Behavioural and Biochemical Responses of Freshwater Bivalve Anodonta marginata Exposed to Dichlorvos
Abstract
1. Introduction
2. Materials and Methods
2.1. Procurement of Dichlorvos, Collection and Acclimatisation of A. marginata
2.2. Toxicity Tests
2.3. Determination of Behavioural and Biochemical Marker
2.3.1. Opening of Shell and Extension of the Foot
2.3.2. Reduced Glutathione (GSH)
2.3.3. Acetylcholinesterase (AChE)
2.4. Determination of Dichlorvos Concentrations in A. marginata After 96 h Exposure
2.5. Data Analyses
3. Results
3.1. Screening of Bivalves for Dichlorvos and Lethal Toxicity
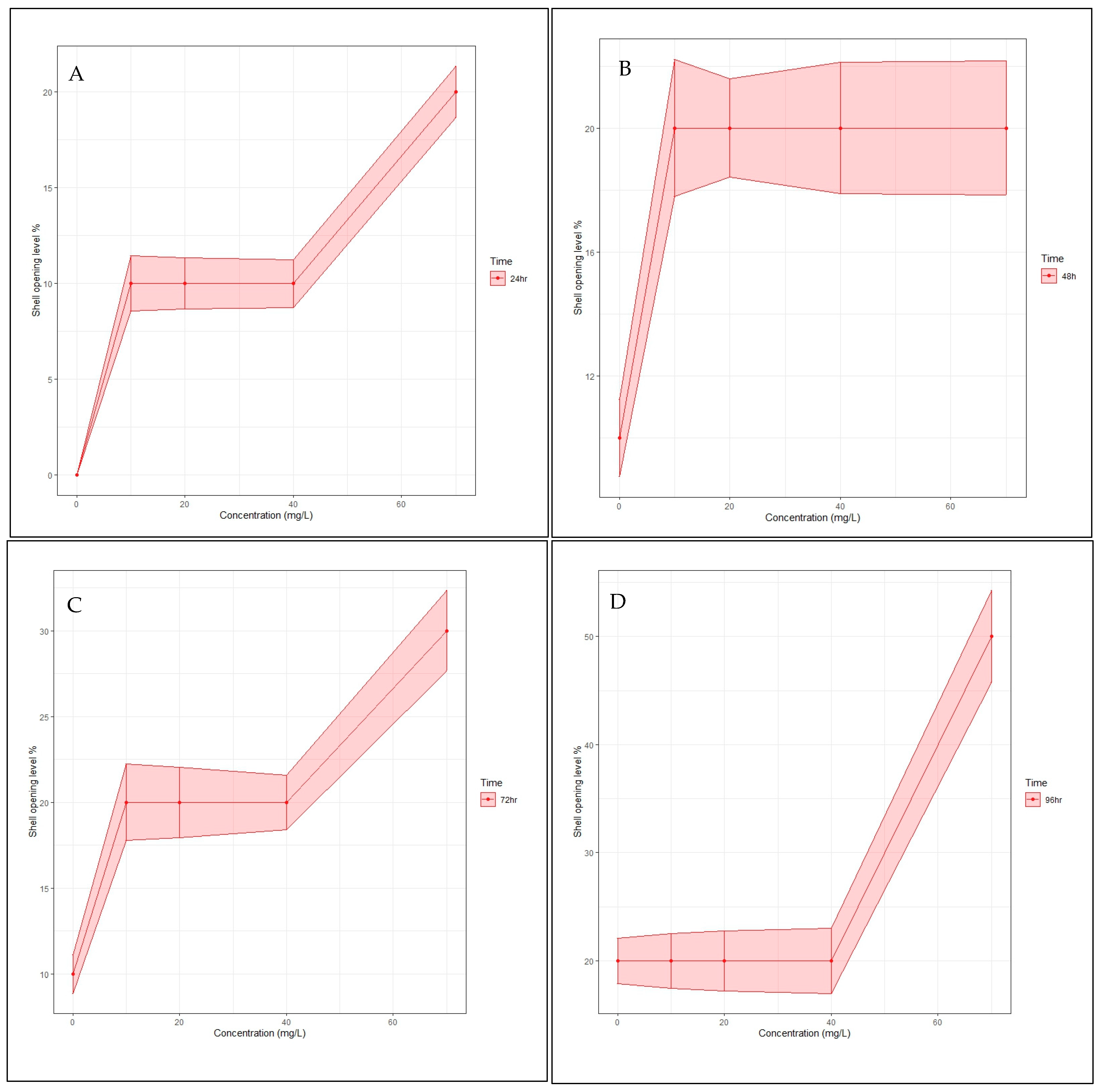
3.2. Behavioural Response/Shell Opening
3.3. Survival Response
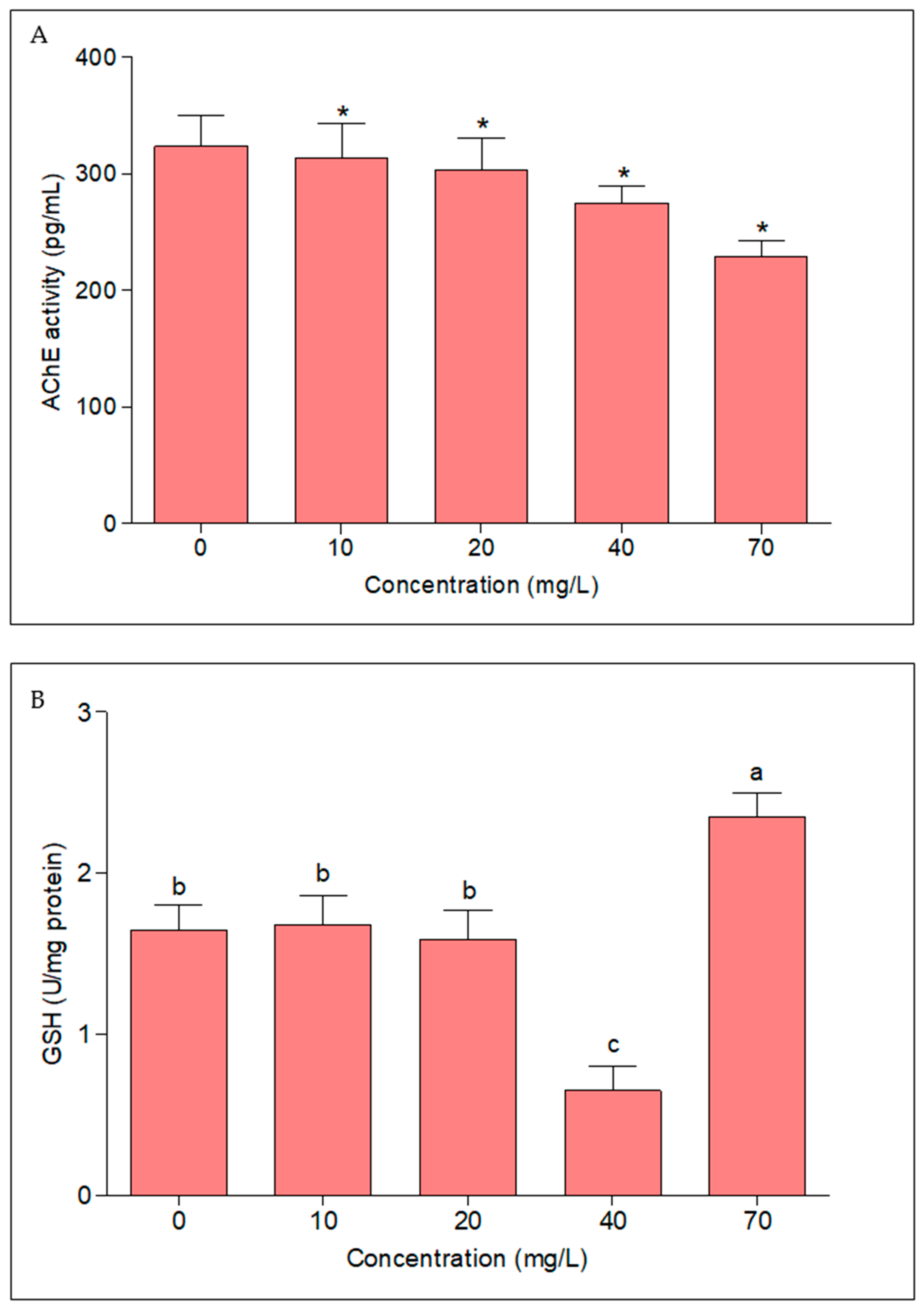
3.4. Acetylcholinesterase (AChE) and Reduced Glutathione (GSH) Activity
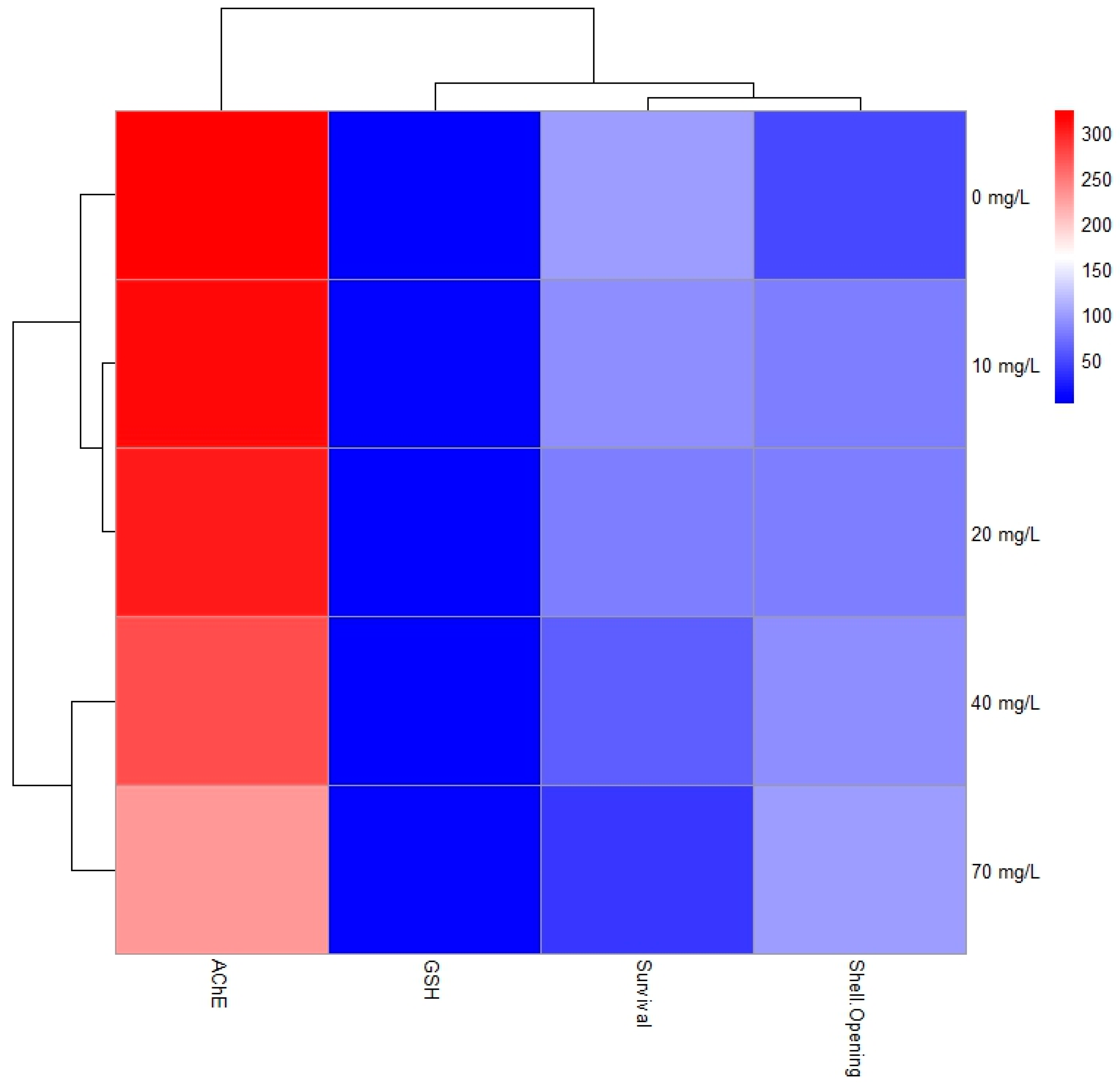
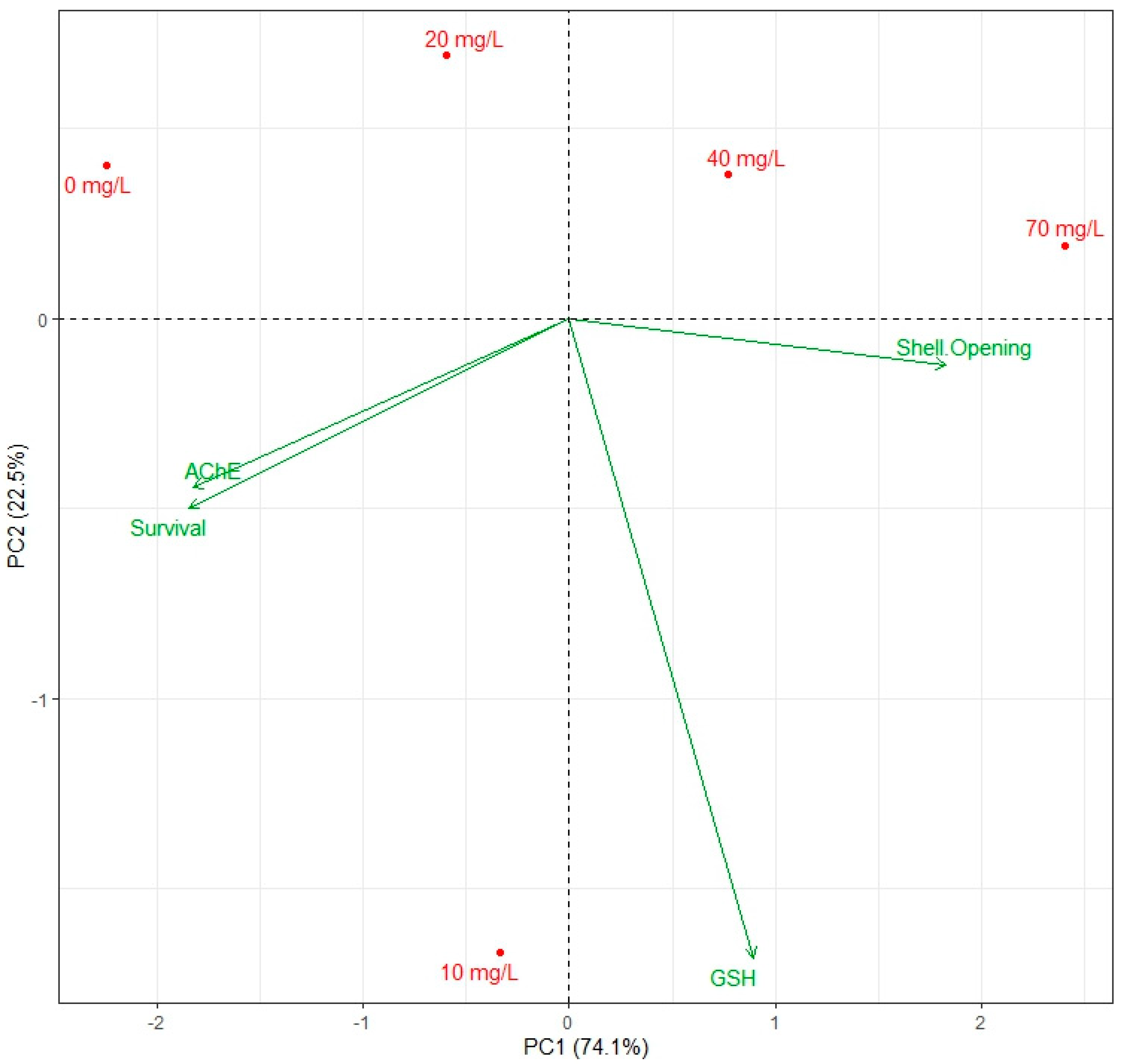
3.5. Correlation Between Behavioural Responses and Biochemical Markers
4. Discussion
4.1. Dichlorvos Toxicity and Effect on Behavioral Responses in A. marginata Shell Opening
4.2. Survival of A. marginata in Response to Dichlorvos
4.3. Effect of Dichlorvos on Acetylcholinesterase (AChE) and Reduced Glutathione (GSH) Activity
4.4. Influence of Dichlorvos on Behavioural Responses and Biochemical Markers
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.F.; Kumar, P.S.; Kabir, M.; Zuhara, F.T.; Mehjabin, A.; Tasannum, N.; Mofijur, M. Threats, challenges, and sustainable conservation strategies for freshwater biodiversity. Environ. Res. 2022, 214, 113808. [Google Scholar] [CrossRef] [PubMed]
- Brodie, J.; Landos, M. Pesticides in Queensland and Great Barrier Reef waterways—Potential impacts on aquatic ecosystems and the failure of national management. Estuar. Coast. Shelf Sci. 2019, 230, 106447. [Google Scholar] [CrossRef]
- Silva, C.; Nunes, B.; Nogueira, A.J.; Goncalves, F.; Pereira, J.L. In vitro test systems supporting the development of improved pest control methods: A case study with chemical mixtures and bivalve biofoulers. Biofouling 2016, 32, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Safana, A.I.; Imam, T.S. Bioaccumulation of heavy metals and oxidative stress biomarkers response in Anodonta marginata from River Challawa Kano State, Nigeria. Biol. Environ. Sci. J. Trop. 2023, 20, 77–94. [Google Scholar]
- Drewek, A.; Rybak, M.; Drzewiecka, K.; Niedzielski, P.; Polak, J.; Klimaszyk, P. The impact of iron coagulant on the behavior and biochemistry of freshwater mussels Anodonta cygnea and Unio tumidus during lake restoration. J. Environ. Manag. 2022, 318, 115535. [Google Scholar] [CrossRef]
- AbuQamar, S.F.; El-Saadony, M.T.; Alkafaas, S.S.; Elsalahaty, M.I.; Elkafas, S.S.; Mathew, B.T.; El-Tarabily, K.A. Ecological impacts and management strategies of pesticide pollution on aquatic life and human beings. Mar. Pollut. Bull. 2024, 206, 116613. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel, R.H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Yadav, I.C.; Devi, N.L. Pesticides Classification and Its Impact on Human and Environment. Environ. Sci. Eng. 2017, 6, 140–158. [Google Scholar]
- Widenfalk, A. Pesticide Bioavailability in Aquatic Sediments: A Literature Review. Citeseer. 2002. Available online: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=d5f10122526c8bb87a63d8e3fe5b6586430e1253 (accessed on 8 December 2024).
- Fitriyani, A.L.; Rahardjo, S.S.; Murti, B. The Effect of Organophosphate Pesticides Exposure and Other Factors Associated with Neuropsychiatric Disorders among Rice Farmers: A Path Analysis Evidence from Sukoharjo, Central Java. J. Epidemiol. Public Health 2020, 5, 182–194. [Google Scholar] [CrossRef]
- Sonone, S.S.; Jadhav, S.; Sankhla, M.S.; Kumar, R. Water Contamination by Heavy Metals and Their Toxic Effect on Aquaculture and Human Health through Food Chain. Lett. Appl. Nanobiosci. 2020, 10, 2148–2166. [Google Scholar]
- Kamal, A.; Ahmad, F.; Shafeeque, M.A. Toxicity of Pesticides to Plants and Non-target Organism: A Comprehensive Review. Iran. J. Plant Physiol. 2020, 10, 3299–3313. [Google Scholar]
- Meftaul, I.M.; Venkateswarlu, K.; Dharmarajan, R.; Annamalai, P.; Megharaj, M. Pesticides in the urban environment: A potential threat that knocks at the door. Sci. Total Environ. 2020, 711, 134612. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kaushik, G.; Dar, M.A.; Nimesh, S.; Lopez-Chuken, U.J.; Villarreal-Chiu, J.F. Microbial degradation of organophosphate pesticides: A review. Pedosphere 2018, 28, 190–208. [Google Scholar] [CrossRef]
- Sousa, J.C.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Bustos, N.J.; Iriel, A.; Cirelli, A.F.; Cedergreen, N. Species sensitivity distribution of dichlorvos in surface water species. Sustain. Environ. Res. 2022, 32, 30. [Google Scholar] [CrossRef]
- Moran, K.; Anderson, B.; Phillips, B.; Luo, Y.; Singhasemanon, N.; Breuer, R.; Tadesse, D. Water quality impairments due to aquatic life pesticide toxicity: Prevention and mitigation in California, USA. Environ. Toxicol. Chem. 2020, 39, 953–966. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Li, J.; Pang, S.; Mishra, S.; Bhatt, P.; Zeng, D.; Chen, S. Emerging technologies for degradation of dichlorvos: A review. Int. J. Environ. Res. Public Health 2021, 18, 5789. [Google Scholar] [CrossRef]
- Baker, B.P.; Green, T.A.; Loker, A.J. Biological control and integrated pest management in organic and conventional systems. Biol. Control 2020, 140, 104095. [Google Scholar] [CrossRef]
- Gu, Y.; Li, G.; Huang, C.; Liu, P.; Hu, G.; Wu, C.; Liu, P. Dichlorvos poisoning caused chicken cerebrum tissue damage and related apoptosis-related gene changes. Sci. Total Environ. 2021, 783, 147051. [Google Scholar] [CrossRef]
- Herbert, L.T.; Cossi, P.F.; Painefilo, J.C.; Gooalons, C.M.; Luquet, C.M.; Kristoff, G. Acute Neurotoxicity Evaluation of Two Anticholinesterasic Insecticides, Independently and in Mixtures, and a Neonicotinoid on a Freshwater Gastropod. Chemosphere 2021, 265, 129107. [Google Scholar] [CrossRef]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Pérez, M.R.; Covantes-Rosales, C.E.; Toledo-Ibarra, G.A.; Mercado-Salgado, U.; Ponce-Regalado, M.D.; Díaz-Resendiz, K.J.G.; Girón-Pérez, M.I. Organophosphorus pesticides as modulating substances of inflammation through the cholinergic pathway. Int. J. Mol. Sci. 2022, 23, 4523. [Google Scholar] [CrossRef] [PubMed]
- Darvesh, S.; Hopkins, D.A.; Geula, C. Neurobiology of butyrylcholinesterase. Nat. Rev. Neurosci. 2003, 4, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C. Neurotoxicity of organophosphate nerve agents. In Advances in Neurotoxicology; Academic Press: Cambridge, MA, USA, 2020; Volume 4, pp. 79–112. [Google Scholar]
- Vega-López, A.; Lara-Vega, I.; Atonal-Brioso, G.; Nájera-Martínez, M. Neurotoxicant effects of bisphenol A, nonylphenol, and tert-butyl phenol in the Nile tilapia (Oreochromis niloticus). Aquat. Toxicol. 2024, 268, 106868. [Google Scholar] [CrossRef]
- Sonchez-Santed, F.; Colomina, M.T.; Hernondez, E.H. Organophosphate Pesticide Exposure and Neurodegeneration. Cortex 2016, 74, 417–426. [Google Scholar] [CrossRef]
- Karua, P.C.; Parida, S.K. Efficacy in Treatment of Moderate to Severe Degree Organophosphorus Poisoning with Fresh Frozen Plasma, Atropine and 2-pyridine Aldoxime Methyl Chloride As Compared to Treatment with Atropine and 2-pyridine Aldoxime Methyl Chloride in Western Odisha. Int. J. Res. Med. Sci. 2020, 8, 3513. [Google Scholar] [CrossRef]
- Logesh, R. A Cross Sectional Study of Relation of Creatine Phospho Kinase-mb Levels with Electrocardiogram Parameters in Organophosphorous Poisoning; Stanley Medical College: Chennai, India, 2022. [Google Scholar]
- Brahma, N.; Gupta, A. Acute toxicity of lead in fresh water bivalves Lamellidens jenkinsianus obesa and Parreysia (Parreysia) corrugata with evaluation of sublethal effects on acetylcholinesterase and catalase activity, lipid peroxidation, and behavior. Ecotoxicol. Environ. Saf. 2020, 189, 109939. [Google Scholar] [CrossRef] [PubMed]
- de Souza Pinheiro, A.; Olimpio da Rocha, G.; Bittencourt de Andrade, J. A SDME/GC–MS methodology for determination of organophosphate and pyrethroid pesticides in water. Microchem. J. 2011, 99, 303–308. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Methods for Measuring the Acute Toxicity of Effluents and Receiving Waters to Freshwater and Marine Organisms, 5th ed.; USEPA: Washington, DC, USA, 2021.
- Serrano, R.; Hernandez, F.; Pena, J.B.; Dosda, V.; Canales, J. Toxicity and bioconcentration of selected organophosphorus pesticides in Mytilus galloprovincialis and Venus gallina. Arch. Environ. Contam. Toxicol. 1995, 29, 284–290. [Google Scholar] [CrossRef]
- Chmist, J.; Szoszkiewicz, K.; Drożdżyński, D. Behavioural responses of Unio tumidus freshwater mussels to pesticide contamination. Arch. Environ. Contam. Toxicol. 2019, 77, 432–442. [Google Scholar] [CrossRef]
- Yasmeen, S.; Pathan, T.S. Behavioural response in freshwater bivalve mollusk, Lamellidens marginalis due to acute toxicity of cadmium chloride. Int. J. Biol. Innov. 2021, 3, 148–153. [Google Scholar] [CrossRef]
- Jakubowska, M.; Normant-Saremba, M. The effect of CO2-induced seawater acidification on the behaviour and metabolic rate of the Baltic clam Macoma balthica. Ann. Zool. Fenn. 2015, 52, 353–367. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, S.; Yue, M.; Zhu, S.; Liu, Q.; Zhao, X.E. Recent advances in analytical methods of oxidative stress biomarkers induced by environmental pollutant exposure. TrAC Trends Anal. Chem. 2023, 160, 116978. [Google Scholar] [CrossRef]
- Van Emon, J.M. Immunoassay and Other Bioanalytical Techniques; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Shiry, N.; Derakhshesh, N.; Alavinia, S.J.; Pouladi, M.; Falco, F.; Faggio, C. Anodonta Cygnea, a Freshwater Swan Mussel, Exposed to Diazinon: Toxicity Thresholds in Behaviour and Physiology. Vet. Res. Commun. 2023, 47, 1303–1319. [Google Scholar] [CrossRef]
- Catania, D. The Influence of Macroalgae on the Proliferation and Regulation of the Benthic Dinoflagellate Ostreopsis cf. ovata Blooms; Universit’ C’te d’Azur: Nice, France, 2017. [Google Scholar]
- Lima, M.G.; Augusto, R.d.; Pinheiro, J.; Thiengo, S.C. Physiology and Immunity of the Invasive Giant African Snail, Achatina (Lissachatina) fulica, Intermediate Host of Angiostrongylus cantonensis. Dev. Comp. Immunol. 2020, 105, 103579. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, J.T.; Beggel, S.; Auerswald, K.; Stoeckle, B.C.; Geist, J. Establishing mussel behavior as a biomarker in ecotoxicology. Aquat. Toxicol. 2016, 170, 279–288. [Google Scholar] [CrossRef]
- Pathiratne, A.; Kroon, F.J. Using Species Sensitivity Distribution Approach to Assess the Risks of Commonly Detected Agricultural Pesticides to Australia’s Tropical Freshwater Ecosystems. Environ. Toxicol. Chem. 2016, 35, 419–428. [Google Scholar] [CrossRef]
- Castro, B.B.; Silva, C.; Macorio, I.P.; Oliveira, B.; Goncalves, F.; Pereira, J.L. Feeding Inhibition in Corbicula Fluminea (of Muller, 1774) As an Effect Criterion to Pollutant Exposure: Perspectives for Ecotoxicity Screening and Refinement of Chemical Control. Aquat. Toxicol. 2018, 196, 25–34. [Google Scholar] [CrossRef]
- Stara, A.; Pagano, M.; Capillo, G.; Fabrello, J.; Sandova, M.; Albano, M.; Zuskova, E.; Velisek, J.; Matozzo, V.; Faggio, C. Acute Effects of Neonicotinoid Insecticides on Mytilus Galloprovincialis: A Case Study with the Active Compound Thiacloprid and the Commercial Formulation Calypso 480 Sc. Ecotoxicol. Environ. Saf. 2020, 203, 110980. [Google Scholar] [CrossRef]
- Maoxiao, P.; Xiaojun, L.; Donghong, N.; Bo, Y.; Tianyi, L.; Zhiguo, D.; Jiale, L. Survival, Growth and Physiology of Marine Bivalve (Sinonovacula constricta) in Long-term Low-salt Culture. Sci. Rep. 2019, 9, 2819. [Google Scholar] [CrossRef]
- Boldina-Cosqueric, I.; Amiard, J.C.; Amiard-Triquet, C.; Dedourge-Geffard, O.; Métais, I.; Mouneyrac, C.; Moutel, B.; Berthet, B. Biochemical, physiological and behavioural markers in the endobenthic bivalve Scrobicularia plana as tools for the assessment of estuarine sediment quality. Ecotoxicol. Environ. Saf. 2010, 73, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Yu, J.; Yang, D.B.; Ra, K.; Kim, K.T.; Hong, G.H.; Shin, K.H. Acetylthiocholine (atc)—Cleaving Cholinesterase (che) Activity As a Potential Biomarker of Pesticide Exposure in the Manila clam, Ruditapes philippinarum, of Korea. Mar. Environ. Res. 2011, 71, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Steeves, L.E.; Filgueira, R.; Guyondet, T.; Chasse, J.; Comeau, L. Past, Present, and Future: Performance of Two Bivalve Species under Changing Environmental Conditions. Front. Mar. Sci. 2018, 5, 184. [Google Scholar] [CrossRef]
- Bui-Nguyen, T.M.; Baer, C.E.; Lewis, J.A.; Yang, D.; Lein, P.J.; Jackson, D.A. Dichlorvos Exposure Results in Large Scale Disruption of Energy Metabolism in the Liver of the Zebrafish, Danio rerio. BMC Genom. 2015, 16, 853. [Google Scholar] [CrossRef]
- Bali, Y.A.; Kaikai, N.E.; Ba-M’hamed, S.; Bennis, M. Learning and memory impairments associated to acetylcholinesterase inhibition and oxidative stress following glyphosate based-herbicide exposure in mice. Toxicology 2019, 415, 18–25. [Google Scholar] [CrossRef]
- Todd, S.W.; Lumsden, E.W.; Aracava, Y.; Mamczarz, J.; Albuquerque, E.X.; Pereira, E.F. Gestational exposures to organophosphorus insecticides: From acute poisoning to developmental neurotoxicity. Neuropharmacology 2020, 180, 108271. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Jhamtani, R.C.; Dahiya, M.S.; Agarwal, R. Oxidative injury caused by individual and combined exposure of neonicotinoid, organophosphate and herbicide in zebrafish. Toxicol. Rep. 2017, 4, 240–244. [Google Scholar] [CrossRef]
- Montory, J.A.; Cubillos, V.M.; Lee, M.R.; Chaparro, O.R.; Gebauer, P.; Cumillaf, J.P.; Cruces, E. The interactive effect of anti-sea lice pesticide azamethiphos and temperature on the physiological performance of the filter-feeding bivalve Ostrea chilensis: A non-target species. Mar. Environ. Res. 2023, 183, 105837. [Google Scholar] [CrossRef]
- Bernal-González, K.G.; Covantes-Rosales, C.E.; Camacho-Pérez, M.R.; Mercado-Salgado, U.; Barajas-Carrillo, V.W.; Girón-Pérez, D.A.; Montoya-Hidalgo, A.C.; Díaz-Resendiz, K.J.G.; Barcelos-García, R.G.; Toledo-Ibarra, G.A.; et al. Organophosphate-pesticide-mediated immune response modulation in invertebrates and vertebrates. Int. J. Mol. Sci. 2023, 24, 5360. [Google Scholar] [CrossRef]

| Conc. (mg/L) | Log of Conc. | Actual Conc. (mg/L) | Total No. of Bivalve Exposed | Mortality | % Mortality | Survival | % Survival | PS | LC50 |
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0 | BDL | 10 | 0 | 0 | 10 | 100 | BDL * | 4.79 |
| 10 | 1 | 0.24 | 10 | 4 | 40 | 6 | 60 | ||
| 20 | 1.301 | 1.94 | 10 | 1 | 10 | 9 | 90 | ||
| 40 | 1.602 | 2.57 | 10 | 6 | 60 | 4 | 40 | ||
| 70 | 1.845 | 6.35 | 10 | 8 | 80 | 2 | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhassan, A.B.; Aljahdali, M.O. Behavioural and Biochemical Responses of Freshwater Bivalve Anodonta marginata Exposed to Dichlorvos. Water 2024, 16, 3572. https://doi.org/10.3390/w16243572
Alhassan AB, Aljahdali MO. Behavioural and Biochemical Responses of Freshwater Bivalve Anodonta marginata Exposed to Dichlorvos. Water. 2024; 16(24):3572. https://doi.org/10.3390/w16243572
Chicago/Turabian StyleAlhassan, Abdullahi Bala, and Mohammed Othman Aljahdali. 2024. "Behavioural and Biochemical Responses of Freshwater Bivalve Anodonta marginata Exposed to Dichlorvos" Water 16, no. 24: 3572. https://doi.org/10.3390/w16243572
APA StyleAlhassan, A. B., & Aljahdali, M. O. (2024). Behavioural and Biochemical Responses of Freshwater Bivalve Anodonta marginata Exposed to Dichlorvos. Water, 16(24), 3572. https://doi.org/10.3390/w16243572







