Abstract
An oceanographic cruise from the southern Adriatic to the northern Ionian Sea in May 2013 allowed us to describe the spatial abundance and distribution of decapod crustacean larval assemblages with a multidisciplinary approach. Seventeen locations on the Apulian and Albanian shelves and offshore waters, including the Strait of Otranto, were sampled by a BIONESS electronic multinet. A swarm of zoeae (11 Brachyura taxa, mostly at first instar, with Xantho granulicarpus at 87%) was recorded in the neuston of the Italian side. Decapod larvae were concentrated in the first 20–30 m surface layer, strongly linked to the thermocline and generally above the Deep Chlorophyll Maximum (DCM), suggesting that they are carried by surface water circulation. The migratory behavior of decapod larvae in coastal stations is quite regular at between 20 and 60 m depths and independent of the time of day. In offshore stations, migration is compatible with the day–night cycle, where a minimum Weighted Mean Depth (WMD) value is evident at about 20 m at night. The availability of four satellite-tracked surface drifters in the same area and during the period of larvae presence presented a possibility to explore the link between the geographic dispersal of larvae and their surface circulation in successive days. Only one drifter crossed the south Adriatic, passing from the Italian to the Balkan neritic area, taking about 40 days. The actual genetic homogeneity of many Brachyura coastal species populations on opposite sides of the Adriatic Sea suggests the existence of a genetic connection that does not rely exclusively on larvae circulation and appears to be fueled by additional strategies of biological communication.
1. Introduction
The existence of planktonic stages, generally larvae, in the life cycle of marine benthos species has been considered the main cause of the observed geographic distribution and/or genetic inter-population connectivity, mainly for sessile neritic species [1,2]. Supply-side ecology [3,4], founded on larvae abundance and mobility, is key to the interpretation of community dynamics and population connectivity along shorelines. Larvae, in addition, have survival rates and persistence times in the plankton phase (the Pelagic Larval Duration, PLD), which only in a few cases justify high dispersal possibility (the so-called teleplanic larvae, sensu [5]). More commonly, the PLD does not allow for dispersal on large spatial scales. Under laboratory conditions, the PLD of Brachyura larvae (zoea) has been observed to be inversely correlated with water temperature [6,7,8,9,10,11].
The debate on the likelihood of marine larvae produced in a local population returning to that population (self-recruitment or retention) or migrating to another population (export) is open [12]. Moreover, hydrodynamic models and genetic structure data indicate that the average scale of dispersal can vary widely, even within a given species, at different locations in space and time [12,13,14]. Larval dispersal prediction requires knowledge of the processes regulating the phenomenon and of the spatial and temporal scales over which it occurs [15]. Estimates of marine larval dispersal, which ranges from a few meters to hundreds of kilometers [16,17,18,19,20], are well correlated with the PLDs of many organisms, including Decapoda, even though exceptions do exist [21,22,23]. Furthermore, larval behavior significantly affects their dispersal; e.g., larvae occupying very near-bottom waters typically undergo short-distance dispersal [12].
Small basins, where the presence of the continental shelf affects local circulation, can be the stage for coast-to-coast exchanges for species propagules represented by planktonic larvae, more than large oceanic areas [24,25]. The South Adriatic Sea is a very good example, as it is only 76 km wide at its narrowest point (the Otranto Strait), thus suggesting a possible enhancement of connectivity between benthic communities on opposite sides of the basin. Bray et al. [26] predicted that larvae of coastal benthos in the Adriatic Sea are able to pass from the East to the West sides of the basin by following the surface currents, with Apulia (the southwest coast of the Basin) acting as a sink area.
Among Crustacea, Decapoda Brachyura is a good candidate for studies on the dispersal capability of coastal benthos using larvae. These larvae (zoeae and megalopae) are reported as typical of the uppermost layer of the seawater (neuston) [27], although they undertake daily vertical migrations, and this enables prediction of their traveling routes driven by surface currents. Dos Santos et al. [28] described a general tendency among Decapoda larvae to persist in the vicinity of their birth sites, with larvae of coastal species accumulating in coastal sites and those of the neritic species having a larger spatial distribution. Long-term studies have highlighted that climate change has led to a change in the timing of biological events, although few studies have investigated the effects that such events may have on the phenology of marine and estuarine larval species [29,30]. Inter-annual, cross-shore, and alongshore differences in decapod larvae distribution have been established as closely affected by local hydrodynamic conditions of adult sites [31,32,33], suggesting the existence of a strategy driven by the necessity of adults to persist in the same area more than to be dispersed elsewhere by currents. Studies by Torres et al. [34,35] suggest that larvae of coastal/neritic species living in shallow waters undertake daily vertical migrations (involving the neuston) that are smaller than those of mesopelagic and/or deep bottom species. The two-dimensional space represented by the sea surface is the migration field for those larvae that stay in the surface layer, at least for part of the day (generally nighttime). Vertical migration, with a shifting presence during the day, in different water layers that possibly move at different speeds and/or in different directions [28] might also have to be taken into account. More recently, 12 published contributions to a Special Issue addressed a variety of topics, including the diversity, biology, and ecology of Decapoda crustacea [36].
Despite the high number of studies investigating decapod larvae abundances in coastal and shelf areas [34,37,38,39], such studies remain scarce in slope and offshore areas [35,40,41,42]. As for the Mediterranean Sea, most of these studies on decapod larvae abundances were carried out in the western part [34,35,41,42,43,44,45] in summer and autumn–winter periods. Referring to the Adriatic Sea, very few studies have been carried out specifically on decapod larvae distribution [46,47,48], while a few references are available in some zooplankton papers [49,50]. During the same spring oceanographic cruise reported in this paper, spatial variations in neuston biodiversity patterns were investigated [51]; drifter tracks are also available [52].
This study aims to understand the spatial abundance and distribution of decapod crustacean larval assemblages in seventeen coastal, shelf, and offshore locations spanning from the southern Adriatic to the northern Ionian basin during the spring period of the year 2013. The study aims to assess the fine-scale vertical distribution and migration behavior of decapod larvae in relation to environmental conditions. Here, attention has been focused on the swarm of Decapoda larvae recorded in the neuston at stations “Penna Grossa” (PGR) and S1351. This experiment is useful for clarifying what role, if any, larvae play in the geographic distribution of species and whether they represent the best connection between the populations inhabiting opposite sides of a small coastal basin like the Adriatic Sea [26,53].
2. General Environmental Patterns in the Region
The Southern Adriatic Sea is enclosed between the Italian and the Balkan coast. It is characterized by a wide depression that is more than 1200 m deep [54]. South of the Gargano Promontory, the Italian coast is low and has a wide and sandy shelf, whereas the eastern coast is generally irregular, with several river mouths and a shelf that narrows from north to south, where the Otranto Straits connects the Adriatic to the Ionian Sea.
Previous climatological studies highlighted a general west-to-east positive gradient for salinity (38 to 38.4) due to the presence of large freshwater discharges in the northwestern Adriatic basin (from the Po River) flowing southeastward, and saltier inputs from the Ionian Sea along the eastern coast. Temperature fields show a seasonal excursion exceeding 10 degrees (12–14 °C in winter, 24 °C in summer, with generally colder waters along the Italian coast) [55] and exhibit frontal structures characterized by large-scale patchiness in spring and summer during the spring–summer seasons. Seasonal thermoclines extend down to approximately 75 m [54]. Physical and geochemical properties of the surface layer are subject to seasonal variability induced by modulations in wind forcing and offshore transport of freshwater inflows [56]. Winter weather conditions often cause heat loss and water evaporation that can induce dense water formation by open-ocean deep convection [57].
The South Adriatic Sea is characterized by two surface coastal currents: the Western Adriatic Current (WAC), which flows southeastwards along the Italian coast, and the Eastern Adriatic Current (EAC), which enters the basin along the Balkan coast and flows northwestwards. The former carries Adriatic Surface Water (ASW), which is fresher and nutrient-rich due to river inputs in the North Adriatic [58]; the latter carries Ionian Surface Water (ISW), which is relatively warmer and saltier [59]. The EAC forms the South Adriatic Gyre (SAG), a permanent topographically constrained cyclonic circulation feature in the Southern Adriatic whose strength is modulated by large-scale climate-driven patterns [60]. In periods characterized by an Ionian cyclonic circulation phase (such as at the beginning of 2013 [61]), the higher vorticity of the local wind forcing, correlated with more frequent southerlies over the Southern Adriatic, has a dominant effect in sustaining the SAG strength.
In April ([62], Figure 3d) and May 2013, the wind regime in the Southern Adriatic showed dominant components towards east (1.0 m s−1) and north (0.33 m s−1) [63], with a clear influence on the monthly averaged SAG pattern and transient connectivity at the surface in the region, as highlighted in the model reanalysis [64] (Figure 1).
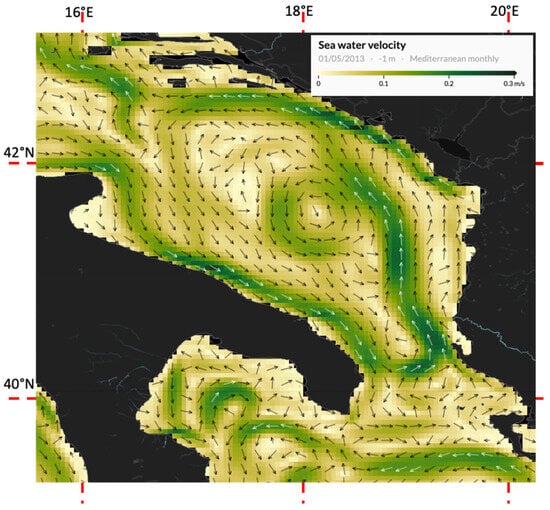
Figure 1.
May 2013: Estimated monthly averaged surface velocities in the Southern Adriatic (model reanalysis, modified from CMEMS-Copernicus Marine Environment Monitoring Service). Main circulation patterns (WAC, EAC, SAG) can be recognized.
3. Materials and Methods
An oceanographic cruise in the southern Adriatic Sea, between the Italian and the Albanian–Greek coasts (Figure 2), was carried out aboard R/V Urania from 8 to 21 May 2013 in the framework of the EU FP7 CoCoNET project [65].
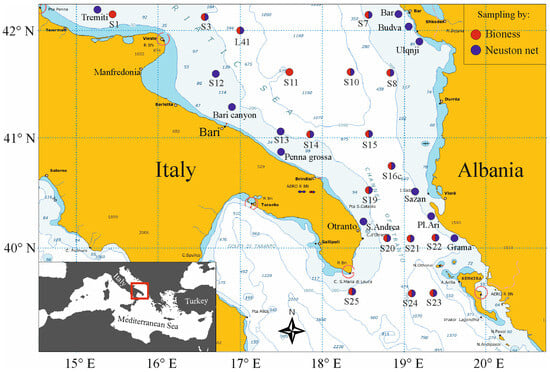
Figure 2.
Map of the study area, with the BIONESS multinet and Neuston net sampling locations (red and blue dots, respectively).
3.1. BIONESS Sampling
To investigate the horizontal and vertical distributions of zooplankton, especially decapod larvae, seventeen locations on the Apulian and Albanian shelves and offshore waters, including the Strait of Otranto, were sampled (Figure 2). The samples were collected in late spring using a BIONESS (Bedford Institute of Oceanography Net and Environmental Sampling System [66]), a multiple-opening and closing-net sampler equipped with ten nets (200 µm mesh size) with a mouth area of 0.25 m2. Vertical profiles of temperature (°C), salinity, and Chla (μg l−1) gathered by a multi-parametric probe (SBE 911 plus, SeaBird Electronics, Bellevue, WA, USA) with a fluorescence sensor (Seapoint Chlorophyll Fluorometer, Seapoint Sensor Inc, Exeter, NH, USA) mounted on its frame were processed with the Ocean Data View (ODV) software (5.7.2 version) to obtain on board real-time vertical profiles. Flow velocity and filtration efficiency were monitored by internal and external flowmeters (GO2031H) (General Oceanics, Miami, FL, USA).
The BIONESS was towed at a speed of 1.5–2 m s−1 along an oblique path, allowing very detailed resolution of the zooplankton vertical distribution. During each tow, a maximum of nine depth intervals were sampled. During the first downcast, the thermocline, pycnocline, halocline, and Deep Chlorophyll Maximum (DCM) layer thickness were analyzed in order to decide on the sampling layers. A total of 136 zooplankton samples were collected from several layers between the surface and a few meters above the seabed along a 0–1100 m water column (Table 1). Five 20 m thick layers (0–20 m, 20–40 m, 40–60 m, 60–80 m, 80–100 m) in the first 100 m were sampled, followed by wider ones down to the maximum depth reached. Filtered water volumes varied between 25 and 108 m3, generally increasing with depth. On board, each sample was preserved in a 4% borax-buffered formaldehyde and seawater solution. Sampling details are shown in Table 1. Sunrise and sunset times were 05:49 and 20:45 (GMT +2:00).

Table 1.
BIONESS sampling details.
3.2. Neuston Collection
A neuston net (with a 1 × 0.5 m rectangular mouth opening and a 200 µm mesh size), equipped with lateral buoys to float on the sea surface with the upper border 5 cm above sea level, was used to collect neuston in a total of 27 stations (Figure 2). The net was towed at a speed of 1 kn. The presence of a flowmeter at the center of the mouth allowed us to measure the volume of filtered water at each sampling collection. The filtered volume (m3) of each sample was calculated by approximately correcting the value by an average of 90% of the net mouth surface (on the basis of the non-complete submersion of the net mouth). At each sample collection, the neuston was immediately stored in 50 mL Falcon tubes with 95% ethanol (final concentration: 80–90%). At each station, abundance data represent the average of two neuston collections. For more sampling details, see Liparoto et al. [51]. Environmental parameters on Brachyura larvae swarm sites are shown in Table 2. Temperature, salinity, and dissolved oxygen (CTD) vertical profiles were obtained by a multiparametric probe incorporated into a carousel of 5 L Niskin bottles. Due to the mostly superficial presence of Brachyura larvae, only surface water characteristics (above the thermocline) were used to calculate its pelagic life duration (PLD).

Table 2.
Hydrological parameters (salinity, temperature, dissolved oxygen) of the seawater in the South Adriatic Sea at stations Penna Grossa (PGR) and S13, and average values over the whole basin.
3.3. Laboratory Analysis
In the laboratory, a qualitative–quantitative analysis of both mesozooplankton and neuston samples was performed. BIONESS sub-samples, ranging from 1/10 to 1/25, were obtained with a Folsom Splitter and Stempel pipettes from the original sample and analyzed for species identification and specimen counts, depending on total sample richness, while identification of rare species was carried out on the entire sample. For the neuston, mesozooplankton and decapod larvae were sorted and quantified. The abundance in each stratum was calculated by dividing the total number by the filtered water volume and expressed as individuals m−3 or individuals times 100 m−3. For the entire water column, the total abundance at each station was expressed as a weighted mean, summing all counted individuals and dividing by the total volume of seawater filtered (m3), multiplied by the water column thickness, and expressed as individuals m−2. Zooplankton biomass was estimated from a 250 mL aliquot obtained with a Folsom Splitter from the original sample. The aliquots were filtered and weighed according to Tranter (1962) [67]. Biomass was given in wet and dry weights and expressed as mg m−3 of filtered seawater. At stations PGR and S13 (Table 2), Brachyura larvae (zoeae and megalopae) were found to be dominant in the remaining zooplankton community [51]. Such abundant populations were chosen for the study of Brachyura dispersion and, consequently, the larvae were identified at higher taxonomic levels using the guides given by [68,69,70,71,72]. Larvae derived from BIONESS collections were roughly identified as Decapoda, containing not only Brachyura but also Anomura and shrimp larvae, to a lesser extent. BIONESS samples were not collected in PGR and S13 coastal stations (those interested in the Brachyura swarm), but their results have been used to give a general picture of decapod larvae abundance (ind. m−3) and distribution in the whole south Adriatic basin.
3.4. Statistical Analysis
To assess the vertical partitioning of the decapod larvae by daytime and nighttime abundances, the Weighted Mean Depth (WMD) was calculated according to the equation WMD = Σ(ni × zi × di)/Σ(ni × zi), where ni is the number of ind. 100 m−3 in the i-th layer, di is the depth of sample i (center of the depth interval), and zi is the thickness of the layer [73,74]. The thicknesses of the thermocline and halocline layers were visually estimated based on the vertical profiles of temperature and salinity at each station; likewise, the depth at which fluorescence was highest was considered the DCM depth. The correlations between these parameters and the abundance of decapod larvae in the seventeen stations were tested using the Kendall rank-based test, a non-parametric test that does not rely on any assumptions of the distribution of the variables under analysis. Kendall’s Tau coefficient is a measure of rank correlation between two variables. In our case, since the Shapiro–Wilks normality test is violated (by the variables “abundance of decapod larvae” and “Thermocline thickness”), the Kendall rank-based correlation method is used.
Significant differences between groups of data (temperature, salinity, and fluorescence in the 0–100 m layer in the three regions of the Italian shelf, Pelagic waters, and the Albanian coast) were assessed using one-way ANOVA.
3.5. Surface Drifters
Lagrangian, i.e., water-following, instruments are best suited to studying transport and dispersion in marine environments [75,76]. A total of 26 CODE drifters (for a description, see Kalampokis et al. [77] or Zambianchi et al. [52]) were employed to reconstruct the surface water circulation in the south Adriatic during the cruise. Their 2D motion was tracked by satellite. The present study only took into consideration the tracks of 4 drifters transiting in the same area of the Brachyura larvae swarm (stations PGR and S13) at the same period of their localization, i.e., from 2 days before to 10 days after their collection (Table 3). This area is defined as a rectangular box (0.7° Lat × 0.4° Lon) around the two selected stations (PGR and S13), which are considered the starting points for larvae drift (see Table 3).

Table 3.
Identification codes for the drifters entering the box area containing the stations where Brachyura larvae were abundant in the neuston (date of collection, 17 May 2013). Only drifters entering the box in the period 15–27 May 2013 were considered.
Based on the water temperature, a PLD of 40 days has been established for the larvae (zoeae, mostly of the first instar) found in May 2013 at stations PGR and S13 (surface water temperatures of 18.44 and 19.69 °C; average for the whole basin, 18.97 °C, and never more than 21.15 °C in the other stations).
The CODE drifter motion represents the uppermost 1 m of the sea surface circulation. Their GPS positions were accurate to a 10 m order of magnitude and were transmitted/recorded every 15 min. Raw data were edited to remove spikes and errors and are available at http://www.coconet-fp7.eu (accessed on 3 August 2024).
4. Results
4.1. Hydrographic Conditions
As expected, clear differences emerged for environmental parameters in the three regions. Considering the 0–100 m layer, the Italian coast was occupied by colder and less salty waters than the Balkan coast. Mean temperature exhibited a west-to-east gradient (Italian shelf: 15.3 °C; Pelagic waters: 15.7 °C; Albanian shelf: 16.1 °C) with significant differences among the regions (ANOVA, p < 0.001). Salinity was significantly lower and more variable in the Italian shelf (38.8 ± 0.21) than in pelagic waters (38.9 ± 0.06) and the Albanian shelf (38.9 ± 0.06) (ANOVA, p < 0.001).
Chlorophyll fluorescence in the 0–100 m layer showed an opposite longitudinal gradient, with maxima on the Italian shelf (0.42 ± 0.22 mg m−3), lower values in pelagic waters (0.42 ± 0.26), and minima along the Albanian coast (0.38 ± 0.26), with significant differences between the regions (ANOVA, p = 0.029).
The vertical structure of the water column sampled in mid-May at Apulian, offshore, and Albanian stations showed clear site-dependent differences. Temperature and salinity profiles (Figure 3) display an evident stratified temperature and a well-defined thermocline between 20 and 40 m that separates the upper layer from the underlying layers, with a difference of about 4–5° C between the upper (warmer) and the lower (colder) layers. A clear halocline was evident deeper (18–26 m) on the Italian side than on the Albanian one (5–15 m). Horizontal and vertical variability of thermohaline characteristics showed strong differences between stations and along the sampled water column.
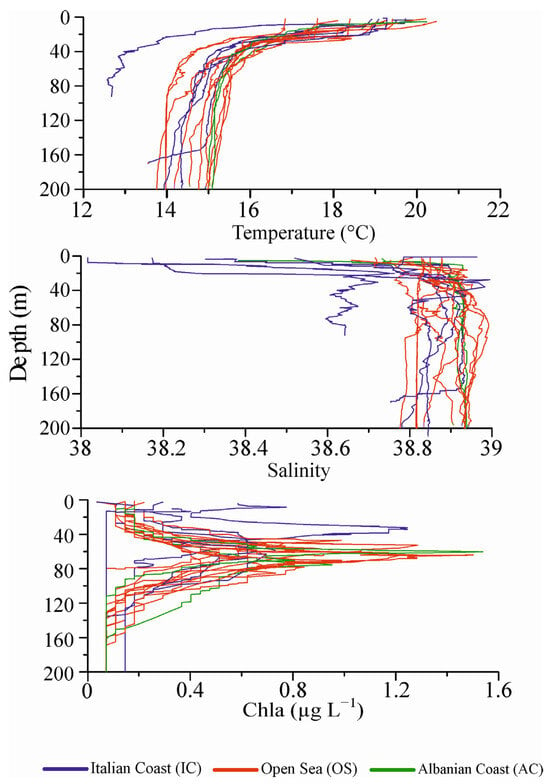
Figure 3.
Potential temperature, salinity, and fluorescence values of vertical profiles at all sampled stations, from the surface down to a 200 m depth (IC: S1, S3, S25, S19, S14 stations; OS: L41, S10, S15, S16c, S22, S23, S21, S24, S20, S11 stations; AC: S7, S8 stations).
Fluorescence profiles showed maxima in the layer between the 50 m and 80 m depth for all the stations (Figure 4) except St. S1, which showed the highest chlorophyll a concentration at about 35 m (1.17 mg m−3). A different depth of the DCM was detected in the different sites. Generally, in the areas close to the coast and in the Strait of Otranto, this maximum was found at about 60 m, but with very different max chlorophyll a values of 0.696 mg m−3, 1.54 mg m−3, and 1.12 mg m−3 in the Apulian and Albanian sides and in the middle of the Strait, respectively.
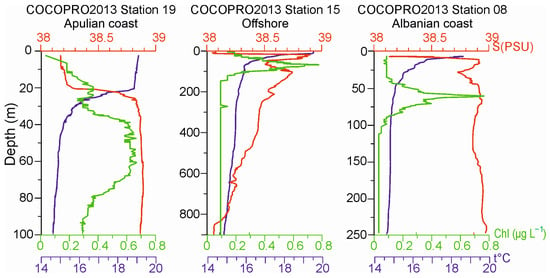
Figure 4.
Vertical profiles of temperature (°C), salinity, and Chla (μg L−1) at stations S19, S15, and S08 in the CoCoNet cruise, 2013, as representatives of Italian, central South Adriatic, and Balkan coast, respectively. Please note that depth scales vary.
4.2. Spatial Distribution of Zooplankton and Decapod Larvae
The total zooplankton mean abundance was 442 ± SD 258 ind. m−3. Copepods were the most abundant taxon, representing 72 to 91% of the total zooplankton, with a mean abundance of 401 ± 236 ind. m−3. Zooplankton abundance and biomass (dry mass) were higher on the Italian coast (408 ± 811.7 ind. m−3 and 9.7 ± 15.5 mg m−3, respectively) than on the Albanian coast (219 ± 53.0 ind. m−3 and 5.4 ± 2.7 mg m−3). Spring holoplankton was the main component of the zooplankton (85–98%). Abundance peaks of the most representative species occurred at chlorophyll maximum depths of between 20–40 m and 60–80 m.
Meroplankton percentage increased along the Albanian coasts, mostly due to bivalve and polychaete larvae. Crustacean decapod larvae represented less than 0.7% of the zooplankton community and about 7.2% of the meroplankton. Decapod larvae densities were higher along the coastal and continental shelf waters rather than in offshore pelagic waters (Figure 5). In the integrated 0–100 m layer, abundance values were highly variable (0.44 to 88.89 ind. m−2 at stations 23 and 19); abundance at the stations, in decreasing order, were as follows: stations S7 (45.16 ind. m2), S22 (26.11), S14 (16.89), and S3 (16.22). No decapod larvae were found in four stations: L41, S16c, S20, and S21.
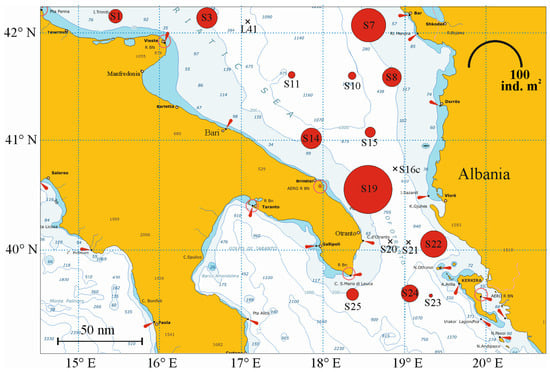
Figure 5.
Representation of decapod larvae abundance. Values represent the integrated 0–100 m surface layer and were measured in a 1 m2 water column and at a depth of 100 m. BIONESS sampling was not carried out at stations PGR and S13 (swarmed by Brachyura larvae).
The vertical distribution of the decapod larvae is shown in Figure 6. Among the nine stations with a depth greater than 700 m, very few decapod larvae were found in the layers between 400 and 800 m depths in only two stations (S22 and S23). More than 95% of the larvae occupied the 100 m surface layer. Higher larvae concentrations occurred in the 0–20 m layer, just over the thermocline, in station 19 along the Italian coast (about 436 ind. 100 m−3), and in the 20–60 m layer, below the thermocline and over the DCM, in station S7 on the Albanian side (113 ind. 100 m−3). In the other stations, the concentration of decapod larvae was lower than 70 ind. 100 m−3.
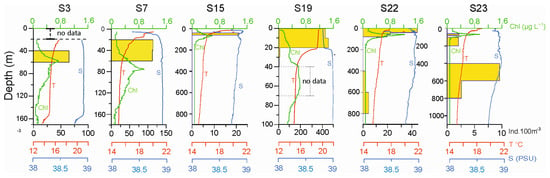
Figure 6.
Vertical distribution of crustacean decapod larvae in six selected stations.
4.3. Diel Vertical Migration
To examine temporal changes in the vertical distribution of decapod larvae abundance (%) in the absence of daily vertical catches in a fixed station, ten coastal and offshore BIONESS samples were chosen and sorted according to daily sampling time (Figure 7). Decapod larvae showed clear diel vertical migration, which did not appear to be affected by the differences between inshore and shelf stations’ physical conditions.
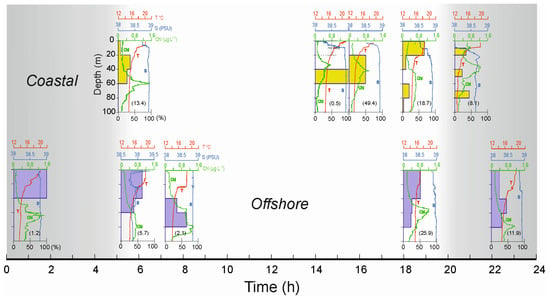
Figure 7.
Vertical distribution of decapod larvae (as a percentage of total numbers) from the BIONESS hauls of five coastal (yellow) and offshore (purple) stations selected according to sampling time. Numbers in brackets are average abundance in ind. m−3 for the entire water column at each sampling time.
In the morning, between 07:00 and 08:00 h, about one hour after sunrise, the whole community of decapod larvae was distributed between the 40 and 80 m depth, with the greatest percentage between 60 and 80 m. At the beginning of the afternoon, between 14:00 and 16:00 h, the community remained between 40 and 60 m, even though some of them were distributed up to 20 m. Between 18:00 and 21:00 h, just before nighttime, their distribution was almost bimodal, with the highest percentage in the first 20 m (coastal) or 40 m (offshore) and a low number of individuals down to 80 m. Between 22:00 and 24:00, at the beginning of the nocturnal period, the community occupied almost the whole water column between the surface and the 80 m depth, with about 50% occurring between 60 and 80 m. Between midnight and 02:00 h, their distribution entirely occupied the layer between the surface and 40 m. Before sunrise, between 05:00 and 06:00, migration to deeper layers was evident and ended after sunrise. Figure 8 shows the WMD trend both in coastal (bottom, around 100 m depth) and offshore (bottom, around 600 m depth) stations. The migratory behavior of decapod larvae in coastal stations appears quite regular between a depth of 20 and 60 m and is time independent. In offshore stations, on the other hand, migration is classically compatible with the day–night cycle, where a minimum WMD value is evident at about 20 m at night and becomes gradually deeper up to late afternoon (about 19.00–20.00 h) when migration towards the surface layers begins.
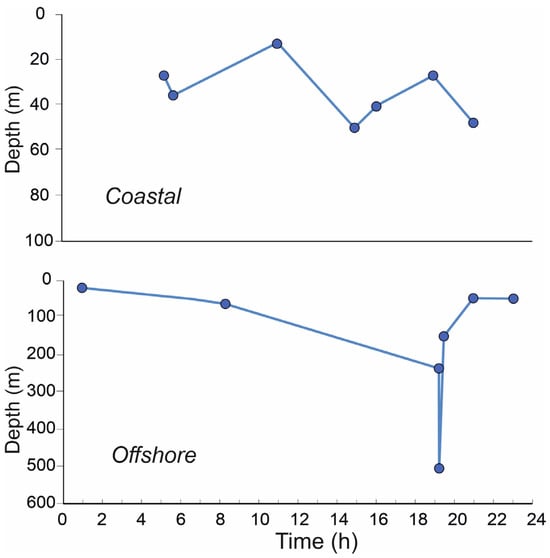
Figure 8.
Vertical profiles of decapod larvae WMD values in coastal and offshore stations, according to the daily sampling time.
4.4. Vertical Larvae Distribution in Relation to Environmental Variables
Decapod larvae abundance appears significantly and inversely correlated with the thermocline layer thickness (Tau = −0.51, Kendall rank-based test, p < 0.01) and is therefore lower in offshore waters than in coastal waters (Figure 9). However, this abundance is not significantly correlated either with the DCM depth or with the halocline thickness (p > 0.05 Kendall rank-based test).
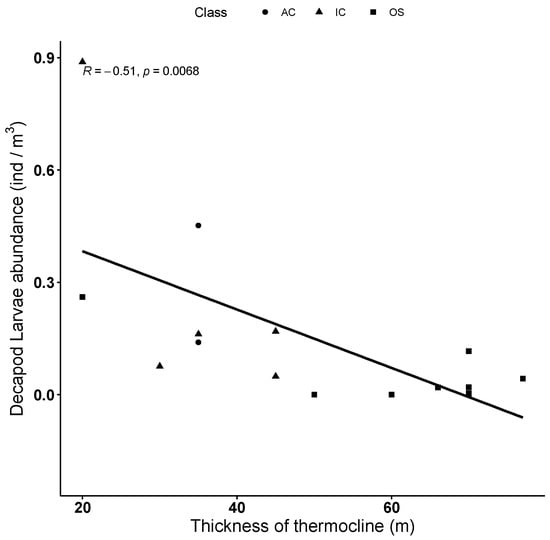
Figure 9.
Relationship between decapod larvae abundance and thermocline thickness in each station (AC—Albanian Coast, IC—Italian Coast, OS—Open Sea).
4.5. Neuston Collection
Decapod larvae have been collected in the neustons of many South Adriatic stations in the same cruise (see Liparoto et al. [51]). Among Decapoda (Table 4), Brachyura larvae (zoeae and megalopae) were considerably abundant (about 0–50 cm below the sea surface) in isolated coastal stations. In May 2013, their abundance ranged between 0 and 3.75 ind. m−3 in the whole neuston collection, but stations PGR and S13 (on the Italian side of the basin) showed concentrations of 93.02 and 231.00 ind. m−3, respectively. The highest number of Natantia larvae (593.97 ind. m−3) was recorded in station S13. In total, the average mean of the four groups of Decapoda was 34.82 ind. m−3 ± 150.29. The average abundance values of decapod larvae obtained from fifteen neuston stations, twenty-three mesozooplankton (WP2) stations, and seventeen multilayered (BIONESS) collections were 1.87 and 0.34 ind. m−3 in the neuston and water column, respectively.

Table 4.
Decapod larvae abundance (ind. m−3) in the whole neuston collection.
The detailed analysis of data from the two neuston-rich stations (S13 and PNG) allowed the recognition of Brachyura (Table 5) as the main component of the whole decapod assemblage. The identified larvae were mainly zoea-l instar belonging to a total of 11 species, largely (87%) represented by Xantho granulicarpus.

Table 5.
Larvae identified in the two cruises and relative instars (Z1–Z6 = zoea stage, instars 1–6; M = megalopa stage; undet. = not determined). Reported numbers indicate percentages of the total larvae.
4.6. Transport by Surface Currents
The basin and sub-basin-wide circulation described by CODE drifters deployed in the framework of the May 2013 cruise has been discussed in two recent papers [78,79]. Lagrangian surface trajectories accurately depict an overall cyclonic circulation dominated by a northwestward coastal current along the Balkan coast, commonly known as EAC, and the southeastward WAC along the Italian coasts. West–east connections are guaranteed by three cyclonic re-circulations localized in three corresponding morphologically distinct sub-basins of the Adriatic Sea (northern, central, and southern).
In consideration of the approximate PLDs evaluated as described above, we considered the backward and forward destinies of surface drifters passing by stations with high concentrations of larvae for a drifting time of 40 days (as often performed in physical–biological studies [80,81]). As mentioned in the Materials and Methods Section, we broadened these high concentration points into a 0.7° Lat by 0.4° Lon rectangular region (Figure 10). The drifter trajectories passing through the box (and so possibly the zoeae tracks) are characterized by a local southeastward direction off the Apulian coasts, roughly following the WAC. Two of them (drifters A and B) eventually turn eastwards, following the southernmost branch of the SAG, but only B reaches the continental shelf (i.e., depths less than 200 m) on the opposite side of the basin, whereas A is trapped by higher bathymetry (Figure 10).
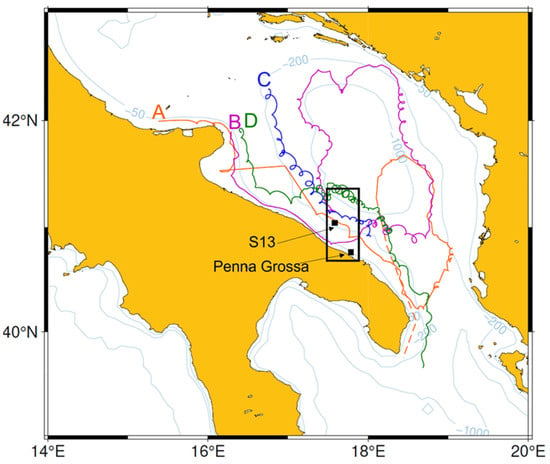
Figure 10.
Trajectories of 4 drifters passing close to stations PGR and S13 (black squares in the rectangular area) at the time of the Brachyura larvae swarm. Trajectory starting points are marked by the corresponding drifter names (letters A to D). Dashed lines refer to poorly sampled track segments.
5. Discussion
The vertical profiles of temperatures in May 2013 showed a thermocline between the 20 and 40 m depths and generally above the DCM. Larvae of decapods (collected with BIONESS in the whole basin) appeared strongly linked to this layer, suggesting that they were involved in surface water circulation. Among Decapoda, larvae of Brachyura are reported as typical components of the neuston [27] and persist in the first 50 m if they belong to coastal benthic species [28]. Also, in the present study, multilayer samplings carried out over the whole studied area suggested that Decapoda larvae prefer the uppermost water layers (0–40 m). The aggregation of such a rich plankton component at the sea surface justifies a prediction of their horizontal distribution with time, based on surface drifter movements. The availability of a set of surface drifters deployed during the same cruise when zooplankton and neuston were collected allowed us to assess the destiny of surface water masses and, indirectly, the destiny of their content in terms of larval populations. Although possible exceptions exist, water masses above the thermocline can be considered as homogenous from the dynamical point of view. Our assessment was based on a definition of PLD derived from the literature (albeit for different species). The maximum PLD relative to the larval stage/age and to the sea surface temperature in May 2013 was 45 days (mainly zoeae). Surface drifters transiting through the area where the sampling stations were located moved back and forth for a total of 40 days in order to reconstruct a possible larvae dispersal path. Even though the PLD of the species found in abundance during the considered period (Xantho granulicarpus) is not known, data from the literature suggest that west–east crossings of the South Adriatic Sea may occur. The main species represented in the samples in the present study is common along the Mediterranean coastline, and inter-population genetic connections among crab species are documented [53]. It is known that the littoral crab Pachygrapsus marmoratus is genetically uniform in the whole Mediterranean Sea [82], even when the compared populations are separated by thousands of kilometers. Schiavina et al. [83], however, established that coastal crabs of different species are genetically related and grouped in the three areas (north, central, and south) of the Adriatic Sea, independent of their collocation on the Italian or Balkan coastlines. This mirrors the circulation of the Adriatic, which can be summarized as an overall cyclonic circuit, further subdivided, both morphologically and dynamically, into three sub-basins and three corresponding cyclonic re-circulations. In terms of the transport of passive particles, an asymmetry has been observed in the zonal exchange, with a preferential east-to-west surface connection as opposed to the opposite west-to-east one [78]. This is witnessed by the successive results of Bray et al. [26], who assessed a preferential transfer of larvae from the eastern to the western coast, with the southwestern coast (i.e., the Apulian one) functioning mainly as a sink area. Fraser et al. [84] demonstrated that for many coastal taxa, transoceanic transport and landfall occur thanks to passive rafting of adults on buoyant objects more than larvae drift. On this basis, Treml et al. [85] predicted that for 95% of coral reef species, the larval settlement occurs within 155 km of the source population and/or within 13 days.
Most decapod crustaceans exhibit a complex life cycle with pelagic larval stages and benthic juvenile stages, although some species remain pelagic throughout their lives [35]. Referring to decapod Brachyura larvae found in this study, the adult crab P. marmoratus is a species with a long larval duration and a high fecundity, similar to the Mediterranean shore crab C. aestuarii studied by Schiavina et al. (2014) [83] in the Adriatic and Ionian seas. P. marmoratus breeds from late spring to late summer, depending on the geographic area [86,87]; thus, megalopae settlement peaks between June and October [88] and seems to follow a semilunar cycle. C. aestuarii planktonic larval stages remain in the water column for approximately four weeks before settlement [89]. The portunid crab Liocarcinus depurator presents sexual dimorphism, and its reproductive period in Mediterranean populations mainly takes place in autumn–winter [25], although ovigerous females are recorded throughout the year [90]. Larval development consists of two phases: five zoea stages and one megalopa stage [91]. Planktonic larval duration, although highly dependent on temperature, is estimated to be at least five weeks [92]. Juvenile crabs grow rapidly, and sexual maturity is reached during their first year of life [90]. Among the most prevalent demersal decapod species, Liocarcinus depurator accounted for most of the total biomass in the Southern Adriatic area [93]. Slope assemblages were homogeneously distributed throughout the area, while differences between the western and eastern zones were mostly on the shelf bottoms, which are influenced by other variables in addition to depth. The Acanthonyx lunulatus crab is reported in several places around the Mediterranean and Adriatic Sea [94].
Although very abundant specimens of Xantho granulicarpus Z2 larvae were found in this study, a rare adult species was reported in the northwestern Adriatic [95,96]. The species is strictly Mediterranean, living at depths of 0 to 10 m or 100 m [97] on seabeds with rocks, stones, and coarse sediments. The larval series included four zoeal and one megalopal stage, typical of the vasta majority of Xanthidae species in the subfamily Xanthinae [98]. The duration of each zoeal stage ranged from 2 to 4 days, that of the megalopa averaged 10 days, and the first crab stage was reached after 23 days. Considering that in this study only the larval stages of the second zoea (Z2) were collected on 17 May and that this planktonic larval period (the dispersal phase) lasts approximately 4–8 days, we can assume that the spawning of the ovigerous adult females occurred in the first week of May. Given the considerable difficulty in finding studies on the reproductive biology, population structure, and life history traits of adult Xantho granulicarpus, we thought it would be useful to compare our study with data from a similar study carried out on the related species Xantho poressa between March 2007 and April 2008 [99]. The annual reproductive cycle was seasonal, and successive peaks in the abundance of ovigerous females were observed in late spring and summer. The number of eggs carried by a female ranged from 2400 to about 14,500 and was correlated with female size. More recently [100], similar results have been found for the population structure and fecundity of the Xanthid crab Leptodius exaratus. The population breeds year-round; however, the maximum percentage occurrence of ovigerous females was observed from December to April, which indicates the peak breeding season. Rare X. granulicarpus specimens were reported along the Albanian coast and, for the first time, on the Tunisian coast [101,102]. Bianchi et al. (2022) [103] did not mention X. granulicarpus among the decapod crustaceans found in Mediterranean marine caves, while they found Pachygrapsus marmoratus, Pilumnus hirtellus, and Xantho pilipes (only one individual).
To get an indication of the vertical distribution of Brachyura larvae, the multilayer BIONESS samplings were used as a reference, although they were not carried out in the stations rich in Brachyura larvae. Larvae of BIONESS samples appeared, in general, to be scantly concentrated, confirming the exceptionality of the results from stations PGR and S13. From the analysis of the whole sample set collected, it is evident that decapod larvae swarms were significantly present only at stations PGR and S13. The present study shows that Brachyura larvae generated there mostly disperse alongshore in the southeastward direction. The west–east coast neritic crab species connection based on larvae dispersal is possible but weak because it is based on only a quarter of the identified dispersal paths, and because the survival rate of Brachyura larvae after 40 days is likely very low. It is possible that Brachyura use other solutions than planktonic larvae to disperse in large geographic areas. The discontinuous geographic presence of corals in isolated Pacific atolls has not been justified with larvae dispersal but with the rafting of the benthic phase on buoyant pumice [104].
The role of nonplanktonic stages in the geographic distribution of neritic benthic organisms and in the connectivity of distant populations has been investigated in further depth, taking into consideration viable fragments (the so-called asexually produced propagules). These are sometimes more abundant than larval stages in coastal plankton [105,106,107]. Additionally, resting stages might allow species to perform long travels and/or to be relatively insensitive to ecological barriers [108]. Whatever the nature of the propagules, their dispersal mechanisms represent an open question, the main problem being a quantitative assessment of the phenomenon. Such alternative dispersal strategies justify species distribution and genetic flow between populations more than that attributable to larvae.
6. Conclusions
The present study proposes a detailed general framework for Brachyura larvae (Xantho granulicarpus) circulation. The PLD obtained from the literature, based on larval age and water temperature and the study of drifter motion in the southern Adriatic, suggested that zonal coast-to-coast crossing of larvae from the Italian side to the Balkan side is possible in the studied period and at the investigated latitude, but it appears to not be sufficiently reliable to ensure inter-population connectivity; in particular, most drifters (here considered as proxies for larvae in the surface layer) moved mostly along the shore (southeastwards) and crossed the basin in only one of four cases, in agreement with Carlson et al.’s experimental and numerical findings [78]. Finally, the high mortality that affects crab larvae might further reduce the drifting survivors to a negligible number. All these considerations suggest that the huge recorded swarm of Xantho granulicarpus larvae on the Apulian side of the southern Adriatic cannot justify the genetic connectivity of the two populations on opposite sides of the basin, and other mechanisms are probably responsible for this connection.
The present study, conducted directly in the field, adds information on newly considered species, areas, and/or seasons. The connectivity of Brachyura populations cannot be attributed solely to planktonic larvae, and other mechanisms probably play a role in the dispersal of this and many other species.
Author Contributions
Conceptualization, A.G., G.B. and L.G.; Laboratory analysis, R.M., A.G., S.V. and Y.G.; Statistical analysis, A.B.; Oceanography, P.C. and E.Z.; Writing—original draft preparation, A.G., R.M., G.B., L.G. and E.Z.; Writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The Neuston and BIONESS data presented in this study are available from Genuario Belmonte and Roberta Minutoli, respectively.
Acknowledgments
The research was carried out in the framework of the FP7 Ocean 11 Program project “towards COast to COast NETworks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential” (COCONET; responsible party: Ferdinando Boero) under Grant Agreement No. 287844. Special thanks are extended to the crew of RV Urania, to the colleagues who shared the cruise experience with us, and particularly to Giuseppe Arena (UNIME) for the BIONESS management during the oceanographic campaign and Stefano Aliani and Mireno Borghini (CNR La Spezia, Italy), who organized and assumed responsibility for the cruise. Special thanks are also extended to Daniela Pessani and Giorgia Di Muzio of the University of Turin for the diagnosis of the decapod Brachyura larvae.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scheltema, R.S. Passive dispersal of planktonic larvae and the biogeography of tropical sublittoral invertebrate species. In Marine Eutrophication and Population Dynamics; Colombo, G., Ferrari, I., Ceccherelli, V.U., Rossi, R., Eds.; Olsen & Olsen: Fredensborg, Denmark, 1992; pp. 195–202. [Google Scholar]
- Giangrande, A.; Geraci, S.; Belmonte, G. Life-cycle and life-history diversity in marine invertebrates and the implications in community dynamics. Oceanogr. Mar. Biol. Annu. Rev. 1994, 32, 305–333. [Google Scholar]
- Gaines, S.; Roughgarden, J. Larval settlement rate: A leading determinant of structure in an ecological community of the marine intertidal zone. Proc. Natl. Acad. Sci. USA 1985, 83, 3707–3711. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R. Supply-side ecology. Science 1986, 234, 25–27. [Google Scholar] [CrossRef] [PubMed]
- Scheltema, R.S. On dispersal and planktonic larvae of benthic invertebrates: An eclectic overview and summary of problems. Bull. Mar. Sci. 1986, 39, 290–322. [Google Scholar]
- Sulkin, S.D.; Mckeen, G.L. Laboratory study of survival and duration of individual zoeal stages as a function of temperature in the brachyuran crab Cancer magister. Mar. Biol. 1989, 103, 31–37. [Google Scholar] [CrossRef]
- Sastry, A. Culture of brachyuran crab larvae using a re-circulating sea-water system in the laboratory. Helgol. Mar. Res. 1970, 20, 406–416. [Google Scholar] [CrossRef]
- Bliss, D.E.; Vernberg, F.; Vernberg, W. Biology of the Crustacea. 7: Behaviour and Ecology; Academic Press: London, UK, 1983. [Google Scholar]
- Nagaraj, M. Combined effects of temperature and salinity on the zoeal development of then green crab, Carcinus maenas (Linnaeus, 1758) (Decapoda: Portunidae). Sci. Mar. 1993, 57, 1–8. [Google Scholar]
- Lough, R.G. Dynamics of Crab Larvae (Anomura, Brachyura) off the Central Oregon Coast, 1969–1971. Ph.D. Thesis, Oregon State University, Corvallis, OR, USA, 1975. [Google Scholar]
- Miller, S.H. Larval Behavior and Natural Trace Element Signatures as Indicators of Crustacean Population Connectivity. Ph.D. Thesis, University of California, Los Angeles, CA, USA, 2011. [Google Scholar]
- Kinlan, B.P.; Gaines, S.D.; Lester, S.E. Propagule dispersal and the scales of marine community process. Divers. Distrib. 2005, 11, 139–148. [Google Scholar] [CrossRef]
- Cowen, R.K.; Paris, C.B.; Olson, D.B.; Fortuna, J.L. The role of long distance dispersal versus local retention in replenishing marine populations. Gulf Caribb. Res. 2003, 14, 129–137. [Google Scholar] [CrossRef][Green Version]
- Sotka, E.E.; Wares, J.P.; Barth, J.A.; Grosberg, R.K.; Palumbi, S.R. Strong genetic clines and geographical variation in gene flow in the rocky intertidal barnacle Balanus glandula. Mol. Ecol. 2004, 13, 2143–2156. [Google Scholar] [CrossRef]
- Landeira, J.M.; Fatira, E.; Cuesta, J.A.; Schubart, C.D.; Moreno-Borges, S.; Rodrıguez, A. Larval dynamics suggest phenological strategies and positive effect of marine protected areas controlling indigenous and non-indigenous crab populations. Front. Mar. Sci. 2024, 11, 1371782. [Google Scholar] [CrossRef]
- Scheltema, R.S. Larval dispersal as a means of genetic exchange between geographically separated populations of shallow-water benthic marine gastropods. Biol. Bull. 1971, 140, 284–322. [Google Scholar] [CrossRef]
- Roughgarden, J.; Gaines, S.; Possingham, H.P. Recruitment dynamics in complex life cycles. Science 1988, 241, 1460–1466. [Google Scholar] [CrossRef] [PubMed]
- Botsford, L.W.; Moloney, C.L.; Hastings, A.; Largier, J.L.; Powell, T.M.; Higgins, K.; Quinn, J.F. The influence of spatially and temporally varying oceanographic conditions on meroplanktonic metapopulations. Deep. Sea Res. II 1994, 41, 107–145. [Google Scholar] [CrossRef]
- Cowen, R.K.; Lwiza, K.M.M.; Sponaugle, S.; Paris, C.B.; Olson, D.B. Connectivity of MarinePopulations: Open or Closed? Science 2000, 287, 857–859. [Google Scholar] [CrossRef]
- Kinlan, B.P.; Gaines, S.D. Propagule dispersal in marine and terrestrial environments: A community perspective. Ecology 2003, 84, 2007–2020. [Google Scholar] [CrossRef]
- Shanks, A.L.; Grantham, B.A.; Carr, M. Propagule dispersal distance and the size and spacing of marine reserves. Ecol. Appl. 2003, 13, S159–S169. [Google Scholar] [CrossRef]
- Shanks, A.L. Pelagic larval duration and dispersal distance revisited. Biol. Bull. 2009, 216, 373–385. [Google Scholar] [CrossRef]
- Siegel, D.A.; Kinlan, B.P.; Gaylord, D.; Gaines, S.D. Lagrangian descriptions of marine larval dispersion. Mar. Ecol. Prog. Ser. 2003, 260, 83–96. [Google Scholar] [CrossRef]
- Pires, R.F.T.; Pan, M.; Santos, A.M.P.; Peliz, Á.; Boutov, D.; dos Santos, A. Modelling the variation in larval dispersal of estuarine and coastal ghost shrimp: Upogebia congeners in the Gulf of Cadiz. Mar. Ecol. Prog. Ser. 2013, 492, 153–168. [Google Scholar] [CrossRef]
- Ojeda, V.; Serra, B.; Lagares, C.; Rojo Francàs, E.; Sellés, M.; Marco Herrero, E.; García, E.; Farré, M.; Arenas, C.; Abelló, P.; et al. Interannual fuctuations in connectivity among crab populations (Liocarcinus depurator) along the Atlantic Mediterranean transition. Sci. Rep. 2022, 12, 9797. [Google Scholar] [CrossRef] [PubMed]
- Bray, L.; Kassis, D.; Hall-Spencer, J.M. Assessing larval connectivity for marine spatial planning in the Adriatic. Mar. Environ. Res. 2017, 125, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, Y.P. Neuston of Seas and Oceans. In The Sea Surface and the Global Change; Liss, P.S., Duce, R.A., Eds.; University Press: Cambridge, UK, 1997; pp. 371–382. [Google Scholar]
- dos Santos, A.; Santos, A.M.P.; Conway, D.V.P.; Bartilotti, C.; Lourenço, P.; Queiroga, H. Diel vertical migration of decapod larvae in the Portuguese coastal upwelling ecosystem: Implications for offshore transport. Mar. Ecol. Prog. Ser. 2008, 359, 171–183. [Google Scholar] [CrossRef]
- Émond, K.; Sainte-Marie, B.; Bêty, J. Long-term trends and drivers of larval phenology and abundance of dominant brachyuran crabs in the Gulf of St. Lawrence (Canada). Fish. Oceanogr. 2020, 29, 185–200. [Google Scholar] [CrossRef]
- González Ortegón, E.; de Carvalho Souza, G.F.; Vilas, C.; Baldó, F.; Cuesta, J.A. Trends in the decapod crustacean community at the southernmost estuary of the Atlantic coast of Europe. Sci. Rep. 2023, 13, 22857. [Google Scholar] [CrossRef] [PubMed]
- Pochelon, P.N.; Pires, R.F.T.; Dubert, J.; Nolasco, R.; Santos, A.M.P.; Queiroga, H.; dos Santos, A. Decapod larvae distribution and species composition off the southern Portuguese coast. Cont. Shelf Res. 2017, 151, 53–61. [Google Scholar] [CrossRef]
- Monteiro, M.; Azeiteiro, U.M.; Cruz, J.; Maia, S.; Leandro, S.M.; Marques, S.C. Distribution and composition of Decapod larvae assemblages on the Berlengas archipelago and Peniche coast (western coast of Portugal). Reg. Stud. Mar. Sci. 2024, 70, 103354. [Google Scholar] [CrossRef]
- Monteiro, M.; Pardal, M.A.; Azeiteiro, U.M.; Cardoso Pereira, S.; Vaz, N.; Lígia Primo, A.; Ramirez-Romero, E.; Molinero, J.-C.; Cotrim Marques, S. Climate-driven shifts in decapod larvae assemblages in a temperate estuary. Mar. Environ. Res. 2024, 198, 106526. [Google Scholar] [CrossRef]
- Torres, A.P.; Dos Santos, A.; Balbín, R.; Alemany, F.; Massutí, E.; Reglero, P. Decapod crustacean larval communities in the Balearic Sea (western Mediterranean): Seasonal composition, horizontal and vertical distribution patterns. J. Mar. Syst. 2014, 138, 112–126. [Google Scholar] [CrossRef]
- Torres, A.P.; Reglero, P.; Hidalgo, M.; Abello, P.; Simao, D.S.; Alemany, F.; Massutí, E.; Dos Santos, A. Contrasting patterns in the vertical distribution of decapod crustaceans throughout ontogeny. Hydrobiologia 2018, 808, 37–152. [Google Scholar] [CrossRef]
- Briones-Fourzán, P.; Hendrickx, M.E. Ecology and Diversity of Marine Decapod Crustaceans. Divers. Editor. 2022, 14, 614. [Google Scholar] [CrossRef]
- Bartilotti, C.; dos Santos, A.; Castro, M.; Peliz Santos, A.M.P. Decapod larval retention within distributional bands in a coastal upwelling ecosystem. Mar. Ecol. Prog. Ser. 2014, 507, 233–247. [Google Scholar] [CrossRef]
- de Santana, C.S.; Schwamborn, R.; Neumann-Leitão, S.; de Jesus Flores Montes, M.; de Albuquerque Lira, S.M. Spatio-temporal variation of planktonic decapods along the leeward coast of the Fernando de Noronha archipelago Brazil. Braz. J. Oceanogr. 2018, 66, 1–14. [Google Scholar] [CrossRef]
- de Lima, F.A.; Butturi-Gomes, D.; das Neves Pantoja, M.H.; Martinelli-Lemos, J.M. Larval dispersal of Brachyura in one of the largest estuarine/marine systems in the world. PLoS ONE 2022, 17, e0252695. [Google Scholar] [CrossRef]
- Landeira, J.M.; Brochier, T.; Mason, E.; Lozano-Soldevilla, F.; Hernández-León, S.; Barton, E.D. Transport pathways of decapod larvae under intense mesoscale activity in the Canary-African coastal transition zone: Implications for population connectivity. Sci. Mar. 2017, 81, 299–315. [Google Scholar] [CrossRef]
- Carreton, M.; Boné, A.; Rotllant, G.; Guerao, G.; Bahamon, N.; Roldán, M.I.; Dos Santos, A. Decapod crustacean larval community structure of the submarine canyon off Blanes (NW Mediterranean Sea). Sci. Mar. 2020, 84, 71–82. [Google Scholar] [CrossRef]
- Carreton, M.; Rotllant, G.; Castejòn, D.; Bahamòn, N.; Company, J.B. Summer decapod crustacean larval communities along the eastern Spanish Mediterranean coast. PLoS ONE 2022, 17, e0275892. [Google Scholar] [CrossRef]
- Hidalgo, M.; Reglero, P.; Álvarez-Berastegui, D.; Torres, A.P.; Álvarez, I.; Rodriguez, J.M.; Carbonell, A.; Zaragoza, N.; Tor, A.; Goñi, R.; et al. Hydrographic and biological components of the seascape structure the meroplankton community in a frontal system. Mar. Ecol. Prog. Ser. 2014, 505, 65–80. [Google Scholar] [CrossRef]
- Mallol, S.; Mateo-Ramírez, A.; Alemany, F.; Álvarez-Berastegui, D.; Díaz, D.; López-Jurado, J.L.; Goñi, R. Abundance and distribution of scyllarid phyllosoma larvae (decapoda: Scyllaridae) in the Balearic Sea (Western Mediterranean). J. Crustac. Biol. 2014, 34, 442–452. [Google Scholar] [CrossRef]
- Carbonell, A.; Aparicio-González, A.; Papiol, V.; Cartes, J.E. Composition and distribution of the larval decapod community in the deep sea of the Western Mediterranean Sea Balearic Sub-basin. Fish. Oceanogr. 2021, 30, 205–218. [Google Scholar] [CrossRef]
- Kurian, C.V. Larvae of Decapod Crustacea from the Adriatic Sea. Acta Adriat. 1956, 6, 1–108. [Google Scholar]
- Lucic, D. Annual variability of the decapod larvae community in the shallow waters of the southern Adriatic. Acta Adriat. 1998, 39, 25–30. [Google Scholar]
- Di Muzio, G.; Belmonte, G.; Pessani, D. Biodiversity and distribution of crustacean decapod larvae in South Adriatic and Otranto Channel. Biol. Mar. Mediterr. 2016, 23, 283–284. [Google Scholar]
- Belmonte, G.; Scirocco, T.; Denitto, F. Zooplankton composition in Lake Varano (Adriatic Sea coast, Italy). Ital. J. Zool. 2011, 78, 370–378. [Google Scholar] [CrossRef]
- Fanelli, E.; Menicucci, S.; Malavolti, S.; De Felice, A.; Leonori, I. Spatial changes in community composition and food web structure of mesozooplankton across the Adriatic basin (Mediterranean Sea). Biogeosciences 2022, 19, 1833–1851. [Google Scholar] [CrossRef]
- Liparoto, A.; Mancinelli, G.; Belmonte, G. Spatial variation in biodiversity patterns of neuston in the Western Mediterranean and Southern Adriatic Seas. J. Sea Res. 2016, 129, 12–21. [Google Scholar] [CrossRef]
- Zambianchi, E.; Trani, M.; Falco, P. Lagrangian Transport of Marine Litter in the Mediterranean Sea. Front. Environ. Sci. 2017, 5, 5. [Google Scholar] [CrossRef]
- Marino, I.A.M.; Schiavina, M.; Aglieri, G.; Bevilacqua, S.; Boscari, E.; Congiu, L.; Faggion, S.; Kruschel, C.; Papetti, C.; Patarnello, T.; et al. Assessment of connectivity patterns of the marbled crab Pachygrapsus marmoratus in the Adriatic and Ionian seas through combination of genetic data and Lagrangian simulations. Front. Mar. Sci. 2022, 9, 944851. [Google Scholar] [CrossRef]
- Artegiani, A.; Bregant, D.; Paschini, E.; Pinardi, N.; Raicich, F.; Russo, A. The Adriatic Sea general circulation. Part I: Air–sea interactions and water mass structure. J. Phys. Oceanogr. 1997, 27, 1492–1514. [Google Scholar] [CrossRef]
- Artegiani, A.; Bregant, D.; Paschini, E.; Pinardi, N.; Raicich, F.; Russo, A. The Adriatic Sea general circulation, Part II: Baroclinic circulation structure. J. Phys. Oceanogr. 1997, 27, 1515–1532. [Google Scholar] [CrossRef]
- Lipizer, M.; Partescano, E.; Rabitti, A.; Giorgetti, A.; Crise, A. Qualified temperature, salinity and dissolved oxygen climatologies in a changing Adriatic Sea. Ocean Sci. 2014, 10, 771–797. [Google Scholar] [CrossRef]
- Gačić, M.; Civitarese, G.; Miserocchi, S.; Cardin, V.; Crise, A.; Mauri, E. The open-ocean convention in the Southern Adriatic: A controlling mechanism of the spring phytoplankton bloom. Cont. Shelf Res. 2002, 22, 1897–1908. [Google Scholar] [CrossRef]
- Fonda-Umani, S. Successioni fitoplanctoniche, micro e mesozoplanctoniche nell’Alto Adriatico. In Atti V Congresso SITE; Marchetti, R., Cotta Ramusino, M., Eds.; Italian Society of Ecology: Parma, Italy, 1992; pp. 221–246. [Google Scholar]
- Zore-Armanda, M. The system of currents in the Adriatic Sea. Stud. Rev. Gen. Fish. Counc. Mediterr. 1968, 34, 1–48. [Google Scholar]
- Shabrang, L.; Menna, M.; Pizzi, C.; Lavigne, H.; Civitarese, G.; Gačić, M. Long-term variability of the South Adriatic circulation and phytoplankton biomass in relation to large-scale climatic pattern. Ocean Sci. Discuss. 2015, 12, 203.226. [Google Scholar] [CrossRef]
- Gačić, M.; Civitarese, G.; Kovačević, V.; Ursella, L.; Bensi, M.; Menna, M.; Cardin, V.; Poulain, P.M.; Cosoli, S.; Notarstefano, G.; et al. Extreme winter 2012 in the Adriatic: An example of climatic effect on the BiOS rhythm. Ocean Sci. 2014, 10, 513–522. [Google Scholar] [CrossRef]
- Specchiulli, A.; Bignami, F.; Marini, M.; Fabbrocini, A.; Scirocco, T.; Campanelli, A.; Penna, P.; Santucci, A.; D’Adamo, R. The role of forcing agents on biogeochemical variability along the southwestern Adriatic coast: The Gulf of Manfredonia case study. Estuar. Coast. Shelf Sci. 2016, 183, 136–149. [Google Scholar] [CrossRef]
- CMEMS. Global Ocean Monthly Mean Sea Surface Wind and Stress from Scatterometer and Model, Product ID: WIND_GLO_PHY_CLIMATE_L4_MY_012_003. 2024. Available online: https://data.marine.copernicus.eu/product/WIND_GLO_PHY_CLIMATE_L4_MY_012_003/description (accessed on 10 July 2024).
- CMEMS. Mediterranean Sea Physics Reanalysis, Product ID: MEDSEA_MULTIYEAR_PHY_006_004. 2024. Available online: https://data.marine.copernicus.eu/product/MEDSEA_MULTIYEAR_PHY_006_004/description?view=-&option=-&product_id=- (accessed on 10 July 2024).
- Boero, F.; Foglini, F.; Fraschetti, S.; Goriup, P.; Macpherson, E.; Planes, S. COCONET CONSOTIUM. CoCoNet: Towards Coast to Coast Networks of marine protected areas (from the shore to the high and deep sea), coupled with sea-based wind energy potential. SCIRES-IT 2016, 6, 1–95. [Google Scholar]
- Sameoto, D.D.; Saroszynsky, L.O.; Fraser, W.B. BIONESS, a new design in multiple net zooplankton sampler. J. Fish. Res. Board Can. 1980, 3, 722–724. [Google Scholar] [CrossRef]
- Tranter, D.J. Zooplankton abundance in Australasian waters. Aust. J. Mar. Freshw. Res. 1962, 13, 106–142. [Google Scholar] [CrossRef]
- Pessani, D.; Burri, R.; Salton, L. A key for the identification of the known larval stages of the Mediterranean Brachyura. Invertebr. Reprod. Dev. 1998, 33, 191–199. [Google Scholar] [CrossRef]
- Pessani, D.; Tirelli, T.; Flagella, S. Key for the Identification of Mediterranean Brachyuran megalopae. Mediterr. Mar. Sci. 2004, 5, 53–64. [Google Scholar] [CrossRef]
- De Melo Dos Santos, A.M. Larvas de Crustáceos Decápodes ao largo da Costa Portuguesa; Tese apresentada à Faculdade de Ciências da Universidade de Lisboa para a obtenção do grau de Doutor: Lisboa, Portugal, 1999; p. 262. [Google Scholar]
- Pohle, G.; Mantelatto, F.L.M.; Negreiros-Fransozo, M.L.; Fransozo, A. Larval Decapoda (Brachyura). In South Atlantic Zooplankton; Boltovskoy, V.D., Ed.; Backhuys Publishers: Leiden, The Netherlands, 1999; pp. 1281–1351. [Google Scholar]
- Rodrigues dos Santos Bento, M.A. Keys and Bibliography for the Identification of Larval Stages of Brachyuran Crabs from the Western Indian Ocean; Mestrado em Ecologia Marinha, Universidade de Lisboa Faculdade de Ciências Departamento de Biologia Animal: Lisboa, Portugal, 2017; p. 49. [Google Scholar]
- Barange, M. Vertical migration and habitat partitioning of six euphausiid species in the northern Benguela upwelling system. J. Plankton Res. 1990, 12, 1223–1237. [Google Scholar] [CrossRef]
- Andersen, V.; Sardou, J. The die1 migrations and vertical distributions of zooplankton and micronekton in the Northwestern Mediterranean Sea, Euphausiids, mysids, decapod and fishes. J. Plankton Res. 1992, 14, 112–1554. [Google Scholar]
- Davis, R.E. Observing the general circulation with floats. Deep. Sea Res. Part A Oceanogr. Res. Pap. 1991, 38, S531–S571. [Google Scholar] [CrossRef]
- Corrado, R.; Lacorata, G.; Palatella, L.; Santoleri, R.; Zambianchi, E. General characteristics of relative dispersion in the ocean. Sci. Rep. 2017, 7, 46291. [Google Scholar] [CrossRef]
- Kalampokis, A.; Uttieri, M.; Poulain, P.M.; Zambianchi, E. Validation of HF radar-derived currents in the Gulf of Naples with Lagrangian data. IEEE Geosci. Remote Sens. Lett. 2016, 13, 1452–1456. [Google Scholar] [CrossRef]
- Carlson, D.F.; Griffa, A.; Zambianchi, E.; Suaria, G.; Corgnati, L.; Magaldi, M.G.; Poulain, P.-M.; Russo, A.; Bellomo, L.; Mantovani, C.; et al. Observed and modeled surface Lagrangian transport between coastal regions in the Adriatic Sea with implications for marine protected areas. Cont. Shelf Res. 2016, 118, 23–48. [Google Scholar] [CrossRef]
- Sciascia, R.; Berta, M.; Carlson, D.F.; Griffa, A.; Panfili, M.; La Mesa, M.; Corgnati, L.; Mantovani, C.; Domenella, E.; Fredj, E.; et al. Linking sardine recruitment in coastal areas to ocean currents using surface drifters and HF radar: A case study in the Gulf of Manfredonia, Adriatic Sea. Ocean Sci. 2018, 14, 1461–1482. [Google Scholar] [CrossRef]
- Batchelder, H.P. Forward-in-time-/backward-in-time-trajectory (FITT/BITT) modeling of particles and organisms in the coastal ocean. J. Atmos. Ocean. Technol. 2006, 23, 727–741. [Google Scholar] [CrossRef]
- Cianelli, D.; D’Alelio, D.; Uttieri, M.; Sarno, D.; Zingone, A.; Zambianchi, E.; Ribera D’alcalà, M. Disentangling physical and biological drivers of phytoplankton dynamics in a coastal system. Sci. Rep. 2017, 20, 15868. [Google Scholar] [CrossRef]
- Fratini, S.; Ragionieri, L.; Deli, T.; Harrer, A.; Marino, I.A.M.; Cannicci, S.; Zane, L.; Schubart, C.D. Unravelling population genetic structure with mitochondrial DNA in a notional panmictic coastal crab species: Sample size makes the difference. BMC Evol. Biol. 2016, 16, 150. [Google Scholar] [CrossRef] [PubMed]
- Schiavina, M.; Marino, I.A.M.; Zane, L.; Melià, P. Matching oceanography and genetics at the basin scale. Seascape connectivety of the Mediterranean shore crab in the Adriatic. Mol. Ecol. 2014, 23, 5496–5507. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.I.; Nikula, R.; Waters, G.M. Oceanic rafting by a coastal community. Proc. R. Soc. 2011, B 278, 649–655. [Google Scholar] [CrossRef]
- Treml, E.A.; Roberts, J.J.; Chao, Y.; Halpin, P.N.; Possingham, H.P.; Rigin, C. Reproductive output and Duration of the Pelagic Larval Stage determine seascape-wide connectivity of marine populations. Integr. Comp. Biol. 2012, 52, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Zariquiey Alvarez, R. Crustaceos Decapodos Ibericos; Investigación Pesquera: Barcelona, Brazil, 1968; Volume 32, pp. 1–510. [Google Scholar]
- Ingle, R.W. British Crabs; Oxford University Press: London, UK, 1980. [Google Scholar]
- Flores, A.A.V.; Cruz, J.; Paula, J. Temporal and spatial patterns of settlement of brachyuran crab megalopae at a rocky coast in central Portugal. Mar. Ecol. Prog. Ser. 2002, 229, 207–220. [Google Scholar] [CrossRef]
- Cuesta, J.A.; Rodriguez, A. Zoeal stages of the intertidal crab Pachygrapsus marmoratus (Fabricius) (Brachyura, grapsidae) reared in the laboratory. Hydrobiologia 2000, 436, 119–130. [Google Scholar] [CrossRef]
- Abelló, P. Reproduction and moulting in Liocarcinus depurator (Linnaeus, 1758) (Brachyura: Portunidae) in the Northwestern Mediterranean Sea. Sci. Mar. 1989, 53, 127–134. [Google Scholar]
- Guerao, G.; Abelló, P.; Dos Santos, A. Morphological variability of the megalopa of Liocarcinus depurator (Brachyura: Portunidae) in Mediterranean and Atlantic populations. J. Nat. Hist. 2006, 40, 1851–1866. [Google Scholar] [CrossRef]
- Marco-Herrero, E.; Drake, P.; González-Gordillo, J.I.; Cuesta, J.A. Larval development of the pea crab Afropinnotheres monodi Manning, 1993 (Decapoda, Pinnotheridae) using plankton-collected and laboratory-reared specimens: Efects of temperature. Mar. Biol. Res. 2016, 12, 43–55. [Google Scholar] [CrossRef]
- Ungaro, N.; Marano, C.A.; Ceriola, L.; Martino, M. Distribution of demersal crustaceans in the southern Adriatic Sea. Acta Adriat. 2005, 46, 27–40. [Google Scholar]
- Stevic, Z.; Gallil, B.S. Cheklist of the Mediterranean Brachyuran Crabs. Acta Adriat. 1994, 34, 65–76. [Google Scholar]
- Froglia, C. Checklist Della Fauna Marina Italiana—Decapoda. 2006. Available online: www.sibm.it (accessed on 19 June 2024).
- Froglia, C. Crustacea, Malacostraca, Decapoda. Biol. Mar. Mediterr. 2010, 17, 519–534. [Google Scholar]
- Noël, P.Y. Clé Préliminaire D’identification des Crustacea Decapodea de France et des Principales Autres Espèces d’Europe; Muséum National d’Histoire Naturelle: Paris, France, 1992; p. 146. [Google Scholar]
- Rodriguez, A.; Martin, J.W. Larval development of the crab Xantho poressa (Decapod:Xanthidae) reared in the Laboratory. J. Crustac. Biol. 1997, 17, 98110. [Google Scholar] [CrossRef]
- Spivak, E.D.; Arevalo, E.; Cuesta, J.A.; Gonzalez-Gordillo, I. Population structure and reproductive biology of the stone crab Xantho poressa (Crustacea: Decapoda: Xanthidae) in the ‘Corrales de Rota’ (south-western Spain), a human-modified intertidal fishing area. J. Mar. Biol. Assoc. United Kingd. 2010, 90, 323–334. [Google Scholar] [CrossRef]
- Patel, K.; Patel, H.; Gosavi, S.; Vachhrajani, K.; Trivedi, J. Population structure and fecundity of the Xanthid crab Leptodius exaratus (H. Milne Edwards, 1834) on the rocky shore of Gujarat state, India. PeerJ 2024, 12, e16916. [Google Scholar] [CrossRef]
- Vaso, A.; Gjiknuri, L. Decapod Crustaceans of the Albanian Coast. Crustaceana 1993, 65, 390–407. [Google Scholar] [CrossRef]
- Zaabar, W.; Achouri, M.S. Inventory, Systematic and Biogeography of Brachyuran Crabs (Crustacea: Decapoda: Brachyura) on the Tunisian Coast. Open J. Ecol. 2023, 13, 711–730. [Google Scholar] [CrossRef]
- Bianchi, C.N.; Gerovasileiou, V.; Morri, C.; Froglia, C. Distribution and ecology of decapod crustaceans in Mediterranean marine caves: A review. Diversity 2022, 14, 176. [Google Scholar] [CrossRef]
- Jokiel, P.L. Rafting of reef corals and other organisms at Kwajalein Atoll. Mar. Biol. 1989, 101, 483–493. [Google Scholar] [CrossRef]
- Pati, A.C.; Belmonte, G.; Fanelli, G.; Giangrande, A.; Gravili, C.; Saracino, O.; Boero, F. Interkingdom convergence in the architecture of asexual propagules. Biol. Mar. Mediterr. 1998, 5, 365–366. [Google Scholar]
- Moscatello, S.; Belmonte, G. The plankton of a shallow submarine cave (‘Grotta di Ciolo’, Salento Peninsula, SE Italy). Mar. Ecol. 2007, 28 (Suppl. 1), 47–59. [Google Scholar] [CrossRef]
- Pati, A.C.; Belmonte, G. Asexually Generated Propagules from Subtidal Sessile Benthic Organisms. Prog. Aqua Farming Mar. Biol. 2018, 1, 180002. [Google Scholar]
- Belmonte, G.; Rubino, F. Resting cysts from coastal marine plankton. Oceanogr. Mar. Biol. Annu. Rev. 2019, 57, 1–88. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).