1. Introduction
Thermal conditions are one of the most important parameters determining the functioning of inland ecosystems. This is why the first classifications of lakes considering water temperature were created in the 19th century. The most common classification, based on the lake’s location in a specific climatic zone and the number of water circulations during the year, was the Forel typology from 1882 [
1,
2], dividing lakes into polar, temperate, and tropical. According to this division, the water temperature in polar lakes is always below 4 °C, in tropical lakes it is higher than 4 °C, and in temperate lakes it reaches 4 °C twice a year (in spring and autumn). In 1936, Yoshimura added subpolar lakes (with temperatures above 4 °C with vertical gradients and a thermocline close to the surface) and subtropical lakes (with water temperatures above 4 °C at the bottom all year round, and uniform in winter with large amplitudes at the surface) to the Forel classification. Findenegg was the first to introduce mixing into the lake typology. Based on studies of the range of circulation in subalpine reservoirs in Corinth, he distinguished holomictic lakes and meromictic lakes (mixing does not occur in the entire water column) [
3,
4]. In later years, Passowicz, Olszewski, and Wiszniewski supplemented this taxonomy with tachymictic lakes (rapid water mixing), bradymictic lakes (slow water mixing), eumictic lakes (intermediate type between tachy- and brady-mixing), and polymictic lakes (often mixed) [
3].
An exciting and rare mictic type is the meromictic lake [
5,
6]. Most deep lakes experience seasonal stratification and complete circulation, and meromixis is a condition where the lake does not mix completely. All surface waters are heated by the sun and mixed by the wind [
7,
8]. In shallow lakes, wind-driven currents keep the water circulating all year round, but in deep lakes, during the spring warming period, there comes a period when the currents can no longer overcome the density differences that arise between the surface and bottom water layers The lake is divided into an epilimnion (top layer) and a hypolimnion (bottom layer), with an intermediate boundary layer called the metalimnion or thermocline. Later, as the air cools, the density difference decreases, currents penetrate deeper, and the lake eventually returns to full circulation (holomixis).
In meromictic lakes, the bottom water usually contains dissolved salts, so the density difference between the upper and lower water layers is amplified by both salinity and water temperature [
9]. This effect may be aided by the size, shape, and depth of the lake basin. If the lake has a small surface area and is very deep and is surrounded by high wooded hills, the effect of wind is limited and the reservoir tends to maintain permanent stratification [
10,
11].
The commonly used systematics of meromictic lakes was developed by Hakala [
12], who connected existing classifications and took into account the anthropogenic impact and the reservoir’s morphometry. He listed the following types (and at the same time the reasons for the limited water dynamics): inflow of salty or brackish water above the freshwater layer and its concentration at the bottom of the reservoir (ectogenic meromixis according to Hutchinson), inflow of fresh water to lakes with significant salinity (crenogenic lakes in Hutchinson’s approach), and inflow of biogenic salts and turbid water from the catchment and diffuse sources and watercourses. In that reservoir, the delivered load leads to stabilization and deoxidation in the hypolimnion, the morphology of the lake, and the catchment.
Meromictic lakes are characterized by the presence of a mixolimnion (the upper layer of water that is subject to mixing by the wind) and a monimolimnion (the lower layer of high-density water that is not mixed by the wind), along with an intermediate boundary layer called the chemocline (a thin layer, but characterized by the highest chemical or density gradient) [
13,
14]. The mixolimnion includes typical stagnation zones in holomictic lakes and undergoes stratification (epilimnion and hypolimnion) and subsequent circulation. The monimolimnion remains isolated from the atmosphere and is not subject to circulation [
15,
16].
The group of meromictic lakes is not very well represented in Poland. Usually, meromixis is human-caused and occurs in former industrial sites or flooded mines [
17,
18]. Klasztorne Małe Lake in Kartuzy is an example of a meromictic water body, where meromixis is not its natural state but is the effect of mass pollution of the lake with domestic and industrial sewage. Studies of Klasztorne Małe Lake conducted in 1888 showed that, under natural conditions, this reservoir was subject to spring and autumn circulation and the lake was classified as a holomictic type [
19]. The next available data, dating back to the 1950s, when the lake was already functioning as a sewage receiver, indicated a lack of spring circulation and a tendency towards meromixis. The annual amplitude of temperature fluctuations in the bottom water layers was then 2.3 °C. In the following years, this amplitude decreased and did not exceed 0.5 °C. Another indicator of the anthropogenic and biogenic meromictic activity of Klasztorne Małe Lake is its relative depth of only 0.054. Meromictic lakes usually have a significant depth with a relatively small surface of the water surface, which is described usually with a high relative depth value [
20]. In water bodies with average values of relative depth, the limitation of water dynamics is always related to sewage pollution and the resulting excessive primary production or the inflow of saline waters with increased density. Thus, the meromixis of Klasztorne Małe Lake developed quickly and became permanent due to a specific type of mero eutrophy related to the process of pollution of the lake with sewage and consisting of filling the small volume of the lake with sewage.
The process of degradation of the lake caused by the inflow of untreated sewage lasted for over 100 years. In the mid-1970s, the water and sewage management in Kartuzy was reorganized, the first stage of which consisted of directing part of the pollutants outside the catchment area through a combined sewer. In the early 1980s a mechanical/biological treatment plant was put into operation; however, due to its insufficient capacity, raw sewage from the emergency overflow of the sanitary network was often discharged into Klasztorne Małe Lake. A common practice at that time was the illegal discharge of pollutants into lakes through storm sewers, especially from areas of scattered single-family housing in the western part of the city.
Intensive actions aimed at reducing the supply of pollutants to the lakes were undertaken in the 1990s. They consisted primarily of covering almost the entire city with a sanitary network, modernizing and expanding the sewage treatment plant, and also partially dividing the combined sewer system. In 2018, another modernization of the sewage network was carried out, consisting of replacing the pipes and equipping the existing rainwater collectors with appropriate pre-treatment device separators.
Due to the very poor ecological condition of Klasztorne Małe Lake in 2021 and 2022, its restoration process was carried out using the phosphorus inactivation method.
This study aims to answer the question of whether the applied restoration method can influence the change of the water chemistry of a degraded meromictic lake and whether it can cause a return to the holomictic circulation type from before the pollution period.
2. Description of Research Object
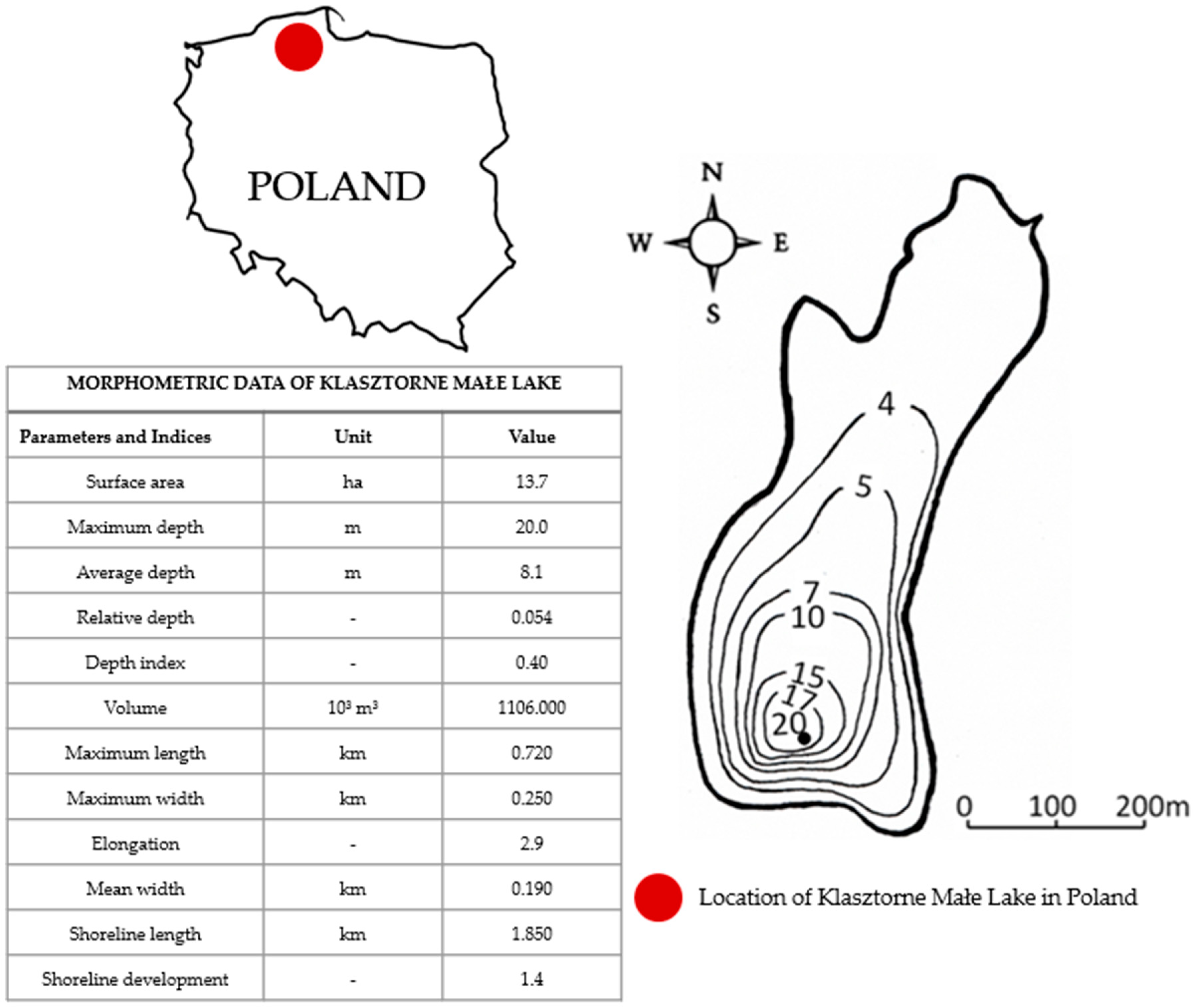
Klasztorne Małe Lake (KML) is located in Kartuzy in the Pomeranian Lakeland (northern Poland) (
Figure 1) [
21]. The relief of this area is strongly undulating, in places similar to a typical mountain relief, with significant terrain variations. The relief of this area was shaped by several different factors, among which the decisive role was played by the Scandinavian ice sheet and fluvioglacial waters, and the subsequent erosion and accumulation activity of rivers. The groundwater level is by the geological structure of the area. In the area of gutter depressions and septic depressions, blackwater occurs below 2 m. The remaining part of the area has a groundwater table at a depth of 2–3 m, and, in many places, there is no water even at a depth of 4.5 m [
22]. The area of the Kashubian Lake District is covered with Quaternary sediments of glacial origin, approximately 200 m thick, under which there are Tertiary sediments of marine origin and, below, Precambrian sediments composed of granites, diorites, and granodiorites. In the Kashubian Lake District, there are mineral resources such as boulder clay, sands, gravels, erratic boulders, peats, and layered clay (
https://pl.wikipedia.org/wiki/Pojezierze_Kaszubskie (accessed on 15 September 2024)).
KML has a bowl shape, typical of ribbon lakes. It probably owes its formation to the work of subglacial waters operating at the front of the glacier. KML is described by the following geographic coordinates: 54°20′21″ N, 18°11′35″ E. The lake is located at an altitude of 203 m above sea level in the Klasztorna Struga–Mała Słupina–Radunia–Motława–Martwa Wisła–Bałtyk river basin. KML has an area of 13.7 ha and a maximum depth of 20 m, with an average depth of 8.1 m. Its basin contains1106.0 thousand m3. The water body has an elongated shape in the north/south direction. The 1850 m long shoreline of the lake is poorly developed (K 1.4). The lake basin, with a shape close to a paraboloid (Wg—0.4), is strongly sunk into the substrate, as evidenced by the high value of the relative depth—0.054. KML is a flow-through water body. In the southern part, it is connected to Karczemne Lake via a drainage ditch, and, in the northern part, there is an outflow to Klasztorne Duże Lake.
At the beginning of the 20th century, Klasztorne Małe was a lake used for recreational purposes, equipped with a kayaking marina and a swimming area. Before World War I, due to the expansion of the city of Kartuzy and the growing amount of pollution, the analyzed lake was transformed into a sewage receiver. Sanitary sewage, stormwater sewage, sewage from dairies, slaughterhouses, fruit and vegetable processing plants, and hospitals were discharged into the lake without treatment or after passing through inefficient treatment devices. Improper water and sewage management in the city contributed to the total degradation of Klasztorne Małe Lake. The pollution of the lake was associated with the loss of landscape, and recreational and economic values. Protective measures in the lake catchment area have been implemented since the 1980s, but it was not until 2018 that the inflow of pollutants to the lake was definitively cut off. The protection of the lake contributed to reducing the external load to the level permissible according to the Vollenweider criteria [
23], but did not change the water quality in the lake. A necessary action that could change the trophic state in the analyzed reservoir was an appropriately designed restoration. The restoration project, together with water legal documentation, was prepared by the Department of Water Protection Engineering and Environmental Microbiology at the University of Warmia and Mazury in Olsztyn [
24]. It was planned to use a phosphorus inactivation method, which consists of reducing the availability of this element for primary producers (mainly planktonic algae and cyanobacteria) using coagulants. The mechanism of action of coagulants introduced into the lake is two-fold. Firstly, phosphates are precipitated from the water column by sorption or binding them to salts of a given metal originating from a specific type of preparation (FePO
4, AlPO
4). Secondly, phosphorus compounds are immobilized in bottom sediments by improving their sorption capacity and as a result of sedimentation at the bottom of coagulant flocs, create a barrier that additionally prevents the release of phosphorus from sediments into the water. Two types of coagulants were used for the restoration of KML—iron PIX 111 and aluminum PAX 18—which were applied sequentially. The iron coagulant was distributed in the coastal parts of the lake, where the bottom water is oxygenated all year round (the 2 m isobath was the boundary of the PIX 111 introduction zone), while the aluminum coagulant, insensitive to low redox potential, was applied in the remaining part of the lake, so that after the sedimentation process it settled on the deep sediments, located under the water of the monimolimnion permanently deprived of oxygen.
During the restoration, 75.3 tons of PAX 18 and 28.2 tons of PIX 111 were introduced into the KML during four applications, which took place in March 2021 and 2022 and in November 2021 and 2022. The doses of the preparations were selected based on the phosphorus content in the water and bottom sediments. In each of the four stages, the amount of the preparation used in terms of the volume of water ranged from 15 to 39 mL/m
3 of water [
24].
Coagulants were applied to the lake using the surface method, from specially designed floating units. On the platform, there was a coagulant tank equipped with a release control valve and a transfer hose to the application grid. The application grid consisted of perforated plastic pipes, which had a supporting structure made of steel sections and a float system with adjustable grid immersion depth. From the storage tank, the coagulant was transferred by gravity through a transfer hose to the feeding system—a perforated grid. The coagulant dosing system was designed to allow for the regulation of the intensity of their delivery through holes in the application grid. The floating platform was powered by a combustion engine. The dosing grid was placed under the water surface, which, in combination with the way the floating unit moved, allowed for the cloud of coagulant flocs to be evenly mixed in the application zone and prevented aeration of the forming flocs and their flotation. Coagulants were evenly distributed in designed zones. Precise application was possible thanks to the positioning of vessels by the satellite navigation system.
3. Methods
The research of KML was carried out monthly from January to December 2018 and from January 2020 to December 2023. According to the guidelines used in lake monitoring research, water samples for laboratory tests were collected at the deepest point of the lake (20.0 m), which was located according to bathymetric map and GPS. At the measuring station, water samples (480) were collected using a 3.5 L Ruttner sampler (Geomor Technik, Szczecin, Poland) into 2 L plastic bottles from 1 m below the surface (miksolimnion) and 1 m above the bottom (monimolimnion). At the measuring station, at every meter of depth, water temperature, oxygen content, chlorophyll a, and conductivity were measured using a YSI 6600V2 multiparameter sensor (Yellow Springs, OH, USA).
The scope of the water analysis included phosphorus and nitrogen compounds, Ca (calcium), Mg (magnesium), Fe (iron), Mn (manganese), Cl (chloride), Al (aluminum), BOD
5 (biochemical oxygen demand), and TOC (total organic carbon). Chemical analyses of water were performed according to standard methods [
25].
The obtained results of the selected water quality parameters were statistically analyzed (one-way ANOVA,
p = 0.05, Tukey’s HSD) using the Statistica 13.3 software package [
26]. The alternative tested hypothesis presumed the presence of significant differences in the content of selected parameters of water between the control year (2018, 2020; before restoration), experimental years (2021, 2022; restoration treatments), and after end of restoration (2023) (
Table 1).
Results of pH, BOD5, TOC, chlorophyll a, and Secchi disc visibility (SDV) are presented in the table containing annual means, standard deviation, and minimum and maximum values.
4. Results
Thermal values in KML indicate the meromictic nature of this reservoir. This means there are two thermal layers in the lake: the mixolimnion, reaching a depth of 14 m, which is subject to mixing according to the annual cycle, and the monimolimnion located below, which never mixes with the water above.
During the studies conducted between 2018 and 2022, the beginnings of thermal stratification were already visible in April in the mixolimnion. Depending on weather conditions, the temperature of the upper layer of the mixolimnion ranged from 4.7 to 6.3 °C (
Figure 2). A thermocline formed between 7 and 8 m deep. The waters of the monimolimnion were characterized by temperatures oscillating around 4.6 °C at that time. At the peak of summer stagnation in the mixolimnion, a distinct epilimnion layer could be distinguished, with a thickness of 1 to 2 m and a temperature of 21.8 to 25.9 °C (
Figure 2). Below this, there was a metalimnion with a maximum gradient of 6 °C/m (2020). Under the metalimnion layer, there was a hypolimnion, the lower layer of which was a monimolimnion with a temperature between 4.5 and 5.5 °C. In the autumn, the lake cooled down; however, in the indicated period of the study, no full homothermy was recorded (
Figure 2).
In the 2023 research cycle, limited mixing of the lake resulting from the water density system was not confirmed. In April, only a small temperature difference was visible between the surface and bottom water layers (
Figure 2). Summer stratification lasted from May to October with an epilimnion of 3 to 6 m thickness. Below this, there was a thermocline, cutting off with a gradient of maximum value of 6.9 °C/m, under which the hypolimnion layer was located. The water temperature of this layer was stable at 3.7–5.4 °C throughout the research period.
The poor water dynamics of KML and its long-term pollution with various types of sewage caused the oxygen conditions in this reservoir to be very poor. In the years 2018–2022, in the spring before the formation of thermal stratification in the mixolimnion waters, the presence of oxygen was recorded to a maximum depth of 13 m, and in 2021 only to 6 m (
Figure 3). Oxygen concentrations ranged between 0.9 and 17.2 mg O
2/L. In the following months of the year, and especially in the peak period of summer stagnation, dissolved oxygen was found only in surface waters up to 3 m deep, and below that the entire mass of water was deoxygenated (
Figure 3). It should be noted that oxygen concentrations in this small mass of water were very high, reaching 200% oxygen saturation. Autumn water circulation improved oxygen conditions, but only to a depth of several meters. After the end of restoration treatments, in 2023, a slight improvement in the oxygenation of the reservoir water was noted. In the spring, the oxygenated water layer was 16 m thick (
Figure 3). Deoxygenation progressing from the bottom meant that, by the end of summer, oxygen was present in the water layer to a depth of 6 m. It is worth noting that the range of the oxygenated water layer increased, and its oxygen saturation did not exceed 150%.
Studies on KML conducted between 2018 and 2022 showed a very clear conductivity stratification, with an increase in values towards the monimolimnion (
Figure 4). In the upper water layer, electrolytic conductivity varied between 278 and 509 µS/cm, while in the lower layers between 494 and 1166 µS/cm. After restoration, it was noticed that the differences between the mixolimnion and the monimolimnion were no longer so visible. In 2023, in the mixolimnion, water conductivity varied between 463 and 507 µS/cm, and in the monimolimnion between 612 and 769 µS/cm (
Figure 4).
The analysis of the results of the nitrogen compound amounts in the KML water showed highly statistically significant differences between the data obtained before restoration and after the application of coagulants. Only in the ammonium nitrogen concentration in the miksolimnion layer were no statistically significant differences found (
Table 1). Before the restoration treatments, the average content of ammonium nitrogen in the miksolimnion was 0.346 mg N/L (±0.364), nitrate nitrogen 0.271 mg N/L (±0.245), organic nitrogen 1.351 mg N/L (±0.482), and total nitrogen 2.035 mg N/L (±0.171). After restoration, the average values of ammonia changed to 0.314 mg N/L (±0.349), nitrate nitrogen to 0.076 (±0.047), organic nitrogen to 1.344 mg N/L (0.469), and total nitrogen to 1.740 mg N/L (±0.718) (
Figure 5). The monimolimnion water was much richer in the nitrogen compounds. The average content of ammonium nitrogen in 2018 was 10. 209 mg N/L (±4.825), nitrate nitrogen 0.241 mg N/L (±0.167), organic nitrogen 9.378 mg N/L (4.089), and total nitrogen 19.829 mg N/L (±1.983) (
Figure 6). The applied restoration treatments caused an obvious change in the content of nitrogen compounds in the monimolimnion layer. In 2023, the average content of ammonium nitrogen dropped to 3.893 mg N/L (±1.856), nitrate nitrogen changed to an average of 0.122 mg N/L (±0.049), the average amount of organic nitrogen reduced to 2.575 (±0.823), and the average TN in the last year of the study was three times lower at 6.600 mg N/L (±2.206) (
Figure 6).
Interpretation of the obtained research results showed that the restoration carried out caused highly statistically significant differences in the content of P compounds in both the mixolimnion and monimolimnion waters of the studied lake (
Table 1). Before restoration, the average phosphate concentration in the mixolimnion water was 0.211 mg P/L (±0.131), organic phosphorus 0.312 mg P/L (±0.076), and total phosphorus 0.522 mg P/L (0.204) (
Figure 7). After phosphorus inactivation, the average phosphate concentrations changed to 0.005 mg P/L (±0.005) (98% reduction), organic phosphorus to 0.066 mg P/L (±0.033) (79% reduction), and total phosphorus to 0.076 mg P/L (±0.035) (85% reduction) (
Figure 7). In the monimolimnion water before restoration, the average PO
4 content was 9.679 mg P/L (2.867), organic phosphorus 9.631 mg P/L (±3.445), and total phosphorus 19.310 mg P/L (±6.134) (
Figure 8). After the application of PIX 111 and PAX 18 in the monimolimnion, the average PO
4 content dropped to 0.011 (±0.012) (reduction of 99.9%), organic phosphorus to 0.086 mg P/L (±0.068) (reduction of 99.9), and TP to 0.098 mg P/L (±0.063) (reduction of 99.5%) (
Figure 8).
Statistical analysis of the results showed highly significant statistical differences in Ca content in both the mixolimnion and monimolimnion waters of KML (
Table 1). In KML, in the period preceding the restoration, a clear vertical stratification was observed in the occurrence of Ca, with an increased value towards the bottom. In the surface layers of KML in 2018, the average Ca content was 40.9 mg Ca/L (±7.8), and in the monimolimnion 46.3 mg Ca/L (±2.0) (
Figure 9). After the implementation of restoration activities, an increase in the average Ca concentration was observed in the entire volume of water, along with a reduction in the differences in its content between the surface and the bottom (
Figure 9).
Analysis of results on the changes in Mg content in the water of the KML showed statistically significant differences only in the bottom water (
Table 1). Both before and after the restoration process, the average Mg concentrations generally did not exceed 25 mg Mg/L (
Figure 9). Before the restoration in KML, a clear vertical stratification was observed in the Mg concentrations, with an increase in value towards the bottom, and after the lake restoration process, the average Mg concentrations in the monimolimnion of this water body decreased significantly, and no significant differences were noted between the surface and the bottom.
In the case of iron, the applied restoration caused statistically significant differences only in the content of this element in the monimolimnion water. Before restoration, the average content of iron on the surface was 0.142 mg Fe/L (±0.051), and at the bottom 27.208 mg Fe/L (±6.879) (
Figure 10). After restoration, the average content of this metal on the surface layers of water dropped to 0.116 mg Fe/L (±0.039) and in the monimolimnion to 2.6 mg Fe/L (±0.876) (
Figure 10).
Restoration activities caused highly statistically significant differences in manganese content in the entire volume of KML water (
Table 1). In 2018, the average amount of manganese in the surface water layers was 0.275 mg Mn/L (±0.087), and in the monimolimnion 4.010 mg Mn/L (±0.597) (
Figure 10). After the application of coagulants, the average manganese content at the surface dropped to 0.142 mg Mn/L (±0.051) and at the bottom to 1.817 mg Mn/L (0.499) (
Figure 10).
Statistical analysis of the data showed highly significant differences in chloride content in the entire KML water mass (
Table 1). Before restoration, in 2018, the average amount of chloride in the mixolimnion layer was 72 mg Cl/L (±16), and in the monimolimnion layer 87 mg Cl/L (±11). After introducing PIX 111 and PAX 18 into the reservoir waters, the average chloride concentration at the surface increased to 87 mg Cl/L (±4), and at the bottom to 100 mg Cl/L (±8) (
Figure 11).
During research, the surface water layer of the restored lake did not show significant changes in pH (
Table 2). In all the research years (before restoration, during sequential application of coagulants), the pH of the surface water layers varied from 7.10 to 9.78 (
Table 2). It should be noted that, after restoration, the maximum values of pH were lower and did not exceed 9.15 pH, i.e., the range for eutrophic waters. In the bottom water of the studied lake, the differences in pH values were higher. The average pH of the bottom water of this water body changed from 7.35 pH to 6.89 pH.
Before restoration, in 2018, the average BOD
5 value in surface water layer was 7.6 mg O
2/L (±2.4), and in near bottom water layer 45.8 mg O
2/L (±13.9) (
Table 2). After restoration treatments, the average BOD
5 in miksolimnion decreased to 4.8 mg O
2/L (±2.4), and in monimolimnion to 11.9 mg O
2/L (±4.8). Before restoration treatments, the mean TOC concentration throughout the entire volume of KML oscillated at 14.3 mg C/L (
Table 2). In 2023, after restoration treatments, the average total organic carbon concentration in KML decreased by about 50% (
Table 2).
In 2018, the mean value of SDV in KML was 0.60 m (±0.36) and chlorophyll a was 45.02 µg/L (±16.79). After restoration treatments, in 2023, the average value of SDV in KML was 1.32 m (±0.35) and chlorophyll a was 19.36 µg/L (±10.15).
During the research on the water of the restored lake, trace amounts of aluminum were detected only during the application of the PAX 18 coagulant. During application, the concentration of aluminum in the water did not exceed 0.05 mg Al/L, while the permissible content of this metal in surface waters, safe for aquatic organisms, is 0.3 mg Al/L.
5. Discussion
The consequences of anthropogenic degradation of water bodies are particularly acute in countries with small water resources. This indicates the need not only to protect existing “clean water” resources but also to urgently seek ways to improve the quality of water in degraded bodies of water. Numerous attempts that have been made around the world to restore lakes by eliminating the cause of their pollution (believed to be the excessive inflow of nutrients from the catchment areas) often have not produced the expected results. This applies especially to hypertrophic lakes in which an internal source of supply has been activated [
27]. In such cases, a decrease in trophy can only be expected after the application of specific restoration methods, consisting of removing nutrients from the circulation and immobilizing them in bottom sediments [
28]. KML has been degraded as a result of its transformation into a sewage receiver. In addition to significant water pollution by sewage, an additional effect of the inflow of pollutants is the deterioration of water dynamics and the lake’s transition into a state of meromictic. The thickness of the epilimnion in lakes depends on the intensity of vertical movements of water masses and is taken as a comparative indicator of the degree of water mixing [
29,
30]. The theoretical range of the epilimnion calculated from the formula E = 4.4√D, where D is the average effective length of the lake axis, for KML was 3.2 m and was almost 1.5 m higher than the actual range of the epilimnion found during the studies. Another indicator, which is the ratio of the theoretical range of the epilimnion to the maximum depth, characterizes the intensity of water exchange [
1,
2,
31]. This coefficient was 0.19 for KML and, in the Patalas scheme [
29], corresponded to the V degree of statics. Both indicators confirm that, in natural conditions, KML was a bradymictic reservoir, and the weakening of water movements and its meromixis was the effect of external factors related to the excessive inflow of sewage. Throughout the hydrological year, a layer of water that was not subject to circulation—the monimolimnion—was below the depth of 14 m. Above the monimolimnion, there was a mixolimnion, in which, during the summer stagnation period, there was an epilimnion with a thickness of 1 to 2 m, a metalimnion with a gradient of up to 6 °C/m, and a hypolimnion, the lower part of which passed into a thermally stable monimolimnion. Before restoration, the annual temperature amplitude in the monimolimnion was 0.4 °C.
After the reclamation activities were carried out, the thermal systems recorded in KML did not confirm the limited mixing of the lake resulting from the density system of the water. Summer stratification lasted from May to October, with an epilimnion of 3 to 6 m in thickness. Below this, there was a thermocline cut off by a gradient with a maximum value of 6.9 °C/m, under which there was a layer of hypolimnion. The water temperature of this layer varied between 3.7 and 5.4 °C, so the annual amplitude increased to 0.8 °C. This situation may be related to the fact that, as a result of the application of coagulants, a significant amount of suspensions and salts that caused an increase in the density of the water layers above the bottom, was sedimented into the bottom sediments. After restoration treatments, it was noticed that the differences in water conductivity, indicating the amount of salts in water capable of dissociation, were no longer so visible between the mixolimnion and the monimolimnion. In 2023, the water conductivity in the mixolimnion varied from 463 to 507 µS/cm, while in the monimolimnion it varied from 612 to 769 µS/cm. These values are still high, typical for lakes with high fertility [
32].
Many researchers claim that one of the most important parameters indicating the condition of the lake is oxygen conditions [
33,
34,
35]. The poor dynamics of the waters of KML and its long-term pollution with various types of sewage caused the oxygen conditions in this reservoir to be very bad. The lack of autumn and spring water circulation caused anaerobic conditions to prevail in the monimolimnion layer all year round. In the years 2018–2022, in spring before the formation of thermal stratification in the mixolimnion water, the presence of oxygen was recorded to a maximum depth of 13 m, and in 2021 only to 6 m. In the following months of the year, and especially in the peak period of summer stagnation, dissolved oxygen was present only in surface waters to a depth of 3 m, and below that the entire water mass was deoxygenated. It should be noted that oxygen concentrations in this small mass of water (up to 3 m depth) were very high, reaching 200% oxygen saturation. This situation was related to the high nutrient concentrations and increased primary production in the water. The water saturation with oxygen was accompanied by high amounts of chlorophyll a (64.5 µg/L), BOD
5 (11.7 mg O
2/L) and low water transparency (SDV 0.3 m). In turn, the main cause of oxygen depletion in the lower parts of water was certainly the bottom sediments, because the oxygen demand (SOD) of the bottom sediments according to Sehgal and Welch [
36] constitutes 96% of the total oxygen demand in the lakes. In the depth of the lake and the bottom sediments, an extremely high amount of organic matter was accumulated (BOD
5 90.1 mg O
2/L), the decomposition of which caused rapid depletion of oxygen. Due to the meromictic nature of the reservoir, for many years the decomposition of organic matter took place in anaerobic conditions, and its effect was the accumulation of products of incomplete decomposition of matter in the monimolimnion waters (ammonia, hydrogen sulfide). After restoration, a slight improvement in the oxygenation of the reservoir water was noted in 2023. In spring, the oxygenated water layer was 16 m thick, but the deoxygenation progressing from the bottom, associated with the still high oxygen demand of the bottom sediments, despite the drop in the BOD
5 value to 21.9 mg O
2/L, meant that, at the end of summer, oxygen was present only in the water layer up to 7 m deep. It is worth noting that the range of the oxygenated water layer increased and its oxygen saturation did not exceed 150%. This is related to the limited availability of biogenic compounds, which was achieved after the application of coagulants. As a result of the treatments, the amount of chlorophyll decreased to values typical for eutrophic lakes (19.6 µg/L), the average annual BOD
5 value decreased (4.8 mg O
2/L), and the transparency of water increased to 2.0 m. A similar situation was recorded in Lake Długie in Olsztyn after the recultivation of this reservoir using artificial aeration and phosphorus inactivation [
37].
The long-term use of KML as a sewage receiver was reflected in the high nutrient concentration. Total phosphorus concentrations in the monimolimnion water reached 30 mg P/L and total nitrogen up to 65 mg N/L. Nutrients flowing into water reservoirs are partially withdrawn from circulation due to sedimentation and accumulation in organisms. Mainly insoluble forms of N and P that “fall out” of circulation are those that, together with other elements, create insoluble easily sinking particles, along with forms that are taken up by aquatic organisms and then fall to the bottom as feces, dead individuals, or their remains [
38,
39]. After the sewage inflow was cut off, the lake water self-purification processes did not occur because the bottom layers of water were deoxygenated all year round. Solim and Wanganeo [
40] claim that, in such conditions, there is no precipitation and binding of phosphorus in the bottom sediments and there is no nitrification of ammonium nitrogen formed during the mineralization of organic matter. In such cases, an appropriate restoration method should be implemented. Among the biogenic substances, the factor determining the degree of trophy of the lake and the associated abundance of phytoplankton is phosphorus, which occurs in natural waters in the form of numerous mineral and organic compounds in dissolved, colloidal, and suspended states [
41,
42]. Phosphorus occurs in various forms in bottom sediments and interstitial water. In the upper layers of sediments, soluble forms of phosphorus are present, which are formed in the processes of destruction of organic compounds, along with being a result of desorption from complexes with iron, manganese, calcium, and aluminum. The share of insoluble forms, permanently removed from the circulation in the reservoir, increases deeper into the sediment [
43]. Boers and de Bles [
44] believe that soluble forms of phosphorus pass from the solid phase of the sediments to the liquid phase and from there through the contact zone of sediments and water, the so-called interphase, they are released to the water above the bottom. Phosphorus exchange processes between sediments and water are mainly dependent on the oxygen concentration in the bottom waters [
45]. When the oxygen concentration is 2 mg O
2/L and below, the redox potential decreases to 200 mV. The decrease in potential in sediments allows the reduction in manganese (IV) to soluble manganese (II), releasing the phosphorus bound to it. Below a potential of 200 mV, insoluble iron in the +3 oxidation state, to which phosphorus is permanently bound, is reduced to iron (II), and then both iron and phosphorus pass into the solution. In the KML, the monimolimnion water was constantly anoxic, which favored the enrichment of this layer with phosphorus from the bottom sediment. Wang et al. [
46] and Lean et al. [
47] draw attention to the enormous influence of Fe presence in water on the precipitation of phosphate phosphorus from the water column to the bottom sediment. These authors emphasize that the higher the iron concentrations, the more phosphorus will be accumulated in sediments. According to Wiśniewski [
48], the most important role in the precipitation of phosphorus from water is played by the Fe/P ratio. If this ratio is higher than 3, then the spontaneous precipitation of phosphorus into sediments occurs, while if it is lower than 0.5, then even in conditions of good water oxygenation, phosphorus release from sediments will be noted. In KML, iron concentrations in the monimolimnion waters were high, exceeding 38 mg Fe/L, but the Fe/P ratio oscillated around 2. Such conditions, combined with the constant deoxygenation of water, did not favor the binding of phosphorus with iron. In the monimolimnion waters, quite high concentrations of manganese were also recorded—over 7 mg Mn/L—which is also considered a factor binding phosphorus. However, manganese occurred in this reservoir in much smaller quantities than iron, so it did not have a significant role in the precipitation of phosphorus into the sediment.
In addition to iron and manganese, many researchers mention calcium, or rather calcium carbonate CaCO
3, as a factor that can participate in phosphorus binding in bottom sediments. According to Koschel et al. [
49], calcite is formed as a result of combining phosphates with calcium. However, this process is most intensive in hard waters. Experiments conducted by Danen-Louwerse et al. [
50] have shown that the precipitation of phosphates with calcium carbonate occurs when phosphorus concentrations are lower than 0.3 mg P/L and the pH of the water is higher than 8.5. In the studied KML, the pH in the bottom parts of the water remained at 6 pH, and the phosphorus concentrations reached 30 mg P/L. Calcium compounds did not play any role in the precipitation of phosphorus into the bottom sediments. Taking into account the above-described environmental conditions prevailing in the water of KML, it was found that the optimal method of restoring this reservoir would be the inactivation of phosphorus, using the following two types of coagulants: iron PIX 111, which was dosed in the coastal areas of the lake, i.e., in the zone of well-oxygenated water, and aluminum PAX 18, which was applied in the zone of water lying above the deoxygenated bottom and the monimolimnion. Aluminum is an element that forms permanent bonds with phosphorus even in anaerobic conditions and with low redox potential [
51]. After the application of coagulants, almost complete precipitation of phosphates from the water column (over 90%) occurred and, as a result, the total amount of phosphorus in the reservoir decreased (over 90%). After remediation, the average content of phosphates in the mixolimnion layer was 0.005 mg P/L, and total phosphorus was 0.076 mg P/L. In turn, at the bottom, the average concentration of phosphates was 0.011 mg P/L and total phosphorus 0.098 mg P/L.
Studies have shown that, even though phosphorus inactivation does not directly affect the content of nitrogen compounds, a several-fold decrease in the concentration of total nitrogen was achieved in the lake. Before restoration, the average content of total nitrogen in the monimolimnion layer was 45 mg N/L, while after reclamation it dropped to about 7 mg N/L. This can be explained by the fact that, as a result of phosphorus precipitation from the water column, there was a radical decrease in primary production and, as a result, there was a decrease in the amount of organic nitrogen. In such a situation, the amount of dead organic matter falling towards the bottom is smaller. This in turn entails a decrease in the amount of ammonium nitrogen, which is a product of its decomposition. Ammonification can occur both in aerobic and anaerobic conditions, in a wide range of pH and temperature, but the intensity of this process depends on fluctuations in the number of appropriate physiological groups of bacteria [
52]. The ammonification process is most intensive when the water temperature is 13–17 °C, and the temperature of the KML monimolimnion water usually did not exceed 6 °C, which inhibited this process. It is also worth noting that, after phosphorus inactivation, the N/P ratio increased to the range of 13–52. Such a ratio of the two main nutrients does not favor cyanobacterial blooms [
15].
In KML, after restoration, the organic matter production processes decreased significantly. Before restoration, the average BOD5 value exceeded 90 mg O2/L, while after the completion of restoration, it averaged 8 mg O2/L. The amount of chlorophyll also decreased, the average concentration of which before restoration was 64 µg/L and in 2023 19.6 µg/L. Light conditions in the lake improved significantly. The Secchi disc visibility (SDV) did not fall below 1 m.
As a result of the use of PIX 111 (iron chloride) and PAX 18 (polyaluminum chloride), the chloride content in KML increased slightly.