Abstract
Drinking water requires excellent physico-chemical quality. It must therefore not contain any substance which is harmful, or which may harm the health of the consumer. The drinking water supply of Bangeli canton (Togo) is provided by ground water and surface water which have been polluted by several industrial discharges as a result of Togo’s intensive industrialization in the last few decades. In order to contribute to the control of drinking water in this locality, our study focused on the state of metal pollution in the waters of this canton. To assess the spatiotemporal evolution of the metallic contamination in Bangeli waters, surface and groundwater samples were taken during dry and rainy seasons in the last three years. This comparative study will allow for a more in-depth assessment of the study area. These samples were analysed by ICP-MS for heavy metals such as Fe, Pb, Cr, Zn, Cu, Co, As, Mn, Sb, Ni, and Cd. The mean values obtained for these metals from groundwater are, respectively, 1144.87; 2.53; 3.42; 3.63; 6.49; 0.69; 143.76; 160.03; 5.67; and 0.036 µg/L. Except for Fe, Sb, and Mn, all the other metals have values below WHO guidelines. The heavy metal pollution index (HPI) applied to these waters gave values between 31.49 and 307.51, with 88.66% of samples below the limit value (100). Health risk assessment factors, including average daily doses, hazard quotient, carcinogenic risks, and hazard index indices, were computed for children and adults. Finally, the HPI index of Fe and Sb in both child and adult cases showed a value greater than the safe limits, which causes harmful health hazards and potential non-carcinogenic health risks to humans. However, carcinogenic risk for Cr, Cd, Pb, and As is less than the limit value (10−4), indicating that there is no risk of cancer. The interpretation of PCA results made it possible to understand that mining has contributed to the pollution of some water resources in Bangeli, and cluster analysis (CA) applied to the data showed that the sampling points can be grouped into four groups, which were primarily formed by extravagant concentrations of Fe, Mn, Cu, and Sr.
1. Introduction
The quality of surface and ground waters can result from natural and anthropogenic constraints as well as from the management and economy of these waters [1]. Given the socio-economic and demographic development of developing countries, particular interest is given to the protection and sustainable conservation of hydrographic systems. The main cause of the degradation of water quality remains human activity: industry, domestic waste, agriculture, and transport [2]. Several studies have proven that these anthropogenic activities can cause pollution of water resources by heavy metals [3,4,5,6,7]. The contamination of aquatic systems by heavy metals is a serious issue globally, due to the toxic potential and accumulation of these contaminants in aquatic habitats, leading to ecological impacts and problems for human health [8].
Togo is not immune to the scourge of water pollution by heavy metals. Indeed, several industries have been booming in recent years. These industries generate effluents and waste that contain heavy metals [9]. A clear example is the mining industry, as its activities generate waste containing heavy metals, which are often dispersed by wind or water [10]. Heavy metals from these industries are mostly at high levels and constitute a great danger to public health. The extent and degree of heavy metal contamination in mining areas vary according to the geochemical characteristics and mineralization degree of the tailings (mining residues) [10], so it is important to understand the study area and the nature of mining activity when assessing heavy metal pollution in a specific zone. Thus, several researchers have proven that the waters of abandoned mine sites are polluted by heavy metals such as Pb, Cd, Zn, Cu, Cr, and As [2,11]. The release of these heavy metals in the water is linked to several factors, in particular the pH, the redox potential, the form in which they occur in the environment, the microorganisms found there, or the cation exchange capacity [2]. In fact, numerous studies have investigated the sources and pathways of the release of heavy metals into the environment, which is essential to understand the situation from the base [12,13,14]. Additionally, these types of investigations typically include health risk assessments and offer valuable overviews of the hazards associated with heavy metal exposure [5,15,16]. However, there are not many cases of studies about specific areas currently undergoing an intensive process of industrialization, as is the case with Togo.
Heavy metals are generally considered to be metals whose density exceeds 5 g/cm3. Heavy metals are among the main contaminants in water sources. The following make up those heavy metals and metalloids known to be environmentally hazardous: copper, silver, zinc, cadmium, lead, chromium, iron, nickel, tin, arsenic, selenium, molybdenum, cobalt, manganese, and aluminium [17,18]. Some of these heavy metals are essential for the growth, development, and health of living organisms, while others are well-known, highly water-soluble, toxic and carcinogenic agents. They pose a threat to humans (cancer, organ damage, nervous system damage, and, in extreme cases, death) as well as to the fauna and flora of the receiving water [17,18,19]. They are neither biologically nor chemically degradable in the environment but accumulate in the human body, reducing growth and development [5,20]. Indeed, heavy metals play a crucial role in the field of ecotoxicology due to their high persistence, bioaccumulation, and biomagnification in the food chain of aquatic bodies [21]. This leads to episodes of heavy metal contamination occurring far from the source of emission of these pollutants. Human exposure to heavy metals occurs primarily through three routes: ingestion, inhalation, or skin contact [22]. It is worth noting that when heavy metals are present in low concentrations they are not harmful to health. However, they can cause serious health problems when they occur in high concentrations and over a prolonged period of exposure [21,23]. Refs. [8,23] conducted a literature review of 147 studies worldwide related to heavy metal assessments and concluded that aquatic systems are highly contaminated by heavy metals. Furthermore, they stated that elements such as As, Co, Cr, Ni, and Cd exhibited the highest levels of toxicity and cancer risk through oral ingestion. Therefore, considering the hazardous nature of heavy metals, it is necessary to carry out preventive detection and effective studies on health risks in specific areas where contamination issues may arise due to anthropogenic activities such as mining. Conversely, the lack of environmental monitoring will lead to serious complications in the future due to the adverse effects of heavy metals [23,24].
The adverse health effects of long-term exposure to heavy metals may lead to a variety of cancers, mental retardation and neurological, cardiovascular, kidney, and bone diseases [12,15,16,25,26]. For these reasons, chronic exposure to a mixture of metal(loid)s must be seen as a major public health concern. Also, a systematic human health risk assessment for individuals exposed to toxic metal(loid)s through groundwater consumption is obligatory. Health risk assessment involves identifying the potential of a risk source to introduce risk agents into the environment, estimating the amount of risk agents that come into contact with human–environment boundaries, and quantifying the health consequences of exposure [15,16,26,27,28,29,30,31,32]. Water contaminated by these pollutants therefore requires depollution before its use.
Bangeli is a commune in the prefecture of Bassar, within which there is an open-pit mine whose main activity was iron extraction. The solid waste generated by this activity is rich in heavy metals such as Pb, Cd, Mn, and Fe [33]. They are stored at the foot of the hill called Bangeli Mountain, upstream of the water retention basin built on the site of Ledjole River. This river drains water during the rainy period, the use of which is diverse: market gardening, laundry, or watering of animals. It is therefore necessary to know the state of metal pollution of the waters of this municipality, in order to avoid the consequences for health. Therefore, this study aims to comprehensively evaluate the metal pollution status of the waters in Bangeli canton. Our specific objectives are to identify potential sources of pollution, assess the risks posed within human-environment limits, and quantify the health consequences of these exposures.
2. Methodology
2.1. Study Area
The prefecture of Bassar is located in zone IV of the ecological map of Togo. Within this prefecture is the canton of Bangeli, located approximately 36 km west of the city of Bassar (Figure 1). It is located between 09°42′19″ North latitude and 0°62′43″ East longitude. Iron working has been carried out for decades in an artisanal way. The Bangeli iron mine was operated by MM Mining SA, on the basis of an investment agreement dated 7 August 2006 with the Togolese State. Mining activities are expected to cover an area of approximately 3708 km2 in the Buem structural unit and approximately 11,621 km2 in the Atakora structural unit [34]. The reserve is estimated at 500 million tons and is located at a depth of about 10 to 30 m from the ground surface. Iron ore is mainly concentrated over a length of 50 km at the Bangeli hill, with iron proportions varying between 35 and 55%. The ore occurs as hematite (Fe2O3), with traces of titanium (Ti), aluminium, manganese (Mn), and water (H2O) [33,35]. Around the mine, which has been abandoned since 2017, there are large quantities of waste rock and ores abandoned in the open pit which are dispersing into the environment. The water from upstream was used to wash the ores before transporting them. Other authors [34] have shown that the sediments of the water reservoir, the waste rock, and the raw ores abandoned on the site are polluted with Pb, Cd, As, Mn, and Fe, with respective average values of 52.4 mg/kg, 34.8 mg/kg, 53.68 mg/kg, 2503.6 mg/kg, and 383,742 mg/kg. The area is built on Precambrian metasedimentary rock and is characterized by significant annual rainfall ranging from 1300 to 1500 mm, varied and diversified soils, and a climate of the Guinean Sudanese type [34]. The region benefits from a Guinean tropical climate with two seasons: a dry season, which lasts from October to April, and a rainy season (the monsoon), which lasts from April to October. The annual thermal averages vary from 26.4 °C to 28.3 °C, and the climate is characterized by a monomodal rainfall regime. The length of the vegetative period is 206 days, with an evapotranspiration of 1700 mm. Solar radiation per day is greater than 500 cal/cm2 [36].
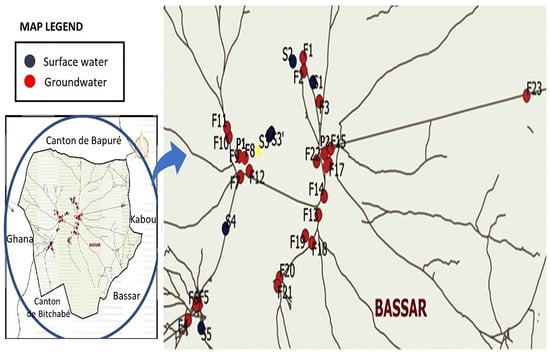
Figure 1.
Study area, indicating sampling points.
2.2. Sampling and Analysis
A total of five sampling campaigns were carried out, respectively, in August (2021 and 2022) and April (2021, 2022, and 2023). This specific selection was driven by the distinct climatic conditions observed in Togo during these months. August is characterized by a significant amount of precipitation, representing the peak of the rainy season. In contrast, April experiences minimal rainfall. By choosing these months, we aimed to capture the contrasting environmental dynamics associated with the wet and dry seasons, allowing for a comprehensive understanding of how heavy metal concentrations vary under different climatic conditions. The samples were collected in 23 human-powered boreholes designated by Fi, two wells (P1 and P2), and surface water (Sp). Boreholes and wells are used as sources of drinking water. The surface waters are used for washing dishes, bathing, and laundry; the surface water consists of ponds, a dam (S3), and a stream (S4). The choice of these sites results from their frequentation by the population, their accessibility, their position in relation to the abandoned site (upstream, downstream, more or less than 2 km distant), and the availability of water in all seasons. Besides, these sample points are near from the former mining sites (represented by the yellow circle in Figure 1). The samples were collected in polyethylene bottles, after pumping for 10 min for borehole water. As far as surface waters are concerned, a polyethylene plastic container was used to take the samples; well water was collected using a bucket with a rope. The bottles were washed beforehand with a nitric acid solution (10%), then rinsed with distilled water. At each sampling point, the bottle was rinsed three times with water from the sampling point before it was filled, in order to avoid oxidation of trace elements in the presence of oxygen. Surface water is used particularly for market gardening, laundry, watering, washing up, and even sometimes human food in times of drought. Groundwater is used as drinking water.
The samples were acidified with 0.5% of nitric acid on arrival at the laboratory and then filtered on filter paper at 0.45 µm [37,38,39]. Acidification helps dissolve metals associated with particulate matter, and filtration removes any solid particles, ensuring that the analysis mainly captures the metal content in the liquid or soluble phase of the samples. Then, the samples were sent to the laboratory of the Scientific Instrumentation Centre (CIC) of Granada University for the determination of major elements and metallic traces. The analyses of metals such as Co, As, Cd, Cu, Mn, Sb, Cr, Ni, Zn, Sr, Pb, and Al were performed by ICP-MS; these metals were in dissolved form. The detection limit varies from 0.5 to 0.01 µg/L, depending on the metals. The precision was better for 62% and 65% analyte concentrations of 50 and 5 ppm, respectively.
Parameters such as pH, temperature, and conductivity were determined in situ, using a Sartorius pH meter PT-10 and a portable conductivity meter, brand Elmettron, type CC—411 from the LaCOSE laboratory (Kara University). Each device directly gives the value of the parameter and specifies the temperature of the sample once the probe is immersed.
2.3. Heavy Metal Pollution Index
In order to estimate the water contamination, a metal contamination quantification tool was used: HPI. This index has been used by several authors to assess the level of pollution [40,41,42,43]. It provides information and advice needed by policy makers on the level of pollution of an aquatic ecosystem [40,44,45]. HPI values were calculated according to Equation (1):
where: Qi is the sub-index of the ith parameter;
Wi: weighting unit of the ith parameter; n is the number of parameters considered;
Mi is the value of the heavy metal content of the ith parameter;
Si and li are the standards and ideal values, respectively, for drinking water taken from WHO (2017) for the HMs (μg/L). A value of HPI below 100 represents low pollution of HMs, while 100 is the threshold value at which harmful health consequences are probable. An HPI value greater than 100 indicates the water is unsuitable for consumption [42,43].
2.4. Human Health Risk Assessment
The human health risk assessment (HHRA) of each metal(loid) involves data compilation and examination, exposure and toxicity determination, and risk categorization. The initial step involved a comprehensive review and compilation of relevant data on heavy metal concentrations, exposure pathways, and toxicity profiles. The existing literature, particularly works by [8,12,30], served as foundational references to ensure a thorough understanding of the variables influencing human health risk. Exposure determination is estimated by considering the daily exposure value of a human body to heavy metals. The key exposure routes include direct ingestion, inhalation, and dermal absorption [46,47]. The inhalation route being negligible [48,49], we have determined exposure by employing ingestion and dermal routes, since these are the two important routes of HM exposure from an aquatic ecosystem [8,30]. The exposure assessment was computed using equations from [8,27]:
where ADDi (µg/kg/day) and ADDd (µg/kg/day) are the average daily doses through ingestion and dermal absorption of water, respectively. In Equations (4) and (5), Ci is the concentration of the HM (µg/L), and Kp is the dermal permeability coefficient (cm/h) of a heavy metal in water, IR is the ingestion rate, EF represents exposure frequency, ED is exposure duration, BW indicates body weight, AT is the average time for non-carcinogens, SA represents exposed skin area, ET represents exposure time, and CF is the conversion factor [30,50].
The hazard quotient (HQ) is an estimate of the toxicity potential posed by an element from direct ingestion or dermal absorption routes, which can be calculated using the equations:
where RfDi and RfDd are the ingestion and oral/dermal reference doses (mg/kg/d), respectively, HQi is the hazard quotient through ingestion, and HQd is the hazard quotient through dermal absorption [8,27,30]. In general, RfD is an approximate estimate of daily exposure to humans (including sensitive subgroups) that is likely to have any noticeable risk of harmful effects during a lifetime. The RfD estimate may have an uncertainty spanning perhaps an order of magnitude; its values are those used by [8,27,30]. The exposed population is assumed to be safe when the HQ is lower than 1 [31,32,46]. The hazard index (HI) is the overall potential for non-carcinogenic effects posed by more than one contaminant via ingestion or dermal pathways, which can be estimated from the equation:
This index categorizes health risks into two types: HI < 1 indicates a low detrimental impact of HMs on human health, while HI > 1 represents greater chances of harmful health effects [12,27]. The carcinogenic risks (CR) of HMs were calculated to evaluate the chances of an individual developing cancer during their life span due to their contact with potential carcinogens. The slope factor (SF) is a toxicity value that describes the association between dose and response. Of the selected heavy metals, Cd, Cr, Pb, and As have significant cancer risks. SF (mg/kg/d) values are those used by [8] (Cr = 0.5; Cd = 15; Pb = 1.7; and As = 1.5). The CR was computed by:
where CRi and CRd are the carcinogenic risks for ingestion and dermal absorption pathways, respectively [27].
2.5. Statistical Analysis
An exhaustive statistical analysis was used to study the behaviour of heavy metals in the environment. All the statistical techniques were employed with R software; specifically, the R Commander package was used, due to its push-button menus which make statistical tests, plots and data manipulation easily accessible [51]. First of all, the Shapiro–Wilk test was conducted to check the normality of the data before proceeding with the rest of the analyses. Then, a Pearson correlation matrix was performed, in order to see the relationships between concentrations of heavy metals and physical-chemical parameters, based on Pearson coefficient (r). Therefore, this test was essential to understand behaviour of the heavy metals in the study zone. Additionally, p-values were observed, in order to prove the significance of the correlations by Holm’s method.
On the other hand, ANOVA with the Tukey test was performed in order to compare the means of heavy metals and physical-chemical parameter concentrations between seasons and type of water (surface and groundwater). For this purpose, it was necessary to add new qualitative variables to the dataset which group the two seasons of the year (dry and rain), type of water, and the zones chosen, according to the date at which each sample was taken and its proximity in relation to the mining activity of the study zone. In this case, the ANOVA-Tukey test was truly convenient for the study, since it enabled us to compare entirely different situations regarding the presence of HMs in the study area.
Finally, principal component analysis (PCA) and hierarchical cluster analysis (HCA) were performed, since they are effective multivariate statistical methods to organize datasets with a huge number of variables and observations. This was a crucial test for our study, as it involved the analysis of multiple variables. PCA synthesizes the information of the dataset, so the objective was to reduce the number of variables by losing as little information as possible. To achieve this, Kaiser’s criterion was used to choose the principal components (PCs) which have values of variance greater than 1. On the other hand, HCA creates data groups based on their similarity, so it is a nice way to classify the observations of the dataset in order to find some reason why such a grouping has been generated. Ward’s method was used to perform the HCA, due to it being a method which obtains a high agglomerative coefficient (reliability of the classification).
3. Results and Discussion
3.1. Spatial Variation of Heavy Metals in Water
The field parameters showed a variability of values depending on the season. Figure 2 shows the results for temperature (a), pH (b), and electrical conductivity (c) in every sample point. The analysis of these results shows, on the one hand, a heterogeneity between the values of the conductivity according to the seasons and the sampling points (Figure 2a). Indeed, the electrical conductivity values of water decrease from the dry season to the rainy season and oscillate from 378 to 1443 µs/cm in the rainy season, and from 370 to 2575 µs/cm in the dry season for groundwater. On the other hand, for surface waters, this parameter evolves from 50.3 to 179.56 µs/cm in the rainy season, and from 95.75 to 542.5 µs/cm in the dry season. This variability can be explained by the contribution of ions by runoff and infiltration water during the rainy period. The seasonal variations observed are on the same order as those obtained by [52], a study carried out on River Benue water at Makurdi. A decrease in electrical conductivity in the rainy season would be linked to the dilution effect. On the other hand, there is a significant decrease in temperature from the rainy season to the dry season, with values that oscillate between 25.8 and 29 °C in the rainy season and from 28.5 to 35.7 °C in the dry season (Figure 2c). Between 10 and 30 °C, the temperature has only a negligible effect on the mobility of metals. However, in a mining environment, a water temperature higher than 30 °C could influence acid mine drainage (AMD) because it can accelerate the oxidation reactions of minerals [53]. The variation in temperature observed would be linked to the level of sunshine. Figure 2b also presents the variations of pH according to the seasons and the sampling points. Analysis of graph (b) in Figure 2 shows that pH is between 6.51 and 7.31 in the rainy season and 4.6 to 7.1 in the dry season. These results show that 26% of the samples in the dry season have a pH lower than 6.5, which represents the guideline value of the [54,55]. These waters are therefore acidic and can lead to the dissolution of metals [11]. Similar seasonal variations in pH and conductivity have been obtained by other researchers [37,56]. Therefore, key field parameters such as temperature, pH, and EC are influenced by seasonal changes and sample points in the study zone, which can have an impact on HM behaviour in the environment.
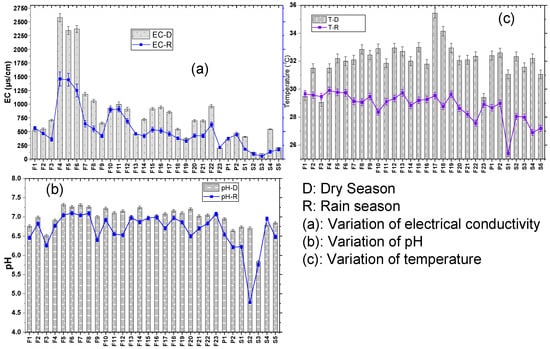
Figure 2.
Variations of pH, conductivity, and temperature of the waters studied.
Table 1 presents the content of each HM (Heavy Metal) analysed, in groundwater as well as surface water sample points. Additionally, the established limit values set by the EU and WHO are shown, to be able to contrast them. Analysis of this table shows that Fe, Sr, Sb, and Mn are the most important metals in the water. Concentrations of metals such as As (<4.5 µg/L), Cd (<0.5 µg/L), Pb (<6.5 µg/L), Cu (<61.7 µg/L), Cr (<20 µg/L), and Zn (<2852.25 µg/L) are all below the WHO and EU limit values; Al has not been detected in groundwater, but exists in trace amounts in surface water (<0.55 µg/L). Except for sample S5 (112.78 µg/L), all the other samples have values below 50 µg/L. The low levels found symbolize that the mine did not have such an impact on these waters for these metals. This testifies to the non-pollution of water by this metal. This result agrees with those of other authors [34]. Indeed, these authors reported the non-detection of metals such as copper, nickel, and lead during their work in the same zone. However, their analysis device had a detection limit of 5 µg/L for copper and 30 µg/L for nickel and lead, not allowing them to obtain a value. No limit value has been set by the organizations for Strontium (Sr), although its concentrations are high (7.46 to 1359.70 µg/L). For cobalt, the concentrations of vary between 0.1 and 11.52 µg/L. Similar results were obtained by [44] in western Côte d’Ivoire during the assessment of HM contamination of Zouan-Hounien groundwater.

Table 1.
Average concentration (µg/L) and guideline value of each HM in the waters of the study environment.
Iron and manganese play an important role in regulating the biochemical cycle of plants and animals [57]. Iron is one of the most abundant metals in the waters sampled. The Fe concentration varies from 8 to 20,475 µg/L (mean = 1144.87 µg/L) for groundwater and from 40 to 835,843 µg/L (average of 115,548.15 µg/L) for surface waters. This high value of Fe is a pivotal point for our study, since Fe is well-linked with mining activity. Sampling points where the iron content exceeds the desirable limit value of [54] (300 µg/L), respectively, represent 56.52% (13/23) for groundwater and 80% (4/5) for surface water; but the same groundwater pollution rate for Fe is 66.52% compared to EU standards (2022). S3 and S5 have iron levels lower than that obtained for some underground water (6307.66 µg/L and 40 µg/L, respectively); this difference would be linked to the aerobic oxidation of iron and precipitation. These waters, with iron concentration exceeding the value, are likely to present a bad appearance, an unpleasant taste, or promote the growth of iron-oxidizing bacteria [57]. This significant pollution by iron of water resources in the canton would come from the mining waste. The Mn concentration varies from 5.76 to 431.72 µg/L (average 143.77 µg/L) in groundwater. For surface water, Mn varies from 11.27 to 2693.48 µg/L (Table 1). A total of 52.17% of groundwater samples and 59% of surface water samples have Mn levels exceeding the WHO guideline value. Similar results have already been obtained in the field by other researchers [34]. Excessive levels may result in nervous system breakdown and psychological problems in humans [26]. Several studies have shown that these two metals are the most abundant in the earth’s crust; their presence in water comes mainly from soil leaching, the dissolution of rocks and ores, and industrial discharges [58,59]. In general, the highest concentration of Mn and Fe being found in hand pump water samples indicates the poor quality and maintenance of hand pumps [60]. Since the mine stopped, the solid waste and ore, left on the site on the side of a steep mountain, are left to the rain and wind, which can drain them towards water resources. In addition, the work of [33] showed that the soils of the mining area, the mining waste, and the sediments of the stream receiving drain water (sampling point S3) are polluted by Fe and other metals (Mn, Pb, Cd, Al, Cr, and Cu). Indeed, the study area is located in the Bassar structural unit with silico-ferruginous rocks (40–70% de Fe2O3) [61]. Strong leaching of these soils by the rains will lead to infiltration and accumulation of heavy metals in the waters [44]. This pollution may also be due to the corroded nature of the iron pipes of hand pumps, or due to the presence of more iron in the ground strata bearing the ground water. On the other hand, the antimony concentrations greatly exceed the guideline value of the WHO [55] for all samples. The Sb content varies from 25.79 to 401.63 µg/L for groundwater and from 26.23 to 516.47 µg/L for surface water (Table 1). Most antimony is found in the soil, where it binds strongly to particles that contain iron, manganese, or aluminium [62]. The pollution of this metal in the study area is too general, so its presence may be natural.
Therefore, this analysis reveals elevated concentrations of certain heavy metals in the water sources, notably Fe, Sr, Sb, and Mn, originating from a former mining site. While metals such as As, Cd, Pb, Cu, Cr, and Zn remain below established limits, others like antimony (Sb) greatly surpass guideline values. The potential health and environmental risks necessitate urgent intervention. Proposed countermeasures include continuous monitoring, remediation of the mining site, community education, infrastructure improvement, and the exploration of water treatment technologies. Collaboration with environmental authorities is crucial for effective implementation. Addressing these concerns is imperative to safeguard water quality and protect the well-being of the local population.
In relation to environmental aspects, the high concentrations of iron and manganese, particularly in surface waters, indicate pollution likely stemming from mining activities, posing a threat to water quality. Excessive levels of these metals can impact human health and ecosystem stability. Antimony concentrations exceeding guidelines suggest potential contamination, emphasizing the need for further investigation. The cumulative impact of heavy metals raises concerns about ecosystem disruption and underscores the importance of continuous monitoring and remediation efforts. Addressing these issues is crucial for safeguarding both environmental integrity and human well-being in the study area.
3.2. Heavy Metal Pollution Indices (HPI) in Groundwater and Surface Water
The HPI has been determined by taking the mean concentration of heavy metals during all the study period using Equations (1)–(3). Table 2 presents detailed calculations of HPI for every HM analysed in this study. The different mean values of the HPI for each sample are shown in Figure 3 in order to visually assess the outcome of this index. This analysis allows us to define three classes representing the quality of the studied waters: waters of excellent quality (HPI < 50) which represent 20% of all the samples; good quality waters (50 < HPI < 100) or 66.66% of the samples; and low-quality waters which represent 13.33%. This last class is made up of samples F10, F15, P2, and S1, with the respective HPI values of 108.41; 115.54; 109.97; and 153.54. These values exceed the limit value (100) defined as the critical value by [44,60,63]; these four water sampling points therefore present heavy metal pollution. These results show that the waters of the study environment are on the whole of good quality. The low pollution of certain surface water points by MTEs can be explained by aerobic oxidation of most of them. Similar results have been obtained by other researchers [56].

Table 2.
Calculation of heavy metal pollution index (HPI) for groundwater of Bangeli.
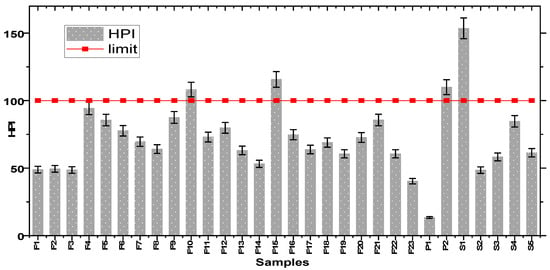
Figure 3.
Heavy metal pollution index (HPI) for samples.
This research show that Fe, Mn, and Sb are the main contaminants responsible for polluting the water in Bangeli canton. The presence of these heavy metals, especially in points exceeding the critical HPI limit, signifies the need for their removal to make the water suitable for human purposes.
3.3. Human Health Risk Assessment
The HQ, HI, and CR (cancer risk) for the drinking water pathway with respect to adults and children are quantified in Table 3 and Figure 4, using the arithmetic mean of the metal(loid) concentrations for all the drinking water samples of the study area. The results of the non-carcinogenic risks as depicted by HQ for children indicated on the one hand that Sb, followed by Fe and Mn, showed higher risks via the ingestion route, and Sb and Sr via the dermal route (HQ > 1). On the other hand, the values of HI for Sb, Sr, Fe, and Mn exceed 1. Those HMs exhibit the highest risks of contamination.

Table 3.
Assessment of potential health risks posed by various heavy metals.
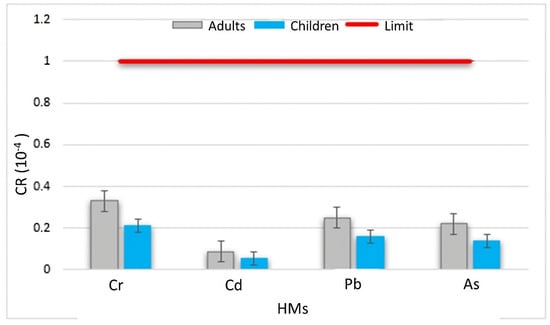
Figure 4.
Carcinogenic risk (CR) of various heavy metals in Bangeli waters.
Except for Sb (24.40), the hazard quotient (HQ) and hazard index (HI) results for all HMs display the lowest human health risks through the dermal or ingestion route (HI < 1) for adults. The hazard index was found to be higher than 1 for Sb, indicating a detrimental effect of Sb on the health of both children and adults. Since the groundwater of the study area is not contaminated with a single metal, the potential risks of the combined effect of all the 12 metals (loid)s through ingestion of drinking water was assessed using HI and calculated to be 24.40 for adult and 63.67 for children. This suggests a potential risk to human health due to consumption of the waters of Bangeli district as drinking water.
The carcinogenic risks determined for all HMs (Figure 4) showed a low risk of cancer, either via the oral or dermal route pathway, for both children and adults. The CR index was estimated for only As, Cr, Cd, and Pb, due to the availability of carcinogenic slope factors (SFs) for these elements. The cumulative range of CR was 2.2 × 10−5 to 0.87 × 10−4 (mean 0.72 × 10−4) for adults and 0.055 × 10−4 to 0.21 × 10−4 (mean 0.14 × 10−4) for children. Moreover, the cumulative CR value did not exceed the threshold range of 10−6 to 10−4. According to [28,29,30,46], the mean of total CR values due to drinking the water from Bangeli canton was less than 10−4, which supported our study. For every HM, the carcinogenic risks for different populations vary greatly, generally in the order of adults > children. The reason that the carcinogenic risk for children is less than that for adults lies in the shorter duration of exposure for children. The same results were obtained by many authors, e.g., Isa Baba [46]. Hence, the studied values did not possess any threat to local residents. In view of the fact that the cancer risk did not exceed the target risk of 10−4, it can be thus be considered ‘acceptable’ and suggests that the ingestion of these waters over a long lifetime will not increase the probability of cancer for the consumers. The human health risk assessment does not only depend on the concentration of metal(loid) in drinking water, but also on the water consumption rate. The adverse carcinogenic and non-carcinogenic risk that has been calculated for the study area can also be related to the higher consumption of water due to an inhabitant’s occupation and/or the climate. Due to the tropical climate of the study area, the daily water intake is higher as compared to regions in colder climates [12,64]. In addition, the exposure parameters employed in the study were taken from the USEPA 2005 and from other countries [12,27,65]; they might be different for Togo conditions. Therefore, for further precise risk characterisation, risk assessment approaches may be modified according to the investigation of the risk levels in Bangeli canton. In fact, these significant findings contribute to the overall understanding of heavy metal variations in Bangeli canton, emphasizing the complexity of factors influencing health risks. The importance of considering both carcinogenic and non-carcinogenic risks in water quality assessments should be noted, providing valuable insights for environmental and public health management in the region.
3.4. Statistical Analysis
With regard to correlations, only two significant correlations were found, between Cu–Cd (r = 0.66 and p-value = <0.001) and Sb–Pb (r = −0.6 and p-value = 0.0003) (Table 4). The high positive correlation between Cu–Cd may explain similar hydro-chemical characteristics or the existence of a common anthropogenic source/origin of these metals in the environment [12]. On the other hand, the negative correlation between Sb–Pb indicates that originated from different sources [21]. Additionally, it was observed that there are many negative correlations in the dataset in comparison with some works [10,12]. However, no significant correlations were found between the remaining HMs. According to [8], this is an indication of multiple points of origin for the HMs, which makes sense considering the diversity of human activities and environments in the study zone. The fact that Cd and Pb are significantly correlated HMs in the dataset is interesting, because it emphasises that mining activity really generates an impact in the zone, since the presence of Cd and Pb suggests anthropogenic activity [66]. Therefore, the Pearson correlation matrix has highlighted the interconnections between specific heavy metals and their potential sources, shedding light on the anthropogenic and environmental factors contributing to heavy metal variations in the study area. These results contribute significantly to the broader understanding of heavy metal contamination and can inform targeted environmental management strategies in regions with similar characteristics.

Table 4.
Pearson correlation matrix between concentrations of HMs.
On the other hand, ANOVA was used to study the differences in relation to seasonal variations and type of water. In the case of seasonal variations, it was observed that only the concentration of Pb and Sb had significant differences between the dry and rainy seasons (Table 5). Ref. [67] also performed an ANOVA, but they filtered the data into Pre-Monsoon, Monsoon, and Post-Monsoon. They also obtained significant seasonal differences with only two HMs (Pb and Fe). However, the remaining HMs did not show a significant variability among seasons. Ref. [68] also did not obtain significant differences with any HMs, which demonstrates that most HMs are available in the water in a stable way. In the case of Pb, high values during the dry season are attributed to the high evaporation rate of surface water followed by high temperature, and low flow condition of the water bodies leading the accumulation of HMs [67]. On the other hand, the HMs with significant differences according to the type of water were Fe and Sr. In the case of Sr, the groundwater concentration is higher than in the surface, so this element is clearly affected by the filtration processes. This shows the dynamic nature of heavy metal concentrations in water, highlighting the interplay between environmental factors, hydrogeological processes, and seasonal variations.

Table 5.
ANOVA study for season variations and type of water.
PCA shows five principal components (PCs) with eigenvalues higher than 1, representing 72.1% of the total variance of the dataset. Therefore, according to the Kaiser criterion, when analysing the behaviour of metals with the physico-chemical variables, the number of correlations can be reduced using these five PCs. The representation of the variance and the affinity of each PC with heavy metals is shown in Table 6. It can be observed that PC1 has a higher representation of the dataset variance (21.75%), and thus encompasses the highest number of heavy metals (HMs). Probably, the HMs of PC1 (Sb, Cd, Sr, Co, Cu, and Fe) are quite strongly related to mining activity in the study zone, since they share characteristics of formation or certain behaviours in correspondence with environmental variables. In fact, Table 6 also shows the correlation with the EC, temperature, and pH of each PC, and PC1 is moderately related to EC (r = 0.63 and p-value = 0.005). It means that the weathering and dissolution processes have a significant impact on these HMs [69]. Perhaps, during the activity of the mining industry in the study area, there was an uncontrolled disposal of mining wastes, and, as a result of the climate conditions, there were processes of dissolution which eventually caused the HMs to be filtrated into the groundwater. This suggests that these metals in solution may precipitate or adsorb onto the surface of precipitated hydrated sulphates during alteration processes [10]. In contrast, Ref. [70] obtained a PC1 with a strong correlation with Fe and Mn, and they suggested that it could be controlled by geogenic factors. This is interesting, because in our PC1 the Fe and Co have a negative correlation, which may mean that these metals have a different source of origin than the rest, probably geogenic. In this sense, PC2 had a positive correlation with Pb and negative with Sr, so it is possible that PC2 is influenced by mixed factors (natural and anthropogenic). PC3 and PC4 are clearly affected by mixed factors, because Cr and As are associated with anthropogenic origins such as metal processing industries, leaching of e-wastes, fossil fuel combustion, and industrial influents [69], whereas Mn and Zn have natural origins from weathering and dissolution of parent rocks. On the other hand, PC5 showed strong correlation with solely Ni, which is inversely related to the pH level (r = −0.53 and p-value = 0.037). So, PC5 can purely be attributed to anthropogenic sources such as vehicle emissions, industrial effluents, and landfill pollution [70]. This negative correlation with pH can be explained by the precipitation of HMs at higher pH and high solubility at low pH [69]. Ref. [10] also obtained negative correlations between pH and some elements, and attributed it to an origin of the elements based on pyrite oxidation.

Table 6.
PCs chosen by Kaiser’s criterion and their characteristics.
Additionally, PCA shows two different plots: the distribution of variables (HMs) (Figure 5a) and the spatial distribution of sampling points (Figure 5b). Based on the distribution of variables with respect to PC1 and PC2, HMs that appear closer together share similar behaviours and requirements. The further apart they are, the greater the difference between the elements. Therefore, at first glance, it can be observed that Cr, Ni, and Sb are grouped together, indicating that their formation or retention in the environment may be caused by the same factor. The same pattern can be observed with other groups of elements such as Mn, Fe, and Co, which are located on the opposite side of the plot, indicating that their behaviour in the environment is completely different from the previous group. This association highlights the important role played by mineral dissolution (Fe/Mn oxides and hydroxides) in water chemistry [13]. On the other hand, the arrangement of Cu and Cd is virtually identical. According to [14], these HMs are commonly originated from the leaching of phosphate fertilizers in agricultural lands. In contrast Figure 5a shows that Pb, Zn, and Sr do not share similarities with other HMs and are more isolated.
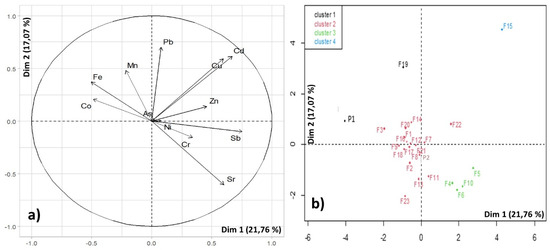
Figure 5.
Distribution of heavy metals in the study area.
On the other hand, Figure 5b groups the sample points in relation to their characteristics. The samples that appear closer together exhibit a greater similarity in their HM composition, and, therefore are more similar in their environmental characteristics. It can be observed that HCA automatically differentiates between four distinct groups based on the sampling points. It is true that some sampling points coincide in the same group due to their proximity, such as F4, F5, and F6, indicating that in that area HMs are influenced by the same factors. However, other sampling points are close on the factor map but do not coincide in reality. This may be because different areas are affected by the same factors. Ref. [10] also conducted a factor map with their sampling points, and the dispersion of their points depended on the content of jarosite and carbonates in the soils, so analysing these compounds for future investigations could be interesting.
Table 7 shows the means of each HM in relation to the four cluster groups. Firstly, it can be observed that the first group consists of only two sampling points (P1 and F19), which are characterized by high concentrations of Fe and Co, as well as relatively low concentrations of Sr compared to the other groups. Secondly, the second group is the largest one, as it has the highest number of samples (F1, F2, F3, F7, F8, F9, F11, F12, F13, F16, F17, F18, F20 F21, F22, F23, and P2), and does not stand out for having extraordinary concentrations of any HM within the scope of this study. Therefore, it can be inferred that the mean concentrations exhibited by this group would be considered normal for the study area. On the other hand, the third group consists of four sampling points (F4, F5, F6, and F10) and is characterized by high mean concentrations of Sr, Zn, and Cr. Lastly, the fourth group consists of a single sampling point (F15) that exhibits a high concentration of Cu compared to the others. Ref. [14] also obtained the same result and attributed it to the application of fungicides or algaecides in agricultural lands. Taking everything into consideration, these findings contribute to a more nuanced understanding of heavy metal variations in the study area, shedding light on the complex interplay between natural geological factors and anthropogenic activities.

Table 7.
Means of HMs for each cluster group.
4. Conclusions
This study made it possible to assess the level of contamination of surface and underground water in Bangeli canton by metallic trace elements and the origin of this pollution. The temperature and electrical conductivity of the waters vary according to the seasons, with an average neutral pH and high conductivity for groundwater (370 to 2575 µs/cm) and low for surface water (50–179.56 µs/cm). The Fe, Mn, and Sb levels greatly exceed the WHO guideline values and the EU standard for the majority of the samples. The calculation of the heavy metal pollution index shows that, except for four sampling points (F10, F15, P1 and S1), all the samples have values below the limit value (100). The HI developed in this paper showed that Sb, Fe, Cr, and Sr exhibit maximum toxicity in the water bodies, especially Sb, which is the major contaminant that affects human health via both ingestion and dermal pathways. HMs showed low cancer risks through both pathways. Therefore, the results of this paper will be helpful to local authorities in developing strategies to mitigate the problems of HM pollution in water. Then, the statistical study made it possible to highlight the source of the water pollution in the study area and to understand the behaviour of the HMs, showing a great variability in the studied area. The relationships between Cd–Cu and Sb–Pb showed great significance, indicating the presence of human impact. PCA grouped HMs into five PCs, and it showed that EC was the most relevant parameter in HM distribution. On the other hand, CA grouped the sampling points into four groups, which demonstrated that Fe, Mn, Sr, and Cu have an irregular distribution in the study area. Finally, this study allowed us to obtain an idea of the metallic pollution of the waters of Bangeli. Therefore, more appropriate measures can be taken in order to improve the management of drinking water in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16030471/s1.
Author Contributions
Conceptualization: B.T.B.; methodology: B.T.B. and A.J.A.-B.; software: B.T.B. and A.J.A.-B.; investigation: B.T.B. and A.J.A.-B.; resources: F.O.; data curation: B.T.B.; writing—original draft preparation: B.T.B. and A.J.A.-B.; writing—review and editing: F.O.; supervision: F.O., J.G.-L. and G.B.; project administration: F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article or Supplementary Material.
Acknowledgments
The results of this research were obtained through a collaboration between the University of Kara (Togo) and the University of Granada (Spain), thanks to the Development Cooperation Initiatives Centre (CICODE). Specifically, we extend our sincere appreciation to its director, Domingo Barrera Rosilio, for his support during the research period.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Akil, A.; Hassan, T.; Lahcen, B.; Abderrahim, L. Etude de la qualité physico-chimique et contamination métallique des eaux de surface du bassin versant de Guigou, Maroc. Eur. Sci. J. 2014, 10, 84–94. [Google Scholar]
- Abid, M.G.B. Contamination Métallique Issue des Déchets de l’ancien Site Minier de Jebel Ressas: Modélisation des Mécanismes de Transfert et Conception de Cartes d’aléa Post-Mine Dans un Contexte Carbonaté et Sous un Climat Semi-Aride. Evaluation du Risque Pour la sa. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2012. [Google Scholar]
- Bawa, M.M.L.; Djaneye-Boundjou, G.; Boukari, Y. Caractérisation de deux effluents industriels au Togo: étude d’impact sur l’environnement. Afrique Sci. Rev. Int. Des Sci. Technol. 2010, 2, 57–68. [Google Scholar] [CrossRef]
- Hakkou, R.; Benzaazoua, M.; Bussière, B.; Rachid, H.; Mostafa, B. Evaluation de la qualité des eaux de ruissellement dans la mine abandonnée de Kettara (Maroc). In Proceedings of the Congres International sur le Theme: Gestion Intégrée des Ressources en Eaux et Défis du Développement Durable (GIRE3D), Marrakech, Morocco, 23–25 May 2006; pp. 1–6. [Google Scholar]
- Belkhiri, L.; Mouni, L.; Narany, T.S.; Tiri, A. Evaluation of potential health risk of heavy metals in groundwater using the integration of indicator kriging and multivariate statistical methods. Groundw. Sustain. Dev. 2017, 4, 12–22. [Google Scholar] [CrossRef]
- Dan-Badjo, A.T.; Tidjani, D.; Idder, T.; Guero, Y.; Lamso, N.D.; Matsallabi, A.; Ambouta, J.; Feidt, C.; Sterckeman, T.; Echevarria, G. Diagnostic de la contamination des eaux par les éléments traces métalliques dans la zone aurifère de Komabangou–Tillabéri, Niger. Int. J. Biol. Chem. Sci. 2014, 8, 2849–2857. [Google Scholar] [CrossRef]
- Saddik, M.; Fadili, A. Assessment of heavy metal contamination in surface sediments along the Mediterranean coast of Morocco. Environ. Monit. Assess. 2019, 191, 197. [Google Scholar] [CrossRef]
- Kumar, R.N.; Solanki, R. Seasonal variation in heavy metal contamination in water and sediments of river Sabarmati and Kharicut canal at Ahmedabad, Gujarat. Environ. Monit. Assess. 2012, 185, 359–368. [Google Scholar] [CrossRef]
- Zoulgami, S.; Gnazou, M.; Kodom, T.; Djaneye-Boundjou, G.; Bawa, L. Physico-chemical study of groundwater in the Northeast of Kara region (Togo). Int. J. Biol. Chem. Sci. 2015, 9, 1711. [Google Scholar] [CrossRef]
- Navarro, M.C.; Pérez-Sirvent, C.; Martínez-Sánchez, M.J.; Vidal, J.; Tovar, P.J.; Bech, J. Abandoned mine sites as a source of contamination by heavy metals: A case study in a semi-arid zone. J. Geochemical Explor. 2008, 96, 183–193. [Google Scholar] [CrossRef]
- Baghdad, B.; Naimi, M.; Bouabdli, A.; Sonnet, P.; Garcia, S.; Bounakhla, M.; Inigo Inigo, A.C. Evaluation de la contamination et évolution de la qualité des eaux au voisinage d ’ une mine abandonnée d’ extraction de plomb (Zaida-Haute Moulouya-Maroc). In Proceedings of the 12ème Conférence Interrégionale Enviro Water, ANAFIDE (Association Nationale des Améliorations Foncières, de l’Irrigation, du Drainage et de l’Environnement) et CIGR, Marrakech, Morocco, 9–11 November 2012. [Google Scholar]
- Giri, S.; Singh, A.K. Spatial distribution of metal(loid)s in groundwater of a mining dominated area: Recognising metal(loid) sources and assessing carcinogenic and non-carcinogenic human health risk. Int. J. Environ. Anal. Chem. 2016, 96, 1313–1330. [Google Scholar] [CrossRef]
- Rajmohan, N.; Masoud, M.H.Z.; Niyazi, B.A.M.; Alqarawy, A.M. Appraisal of trace metals pollution, sources and associated health risks using the geochemical and multivariate statistical approach. Appl. Water Sci. 2023, 13, 113. [Google Scholar] [CrossRef]
- Micó, C.; Recatalá, L.; Peris, M.; Sánchez, J. Assessing heavy metal sources in agricultural soils of an European Mediterranean area by multivariate analysis. Chemosphere 2006, 65, 863–872. [Google Scholar] [CrossRef]
- Duan, B.; Zhang, W.; Zheng, H.; Wu, C.; Zhang, Q.; Bu, Y. Comparison of health risk assessments of heavy metals and as in sewage sludge from wastewater treatment plants (WWTPs) for adults and children in the urban district of Taiyuan, China. Int. J. Environ. Res. Public Health 2017, 14, 1194. [Google Scholar] [CrossRef]
- Kharazi, A.; Leili, M.; Khazaei, M.; Alikhani, M.Y.; Shokoohi, R. Human health risk assessment of heavy metals in agricultural soil and food crops in Hamadan, Iran. J. Food Compos. Anal. 2021, 100, 103890. [Google Scholar] [CrossRef]
- Ullah, A.; Heng, S.; Munis, M.F.H.; Fahad, S.; Yang, X. Phytoremediation of heavy metals assisted by plant growth promoting (PGP) bacteria: A review. Environ. Exp. Bot. 2015, 117, 28–40. [Google Scholar] [CrossRef]
- Jacob, J.M.; Karthik, C.; Saratale, R.G.; Kumar, S.S.; Prabakar, D.; Kadirvelu, K.; Pugazhendhi, A. Biological approaches to tackle heavy metal pollution: A survey of literature. J. Environ. Manag. 2018, 217, 56–70. [Google Scholar] [CrossRef]
- Yuan, L.; Zhi, W.; Liu, Y.; Karyala, S.; Vikesland, P.J.; Chen, X.; Zhang, H. Lead toxicity to the performance, viability, community composition of activated sludge microorganisms. Environ. Sci. Technol. 2015, 49, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Nada, N. Methods of removing heavy metals from industrial wastewater. J. Multidiscip. Eng. Sci. Stud. 2015, 1, 12–18. [Google Scholar]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, I.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef] [PubMed]
- Gairoard, S. Contribution à l’étude de l’ Impact des Anciens Travaux Miniers de Charbon sur les eaux Souterraines: Application a la Région d’Ales (Gard). Ph.D. Thesis, Université de Lorraine, Lorraine, France, 2009. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Sadat, A.W.; N’goran, E.B.Z.; Siaka, S.; Parinet, B. Intérêt de l’analyse multidimensionnelle pour l’évaluation de la qualité physico-chimique de l’eau d’un système lacustre tropical: Cas des lacs de Yamoussoukro (Côte d’Ivoire). J. Appl. Biosci. 2011, 38, 2573–2585. [Google Scholar]
- Zheng, X.; Zhao, W.; Yan, X.; Shu, T.; Xiong, Q.; Chen, F. Pollution characteristics and health risk assessment of airborne heavy metals collected from Beijing bus stations. Int. J. Environ. Res. Public Health 2015, 12, 9658–9671. [Google Scholar] [CrossRef]
- Ahmed, A.S.S.; Hossain, M.B.; Babu, S.M.O.F.; Rahman, M.M.; Sarker, M.S.I. Human health risk assessment of heavy metals in water from the subtropical river, Gomti, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100416. [Google Scholar] [CrossRef]
- Sharma, S.D. Risk assessment via oral and dermal pathways from heavy metal polluted water of Kolleru lake—A Ramsar wetland in Andhra Pradesh, India. Environ. Anal. Health Toxicol. 2020, 35, 11. [Google Scholar] [CrossRef]
- Gnonsoro, U.P.; Assi, Y.E.D.A.; Sangare, N.S.; Kouakou, Y.U.; Trokourey, A. Health Risk Assessment of Heavy Metals (Pb, Cd, Hg) in Hydroalcoholic Gels of Abidjan, Côte d’Ivoire. Biol. Trace Elem. Res. 2022, 200, 2510–2518. [Google Scholar] [CrossRef]
- Niknejad, H.; Ala, A.; Ahmadi, F.; Mahmoodi, H.; Saeedi, R.; Gholami-Borujeni, F.; Abtahi, M. Carcinogenic and non-carcinogenic risk assessment of exposure to trace elements in groundwater resources of Sari city, Iran. J. Water Health 2023, 21, 501–513. [Google Scholar] [CrossRef]
- USEPA. Risk Assessment Guidance for Superfund (RAGS). Volume I. Human Health Evaluation Manual (HHEM). Part E. Supplemental Guidance for Dermal Risk Assessment. USEPA vol. 1, no. 540/R/99/005. 2004. Available online: https://www.epa.gov/risk/risk-assessment-guidance-superfund-rags-part-e (accessed on 25 January 2024).
- Seleem, E.M.; Mostafa, A.; Mokhtar, M.; Salman, S.A. Risk assessment of heavy metals in drinking water on the human health, Assiut City, and its environs, Egypt. Arab. J. Geosci. 2021, 14, 427. [Google Scholar] [CrossRef]
- Alidadi, H.; Belin, S.; Sany, T.; Zarif, B.; Oftadeh, G.; Mohamad, T. Health risk assessments of arsenic and toxic heavy metal exposure in drinking water in northeast Iran. Environ. Health Prev. Med. 2019, 24, 59. [Google Scholar] [CrossRef] [PubMed]
- Tchanadema, M.; Ayah, M.; Kodom, T.; Nambo, P.; Bawa, L.M.; Djaneye-Boundjou, G. Risks of chemical pollution on the environment by solid mine waste at the semi-industrial iron mining site in Bandjeli, Togo. J. Mater. Environ. Sci. 2021, 12, 1057–1070. [Google Scholar]
- Ogouvidé, A.; Batcha, O.; Gnon, B. Modern and Traditional Methods of Water Resource Management in Africa. In Proceedings of the Water Perspectives in Emerging Countries, Durban, South Africa, 5–9 May 2019; pp. 8–10. [Google Scholar] [CrossRef]
- de Barros, P.L.; Iles, L.; Frame, L.D.; Killick, D. The Early Iron Metallurgy of Bassar, Togo: Furnaces, metallurgical remains and iron objects. Azania 2020, 55, 3–43. [Google Scholar] [CrossRef]
- Djeri, B.; Ameyapoh, Y.; Karou, D.S.; Anani, K.; Soncy, K.; Adjrah, Y.; Souza, C. Assessment of microbiological qualities of yam chips marketed in Togo. Adv. J. Food Sci. Technol. 2010, 2, 236–241. [Google Scholar]
- El Hachimi, M.L.; El Hanbali, M.; Fekhaoui, M.; Bouabdli, A.; El Founti, L.; Saïdi, N. Impact d’un site minier abandonné sur l’environnement: Cas de la mine de Zeïda (Haute Moulouya, Maroc). Bull. l’institut Sci. Rabat Sect. Sci. la Terre 2005, 27, 93–100. [Google Scholar]
- Hamid, T.; Chakit, M. Evaluating Metallic Pollution Caused By Iron, Copper, Lead and Cadmium of Oum Er-Rabia River Water. J. Bio Innov. 2016, 5, 59–67. [Google Scholar]
- Rodier, J.; Legube, B. L’Analyse De L’Eau, 9th ed.; Dépôts et Sédiments; Dunod: Malakoff, France, 2009; p. 10. [Google Scholar]
- Tiwari, A.K.; De Maio, M.; Singh, P.K.; Mahato, M.K. Evaluation of Surface Water Quality by Using GIS and a Heavy Metal Pollution Index (HPI) Model in a Coal Mining Area, India. Bull. Environ. Contam. Toxicol. 2015, 95, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Prakash, B.P.; Singh, P.K.; Tiwari, A.K.; Kumar, B.; Kumar, A. Assessment of heavy metal pollution index for groundwater around Jharia coalfield region, India. J. Biodivers. Environ. Sci. 2015, 33, 33–39. [Google Scholar]
- Prasad, M.; Sunitha, V.; Reddy, Y.S.; Suvarna, B.; Reddy, B.M.; Reddy, M.R. Data on water quality index development for groundwater quality assessment from Obulavaripalli Mandal, YSR district, A.P India. Data Brief 2019, 24, 103846. [Google Scholar] [CrossRef] [PubMed]
- Mambenga, V.I. Approche de Caractérisation Géochimique et Géo-Environnementale d’un Projet Minier Dans le Contexte de Fond Géochimique Naturellement Élevé et/ou Anthropisé: Application aux Secteurs Miniers Siscoe-Sullivan-Marban, Val-d’Or, Canada. Ph.D. Thesis, Université du Québec en Abitibi-Témiscamingue, Rouyn-Noranda, QC, Canada, 2020. [Google Scholar]
- Djade, P.J.O.; Traore, A.; Koffi, K.J.T.; Keumean, K.N.; Soro, G. Evaluation du niveau de contamination des eaux souterraines par les éléments traces métalliques dans le département de Zouan-Hounien (Ouest de la Côte d’Ivoire). J. Appl. Biosci. 2020, 150, 15457–15468. [Google Scholar] [CrossRef]
- Bhutiani, R.; Kulkarni, D.B.; Khanna, D.R.; Gautam, A. Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energy Ecol. Environ. 2017, 2, 155–167. [Google Scholar] [CrossRef]
- Isa, B.K.; Amina, S.B.; Aminu, U.; Sabo, Y. Health risk assessment of heavy metals in water, air, soil and fish. African J. Pure Appl. Chem. 2015, 9, 204–210. [Google Scholar] [CrossRef]
- Mahato, M.K.; Singh, G.; Singh, P.K.; Singh, A.K.; Tiwari, A.K. Assessment of Mine Water Quality Using Heavy Metal Pollution Index in a Coal Mining Area of Damodar River Basin, India. Bull. Environ. Contam. Toxicol. 2017, 99, 54–61. [Google Scholar] [CrossRef]
- Yakamercan, E.; Aygün, A. Health risk assessment of metal(loid)s for land application of domestic sewage sludge in city of Bursa, Türkiye. Environ. Monit. Assess. 2023, 195, 733. [Google Scholar] [CrossRef]
- Yu, G.; Wang, X.; Liu, J.; Jiang, P.; You, S.; Ding, N.; Guo, Q.; Lin, F. Applications of Nanomaterials for Heavy Metal Removal from Water and Soil: A Review. Sustainability 2021, 13, 713. [Google Scholar] [CrossRef]
- Yakamercan, E.; Aygün, A. Ecological risk assessment of domestic sewage sludge: A case study. Sigma J. Eng. Nat. Sci. 2021, 39, 422–433. [Google Scholar] [CrossRef]
- Borges, E.M. Hypothesis Tests and Exploratory Analysis Using R Commander and Factoshiny. J. Chem. Educ. 2023, 100, 267–278. [Google Scholar] [CrossRef]
- Iwar, R.T.; Utsev, J.T.; Hassan, M. Assessment of heavy metal and physico-chemical pollution loadings of River Benue water at Makurdi using water quality index (WQI) and multivariate statistics. Appl. Water Sci. 2021, 11, 124. [Google Scholar] [CrossRef]
- Yapi, Y.H.A.; Dongui, B.K.; Trokourey, A.; Barima, Y.S.S.; Essis, Y.; Etheba, P. Evaluation de la pollution métallique des eaux souterraines et de surface dans un environnement minier aurifère à Hiré (Côte d’Ivoire). Int. J. Biol. Chem. Sci. 2014, 8, 1281–1289. [Google Scholar] [CrossRef][Green Version]
- (Orgnisation Mondiale de la Santé) OMS. Directives De Qualité Pour L’Eau De Boisson Quatrième Édition Intégrant Le Premier Additif. 2017. Volume 1. Available online: https://www.who.int/fr/publications-detail/9789241549950 (accessed on 25 January 2024).
- Directive (Ue) Du Parlement Européen Et Du Conseil Relative a La Qualite Des Eaux Destinees a La Consommaiton Humaine. 2020. pp. 1–62. Available online: https://eur-lex.europa.eu/legal-content/FR/LSU/?uri=CELEX:32020L2184 (accessed on 25 January 2024).
- Lghoul, M.; Maqsoud, A.; Hakkou, R.; Kchikach, A. Hydrogeochemical behavior around the abandoned Kettara mine site, Morocco. J. Geochem. Explor. 2014, 144, 456–467. [Google Scholar] [CrossRef]
- Wagh, V.M.; Panaskar, D.B.; Mukate, S.V.; Gaikwad, S.K.; Muley, A.A.; Varade, A.M. Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik, India. Model. Earth Syst. Environ. 2018, 4, 969–980. [Google Scholar] [CrossRef]
- Fatombi, J.K.; Avocznh, G.; Topanou, N.; Aminou, T.; Josse, R.G. Elimination du fer et du manganèse d’une eau de surface par les graines de Moringa oleifera. Int. J. Biol. Chem. Sci. 2013, 7, 1379–1391. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Nasirizadeh, N.; Dashti, Z.; Babanezhad, E. Removal of Fe(II) from aqueous solution using pomegranate peel carbon: Equilibrium and kinetic studies. Int. J. Ind. Chem. 2013, 4, 19. [Google Scholar] [CrossRef]
- Prasad, B.; Kumari, P.; Bano, S.; Kumari, S. Ground water quality evaluation near mining area and development of heavy metal pollution index. Appl. Water Sci. 2014, 4, 11–17. [Google Scholar] [CrossRef]
- Le Cocq, A. Carte Pédologique et Capacité Agronomique des Sols du Togo. Bassar. 1986. Available online: https://horizon.documentation.ird.fr/exl-doc/pleins_textes/pleins_textes_5/notexp/31639.pdf (accessed on 25 January 2024).
- INERIS. Antimoine Et Ses Dérivés. 2007. Available online: https://substances.ineris.fr/fr/substance/getDocument/2712 (accessed on 25 January 2024).
- Prasad, B.; Bose, J.M. Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower himalayas. Environ. Geol. 2001, 41, 183–188. [Google Scholar] [CrossRef]
- Shehu, A.; Vasjari, M.; Duka, S.; Vallja, L.; Broli, N.; Cenolli, S. Assessment of health risk induced by heavy metal contents in drinking water. J. Water Sanit. Hyg. Dev. 2022, 12, 816–827. [Google Scholar] [CrossRef]
- Tiri, A.; Belkhiri, L.; Mouni, L. Evaluation of surface water quality for drinking purposes using fuzzy inference system. Groundw. Sustain. Dev. 2018, 6, 235–244. [Google Scholar] [CrossRef]
- Singh, R.; Majumder, C.B.; Vidyarthi, A.K. Assessing the impacts of industrial wastewater on the inland surface water quality: An application of analytic hierarchy process (AHP) model-based water quality index and GIS techniques. Phys. Chem. Earth Parts A/B/C 2023, 129, 103314. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Gupta, A.; Garg, J.K. Evaluation of heavy metal contamination using environmetrics and indexing approach for River Yamuna, Delhi stretch, India. Water Sci. 2017, 31, 52–66. [Google Scholar] [CrossRef]
- Acharya, P.; Muduli, P.R.; Das, M. Assessment of heavy metal accumulation in Penaeus monodon and its human health implications. Mar. Pollut. Bull. 2023, 188, 114632. [Google Scholar] [CrossRef] [PubMed]
- Rajan, S.; Nandimandalam, J.R. Environmental health risk assessment and source apportion of heavy metals using chemometrics and pollution indices in the upper Yamuna river basin, India. Chemosphere 2024, 346, 140570. [Google Scholar] [CrossRef] [PubMed]
- Krati, S.; Raju, N.J.; Singh, N.; Sreekesh, S. Heavy metal pollution in groundwater of urban Delhi environs: Pollution indices and health risk assessment. Urban Clim. 2022, 45, 101233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).