Electrochemically Coupled Anaerobic Membrane Bioreactor Facilitates Remediation of Microplastic-Containing Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactor Construction
2.2. Sources of Seed Sludge and Microplastics
2.3. Reactor Operation and Physicochemical Characterization
2.4. Enzyme Activity Determination
2.5. Microbial Community Analysis and Functional Prediction
3. Results and Discussion
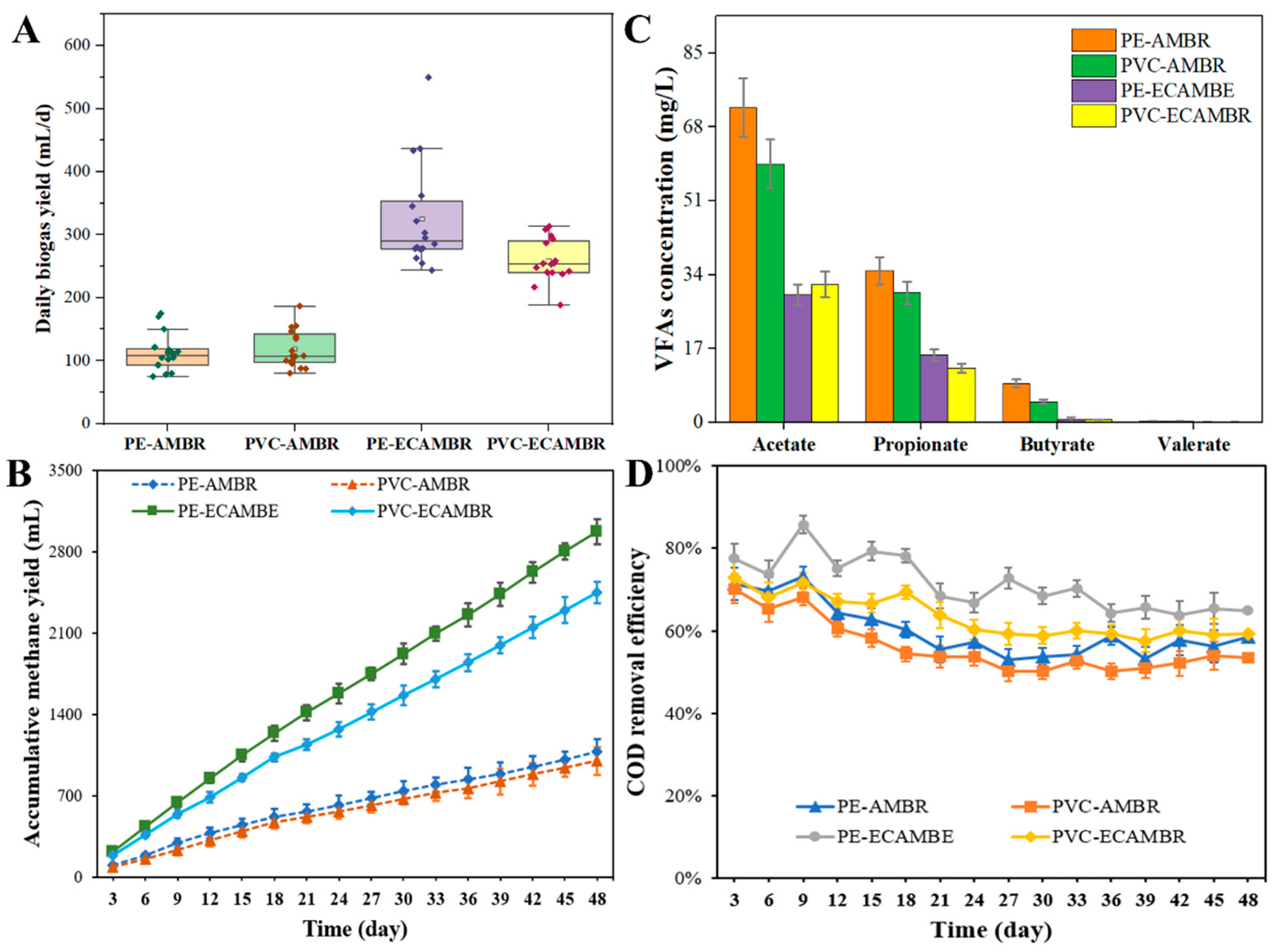
3.1. Fermentation Properties During Wastewater Treatment
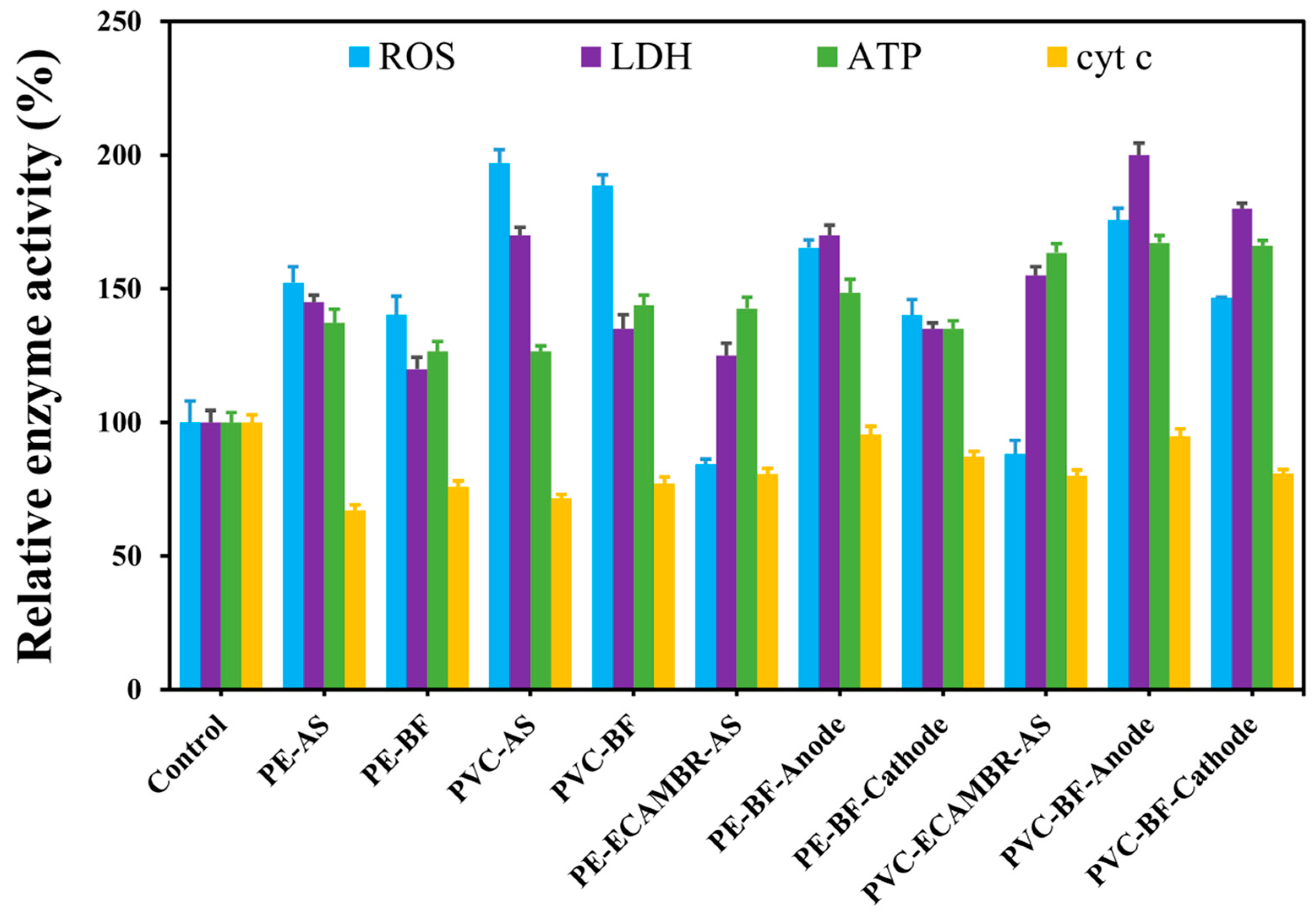
3.2. Shifts in Cell Viability and Enzyme Activity
3.3. Changes in Microbial Resistance
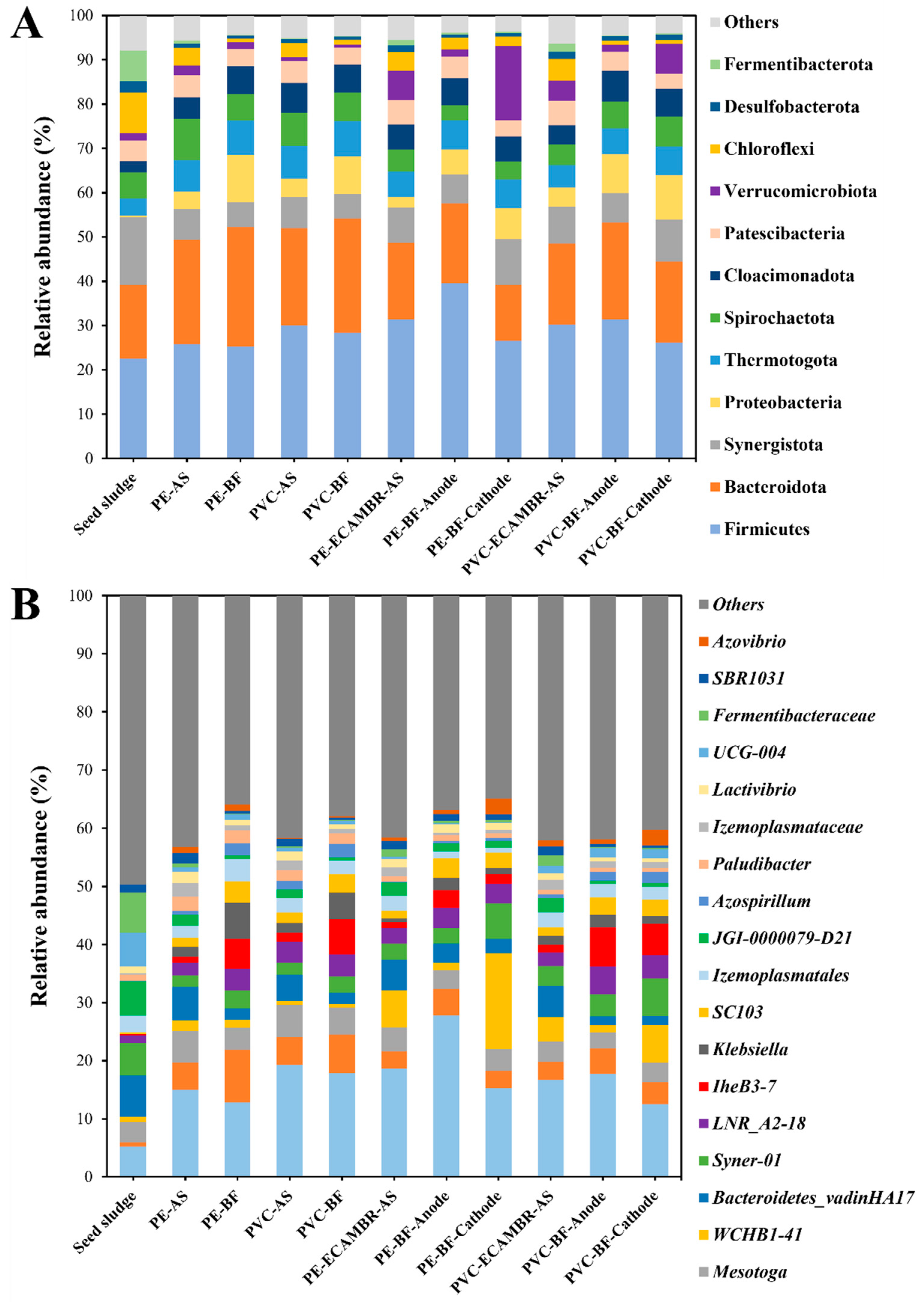
3.4. Microbial Community Structure and Function Predictive Analysis
3.5. Environmental Impacts and Future Perspectives
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Omura, T.; Isobe, N.; Miura, T.; Ishii, S.; Mori, M.; Ishitani, Y.; Kimura, S.; Hidaka, K.; Komiyama, K.; Suzuki, M.; et al. Microbial Decomposition of Biodegradable Plastics on the Deep-Sea Floor. Nat. Commun. 2024, 15, 568. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Mao, M.; Feng, M.; Peng, T.; Long, X.; Yang, F. Fate, Abundance and Ecological Risks of Microcystins in Aquatic Environment: The Implication of Microplastics. Water Res. 2024, 251, 121121. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, Y.; Sun, Y.; Xie, K.; Zeng, Q.; Hao, Y.; Yang, Q.; Pu, Y.; Shi, S.; Gong, Z. Deterioration of Sludge Characteristics and Promotion of Antibiotic Resistance Genes Spread with the Co-Existing of Polyvinylchloride Microplastics and Tetracycline in the Sequencing Batch Reactor. Sci. Total Environ. 2024, 906, 167544. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Jin, B.; Su, Y.; Zhang, Y. Long-Term Effect of Polyethylene Microplastics on the Bioelectrochemical Nitrogen Removal Process. Chem. Eng. J. 2023, 466, 143172. [Google Scholar] [CrossRef]
- Bodzek, M.; Pohl, A. Removal of Microplastics in Unit Processes Used in Water and Wastewater Treatment: A Review. Arch. Environ. Prot. 2022, 48, 102–128. [Google Scholar] [CrossRef]
- Sheriff, I.; Yusoff, M.S.; Halim, H.B. Microplastics in Wastewater Treatment Plants: A Review of the Occurrence, Removal, Impact on Ecosystem, and Abatement Measures. J. Water Process Eng. 2023, 54, 104039. [Google Scholar] [CrossRef]
- Wang, S.; Xu, M.; Jin, B.; Wünsch, U.J.; Su, Y.; Zhang, Y. Electrochemical and Microbiological Response of Exoelectrogenic Biofilm to Polyethylene Microplastics in Water. Water Res. 2022, 211, 118046. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity Assessment of Microplastic (MPs); a Threat to the Ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Wei, J.; Chen, M.; Wang, J. Insight into Combined Pollution of Antibiotics and Microplastics in Aquatic and Soil Environment: Environmental Behavior, Interaction Mechanism and Associated Impact of Resistant Genes. TrAC—Trends Anal. Chem. 2023, 166, 117214. [Google Scholar] [CrossRef]
- Yousefzadeh, S.; Ahmadi, E.; Gholami, M.; Ghaffari, H.R.; Azari, A.; Ansari, M.; Miri, M.; Sharafi, K.; Rezaei, S. A Comparative Study of Anaerobic Fixed Film Baffled Reactor and Up-Flow Anaerobic Fixed Film Fixed Bed Reactor for Biological Removal of Diethyl Phthalate from Wastewater: A Performance, Kinetic, Biogas, and Metabolic Pathway Study. Biotechnol. Biofuels 2017, 10, 139. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E. A New Sponge-GAC-Sponge Membrane Module for Submerged Membrane Bioreactor Use in Hospital Wastewater Treatment. Biochem. Eng. J. 2018, 133, 130–139. [Google Scholar] [CrossRef]
- Khalidi-idrissi, A.; Madinzi, A.; Anouzla, A.; Pala, A.; Mouhir, L.; Kadmi, Y.; Souabi, S. Recent Advances in the Biological Treatment of Wastewater Rich in Emerging Pollutants Produced by Pharmaceutical Industrial Discharges. Int. J. Environ. Sci. Technol. 2023, 20, 11719–11740. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Guo, W.; Ngo, H.H.; Zhang, X.; Chen, C.; Chen, Z.; Cheng, D.; Ni, S.Q.; Wang, Q. Recent Advances in Attached Growth Membrane Bioreactor Systems for Wastewater Treatment. Sci. Total Environ. 2022, 808, 152123. [Google Scholar] [CrossRef]
- Zakaria, B.S.; Azizi, S.M.M.; Pramanik, B.K.; Hai, F.I.; Elbeshbishy, E.; Dhar, B.R. Responses of Syntrophic Microbial Communities and Their Interactions with Polystyrene Nanoplastics in a Microbial Electrolysis Cell. Sci. Total Environ. 2023, 903, 166082. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhao, S.; Qiu, T.; Cui, Q.; Yang, Y.; Li, L.; Chen, J.; Huang, M.; Zhan, A.; Fang, L. Interaction of Microplastics with Heavy Metals in Soil: Mechanisms, Influencing Factors and Biological Effects. Sci. Total Environ. 2024, 918, 170281. [Google Scholar] [CrossRef]
- Li, Z.H.; Yuan, L.; Yang, C.W.; Wang, R.; Sheng, G.P. Anaerobic Electrochemical Membrane Bioreactor Effectively Mitigates Antibiotic Resistance Genes Proliferation under High Antibiotic Selection Pressure. Environ. Int. 2022, 166, 107381. [Google Scholar] [CrossRef]
- Tang, Y.; Xie, H.; Sun, J.; Li, X.; Zhang, Y.; Dai, X. Alkaline Thermal Hydrolysis of Sewage Sludge to Produce High-Quality Liquid Fertilizer Rich in Nitrogen-Containing Plant-Growth-Promoting Nutrients and Biostimulants. Water Res. 2022, 211, 118036. [Google Scholar] [CrossRef]
- Foo, K.; Liang, Y.Y.; Tan, C.K.; Fimbres Weihs, G.A. Coupled Effects of Circular and Elliptical Feed Spacers under Forced-Slip on Viscous Dissipation and Mass Transfer Enhancement Based on CFD. J. Membr. Sci. 2021, 637, 119599. [Google Scholar] [CrossRef]
- Tang, K.H.D. Bioaugmentation of Anaerobic Wastewater Treatment Sludge Digestion: A Perspective on Microplastics Removal. J. Clean. Prod. 2023, 387, 135864. [Google Scholar] [CrossRef]
- Flemming, H.C.; van Hullebusch, E.D.; Little, B.J.; Neu, T.R.; Nielsen, P.H.; Seviour, T.; Stoodley, P.; Wingender, J.; Wuertz, S. Microbial Extracellular Polymeric Substances in the Environment, Technology and Medicine. Nat. Rev. Microbiol. 2024. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Bioelectrochemical Systems—A Potentially Effective Technology for Mitigating Microplastic Contamination in Wastewater. J. Clean. Prod. 2024, 450, 141931. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Bioelectrochemical Anaerobic Digestion Mitigates Microplastic Pollution and Promotes Methane Recovery of Wastewater Treatment in Biofilm System. J. Hazard. Mater. 2024, 472, 134488. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hadji-Thomas, A.; Adekunle, A.; Raghavan, V. The Exploitation of Bio-Electrochemical System and Microplastics Removal: Possibilities and Perspectives. Sci. Total Environ. 2024, 930, 172737. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Chen, X.; Ni, B.J. Different Pathways of Microplastics Entering the Sludge Treatment System Distinctively Affect Anaerobic Sludge Fermentation Processes. Environ. Sci. Technol. 2021, 55, 11274–11283. [Google Scholar] [CrossRef]
- Niu, C.; Zhang, Z.; Cai, T.; Pan, Y.; Lu, X.; Zhen, G. Sludge Bound-EPS Solubilization Enhance CH4 Bioconversion and Membrane Fouling Mitigation in Electrochemical Anaerobic Membrane Bioreactor: Insights from Continuous Operation and Interpretable Machine Learning Algorithms. Water Res. 2024, 264, 122243. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Zeng, S.; Pan, X.; Cheng, H.; Liu, L.; Luo, F. Bioelectrochemistry Explore the Difference between the Single-Chamber and Dual-Chamber Microbial Electrosynthesis for Biogas Production Performance. Bioelectrochemistry 2021, 138, 107726. [Google Scholar] [CrossRef]
- Chen, K.H.; Feng, J.; Bodelier, P.L.E.; Yang, Z.; Huang, Q.; Delgado-Baquerizo, M.; Cai, P.; Tan, W.; Liu, Y.R. Metabolic Coupling between Soil Aerobic Methanotrophs and Denitrifiers in Rice Paddy Fields. Nat. Commun. 2024, 15, 3471. [Google Scholar] [CrossRef]
- Luo, T.; Dai, X.; Chen, Z.; Wu, L.; Wei, W.; Xu, Q.; Ni, B.J. Different Microplastics Distinctively Enriched the Antibiotic Resistance Genes in Anaerobic Sludge Digestion through Shifting Specific Hosts and Promoting Horizontal Gene Flow. Water Res. 2023, 228, 119356. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Fessler, M.; Jin, B.; Su, Y.; Zhang, Y. Insights into the Impact of Polyethylene Microplastics on Methane Recovery from Wastewater via Bioelectrochemical Anaerobic Digestion. Water Res. 2022, 221, 118844. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, Q. Dominant Factors Analyses and Challenges of Anaerobic Digestion under Cold Environments. J. Environ. Manag. 2023, 348, 119378. [Google Scholar] [CrossRef]
- Geng, H.; Xu, Y.; Liu, R.; Yang, D.; Dai, X. Cation Exchange Resins Enhance Anaerobic Digestion of Sewage Sludge: Roles in Sequential Recovery of Hydrogen and Methane. Water Res. 2024, 248, 120897. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, Q. Potential Application of Bioelectrochemical Systems in Cold Environments. Sci. Total Environ. 2024, 927, 172385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Adams, C.A.; Wang, F.; Sun, Y.; Zhang, S. Interactions between Microplastics and Soil Fauna: A Critical Review. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3211–3243. [Google Scholar] [CrossRef]
- Mohammad Mirsoleimani Azizi, S.; Hai, F.I.; Lu, W.; Al-Mamun, A.; Ranjan Dhar, B. A Review of Mechanisms Underlying the Impacts of (Nano)Microplastics on Anaerobic Digestion. Bioresour. Technol. 2021, 329, 124894. [Google Scholar] [CrossRef]
- Zheng, P.; Li, Y.; Cheng, Y.; Shen, J. Mechanism Involved in Polyvinyl Chloride Nanoplastics Induced Anaerobic Granular Sludge Disintegration: Microbial Interaction Energy, EPS Molecular Structure, and Metabolism Functions. Environ. Sci. Technol. 2024, 58, 11542–11553. [Google Scholar] [CrossRef]
- Zantis, L.J.; Borchi, C.; Vijver, M.G.; Peijnenburg, W.; Di Lonardo, S.; Bosker, T. Nano- and Microplastics Commonly Cause Adverse Impacts on Plants at Environmentally Relevant Levels: A Systematic Review. Sci. Total Environ. 2023, 867, 161211. [Google Scholar] [CrossRef]
- Schubert, T.; Adrian, L.; Sawers, R.G.; Diekert, G. Organohalide Respiratory Chains: Composition, Topology and Key Enzymes. FEMS Microbiol. Ecol. 2018, 94, fiy035. [Google Scholar] [CrossRef]
- Ahmadi, S.; Rezae, A.; Ghosh, S.; Malloum, A.; Banach, A. A Review on Bioelectrochemical Systems for Emerging Pollutants Remediation: A Computational Approaches. J. Environ. Chem. Eng. 2023, 11, 110021. [Google Scholar] [CrossRef]
- Yang, K.; Ji, M.; Liang, B.; Zhao, Y.; Zhai, S.; Ma, Z.; Yang, Z. Bioelectrochemical Degradation of Monoaromatic Compounds: Current Advances and Challenges. J. Hazard. Mater. 2020, 398, 122892. [Google Scholar] [CrossRef]
- Kumar, M.; Xiong, X.; He, M.; Tsang, D.C.W.; Gupta, J.; Khan, E.; Harrad, S.; Hou, D.; Ok, Y.S.; Bolan, N.S. Microplastics as Pollutants in Agricultural Soils. Environ. Pollut. 2020, 265, 114980. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Fimbres Weihs, G.A.; Fletcher, D.F. CFD Study of the Effect of Unsteady Slip Velocity Waveform on Shear Stress in Membrane Systems. Chem. Eng. Sci. 2018, 192, 16–24. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, H.; Zhou, S.; Sun, L.; Lu, H. Enhanced Performance and Electron Transfer of Sulfur-Mediated Biological Process under Polyethylene Terephthalate Microplastics Exposure. Water Res. 2022, 223, 119038. [Google Scholar] [CrossRef] [PubMed]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.M.; Lin, Y.M. Chemical Characterization Methods for the Analysis of Structural Extracellular Polymeric Substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y. Effects of Microplastics on Wastewater and Sewage Sludge Treatment and Their Removal: A Review. Chem. Eng. J. 2020, 382, 122955. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Wei, W.; Sun, J.; Xu, Q.; Ni, B.J. Long-Term Effects of Polyvinyl Chloride Microplastics on Anaerobic Granular Sludge for Recovering Methane from Wastewater. Environ. Sci. Technol. 2020, 54, 9662–9671. [Google Scholar] [CrossRef]
- Cheng, X.; Wei, Z.; Cao, W.; Feng, Q.; Liu, J.; Wu, Y.; Feng, L.; Wang, D.; Luo, J. Untangling the Interplay of Dissolved Organic Matters Variation with Microbial Symbiotic Network in Sludge Anaerobic Fermentation Triggered by Various Pretreatments. Water Res. 2024, 260, 121930. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Wu, Z.; Zhou, J.; Zhou, L.; Lu, Y.; Liu, X.; Wu, W. Groundwater Contaminated with Short-Chain Chlorinated Paraffins and Microbial Responses. Water Res. 2021, 204, 117605. [Google Scholar] [CrossRef]
- Muenmee, S.; Chiemchaisri, W.; Chiemchaisri, C. Microbial Consortium Involving Biological Methane Oxidation in Relation to the Biodegradation of Waste Plastics in a Solid Waste Disposal Open Dump Site. Int. Biodeterior. Biodegrad. 2015, 102, 172–181. [Google Scholar] [CrossRef]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct Community Structure and Microbial Functions of Biofilms Colonizing Microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The Role of Microbial Amino Acid Metabolism in Host Metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef]
- Wang, K.; Yang, S.; Yu, X.; Bai, M.; Ye, H.; Xu, Y.; Zhao, L.; Wu, D.; Li, X.; Weng, L.; et al. Microplastics Degradation Stimulated by In-Situ Bioelectric Field in Agricultural Soils. Environ. Int. 2023, 177, 108035. [Google Scholar] [CrossRef] [PubMed]
- Fischer, F. Photoelectrode, Photovoltaic and Photosynthetic Microbial Fuel Cells. Renew. Sustain. Energy Rev. 2018, 90, 16–27. [Google Scholar] [CrossRef]
- Li, N.; Zhang, X.; Zhao, M.; Zhang, Y.; Yuan, Y.; Lu, X.; Zhang, H.; Sun, J. Integrating Solar Photovoltaic Capacitor into Algal-Bacterial Photo-Bioelectrochemical System towards All-Weather Synchronous Enhanced Antibiotic and Nitrogen Removal from Wastewater. J. Clean. Prod. 2020, 272, 122661. [Google Scholar] [CrossRef]




| α-Index | Seed Sludge | PE-AS | PE-BF | PVC-AS | PVC-BF | PE-ECAMBR-AS | PE-BF-Anode | PE-BF-Cathode | PVC-ECAMBR-AS | PVC-BF-Anode | PVC-BF-Cathode |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD | 53.66891 | 47.56555 | 45.95122 | 47.84379 | 45.85557 | 59.87742 | 50.77436 | 53.14066 | 59.41979 | 47.95153 | 51.16805 |
| Chao | 447.5831 | 388.6456 | 373.6525 | 397.0422 | 363.7217 | 525.8259 | 444.0042 | 469.3273 | 535.8279 | 400.6781 | 425.9901 |
| Shannon | 6.927494 | 6.845406 | 6.593982 | 6.886326 | 6.622241 | 7.249317 | 6.937331 | 6.742847 | 7.393404 | 6.862342 | 6.862443 |
| Simpson | 0.981332 | 0.981839 | 0.977834 | 0.981896 | 0.979571 | 0.985561 | 0.980886 | 0.978289 | 0.987962 | 0.982458 | 0.982439 |
| Coverage | 0.999633 | 0.999671 | 0.999707 | 0.999761 | 0.999798 | 0.999535 | 0.999691 | 0.999553 | 0.999617 | 0.999688 | 0.999622 |
| Ace | 446.8139 | 385.5495 | 372.6485 | 396.3976 | 360.9708 | 523.4619 | 441.0679 | 465.1366 | 531.8799 | 398.0596 | 421.3268 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, K.; Yin, H.; Ding, Z.; Xu, N.; Fan, Y. Electrochemically Coupled Anaerobic Membrane Bioreactor Facilitates Remediation of Microplastic-Containing Wastewater. Water 2024, 16, 3236. https://doi.org/10.3390/w16223236
Zhou K, Yin H, Ding Z, Xu N, Fan Y. Electrochemically Coupled Anaerobic Membrane Bioreactor Facilitates Remediation of Microplastic-Containing Wastewater. Water. 2024; 16(22):3236. https://doi.org/10.3390/w16223236
Chicago/Turabian StyleZhou, Kunpeng, Huilin Yin, Zhenyu Ding, Nuchao Xu, and Yun Fan. 2024. "Electrochemically Coupled Anaerobic Membrane Bioreactor Facilitates Remediation of Microplastic-Containing Wastewater" Water 16, no. 22: 3236. https://doi.org/10.3390/w16223236
APA StyleZhou, K., Yin, H., Ding, Z., Xu, N., & Fan, Y. (2024). Electrochemically Coupled Anaerobic Membrane Bioreactor Facilitates Remediation of Microplastic-Containing Wastewater. Water, 16(22), 3236. https://doi.org/10.3390/w16223236





