Enhanced Peroxydisulfate (PDS) Activation for Sulfamethoxazole (SMX) Degradation by Modified Sludge Biochar: Focusing on the Role of Functional Groups
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Experimental Procedures
2.3. Analytical Methods
2.4. Theoretical Calculation
3. Results and Discussion
3.1. Degradation of SMX in the TSBC/PDS System
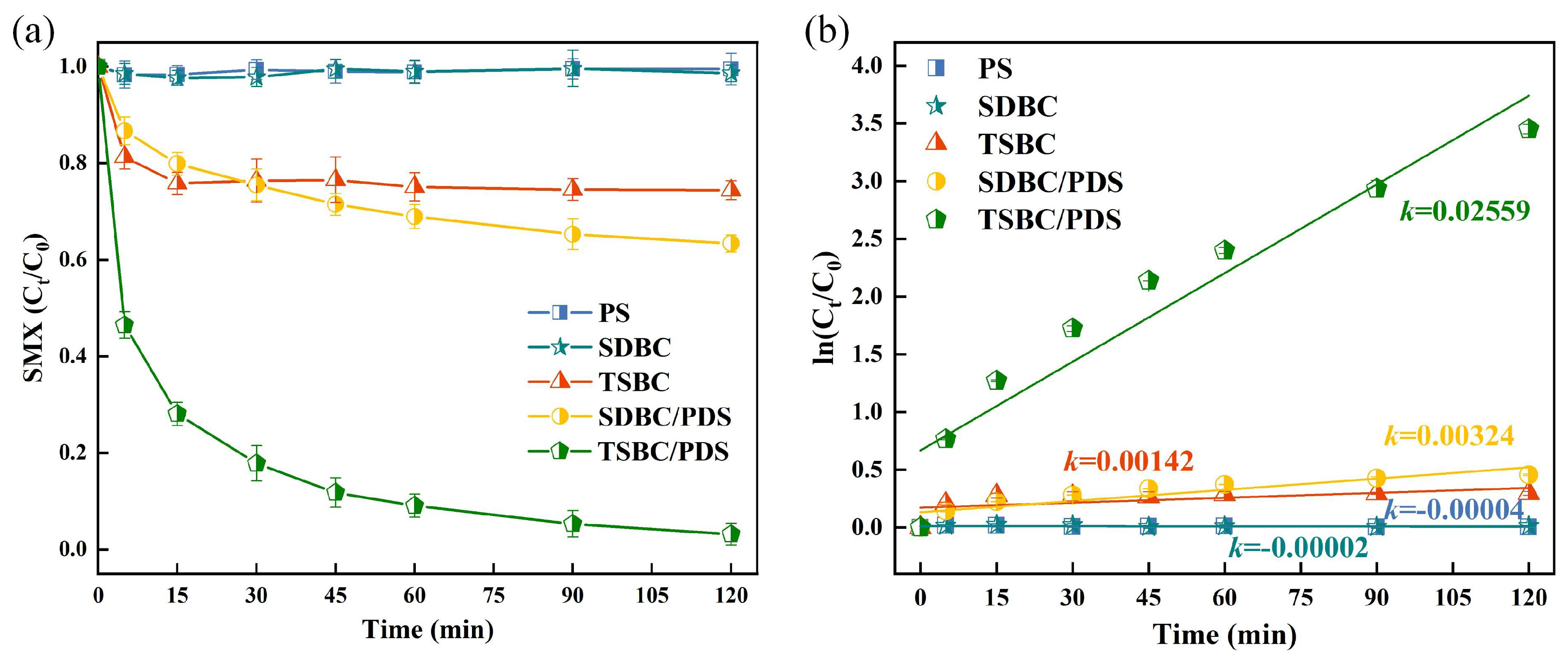
3.1.1. SMX Degradation Efficiency
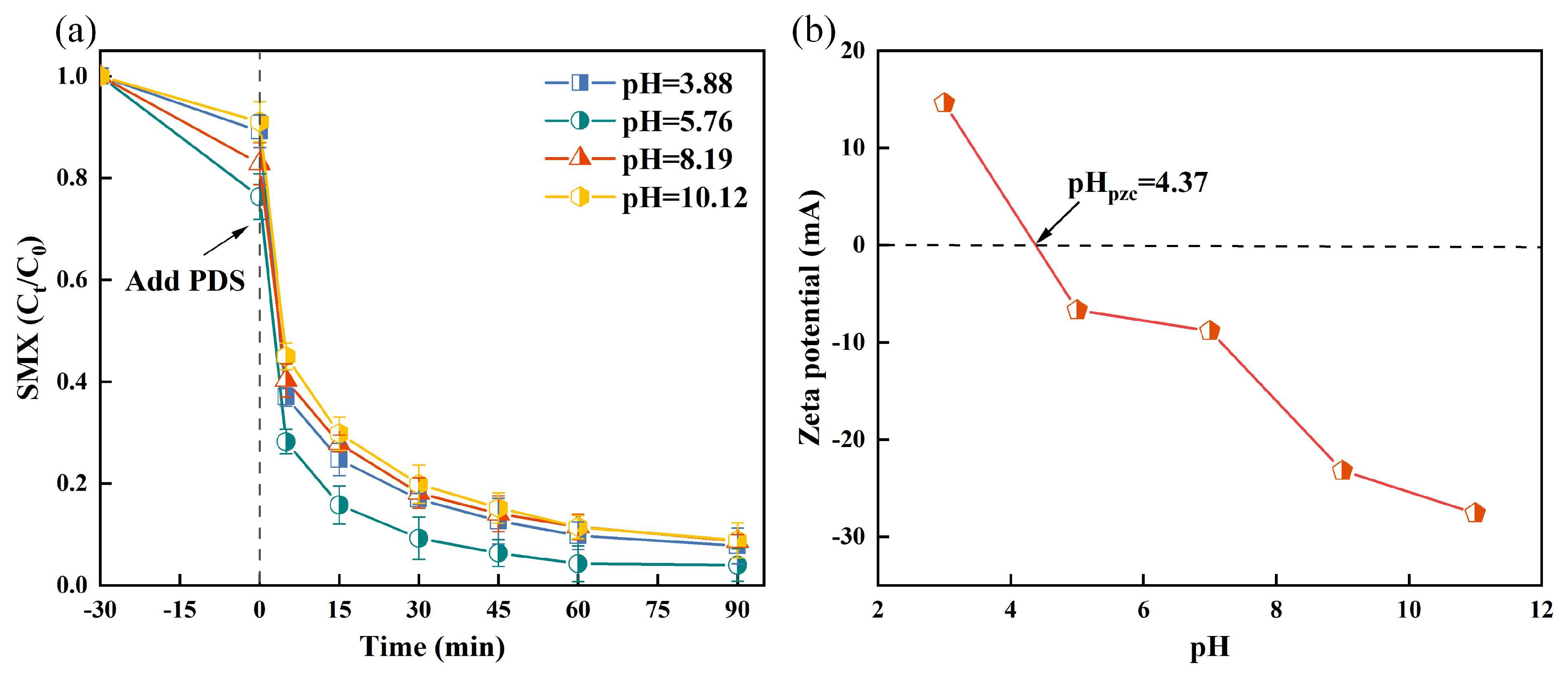
3.1.2. Effect of pH on SMX Degradation
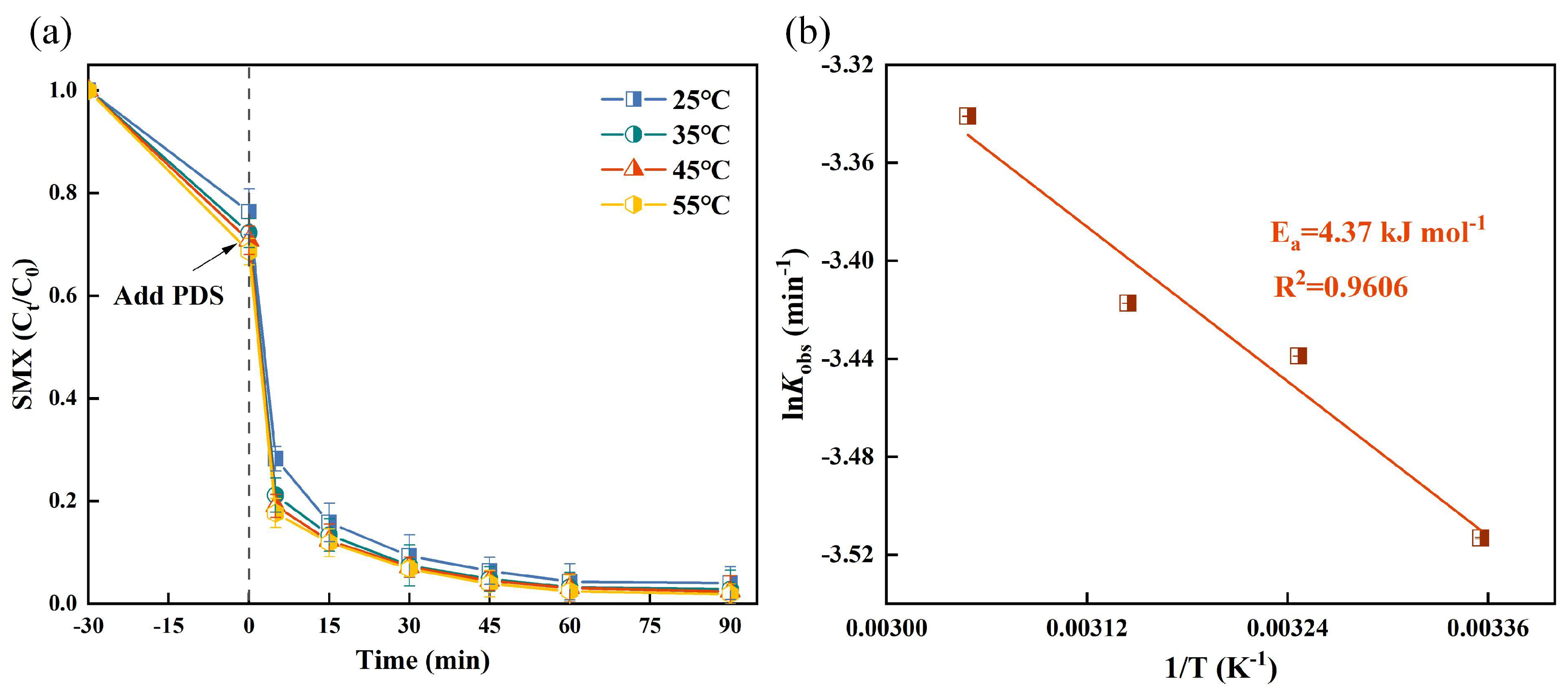
3.1.3. Effect of Temperature on SMX Degradation
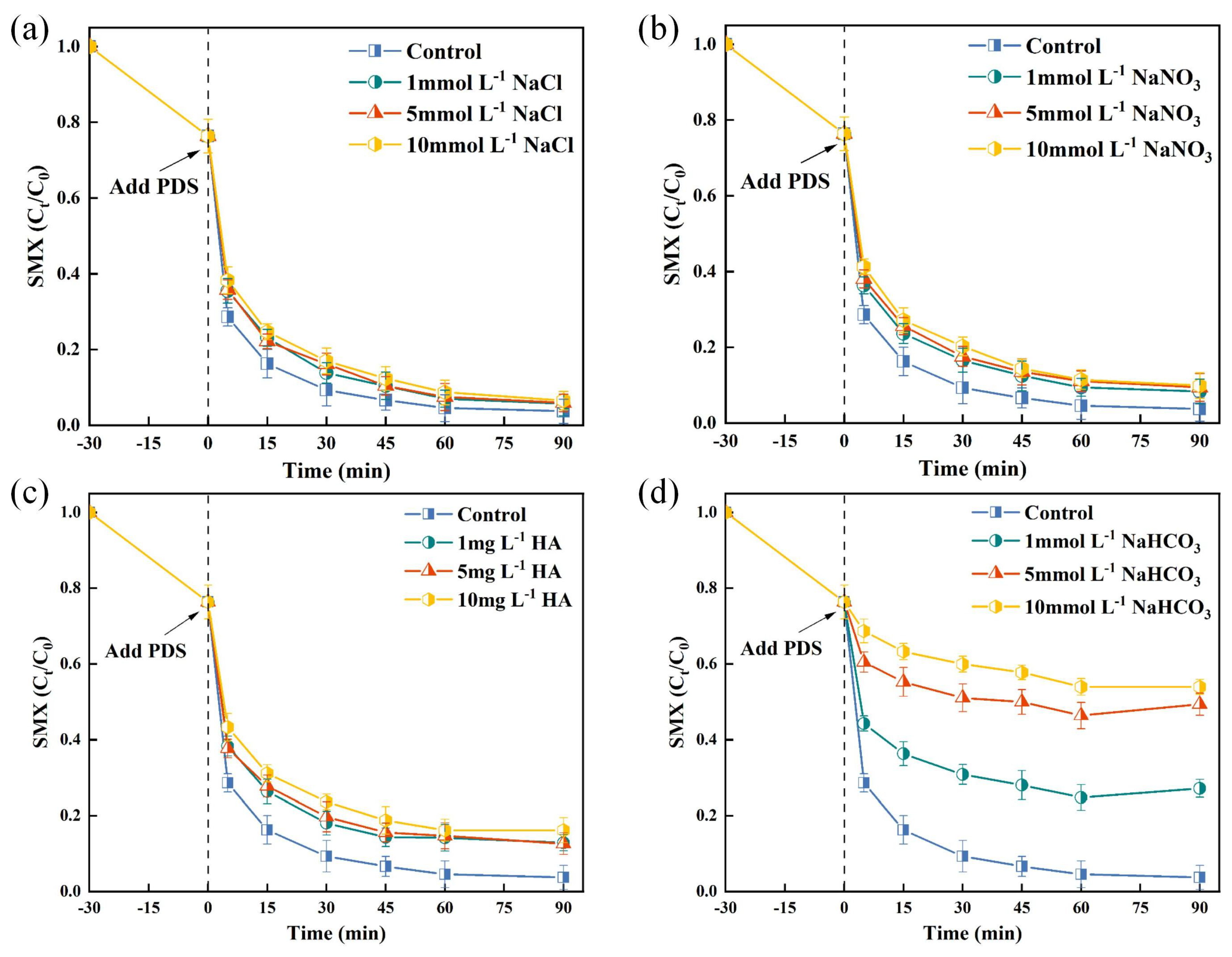
3.1.4. Effect of Natural Water Constituents on SMX Degradation
3.2. Degradation Mechanism Analysis
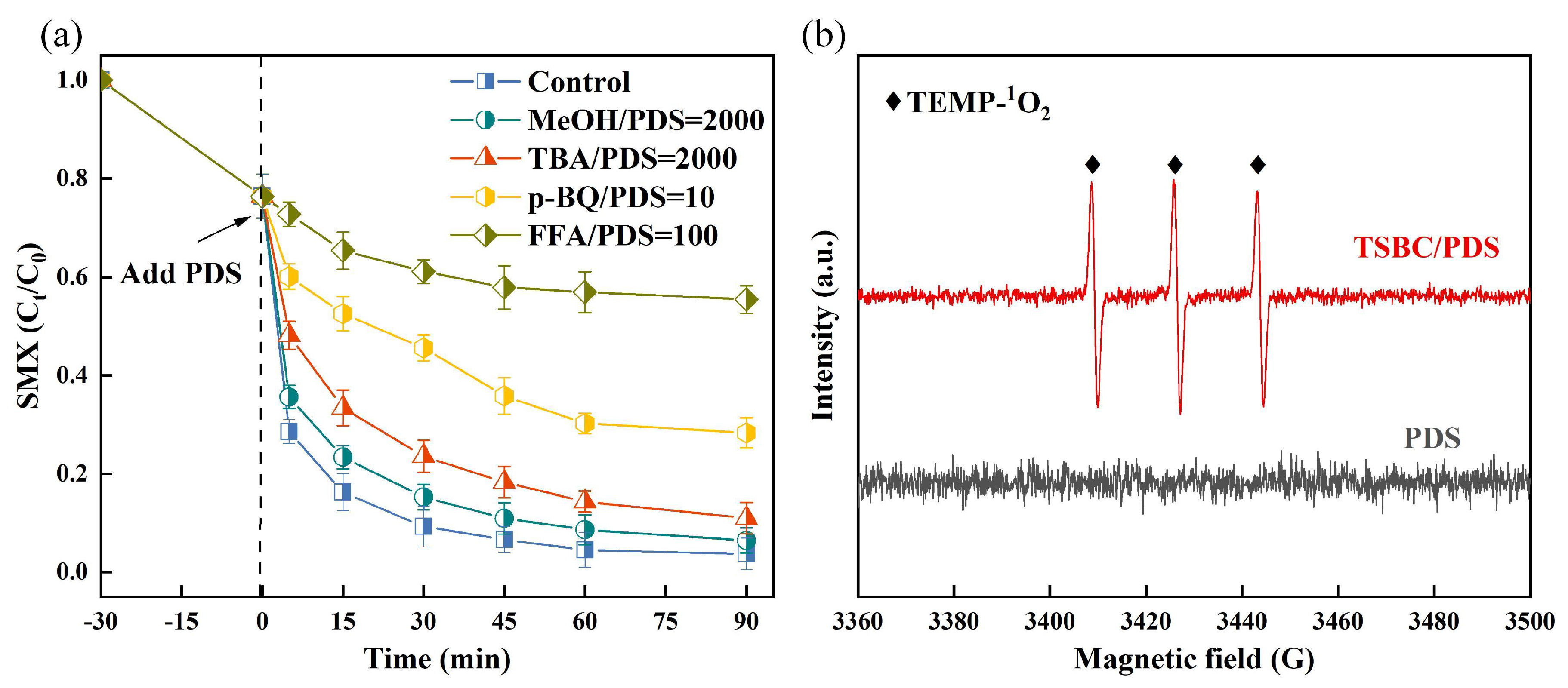
3.2.1. Identification in the Reactive Oxygen Species
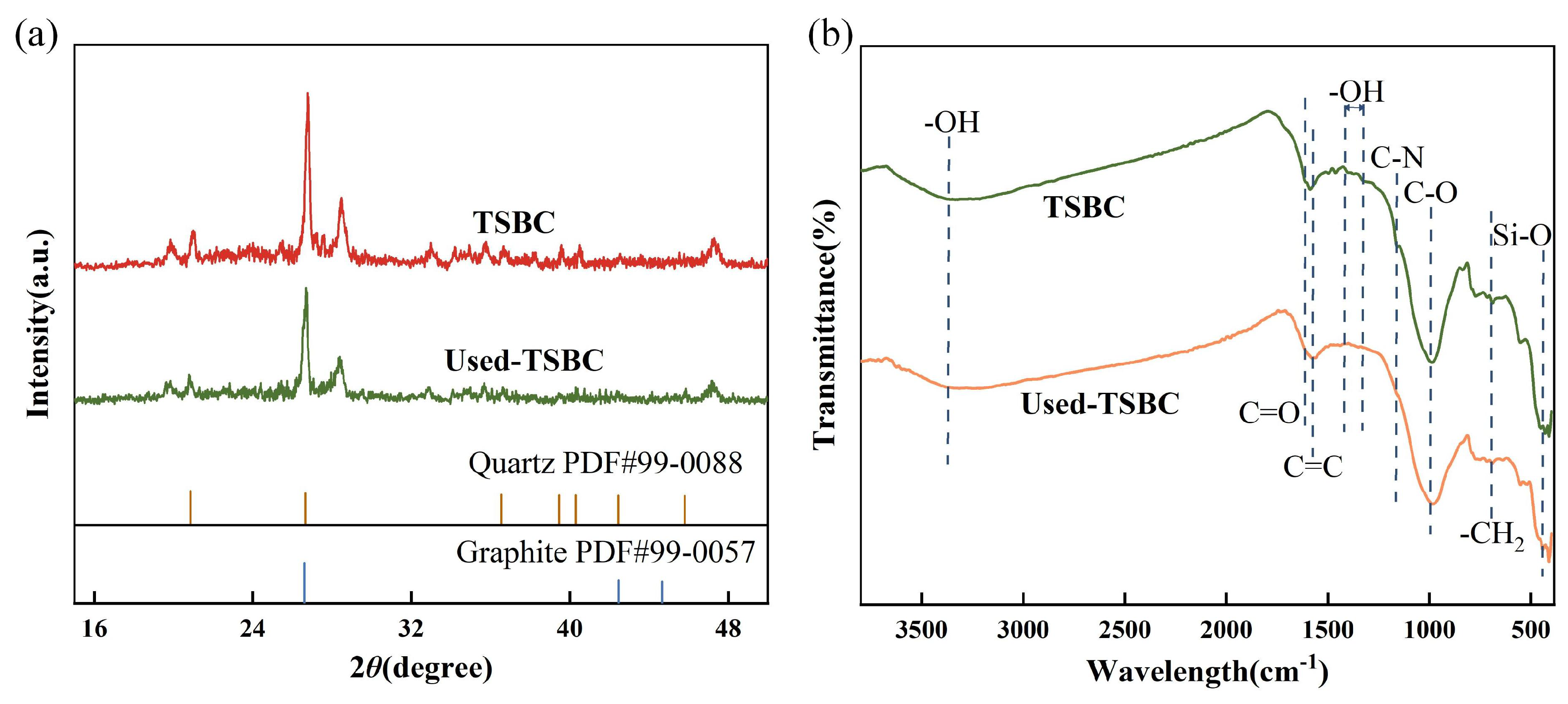
3.2.2. Identification in the Active Sites
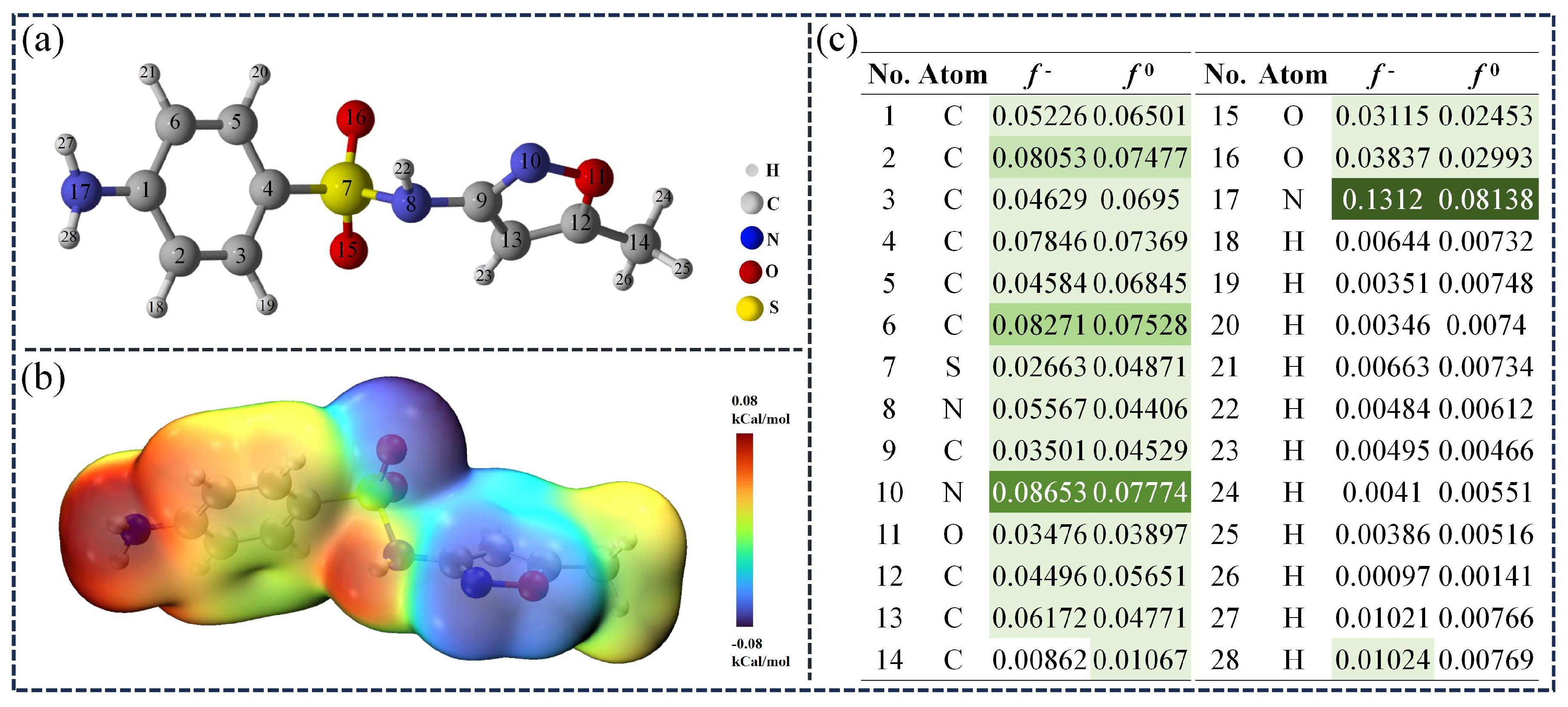
3.2.3. Theoretical Calculation
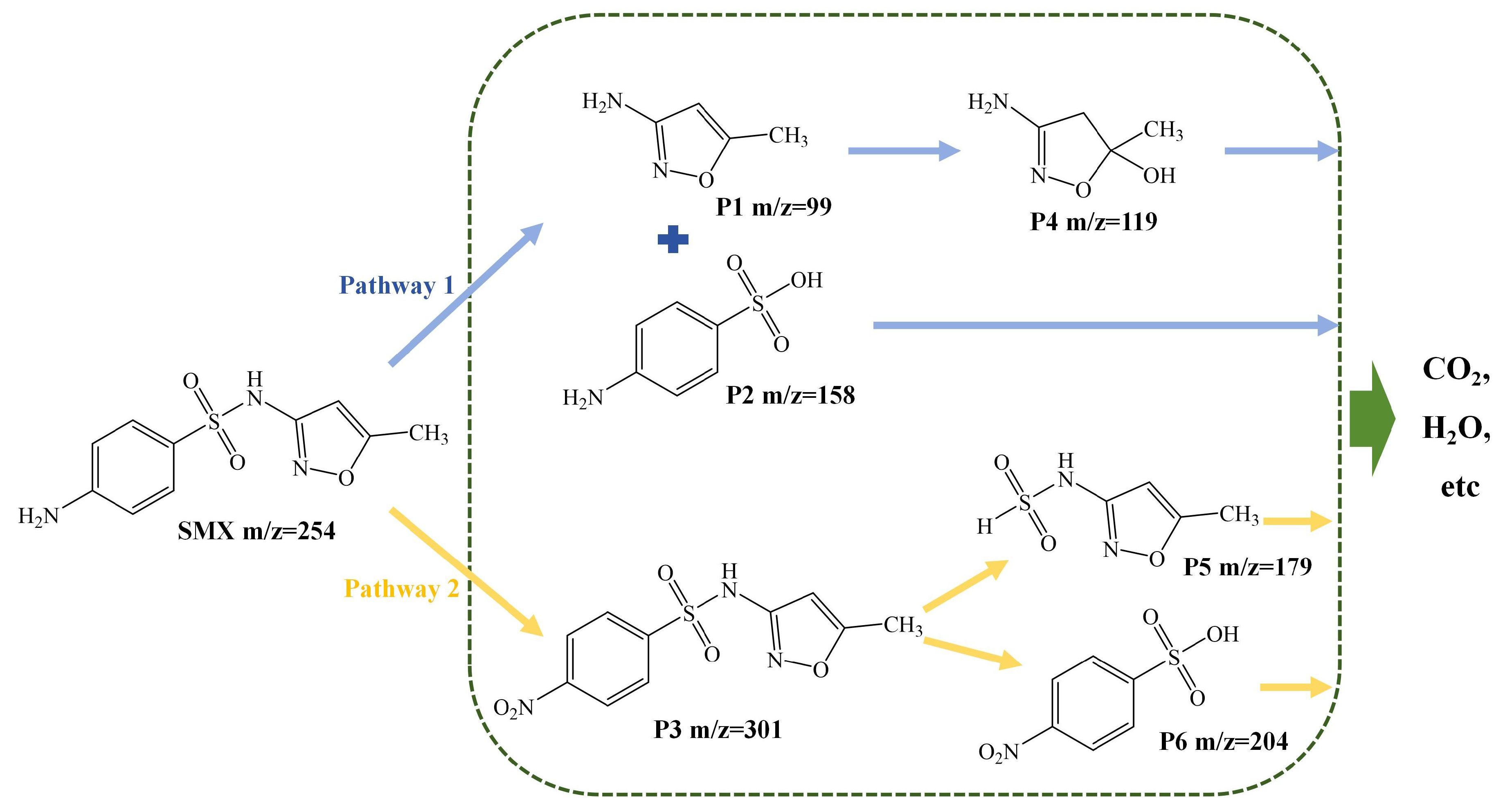
3.3. Degradation Pathways of SMX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, B.; He, Y.; Shi, W.; Liu, L.; Li, L.; Liu, C.; Lens, P.N. Biotransformation of sulfamethoxazole (SMX) by aerobic granular sludge: Removal performance, degradation mechanism and microbial response. Sci. Total Environ. 2023, 858, 159771. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; He, L.; Shen, S.; Li, Y.; He, Y.; Chen, Z.; Zhang, D.; Chen, Z.; Chen, X.; Wu, L.; et al. Unveiling the mechanisms of peracetic acid activation by iron-rich sludge biochar for sulfamethoxazole degradation with wide adaptability. J. Environ. Manag. 2023, 347, 119119. [Google Scholar] [CrossRef] [PubMed]
- Shang, K.; Morent, R.; Wang, N.; Wang, Y.; Peng, B.; Jiang, N.; Lu, N.; Li, J. Degradation of sulfamethoxazole (SMX) by water falling film DBD Plasma/Persulfate: Reactive species identification and their role in SMX degradation. Chem. Eng. J. 2022, 431, 133916. [Google Scholar] [CrossRef]
- Szymańska, U.; Wiergowski, M.; Sołtyszewski, I.; Kuzemko, J.; Wiergowska, G.; Woźniak, M.K. Presence of antibiotics in the aquatic environment in Europe and their analytical monitoring: Recent trends and perspectives. Microchem. J. 2019, 147, 729–740. [Google Scholar] [CrossRef]
- Guan, C.; Jiang, J.; Pang, S.; Ma, J.; Chen, X.; Lim, T.T. Nonradical transformation of sulfamethoxazole by carbon nanotube activated peroxydisulfate: Kinetics, mechanism and product toxicity. Chem. Eng. J. 2019, 378, 122147. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Qu, Y.; Zhang, X.; Zhang, Q.; Yang, D.; Wang, Q.; Dong, Z.; Zhao, J. Intestinal microbiota perturbations in the gastropod Trochus niloticus concurrently exposed to ocean acidification and environmentally relevant concentrations of sulfamethoxazole. Chemosphere 2023, 311, 137115. [Google Scholar] [CrossRef]
- Bai, Y.; Ruan, X.; Wang, F.; Antoine, G.; van der Hoek, J.P. Sulfonamides removal under different redox conditions and microbial response to sulfonamides stress during riverbank filtration: A laboratory column study. Chemosphere 2019, 220, 668–677. [Google Scholar] [CrossRef]
- Lu, W.; Chen, N.; Feng, C.; An, N.; Dong, Y. Peracetic acid-based electrochemical treatment of sulfamethoxazole and real antibiotic wastewater: Different role of anode and cathode. J. Hazard. Mater. 2024, 463, 132819. [Google Scholar] [CrossRef]
- Yu, X.; Jin, X.; Li, M.; Yu, Y.; Liu, H.; Zhou, R.; Yin, A.; Shi, J.; Sun, J.; Zhu, L. Mechanism and security of UV driven sodium percarbonate for sulfamethoxazole degradation using DFT and metabolomic analysis. Environ. Pollut. 2023, 323, 121352. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chu, K.; Sun, S.; Chen, H.; Song, B.; Wang, J.; Liu, Z.; Zhu, L. Synthesis of magnetic core-shell Fe3O4-Mn3O4 composite for degradation of sulfadiazine via peroxymonosulfate activation: Characterization, mechanism and toxicity analysis. J. Environ. Chem. Eng. 2023, 11, 109230. [Google Scholar] [CrossRef]
- Wang, L.L.; Jiang, S.F.; Huang, J.; Jiang, H. Oxygen-doped biochar for the activation of ferrate for the highly efficient degradation of sulfadiazine with a distinct pathway. J. Environ. Chem. Eng. 2022, 10, 108537. [Google Scholar] [CrossRef]
- Xiao, G.; Xu, T.; Faheem, M.; Xi, Y.; Zhou, T.; Moryani, H.T.; Bao, J.; Du, J. Evolution of singlet oxygen by activating peroxydisulfate and peroxymonosulfate: A review. Int. J. Environ. Res. Public Health 2021, 18, 3344. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, X.; Xi, S.; Miao, S.; Ding, J.; Cai, W.; Liu, S.; Yang, X.; Yang, H.; Gao, J.; et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient Fenton-like catalysis. J. Am. Chem. Soc. 2018, 140, 12469–12475. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, J. Kinetics of PMS activation by graphene oxide and biochar. Chemosphere 2020, 239, 124812. [Google Scholar] [CrossRef]
- Fang, Z.; Zhou, Z.; Xue, G.; Yu, Y.; Wang, Q.; Cheng, B.; Ge, Y.; Qian, Y. Application of sludge biochar combined with peroxydisulfate to degrade fluoroquinolones: Efficiency, mechanisms and implication for ISCO. J. Hazard. Mater. 2022, 426, 128081. [Google Scholar] [CrossRef]
- Duan, X.; Zhang, C.; Wang, S.; Ren, N.Q.; Ho, S.H. Graphitic biochar catalysts from anaerobic digestion sludge for nonradical degradation of micropollutants and disinfection. Chem. Eng. J. 2020, 384, 123244. [Google Scholar]
- Wang, T.; Zhou, Y.; Xue, Y.; Sang, T.; Ren, L.; Chen, S.; Liu, J.; Mei, M.; Li, J. Pyrolysis of hydrothermally dewatering sewage sludge: Highly efficient peroxydisulfate activation of derived biochar to degrade diclofenac. Environ. Pollut. 2022, 313, 120176. [Google Scholar] [CrossRef]
- Hung, C.M.; Chen, C.W.; Huang, C.P.; Lam, S.S.; Dong, C.D. Peroxymonosulfate activation by a metal-free biochar for sulfonamide antibiotic removal in water and associated bacterial community composition. Bioresour. Technol. 2022, 343, 126082. [Google Scholar] [CrossRef]
- Yu, J.; Tang, L.; Pang, Y.; Zeng, G.; Wang, J.; Deng, Y.; Liu, Y.; Feng, H.; Chen, S.; Ren, X. Magnetic nitrogen-doped sludge-derived biochar catalysts for persulfate activation: Internal electron transfer mechanism. Chem. Eng. J. 2019, 364, 146–159. [Google Scholar] [CrossRef]
- Yin, R.; Guo, W.; Wang, H.; Du, J.; Wu, Q.; Chang, J.S.; Ren, N. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism. Chem. Eng. J. 2019, 357, 589–599. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, H.; Murugananthan, M.; Sun, P.; Dionysiou, D.D.; Zhang, K.; Khan, A.; Zhang, Y. Glucose and melamine derived nitrogen-doped carbonaceous catalyst for nonradical peroxymonosulfate activation. Carbon 2020, 156, 399–409. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Li, J.; Xue, Y.; Liu, J.; Mei, M.; Hou, H.; Chen, S. Co-pyrolysis behavior of sewage sludge and rice husk by TG-MS and residue analysis. J. Clean. Prod. 2020, 250, 119557. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, J.; Wang, M.; Naidu, R.; Liu, Y.; Man, Y.B.; Liang, X.; Wong, M.H.; Christie, P.; Zhang, Y.; et al. Co-pyrolysis of sewage sludge and rice husk/bamboo sawdust for biochar with high aromaticity and low metal mobility. Environ. Res. 2020, 191, 110034. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Liu, Q.; Zhang, X.; Cao, L.; Hu, B.; Shao, J.; Ding, F.; Guo, X.; Gao, B. Synergetic effect of co-pyrolysis of sewage sludge and lignin on biochar production and adsorption of methylene blue. Fuel 2022, 324, 124587. [Google Scholar] [CrossRef]
- Wang, R.; Duan, X.; Wang, S.; Ren, N.Q.; Ho, S.H. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation. Water Res. 2020, 187, 116390. [Google Scholar]
- Yin, Q.; Liu, M.; Ren, H. Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 2019, 249, 109410. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, J.; Liu, Z.; Liu, M. Degradation of tetracycline by peroxydisulfate activation on sludge-derived biochar modified with tannin extract. Process. Saf. Environ. Prot. 2023, 173, 472–484. [Google Scholar] [CrossRef]
- Tan, J.; Chen, X.; Shang, M.; Cui, J.; Li, D.; Yang, F.; Zhang, Z.; Zhang, H.; Wu, Q.; Li, Y.; et al. N-doped biochar mediated peroxydisulfate activation for selective degradation of bisphenol A: The key role of potential difference-driven electron transfer mechanism. Chem. Eng. J. 2023, 468, 143476. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Zhou, H. Preparation of N-doped biochar from sewage sludge and melamine for peroxymonosulfate activation: N-functionality and catalytic mechanisms. Sci. Total Environ. 2020, 744, 140862. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Z.; Chen, W.; Chen, Y.; Wang, X.; Chen, H. Role of porous structure and active O-containing groups of activated biochar catalyst during biomass catalytic pyrolysis. Energy 2020, 210, 118646. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, L.; Chu, H.; Wang, J.F.; Wang, T.; Ma, Y.; Wang, C.C. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@ NCx: Singlet oxygen evolution and electron-transfer mechanisms. Appl. Catal. B Environ. 2023, 321, 122034. [Google Scholar] [CrossRef]
- Xu, K.; Lin, Q.; Fan, X.; Zheng, J.; Liu, Y.; Ma, Y.; He, J. Enhanced degradation of sulfamethoxazole by activation of peroxodisulfate with red mud modified biochar: Synergistic effect between adsorption and nonradical activation. Chem. Eng. J. 2023, 460, 141578. [Google Scholar] [CrossRef]
- Wang, H.; Guo, W.; Liu, B.; Si, Q.; Luo, H.; Zhao, Q.; Ren, N. Sludge-derived biochar as efficient persulfate activators: Sulfurization-induced electronic structure modulation and disparate nonradical mechanisms. Appl. Catal. B Environ. 2020, 279, 119361. [Google Scholar] [CrossRef]
- CYLview20. Available online: https://www.cylview.org/index.html (accessed on 15 December 2023).
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Zhang, K.; Min, X.; Zhang, T.; Xie, M.; Si, M.; Chai, L.; Shi, Y. Selenium and nitrogen co-doped biochar as a new metal-free catalyst for adsorption of phenol and activation of peroxymonosulfate: Elucidating the enhanced catalytic performance and stability. J. Hazard. Mater. 2021, 413, 125294. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Zhang, Y.; Shi, J.; Xu, L.; Wang, Y.; Jin, P. A new application pattern for sludge-derived biochar adsorbent: Ideal persulfate activator for the high-efficiency mineralization of pollutants. J. Hazard. Mater. 2021, 419, 126343. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, D.; Wang, S.; Zhang, R.; Wang, Y.; Liu, M. Activation of peroxymonosulfate by nitrogen-doped porous carbon for efficient degradation of organic pollutants in water: Performance and mechanism. Sep. Purif. Technol. 2022, 280, 119791. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Functionalization, pH, and ionic strength influenced sorption of sulfamethoxazole on graphene. J. Environ. Chem. Eng. 2014, 2, 310–315. [Google Scholar] [CrossRef]
- Shi, Q.; Li, A.; Qing, Z.; Li, Y. Oxidative degradation of Orange G by persulfate activated with iron-immobilized resin chars. J. Ind. Eng. Chem. 2015, 25, 308–313. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, Y.; Kong, D.; Lu, J.; Zhou, Q. Kinetic and mechanistic investigations of the degradation of sulfamethazine in heat-activated persulfate oxidation process. J. Hazard. Mater. 2015, 300, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Berruti, I.; López, M.I.P.; Oller, I.; Laurenti, E.; Minella, M.; Calza, P. The reactivity of peroxymonosulfate towards sulfamethoxazole. Catal. Today 2023, 413, 113975. [Google Scholar] [CrossRef]
- Du, A.; Fu, H.; Wang, P.; Zhao, C.; Wang, C.C. Enhanced catalytic peroxymonosulfate activation for sulfonamide antibiotics degradation over the supported CoSx-CuSx derived from ZIF-L (Co) immobilized on copper foam. J. Hazard. Mater. 2022, 426, 128134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zou, X.; Mao, J.; Wu, X. Decomposition of sulfadiazine in a sonochemical Fe0-catalyzed persulfate system: Parameters optimizing and interferences of wastewater matrix. Appl. Catal. B Environ. 2016, 185, 31–41. [Google Scholar] [CrossRef]
- Liu, B.; Guo, W.; Wang, H.; Si, Q.; Zhao, Q.; Luo, H.; Ren, N. B-doped graphitic porous biochar with enhanced surface affinity and electron transfer for efficient peroxydisulfate activation. Chem. Eng. J. 2020, 396, 125119. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, X.; Liu, S.; Liu, Y.; Zeng, G.; Ye, S.; Yin, Z.; Hu, X.; Liu, N. Catalytic degradation of estrogen by persulfate activated with iron-doped graphitic biochar: Process variables effects and matrix effects. Chem. Eng. J. 2019, 378, 122141. [Google Scholar] [CrossRef]
- Yu, Y.; Li, N.; Lu, X.; Yan, B.; Chen, G.; Wang, Y.; Duan, X.; Cheng, Z.; Wang, S. Co/N co-doped carbonized wood sponge with 3D porous framework for efficient peroxymonosulfate activation: Performance and internal mechanism. J. Hazard. Mater. 2022, 421, 126735. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, P.; Chen, T.; Gao, K.; Xiong, Y.; Lu, Y.; Dionysiou, D.D.; Wang, D. Efficient 1O2 production from H2O2 over lattice distortion controlled spinel ferrites. Appl. Catal. B Environ. 2024, 343, 123468. [Google Scholar] [CrossRef]
- Pei, X.; Peng, X.; Jia, X.; Wong, P.K. N-doped biochar from sewage sludge for catalytic peroxydisulfate activation toward sulfadiazine: Efficiency, mechanism, and stability. J. Hazard. Mater. 2021, 419, 126446. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S.; et al. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on π–π electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, L.; Wang, R.; Li, G.; Rao, P.; Ju, M.; Jian, L.; Guo, X.; Che, L. Recyclable nitrogen-doped biochar via low-temperature pyrolysis for enhanced lead (II) removal. Chemosphere 2022, 286, 131666. [Google Scholar] [CrossRef] [PubMed]
- Sang, F.; Yin, Z.; Wang, W.; Almatrafi, E.; Wang, Y.; Zhao, B.; Gong, J.; Zhou, C.; Zhang, C.; Zeng, G.; et al. Degradation of ciprofloxacin using heterogeneous Fenton catalysts derived from natural pyrite and rice straw biochar. J. Clean. Prod. 2022, 378, 134459. [Google Scholar] [CrossRef]
- Yang, F.; Jin, C.; Wang, S.; Wang, Y.; Wei, L.; Zheng, L.; Gu, H.; Lam, S.S.; Naushad, M.; Li, C.; et al. Bamboo-based magnetic activated carbon for efficient removal of sulfadiazine: Application and adsorption mechanism. Chemosphere 2023, 323, 138245. [Google Scholar] [CrossRef]
- Sun, P.; Liu, H.; Feng, M.; Guo, L.; Zhai, Z.; Fang, Y.; Zhang, X.; Sharma, V.K. Nitrogen-sulfur co-doped industrial graphene as an efficient peroxymonosulfate activator: Singlet oxygen-dominated catalytic degradation of organic contaminants. Appl. Catal. B Environ. 2019, 251, 335–345. [Google Scholar] [CrossRef]
- Meghani, R.; Lahane, V.; Kotian, S.Y.; Lata, S.; Tripathi, S.; Ansari, K.M.; Yadav, A.K. Valorization of Ginger Waste-Derived Biochar for Simultaneous Multiclass Antibiotics Remediation in Aqueous Medium. ACS Omega 2023, 8, 11065–11075. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. Nitrogen functionalized biochar as a renewable adsorbent for efficient CO2 removal. Energy Fuels 2018, 32, 11742–11748. [Google Scholar] [CrossRef]
- Li, W.; Nie, C.; Wang, X.; Ye, H.; Li, D.; Ao, Z. Alkaline lignin-derived N-doped biochars as peroxymonosulfate activators for acetaminophen degradation: Performance and catalytic bridging mediated Electron-Transfer mechanism. Sep. Purif. Technol. 2023, 323, 124418. [Google Scholar] [CrossRef]
- Wu, W.; Wang, R.; Chang, H.; Zhong, N.; Zhang, T.; Wang, K.; Ren, N.; Ho, S.H. Rational electron tunning of magnetic biochar via N, S co-doping for intense tetracycline degradation: Efficiency improvement and toxicity alleviation. Chem. Eng. J. 2023, 458, 141470. [Google Scholar] [CrossRef]
- Wang, A.; Ni, J.; Wang, W.; Liu, D.; Zhu, Q.; Xue, B.; Chang, C.C.; Ma, J.; Zhao, Y. MOF Derived Co- Fe nitrogen doped graphite carbon@ crosslinked magnetic chitosan Micro- nanoreactor for environmental applications: Synergy enhancement effect of adsorption- PMS activation. Appl. Catal. Environ. 2022, 319, 121926. [Google Scholar] [CrossRef]
- Byambaa, B.; Seid, M.G.; Song, K.G.; Kim, E.J.; Lee, D.; Lee, C. Insight into disparate nonradical mechanisms of peroxymonosulfate and peroxydisulfate activation by N-doped oxygen-rich biochar: Unraveling the role of active sites. Chemosphere 2024, 346, 140563. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Yan, H.; Liang, Y.; Wang, H.; Duan, P.; Lai, B. Nitrogen doped biochar derived from algae as persulfate activator for the degradation of tetracycline: Role of exogenous N doping and electron transfer pathway. Sep. Purif. Technol. 2023, 318, 123970. [Google Scholar] [CrossRef]
- Hu, J.; Li, X.; Liu, F.; Fu, W.; Lin, L.; Li, B. Comparison of chemical and biological degradation of sulfonamides: Solving the mystery of sulfonamide transformation. J. Hazard. Mater. 2022, 424, 127661. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Peng, W.; Liao, P.; Xiao, Y.; Xie, W.; Qian, A.; Ning, Y.; Zhang, N.; Niyuhire, E.; Cui, H.J. Natural sediments activating peroxydisulfate for in situ degradation of antibiotics under anoxic conditions: The critical role of surface-adsorbed and structural Fe (II). Chem. Eng. J. 2023, 472, 144938. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhou, Z.W.; Li, X.; Yang, Y.L.; Gao, J.F.; Yu, R.; Wang, H.P.; Wang, N. Synergistically boosting sulfamerazine degradation via activation of peroxydisulfate by photocatalysis of Bi2O3-TiO2/PAC under visible light irradiation. Chem. Eng. J. 2022, 428, 132613. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Lin, J.; Yang, Y.; Liu, M.; Liu, Y. Enhanced Peroxydisulfate (PDS) Activation for Sulfamethoxazole (SMX) Degradation by Modified Sludge Biochar: Focusing on the Role of Functional Groups. Water 2024, 16, 505. https://doi.org/10.3390/w16030505
He Y, Lin J, Yang Y, Liu M, Liu Y. Enhanced Peroxydisulfate (PDS) Activation for Sulfamethoxazole (SMX) Degradation by Modified Sludge Biochar: Focusing on the Role of Functional Groups. Water. 2024; 16(3):505. https://doi.org/10.3390/w16030505
Chicago/Turabian StyleHe, Yuting, Jiantao Lin, Yuchuan Yang, Minghua Liu, and Yifan Liu. 2024. "Enhanced Peroxydisulfate (PDS) Activation for Sulfamethoxazole (SMX) Degradation by Modified Sludge Biochar: Focusing on the Role of Functional Groups" Water 16, no. 3: 505. https://doi.org/10.3390/w16030505
APA StyleHe, Y., Lin, J., Yang, Y., Liu, M., & Liu, Y. (2024). Enhanced Peroxydisulfate (PDS) Activation for Sulfamethoxazole (SMX) Degradation by Modified Sludge Biochar: Focusing on the Role of Functional Groups. Water, 16(3), 505. https://doi.org/10.3390/w16030505






