Inhibition of Polycyclic Aromatic Hydrocarbons Formation During Supercritical Water Gasification of Sewage Sludge by H2O2 Combined with Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Apparatus and Procedure
2.3. Products Analysis
2.4. Data Interpretation
3. Results and Discussion
3.1. Effects of Catalysts on the Distribution of PAHs in the SCWG Products
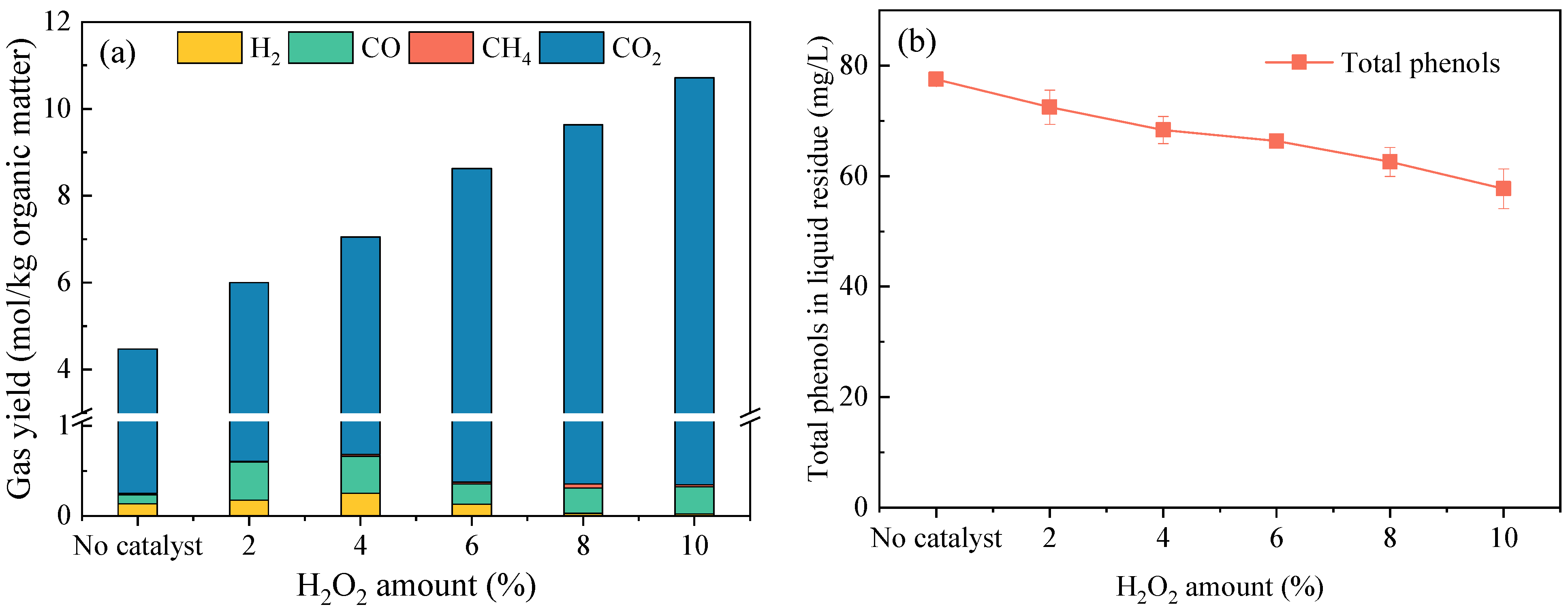
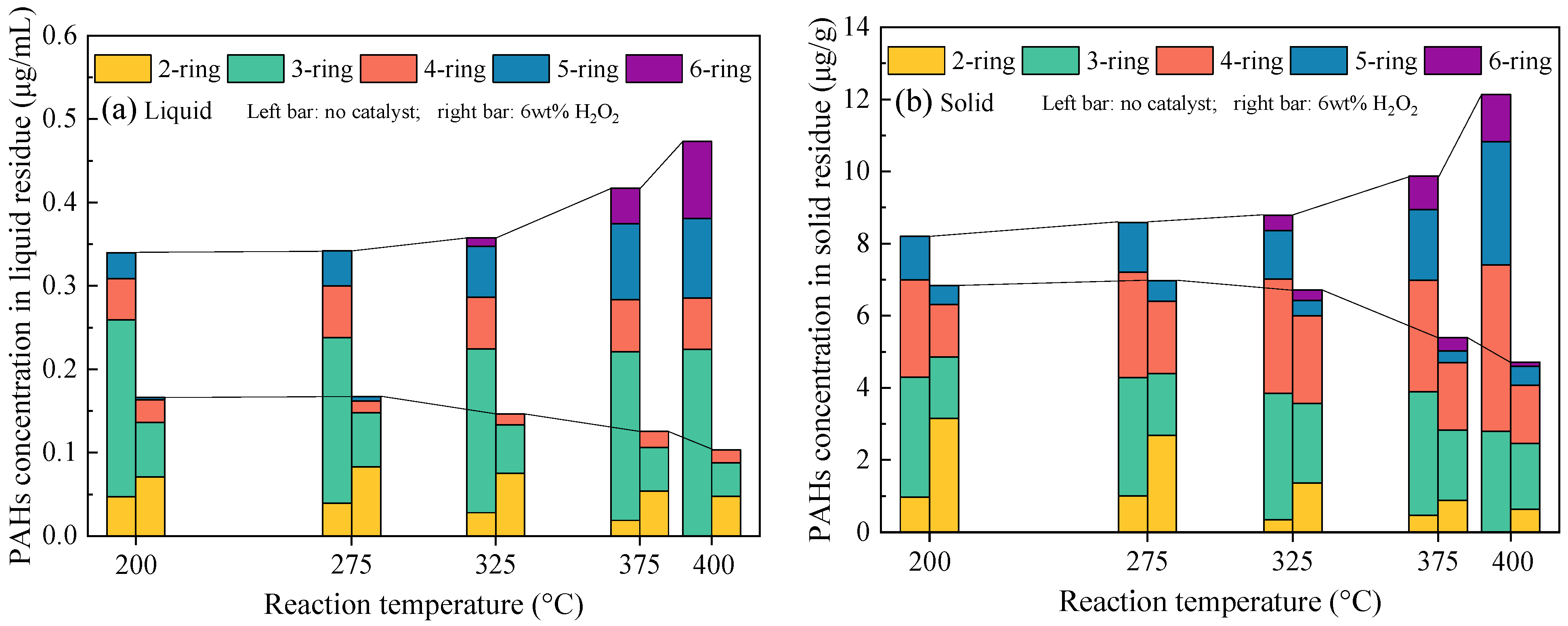
3.2. Effects of the H2O2 Amount on Alterations in PAHs Distribution in the SCWG Products
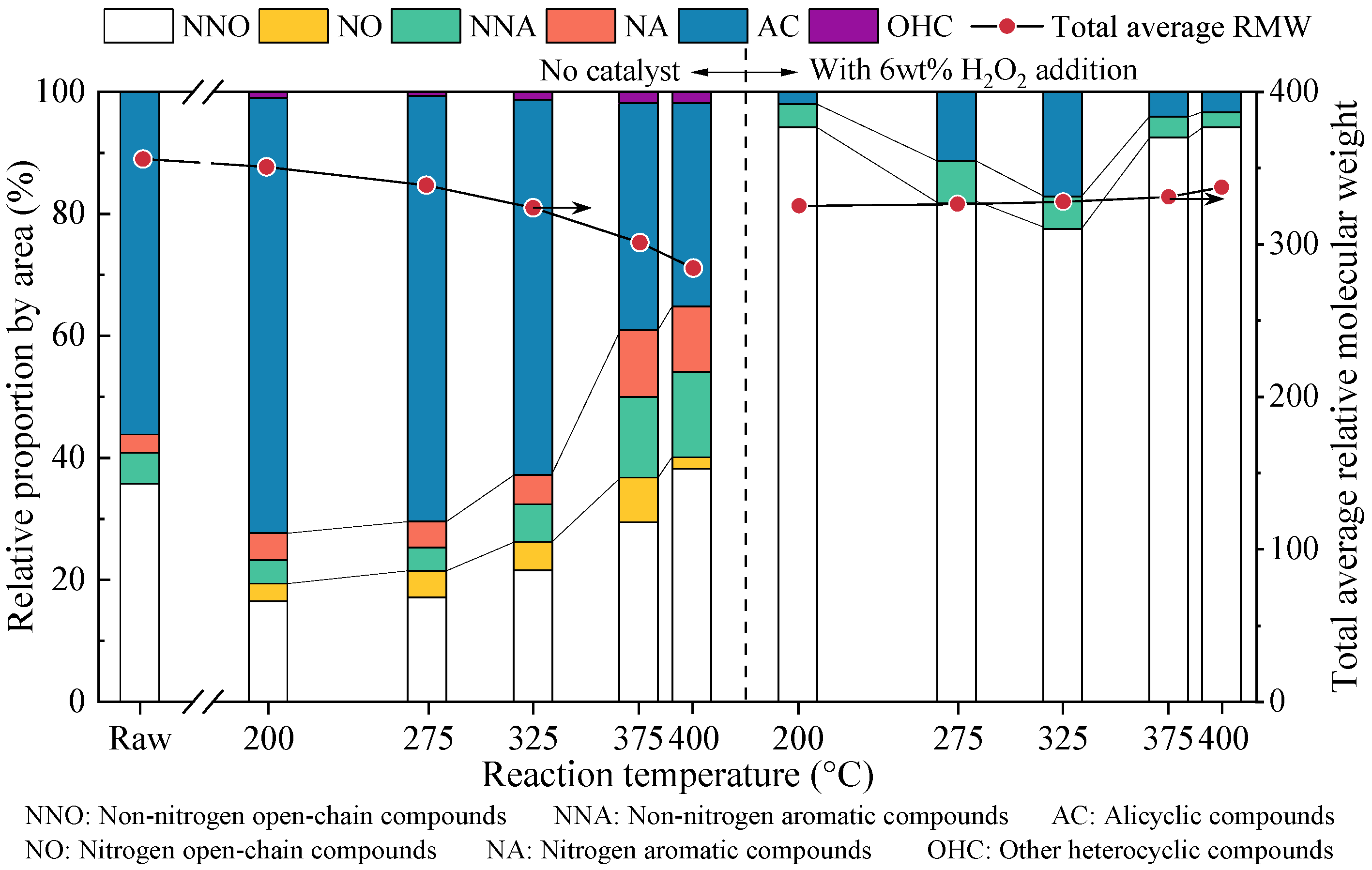
3.3. Inhibition Mechanism of H2O2 on PAHs Formation During Heating Stage of SCWG Process
3.4. Mechanism of Combined Catalysts on PAHs Formation and Gas Production
4. Conclusions
- Except for the NaNO3 catalyst, the addition of the other five catalysts (Ni, NaOH, Na2CO3, H2O2, and KMnO4) reduced PAH concentrations in the liquid and solid residues. In particular, the addition of Ni and H2O2 significantly lowered the PAH concentrations. The PAH concentrations in the liquid residue dropped from 0.39 μg/mL to 0.29 and 0.26 μg/mL while that in the solid residue was reduced from 12.5 μg/g to 7.1 and 6.3 μg/g, respectively.
- The PAH concentrations in both the liquid and solid residues decreased sharply with increases in the amount of H2O2, leading to decreases of 91% and 88%, respectively, with a loading of 10 wt%. As the H2O2 amount increased to 8 wt%, 5-ring and 6-ring PAHs were not detected in the residues. The H2O2 addition inhibited PAH formation by promoting the ring-opening reaction of existing aromatic compounds in the raw sludge and limiting the polymerization of open-chain intermediates.
- Both NaOH + H2O2 and Ni + H2O2, acting as combined catalysts, greatly decreased the PAH concentrations while raising the hydrogen yield. The H2O2 generated ring-opening intermediates that were further gasified by the NaOH or Ni. Thus, these combinations exhibited a synergistic effect.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalski, Z.; Makara, A.; Kulczycka, J.; Generowicz, A.; Kwaśnicki, P.; Ciuła, J.; Gronba-Chyła, A. Conversion of Sewage Sludge into Biofuels via Different Pathways and Their Use in Agriculture: A Comprehensive Review. Energies 2024, 17, 1383. [Google Scholar] [CrossRef]
- Das, P.; Khan, S.; AbdulQuadir, M.; Thaher, M.; Waqas, M.; Easa, A.; Attia, E.S.M.; Al-Jabri, H. Energy recovery and nutrients recycling from municipal sewage sludge. Sci. Total Environ. 2020, 715, 136775. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.X.; Tan, Y.; Ma, X.Q.; Li, B.; Chen, Y.X.; Zhang, B.; Osman, S.M.; Luo, J.Y.; Luque, R. Valorization of sewage sludge for facile and green wood bio-adhesives production. Environ. Res. 2023, 239, 117421. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Feng, A.; Wang, L.; Wang, M.; Hu, J.; Fan, Y. Coupling of hydrothermal pretreatment and supercritical water gasification of sewage sludge for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 17914–17925. [Google Scholar] [CrossRef]
- Yan, M.; Cui, J.; Li, T.; Feng, H.; Hantoko, D.; Kanchanatip, E. Transformation and distribution of nitrogen and phosphorus in sewage sludge during supercritical water gasification. Fuel 2023, 332, 125918. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Wang, S.; Zhao, S.; Zhang, B. Supercritical water gasification and partial oxidation of municipal sewage sludge: An experimental and thermodynamic study. Int. J. Hydrogen Energy 2021, 46, 89–99. [Google Scholar] [CrossRef]
- Hoang, S.A.; Bolan, N.; Madhubashani, A.M.P.; Vithanage, M.; Perera, V.; Wijesekara, H.; Wang, H.; Srivastava, P.; Kirkham, M.B.; Mickan, B.S.; et al. Treatment processes to eliminate potential environmental hazards and restore agronomic value of sewage sludge: A review. Environ. Pollut. 2022, 293, 118564. [Google Scholar] [CrossRef]
- Meng, X.Z.; Venkatesan, A.K.; Ni, Y.L.; Steele, J.C.; Wu, L.L.; Bignert, A.; Bergman, Å.; Halden, R.U. Organic Contaminants in Chinese Sewage Sludge: A Meta-Analysis of the Literature of the Past 30 Years. Environ. Sci. Technol. 2016, 50, 5454–5466. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.C.; Meng, X.-Z.; Venkatesan, A.K.; Halden, R.U. Comparative meta-analysis of organic contaminants in sewage sludge from the United States and China. Sci. Total Environ. 2022, 821, 153423. [Google Scholar] [CrossRef]
- Xu, Z.X.; Dou, R.; Gao, F.; Chen, Y.X.; Leng, L.J.; Osman, S.M.; Luque, R. Bio-adhesives derived from sewage sludge via hydrothermal carbonization: Influence of aqueous phase recycling. Chem. Eng. J. 2024, 490, 151685. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Zhang, H.W.; Su, Y.; Fan, Y.J. Polycyclic aromatic hydrocarbon formation from gasification of sewage sludge in supercritical water: The concentration distribution and effect of sludge properties. J. Supercrit. Fluids 2016, 113, 112–118. [Google Scholar] [CrossRef]
- Gong, M.; Wang, Y.L.; Fan, Y.J.; Zhu, W.; Zhang, H.W.; Su, Y. Polycyclic aromatic hydrocarbon formation during the gasification of sewage sludge in sub- and supercritical water: Effect of reaction parameters and reaction pathways. Waste Manag. 2018, 72, 287–295. [Google Scholar] [CrossRef]
- Li, Z.; Gong, M.; Wang, M.; Feng, A.; Wang, L.; Ma, P.; Yuan, S. Influence of AlCl3 and oxidant catalysts on hydrogen production from the supercritical water gasification of dewatered sewage sludge and model compounds. Int. J. Hydrogen Energy 2021, 46, 31262–31274. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhu, W.; Li, M.; Zhang, H.W.; Gong, M. Quantitative analysis of polycyclic aromatic hydrocarbons in solid residues from supercritical water gasification of wet sewage sludge. Appl. Energy 2013, 102, 476–483. [Google Scholar] [CrossRef]
- Zhang, L.H.; Xu, C.B.; Champagne, P. Energy recovery from secondary pulp/paper-mill sludge and sewage sludge with supercritical water treatment. Bioresour. Technol. 2010, 101, 2713–2721. [Google Scholar] [CrossRef]
- Qian, L.L.; Wang, S.Z.; Xu, D.H.; Guo, Y.; Tang, X.Y.; Wang, L.F. Treatment of sewage sludge in supercritical water and evaluation of the combined process of supercritical water gasification and oxidation. Bioresour. Technol. 2015, 176, 218–224. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, Y.B.; Zhu, Y.; Peng, C.; Wang, T.F.; Zeng, G.M.; Wu, D.B.; Zhao, X. Distribution and Conversion of Polycyclic Aromatic Hydrocarbons during the Hydrothermal Treatment of Sewage Sludge. Energy Fuels 2017, 31, 9542–9549. [Google Scholar] [CrossRef]
- Ren, J.; Liu, Y.L.; Zhao, X.Y.; Cao, J.P. Biomass thermochemical conversion: A review on tar elimination from biomass catalytic gasification. J. Energy Inst. 2020, 93, 1083–1098. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhu, W.; Gong, M.; Zhang, H.W. Direct gasification of dewatered sewage sludge in supercritical water. Part 1: Effects of alkali salts. Int. J. Hydrogen Energy 2013, 38, 3963–3972. [Google Scholar] [CrossRef]
- Yeletsky, P.M.; Reina, T.R.; Bulavchenko, O.A.; Saraev, A.A.; Gerasimov, E.Y.; Zaikina, O.O.; Bermúdez, J.M.; Arcelus-Arrillaga, P.; Yakovlev, V.A.; Millan, M. Phenanthrene catalytic cracking in supercritical water: Effect of the reaction medium on NiMo/SiO2 catalysts. Catal. Today 2019, 329, 197–205. [Google Scholar] [CrossRef]
- Reina, T.R.; Yeletsky, P.; Bermúdez, J.M.; Arcelus-Arrillaga, P.; Yakovlev, V.A.; Millan, M. Anthracene aquacracking using NiMo/SiO2 catalysts in supercritical water conditions. Fuel 2016, 182, 740–748. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Wei, C.H.; Shi, H.S.; Wu, C.F.; Chai, X.S. Partial oxidative gasification of phenol for hydrogen in supercritical water. Appl. Energy 2011, 88, 2612–2616. [Google Scholar] [CrossRef]
- Smara, M.; Khalladi, R.; Moulai-Mostefa, N.; Madi, K.; Mansour, D.; Lekmine, S.; Benslama, O.; Tahraoui, H.; Zhang, J.; Amrane, A. Efficiency of Hydrogen Peroxide and Fenton Reagent for Polycyclic Aromatic Hydrocarbon Degradation in Contaminated Soil: Insights from Experimental and Predictive Modeling. Processes 2024, 12, 621. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Williams, P.T. Flameless supercritical water incineration of polycyclic aromatic hydrocarbons. Int. J. Energy Res. 2006, 30, 523–533. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Wang, S.Z.; Zhao, G.Y.; Guo, Y.F.; Guo, Y. Hydrogen production by partial oxidation gasification of a phenol, naphthalene, and acetic acid mixture in supercritical water. Int. J. Hydrogen Energy 2016, 41, 2238–2246. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.Z.; Gong, Y.M.; Xu, D.H.; Tang, X.Y.; Ma, H.H. Partial oxidation of municipal sludge with activited carbon catalyst in supercritical water. J. Hazard. Mater. 2010, 180, 137–144. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Zhang, X.; Zhang, R.; Ding, L. Formation and inhibition of polycyclic aromatic hydrocarbons from the gasification of cyanobacterial biomass in supercritical water. Chemosphere 2020, 253, 126777. [Google Scholar] [CrossRef]
- Zhong, J.; Zhu, W.; Wang, C.; Mu, B.; Lin, N.; Chen, S.; Li, Z. Transformation mechanism of polycyclic aromatic hydrocarbons and hydrogen production during the gasification of coking sludge in supercritical water. Chemosphere 2022, 300, 134467. [Google Scholar] [CrossRef]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Reaction products from the subcritical water gasification of food wastes and glucose with NaOH and H2O2. Bioresour. Technol. 2010, 101, 6812–6821. [Google Scholar] [CrossRef]
- Wang, C.Y.; Zhu, W.; Gong, M.; Su, Y.; Fan, Y.J. Influence of H2O2 and Ni catalysts on hydrogen production and PAHs inhibition from the supercritical water gasification of dewatered sewage sludge. J. Supercrit. Fluids 2017, 130, 183–188. [Google Scholar] [CrossRef]
- Gong, M.; Wang, S.; Hu, J.; Fan, Y. Effect of CuSO4 on the behavior of nitrogen during supercritical water gasification of microalgal biomass. J. Environ. Chem. Eng. 2024, 12, 113737. [Google Scholar] [CrossRef]
- Gong, M.; Hu, J.; Xu, Q.; Fan, Y. Catalytic gasification of Enteromorpha prolifera for hydrogen production in supercritical water. Process. Saf. Environ. 2023, 175, 227–237. [Google Scholar] [CrossRef]
- Guan, Q.Q.; Wei, C.H.; Ning, P.; Tian, S.L.; Gu, J.J. Catalytic gasification of algae Nannochloropsis sp. in sub/supercritical water. Procedia Environ. Sci. 2013, 18, 844–848. [Google Scholar] [CrossRef]
- Jin, H.; Liu, S.K.; Wei, W.W.; Zhang, D.M.; Cheng, Z.N.; Guo, L.J. Experimental investigation on hydrogen production by anthracene gasification in supercritical water. Energy Fuels 2015, 29, 6342–6346. [Google Scholar] [CrossRef]
- Yang, B.; Cheng, Z.; Tang, Q.; Shen, Z. Nitrogen transformation of 41 organic compounds during SCWO: A study on TN degradation rate, N-containing species distribution and molecular characteristics. Water Res. 2018, 140, 167–180. [Google Scholar] [CrossRef]
- Gong, X.; Xu, D.; Diao, Y.; Yang, L.; Wang, S.; Zhao, J. Nitrogen removal mechanisms and effect enhancement of N-containing organic matters in supercritical water. J. Clean. Prod. 2024, 434, 139974. [Google Scholar] [CrossRef]
- Su, H.; Yan, M.; Wang, S. Recent advances in supercritical water gasification of biowaste catalyzed by transition metal-based catalysts for hydrogen production. Renew. Sustain. Energy Rev. 2022, 154, 111831. [Google Scholar] [CrossRef]
- Su, W.; Zhao, M.; Xing, Y.; Ma, H.; Liu, P.; Li, X.; Zhang, H.; Wu, Y.; Xia, C. Supercritical water gasification of hyperaccumulators for hydrogen production and heavy metal immobilization with alkali metal catalysts. Environ. Res. 2022, 214, 114093. [Google Scholar] [CrossRef]
- Muangrat, R.; Onwudili, J.A.; Williams, P.T. Reactions of different food classes during subcritical water gasification for hydrogen gas production. Int. J. Hydrogen Energy 2012, 37, 2248–2259. [Google Scholar] [CrossRef]
- Xu, D.H.; Wang, S.Z.; Guo, Y.; Tang, X.Y.; Gong, Y.M.; Ma, H.H. Catalyzed partial oxidative gasification of phenol in supercritical water. Ind. Eng. Chem. Res. 2011, 50, 4301–4307. [Google Scholar] [CrossRef]





| Moisture Content (wt.%) | pH | Proximate Analysis (wt%) 1 | Ultimate Analysis (wt%) 1 | HHV (MJ/kg) 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VM | FC | Ash | C | H | N | S | O 2 | ||||||||
| 77.05 | 7.50 | 27.84 | 2.19 | 69.97 | 12.11 | 2.08 | 1.82 | 1.09 | 12.93 | 4.91 | |||||
| Heavy Metal Content (mg/kg) 1 | Fe | Ni | Cu | Zn | Cr | Pb | As | ||||||||
| 23,201 | 27.6 | 128 | 1254 | 45.8 | 44.7 | - | |||||||||
| PAHs Content (μg/g) 1,4 | 2-Ring | 3-Ring | 4-Ring | 5-Ring | 6-Ring | ||||||||||
| 0.35 | 2.80 | 3.38 | 0.71 | N.D | |||||||||||
| Feedstock | Experimental Conditions | Catalyst | Effects on H2 Yield | Effects on PAH Concentrations in Solid Residues | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Without Catalyst (mol/kg OM) | With Catalyst (mol/kg OM) | Increase Multiple | Without Catalyst (μg/g) | With Catalyst (μg/g) | Decrease Percentage | ||||
| DSS | 400 °C, 10min | 2.5 wt% Ni + 2.5 wt% H2O2 | 0.13 | 3.88 | 27.9 | 11.04 | 6.20 | 44% | This work |
| 2.5 wt% NaOH + 2.5 wt% H2O2 | 0.13 | 1.75 | 12.0 | 11.04 | 7.99 | 28% | |||
| DSS | 400 °C, 60min | 2.5 wt% Ni + 2.5 wt% H2O2 | 0.29 | 0.83 | 1.86 | 1.27 | 0.66 | 48% | [30] |
| Cyanobacterial biomass | 400 °C, 10min | 1 wt% H2O2 | 0.17 | 0.50 | 1.94 | 10.54 | 3.26 | 69% | [27] |
| Coking sludge | 400 °C, 30min | 8 mmol KOH (about 1.2 wt%) | 0.036 | 0.38 | 9.56 | 263.1 | 164.66 | 37% | [28] |
| 2 mmol H2O2 (about 0.4 wt%) | 0.036 | 0.045 | 0.25 | 263.1 | 224.19 | 15% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Q.; Yan, F.; Fan, Y.; Gong, M. Inhibition of Polycyclic Aromatic Hydrocarbons Formation During Supercritical Water Gasification of Sewage Sludge by H2O2 Combined with Catalyst. Water 2024, 16, 3235. https://doi.org/10.3390/w16223235
Xu Q, Yan F, Fan Y, Gong M. Inhibition of Polycyclic Aromatic Hydrocarbons Formation During Supercritical Water Gasification of Sewage Sludge by H2O2 Combined with Catalyst. Water. 2024; 16(22):3235. https://doi.org/10.3390/w16223235
Chicago/Turabian StyleXu, Qiao, Fenfen Yan, Yujie Fan, and Miao Gong. 2024. "Inhibition of Polycyclic Aromatic Hydrocarbons Formation During Supercritical Water Gasification of Sewage Sludge by H2O2 Combined with Catalyst" Water 16, no. 22: 3235. https://doi.org/10.3390/w16223235
APA StyleXu, Q., Yan, F., Fan, Y., & Gong, M. (2024). Inhibition of Polycyclic Aromatic Hydrocarbons Formation During Supercritical Water Gasification of Sewage Sludge by H2O2 Combined with Catalyst. Water, 16(22), 3235. https://doi.org/10.3390/w16223235






