Debating the Rules: An Experimental Approach to Assess Cyprinid Passage Performance Thresholds in Vertical Slot Fishways
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Note
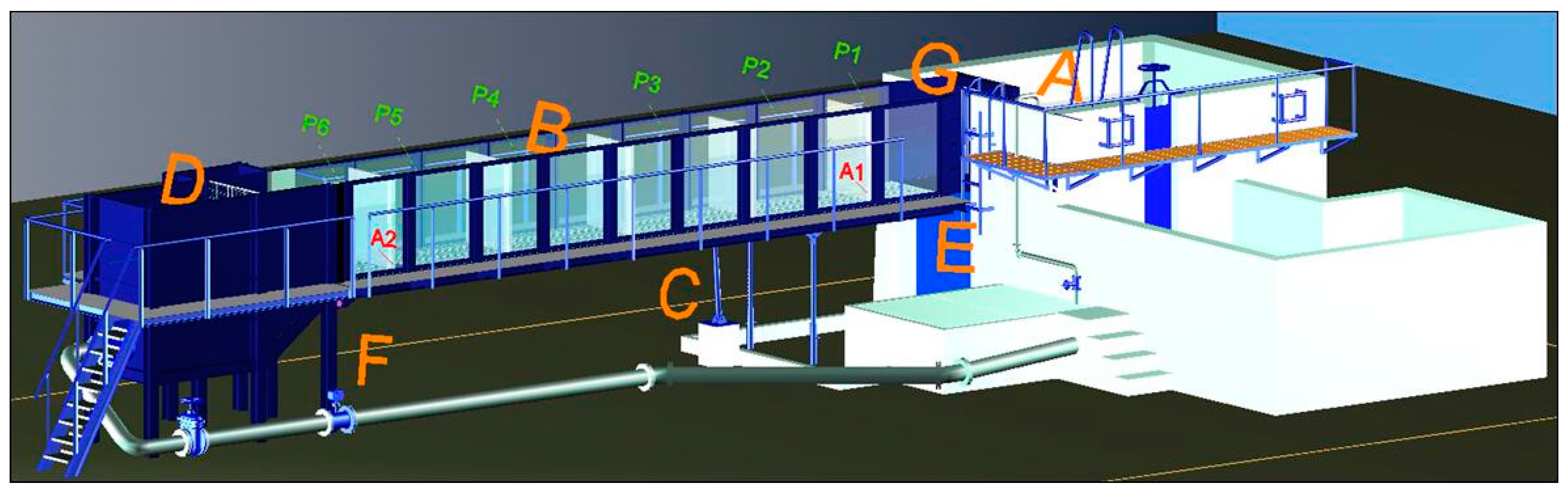
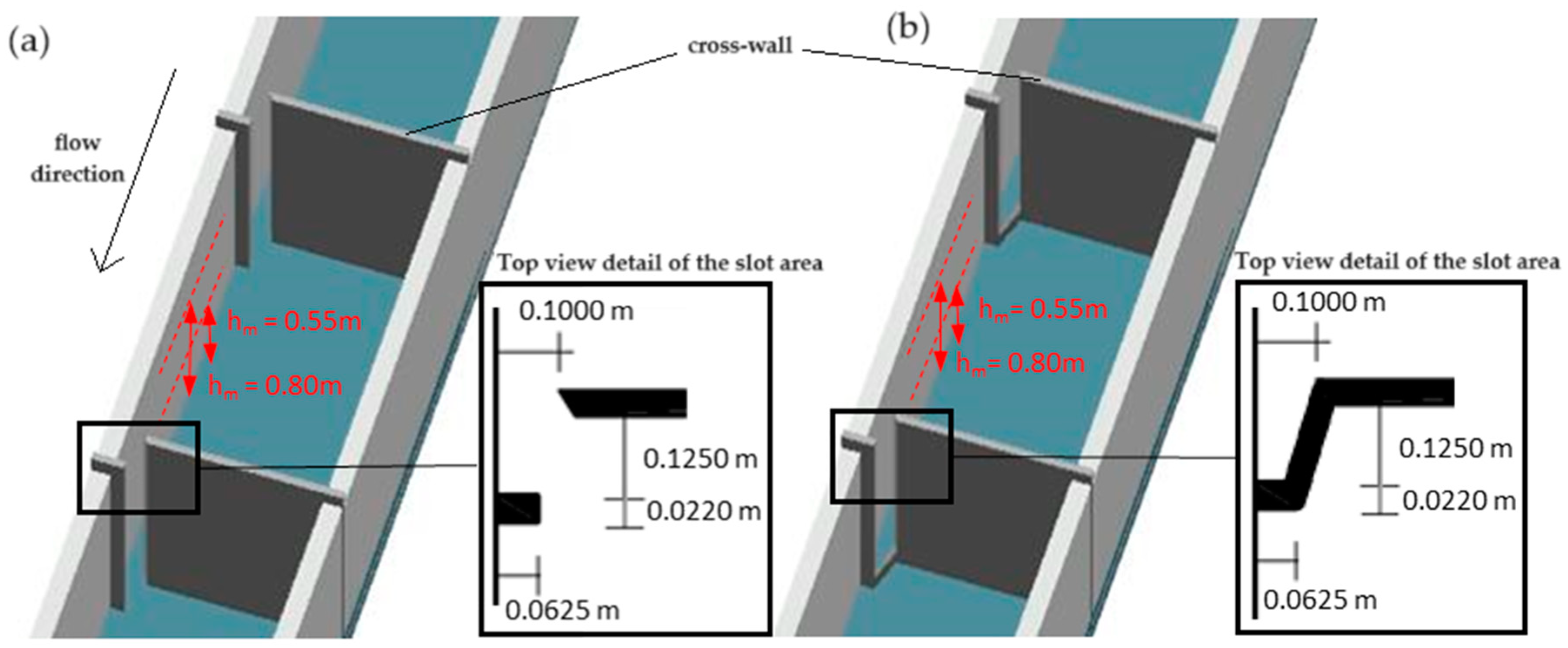
2.2. Experimental Fishway Setup
2.3. Fish Collection, Tagging and Trials
2.4. Numerical Modelling
2.5. Data Analysis
3. Results
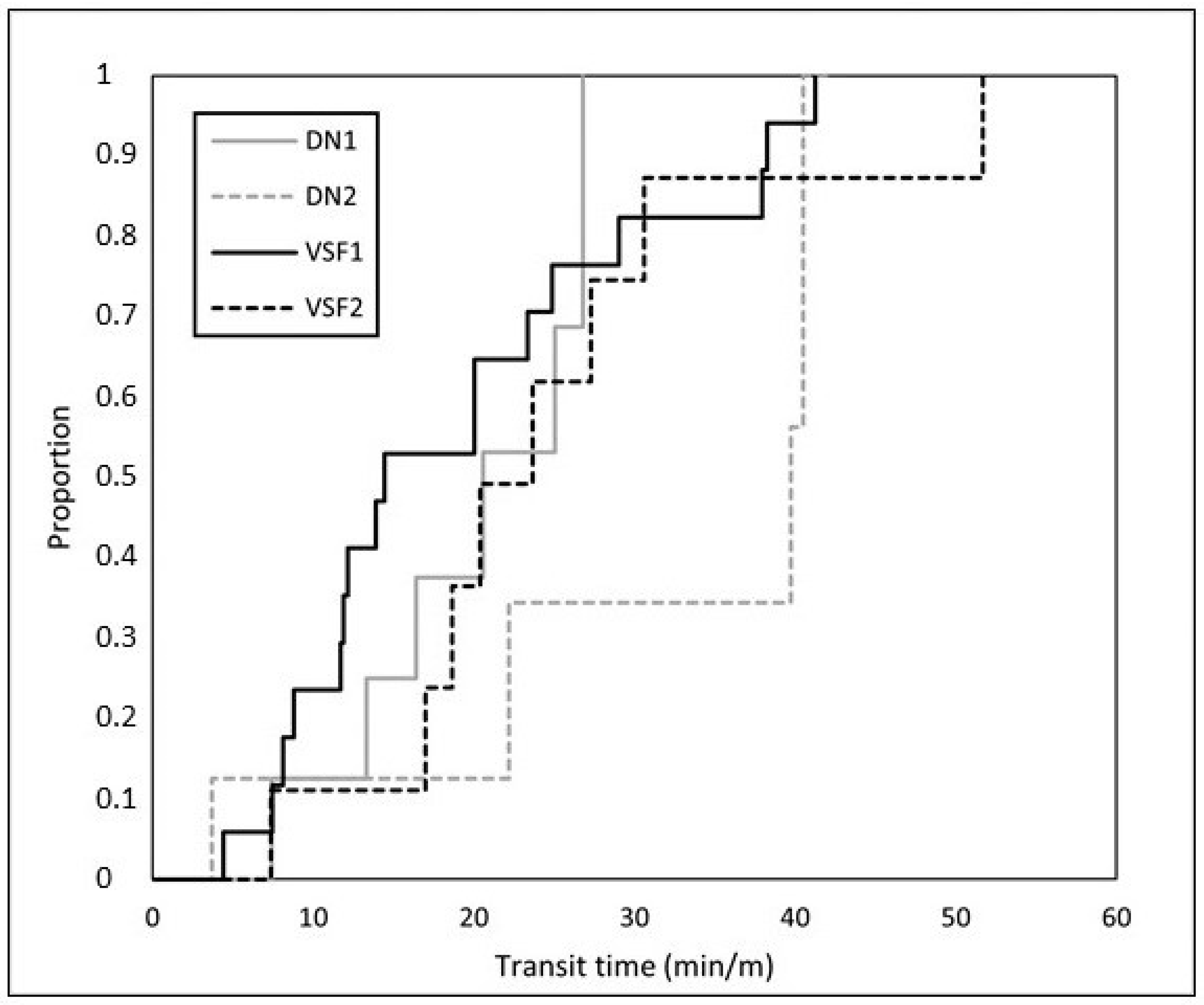
3.1. Fish Trials
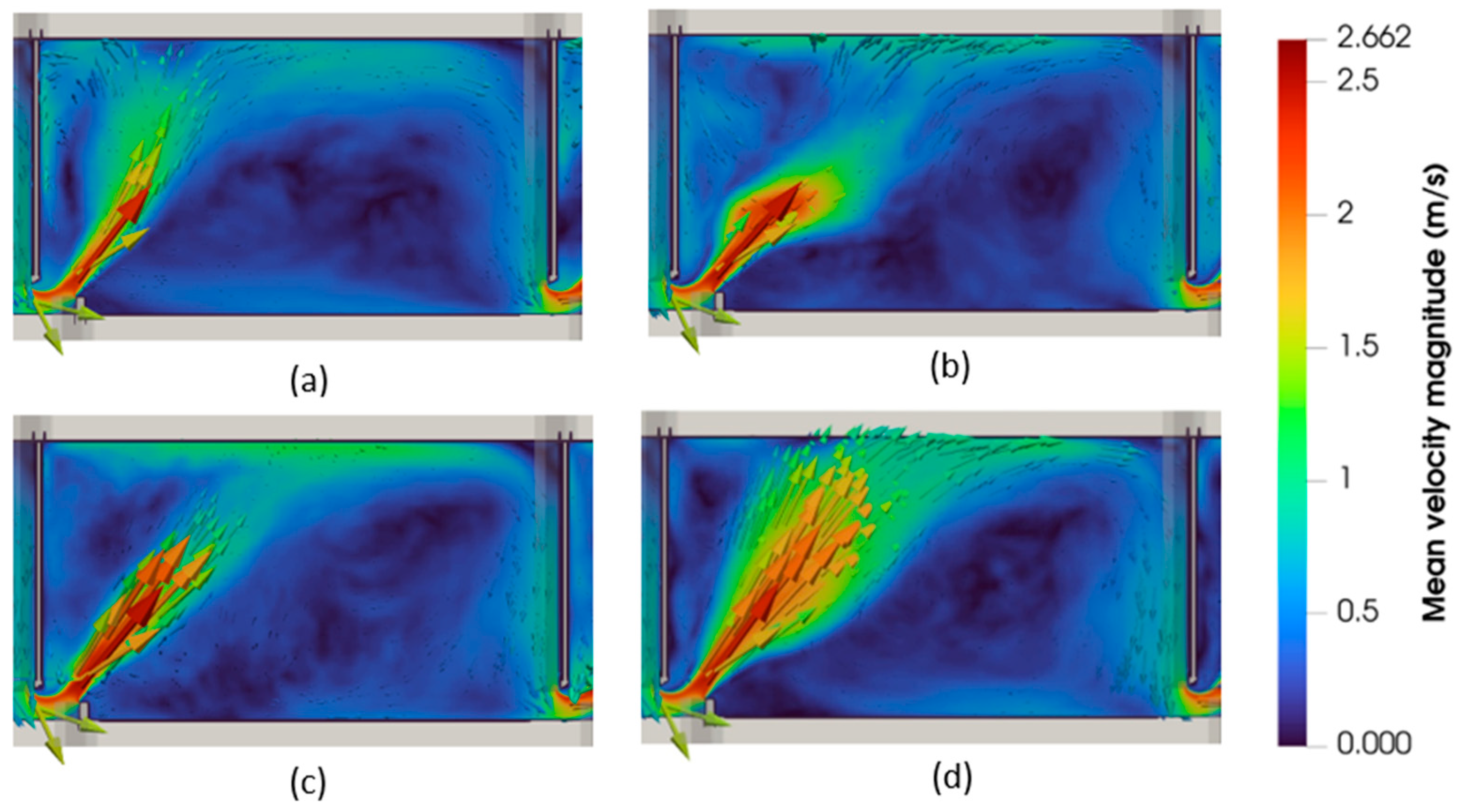
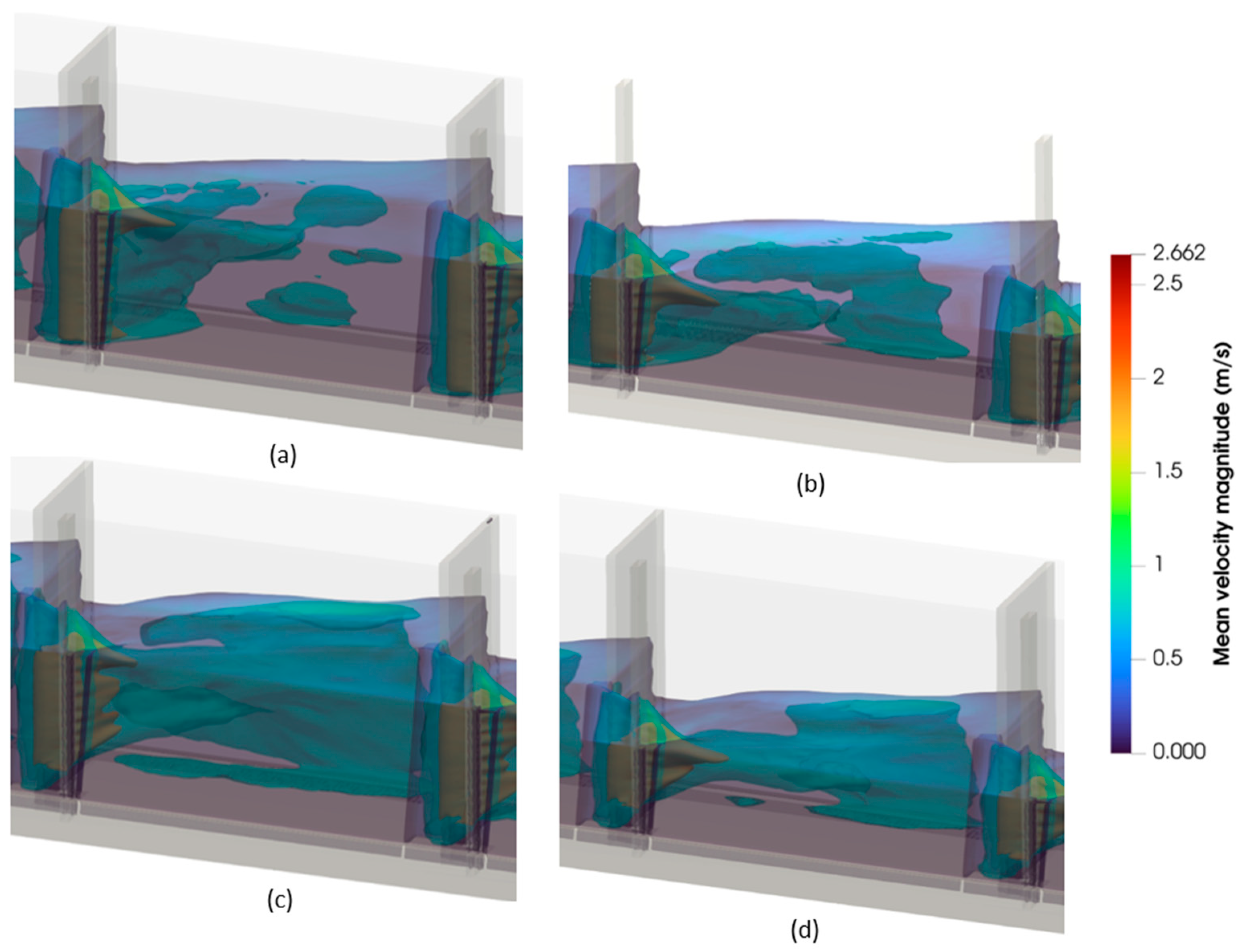
3.2. Hydrodynamic Characterization
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grill, G.; Lehner, B.; Thieme, M.; Geenen, B.; Tickner, D.; Antonelli, F.; Babu, S.; Borrelli, P.; Cheng, L.; Crochetiere, H.; et al. Mapping the World’s Free-Flowing Rivers. Nature 2019, 569, 215–221. [Google Scholar] [CrossRef]
- Belletti, B.; Garcia de Leaniz, C.; Jones, J.; Bizzi, S.; Börger, L.; Segura, G.; Castelletti, A.; van de Bund, W.; Aarestrup, K.; Barry, J.; et al. More than One Million Barriers Fragment Europe’s Rivers. Nature 2020, 588, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.-I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.-H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.J.; Carlson, A.K.; Creed, I.F.; Eliason, E.J.; Gell, P.A.; Johnson, P.T.J.; Kidd, K.A.; MacCormack, T.J.; Olden, J.D.; Ormerod, S.J.; et al. Emerging Threats and Persistent Conservation Challenges for Freshwater Biodiversity. Biol. Rev. 2019, 94, 849–873. [Google Scholar] [CrossRef] [PubMed]
- McCartney, M. Living with Dams: Managing the Environmental Impacts. Water Policy 2009, 11, 121–139. [Google Scholar] [CrossRef]
- Dopico, E.; Arboleya, E.; Fernandez, S.; Borrell, Y.; Consuegra, S.; de Leaniz, C.G.; Lázaro, G.; Rodríguez, C.; Garcia-Vazquez, E. Water Security Determines Social Attitudes about Dams and Reservoirs in South Europe. Sci. Rep. 2022, 12, 6148. [Google Scholar] [CrossRef]
- Lucas, M.C.; Baras, E.; Thom, T.J.; Duncan, A.; Slavik, O. Migration of Freshwater Fishes; Blackwell Science Ltd.: Hoboken, NJ, USA, 2001; ISBN 978-0-632-05754-2. [Google Scholar]
- Lennox, R.J.; Paukert, C.P.; Aarestrup, K.; Auger-Méthé, M.; Baumgartner, L.; Birnie-Gauvin, K.; Bøe, K.; Brink, K.; Brownscombe, J.W.; Chen, Y.; et al. One Hundred Pressing Questions on the Future of Global Fish Migration Science, Conservation, and Policy. Front. Ecol. Evol. 2019, 7, 286. [Google Scholar] [CrossRef]
- Silva, A.T.; Lucas, M.C.; Castro-Santos, T.; Katopodis, C.; Baumgartner, L.J.; Thiem, J.D.; Aarestrup, K.; Pompeu, P.S.; O’Brien, G.C.; Braun, D.C.; et al. The Future of Fish Passage Science, Engineering, and Practice. Fish Fish. 2018, 19, 340–362. [Google Scholar] [CrossRef]
- Communication from the Commission to The European Parliament, the Council, the European Economic And Social Committee and the Committee of the Regions Eu Biodiversity Strategy for 2030 Bringing Nature Back Into Our Lives; 2020. Available online: https://environment.ec.europa.eu/strategy/biodiversity-strategy-2030_en (accessed on 20 October 2023).
- Katopodis, C.; Williams, J.G. The Development of Fish Passage Research in a Historical Context. Ecol. Eng. 2012, 48, 8–18. [Google Scholar] [CrossRef]
- Keefer, M.L.; Jepson, M.A.; Clabough, T.S.; Caudill, C.C. Technical Fishway Passage Structures Provide High Passage Efficiency and Effective Passage for Adult Pacific Salmonids at Eight Large Dams. PLoS ONE 2021, 16, e0256805. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Brand, D.A. Non-Salmonids in a Salmonid Fishway: What Do 50 Years of Data Tell Us about Past and Future Fish Passage? Fish. Manag. Ecol. 2007, 14, 319–332. [Google Scholar] [CrossRef]
- Noonan, M.J.; Grant, J.W.A.; Jackson, C.D. A Quantitative Assessment of Fish Passage Efficiency. Fish Fish. 2012, 13, 450–464. [Google Scholar] [CrossRef]
- Ovidio, M.; Sonny, D.; Watthez, Q.; Goffaux, D.; Detrait, O.; Orban, P.; Nzau Matondo, B.; Renardy, S.; Dierckx, A.; Benitez, J.-P. Evaluation of the Performance of Successive Multispecies Improved Fishways to Reconnect a Rehabilitated River. Wetl. Ecol. Manag. 2020, 28, 641–654. [Google Scholar] [CrossRef]
- Segurado, P.; Branco, P.; Ferreira, M.T. Prioritizing Restoration of Structural Connectivity in Rivers: A Graph Based Approach. Landsc. Ecol. 2013, 28, 1231–1238. [Google Scholar] [CrossRef]
- Branco, P.; Segurado, P.; Santos, J.M.; Ferreira, M.T. Prioritizing Barrier Removal to Improve Functional Connectivity of Rivers. J. Appl. Ecol. 2014, 51, 1197–1206. [Google Scholar] [CrossRef]
- Rajaratnam, N.; Van der Vinne, G.; Katopodis, C. Hydraulics of Vertical Slot Fishways. J. Hydraul. Eng. 1986, 112, 909–927. [Google Scholar] [CrossRef]
- Clay, C.H. Design of Fishways and Other Fish Facilities, 2nd ed.; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Larinier, M. Environmental Issues, Dams and Fish Migration. Dams, Fish and Fisheries: Opportunities, Challenges and Conflict Resolution, MARMULLA G. 2001, pp. 45–84. Available online: https://hal.inrae.fr/hal-02582630 (accessed on 15 September 2023).
- Larinier, M. Fishways—General considerations. Bull. Fr. Pêche Piscic. 2002, 364, 21–27. [Google Scholar] [CrossRef]
- Rodríguez, T.T.; Agudo, J.P.; Mosquera, L.P.; González, E.P. Evaluating Vertical-Slot Fishway Designs in Terms of Fish Swimming Capabilities. Ecol. Eng. 2006, 27, 37–48. [Google Scholar] [CrossRef]
- Chorda, J.; Maubourguet, M.M.; Roux, H.; Larinier, M.; Tarrade, L.; David, L. Two-Dimensional Free Surface Flow Numerical Model for Vertical Slot Fishways. J. Hydraul. Res. 2010, 48, 141–151. [Google Scholar] [CrossRef]
- Romão, F.; Quaresma, A.L.; Branco, P.; Santos, J.M.; Amaral, S.; Ferreira, M.T.; Katopodis, C.; Pinheiro, A.N. Passage Performance of Two Cyprinids with Different Ecological Traits in a Fishway with Distinct Vertical Slot Configurations. Ecol. Eng. 2017, 105, 180–188. [Google Scholar] [CrossRef]
- Bravo-Córdoba, F.J.; García-Vega, A.; Fuentes-Pérez, J.F.; Fernandes-Celestino, L.; Makrakis, S.; Sanz-Ronda, F.J. Bidirectional Connectivity in Fishways: A Mitigation for Impacts on Fish Migration of Small Hydropower Facilities. Aquat. Conserv. Mar. Freshw. Ecosyst. 2023, 33, 549–565. [Google Scholar] [CrossRef]
- Bombač, M.; Novak, G.; Mlačnik, J.; Četina, M. Extensive Field Measurements of Flow in Vertical Slot Fishway as Data for Validation of Numerical Simulations. Ecol. Eng. 2015, 84, 476–484. [Google Scholar] [CrossRef]
- Soom, J.; Pattanaik, V.; Leier, M.; Tuhtan, J.A. Environmentally Adaptive Fish or No-Fish Classification for River Video Fish Counters Using High-Performance Desktop and Embedded Hardware. Ecol. Inform. 2022, 72, 101817. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; García-Vega, A.; Bravo-Córdoba, F.J.; Sanz-Ronda, F.J. A Step to Smart Fishways: An Autonomous Obstruction Detection System Using Hydraulic Modeling and Sensor Networks. Sensors 2021, 21, 6909. [Google Scholar] [CrossRef]
- Romão, F.; Quaresma, A.L.; Santos, J.M.; Amaral, S.D.; Branco, P.; Pinheiro, A.N. Multislot Fishway Improves Entrance Performance and Fish Transit Time over Vertical Slots. Water 2021, 13, 275. [Google Scholar] [CrossRef]
- Ballu, A.; Pineau, G.; Calluaud, D.; David, L. Experimental-Based Methodology to Improve the Design of Vertical Slot Fishways. J. Hydraul. Eng. 2019, 145, 04019031. [Google Scholar] [CrossRef]
- DVWK; Fisheries and Aquaculture Management Division. Fish Passes: Design, Dimensions and Monitoring; FAO/DVWK: Rome, Italy, 2002; ISBN 978-92-5-104894-8. [Google Scholar]
- Baudoin, J.M.; Burgun, V.; Chanseau, M.; Larinier, M.; Ovidio, M.; Sremski, W.; Steinbach, P.; Voegtle, B. The ICE Protocol for Ecological Continuity—Assessing the Passage of Obstacles by Fish; Concepts, Design and Application; Onema: Vincennes, France, 2014. [Google Scholar]
- Larinier, M. Fish Passage Experience at Small-Scale Hydro-Electric Power Plants in France. Hydrobiologia 2008, 609, 97–108. [Google Scholar] [CrossRef]
- Pena, L.; Puertas, J.; Bermúdez, M.; Cea, L.; Peña, E. Conversion of Vertical Slot Fishways to Deep Slot Fishways to Maintain Operation during Low Flows: Implications for Hydrodynamics. Sustainability 2018, 10, 2406. [Google Scholar] [CrossRef]
- Connor, J.; Stuart, I.; Jones, M. Guidelines for the Design, Approval and Construction of Fishways; Arthur Rylah Institute for Environmental Research: Heidelberg, Germany; Department of Environment, Land, Water and Planning: Victoria, BC, Canada, 2017.
- Armstrong, G.S.; Great Britain Environment Agency. Environment Agency Fish Pass Manual: Guidance Notes on the Legislation, Selection and Approval of Fish Passes in England and Wales; Environment Agency: Bristol, UK, 2010.
- Hermoso, V.; Clavero, M. Threatening Processes and Conservation Management of Endemic Freshwater Fish in the Mediterranean Basin: A Review. Mar. Freshw. Res. 2011, 62, 244–254. [Google Scholar] [CrossRef]
- Bravo-Córdoba, F.J.; Valbuena-Castro, J.; García-Vega, A.; Fuentes-Pérez, J.F.; Ruiz-Legazpi, J.; Sanz-Ronda, F.J. Fish Passage Assessment in Stepped Fishways: Passage Success and Transit Time as Standardized Metrics. Ecol. Eng. 2021, 162, 106172. [Google Scholar] [CrossRef]
- CEN—EN 14011; Water Quality—Sampling of Fish with Electricity. GlobalSpec: New York, NY, USA. Available online: https://standards.globalspec.com/std/282064/en-14011 (accessed on 6 September 2023).
- Rodriguez-Ruiz, A.; Granado-Lorencio, C. Spawning Period and Migration of Three Species of Cyprinids in a Stream with Mediterranean Regimen (SW Spain). J. Fish Biol. 1992, 41, 545–556. [Google Scholar] [CrossRef]
- Hammer, C. Fatigue and Exercise Tests with Fish. Comp. Biochem. Physiol. Part A Physiol. 1995, 112, 1–20. [Google Scholar] [CrossRef]
- Plaut, I. Critical Swimming Speed: Its Ecological Relevance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 131, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Mateus, C.S.; Quintella, B.R.; Almeida, P.R. The Critical Swimming Speed of Iberian Barbel Barbus Bocagei in Relation to Size and Sex. J. Fish Biol. 2008, 73, 1783–1789. [Google Scholar] [CrossRef]
- Castro-Santos, T.; Haro, A.; Walk, S. A Passive Integrated Transponder (PIT) Tag System for Monitoring Fishways. Fish. Res. 1996, 28, 253–261. [Google Scholar] [CrossRef]
- Ostrand, K.G.; Zydlewski, G.B.; Gale, W.L.; Zydlewski, J.D. Long Term Retention, Survival, Growth, and Physiological Indicators of Salmonids Marked with Passive Integrated Transponder Tags. Am. Fish. Soc. Symp. 2011, 76, 1–11. [Google Scholar]
- Ficke, A.D.; Myrick, C.A.; Kondratieff, M.C. The Effects of PIT Tagging on the Swimming Performance and Survival of Three Nonsalmonid Freshwater Fishes. Ecol. Eng. 2012, 48, 86–91. [Google Scholar] [CrossRef]
- Romão, F.; Branco, P.; Quaresma, A.L.; Amaral, S.D.; Pinheiro, A.N. Effectiveness of a Multi-Slot Vertical Slot Fishway versus a Standard Vertical Slot Fishway for Potamodromous Cyprinids. Hydrobiologia 2018, 816, 153–163. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Jud, N. The Swimming and Jumping Ability of Three Small Great Plains Fishes: Implications for Fishway Design. Trans. Am. Fish. Soc. 2011, 140, 1521–1531. [Google Scholar] [CrossRef]
- Weihs, D. Hydromechanics of Fish Schooling. Nature 1973, 241, 290–291. [Google Scholar] [CrossRef]
- Boyd, G.L.; Parsons, G.R. Swimming Performance and Behavior of Golden Shiner, Notemigonus Crysoleucas, While Schooling. Copeia 1998, 1998, 467–471. [Google Scholar] [CrossRef]
- Johansen, J.L.; Vaknin, R.; Steffensen, J.F.; Domenici, P. Kinematics and Energetic Benefits of Schooling in the Labriform Fish, Striped Surfperch Embiotoca Lateralis. Mar. Ecol. Prog. Ser. 2010, 420, 221–229. [Google Scholar] [CrossRef]
- Goerig, E.; Castro-Santos, T. Is Motivation Important to Brook Trout Passage through Culverts? Can. J. Fish. Aquat. Sci. 2017, 74, 885–893. [Google Scholar] [CrossRef]
- FLOW-3D®. Users Manual; Flow Science Inc.: Santa Fe, NM, USA, 2019; Available online: https://www.flow3d.com/ (accessed on 10 May 2023).
- Duguay, J.M.; Lacey, R.W.J. Numerical Study of an Innovative Fish Ladder Design for Perched Culverts. Can. J. Civ. Eng. 2016, 43, 173–181. [Google Scholar] [CrossRef]
- Feurich, R.; Boubée, J.; Olsen, N.R.B. Improvement of Fish Passage in Culverts Using CFD. Ecol. Eng. 2012, 47, 1–8. [Google Scholar] [CrossRef]
- Kim, S.; Yu, K.; Yoon, B.; Lim, Y. A Numerical Study on Hydraulic Characteristics in the Ice Harbor-Type Fishway. KSCE J. Civ. Eng. 2012, 16, 265–272. [Google Scholar] [CrossRef]
- Kolden, E.; Fox, B.D.; Bledsoe, B.P.; Kondratieff, M.C. Modelling Whitewater Park Hydraulics and Fish Habitat in Colorado. River Res. Appl. 2016, 32, 1116–1127. [Google Scholar] [CrossRef]
- Quaresma, A.L.; Pinheiro, A.N. Modelling of Pool-Type Fishways Flows: Efficiency and Scale Effects Assessment. Water 2021, 13, 851. [Google Scholar] [CrossRef]
- Shahabi, M.; Ahadiyan, J.; Ghomeshi, M.; Narimousa, M.; Katopodis, C.; Azizi Nadian, H. Numerical Study of the Effect of a V-Shaped Weir on Turbulence Characteristics and Velocity in V-Weir Fishways. River Res. Appl. 2023, 39, 21–34. [Google Scholar] [CrossRef]
- Hirt, C.W.; Nichols, B.D. Volume of Fluid (VOF) Method for the Dynamics of Free Boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Yao, G.F. Development of New Pressure-Velocity Solvers in FLOW-3D; Flow Science Technical Note, Flow Science, Inc.: Los Alamos, NM, USA, 2004. [Google Scholar]
- Barrett, R.; Berry, M.; Chan, T.F.; Demmel, J.; Donato, J.; Dongarra, J.; Eijkhout, V.; Pozo, R.; Romine, C.; van der Vorst, H. Templates for the Solution of Linear Systems: Building Blocks for Iterative Methods; Other Titles in Applied Mathematics; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1994; ISBN 978-0-89871-328-2. [Google Scholar]
- Souders, D.T.; Hirt, C.W. Modeling Roughness Effects in Open Channel Flow; Flow Science Technical Note; Flow Science, Inc.: Santa Fe, NM, USA, 2002. [Google Scholar]
- Fuentes-Pérez, J.F.; Eckert, M.; Tuhtan, J.A.; Ferreira, M.T.; Kruusmaa, M.; Branco, P. Spatial Preferences of Iberian Barbel in a Vertical Slot Fishway under Variable Hydrodynamic Scenarios. Ecol. Eng. 2018, 125, 131–142. [Google Scholar] [CrossRef]
- Marriner, B.A.; Baki, A.B.M.; Zhu, D.Z.; Thiem, J.D.; Cooke, S.J.; Katopodis, C. Field and Numerical Assessment of Turning Pool Hydraulics in a Vertical Slot Fishway. Ecol. Eng. 2014, 63, 88–101. [Google Scholar] [CrossRef]
- Quaranta, E.; Katopodis, C.; Revelli, R.; Comoglio, C. Turbulent Flow Field Comparison and Related Suitability for Fish Passage of a Standard and a Simplified Low-Gradient Vertical Slot Fishway. River Res. Appl. 2017, 33, 1295–1305. [Google Scholar] [CrossRef]
- Celik, I.B.; Cehreli, Z.N.; Yavuz, I. Index of Resolution Quality for Large Eddy Simulations. J. Fluids Eng. 2005, 127, 949–958. [Google Scholar] [CrossRef]
- Pope, S.B. Turbulent Flows. Available online: https://www.cambridge.org/highereducation/books/turbulent-flows/C58EFF59AF9B81AE6CFAC9ED16486B3A (accessed on 26 November 2023).
- Bermúdez, M.; Puertas, J.; Cea, L.; Pena, L.; Balairón, L. Influence of Pool Geometry on the Biological Efficiency of Vertical Slot Fishways. Ecol. Eng. 2010, 36, 1355–1364. [Google Scholar] [CrossRef]
- Barton, A.F.; Keller, R.J.; Katopodis, C. Verification of a Numerical Model for the Prediction of Low Slope Vertical Slot Fishway Hydraulics. Australas. J. Water Resour. 2009, 13, 53–60. [Google Scholar] [CrossRef]
- Bombač, M.; Četina, M.; Novak, G. Study on Flow Characteristics in Vertical Slot Fishways Regarding Slot Layout Optimization. Ecol. Eng. 2017, 107, 126–136. [Google Scholar] [CrossRef]
- Fuentes-Pérez, J.F.; Silva, A.T.; Tuhtan, J.A.; García-Vega, A.; Carbonell-Baeza, R.; Musall, M.; Kruusmaa, M. 3D Modelling of Non-Uniform and Turbulent Flow in Vertical Slot Fishways. Environ. Model. Softw. 2018, 99, 156–169. [Google Scholar] [CrossRef]
- Allison, P.D. Survival Analysis Using the SAS System: A Practical Guide; SAS Institute: Cary, NC, USA, 1995. [Google Scholar]
- Castro-Santos, T.; Haro, A. Quantifying Migratory Delay: A New Application of Survival Analysis Methods. Can. J. Fish. Aquat. Sci. 2003, 60, 986–996. [Google Scholar] [CrossRef]
- Castro-Santos, T.R.; Perry, R.W. Time-to-Event Analysis as a Framework for Quantifying Fish Passage Performance. In Telemetry Techniques: A User Guide for Fisheries Research; American Fisheries Society: Bethesda, MD, USA, 2012; pp. 427–452. [Google Scholar]
- Silva, A.T.; Santos, J.M.; Ferreira, M.T.; Pinheiro, A.N.; Katopodis, C. Effects of Water Velocity and Turbulence on the Behaviour of Iberian Barbel (Luciobarbus bocagei, Steindachner 1864) in an Experimental Pool-Type Fishway. River Res. Appl. 2011, 27, 360–373. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Bravo-Córdoba, F.J.; Fuentes-Pérez, J.F.; Castro-Santos, T. Ascent Ability of Brown Trout, Salmo Trutta, and Two Iberian Cyprinids—Iberian Barbel, Luciobarbus bocagei, and Northern Straight-Mouth Nase, Pseudochondrostoma Duriense − in a Vertical Slot Fishway. Knowl. Manag. Aquat. Ecosyst. 2016, 417, 10. [Google Scholar] [CrossRef]
- Bravo-Córdoba, F.J.; Fuentes-Pérez, J.F.; García-Vega, A.; Peñas, F.J.; Barquín, J.; Sanz-Ronda, F.J. Brown Trout Upstream Passage Performance for a Fishway with Water Drops between Pools beyond Fish Passage Design Recommendations. Water 2022, 14, 2750. [Google Scholar] [CrossRef]
- Sanz-Ronda, F.J.; Ruiz-Legazpi, J.; Bravo-Córdoba, F.J.; Makrakis, S.; Castro-Santos, T. Sprinting Performance of Two Iberian Fish: Luciobarbus bocagei and Pseudochondrostoma Duriense in an Open Channel Flume. Ecol. Eng. 2015, 83, 61–70. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Quintella, B.R.; Ferreira, A.F.; Romão, F.A.; Almeida, P.R. Swimming Performance and Ecomorphology of the Iberian Barbel Luciobarbus bocagei (Steindachner, 1864) on Permanent and Temporary Rivers. Ecol. Freshw. Fish 2014, 23, 244–258. [Google Scholar] [CrossRef]
- Odeh, M.; Noreika, J.; Haro, A.; Maynard, A.; Castro-Santos, T.; Cada, G. Evaluation of the Effects of Turbulence on the Behaviour of Migratory Fish; US Geological Survey: Reston, VA, USA, 2002; p. 55. [Google Scholar]
- Enders, E.C.; Boisclair, D.; Roy, A.G. The Effect of Turbulence on the Cost of Swimming for Juvenile Atlantic Salmon (Salmo Salar). Can. J. Fish. Aquat. Sci. 2003, 60, 1149–1160. [Google Scholar] [CrossRef]
- Mallen-Cooper, M.; Zampatti, B.; Stuart, I.G.; Baumgartner, L.J. Innovative Fishways—Manipulating Turbulence in the Vertical Slot Design to Improve Performance and Reduce Cost; Murray Darling Basin Authority: Canberra, NSW, Australia, 2008. [Google Scholar]
- Hinch, S.G.; Bratty, J. Effects of Swim Speed and Activity Pattern on Success of Adult Sockeye Salmon Migration through an Area of Difficult Passage. Trans. Am. Fish. Soc. 2000, 129, 598–606. [Google Scholar] [CrossRef]
- Alexandre, C.M.; Ferreira, T.F.; Almeida, P.R. Fish Assemblages in Non-Regulated and Regulated Rivers from Permanent and Temporary Iberian Systems. River Res. Appl. 2013, 29, 1042–1058. [Google Scholar] [CrossRef]
- Laine, A.; Jokivirta, T.; Katopodis, C. Atlantic Salmon, Salmo salar L., and Sea Trout, Salmo trutta L., Passage in a Regulated Northern River—Fishway Efficiency, Fish Entrance and Environmental Factors. Fish. Manag. Ecol. 2002, 9, 65–77. [Google Scholar] [CrossRef]
- Betancur-R, R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Phylogenetic Classification of Bony Fishes. BMC Evol. Biol. 2017, 17, 162. [Google Scholar] [CrossRef]
- Vavalidis, T.; Zogaris, S.; Economou, A.N.; Kallimanis, A.S.; Bobori, D.C. Changes in Fish Taxonomy Affect Freshwater Biogeographical Regionalisations: Insights from Greece. Water 2019, 11, 1743. [Google Scholar] [CrossRef]


| Configuration | VSF1 | VSF2 | DN1 | DN2 |
|---|---|---|---|---|
| I (%) | 15.2 | |||
| L (m) | 1.85 | |||
| B (m) | 1.00 | |||
| b (m) | 0.10 | |||
| Δh (m) | 0.28 | |||
| Q (Ls−1) | 81 | 110 | 71 | 99 |
| hm (m) | 0.55 | 0.80 | 0.55 | 0.80 |
| Cd | 0.50 | 0.50 | 0.50 | 0.50 |
| Pv (Wm−3) | 222 | 208 | 194 | 187 |
| β ± SE | p-Value | HR | |
|---|---|---|---|
| Time between the beginning and the first attempt (n = 100) | |||
| Body length (cm) | 0.110 ± 0.056 | 0.050 | 1.116 |
| Condition factor | −4.542 ± 2.098 | 0.030 | 0.011 |
| Trial = DN1 | −0.810 ± 0.404 | 0.045 | 0.445 |
| Trial = DN2 | −0.708 ± 0.379 | 0.062 | 0.493 |
| Trial = VSF1 | Reference level | ||
| Trial = VSF2 | −0.868 ± 0.418 | 0.038 | 0.420 |
| Transit time per meter of ascended height (n = 47) | |||
| Body length (cm) | 0.236 | ||
| Condition factor | 0.379 | ||
| Trial = DN1 | −0.352 ± 0.485 | 0.467 | 0.703 |
| Trial = DN2 | −1.851 ± 0.630 | 0.003 | 0.157 |
| Trial = VSF1 | Reference level | ||
| Trial = VSF2 | −0.472 ± 0.452 | 0.296 | 0.624 |
| Fishway Configuration | VSF1 | VSF2 | DN1 | DN2 |
|---|---|---|---|---|
| Q (Ls−1) | 81 | 110 | 71 | 99 |
| Uaverage (ms−1) | 0.41 | 0.46 | 0.45 | 0.45 |
| Umax (ms−1) | 2.52 | 2.48 | 2.50 | 2.54 |
| Vol.U≤0.81 ms−1 (%) | 91 | 90 | 85 | 80 |
| TKEaverage (m2s−2) | 0.079 | 0.078 | 0.077 | 0.073 |
| TKEmax (m2s−2) | 0.73 | 0.76 | 0.62 | 0.72 |
| Vol.TKE≤0.05 m2s−2 (%) | 54 | 51 | 44 | 37 |
| RSSaverage (Pa) | 86 | 97 | 105 | 98 |
| RSSmax (Pa) | 1151 | 1038 | 993 | 1197 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romão, F.; Quaresma, A.L.; Simão, J.; Bravo-Córdoba, F.J.; Viseu, T.; Santos, J.M.; Sanz-Ronda, F.J.; Pinheiro, A.N. Debating the Rules: An Experimental Approach to Assess Cyprinid Passage Performance Thresholds in Vertical Slot Fishways. Water 2024, 16, 439. https://doi.org/10.3390/w16030439
Romão F, Quaresma AL, Simão J, Bravo-Córdoba FJ, Viseu T, Santos JM, Sanz-Ronda FJ, Pinheiro AN. Debating the Rules: An Experimental Approach to Assess Cyprinid Passage Performance Thresholds in Vertical Slot Fishways. Water. 2024; 16(3):439. https://doi.org/10.3390/w16030439
Chicago/Turabian StyleRomão, Filipe, Ana L. Quaresma, Joana Simão, Francisco J. Bravo-Córdoba, Teresa Viseu, José M. Santos, Francisco J. Sanz-Ronda, and António N. Pinheiro. 2024. "Debating the Rules: An Experimental Approach to Assess Cyprinid Passage Performance Thresholds in Vertical Slot Fishways" Water 16, no. 3: 439. https://doi.org/10.3390/w16030439
APA StyleRomão, F., Quaresma, A. L., Simão, J., Bravo-Córdoba, F. J., Viseu, T., Santos, J. M., Sanz-Ronda, F. J., & Pinheiro, A. N. (2024). Debating the Rules: An Experimental Approach to Assess Cyprinid Passage Performance Thresholds in Vertical Slot Fishways. Water, 16(3), 439. https://doi.org/10.3390/w16030439











