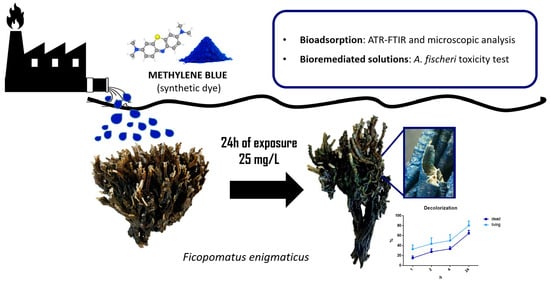
The First Evidence of the Water Bioremediation Potential of Ficopomatus enigmaticus (Fauvel 1923): From Threat to Resource?
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Sampling and Acclimatization
2.2. Methylene Blue Solution
2.3. Bioremediation Experiment
2.4. Expression of the Bioremediation Performance
2.5. Reef Surface Characterization
2.6. Toxicity Test
2.7. Statistical Analysis
3. Results
3.1. Bioremediation Experiment
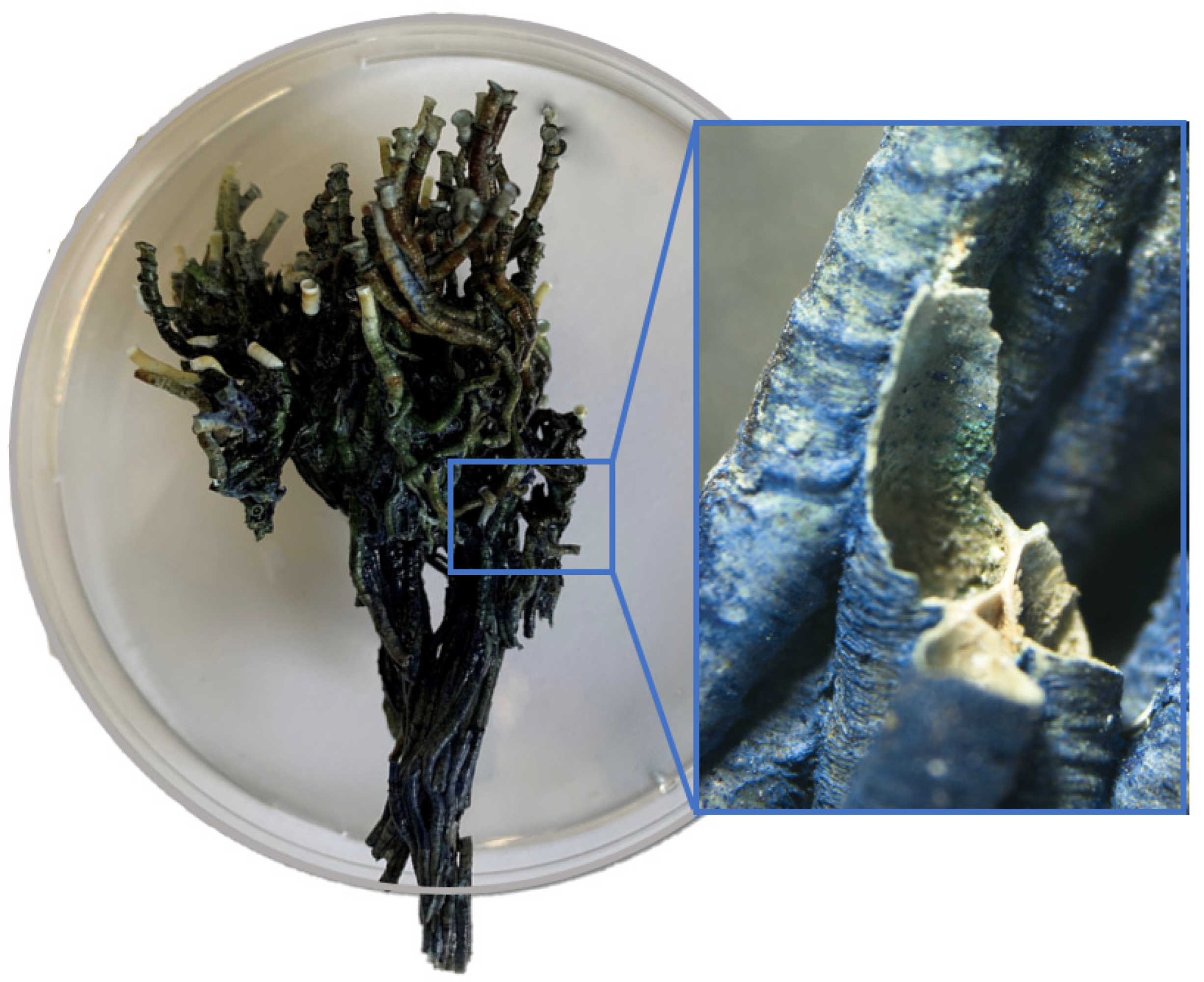
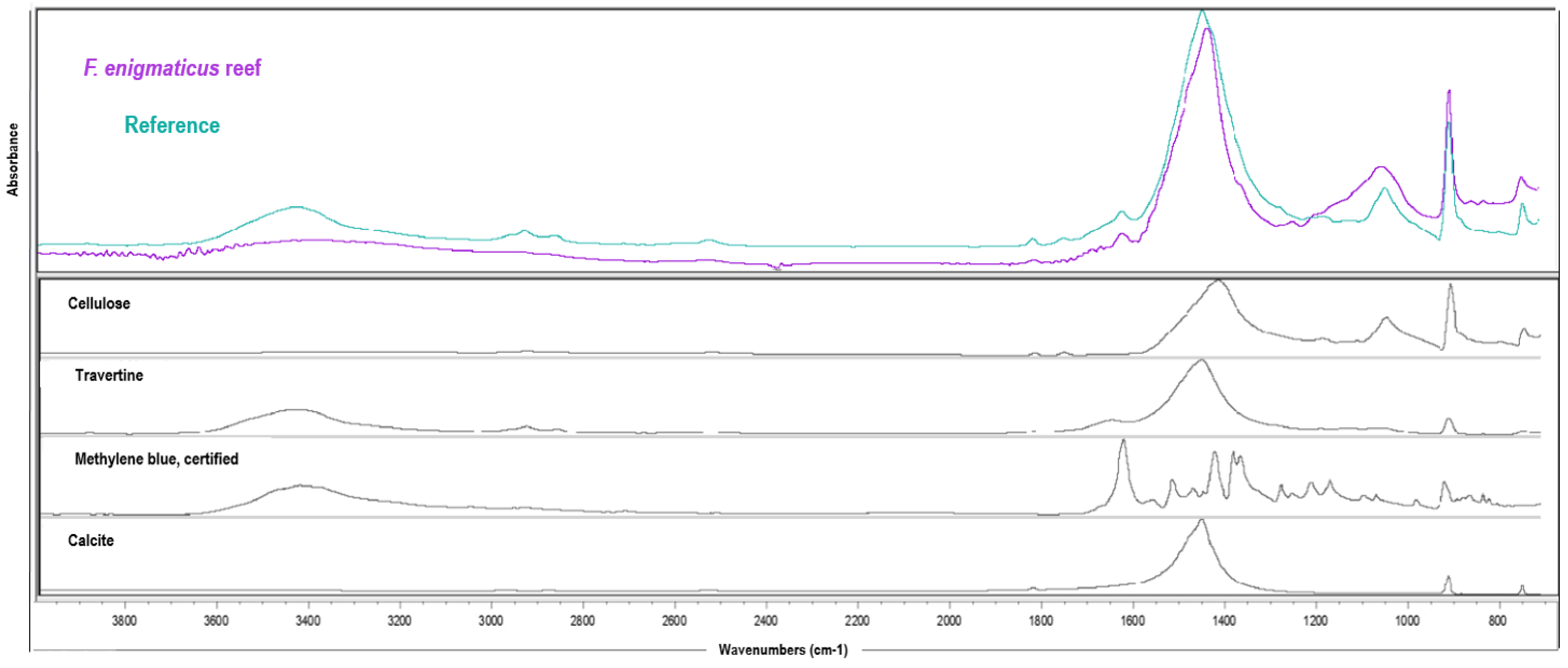
3.2. Reef Surface Characterization
3.3. Toxicity Test
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dittmann, S.; Rolston, A.; Benger, S.N.; Kuptiyanova, E.K. Habitat Requirements, Distribution and Colonisation of the Tubeworm Ficopomatus enigmaticus in the Lower Lakes and Coorong. Report for the South Australian Murray-Darling Basin Natural Resources Management Board. 2009. Available online: https://www.researchgate.net/publication/260981354_Habitat_requirements_distribution_and_colonisation_of_the_tubeworm_Ficopomatus_enigmaticus_in_the_Lower_Lakes_and_Coorong (accessed on 1 December 2023).
- Tebble, N. A source of danger to harbour structures—Encrustation by a tubed marine worm. J. Instit. Munic. Engin. 1953, 80, 259–265. [Google Scholar]
- Fauvel, P. Un nouveau Serpuliden d’eau saumatre Mercierella n. g., enigmatica n. sp. Bull. Soc. Zool. Fr. 1923, 47, 424–430. [Google Scholar]
- DAISIE. Handbook of Alien Species in Europe; Sringer: Dordrecht, The Netherlands, 2009; ISBN 978-1-4020-8279-5. [Google Scholar]
- Charles, M.; Faillettaz, R.; Desroy, N.; Fournier, J.; Costil, K. Distribution, associated species and extent of biofouling “reefs” formed by the alien species Ficopomatus enigmaticus (Annelida, Polychaeta) in marinas. Estuar. Coast. Shelf Sci. 2018, 212, 164–175. [Google Scholar] [CrossRef]
- Schwindt, E.; De Francesco, C.G.; Iribarne, O.O. Individual and reef growth of the invasive reef-building polychaete Ficopomatus enigmaticus in a south-western Atlantic coastal lagoon. J. Mar. Biol. Ass. U.K. 2004, 84, 987–993. [Google Scholar] [CrossRef]
- Bruschetti, M.; Bazterrica, C.; Fanjul, E.; Luppi, T.; Iribarne, O. Effect of biodeposition of an invasive polychaete on organic matter content and productivity of the sediment in a coastal lagoon. J. Sea Res. 2011, 66, 20–28. [Google Scholar] [CrossRef]
- Vaas, K.F. Immigrants among the animals of the delta-area of the SW. Netherlands. Hydrobiol. Bull. 1975, 9, 114–119. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Morri, C. Ficopomatus ‘Reefs’ in the Po River Delta (Northern Adriatic): Their Constructional Dynamics, Biology, and Influences on the Brackish-water Biota. Mar. Ecol. 1996, 17, 51–66. [Google Scholar] [CrossRef]
- Bonifazi, A.; Lezzi, M.; Ventura, D.; Lisco, S.; Cardone, F.; Gravina, M.F. Macrofaunal biodiversity associated with different developmental phases of a threatened Mediterranean Sabellaria alveolata (Linnaeus, 1767) reef. Mar. Environ. Res. 2019, 145, 97–111. [Google Scholar] [CrossRef]
- Heiman, K.W.; Micheli, F. Non-native Ecosystem Engineer Alters Estuarine Communities. Integr. Comp. Biol. 2010, 50, 226–236. [Google Scholar] [CrossRef]
- Schwindt, E.; Iribarne, O.O. Reef of Ficopomatus enigmaticus (polychaeta; Serpulidae) in the Mar Chiquita Coastal Lagoon, Argentina. Boll. Soco Hist. Nat. Balears 1998, 41, 35–40. [Google Scholar]
- Bruschetti, M.; Luppi, T.; Fankul, E.; Rosenthal, A.; Iribarne, O. Grazing effect of the invasive reef-forming polychaete Ficopomatus enigmaticus (Fauvel) on phytoplankton biomass in a SW Atlantic coastal lagoon. J. Environ. Mar. Biol. Ecol. 2008, 354, 212–219. [Google Scholar] [CrossRef]
- Eno, N.C.; Clark, R.A.; Sanderson, W.G. Non-Native Marine Species in British Waters: A Review and Directory; JNCC: Peterborough, UK, 1997; ISBN 86107 442 5.
- Oladoye, P.O.; Ajiboye, T.O.; Omotola, E.O.; Oyewola, O.J. Methylene blue dye: Toxicity and potential elimination technology from wastewater. Results Eng. 2022, 16, 100678. [Google Scholar] [CrossRef]
- Bathia, D.; Sharma, N.R.; Singh, J.; Kanwar, R.S. Biological methods for textile dye removal from wastewater: A Review. Crit. Rev. Environ. Sci. Technol. 2017, 47, 1836–1876. [Google Scholar] [CrossRef]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Zekker, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Ali, S.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Elshikh, M.S.; Al Khulaifi, M.M.; Hadibarata, T.; Yuniarto, A.; Syafiuddin, A. Biotransformation and Detoxification of Antraquione Dye Green 3 using halophilic Hortaea sp. Int. Biodeterior. Biodegrad. 2019, 140, 72–77. [Google Scholar] [CrossRef]
- Lu, G.; Nagbanshi, M.; Gouldau, N.; Jorge, M.M.; Meissner, P.; Jahn, A.; Mockenhaupt, F.P.; Muller, O. Efficacy and safety of methylene blue in the treatment of malaria: A systematic review. BMC Med. 2018, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Pushparajah, M.; Renita, S.M.; Lielbelt, E.L. Methylene Blue an Antidote for Methemoglobinemia and Beyond. Pediatr. Emerg. Care 2021, 37, 474–477. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Liu, Y.; Zhou, R.; Fu, Y.; Chen, J. Degradation of organic pollutants through activating bisulfite with lanthanum ferrite-loaded biomass carbon. RSC Adv. 2023, 13, 24819. [Google Scholar] [CrossRef]
- Lipej, L.; Mavric, B.; Orlando-Bonaca, M.; Malej, A. State of the Art of the Marine Non-Indigenous Flora and Fauna in Slovenia. Mediterr. Mar. Sci. 2012, 13, 243–249. [Google Scholar] [CrossRef]
- De Marchi, L.; Oliva, M.; Freitas, R.; Neto, V.; Figueira, E.; Chiellini, F.; Morelli, A.; Soares, A.M.V.M.; Pretti, C. Toxicity evaluation of carboxylated carbon nanotubes to the reef-forming tubeworm Ficopomatus enigmaticus (Fauvel, 1923). Mar. Environ. Res. 2019, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, F.; Mohamadnia, Z.; Kaboudin, B.; Karimi, Z. Photodegradation of methylene blue with a titanium dioxide/polyacrylamide photocatalyst under sunlight. J. Appl. Polym. Sci. 2016, 43386. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Soegianto, A.; Wahudianto, F.E. Phytoremediation of methylene blue using duckweed (Lemna minor). Heliyon 2019, 5, e02206. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.A.; Morad, N.; Ooi, J.Q. Phytoremediation of Methylene Blue and Methyl Orange Using Eichhornia crassipes. Int. J. Environ. Sci. Dev. 2017, 7, 724–728. [Google Scholar] [CrossRef]
- Contreras, M.; Grande-Tovar, C.D.; Vallejo, W.; Chaves-Lopez, C. Bio-Removal of Methylene Blue from Aqueous Solution by Galactomyces geotrichum KL20A. Water 2019, 11, 282. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Abalde, J.; Torres, E. Efficient removal of dyes from seawater using as biosorbent the dead and living biomass of the microalga Phaeodactylum tricornutum: Equilibrium and kinetics studies. J. Appl. Phycol. 2021, 33, 3071–3090. [Google Scholar] [CrossRef]
- Toth, G.; Hahn, J.; Kriszt, B.; Szoboszlay, S. Acute and chronic toxicity of herbicides and their mixtures measured by Aliivibrio fischeri ecotoxicological assay. Ecotoxicol. Environ. Saf. 2019, 185, 109702. [Google Scholar] [CrossRef] [PubMed]
- Dailianis, S.; Vlastos, D.; Zoppou, C.; Moschopoulou, A.; Antonopoulou, M. Different isoforms of parabens into marine environment: Biological effects on the bacterium Aliivibrio fischeri and the marine mussel Mytilus galloprovincialis. Sci. Total Environ. 2023, 900, 165902. [Google Scholar] [CrossRef]
- UNI EN ISO 11348-2:2019; Qualità dell’acqua—Determinazione dell’effetto inibitorio di campioni acquosi sull’emissione di luce di Vibrio fischeri (prova su batteri luminescenti)—Parte 2: Metodo con batteri disidratati. Ente Nazionale Italiano di Unificazione (UNI): Milano, Italy, 2019.
- Sarioglu, O.F.; Keskin, N.O.S.; Celebioglu, A.; Tekinay, T.; Uyar, T. Bacteria encapsulated electrospun nanofibrous webs for remediation of methylene blue dye in water. Colloids Surf. B Biointerfaces 2017, 152, 245–251. [Google Scholar] [CrossRef]
- Deb, M.; Redkar, N.; Manohar, C.S.; Jagtap, A.S.; Saxena, S.; Shukla, S. Bacillus sp. based nano-bio hybrids for efficient water remediation. Environ. Pollut. 2023, 326, 121490. [Google Scholar] [CrossRef]
- Liu, J.; Su, H.; Xue, J.; Wei, X. Optimization of Decoloration Conditions of Methylene Blue Wastewater by Penicillium P1. Indian. J. Microbiol. 2022, 62, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Lino, V.; Castaldo, R.; Gentile, G.; Manini, P. Reusable melanin-based biosorbents for efficient methylene blue removal: The new frontier of fungi-inspired allomelanin coatings for sustainable water remediation processes. Mater. Today Sustain. 2023, 21, 100283. [Google Scholar] [CrossRef]
- Bouzikri, S.; Ouasfi, N.; Benzidia, N.; Salhi, A.; Bakkas, S.; Khamliche, L. Marine alga “Bifurcaria bifurcata”: Biosorption of Reactive Blue 19 and methylene blue from aqueous solutions. Environ. Sci. Pollut. Res. 2020, 27, 33636–33648. [Google Scholar] [CrossRef]
- Seoane, R.; Santaeufemia, S.; Abalde, J.; Torres, E. Efficient Removal of Methylene Blue Using Living Biomass of the Microalga Chlamydomonas moewusii: Kinetics and Equilibrium Studies. Int. J. Environ. Res. Public. Health 2022, 19, 2653. [Google Scholar] [CrossRef] [PubMed]
- Pathak, V.V.; Kothari, R.; Chopra, A.K.; Singh, D.P. Experimental and kinetic studies for phycoremediation and dye removal by Chlorella pyrenoidosa from textile wastewater. J. Environ. Manag. 2015, 163, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Mehmandost, N.; Goudarzi, N.; Chamjangali, M.A.; Bagherian, G. Removal of methylene blue and crystal violet in binary aqueous solution by magnetic Terminalia catappa kernel shell biosorbent using Box–Behnken design. J. Iran. Chem. Soc. 2022, 19, 3769–3781. [Google Scholar] [CrossRef]
- Saha, P. Assessment on the Removal of Methylene Blue Dye using Tamarind Fruit Shell as Biosorbent. Water Air Soil. Pollut. 2010, 213, 287–299. [Google Scholar] [CrossRef]
- Sharma, A.; Devi, I. A sustainable biosorption technique for treatment of industrial wastewater using snail shell dust (Bellamya bengalensis). Environ. Monit. Assess. 2023, 195, 389. [Google Scholar] [CrossRef]
- Manghabati, H.; Pazuki, G. A study on the decolorization of methylene blue by Spirodela polyrrhiza: Experimentation and modeling. RSC Adv. 2014, 4, 30137. [Google Scholar] [CrossRef]
- Al-Fawwaz, A.; Adbullah, M. Decolorization of Methylene Blue and Malachite Green by Immobilized Desmodesmus sp. Isolated from North Jordan. Int. J. Environ. Sci. Dev. 2016, 7, 95–99. [Google Scholar] [CrossRef]
- Al-Baldawi, I.A.; Abdullah, S.R.S.; Anuar, N.; Hasan, H.A. Phytotransformation of methylene blue from water using aquatic plant (Azolla pinnata). Environ. Technol. Innov. 2018, 11, 15–22. [Google Scholar] [CrossRef]
- Barbosa, P.; Peters, T.M. The effects of vital dyes on living organisms with special reference to Methylene Blue and Neutral Red. Histochem. J. 1971, 3, 71–93. [Google Scholar] [CrossRef] [PubMed]
- Ten Hove, H.A.; Weerdenburg, C.A. A generic revision of the brackish-water serpulid Ficopomatus southern 1921 (polychaeta: Serpulinae), including Mercierella fauvel 1923, Sphaeropomatus treadwell 1934, mercierellopsis rioja 1945 and Neopomatus pillai 1960. Biol. Bull. 1978, 154, 96–120. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hou, J.; Miao, L.; Wu, J. Comparison of adsorption behavior studies of methylene blue by microalga residue and its biochars produced at different pyrolytic temperatures. Environ. Sci. Pollut. Res. 2021, 28, 14028–14040. [Google Scholar] [CrossRef] [PubMed]
- Moghazy, R.M.; Mahmoud, R.H. Microalgal-based macro-hollow loofah fiber bio-composite for methylene blue removal: A promising step for a green adsorbent. Int. J. Biol. Macromol. 2023, 253, 127009. [Google Scholar] [CrossRef] [PubMed]
- Aliani, S.; Bianchi, C.N.; Meloni, R. Scanning electron microscope observations on the tube of the reef-forming serpulid Ficopomatus enigmaticus (Fauvel) (Annelida, Polychaeta). Boll. Zool. 1995, 62, 363–367. [Google Scholar] [CrossRef]
- Daneshvar, E.; Vazirzadeh, A.; Niazi, A.; Kousha, M.; Naushad, M.; Bhatnagar, A. Desorption of Methylene blue dye from brown macroalga: Effects of operating parameters, isotherm study and kinetic modeling. J. Clean. Prod. 2017, 152, 443–453. [Google Scholar] [CrossRef]
- Oladoja, N.A.; Aboluwoye, C.O.; Akinkugbe, A.O. Evaluation of Loofah as a Sorbent in the Decolorization of Basic Dye Contaminated Aqueous System. Ind. Eng. Chem. Res. 2009, 48, 2786–2794. [Google Scholar] [CrossRef]
- Kacem, I.; Laurent, T.; Blanchemain, N.; Neut, C.; Chai, F.; Haulton, S.; Hildebrand, H.F.; Martel, B. Dyeing and antibacterial activation with methylene blue of a cyclodextrin modified polyester vascular graft. J. Biomed. Mater. Res. Part A 2014, 102A, 2942–2951. [Google Scholar] [CrossRef]
- Zhang, X.; Hui, Y.; Fang, C.; Wang, Y.; Han, F.; Lou, X.; Fodjo, E.K.; Cai, Y.; Kong, C. Determination of Methylene Blue and Its Metabolite Residues in Aquatic Products by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. Molecules 2021, 26, 4975. [Google Scholar] [CrossRef]
- Brice, M. Toxicité des colorants metachromatiques pour l’oeuf d’etoil de mer. Archs Biol. 1959, 70, 121–125. [Google Scholar]
- Hakami, A.A.H.; Wabaidur, S.M.; Khan, M.A.; AlOthman, Z.A.; Siddiqui, M.R. Extraction Procedures and Analytical Methods for the Determination of Methylene Blue, Rhodamine B and Crystal Violet—An Overview. Curr. Anal. Chem. 2021, 17, 708–728. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. QUORUM SENSING IN BACTERIA. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Tintiller, F.; Moriou, C.; Petek, S.; Fauchon, M.; Hellio, C.; Saulnier, D.; Ekins, M.; Hooper, J.N.A.; Al-Mourabit, A.; Debitus, C. Quorum Sensing Inhibitory and Antifouling Activities of New Bromotyrosine Metabolites from the Polynesian Sponge Pseudoceratina n. sp. Mar. Drugs 2020, 18, 272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liang, Y.; Liao, X.-J.; Deng, Z.; Xu, S.-H. Isolation of a new butenolide from the South China Sea gorgonian coral Subergorgia suberosa. Nat. Prod. Res. Former. Nat. Prod. Lett. 2014, 28, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Kon-ya, K.; Shimidzu, N.; Adachi, K.; Miki, W. 2,5,6-Tribromo-l-methylgramine, an Antifouling Substance from the Marine Bryozoan Zoobotryon pellucidum. Fish. Sci. 1994, 60, 773–775. [Google Scholar] [CrossRef][Green Version]
- Olguin-Uribe, G.; Abou-Mansour, E.; Boulander, A.; Debard, H.; Francisco, C.; Combaut, G. 6-Bromoindole-3-carbaldehyde, from an Acinetobacter sp. bacterium associated with the ascidian Stomozoa murrayi. J. Chem. Ecol. 1997, 23, 2507. [Google Scholar] [CrossRef]
- Coutinho, M.C.L.; Teixeira, V.L.; Santos, C.S.G. A Review of “Polychaeta” Chemicals and their Possible Ecological Role. J. Chem. Ecol. 2018, 44, 72–94. [Google Scholar] [CrossRef]
- Ianora, A.; Miralto, A. Toxigenic effects of diatoms on grazers, phytoplankton and other microbes: A review. Ecotoxicology 2010, 19, 493–511. [Google Scholar] [CrossRef]


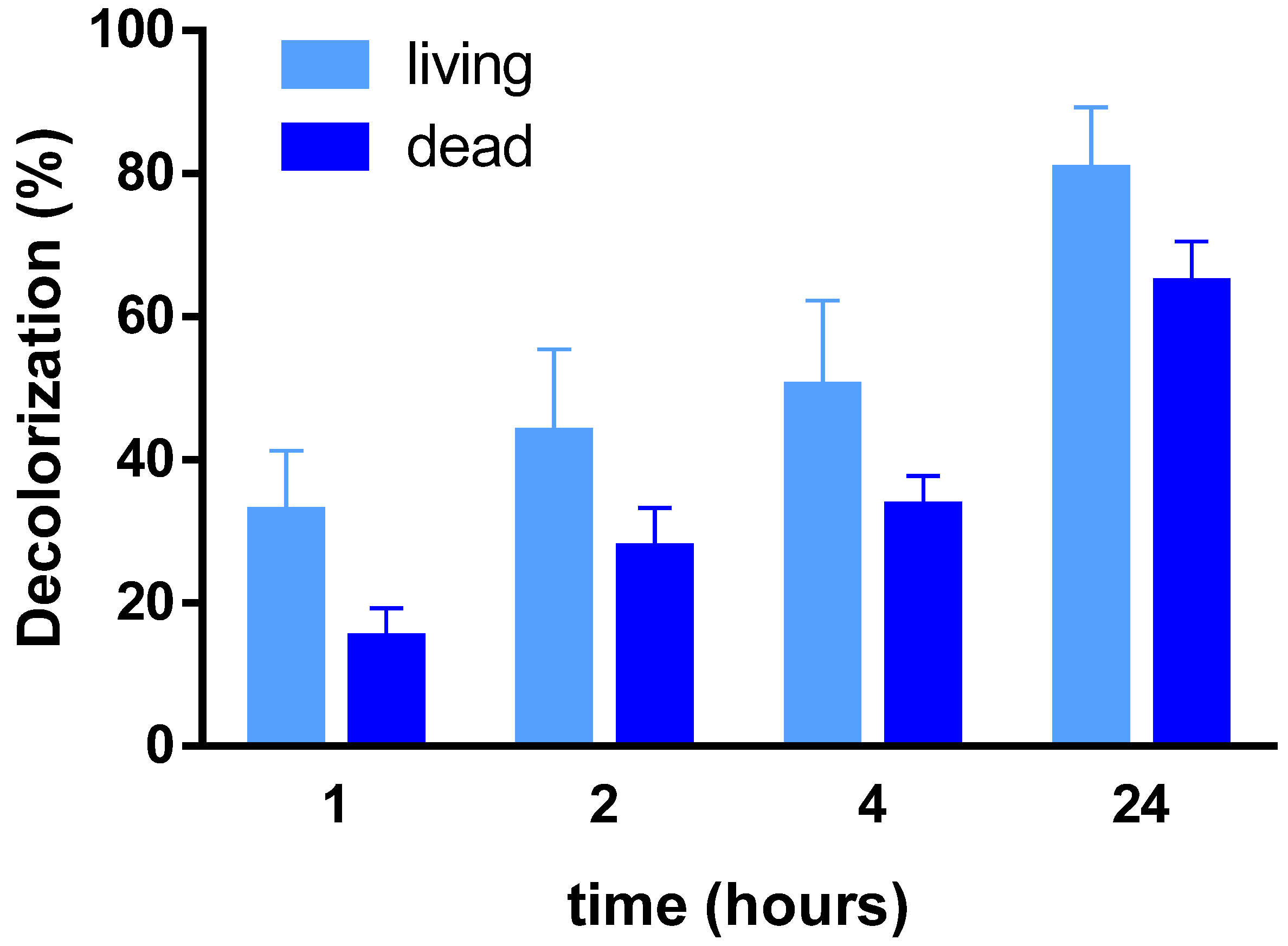
| Reef | 1 h | 2 h | 4 h | 24 h | |
|---|---|---|---|---|---|
| D% | Living | 32.75 ± 8.57 | 43.71 ± 11.73 | 50.20 ± 12.11 | 80.43 ± 8.83 |
| Dead | 15.06 ± 4.19 | 27.66 ± 5.61 | 33.49 ± 4.28 | 64.61 ± 5.94 | |
| Difference | 17.70 ** | 13.44 * | 15.20 * | 15.22 * | |
| qt (mg/g) | Living | 0.17 ± 0.04 | 0.21 ± 0.05 | 0.30 ± 0.08 | 0.53 ± 0.04 |
| Dead | 0.08 ± 0.02 | 0.13 ± 0.02 | 0.15 ± 0.02 | 0.31 ± 0.03 | |
| Difference | 0.09 *** | 0.08 ** | 0.15 ** | 0.22 ** |
| Total Match with Reference (%) | Name Composite | Composite Relative % |
|---|---|---|
| 84.27 | Cellulose | 60.52 |
| Travertine | 22.64 | |
| Calcite | 13.27 | |
| Methylene blue, certified | 3.57 |
| Samples | Residual MB Concentration Tested (mg/L) | Aliivibrio fischeri Bioluminescence Inhibition (%) | |
|---|---|---|---|
| Mean | SD | ||
| CP | 22.50 | 59.8 | 3.4 |
| L2 | 8.39 | 58.2 | 0.0 |
| L1 | 8.04 | 37.2 | 1.5 |
| L5 | 6.09 | 38.5 | 0.0 |
| L8 | 5.43 | 31.3 | 0.1 |
| L7 | 2.99 | 31.1 | 0.0 |
| L4 | 1.89 | 21.5 | 1.4 |
| CNL | 0.00 | 43.4 | 1.5 |
| D1 | 11.61 | 33.0 | 1.5 |
| D2 | 9.58 | 30.5 | 1.9 |
| D3 | 8.76 | 35.1 | 0.0 |
| D5 | 8.04 | 31.3 | 0.7 |
| D4 | 7.90 | 38.2 | 1.8 |
| CND | 0.00 | 33.1 | 1.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccardo, M.; Vellani, V.; Anselmi, S.; Bentivoglio, T.; Provenza, F.; Renzi, M.; Bevilacqua, S. The First Evidence of the Water Bioremediation Potential of Ficopomatus enigmaticus (Fauvel 1923): From Threat to Resource? Water 2024, 16, 368. https://doi.org/10.3390/w16030368
Piccardo M, Vellani V, Anselmi S, Bentivoglio T, Provenza F, Renzi M, Bevilacqua S. The First Evidence of the Water Bioremediation Potential of Ficopomatus enigmaticus (Fauvel 1923): From Threat to Resource? Water. 2024; 16(3):368. https://doi.org/10.3390/w16030368
Chicago/Turabian StylePiccardo, Manuela, Verdiana Vellani, Serena Anselmi, Tecla Bentivoglio, Francesca Provenza, Monia Renzi, and Stanislao Bevilacqua. 2024. "The First Evidence of the Water Bioremediation Potential of Ficopomatus enigmaticus (Fauvel 1923): From Threat to Resource?" Water 16, no. 3: 368. https://doi.org/10.3390/w16030368
APA StylePiccardo, M., Vellani, V., Anselmi, S., Bentivoglio, T., Provenza, F., Renzi, M., & Bevilacqua, S. (2024). The First Evidence of the Water Bioremediation Potential of Ficopomatus enigmaticus (Fauvel 1923): From Threat to Resource? Water, 16(3), 368. https://doi.org/10.3390/w16030368