The Reintroduction of Brown Trout (Salmo trutta fario) in the Upper Scheldt River Basin (Flanders, Belgium): Success or Failure?
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Methodology
2.3. Data Analysis
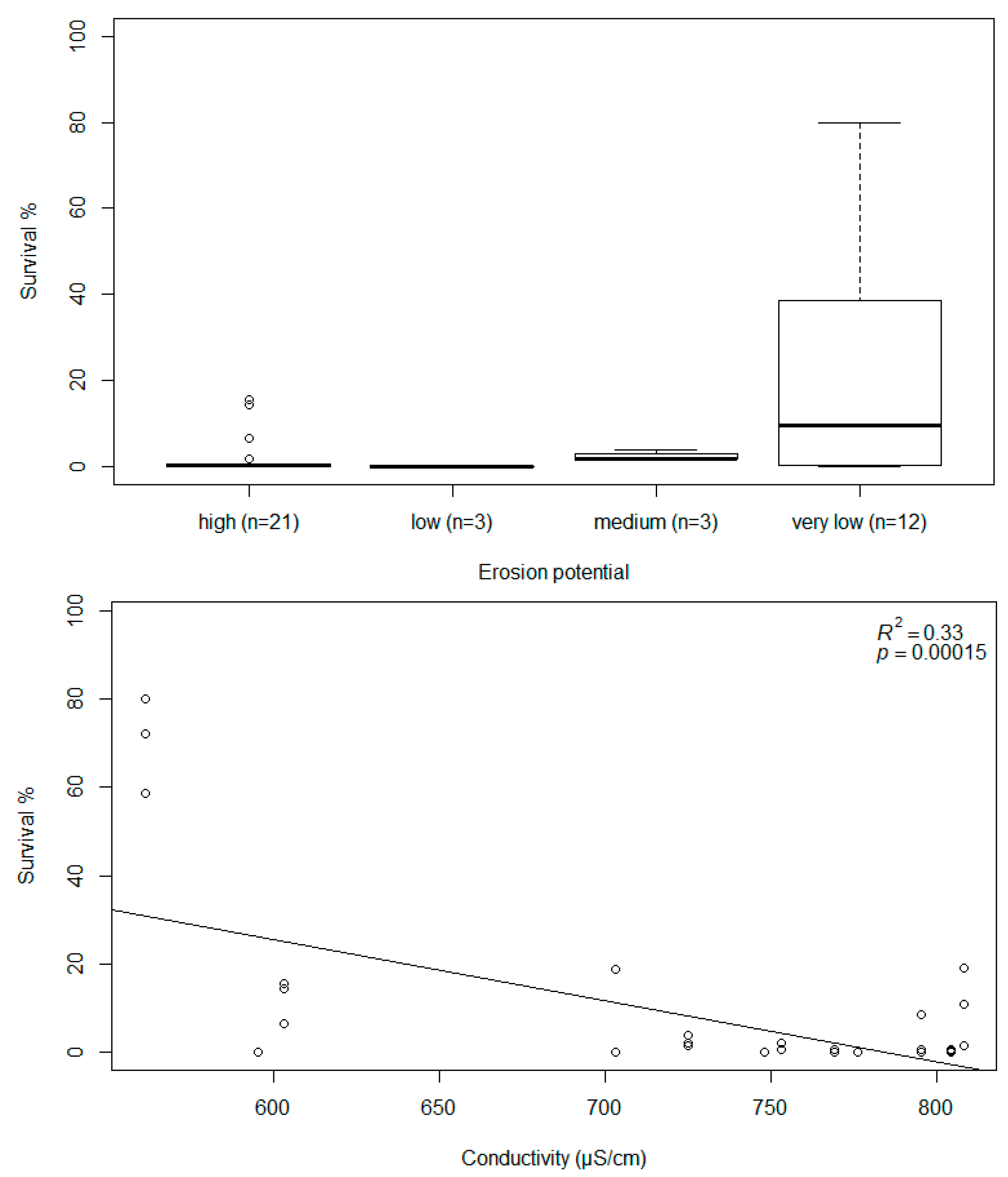
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Albert, J.S.; Destouni, G.; Duke-Sylvester, S.M.; Magurran, A.E.; Oberdorff, T.; Reis, R.E.; Winemiller, K.O.; Ripple, W.J. Scientists’ warning to humanity on the freshwater biodiversity crisis. Ambio 2021, 50, 85–94. [Google Scholar] [CrossRef]
- WWF. Living Planet Report 2020—Bending the Curve of Biodiversity Loss; Almond, R.E.A., Grooten, M., Petersen, T., Eds.; World Wildlife Fund: Gland, Switzerland, 2020. [Google Scholar]
- Reid, A.; Carlson, A.; Creed, I.; Eliason, E.; Gell, P.; Johnson, P.; Kidd, K.; Maccormack, T.; Olden, J.; Ormerod, S.; et al. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biol. Rev. 2018, 94, 849–873. [Google Scholar] [CrossRef]
- Staentzel, C.; Combroux, I.; Barillier, A.; Grac, C.; Chanez, E.; Beisel, J.-N. Effects of a river restoration project along the Old Rhine River (France-Germany): Response of macroinvertebrate communities. Ecol. Eng. 2019, 127, 114–124. [Google Scholar] [CrossRef]
- Boets, P.; Dillen, A.; Mertens, J.; Vervaeke, B.; Van Thuyne, G.; Breine, J.; Goethals, P.; Poelman, E. Do investments in water quality and habitat restoration programs pay off? An analysis of the chemical and biological water quality of a lowland stream in the Zwalm River basin (Belgium). Environ. Sci. Policy 2021, 124, 115–124. [Google Scholar] [CrossRef]
- Stoffers, T.; Collas, F.P.L.; Buijse, A.D.; Geerling, G.W.; Jans, L.H.; Van Kessel, N.; Verreth, J.A.; Nagelkerke, L.A. 30 years of large river restoration: How long do restored floodplain channels remain suitable for targeted rheophilic fishes in the lower river Rhine? Sci. Total Environ. 2021, 755, 142931. [Google Scholar] [CrossRef] [PubMed]
- Boets, P.; Lock, K.; Goethals, P.L.M. Using long-term monitoring to investigate the changes in species composition in the harbour of Ghent (Belgium). Hydrobiologia 2011, 663, 155–166. [Google Scholar] [CrossRef]
- Vlaamse Milieumaatschappij VMM. Fysisch-Chemische Kwaliteit Oppervlaktewater 2019. 2020. Available online: https://www.vmm.be (accessed on 2 September 2023). (In Dutch).
- Haase, P.; Bowler, D.E.; Baker, N.J.; Bonada, N.; Domisch, S.; Marquez, J.R.G.; Heino, J.; Hering, D.; Jähnig, S.C.; Schmidt-Kloiber, A.; et al. The recovery of European freshwater biodiversity has come to a halt. Nature 2023, 620, 582–588. [Google Scholar] [CrossRef]
- Vlaamse Milieumaatschappij VMM. 2020. Available online: https://www.vmm.be/nieuwsbrief/november-2020/vmm-meet-30-jaar-waterkwaliteit# (accessed on 2 September 2023). (In Dutch).
- Dedecker, A.P.; Van Melckebeke, K.; Goethals, P.; De Pauw, N. Development of migration models for macroinverte-brates in the Zwalm river basin as a tool in river assessment and restoration management. Commun. Agric. Appl. Biol. Sci. 2004, 69, 81–84. [Google Scholar]
- Vlaamse Milieumaatschappij VMM. 2023. Available online: https://www.vmm.be/nieuwsbrief/maart-2023/ecologisch-herstel-van-de-zwalm (accessed on 20 January 2024). (In Dutch).
- Forio, M.A.E.; De Troyer, N.; Lock, K.; Witing, F.; Baert, L.; De Saeyer, N.; Rîșnoveanu, G.; Popescu, C.; Burdon, F.J.; Kupilas, B.; et al. Small Patches of Riparian Woody Vegetation Enhance Biodiversity of Invertebrates. Water 2020, 12, 3070. [Google Scholar] [CrossRef]
- Dedecker, A.P.; Van Melckebeke, K.; Goethals, P.L.M.; De Pauw, N. Development of migration models for macroin-vertebrates in the Zwalm River basin (Flanders, Belgium) as tools for restoration management. Ecol. Model. 2007, 203, 72–86. [Google Scholar] [CrossRef]
- Mouton, A.M.; Van Der Most, H.; Jeuken, A.; Goethals, P.L.; De Pauw, N. Evaluation of river basin restoration options by the application of the Water Framework Directive Explorer in the Zwalm River basin (Flanders, Belgium). River Res. Appl. 2009, 25, 82–97. [Google Scholar] [CrossRef]
- Deflem, I.S.; Bennetsen, E.; Opedal, H.; Calboli, F.C.F.; Ovaskainen, O.; Van Thuyne, G.; Volckaert, F.A.M.; Raeymaekers, J.A.M. Predicting fish community responses to environmental policy targets. Biodivers. Conserv. 2021, 30, 1457–1478. [Google Scholar] [CrossRef]
- Boets, P.; Gobeyn, S.; Dillen, A.; Poelman, E.; Goethals, P.L.M. Assessing the suitable habitat for reintroduction of brown trout (Salmo trutta forma fario) in a lowland river: A modelling approach. Ecol. Evol. 2018, 8, 5191–5205. [Google Scholar] [CrossRef] [PubMed]
- Auwerx, J.; (Research Institute for Nature and Forest, Linkebeek, Belgium). Personal communication, 2023.
- Boets, P.; Dillen, A.; Poelman, E. Evaluatie van het visbestand in de Zwalm en de Peerdestokbeek na soortherstel; Provinciaal Centrum voor Milieuonderzoek: Ghent, Belgium, 2021; 8p. (In Dutch)
- Syrjänen, J.; Kiljunen, M.; Karjalainen, J.; Eloranta, A.; Muotka, T. Survival and growth of brown trout Salmo trutta L. embryos and the timing of hatching and emergence in two boreal lake outlet streams. J. Fish Biol. 2008, 72, 985–1000. [Google Scholar] [CrossRef]
- Louhi, P.; Mäki-Petäys, A.; Erkinaro, J. Spawning habitat of Atlantic salmon and brown trout: General criteria and intragravel factors. River Res. Appl. 2009, 24, 330–339. [Google Scholar] [CrossRef]
- Crisp, D.T. Trout and Salmon: Ecology, Conservation and Rehabilitation; Blackwell Science: Oxford, UK, 2000; 212p. [Google Scholar]
- Deschuytter, R. Soortenherstel van reofiele vissoorten in Oost-Vlaanderen. Bachelor’s Thesis, Hogeschool PXL, Diepenbeek, Belgium, 2021; 75p. (In Dutch). [Google Scholar]
- Vlaamse Milieumaatschappij VMM. 2024. Available online: http://geoloket.vmm.be/Geoviews/ (accessed on 20 January 2024).
- Verreycken, H.; Belpaire, C.; Van Thuyne, G.; Breine, J.; Buysse, D.; Coeck, J.; Mouton, A.; Stevens, M.; Neucker, T.V.D.; De Bruyn, L.; et al. IUCN Red List of freshwater fishes and lampreys in Flanders (north Belgium). Fish. Manag. Ecol. 2014, 21, 122–132. [Google Scholar] [CrossRef]
- Verhoeven, R.; Van Poucke, L.; Huygens, M.; Bonosiak, R. Sedimentbalansen in rivieren. In Congres Watersysteemkennis 2006–2007; Universiteit Gent: Ghent, Belgium, 2006; 7p. (In Dutch) [Google Scholar]
- KMI Royal Meteorological Institute. 2023. Available online: https://www.kmi.be (accessed on 14 January 2024).
- Steegen, A.; Rovers, G.; Beuselinck, L.; Nachtergaele, J.; Takken, I.; Poesen, J. Variations in sediment yield from an agri-cultural drainage basin in central Belgium. IAHS Publ. 1998, 249, 177–185. [Google Scholar]
- Ojanguren, A.F.; Braña, F. Thermal dependence of embryonic growth and development in brown trout. J. Fish Biol. 2003, 62, 580–590. [Google Scholar] [CrossRef]
- Cocchiglia, L.; Curran, S.; Hannigan, E.; Purcell, P.J.; Kelly-Quinn, M. Evaluation of the effects of fine sediment inputs from stream culverts on brown trout egg survival through field and laboratory assessments. Inland Waters 2012, 2, 47–58. [Google Scholar] [CrossRef]
- Danner, G.R. Salmonid embryo development and pathology. Am. Fish. Soc. Symp. 2008, 65, 37–58. [Google Scholar]
- Prati, L.; Pavanello, R.; Pesarin, F. Assessment of surface water quality by a single index of pollution. Water Res. 1971, 5, 741–751. [Google Scholar] [CrossRef]
- Giraudoux, P. Pgirmess: Data Analysis in Ecology. 2013. Available online: https://github.com/pgiraudoux/pgirmess (accessed on 20 January 2024).
- Syrjänen, J.T.; Ruokonen, T.J.; Ketola, T.; Valkeajärvi, P. The relationship between stocking eggs in boreal spawning rivers and the abundance of brown trout parr. ICES J. Mar. Sci. 2015, 72, 1389–1398. [Google Scholar] [CrossRef]
- Massa, F.; Baglinière, J.L.; Prunet, P.; Grimaldi, C. Egg-to-fry survival of brown trout (Salmo trutta) and chemical en-vironment in the redd. Cybium 2000, 24, 129–140. [Google Scholar]
- Michel, C.; Wildhaber, Y.S.; Epting, J.; Thorpe, K.L.; Huggenberger, P.; Alewell, C.; Burkhardt-Holm, P. Artificial steps mitigate the effect of fine sediment on the survival of brown trout embryos in a heavily modified river. Freshw. Biol. 2013, 59, 544–556. [Google Scholar] [CrossRef]
- Van den Neucker, T.; Gelaude, E.; Baeyens, R.; Jacobs, Y.; De Maerteleire, N.; Stevens, M.; Mouton, A.; Buysse, D.; Auwerx, J.; Vught, I.; et al. Wetenschappelijke Ondersteuning Herstelprogra’ma’s Kopvoorn, Serpeling, Kwabaal en Beekforel in 2011; Rapporten van Het Instituut voor Natuur-en Bosonderzoek 2012 (19); Instituut voor Natuur-en Bosonderzoek: Brussel, Belgium, 2012. [Google Scholar]
- Goethals, P.; De Pauw, N. Development of a concept for integrated ecological river assessment in Flanders, Belgium. J. Limnol. 2001, 60, 7–16. [Google Scholar] [CrossRef]
- Crisp, D.T.; Carling, P.A. Observation on silting, dimensions and Structures of salmonid redds. J. Fish Biol. 1989, 3, 119–134. [Google Scholar] [CrossRef]
- Acornley, R.M.; Sear, D.A. Sediment transport and siltation of brown trout (Salmo trutta L.) spawning gravels in chalk streams. Hydrol. Process. 1999, 13, 447–458. [Google Scholar] [CrossRef]
- Conallin, J. The Effect of Sedimentation on the Natural Recruitment of Brown Trout (Salmo trutta), in Small Danish Streams; FAO: Rome, Italy, 2003. [Google Scholar]
- Rubin, J.-F.; Glimsäter, C. Egg-to-fry survival of the sea trout in some streams of Gotland. J. Fish Biol. 1996, 48, 585–606. [Google Scholar] [CrossRef]
- Kemp, P.; Sear, D.; Collins, A.; Naden, P.; Jones, I. The impacts of fine sediment on riverine fish. Hydrol. Process. 2011, 25, 1800–1821. [Google Scholar] [CrossRef]
- Alberto, A.; Courtenay SSt-Hilaire, A.; van den Heuvel, M. Factors Influencing Brook Trout (Salvelinus fontinalis) Egg Survival and Development in Streams Influenced by Agriculture. J. FisheriesSciences.com 2017, 11, 9–20. [Google Scholar] [CrossRef]
- Luckenbach, T.; Kilian, M.; Triebskorn, R.; Oberemm, A. Assessment of the developmental success of brown trout (Salmo trutta f. fario L.) embryos in two differently polluted streams in Germany. Hydrobiologia 2003, 490, 53–62. [Google Scholar] [CrossRef]
- Almodóvar, A.; Nicola, G.G.; Ayllón, D.; Elvira, B. Global warming threatens the persistence of Mediterranean brown trout. Glob. Change Biol. 2012, 18, 1549–1560. [Google Scholar] [CrossRef]
- Pulg, U.; Barlaup, B.T.; Sternecker, K.; Trepl, L.; Unfer, G. Restoration of Spawning Habitats of Brown Trout (Salmo trutta) in a Regulated Chalk Stream. River Res. Appl. 2013, 29, 172–182. [Google Scholar] [CrossRef]
- Belgium–CAP Strategic Plan–Flanders. Report N° 2023BE06AFSP001; 2023; 1219p. Available online: https://agriculture.ec.europa.eu/system/files/2023-04/csp-at-a-glance-belgium-flanders_en.pdf (accessed on 5 December 2023).
- Borsuk, M.E.; Reichert, P.; Peter, A.; Schager, E.; Burkhardt-Holm, P. Assessing the decline of brown trout (Salmo trutta) in Swiss rivers using a Bayesian probability network. Ecol. Model. 2006, 192, 224–244. [Google Scholar] [CrossRef]
- Schürings, C.; Feld, C.K.; Kail, J.; Hering, D. Effects of agricultural land use on river biota: A meta-analysis. Environ. Sci. Eur. 2022, 34, 124. [Google Scholar] [CrossRef]
- Forio, M.A.E.; Goethals, P.L.M. An Integrated Approach of Multi-Community Monitoring and Assessment of Aquatic Ecosystems to Support Sustainable Development. Sustainability 2020, 12, 5603. [Google Scholar] [CrossRef]


| Site | Year | Average Survival (%) | Stdev |
|---|---|---|---|
| 2 | 2018–2019 | 10.5 | 8.8 |
| 4 | 2018–2019 | 1.0 | 0.9 |
| 5 | 2018–2019 | 0.0 | 0.0 |
| 6 | 2018–2019 | 0.0 | 0.0 |
| 7 | 2018–2019 | 12.2 | 4.9 |
| Reference | 2018–2019 | 98.0 | 2.0 |
| 1 | 2019–2020 | 2.0 | 3.1 |
| 2 | 2019–2020 | 5.1 | 7.9 |
| 3 | 2019–2020 | 6.8 | 11.7 |
| Reference | 2019–2020 | 76.0 | 3.0 |
| 1 | 2020–2021 | 0.0 | 0.0 |
| 3 | 2020–2021 | 70.2 | 10.9 |
| 8 | 2020–2021 | 2.5 | 1.3 |
| 9 | 2020–2021 | 0.0 | 0.0 |
| 10 | 2020–2021 | 0.0 | 0.0 |
| Reference | 2020–2021 | 49.8 | 38.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boets, P.; Dillen, A.; Auwerx, J.; Zoeter Vanpoucke, M.; Van Nieuwenhuyze, W.; Poelman, E.; Goethals, P. The Reintroduction of Brown Trout (Salmo trutta fario) in the Upper Scheldt River Basin (Flanders, Belgium): Success or Failure? Water 2024, 16, 533. https://doi.org/10.3390/w16040533
Boets P, Dillen A, Auwerx J, Zoeter Vanpoucke M, Van Nieuwenhuyze W, Poelman E, Goethals P. The Reintroduction of Brown Trout (Salmo trutta fario) in the Upper Scheldt River Basin (Flanders, Belgium): Success or Failure? Water. 2024; 16(4):533. https://doi.org/10.3390/w16040533
Chicago/Turabian StyleBoets, Pieter, Alain Dillen, Johan Auwerx, Mechtild Zoeter Vanpoucke, Wim Van Nieuwenhuyze, Eddy Poelman, and Peter Goethals. 2024. "The Reintroduction of Brown Trout (Salmo trutta fario) in the Upper Scheldt River Basin (Flanders, Belgium): Success or Failure?" Water 16, no. 4: 533. https://doi.org/10.3390/w16040533
APA StyleBoets, P., Dillen, A., Auwerx, J., Zoeter Vanpoucke, M., Van Nieuwenhuyze, W., Poelman, E., & Goethals, P. (2024). The Reintroduction of Brown Trout (Salmo trutta fario) in the Upper Scheldt River Basin (Flanders, Belgium): Success or Failure? Water, 16(4), 533. https://doi.org/10.3390/w16040533








