Nacional Del Agua 2010; Instituto de Hidrología, Meteorología y Estudios Ambientales: Bogotá, Colombia, 2010. [Google Scholar]
- Betancur-Vargas, T.; García-Giraldo, D.A.; Vélez-Duque, A.J.; Gómez, A.M.; Flórez-Ayala, C.; Patiño, J.; Ortiz-Tamayo, J.Á. Aguas Subterráneas, Humedales y Servicios Ecosistémicos En Colombia. Biota Colomb. 2017, 18, 1–28. [Google Scholar] [CrossRef][Green Version]
- DNP. Bases Plan Nacional de Desarrollo 2022–2026; Departamento Nacional de Planeación: Bogota, Colombia, 2023.
- Griebler, C.; Avramov, M. Groundwater Ecosystem Services: A Review. Freshw. Sci. 2015, 34, 355–367. [Google Scholar] [CrossRef]
- Olorunfemi, M.O.; Fasuyi, S.A. Aquifer Types and the Geoelectric/Hydrogeologic Characteristics of Part of the Central Basement Terrain of Nigeria (Niger State). J. Afr. Earth Sci. (Middle East) 1993, 16, 309–317. [Google Scholar] [CrossRef]
- Maliva, R.G. Aquifer Characterization and Properties. In Aquifer Characterization Techniques; Springer Hydrogeology; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–24. [Google Scholar]
- Alvarez, R. Geophysical Determination of Buried Geological Structures and Their Influence on Aquifer Characteristics. Geoexploration 1991, 27, 1–24. [Google Scholar] [CrossRef]
- Maskey, S. Models of Groundwater (Saturated Zone) Flow. In Catchment Hydrological Modelling; Elsevier: Amsterdam, The Netherlands, 2022; pp. 101–126. [Google Scholar]
- Pearson, A.; Aitchison-Earl, P. Groundwater: Discovering Our Hidden Resource. Front. Young Minds 2022, 10, 723116. [Google Scholar] [CrossRef]
- Alley, W.M. Ground Water. In Encyclopedia of Inland Waters; Elsevier: Amsterdam, The Netherlands, 2009; pp. 684–690. [Google Scholar]
- Cerón, L.M.; Sarria, J.D.; Torres, J.S.; Soto-Paz, J. Agua Subterránea: Tendencias y Desarrollo Científico. Inf. Tecnol. 2021, 32, 47–56. [Google Scholar] [CrossRef]
- Saleem, Q.M.; Yousif, A. Assessment of Physico-Chemical and Biological Properties of Ground Water of Khulais, Province, Kingdom of Saudi Arabia. Int. J. Sci. Res. Methodol. 2016, 5, 504–521. [Google Scholar]
- IDEAM. Estudio Nacional Del Agua 2014; Instituto de Hidrología, Meteorología y Estudios Ambientales: Bogotá, Colombia, 2015; ISBN 9789588067704.
- IDEAM. Estudio Nacional Del Agua 2022; Instituto de Hidrología, Meteorología y Estudios Ambientales: Bogotá, Colombia, 2023.
- Ramírez, L.D.; Vargas, T.A. Evaluación De La Vulnerabilidad a La Contaminación Por Cuña Marina En Los Acuíferos De San Andrés (Colombia). Bol. Semillas Ambient. 2016, 10, 64–68. [Google Scholar]
- Betancur-Vargas, T.; Duque-Duque, J.C.; Martínez-Uribe, C.; Garcia-Giraldo, D.A.; Villegas-Yepes, P.P.; Paredes-Zuñiga, V. Delimitación de Las Potenciales Zonas de Recarga-Caso de Estudio: Acuífero Multicapa Del Eje Bananero Del Urabá Antioqueño-Colombia. Rev. Politéc. 2020, 16, 41–55. [Google Scholar] [CrossRef]
- Bolaños Chavarría, S.; Betancur Vargas, T. Estado Del Arte Sobre El Cambio Climático y Las Aguas Subterráneas. Ejemplos En Colombia. Rev. Politéc. 2018, 14, 52–64. [Google Scholar] [CrossRef][Green Version]
- Ballesteros, N.; Páez, L.; Luna, N.; Reina, A.; Urrea, V.; Sánchez, C.; Ramírez, A.; Ramirez, J.D.; Muñoz, M. Characterization of Microbial Communities in Seven Wetlands with Different Anthropogenic Burden Using Next Generation Sequencing in Bogotá, Colombia. Sci. Rep. 2023, 13, 16973. [Google Scholar] [CrossRef]
- Meixner, T.; Manning, A.H.; Stonestrom, D.A.; Allen, D.M.; Ajami, H.; Blasch, K.W.; Brookfield, A.E.; Castro, C.L.; Clark, J.F.; Gochis, D.J.; et al. Implications of Projected Climate Change for Groundwater Recharge in the Western United States. J. Hydrol. 2016, 534, 124–138. [Google Scholar] [CrossRef]
- Palacio, A.P.; Betancur, T. Identificación de Fuente y Zonas de Recarga a Un Sistema Acuifero Apartir de Isótopos Estables Del Agua. Caso de Estudio Bajo Cauca Antioqueño. Investigación 2007, 10, 167–181. [Google Scholar]
- Piña, A.; Donado, L.D.; Silva, L.; Pescador, J. Seasonal and Deep Groundwater-surface Water Interactions in the Tropical Middle Magdalena River Basin of Colombia. Hydrol. Process 2022, 36, e14764. [Google Scholar] [CrossRef]
- Patiño-Rojas, S.M.; Jaramillo, M.; Espinosa-Espinosa, C.; Arias-Lopez, M.F. Preferential Groundwater Flow Directions in a Pseudokarst System in Colombia, South America. J. S. Am. Earth Sci. 2021, 112, 103572. [Google Scholar] [CrossRef]
- Malagón, J.P.; Piña, A.; Argüello, S.; Donado, L.D. Análisis Hidrogeoquímico-Multivariado Del Agua Subterránea Del Sistema Acuífero Del Valle Medio Del Magdalena, Colombia: Estudio a Escala Regional. Bol. Soc. Geol. Mex. 2021, 73, A070421. [Google Scholar] [CrossRef]
- Villegas, P.; Paredes, V.; Betancur, T.; Ribeiro, L. Assessing the Hydrochemistry of the Urabá Aquifer, Colombia by Principal Component Analysis. J. Geochem. Explor. 2013, 134, 120–129. [Google Scholar] [CrossRef]
- Granit, I. Microgrids through the Energy-Water-Food Security Nexus in La Guajira, Colombia: Increasing Water and Food Security or Jeopardizing Groundwater Levels? Energy Res. Soc. Sci. 2022, 93, 102814. [Google Scholar] [CrossRef]
- Cortes, J.; Castro, A.; Arboleda, G.; Sepulveda, V.; Piragauta, N.; Higuera, O. Hydrogeological and Hydrogeochemical Evaluation of Groundwaters and Surface Waters in Potential Coalbed Methane Areas in Colombia. Int. J. Coal Geol. 2022, 253, 103937. [Google Scholar] [CrossRef]
- Betancur, T.; Palacio, C.; Gaviria, J.I.; Rueda, M. Methodological Proposal to Assess Groundwater Contamination Danger: Study Case of Bajo Cauca Aquifer (Colombia). Environ. Earth Sci. 2013, 70, 315–328. [Google Scholar] [CrossRef]
- Gómez-Niño, H.I. Análisis de Niveles Piezométricos y Patrones de Captación de Agua Subterránea En El Acuífero Cuaternario de Yopal, Casanare, Colombia. Bol. Geol. 2020, 42, 89–103. [Google Scholar] [CrossRef]
- Agudelo Moreno, L.J.; del Socorro Zuleta Lemus, D.; Lasso Rosero, J.; Agudelo Morales, D.M.; Sepúlveda Castaño, L.M.; Paredes Cuervo, D. Evaluation of Aquifer Contamination Risk in Urban Expansion Areas as a Tool for the Integrated Management of Groundwater Resources. Case: Coffee Growing Region, Colombia. Groundw. Sustain. Dev. 2020, 10, 100298. [Google Scholar] [CrossRef]
- Huerfano-Moreno, G.J.; Rojas-Peña, J.I.; Zapata-Muñoz, Y.L.; Trujillo-González, J.M.; Torres-Mora, M.A.; García-Navarro, F.J.; Jiménez-Ballesta, R. Comparative Assessment of the Quality and Potential Uses of Groundwater in a Typical Rural Settlement in Colombia. Water 2023, 15, 667. [Google Scholar] [CrossRef]
- Vence Márquez, L.; Rivera González, M.; Osorio Bayter, Y.; Castillo Sarabia, A.B. Caracterización Microbiológica y Fisicoquímica de Aguas Subterráneas de Los Municipios de La Paz y San Diego, Cesar, Colombia. Rev. Investig. Agrar. Ambient. 2012, 3, 27. [Google Scholar] [CrossRef][Green Version]
- González-Martínez, F.; Sánchez-Rodas, D.; Varela, N.M.; Sandoval, C.A.; Quiñones, L.A.; Johnson-Restrepo, B. As3MT and GST Polymorphisms Influencing Arsenic Metabolism in Human Exposure to Drinking Groundwater. Int. J. Mol. Sci. 2020, 21, 4832. [Google Scholar] [CrossRef] [PubMed]
- Ortegón, G.P.; Arboleda, F.M.; Candela, L.; Tamoh, K.; Valdes-Abellan, J. Vinasse Application to Sugar Cane Fields. Effect on the Unsaturated Zone and Groundwater at Valle Del Cauca (Colombia). Sci. Total Environ. 2016, 539, 410–419. [Google Scholar] [CrossRef]
- Moreno Méndez, J.O. Los retos del acceso a agua potable y saneamiento básico de las zonas rurales en colombia. Rev. Ing. 2020, 49, 28–37. [Google Scholar] [CrossRef]
- Calao-Ramos, C.R.; Marrugo Negrete, J.L.; Urango Cárdenas, I.; Díez, S. Genotoxicity and Mutagenicity in Blood and Drinking Water Induced by Arsenic in an Impacted Gold Mining Region in Colombia. Environ. Res. 2023, 233, 116229. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Alarcón, J.A.; Alfonso-Pérez, M.P.; Vergara-Gómez, I.; Díaz-Lagos, M.; Martínez-Ovalle, S.A. Removal of Iron and Manganese in Groundwater through Magnetotactic Bacteria. J. Environ. Manag. 2019, 249, 109381. [Google Scholar] [CrossRef]
- Chapelle, F. Ground-Water Microbiology and Geochemistry; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Bazzanti, M.; Della Bella, V.; Grezzi, F. Functional Characteristics of Macroinvertebrate Communities in Mediterranean Ponds (Central Italy): Influence of Water Permanence and Mesohabitat Type. Ann. Limnol.—Int. J. Limnol. 2009, 45, 29–39. [Google Scholar] [CrossRef]
- Lounaci, A.; Brosse, S.; Thomas, A.; Lek, S. Abundance, Diversity and Community Structure of Macroinvertebrates in an Algerian Stream: The Sébaou Wadi. Ann. Limnol.—Int. J. Limnol. 2000, 36, 123–133. [Google Scholar] [CrossRef]
- Bilandžija, H.; Hollifield, B.; Steck, M.; Meng, G.; Ng, M.; Koch, A.D.; Gračan, R.; Ćetković, H.; Porter, M.L.; Renner, K.J.; et al. Phenotypic Plasticity as a Mechanism of Cave Colonization and Adaptation. eLife 2020, 9, e51830. [Google Scholar] [CrossRef] [PubMed]
- Culver, D.C.; Pipan, T. The Biology of Caves and Other Subterranean Habitats; Oxford University Press: Oxford, UK, 2019; ISBN 9780198820765. [Google Scholar]
- Pipan, T.; Culver, D. Convergence and Divergence in the Subterranean Realm: A Reassessment. Biol. J. Linn. Soc. 2012, 107, 1–14. [Google Scholar] [CrossRef]
- Griebler, C.; Lueders, T. Microbial Biodiversity in Groundwater Ecosystems. Freshw. Biol. 2009, 54, 649–677. [Google Scholar] [CrossRef]
- Langwaldt, J.H.; Puhakka, J.A. On-Site Biological Remediation of Contaminated Groundwater: A Review. Environ. Pollut. 2000, 107, 187–197. [Google Scholar] [CrossRef]
- Nicholls, H.C.G.; Rolfe, S.A.; Mallinson, H.E.H.; Hjort, M.; Spence, M.J.; Bonte, M.; Thornton, S.F. Distribution of ETBE-Degrading Microorganisms and Functional Capability in Groundwater, and Implications for Characterising Aquifer ETBE Biodegradation Potential. Environ. Sci. Pollut. Res. 2022, 29, 1223–1238. [Google Scholar] [CrossRef]
- Melo, G.; Paiva, R. Crescimento Vegetativo de Cafeeiros Coffea Arabica Adubados Com Fontes de Fertilizantes Nitrogenados de Liberação Lenta e Controlada Em Tiros-MG. In Proceedings of the SBICafé, Pocos de Caldas, Brazil, 24 September 2017. [Google Scholar]
- Sherpa, R.T.; Reese, C.J.; Montazeri Aliabadi, H. Application of IChip to Grow “Uncultivable” Microorganisms and Its Impact on Antibiotic Discovery. J. Pharm. Pharm. Sci. 2015, 18, 303. [Google Scholar] [CrossRef]
- Otieno, F.A.O.; Olumuyiwa, I.O.; Ochieng, G.M. Groundwater: Characteristics, Qualities, Pollutions and Treatments: An Overview. Int. J. Water Resour. Environ. Eng. 2012, 4, 162–170. [Google Scholar] [CrossRef]
- Solís-Castro, Y.; Zúñiga-Zúñiga, L.A.; Mora-Alvarado, D. La Conductividad Como Parámetro Predictivo de La Dureza Del Agua En Pozos y Nacientes de Costa Rica. Rev. Tecnol. Marcha 2018, 31, 35. [Google Scholar] [CrossRef]
- Ram, A.; Tiwari, S.K.; Pandey, H.K.; Chaurasia, A.K.; Singh, S.; Singh, Y.V. Groundwater Quality Assessment Using Water Quality Index (WQI) under GIS Framework. Appl. Water Sci. 2021, 11, 46. [Google Scholar] [CrossRef]
- Medeiros, R.B.; Berezuk, A.G.; Pinto, A.L.; Silva, C.A. da Calidad de Las Aguas Superficiales En Sistemas Kársticos. Un Estudio de La Cuenca Hidrográfica Del Río Formoso, Bonito, Mato Grosso Do Sul—Brasil. Investig. Geogr. 2022, 78, 107–129. [Google Scholar] [CrossRef]
- Freeze, R.A.; Cherry, J.A. Chapter 9: Groundwater Contamination. In Groundwater; Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1979. [Google Scholar]
- Pandey, P.; Yadav, R. A Review on Volatile Organic Compounds (VOCs) as Environmental Pollutants: Fate and Distribution. Int. J. Plant Environ. 2018, 4, 14–26. [Google Scholar] [CrossRef]
- Peng, X.; Wang, C.; Zhang, K.; Wang, Z.; Huang, Q.; Yu, Y.; Ou, W. Profile and Behavior of Antiviral Drugs in Aquatic Environments of the Pearl River Delta, China. Sci. Total Environ. 2014, 466–467, 755–761. [Google Scholar] [CrossRef]
- Ullah, Z.; Rashid, A.; Ghani, J.; Nawab, J.; Zeng, X.-C.; Shah, M.; Alrefaei, A.F.; Kamel, M.; Aleya, L.; Abdel-Daim, M.M.; et al. Groundwater Contamination through Potentially Harmful Metals and Its Implications in Groundwater Management. Front. Environ. Sci. 2022, 10, 1021596. [Google Scholar] [CrossRef]
- Li, P. To Make the Water Safer. Expo. Health 2020, 12, 337–342. [Google Scholar] [CrossRef]
- Chakraborti, D.; Rahman, M.M.; Mukherjee, A.; Alauddin, M.; Hassan, M.; Dutta, R.N.; Pati, S.; Mukherjee, S.C.; Roy, S.; Quamruzzman, Q.; et al. Groundwater Arsenic Contamination in Bangladesh—21 Years of Research. J. Trace Elem. Med. Biol. 2015, 31, 237–248. [Google Scholar] [CrossRef]
- Al-Hashimi, O.; Hashim, K.; Loffill, E.; Marolt Čebašek, T.; Nakouti, I.; Faisal, A.A.H.; Al-Ansari, N. A Comprehensive Review for Groundwater Contamination and Remediation: Occurrence, Migration and Adsorption Modelling. Molecules 2021, 26, 5913. [Google Scholar] [CrossRef]
- Su, Z.; Wu, J.; He, X.; Elumalai, V. Temporal Changes of Groundwater Quality within the Groundwater Depression Cone and Prediction of Confined Groundwater Salinity Using Grey Markov Model in Yinchuan Area of Northwest China. Expo. Health 2020, 12, 447–468. [Google Scholar] [CrossRef]
- Hansen, B.; Thorling, L.; Schullehner, J.; Termansen, M.; Dalgaard, T. Groundwater Nitrate Response to Sustainable Nitrogen Management. Sci. Rep. 2017, 7, 8566. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wu, J. Hydrogeochemical Characteristics, Groundwater Quality, and Health Risks from Hexavalent Chromium and Nitrate in Groundwater of Huanhe Formation in Wuqi County, Northwest China. Expo. Health 2019, 11, 125–137. [Google Scholar] [CrossRef]
- Adimalla, N.; Wu, J. Groundwater Quality and Associated Health Risks in a Semi-Arid Region of South India: Implication to Sustainable Groundwater Management. Hum. Ecol. Risk Assess. Int. J. 2019, 25, 191–216. [Google Scholar] [CrossRef]
- Nawab, J.; Farooqi, S.; Xiaoping, W.; Khan, S.; Khan, A. Levels, Dietary Intake, and Health Risk of Potentially Toxic Metals in Vegetables, Fruits, and Cereal Crops in Pakistan. Environ. Sci. Pollut. Res. 2018, 25, 5558–5571. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Rashid, A.; Khattak, S.A.; Gao, X.; Jehan, S.; Javed, A. Geochemical Modeling, Fate Distribution, and Risk Exposure of Potentially Toxic Metals in the Surface Sediment of the Shyok Suture Zone, Northern Pakistan. Int. J. Sediment. Res. 2021, 36, 656–667. [Google Scholar] [CrossRef]
- Alsubih, M.; El Morabet, R.; Khan, R.A.; Khan, N.A.; ul Haq Khan, M.; Ahmed, S.; Qadir, A.; Changani, F. Occurrence and Health Risk Assessment of Arsenic and Heavy Metals in Groundwater of Three Industrial Areas in Delhi, India. Environ. Sci. Pollut. Res. 2021, 28, 63017–63031. [Google Scholar] [CrossRef]
- Nawab, J.; Khan, S.; Xiaoping, W. Ecological and Health Risk Assessment of Potentially Toxic Elements in the Major Rivers of Pakistan: General Population vs. Fishermen. Chemosphere 2018, 202, 154–164. [Google Scholar] [CrossRef]
- Koroša, A.; Mali, N. Control of Organic Contaminants in Groundwater by Passive Sampling and Multivariate Statistical Analysis. J. Environ. Manag. 2022, 318, 115440. [Google Scholar] [CrossRef]
- Manamsa, K.; Crane, E.; Stuart, M.; Talbot, J.; Lapworth, D.; Hart, A. A National-Scale Assessment of Micro-Organic Contaminants in Groundwater of England and Wales. Sci. Total Environ. 2016, 568, 712–726. [Google Scholar] [CrossRef]
- Nunes, S.; Tamura, B.M. A Historical Review of Mineral Water. Surg. Cosmet. Dermatol. 2012, 4, 252–258. [Google Scholar]
- Dupont, C.; Campagne, A.; Constant, F. Efficacy and Safety of a Magnesium Sulfate–Rich Natural Mineral Water for Patients with Functional Constipation. Clin. Gastroenterol. Hepatol. 2014, 12, 1280–1287. [Google Scholar] [CrossRef]
- Fornai, M.; Colucci, R.; Antonioli, L.; Ghisu, N.; Tuccori, M.; Gori, G.; Blandizzi, C.; Del Tacca, M. Effects of a Bicarbonate-Alkaline Mineral Water on Digestive Motility in Experimental Models of Functional and Inflammatory Gastrointestinal Disorders. Methods Find. Exp. Clin. Pharmacol. 2008, 30, 261. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, M.; Olivieri, F.; Manghetti, M.; Boccolini, E.; Bellomini, M.; Blandizzi, C.; Bonino, F.; Del Tacca, M. Effects of a Bicarbonate-Alkaline Mineral Water on Gastric Functions and Functional Dyspepsia: A Preclinical and Clinical Study. Pharmacol. Res. 2002, 46, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Bothe, G.; Coh, A.; Auinger, A. Efficacy and Safety of a Natural Mineral Water Rich in Magnesium and Sulphate for Bowel Function: A Double-Blind, Randomized, Placebo-Controlled Study. Eur. J. Nutr. 2017, 56, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Toxqui, L.; Pérez-Granados, A.M.; Blanco-Rojo, R.; Vaquero, M.P. A Sodium-Bicarbonated Mineral Water Reduces Gallbladder Emptying and Postprandial Lipaemia: A Randomised Four-Way Crossover Study. Eur. J. Nutr. 2012, 51, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Crespo, P.-V.; Campos, F.; Leal, M.; Maraver, F. Effects of Sodium Chloride-Rich Mineral Water on Intestinal Epithelium. Experimental Study. Int. J. Environ. Res. Public Health 2021, 18, 3261. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Turba, E.; Mari, C.; Lopilato, S.; Scalabrino, A.; Miglioli, M. Effect of a Mineral Water on Gastric Emptying of Patients with Idiopathic Dyspepsia. Int. J. Clin. Pharmacol. Res. 1999, 19, 53–56. [Google Scholar]
- Evandri, M.G.; Bolle, P. Pharmaco-Toxicological Screening of Commercially Available Italian Natural Mineral Waters. Il Farm. 2001, 56, 475–482. [Google Scholar] [CrossRef]
- Anti, M.; Lippi, M.E.; Santarelli, L.; Gabrielli, M.; Gasbarrini, A.; Gasbarrini, G. Effects of Mineral-Water Supplementation on Gastric Emptying of Solids in Patients with Functional Dyspepsia Assessed with the 13C-Octanoic-Acid Breath Test. Hepatogastroenterology 2004, 51, 1856–1859. [Google Scholar]
- Mennuni, G.; Petraccia, L.; Fontana, M.; Nocchi, S.; Stortini, E.; Romoli, M.; Esposito, E.; Priori, F.; Grassi, M.; Geraci, A.; et al. The Therapeutic Activity of Sulphate-Bicarbonate-Calcium-Magnesiac Mineral Water in the Functional Disorders of the Biliary Tract. Clin. Ter. 2014, 165, e346–e352. [Google Scholar] [PubMed]
- Salminen, L.; Chioralia, G.; Schreiber, S. Distribution of Intravenously Injected Dextrans in the Posterior Part of the Rat Eye. Bibl. Anat. 1977, 16, 69–72. [Google Scholar]
- Coiro, V.; Volpi, R.; Vescovi, P.P. Choleretic and Cholagogic Effect of Sulphuric Sulfate Water from the Springs of Tobiano in Cholestasis in Alcohol Related Liver Diseases. Clin. Ter. 1997, 148, 15–22. [Google Scholar]
- Aptel, I.; Cance-Rouzaud, A.; Grandjean, H. Association between Calcium Ingested from Drinking Water and Femoral Bone Density in Elderly Women: Evidence from the EPIDOS Cohort. J. Bone Miner. Res. 1999, 14, 829–833. [Google Scholar] [CrossRef]
- Pop, M.S.; Cheregi, D.C.; Onose, G.; Munteanu, C.; Popescu, C.; Rotariu, M.; Turnea, M.-A.; Dograru, G.; Ionescu, E.V.; Oprea, D.; et al. Exploring the Potential Benefits of Natural Calcium-Rich Mineral Waters for Health and Wellness: A Systematic Review. Nutrients 2023, 15, 3126. [Google Scholar] [CrossRef]
- Burckhardt, P. The Effect of the Alkali Load of Mineral Water on Bone Metabolism: Interventional Studies. J. Nutr. 2008, 138, 435S–437S. [Google Scholar] [CrossRef]
- Quattrini, S.; Pampaloni, B.; Brandi, M.L. Natural Mineral Waters: Chemical Characteristics and Health Effects. Clin. Cases Min. Bone Metab. 2016, 13, 173–180. [Google Scholar] [CrossRef]
- Aslanabadi, N.; Habibi Asl, B.; Bakhshalizadeh, B.; Ghaderi, F.; Nemati, M. Hypolipidemic Activity of a Natural Mineral Water Rich in Calcium, Magnesium, and Bicarbonate in Hyperlipidemic Adults. Adv. Pharm. Bull. 2014, 4, 303–307. [Google Scholar] [CrossRef]
- Rodgers, A.L. Effect of Mineral Water Containing Calcium and Magnesium on Calcium Oxalate Urolithiasis Risk Factors. Urol. Int. 1997, 58, 93–99. [Google Scholar] [CrossRef]
- Pereira, C.D.; Severo, M.; Araújo, J.R.; Guimarães, J.T.; Pestana, D.; Santos, A.; Ferreira, R.; Ascensão, A.; Magalhães, J.; Azevedo, I.; et al. Relevance of a Hypersaline Sodium-Rich Naturally Sparkling Mineral Water to the Protection against Metabolic Syndrome Induction in Fructose-Fed Sprague-Dawley Rats: A Biochemical, Metabolic, and Redox Approach. Int. J. Endocrinol. 2014, 2014, 384583. [Google Scholar] [CrossRef]
- Halksworth, G.; Moseley, L.; Carter, K.; Worwood, M. Iron Absorption from Spatone (a Natural Mineral Water) for Prevention of Iron Deficiency in Pregnancy. Clin. Lab. Haematol. 2003, 25, 227–231. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albertini, M.C.; Dacha, M.; Teodori, L.; Conti, M.E. Drinking Mineral Waters: Biochemical Effects and Health Implications the State-of-the-Art. Int. J. Environ. Health 2007, 1, 153. [Google Scholar] [CrossRef]
- Ferrier, C. Bottled Water: Understanding a Social Phenomenon. AMBIO J. Hum. Environ. 2001, 30, 118–119. [Google Scholar] [CrossRef]
- Corniello, A.; Guida, M.; Stellato, L.; Trifuoggi, M.; Carraturo, F.; Del Gaudio, E.; Del Giudice, C.; Forte, G.; Giarra, A.; Iorio, M.; et al. Hydrochemical, Isotopic and Microbiota Characterization of Telese Mineral Waters (Southern Italy). Environ. Geochem. Health 2022, 44, 1949–1970. [Google Scholar] [CrossRef]
- Azoulay, A.; Garzon, P.; Eisenberg, M.J. Comparison of the Mineral Content of Tap Water and Bottled Waters. J. Gen. Intern. Med. 2001, 16, 168–175. [Google Scholar] [CrossRef]
- Wynn, E.; Raetz, E.; Burckhardt, P. The Composition of Mineral Waters Sourced from Europe and North America in Respect to Bone Health: Composition of Mineral Water Optimal for Bone. Br. J. Nutr. 2008, 101, 1195–1199. [Google Scholar] [CrossRef]
- Benavides-Guerrero, C.E.; Caro-Caro, L.E.; Mariño-Martínez, J.E. Hacia La Elaboración de Un Modelo Hidrogeológico de La Cuenca Del Río Guachiría (Colombia). Bol. Geol. 2022, 44, 161–182. [Google Scholar] [CrossRef]
- Pineda-Escobar, M.A.; Falla Villa, P.L. Turismo Termal Como Opción de Turismo de Bienestar En Colombia: Un Estudio Exploratorio. Equidad Desarro. 2016, 27, 105–124. [Google Scholar] [CrossRef]
- Esmeral-Barros, C. Las Aguas Minerales Medicinales y su Proyección en el Caribe; Ediciones Corporación Educativa Mayor del Desarrollo Simón Bolívar: Barranquilla, Colombia, 2014. [Google Scholar]
- European Parliament Council of the European Union. Directive 2009/54/EC of the European Parliament and of the Council of 18 June 2009 on the Exploitation and Marketing of Natural Mineral Waters (Recast). Off. J. Eur. Union 2009, L164, 45–58. [Google Scholar]
- Eyzaguirre, F.M.; Castro, F.A.; Vázquez, I. Vademecum III de Aguas Mineromedicinales Españolas; Editorial Complutense, S.A.: Madrid, Spain, 2020. [Google Scholar]
- Fagundo-Castillo, J.; González-Hernández, P. Aguas Naturales, Minerales y Mineromedicinales; Universidad de La Habana: La Habana, Cuba, 2015. [Google Scholar]
- Aguirre Rodríguez, C.J.; Hernández Martínez, N. Actualización Del Médico de Familia En El Tratamiento Termal. Balneoterapia. SEMERGEN—Med. Fam. 2005, 31, 528–532. [Google Scholar] [CrossRef]
- UNESCO. Informe Mundial de Las Naciones Unidas Sobre El Desarrollo de Los Recursos Hídricos 2022: Aguas Subterráneas: Hacer Visible El Recurso Invisible; Datos y Cifras: Hermosillo, Mexico, 2022. [Google Scholar]
- Ore, M.T. Agua: Bien Común y Usos Privados. Riego, Estado y Conflictos En La Achirana Del Inca; Fondo Editorial de la Pontificia Universidad Católica del Perú: Lima, Peru, 2005; ISBN 9972427331. [Google Scholar]
- Murodov, O.U.; Teshayev, U.O.; Amrulloev, O.I.; Islomov, S.U. Determining the Efficiency of the Use of Underground Water in Irrigation of Tarik. Econ. Soc. 2021, 3-1, 187–191. [Google Scholar]
- da Silva Guerra, L.D.; Ribeiro, D.S. Contradições Econômicas Distributivas Entre o Agronegócio e a Agricultura Familiar No Contexto Do Sistema Capitalista Do Cenário (Trans)Pandêmico. JMPHC/J. Manag. Prim. Health Care 2023, 15, e030. [Google Scholar] [CrossRef]
- Alcântara de Assis, A.A.; Petter, C.O.; De Souza, J.C. Economic Viability of a Mineral Water Industry: A Risk Analysis Study. Tecnol. Metal. Mater. Min. 2019, 16, 421–425. [Google Scholar] [CrossRef]
- Wada, Y.; Bierkens, M.F.P. Sustainability of Global Water Use: Past Reconstruction and Future Projections. Environ. Res. Lett. 2014, 9, 104003. [Google Scholar] [CrossRef]
- Porta Mattiuzi, C.D.; Santos Fleischmann, A. Influência de Demandas Hídricas Agrícolas Na Resposta Da Água Subterrânea Na Bacia Do Rio Santa Maria/Rs. Águas Subterrâneas. Available online: https://aguassubterraneas.abas.org/asubterraneas/article/view/28708/18615 (accessed on 16 October 2023).
- Erfurt, P. An Assessment of the Role of Natural Hot and Mineral Springs in Health, Wellness and Recreational Tourism. Ph.D. Thesis, James Cook University, Cairns, Australia, 2011. [Google Scholar]
- Ribeiro Nunes, A. Minería de Agua Mineral: Calidad Para El Consumo Humano y Promoción de La Salud. Rev. Ibero-Am. Humanid. Ciênc. Educ. São Paulo 2022, 8, 341–358. [Google Scholar] [CrossRef]
- Rango, T.; Kravchenko, J.; Atlaw, B.; McCornick, P.G.; Jeuland, M.; Merola, B.; Vengosh, A. Groundwater Quality and Its Health Impact: An Assessment of Dental Fluorosis in Rural Inhabitants of the Main Ethiopian Rift. Environ. Int. 2012, 43, 37–47. [Google Scholar] [CrossRef]
- Mársico, D.P. Aportes a La Perspectiva Geológica e Hidrogeológica Regional En El Sector Centro Este de La Cuenca Chacopampeana; Universidade da Coruña: Coruña, España, 2013. [Google Scholar]
- Díaz, M. Caracterización Hidrogeológica e Hidrogeoquímica Del Extremo Occidental de La Sierra de Gádor y Acuíferos Cercanos; Universidad de Almería: Almería, Spain, 2016. [Google Scholar]
- Celebertti López, D.A.; Castro Morales, Y.A. Caracterización de Sistemas de Producción Bovina Doble Propósito Y de La Producción de Leche Con Enfoque de Género En Matiguas, Matagalpa, Nicaragua 2014; Universidad Nacional Agraria: Managua, Nicaragua, 2018. [Google Scholar]
- Neira, S.P.; Meza, P.A.; Neira, S.P.; Meza, P.A. Aguas de Contacto, Efectos En La Minería y El Medioambiente. Rev. Fac. Derecho 2021, 50, 20215006. [Google Scholar] [CrossRef]
- Saucedo Coley, M.A.; Ríos Reyes, C.A.; Díaz Ramírez, G.A. View of Efecto de Relaves Mineros, Cáscaras de Yuca y Desechos de Cáscara de Arroz En El Desempeño y Durabilidad de Los Ladrillos Cocidos. BISTUA Rev. Fac. Cienc. Básicas 2023, 21, 41. [Google Scholar]
- Toxqui, L.; Vaquero, M. An Intervention with Mineral Water Decreases Cardiometabolic Risk Biomarkers. A Crossover, Randomised, Controlled Trial with Two Mineral Waters in Moderately Hypercholesterolaemic Adults. Nutrients 2016, 8, 400. [Google Scholar] [CrossRef]
- Dupont, C.; Constant, F.; Imbert, A.; Hébert, G.; Zourabichvili, O.; Kapel, N. Time to Treatment Response of a Magnesium- and Sulphate-Rich Natural Mineral Water in Functional Constipation. Nutrition 2019, 65, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, Y.; Khaneghah, A.M.; Hadiani, M.R.; Keramati, H.; Pouya, R.H.; Moradi, B.; da Silva, B.S. Non-Carcinogenic Risk Assessment Induced by Heavy Metals Content of the Bottled Water in Iran. Toxin Rev. 2017, 36, 313–321. [Google Scholar] [CrossRef]
- Bosch, A.C.; O’Neill, B.; Sigge, G.O.; Kerwath, S.E.; Hoffman, L.C. Heavy Metals in Marine Fish Meat and Consumer Health: A Review. J. Sci. Food Agric. 2016, 96, 32–48. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, Mechanism and Health Effects of Some Heavy Metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef]
- Guo, H.; Wen, D.; Liu, Z.; Jia, Y.; Guo, Q. A Review of High Arsenic Groundwater in Mainland and Taiwan, China: Distribution, Characteristics and Geochemical Processes. Appl. Geochem. 2014, 41, 196–217. [Google Scholar] [CrossRef]
- Chakraborti, D.; Mukherjee, S.C.; Pati, S.; Sengupta, M.K.; Rahman, M.M.; Chowdhury, U.K.; Lodh, D.; Chanda, C.R.; Chakraborti, A.K.; Basu, G.K. Arsenic Groundwater Contamination in Middle Ganga Plain, Bihar, India: A Future Danger? Environ. Health Perspect. 2003, 111, 1194–1201. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamauchi, H.; Sun, G.F. Chronic Health Effects in People Exposed to Arsenic via the Drinking Water: Dose-Response Relationships in Review. Toxicol. Appl. Pharmacol. 2004, 198, 243–252. [Google Scholar] [CrossRef]
- Tapio, S.; Grosche, B. Arsenic in the Aetiology of Cancer. Mutat. Res./Rev. Mutat. Res. 2006, 612, 215–246. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of Lead: A Review with Recent Updates. Interdiscip. Toxicol. 2012, 5, 47–58. [Google Scholar] [CrossRef]
- Fagerberg, B.; Borné, Y.; Barregard, L.; Sallsten, G.; Forsgard, N.; Hedblad, B.; Persson, M.; Engström, G. Cadmium Exposure Is Associated with Soluble Urokinase Plasminogen Activator Receptor, a Circulating Marker of Inflammation and Future Cardiovascular Disease. Environ. Res. 2017, 152, 185–191. [Google Scholar] [CrossRef]
- Kobayashi, E.; Suwazono, Y.; Dochi, M.; Honda, R.; Kido, T. Influence of Consumption of Cadmium-Polluted Rice or Jinzu River Water on Occurrence of Renal Tubular Dysfunction and/or Itai-Itai Disease. Biol. Trace Elem. Res. 2009, 127, 257–268. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The Essential Toxin: Impact of Zinc on Human Health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Koedrith, P.; Seo, Y.R. Advances in Carcinogenic Metal Toxicity and Potential Molecular Markers. Int. J. Mol. Sci. 2011, 12, 9576–9595. [Google Scholar] [CrossRef]
- Marmol, F. Lithium: Bipolar Disorder and Neurodegenerative Diseases Possible Cellular Mechanisms of the Therapeutic Effects of Lithium. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1761–1771. [Google Scholar] [CrossRef]
- Neves, M.O.; Marques, J.; Eggenkamp, H.G.M. Lithium in Portuguese Bottled Natural Mineral Waters—Potential for Health Benefits? Int. J. Environ. Res. Public Health 2020, 17, 8369. [Google Scholar] [CrossRef]
- Bellinger, D.C. Lithium in Drinking Water—A Novel Environmental Risk Factor for Autism Spectrum Disorder? JAMA Pediatr. 2023, 177, 563. [Google Scholar] [CrossRef]
- Subba Rao, N.; Sunitha, B.; Sun, L.; Deepthi Spandana, B.; Chaudhary, M. Mechanisms Controlling Groundwater Chemistry and Assessment of Potential Health Risk: A Case Study from South India. Geochemistry 2020, 80, 125568. [Google Scholar] [CrossRef]
- Ghosh, P.; Roy, B.G.; Mukhopadhyay, S.K.; Banerjee, P. Recognition of Fluoride Anions at Low Ppm Level inside Living Cells and from Fluorosis Affected Tooth and Saliva Samples. RSC Adv. 2015, 5, 27387–27392. [Google Scholar] [CrossRef]
- Thapa, R.; Gupta, S.; Gupta, A.; Reddy, D.V.; Kaur, H. Geochemical and Geostatistical Appraisal of Fluoride Contamination: An Insight into the Quaternary Aquifer. Sci. Total Environ. 2018, 640–641, 406–418. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Wang, C.; Dang, X. The Distribution Characteristics and Human Health Risks of High- Fluorine Groundwater in Coastal Plain: A Case Study in Southern Laizhou Bay, China. Front. Environ. Sci. 2022, 10, 901637. [Google Scholar] [CrossRef]
- Ward, M.; Jones, R.; Brender, J.; de Kok, T.; Weyer, P.; Nolan, B.; Villanueva, C.; van Breda, S. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef]
- Khant, N.A.; Kim, H. Review of Current Issues and Management Strategies of Microplastics in Groundwater Environments. Water 2022, 14, 1020. [Google Scholar] [CrossRef]
- Da Silva, V.H.; Murphy, F.; Amigo, J.M.; Stedmon, C.; Strand, J. Classification and Quantification of Microplastics (<100 Μm) Using a Focal Plane Array-Fourier Transform Infrared Imaging System and Machine Learning. Anal. Chem. 2020, 92, 13724–13733. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-Distance Atmospheric Transport of Microplastic Fibres Influenced by Their Shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Singh, S.; Bhagwat, A. Microplastics: A Potential Threat to Groundwater Resources. Groundw. Sustain. Dev. 2022, 19, 100852. [Google Scholar] [CrossRef]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental Exposure to Microplastics: An Overview on Possible Human Health Effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Allen, D.; Allen, S.; Abbasi, S.; Baker, A.; Bergmann, M.; Brahney, J.; Butler, T.; Duce, R.A.; Eckhardt, S.; Evangeliou, N.; et al. Microplastics and Nanoplastics in the Marine-Atmosphere Environment. Nat. Rev. Earth Environ. 2022, 3, 393–405. [Google Scholar] [CrossRef]
- Gastañadui Yica, C.F.; Luján Rojas, J.M.; Llaque Fernández, G.I.; Valderrama Puscan, M.W. Análisis Comparativo de La Normativa Sobre Microplásticos En Perú y Europa, En El Periodo 2005–2020. In Proceedings of the 1st LACCEI International Multi-Conference on Entrepreneurship, Innovation, and Regional Development: “Ideas to Overcome and Emerge from the Pandemic Crisis”, Virtual, 9–10 December 2021; Latin American and Caribbean Consortium of Engineering Institutions: Boca Raton, FL, USA, 2021. [Google Scholar]
- Jin, Y.; Lu, L.; Tu, W.; Luo, T.; Fu, Z. Impacts of Polystyrene Microplastic on the Gut Barrier, Microbiota and Metabolism of Mice. Sci. Total Environ. 2019, 649, 308–317. [Google Scholar] [CrossRef]
- Zektser, I.S.; Everett, L.G. Groundwater Resources of the World and Their Use; United Nations: Paris, France, 2004. [Google Scholar]
- Olalemi, A.O.; Ige, O.M.; James, G.A.; Obasoro, F.I.; Okoko, F.O.; Ogunleye, C.O. Detection of Enteric Bacteria in Two Groundwater Sources and Associated Microbial Health Risks. J. Water Health 2021, 19, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.M.; Parveen, S.; Portier, K.M.; Rose, J.B.; Tamplin, M.L.; Farrah, S.R.; Koo, A.; Lukasik, J. Geographical Variation in Ribotype Profiles of Escherichia coli Isolates from Humans, Swine, Poultry, Beef, and Dairy Cattle in Florida. Appl. Environ. Microbiol. 2003, 69, 1089–1092. [Google Scholar] [CrossRef] [PubMed]
- Totaro, M.; Casini, B.; Valentini, P.; Miccoli, M.; Lopalco, P.L.; Baggiani, A. Identification of Microbial Indicators and Free Living Protozoa in Natural Mineral Water Using Cultural and Molecular Methods. Preprints 2017, 2017080007. [Google Scholar] [CrossRef][Green Version]
- Grabow, W.O.K. Waterborne Diseases: Update on Water Quality Assessment and Control. Water SA 1996, 22, 193–202. [Google Scholar]
- Kim, S.-H.; Cheon, D.-S.; Kim, J.-H.; Lee, D.-H.; Jheong, W.; Heo, Y.-J.; Chung, H.-M.; Jee, Y.; Lee, J.-S. Outbreaks of Gastroenteritis That Occurred during School Excursions in Korea Were Associated with Several Waterborne Strains of Norovirus. J. Clin. Microbiol. 2005, 43, 4836–4839. [Google Scholar] [CrossRef]
- Gallay, A.; De Valk, H.; Cournot, M.; Ladeuil, B.; Hemery, C.; Castor, C.; Bon, F.; Mégraud, F.; Le Cann, P.; Desenclos, J.C. A Large Multi-Pathogen Waterborne Community Outbreak Linked to Faecal Contamination of a Groundwater System, France, 2000. Clin. Microbiol. Infect. 2006, 12, 561–570. [Google Scholar] [CrossRef]
- Beer, K.D.; Gargano, J.W.; Roberts, V.A.; Hill, V.R.; Garrison, L.E.; Kutty, P.K.; Hilborn, E.D.; Wade, T.J.; Fullerton, K.E.; Yoder, J.S. Surveillance for Waterborne Disease Outbreaks Associated with Drinking Water—United States, 2011–2012. MMWR Morb. Mortal Wkly. Rep. 2015, 64, 842–848. [Google Scholar] [CrossRef]
- Cho, H.G.; Lee, S.G.; Kim, W.H.; Lee, J.S.; Park, P.H.; Cheon, D.S.; Jheong, W.H.; Jho, E.H.; Lee, J.B.; Paik, S.Y. Acute Gastroenteritis Outbreaks Associated with Ground-Waterborne Norovirus in South Korea during 2008–2012. Epidemiol. Infect. 2014, 142, 2604–2609. [Google Scholar] [CrossRef]
- Bitton, G.; Farrah, S.R.; Ruskin, R.H.; Butner, J.; Chou, Y.J. Survival of Pathogenic and Indicator Organisms in Ground Water. Groundwater 1983, 21, 405–410. [Google Scholar] [CrossRef]
- Filip, Z.; Demnerova, K. Survival in Groundwater and Ft—Ir Characterization of Some Pathogenic and Indicator Bacteria. In Threats to Global Water Security; Springer: Dordrecht, The Netherlands, 2009; pp. 117–122. [Google Scholar]
- Grisey, E.; Belle, E.; Dat, J.; Mudry, J.; Aleya, L. Survival of Pathogenic and Indicator Organisms in Groundwater and Landfill Leachate through Coupling Bacterial Enumeration with Tracer Tests. Desalination 2010, 261, 162–168. [Google Scholar] [CrossRef]
- IGRAC. Groundwater Monitoring Programmes: A Global Overview of Quantitative Groundwater Monitoring Networks; IGRAC: Delft, The Netherland, 2020. [Google Scholar]
- D’amore, F.; Panichi, C. Geochemistry in Geothermal Exploration. Int. J. Energy Res. 1985, 9, 277–298. [Google Scholar] [CrossRef]
- Goldman, M.; Neubauer, F.M. Groundwater Exploration Using Integrated Geophysical Techniques. Surv. Geophys. 1994, 15, 336–361. [Google Scholar] [CrossRef]
- Shirazi, S.M.; Imran, H.M.; Akib, S.; Yusop, Z.; Harun, Z.B. Groundwater Vulnerability Assessment in the Melaka State of Malaysia Using DRASTIC and GIS Techniques. Environ. Earth Sci. 2013, 70, 2293–2304. [Google Scholar] [CrossRef]
- Rashid, A.; Ayub, M.; Ullah, Z.; Ali, A.; Sardar, T.; Iqbal, J.; Gao, X.; Bundschuh, J.; Li, C.; Khattak, S.A.; et al. Groundwater Quality, Health Risk Assessment, and Source Distribution of Heavy Metals Contamination around Chromite Mines: Application of GIS, Sustainable Groundwater Management, Geostatistics, PCAMLR, and PMF Receptor Model. Int. J. Environ. Res. Public Health 2023, 20, 2113. [Google Scholar] [CrossRef]
- Arun, P.V. A Comparative Analysis of Different DEM Interpolation Methods. Egypt. J. Remote Sens. Space Sci. 2013, 16, 133–139. [Google Scholar] [CrossRef]
- Venkatesan, V.; Muthiah, K.; Karunakaran, K.; Ravikumar, G. GIS Based Multi-Criteria Analysis for Assessment of Groundwater Potential and Land Suitability. Int. J. Earth Sci. Eng. 2010, 3, 207–224. [Google Scholar]
- Díaz-Alcaide, S.; Martínez-Santos, P. Review: Advances in Groundwater Potential Mapping. Hydrogeol. J. 2019, 27, 2307–2324. [Google Scholar] [CrossRef]
- Zainol, N.F.M.; Zainuddin, A.H.; Looi, L.J.; Aris, A.Z.; Isa, N.M.; Sefie, A.; Yusof, K.M.K.K. Spatial Analysis of Groundwater Hydrochemistry through Integrated Multivariate Analysis: A Case Study in the Urbanized Langat Basin, Malaysia. Int. J. Environ. Res. Public Health 2021, 18, 5733. [Google Scholar] [CrossRef]
- IDEAM. Monitoreo y Calidad de Aguas. Available online: http://www.ideam.gov.co/web/siac/monitoreo (accessed on 9 November 2023).
- FAO. Versión Resumida de El Estado de La Seguridad Alimentaria y La Nutrición En El Mundo 2020; FAO; IFAD; UNICEF; WFP; WHO: Roma, Italy, 2020; ISBN 978-92-5-132912-2. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the Projections of the World Water Development Report. NPJ Clean. Water 2019, 2, 15. [Google Scholar] [CrossRef]
- FAO. El Estado Mundial de La Agricultura y La Alimentación; FAO: Roma, Italy, 2017. [Google Scholar]
- Siebert, S.; Döll, P.; Hoogeveen, J.; Faures, J.M.; Frenken, K.; Feick, S. Development and Validation of the Global Map of Irrigation Areas. Hydrol. Earth Syst. Sci. 2005, 9, 535–547. [Google Scholar] [CrossRef]
- UN-Water. Summary Progress. Update 2021: SDG 6—Water and Sanitation for All; UN-Water: Geneva, Switzerland, 2021. [Google Scholar]
- Gobierno de España. RD 1798/2010; Diario Oficial de la Unión Europea: España, Spain, 2011. [Google Scholar]
- Azcoiti, J.M.F. Las Aguas Subterráneas En La Legislación Iberoamericana: Retos y Oportunidades; Fornés Azcoiti, J., Ed.; Publicaciones del Instituto Geológico y Minero de España: Madrid, Spain, 2019; Volume 37, ISBN 978-84-9138-093-1. [Google Scholar]
- WHO Drinking-Water. Available online: https://www.who.int/es/news-room/fact-sheets/detail/drinking-water (accessed on 9 November 2023).
- Programa De Las Naciones Unidas Para El Desarrollo Objetivo 6: Agua Limpia y Saneamiento|Objetivos de Desarrollo Sostenible. Available online: https://www.undp.org/es/sustainable-development-goals/agua-limpia-saneamiento (accessed on 7 November 2023).
- Gobierno Nacional de Colombia. Decreto 1076 de 2015 Sector Ambiente y Desarrollo Sostenible; Gobierno Nacional de Colombia: Bogotá, Colombia, 2015.
- Vélez Otálvaro, M.V.; Otálvaro Hoyos, D.L.; Navarro Cuervo, L.F. Guía Metodológica Para La Formulación de Planes de Manejo Ambiental de Acuíferos; Dirección de Gestión Integral del Recurso Hídrico, Ministerio de Ambiente y Desarrollo Sostenible: Bogotá, Colombia, 2014.
- Ministerio de Ambiente y Desarrollo Sostenible. Resolución 2115; República de Colombia: Bogotá, Colombia, 2007. [Google Scholar]
- Ministerio de Protección Social. Resolución 1618; República de Colombia: Bogotá, Colombia, 2010; Volume 2010. [Google Scholar]
- Ministerio de Agricultura. Decreto 1594 de 1984; República de Colombia: Bogotá, Colombia, 1984; Volume 1984. [Google Scholar]


| Physicochemical Properties | Description |
|---|---|
| Color | There may be coloration due to the presence of colored organic material or chemical elements that when reacting, generate a certain shade, for example, iron and manganese in water may be red. The color varies according to geological characteristics and can be measured by visual comparison with cobalt standards [67]. |
| Odor | It is variable and dependent on the chemical composition of the water, or the elements that make up the water. Likewise, it is complemented by individual perception [67]. |
| Taste | The result of the taste, in addition to being based on the characteristics of a particular water source, is also the combination of the perception of odor, temperature, and the sensation of the taste [67]. |
| Electrical conductivity | It is related to the concentration of salts in solution, in which dissociation generates ions capable of conducting electric charge. In groundwater, the conductivity may be higher due to the number of ions present [68,69] |
| pH | It is generally neutral, around 7; however, it depends on the chemical composition of the adjacent rocks and the presence of acids or bases in the medium [70]. |
| Temperature | It is stable and is influenced by the local climate, but tends to be colder than surface water because it is insulated by the ground [71]. |
| Turbidity | Undisturbed water is usually clear with low turbidity because it passes through natural filtration through the ground. |
| Suspenden solids | They are usually very few, but may be present in unconfined aquifers or during well drilling and development [71]. |
| Total disolved solids (TDS) | They are mainly determined by the geological characteristics of the surrounding environment. TDS are a mixture of organic material and inorganic salts that may be naturally occurring, such as carbonates, chlorides, sulfates, etc., or introduced by anthropogenic contamination [69]. |
| Hardeness | It depends on the concentration of calcium and magnesium in the water. Hard water can cause scaling in pipes, and may require water softening treatments [67]. |
| Contaminants | Contamination is often associated with a variety of sources, including industrial, agricultural or subway storage leaks. Contaminants may include heavy metals, organic pollutants, pesticides and VOCs [72]. |
| Disolved gases | Gases such as O2 and CO2 can be present in water. The former favors the development of aerobic organisms and microorganisms. The latter can be transformed into carbonic acid and modify the pH [71]. |
| Salinity | Salinity is often generated in warm regions or coastal areas. It may be due to the accumulation of salts due to evaporation of water from the Earth’s surface, or by the infiltration of seawater into coastal aquifers [67]. |
| REDOX reactions | Redox conditions are influenced by the availability of different chemical species in the water, the presence of organic material, and microbial activity [73]. |
| Dissolved oxygen | It is an indicator of biochemical processes carried out by organisms and microorganisms in the presence of organic matter. The concentration of dissolved oxygen in groundwater can differ according to depth, pressure, and temperature. Both saturated and anoxic zones can occur [67]. |
| Alkalinity | It is the index of the buffering capacity of water to neutralize the acids present. In groundwater, this capacity is given mostly by carbonates, bicarbonates and hydroxides [69]. |
| Ions | The most common ions in groundwater include [67]: Cations: calcium (Ca2+), magnesium (Mg2+), sodium (Na+), potassium (K+), iron (Fe2+, Fe3+), manganese (Mn2+) and ammonium (NH4+). Anions: sulfate (SO42−), chloride (Cl−), bicarbonate (HCO3−), nitrate (NO3−), carbonate (CO32−), fluoride (F−), phosphate (PO43−), cyanide (CN−) and silicate (SiO32−). The interactions and concentrations of these in water differ according to the geology and conditions determined in each area. |
| Metals | Some metals can be naturally occurring or introduced, some of these are: copper, chromium, cadmium, manganese, arsenic, lead, copper, zinc and mercury [74]. |
| Minerals | The presence of one mineral or another, or the combination of several will depend on the geological conditions of the environment where the water moves. Some of the minerals that can be found in groundwater include: calcite, gypsum, dolomite, quartz, feldspar, iron oxides, manganese oxides, arsenopyrite, realgar, orpiment, phyllosilicate, and selenite [67]. |
| Water Type | Formula | Established Range |
|---|---|---|
| Silicic | SiO2 | >50 mg/L |
| Arsenicas | As | From 0.2 to 0.3 mg/L |
| Boric | Ba | >4 mg/L |
| Fluorics | F− | From 1.0 to 2.0 mg/L |
| Bromics | Br− | >4 mg/L |
| Iodhydric | I− | >1 mg/L |
| Lithics | Li | >1 mg/L |
| Strontium | Sr | >10 mg/L |
| Barium | Ba | >5 mg/L |
| Ferruginous | Fe+2; Fe+3 | >5 g/L |
| Radonics | Rn | >1.82 nCi/L |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranguren-Díaz, Y.; Galán-Freyle, N.J.; Guerra, A.; Manares-Romero, A.; Pacheco-Londoño, L.C.; Romero-Coronado, A.; Vidal-Figueroa, N.; Machado-Sierra, E. Aquifers and Groundwater: Challenges and Opportunities in Water Resource Management in Colombia. Water 2024, 16, 685. https://doi.org/10.3390/w16050685
Aranguren-Díaz Y, Galán-Freyle NJ, Guerra A, Manares-Romero A, Pacheco-Londoño LC, Romero-Coronado A, Vidal-Figueroa N, Machado-Sierra E. Aquifers and Groundwater: Challenges and Opportunities in Water Resource Management in Colombia. Water. 2024; 16(5):685. https://doi.org/10.3390/w16050685
Chicago/Turabian StyleAranguren-Díaz, Yani, Nataly J. Galán-Freyle, Abraham Guerra, Anderson Manares-Romero, Leonardo C. Pacheco-Londoño, Andrea Romero-Coronado, Natally Vidal-Figueroa, and Elwi Machado-Sierra. 2024. "Aquifers and Groundwater: Challenges and Opportunities in Water Resource Management in Colombia" Water 16, no. 5: 685. https://doi.org/10.3390/w16050685
APA StyleAranguren-Díaz, Y., Galán-Freyle, N. J., Guerra, A., Manares-Romero, A., Pacheco-Londoño, L. C., Romero-Coronado, A., Vidal-Figueroa, N., & Machado-Sierra, E. (2024). Aquifers and Groundwater: Challenges and Opportunities in Water Resource Management in Colombia. Water, 16(5), 685. https://doi.org/10.3390/w16050685







