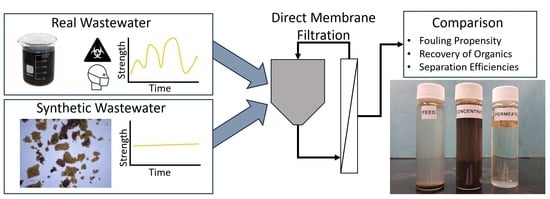
Direct Membrane Filtration of Wastewater: A Comparison between Real and Synthetic Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup and Feedwater
2.2. System Operation
2.3. Membrane Cleaning Procedure
2.4. Monitoring Parameters and Analytical Methods
3. Results
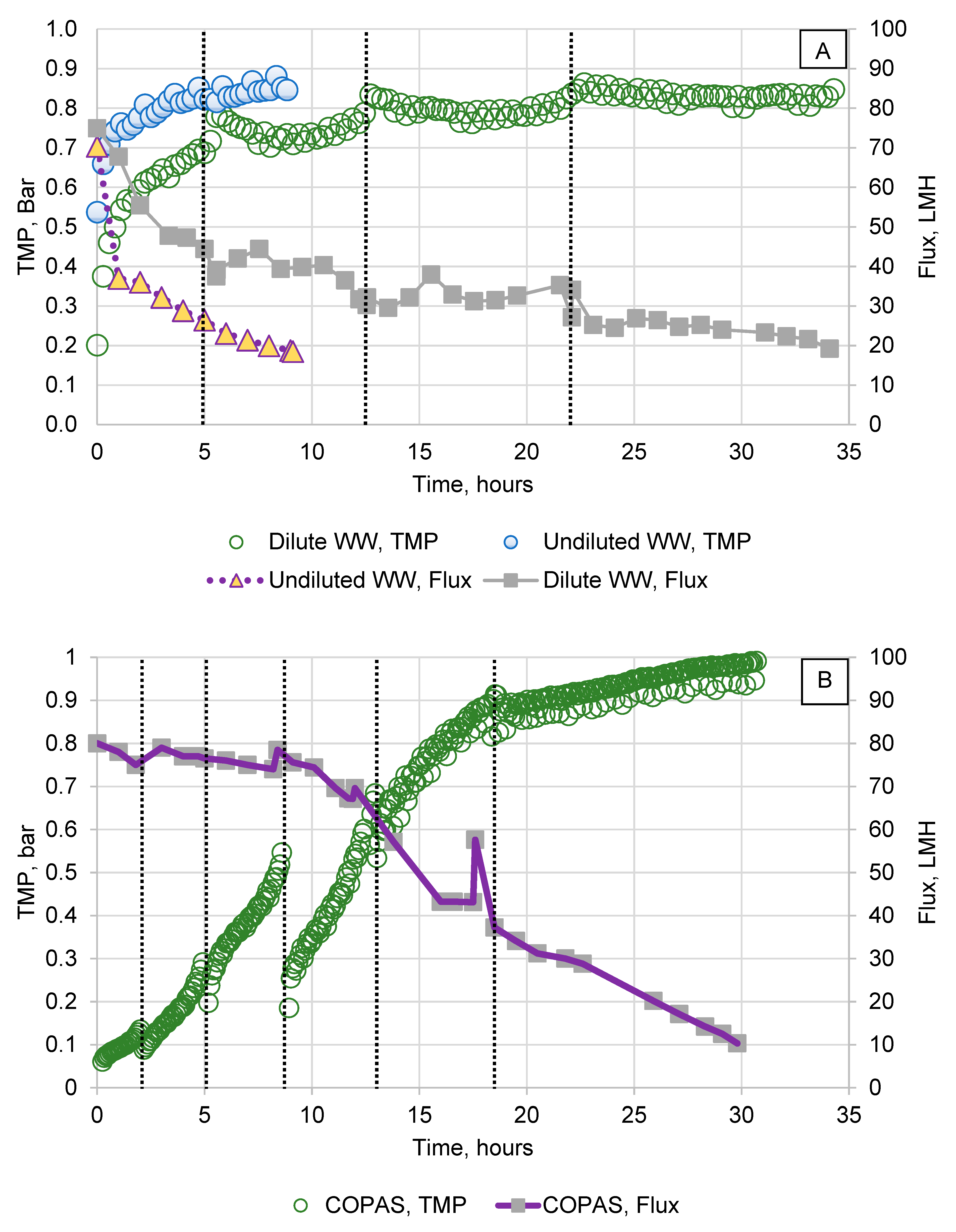
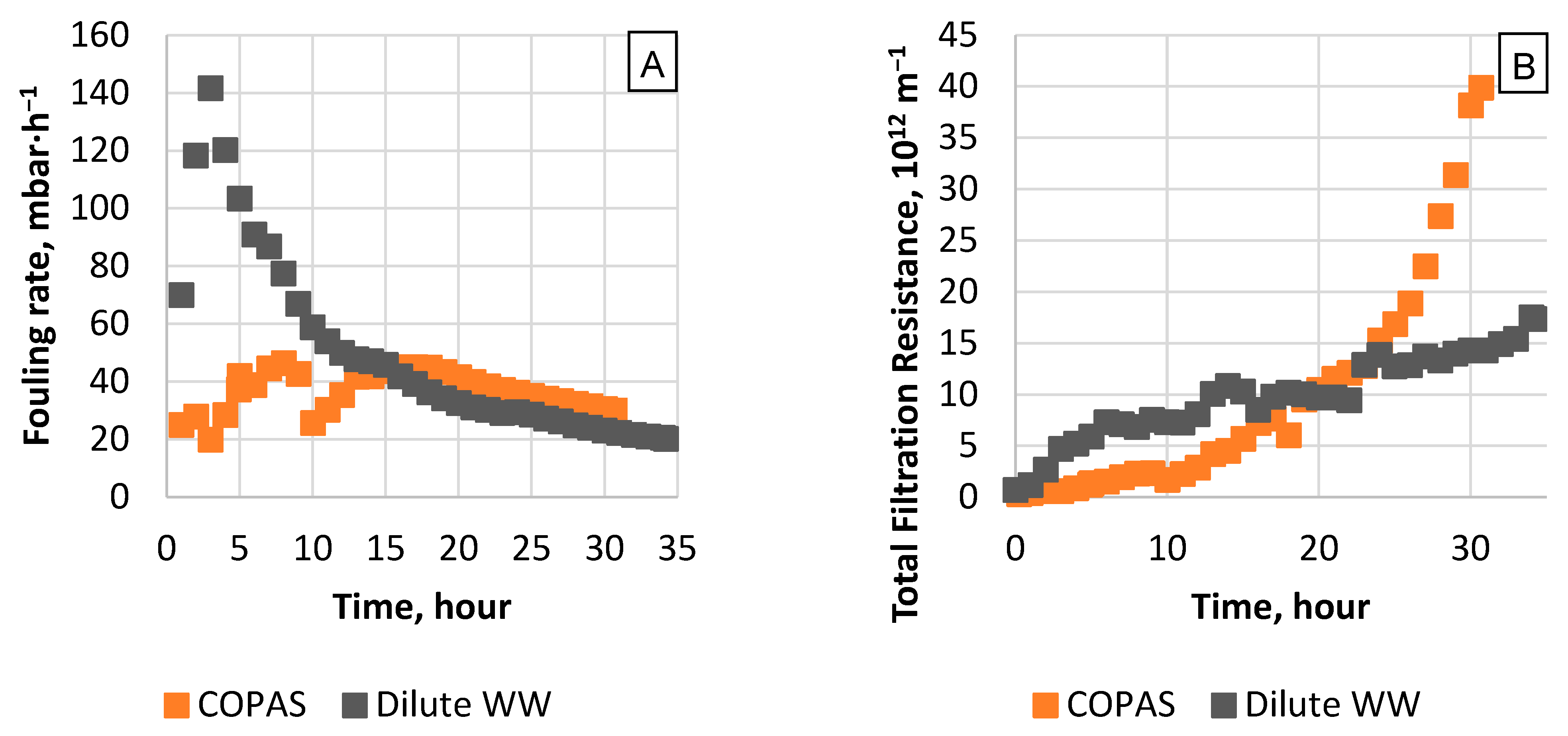
3.1. Membrane Performance and Flux Decline
3.2. Removal and Recovery
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

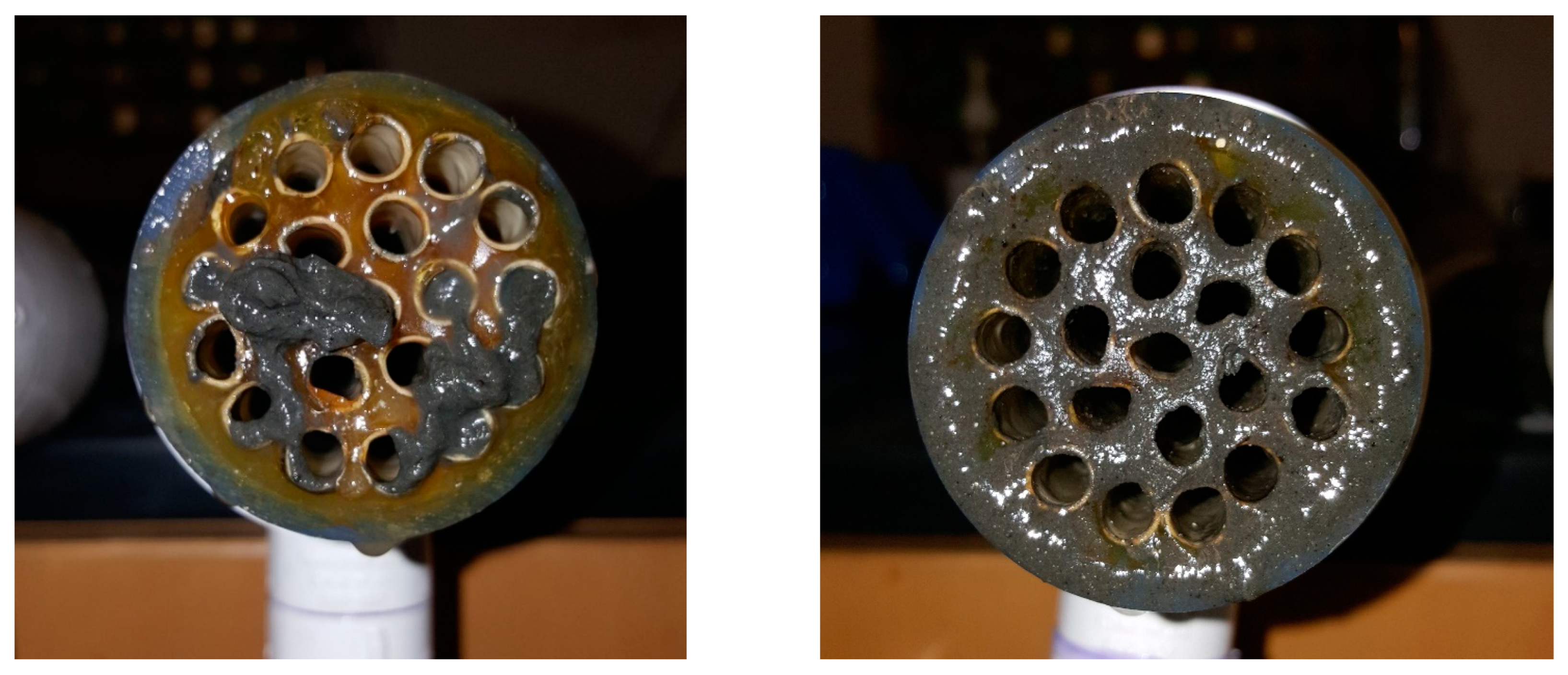
Appendix C
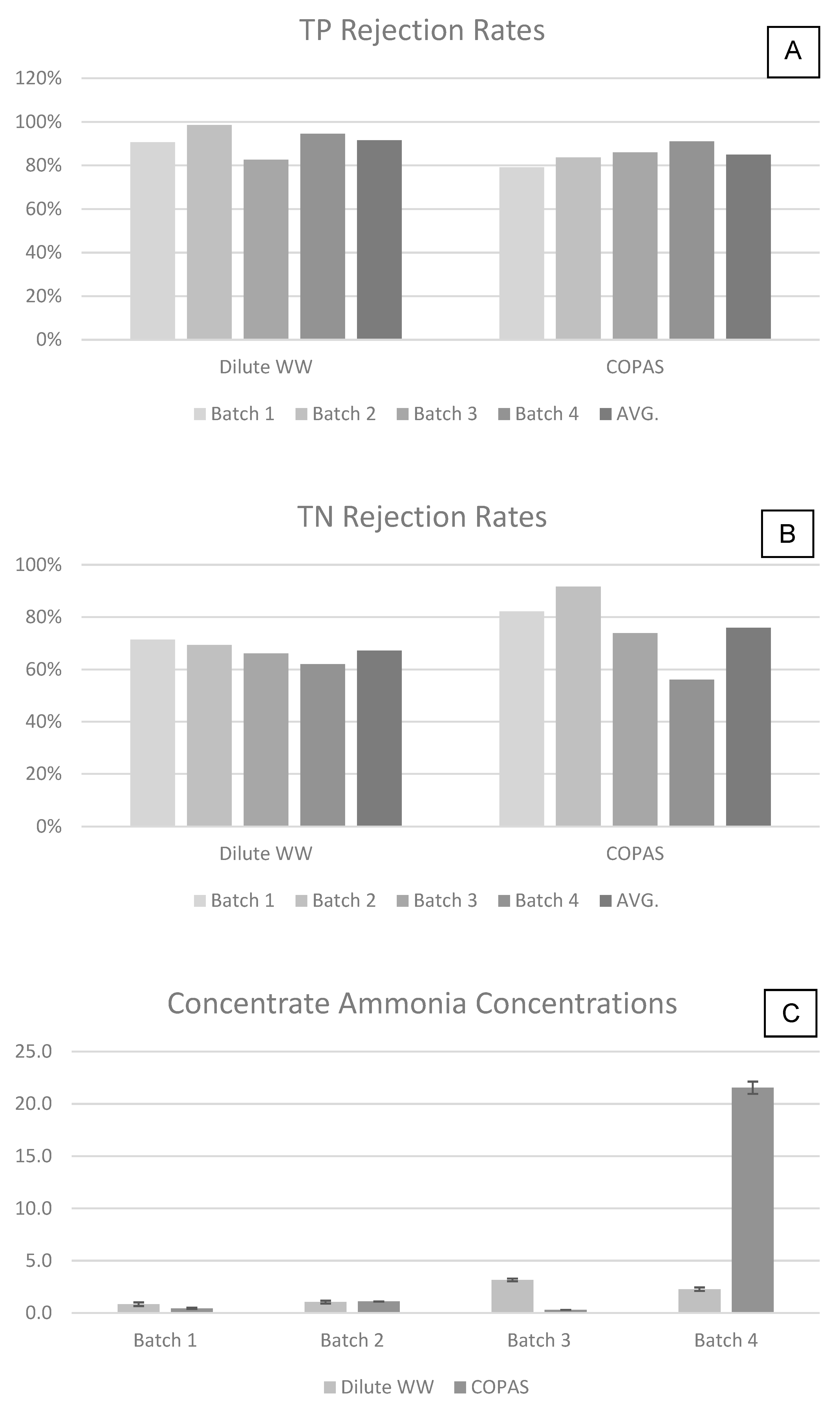
Appendix D
| COPAS | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | |
|---|---|---|---|---|---|
| tCOD in the permeate, mg/L | 19.9 | 35.6 | 24.4 | 20.7 | |
| tCOD in the concentrate, mg/L | 219 | 137 | 326.3 | 68.2 | |
| Diluted WW | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | Undiluted CF10 |
| Concentrate TS, mg/L | 5.9 | 22.1 | 11.3 | 13.2 | 31.1 |
| Feed TSS, mg/L | 5 | 5 | 4 | 5 | 14 |
| Concentrate TSS, mg/L | 26.5 | 49.5 | 42.4 | 7.1 | 70.7 |
| total TS in the feed, mg/L | 13.2 | 12.9 | 16.0 | 11.0 | 10.8 |
| tCOD in the feed, mg/L | 17.1 | 28.2 | 11.8 | 18.5 | 38.5 |
| tCOD in the permeate, mg/L | 6 | 7 | 13 | 8 | 30.6 |
| tCOD in the concentrate, mg/L | 88 | 65 | 32 | 59 | 130 |
| COPAS | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | |
| TP influent, mg/L | 1.0 | 1.0 | 1.0 | 1.0 | |
| TP permeate, mg/L | 0.2 | 1.0 | 0.5 | 0.9 | |
| TP concentrate, mg/L | 7.6 | 1.3 | 3.9 | 6.7 | |
| TN influent, mg/L | 2 | 2 | 2 | 2 | |
| TN permeate, mg/L | 0.5 | 0.4 | 1.9 | 1.7 | |
| TN concentrate, mg/L | 6.0 | 2.7 | 1.4 | 0.8 | |
| NH3 influent, mg/L | NA 1 | NA | NA | NA | |
| NH3 permeate, mg/L | 0.07 | 0.05 | 0.05 | 0.05 | |
| NH3 concentrate, mg/L | 0.05 | 0.00 | 0.00 | 0.58 | |
| Diluted WW | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | Undiluted CF10 |
| TP influent, mg/L | 1.1 | 0.4 | 0.6 | 0.4 | 3.1 |
| TP permeate, mg/L | 0.1 | 0.0 | 0.2 | 0.3 | 0.3 |
| TP concentrate, mg/L | 3.8 | 3.0 | 2.0 | 3.0 | 15.0 |
| TN influent, mg/L | 0.7 | 1.1 | 1.0 | 1.3 | 1.0 |
| TN permeate, mg/L | 0.7 | 0.7 | 1.2 | 1.5 | 1.1 |
| TN concentrate, mg/L | 21.8 | 3.1 | 10.7 | 3.5 | 24.0 |
| NH3 influent, mg/L | 0.17 | 0.10 | 0.13 | 0.08 | 0.11 |
| NH3 permeate, mg/L | 0.17 | 0.31 | 0.13 | 0.12 | 0.57 |
| NH3 concentrate, mg/L | 0.17 | 0.13 | 0.12 | 0.17 | 2.51 |
References
- Mizuta, K.; Shimada, M. Benchmarking Energy Consumption in Municipal Wastewater Treatment Plants in Japan. Water Sci. Technol. 2010, 62, 2256–2262. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, Y.; Luo, J.; Cao, J.; Kang, C.; Wang, S.; Li, K.; Zhao, J.; Aleem, M.; Wang, D. Enhanced Volatile Fatty Acids Production from Waste Activated Sludge with Synchronous Phosphorus Fixation and Pathogens Inactivation by Calcium Hypochlorite Stimulation. Sci. Total Environ. 2020, 712, 136500. [Google Scholar] [CrossRef]
- Uman, A.E.; Usack, J.G.; Lozano, J.L.; Angenent, L.T. Controlled Experiment Contradicts the Apparent Benefits of the Fenton Reaction during Anaerobic Digestion at a Municipal Wastewater Treatment Plant. Water Sci. Technol. 2018, 78, 1861–1870. [Google Scholar] [CrossRef]
- Lateef, S.K.; Soh, B.Z.; Kimura, K. Direct Membrane Filtration of Municipal Wastewater with Chemically Enhanced Backwash for Recovery of Organic Matter. Bioresour. Technol. 2013, 150, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct Membrane Filtration for Wastewater Treatment and Resource Recovery: A Review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Ito, Y.; Hafuka, A.; Kimura, K. Efficient Direct Membrane Filtration (DMF) of Municipal Wastewater for Carbon Recovery: Application of a Simple Pretreatment and Selection of an Appropriate Membrane Pore Size. Water Res. 2022, 221, 118810. [Google Scholar] [CrossRef] [PubMed]
- Men, Y.; Li, Z.; Zhu, L.; Wang, X.; Cheng, S.; Lyu, Y. New Insights into Membrane Fouling during Direct Membrane Filtration of Municipal Wastewater and Fouling Control with Mechanical Strategies. Sci. Total Environ. 2023, 869, 161775. [Google Scholar] [CrossRef]
- Ruigómez, I.; González, E.; Galán, P.; Rodríguez-Sevilla, J.; Vera, L. A Rotating Hollow Fiber Module for Fouling Control in Direct Membrane Filtration of Primary Settled Wastewater. Ind. Eng. Chem. Res. 2019, 58, 16901–16910. [Google Scholar] [CrossRef]
- Horstmeyer, N.; Thies, C.; Lippert, T.; Drewes, J.E. A Hydraulically Optimized Fluidized Bed UF Membrane Reactor (FB-UF-MR) for Direct Treatment of Raw Municipal Wastewater to Enable Water Reclamation with Integrated Energy Recovery. Sep. Purif. Technol. 2020, 235, 116165. [Google Scholar] [CrossRef]
- Gao, Y.; Fang, Z.; Liang, P.; Huang, X. Direct Concentration of Municipal Sewage by Forward Osmosis and Membrane Fouling Behavior. Bioresour. Technol. 2018, 247, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Tal, G.; Hankins, N.P.; Gitis, V. Fouling and Cleaning of Ultrafiltration Membranes: A Review. J. Water Process Eng. 2014, 1, 121–138. [Google Scholar] [CrossRef]
- Uman, A.E.; Bair, R.A.; Yeh, D.H. Assessment of an Anaerobic Membrane Bioreactor (AnMBR) Treating Medium-Strength Synthetic Wastewater under Cyclical Membrane Operation. Membranes 2021, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Azis, K.; Ntougias, S.; Melidis, P. Fouling Control, Using Various Operating Cleaning Methods, Applied on an MBR System through Continuous TMP Monitoring. Desalin. Water Treat. 2019, 167, 343–350. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Tetteh, E.K.; Rathilal, S.; Armah, E.K.; Amo-Duodu, G. Dennis Asante-Sackey Direct Membrane Filtration for Wastewater Treatment. Adv. Mater. Wastewater Treat. Desalin. 2022, 167–193. [Google Scholar] [CrossRef]
- Azis, K.; Ntougias, S.; Melidis, P. Evaluation of Fouling Prevention Methods in a Submerged Membrane Bioreactor Treating Domestic Wastewater. Desalin. Water Treat. 2019, 170, 415–424. [Google Scholar] [CrossRef]
- Kimura, K.; Honoki, D.; Sato, T. Effective Physical Cleaning and Adequate Membrane Flux for Direct Membrane Filtration (DMF) of Municipal Wastewater: Up-Concentration of Organic Matter for Efficient Energy Recovery. Sep. Purif. Technol. 2017, 181, 37–43. [Google Scholar] [CrossRef]
- Nascimento, T.A.; Miranda, M.P. Continuous Municipal Wastewater Up-Concentration by Direct Membrane Filtration, Considering the Effect of Intermittent Gas Scouring and Threshold Flux Determination. J. Water Process Eng. 2021, 39, 101733. [Google Scholar] [CrossRef]
- Penn, R.; Ward, B.J.; Strande, L.; Maurer, M. Review of Synthetic Human Faeces and Faecal Sludge for Sanitation and Wastewater Research. Water Res. 2018, 132, 222–240. [Google Scholar] [CrossRef]
- Kargol, A.K.; Burrell, S.R.; Chakraborty, I.; Gough, H.L. Synthetic Wastewater Prepared from Readily Available Materials: Characteristics and Economics. PLoS Water 2023, 2, e0000178. [Google Scholar] [CrossRef]
- Prieto, A.L.; Criddle, C.S.; Yeh, D.H. Complex Organic Particulate Artificial Sewage (COPAS) as Surrogate Wastewater in Anaerobic Assays. Environ. Sci. 2019, 5, 1661–1671. [Google Scholar] [CrossRef]
- Prieto, A.L.; Futselaar, H.; Lens, P.N.L.; Bair, R.; Yeh, D.H. Development and Start up of a Gas-Lift Anaerobic Membrane Bioreactor (Gl-AnMBR) for Conversion of Sewage to Energy, Water and Nutrients. J. Memb. Sci. 2013, 441, 158–167. [Google Scholar] [CrossRef]
- Hube, S.; Wang, J.; Sim, L.N.; Chong, T.H.; Wu, B. Direct Membrane Filtration of Municipal Wastewater: Linking Periodical Physical Cleaning with Fouling Mechanisms. Sep. Purif. Technol. 2021, 259, 118125. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef]
- Ravazzini, A.M.; van Nieuwenhuijzen, A.F.; van der Graaf, J.H.M.J. Direct Ultrafiltration of Municipal Wastewater: Comparison between Filtration of Raw Sewage and Primary Clarifier Effluent. Desalination 2005, 178, 51–62. [Google Scholar] [CrossRef]
- Yang, Y.; Deng, W.; Zha, L.; Qiu, J.; Hu, Y.; Liu, Y.; Chen, R. Integrated Electroflocculation-Membrane Process for Pre-Concentration of Domestic Wastewater: Filtration Performance, Organics Recovery and Membrane Fouling Characteristics. Resour. Conserv. Recycl. 2023, 199, 107247. [Google Scholar] [CrossRef]
- Jin, Z.; Meng, F.; Gong, H.; Wang, C.; Wang, K. Improved Low-Carbon-Consuming Fouling Control in Long-Term Membrane-Based Sewage Pre-Concentration: The Role of Enhanced Coagulation Process and Air Backflushing in Sustainable Sewage Treatment. J. Memb. Sci. 2017, 529, 252–262. [Google Scholar] [CrossRef]
- Jin, Z.; Gong, H.; Temmink, H.; Nie, H.; Wu, J.; Zuo, J.; Wang, K. Efficient Sewage Pre-Concentration with Combined Coagulation Microfiltration for Organic Matter Recovery. Chem. Eng. J. 2016, 292, 130–138. [Google Scholar] [CrossRef]
- Diamantis, V.I.; Antoniou, I.; Athanasoulia, E.; Melidis, P.; Aivasidis, A. Recovery of Reusable Water from Sewage Using Aerated Flat-Sheet Membranes. Water Sci. Technol. 2010, 62, 2769–2775. [Google Scholar] [CrossRef]
- Jin, Z.; Gong, H.; Wang, K. Application of Hybrid Coagulation Microfiltration with Air Backflushing to Direct Sewage Concentration for Organic Matter Recovery. J. Hazard Mater. 2015, 283, 824–831. [Google Scholar] [CrossRef]
- Gong, H.; Jin, Z.; Wang, X.; Wang, K. Membrane Fouling Controlled by Coagulation/Adsorption during Direct Sewage Membrane Filtration (DSMF) for Organic Matter Concentration. J. Environ. Sci. 2015, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, T.A.; Mejía, F.R.; Fdz-Polanco, F.; Peña Miranda, M. Improvement of Municipal Wastewater Pretreatment by Direct Membrane Filtration. Environ. Technol. 2017, 38, 2562–2572. [Google Scholar] [CrossRef] [PubMed]

| Parameters | COPAS | Diluted WW | Undiluted WW | |||
|---|---|---|---|---|---|---|
| Conc., mg/L | STDev | Conc., mg/L | STDev | Conc., mg/L | STDev | |
| Total Solids (TS) | 417.7 | 15.3 | 705.9 | 94.6 | 2067.1 | 10.8 |
| Volatile Solids (VS) | 307.3 | 10.2 | 277.7 | 57.2 | 1363.8 | 44.6 |
| Total Suspended Solids (TSS) | 198.4 | 12.5 | 165.0 | 25.7 | 1101.7 | 14.1 |
| Volatile Suspended Solids (VSS) | 168.1 | 9.7 | 141.3 | 20.4 | 961.7 | 7.1 |
| Total Chemical Oxygen Demand (tCOD) | 500.0 | 43.0 | 329.4 | 18.9 | 1797.8 | 38.5 |
| Soluble Chemical Oxygen Demand (sCOD) | 83.0 | 4.0 | 76.1 | 9.4 | 529.2 | 14.7 |
| Total Phosphorous (TP) | 30.7 | 0.9 | 10.3 | 0.6 | 49.5 | 3.1 |
| Soluble Phosphorous (sTP) | 7.4 | 0.8 | 2.0 | 0.5 | 11.8 | 0.3 |
| Total Nitrogen (TN) | 14.0 | 1.5 | 10.9 | 2.8 | 52.9 | 4.8 |
| Soluble Nitrogen (sTN) | 3.0 | 0.4 | 4.2 | 1.0 | 25.7 | 1.0 |
| Ammonia (NH3) | ND 1 | NA 2 | 4.2 | 0.1 | 25.6 | 0.1 |
| Parameters | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 |
|---|---|---|---|---|
| Final concentrate volume, L | 5.5 | 5.4 | 5.34 | 3.7 |
| Total filtration time, min | 212 | 220 | 335 | 740 |
| Total permeate volume, L | 56.6 | 56.4 | 56.6 | 58.7 |
| Average effective flux, LMH | 64.1 | 61.5 | 40.5 | 19.0 |
| CF after rinsing, by volume | 10.4 | 10.7 | 11.1 | 15.0 |
| CF after rinsing, by COD | 7.9 | 8.2 | 8.2 | 5.5 |
| Volume reduction after rinsing. % | 90.4 | 90.7 | 91.0 | 93.3 |
| tCOD in the influent, mg/L | 500 | 500 | 500 | 500 |
| tCOD in the permeate, mg/L | 38 | 50.7 | 48.7 | 73.6 |
| tCOD in the concentrate, mg/L | 3956 | 4080 | 4090 | 2744 |
| % tCOD rejection | 93.1% | 90.8% | 91.1% | 86.3% |
| % tCOD in the concentrate | 75.9% | 76.1% | 73.5% | 36.7% |
| % tCOD retained in the CT and membranes | 17.3% | 14.7% | 17.6% | 49.6% |
| % tCOD lost to perm (with deposits in the system) | 6.9% | 9.2% | 8.9% | 13.7% |
| % tCOD lost to perm (without deposits in the system) | 8.3% | 10.8% | 10.8% | 27.3% |
| % solids in the concentrate | 75.9% | 76.1% | 73.5% | 36.7% |
| Diluted WW | Undiluted WW | ||||
|---|---|---|---|---|---|
| Parameters | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | CF10 |
| Final concentrate volume, L | 4.7 | 4.8 | 4.7 | 5.1 | 4.9 |
| Feed TS, mg/L | 634 | 616 | 767 | 806 | 2067 |
| Concentrate TS, mg/L | 2249 | 1787 | 2786 | 2308 | 8517 |
| Feed TSS, mg/L | 173 | 137 | 154 | 197 | 1102 |
| Concentrate TSS, mg/L | 1613 | 1207 | 1586 | 1637 | 7833 |
| Total filtration time, min | 335 | 425 | 575 | 727 | 550 |
| Total permeate production volume, L | 56.9 | 53.9 | 57.0 | 56.8 | 56.8 |
| Average flux, LMH | 40.8 | 30.4 | 23.8 | 18.8 | 24.8 |
| CF after rinsing, by volume | 11.9 | 11.6 | 12.6 | 10.8 | 11.3 |
| CF after rinsing, by COD | 6.6 | 7.3 | 8.6 | 7.5 | 7.3 |
| Volume reduction after rinsing. % | 91.6% | 91.4% | 92.1% | 90.7% | 91.2% |
| tCOD in the influent, mg/L | 303 | 294 | 355 | 366 | 1798 |
| tCOD in the permeate, mg/L | 85 | 57 | 49 | 47 | 310 |
| tCOD in the concentrate, mg/L | 2014 | 2137 | 3057 | 2738 | 13,073 |
| % tCOD rejection | 74.0% | 81.0% | 86.2% | 87.2% | %83 |
| % tCOD in the concentrate | 56% | 63% | 68% | 69% | 64% |
| % TSS retained in the CT and membranes | 20.8% | 23.3% | 18.3% | 22.0% | 36.6% |
| % tCOD retained in the CT and membranes | 18.5% | 19.5% | 19.1% | 19.0% | 20.1% |
| % tCOD lost to perm (with deposits in the system) | 25.7% | 17.7% | 12.7% | 11.7% | 15.7% |
| % tCOD lost to perm (without deposits in the system) | 31.5% | 22.0% | 15.7% | 14.4% | 19.7% |
| % solids in the concentrate | 30.0% | 25.2% | 28.8% | 26.8% | 36.7% |
| % suspended solids in the concentrate | 79.2% | 76.7% | 81.7% | 78.0% | 63.4% |
| COPAS | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | |
|---|---|---|---|---|---|
| TP influent, mg/L | 31 | 31 | 31 | 31 | |
| TP permeate, mg/L | 6.4 | 5.0 | 4.3 | 2.8 | |
| TP concentrate, mg/L | 54.8 | 49.2 | 60.5 | 47.0 | |
| TN influent, mg/L | 14 | 14 | 14 | 14 | |
| TN permeate, mg/L | 2.5 | 1.2 | 3.7 | 4.8 | |
| TN concentrate, mg/L | 138 | 142 | 148 | 130 | |
| NH3 influent, mg/L | ND 1 | ND | ND | ND | |
| NH3 permeate, mg/L | 1.3 | 0.8 | 0.9 | 0.8 | |
| NH3 concentrate, mg/L | 0.5 | 1.1 | 0.3 | 21.5 | |
| TP CF by concentration | 1.8 | 1.6 | 2.0 | 1.5 | |
| TN CF by concentration | 9.8 | 10.1 | 10.5 | 9.2 | |
| TP rejection, % | 79% | 84% | 86% | 91% | |
| TN rejection, % | 82% | 92% | 74% | 66% | |
| Diluted WW | 1st CF10 | 2nd CF10 | 3rd CF10 | 4th CF10 | Undiluted WW CF10 |
| TP influent, mg/L | 10.0 | 7.1 | 10.5 | 13.5 | 49.5 |
| TP permeate, mg/L | 0.9 | 0.1 | 1.8 | 0.7 | 9.0 |
| TP concentrate, mg/L | 48.8 | 93.9 | 114.4 | 86.2 | 398.4 |
| TN influent, mg/L | 3.0 | 1.1 | 7.3 | 5.3 | 25.7 |
| TN permeate, mg/L | 12.4 | 9.4 | 17.8 | 15.4 | 60.0 |
| TN concentrate, mg/L | 100.0 | 72.4 | 110.6 | 94.2 | 415.6 |
| NH3 influent, mg/L | 2.0 | 3.7 | 6.1 | 5.1 | 25.6 |
| NH3 permeate, mg/L | 0.8 | 2.2 | 4.3 | 4.5 | 17.8 |
| NH3 concentrate, mg/L | 0.8 | 1.0 | 3.2 | 2.3 | 18.9 |
| TP CF by concentration | 4.9 | 13.2 | 10.9 | 6.4 | 8.1 |
| TN CF by concentration | 11.7 | 8.7 | 8.2 | 7.0 | 7.9 |
| TP rejection, % | 91% | 99% | 83% | 94% | 82% |
| TN rejection, % | 71% | 69% | 66% | 62% | 63% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uman, A.E.; Bair, R.A.; Yeh, D.H. Direct Membrane Filtration of Wastewater: A Comparison between Real and Synthetic Wastewater. Water 2024, 16, 405. https://doi.org/10.3390/w16030405
Uman AE, Bair RA, Yeh DH. Direct Membrane Filtration of Wastewater: A Comparison between Real and Synthetic Wastewater. Water. 2024; 16(3):405. https://doi.org/10.3390/w16030405
Chicago/Turabian StyleUman, Ahmet Erkan, Robert A. Bair, and Daniel H. Yeh. 2024. "Direct Membrane Filtration of Wastewater: A Comparison between Real and Synthetic Wastewater" Water 16, no. 3: 405. https://doi.org/10.3390/w16030405
APA StyleUman, A. E., Bair, R. A., & Yeh, D. H. (2024). Direct Membrane Filtration of Wastewater: A Comparison between Real and Synthetic Wastewater. Water, 16(3), 405. https://doi.org/10.3390/w16030405