Abstract
Phosphorus removal is critical for effective water treatment and the prevention of eutrophication. This study focuses on the modification of attapulgite, an economical clay material, with zirconium (Zr@ATP) to enhance its phosphorus adsorption capacity. Zr@ATP was comprehensively characterized, and its phosphorus-removal mechanisms were investigated. Additionally, its performance in water treatment was evaluated using a lake water-sediment system. Zr@ATP exhibited a high surface area of 329.29 m2/g. The static adsorption experiments revealed that Zr@ATP achieved a phosphorus-removal efficiency of 95.8% at an adsorbent dosage of 5 g/L. Kinetic studies indicated that the adsorption followed a pseudo-second-order model, with the primary mechanism being chemisorption via ion exchange. Application of Zr@ATP in a lake water-sediment system resulted in an 83.6% reduction in total phosphorus. The chlorophyll concentration significantly decreased from 32.33 μg/L to 8.56 μg/L, and the algal density decreased by 84.6%, effectively inhibiting algal growth. These results suggest that Zr@ATP is a promising adsorbent for sustainable phosphorus removal and eutrophication control in aquatic environments.
1. Introduction
Phosphorus is an essential nutrient for aquatic ecosystems and plays a vital role in biological growth [1]. However, excessive phosphorus from sources such agricultural runoff, industrial effluents, and wastewater discharge often leads to eutrophication, causing algal blooms, hypoxia, and disruption of aquatic life [2,3]. This process impacts water quality, limiting its usability for drinking, recreation, and industrial use [4,5]. Effective phosphorus removal is therefore crucial for preventing eutrophication, restoring ecosystem health, and achieving sustainable water management [6].
Various methods, including biological removal, wetlands, adsorption, and precipitation, are employed to reduce phosphorus concentrations in water [7,8]. Over the past decade, significant progress has been made in the development of phosphorus removal. Traditional methods, such as chemical precipitation using metal salts like alum and ferric chloride, remove phosphorus by forming insoluble metal-phosphate complexes [9,10]. While effective, these methods suffer from several drawbacks, including the production of sludge, secondary pollution, and the need for chemical dosing [11]. Moreover, biological phosphorus removal (BPR), which leverages specific microorganisms to accumulate phosphorus, presents operational challenges, such as the susceptibility of microbes to environmental fluctuations (e.g., such as temperature, pH, and dissolved oxygen levels) [12,13]. Recent advances have focused on developing adsorbent materials, such as biochar, activated alumina, and zeolites, for phosphorus capture [14]. These materials offer distinct advantages, including minimal secondary pollution and the possibility of phosphorus recovery [15]. However, many current adsorbents still face challenges such as low adsorption capacity, poor selectivity, and limited reusability [16]. Furthermore, the high costs associated with the synthesis and regeneration of some advanced adsorbents restrict their widespread application [17], particularly in large-scale water treatment systems.
In light of these challenges, recent research has shifted focus towards enhancing naturally occurring minerals with specific amendments to improve their adsorption capabilities [18,19]. Attapulgite [20] and Zr-based [21] materials are two such candidates that show great promise for phosphorus removal from aqueous solutions. Attapulgite, also known as palygorskite, is a naturally occurring fibrous clay mineral with a unique chain-like structure that imparts a large surface area, high porosity, and excellent adsorption properties [22]. These characteristics make it a promising material for environmental applications, including the removal of heavy metals and organic pollutants from water. In recent years, attapulgite has also gained attention for its potential to remove phosphorus from water bodies.
One of the primary advantages of attapulgite is its low cost and abundant availability, making it an economically viable option for large-scale water treatment [23]. Its high specific surface area allows for effective adsorption of phosphorus species, and its stability under various environmental conditions ensures durability. Moreover, attapulgite has a neutral pH range, making it suitable for application in diverse water bodies without significantly altering the pH of the water.
Despite these advantages, attapulgite alone has limitations in terms of phosphorus-removal efficiency. Its adsorption capacity for phosphorus is relatively low compared to other advanced materials [24], and it lacks selectivity for phosphate ions over competing anions such as sulfate and nitrate. These drawbacks limit its practical application for treating water with high phosphorus concentrations. Therefore, modifications to the attapulgite structure or surface are necessary to improve its adsorption performance.
Zr-based materials have emerged as highly effective adsorbents for phosphorus removal due to their strong affinity for phosphate ions. The high surface charge in aqueous environments allows Zr to form stable complexes with phosphate, leading to high phosphorus-removal efficiency even at low concentrations [25]. Zirconium oxide (ZrO2) and zirconium hydroxide (Zr (OH)4) are particularly noted for their ability to selectively adsorb phosphorus in the presence of other anions, making them suitable for treating complex water matrices. This selectivity is hypothesized to stem from the specific interaction between Zr’s positively charged sites and the lone pairs on phosphate ions, allowing for the formation of inner-sphere complexes that exclude competing anions. Moreover, Zr-based materials offer the advantage of reusability, as the adsorbed phosphorus can be recovered through simple desorption processes, allowing for the regeneration of the adsorbent. This makes Zr an attractive candidate for sustainable phosphorus removal and recovery systems. However, challenges remain in the large-scale application of Zr-based materials. The synthesis of Zr-based adsorbents can be costly, and their use in powder form poses difficulties in terms of material handling and recovery after water treatment. Additionally, while Zr materials demonstrate high selectivity and capacity for phosphorus, their adsorption kinetics can be slow, requiring long contact times for effective phosphorus removal. This limitation may reduce their efficiency in dynamic water treatment systems with high flow rates.
Herein, to overcome the individual limitations of attapulgite ((Mg, Al)2Si4O10(OH)·4(H2O)) and Zr-based materials, in this study, a zirconium-modified attapulgite composite (Zr@ATP) was fabricated via an impregnation method with zirconium oxychloride octahydrate (ZrOCl2·8H2O) and attapulgite. By combining the high surface area and stability of attapulgite with the strong phosphate-binding affinity of Zr, the composite offers a promising solution for efficient phosphorus removal. Finally, this Zr-modified attapulgite material was successfully applied in phosphorus removal in real water bodies.
2. Materials and Methods
2.1. Reagents and Materials
Attapulgite (industrial grade) was purchased from Jiangsu Xuyi Xinyuan Technology Co., Ltd. (Huai’an, China). Zirconium oxychloride octahydrate (analytical grade) was supplied by Aladdin Industrial Corporation. Anhydrous ethanol, potassium dihydrogen phosphate, ascorbic acid, sodium hydroxide, and potassium antimonyl tartrate (analytical grade) were acquired from Xilong Science Co., Ltd. (Shantou, China). Hydrochloric acid and sulfuric acid (analytical grade) were purchased from Lanxi Xuri Chemical Co., Ltd. (Jinhua, China). Potassium persulfate (analytical grade) was obtained from Merck KGaA (Darmstadt, Germany). Ammonium molybdate tetrahydrate (analytical grade) was purchased from Guangdong Xinghua Technology Co., Ltd. (Zhuhai, China).
2.2. Synthesis of Zr@ATP
Alkali Modification [26]: Equal amounts (25 g) of attapulgite and sodium hydroxide (NaOH) were mixed thoroughly and calcined in a muffle furnace at 500 °C for 2 h. After natural cooling, deionized water was added to the mixture, which was then stirred uniformly. The product was washed five times, followed by vacuum filtration and drying at 105 °C.
Acid Modification: A specific quantity (15 g) of the alkali-modified attapulgite was immersed in 75 mL of 3 mol/L hydrochloric acid (HCl) and subjected to shaking in a water bath at 30 °C for 2 h. The mixture was repeatedly centrifuged and washed until chloride ions (Cl−) were no longer detectable (approximately 10 times). The resulting material was dried at 105 °C and ground through a 100-mesh sieve for subsequent use, designated as the acid-modified attapulgite adsorbent.
Zirconium Modification: Zirconium oxychloride octahydrate (ZrOCl2·8H2O) was dissolved in water to prepare a 0.15 mol/L Zr solution. A quantity of 2.0 g of the acid and alkali-modified attapulgite was immersed in varying concentrations of the Zr solution, maintaining a solid-to-liquid ratio of 1:20 (weight of attapulgite, g, to volume of ZrOCl2 solution, mL). The mixture was stirred using a magnetic stirrer for 1 h and allowed to age for 24 h. After centrifugation and three washes, the product was dried and then calcined at 100 °C for 2 h. After natural cooling, the material was sieved through a 100-mesh screen to obtain the Zr@ATP adsorbent.
2.3. Characterization
Characterization of the unmodified attapulgite and Zr@ATP adsorbent materials was conducted using several techniques. Scanning Electron Microscopy (SEM) analysis was performed on an S-4800 instrument (Hitachi, Tokyo, Japan) to examine the surface morphology. The specific surface area and pore structure were determined through nitrogen adsorption-desorption measurements using a BELSORP-mini1 (Microtrac BEL, Osaka, Japan), employing the BET method for surface area calculation. Fourier Transform Infrared Spectroscopy (FTIR) was carried out on a Nicolet iS10 spectrometer (Thermo Scientific, Waltham, MA, USA) to identify functional groups within the materials, scanning in the range of 500–4000 cm−1. These characterization methods provided comprehensive insights into the physical and chemical properties of both the unmodified and modified materials.
2.4. Characterization of Zr@ATP Material for Adsorption of Phosphorus Removal
2.4.1. Adsorbent Dosage Experiment
To prepare the standard phosphate solution, first dry KH2PO4 at 110 °C for 2 h to remove any absorbed moisture. Then, prepare the standard phosphate solution by dissolving dried KH2PO4 in appropriate amounts of distilled water.
Zr@ATP adsorbents were prepared in varying amounts of 0.025 g, 0.04 g, 0.05 g, 0.1 g, 0.15 g, 0.2 g, and 0.25 g, and were added to 50 mL conical flasks containing a 10 mg/L phosphate solution. The flasks were placed in a shaking incubator and agitated at room temperature for 24 h, with a shaking amplitude of 50 mm and an agitation speed of 100 rpm. Following this, the supernatants were collected and centrifuged to separate any suspended particles. The concentration of phosphate ions in the solution was measured using spectrophotometric methods.
The adsorption capacity and efficiency of the Zr@ATP adsorbent for phosphate were calculated using the following equations:
Adsorption Capacity:
where:
C0—Initial concentration of phosphate in the solution, mg/L
Ct—Concentration of phosphate after adsorption, mg/L
V—Volume of the phosphate solution, L
m—Mass of the adsorbent, g
Adsorption Efficiency:
where:
C0—Initial concentration of phosphate, mg/L
Ct—Residual concentration of phosphate after adsorption, mg/L
2.4.2. Adsorption Isotherm Experiments
Phosphate solutions with initial concentrations of 0, 4, 8, 10, 15, and 20 mg/L were prepared. An amount of 0.05 g of the Zr@ATP adsorbent was added to 50 mL of each phosphate solution in conical flasks. These flasks were placed in a shaking incubator and agitated at room temperature for 24 h at an appropriate speed. Following the incubation period, the supernatant was carefully collected and centrifuged to eliminate any suspended particles. The concentration of phosphate ions in the solutions was then quantified using an ammonium molybdate spectrophotometric method.
Specifically, 2 mL of the supernatant was transferred into a 2 mL centrifuge tube and centrifuged at 4000 rpm for 5 min. Then, pipetted 1.5 mL of the supernatant into a 25 mL colorimetric tube and dilute it with deionized water up to the 10 mL mark, then added 2 mL of potassium persulfate for digestion. Allow the sample to cool naturally to room temperature, and then diluted it with deionized water up to the 25 mL mark. Next, added 0.5 mL of ascorbic acid solution to the colorimetric tube and mixed thoroughly. After 30 s, 1 mL of molybdate solution was added and the reaction was allowed to proceed for 15 min. Measured the absorbance of each solution at a wavelength of 700 nm.
The resulting experimental data were analyzed in accordance with the Langmuir, Freundlich and Redlich–Peterson adsorption isotherm models [10] to evaluate the adsorption characteristics of the adsorbent. The suitability of each model for the experimental data was determined by calculating the correlation coefficients (R2).
Langmuir adsorption isotherm model:
The Langmuir isotherm is an empirical model that assumes monolayer adsorption on a homogeneously active site surface, where each adsorption site has the same affinity for the adsorbate. The model posits that as adsorption progresses, the number of available sites decreases, leading to a gradual slowing of the adsorption rate until equilibrium is achieved. The Langmuir equation can be expressed as:
where:
qe represents the amount of adsorbate (mg/g) adsorbed per unit mass of adsorbent at equilibrium; qm indicates the maximum adsorbed amount in saturated conditions (mg/g); Ce denotes the concentration of the adsorbate in solution at equilibrium (mg/L); and KL is the Langmuir constant (L/mg), reflecting the adsorbent’s affinity for the adsorbate.
Freundlich adsorption isotherm model:
The Freundlich model is also an empirical model that characterizes the adsorption process on heterogeneous surfaces, suggesting that the adsorption occurs as a monolayer but without a uniform energy distribution across the adsorption sites. This model is more suitable for lower concentrations of adsorbate and does not provide an estimation of maximum adsorption capacity. The Freundlich equation can be represented as:
Converted to linear form, it is expressed as:
KF and n are Freundlich constants, where KF indicates the adsorption affinity and capacity of the adsorbent. A higher KF suggests a greater maximum adsorption capacity.
The value of n indicates the difficulty of adsorption, with larger values of n representing easier adsorption. Typically, an effective adsorbent will have an n value between 2 and 10.
Redlich–Peterson isotherm models:
The Redlich–Peterson isotherm is a hybrid adsorption model that combines features of both the Langmuir and Freundlich isotherms. It is frequently used to describe adsorption processes where the isotherm behavior may not conform purely to either Langmuir’s homogeneous single-layer or Freundlich’s heterogeneous multilayer assumptions. The model is versatile, providing a good fit over a wide range of adsorbate concentrations, especially when both monolayer and heterogeneous surface characteristics are present in the adsorption process.
The Redlich–Peterson model equation is typically expressed as:
where:
qe is the amount of adsorbate adsorbed per unit mass of adsorbent at equilibrium; Ce is the equilibrium concentration of the adsorbate in solution; KR and aR are constants specific to the isotherm; and g is an exponent that varies between 0 and 1.
This comprehensive analysis allows for a better understanding of the phosphate adsorption characteristics of the Zr@ATP material.
2.4.3. Adsorption Kinetics Experiments
In this study, 0.05 g of both modified and unmodified attapulgite were precisely weighed and added to conical flasks containing 50 mL of a phosphate solution at an initial concentration of 10 mg/L. The flasks were then placed in a shaking incubator and agitated at a suitable speed. Samples of the supernatant were collected at specified time intervals: 5 min, 10 min, 20 min, 30 min, 1 h, 2 h, 4 h, and 24 h.
For each sampling, 2 mL of the supernatant was pipetted into a 2 mL centrifuge tube and centrifuged at 4000 rpm for 5 min. Subsequently, 1.5 mL of the supernatant was transferred into a 25 mL colorimetric tube and diluted with deionized water up to the 10 mL mark. Potassium persulfate was then added for digestion. After naturally cooling to room temperature, the solution was topped up to 25 mL with deionized water. To this, 0.5 mL of ascorbic acid solution was added and mixed thoroughly, followed by the addition of 1 mL of molybdate solution after 30 s. The reaction was allowed to proceed for 15 min, and the absorbance of each solution was measured using an ammonium molybdate spectrophotometric method.
To further investigate the adsorption kinetics of the Zr@ATP material, the experimental results were fitted using Pseudo-First-Order, Pseudo-Second-Order, Weber–Morris (W–M) internal diffusion, and Elovich kinetic models.
Pseudo-First-Order Kinetic Equation:
Pseudo-Second-Order Kinetic Equation:
Weber–Morris (W–M) Internal Diffusion Equation:
Elovich kinetic Equation:
In these equations, qe represents the equilibrium adsorption capacity (mg/g), qt is the adsorption capacity at time t (mg/g), t is the contact time (min), k1 is the pseudo-first-order rate constant (min−1), and k2 is the pseudo-second-order rate constant [g·(mg·min)−1]. This kinetic analysis allows for a deeper understanding of the adsorption dynamics of the modified attapulgite in removing phosphate from water.
2.4.4. Solution pH Experiments
In this study, six aliquots of 50 mL phosphate solutions at an initial concentration of 10 mg/L were prepared. The pH of each solution was adjusted to values of 4, 5, 6, 7, 8, and 9 using 0.1 mol/L hydrochloric acid (HCl) and 0.1 mol/L potassium hydroxide (KOH). Subsequently, 0.05 g of Zr@ATP was added to each flask. The flasks were then placed in a shaking incubator and agitated at an appropriate speed for 24 h at room temperature. After the incubation period, samples were taken and centrifuged to separate the solids. The remaining total phosphorus content in the supernatant was determined using spectrophotometric analysis. This experiment aimed to evaluate the effectiveness of Zr@ATP in removing phosphate under varying pH conditions.
2.5. Phosphorus Removal in Actual Lake Water
To evaluate the phosphorus-removal performance of Zr@ATP in a lake environment, 800 mL of lake water, both with and without sediment, was used to simulate natural conditions. The sediment and water samples were collected from Xingyu Lake in Fuzhou, China. Zr@ATP was added in varying quantities to each sample. Over a 25-day period, the concentrations of total phosphorus, chlorophyll, and algae in the water were monitored and analyzed to assess the material’s effectiveness in reducing phosphorus levels and its impact on lake ecosystem indicators.
3. Results and Discussion
3.1. Characterization of the Zr@ATP Material
The morphological characteristics and surface features of attapulgite and Zr@ATP were compared through SEM analysis to determine the effects of modification. The attapulgite microstructure, characterized by tightly packed rod-like crystals and distinct bundles, exhibited a clear and well-defined fiber morphology (Figure 1A). In contrast, Zr@ATP displayed a more dispersed, sheet-like structure (Figure 1B) following modification, which involved calcination at 500 °C and subsequent acid washing. This treatment effectively increased the specific surface area by removing impurities, thus enhancing its adsorption capacity for phosphorus. The observed granular particles on the Zr@ATP surface likely represent zirconium oxide deposits, indicating successful Zr incorporation and modification of the attapulgite material. Compared to the unmodified attapulgite, Zr@ATP exhibits superior structural and surface characteristics conducive to improved adsorption performance. The surface area and pore structure of the attapulgite and Zr@ATP were analyzed using nitrogen adsorption-desorption isotherms, as shown in Figure 1C,D. The adsorption and desorption curves of both attapulgite and Zr@ATP did not fully overlap, indicating the presence of mesoporous structures. In Figure 1D, the mesopore structure remained intact with distinct H4-type hysteresis loops, indicating the formation of slit-like pores and enhanced porosity of Zr@ATP. Natural clay materials like bentonite and montmorillonite generally have lower surface areas, approximately 30–150 m2/g. [27] The BET surface area calculations reveal that the specific surface area of attapulgite was 123.75 m2/g while the surface area of Zr@ATP increased significantly to 329.29 m2/g. This increase in surface area suggests that the Zr modification process successfully removed impurities and improved pore accessibility, thus enhancing the material’s potential for phosphate adsorption from aqueous environments.
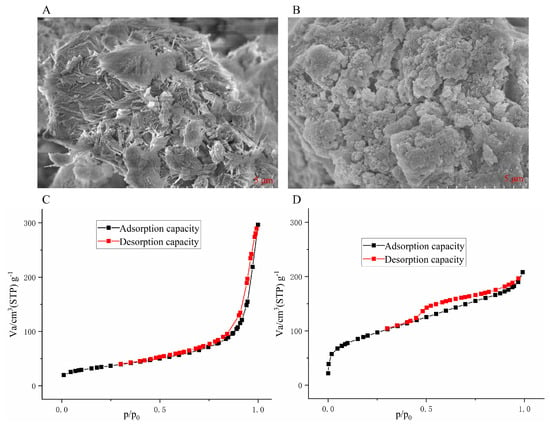
Figure 1.
Characterization of materials (A) SEM of ATP, (B) SEM of Zr@ATP, (C) ATP adsorption-desorption isotherm curve, (D) Zr@ATP adsorption-desorption isotherm curve.
To further characterize the material preparation, we conducted FT-IR and XRD analyses. The FT-IR results are shown in Figure S1a. In ATP, the major forms of water include crystallized water, zeolite water, surface-adsorbed water, and structural water, with decreasing stability. The absorption peak around 1077.09 cm−1 corresponds to the bending vibration of hydroxyl groups (crystallized water). Peaks related to adsorbed water and zeolite water appear around 163.86 cm−1. The absorption peak at 3423.17 cm−1 is caused by the stretching vibration of hydroxyl groups in the internal adsorbed water of attapulgite (structural water). The peak at 475 cm−1 corresponds to the symmetric stretching vibration of Si-O-Si bonds. Compared to ATP, Zr@ATP shows a blue shift (456.10 cm−1) in the vibration peak at 475 cm−1 and a new absorption peak at 796.01 cm−1, indicating the incorporation of zirconium into ATP. Moreover, the absorption peaks at 1077.09 cm−1 and 3423.17 cm−1 become narrower, suggesting that after modification, crystallized water and structural water are reduced, which helps enhance surface adsorption capability and improves the absorption of phosphate ions. It indicated that Zr has been successfully loaded on Zr@ATP. The XRD results (Figure S1b) showed that ATP diffraction peaks are typically observed at 2θ = 20.84°, 30.99°, 35.35°, and 41.15°, while the strong diffraction peak at 26.64° represents quartz in the (011) plane. The characteristic peak of ZrO2 appears at 28.2°, demonstrating that Zr has been effectively loaded onto ATP in the form of ZrO2. Additionally, the Zr@ATP materials exhibited a weakening of the peak intensity at the characteristic peaks compared to ATP. This phenomenon might be attributed to the fact that Zr element was not only distributed on the surface of ATP but also partially entered the pores of the ATP, increasing the interlayer spacing of the material and forming a bilayer structure with a larger surface area. This is consistent with the surface morphology observed in the SEM images and the physical property parameters (specific surface area) of the modified ATP, confirming the successful synthesis of Zr@ATP.
3.2. Adsorption of the Phosphorus Removal Performance
3.2.1. Effect of Zr@ATP Dosage on Phosphorus Removal
In practical applications, the concentration of adsorbent in the solution is a key factor influencing the effectiveness of phosphorus removal. To determine the optimal dosage of the Zr@ATP adsorbent, this study investigated its phosphorus-removal capacity at dosages ranging from 0.50 to 5.00 g/L in 50 mL of a 10 mg/L phosphate solution. The results are shown in Figure 2A. Generally, an increase in the Zr@ATP dosage significantly improved the phosphorus-removal efficiency, yet the adsorption capacity decreased from 8.57 mg/g to 2.10 mg/g as the dosage increased. This is primarily due to the heightened adsorbent dosage resulting in an increase in adsorption sites, while the phosphorus concentration in the solution remains constant, leading to a reduction in phosphorus adsorption per unit mass of Zr@ATP. Specifically, the adsorption efficiency improved sharply from 10% to 90% when the adsorbent dosage was increased to 0.50–2.00 g/L. At a dosage of 5.00 g/L, the phosphorus-removal efficiency reached 95.80%. This dosage is much lower than that of previous report (adsorbing material 7.50 g/L, phosphate removal rate 90%). To balance cost and efficiency, and to avoid measurement errors in the spectrophotometric analysis caused by overly low phosphate concentrations (detection limit: 0.01 mg/L) in the solution after adsorption, an adsorbent dosage of 1.00 g/L was selected for subsequent experiments. The optimal dosage determined in the experiment should be considered a reference point and may require adjustments depending on the specific composition and concentration of phosphorus in the water during applications.
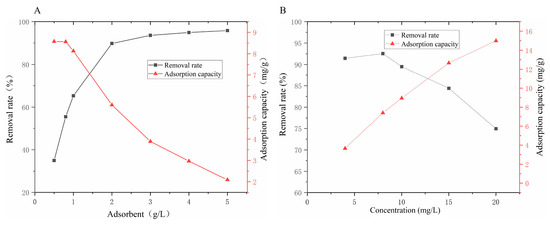
Figure 2.
(A) Effects of different initial adsorbent dosage on phosphorus-removal effect of Zr@ATP, (B) Effects of different initial phosphate concentration on phosphorus-removal effect of Zr@ATP.
3.2.2. Adsorption Isotherms
Isothermal adsorption experiments were conducted to elucidate the phosphate adsorption mechanism of Zr@ATP. Phosphate solutions at concentrations ranging from 4.00 mg/L to 20.00 mg/L were used. The results, shown in Figure 2B, indicated that as phosphate concentration increased, the adsorption capacity of Zr@ATP rose, while the adsorption efficiency declined. The optimal performance was observed at 15.00 mg/L, with an adsorption capacity of 12.66 mg/g and an efficiency of 84.4%.
To model the equilibrium relationship between the adsorbent and phosphate, both the Langmuir, Freundlich and Redlich–Peterson isotherms were applied (Figure S2a–c). The Langmuir model provided a superior fit with an R2 of 0.991 compared to 0.892 for the Freundlich model and 0.905 for Redlich–Peterson, indicating that the adsorption follows a monolayer, chemical adsorption process (Table S1). In the Redlich–Peterson isotherms model, g value close to 1 also suggested that the adsorption behavior was approaching that of a Langmuir isotherm. The maximum adsorption capacity calculated from the Langmuir model was 18.45 mg/g, reinforcing the notion that Zr@ATP exhibits a strong affinity for phosphate through chemical adsorption.
3.2.3. Adsorption Kinetics
In Figure 3, the adsorption kinetics curves for phosphate removal by attapulgite and Zr@ATP were compared. The results clearly demonstrated that Zr@ATP exhibited significantly higher phosphate-removal efficiency at all time points compared to the unmodified attapulgite, confirming the improvement in adsorption performance after Zr modification. The phosphate adsorption by Zr@ATP could be divided into two distinct phases: a rapid initial stage where 80% of the equilibrium adsorption capacity was achieved within 4 h, followed by a slower phase where adsorption rates gradually increased and reached equilibrium at approximately 6 h. After 24 h of adsorption, the removal rate reached 94.4%.
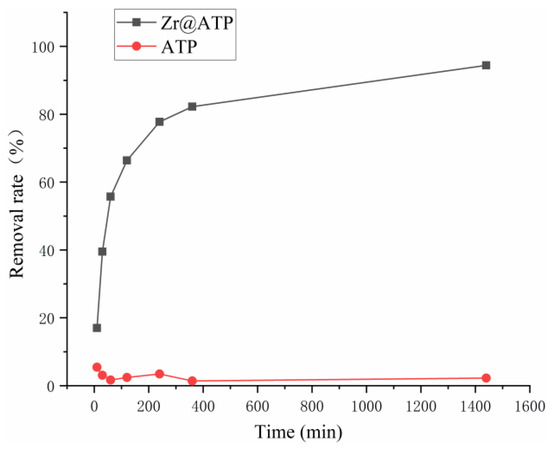
Figure 3.
Adsorption kinetics curves on phosphorus removal of Zr@ATP.
To further investigate the adsorption kinetics, the experimental data in Figure 3 were fitted with kinetic models, as shown in Figure S2. Model parameters are provided in Table S2. The Pseudo-First-Order kinetic model yielded a correlation coefficient of 0.910, and the correlation coefficient of Elovich kinetic models was 0.971, while the Pseudo-Second-Order kinetic model exhibited a much higher correlation coefficient of R2 0.998. Using the Pseudo-First-Order and Pseudo-Second-Order models, the calculated qe (qe,cal) values obtained were 6.60 mg/g and 9.26 mg/g, respectively, with percentage deviations from the experimental value (qe,exp, 9.04 mg/mg) of 27% and 2.4%. This indicates that the Pseudo-Second-Order kinetic model better described the adsorption process, suggesting that the adsorption mechanism of Zr@ATP was primarily governed by chemisorption, predominantly through ion exchange. The involvement of ion exchange between Zr-based complexes played a significant role in enhancing the phosphate-adsorption capacity of the modified attapulgite. And, compared with other reported adsorbent materials (Table S3), this Zr@ATP also demonstrates superior phosphorus-adsorption efficiency [26,28,29,30,31,32].
The adsorption process typically occurs in some stages: (1) external surface adsorption (Instantaneous Stage): initially, phosphate molecules rapidly adsorb onto the external surface of the adsorbent, driven primarily by the concentration gradient. This stage is rapid and occurs within the first 2 h in this study (as shown in Figure S2c). (2) Gradual adsorption (Intra-particle Diffusion Stage): following the initial phase, the adsorption rate slows due to intra-particle diffusion limitations. In this study, the phase, extending from 2 to 24 h, involves the gradual migration of phosphate into the adsorbent’s inner pores. (3) Equilibrium Stage: after 24 h, the adsorption sites on the adsorbent surface reach saturation, leading to equilibrium, and intra-particle diffusion ceases, indicating no further significant adsorption. In this study, the Weber–Morris adsorption curve for phosphorus on Zr@ATP, shown in Figure S3c, reveals a multi-linear qt versus t0.5 relationship, indicating that both intra-particle diffusion and film diffusion jointly govern the adsorption rate. In the initial stage, the adsorption line closely approaches the origin, suggesting a rapid adsorption rate dominated by intra-particle diffusion as the rate-limiting step. In the second stage, however, the line segment does not pass through the origin and shows a higher intercept, implying that film diffusion becomes the rate-limiting step for phosphorus adsorption by Zr@ATP during this period. The rapid phosphate uptake within the first 2 h indicates high removal efficiency with shorter contact times, particularly for applications where exposure duration is limited to less than 2 h.
3.2.4. pH Effect
The pH of the solution plays a vital role in influencing the speciation of phosphate ions in water, which in turn affects the adsorption efficiency of materials used for phosphorus removal. Depending on the pH, phosphorus (P) exists in various ionic forms, such as H3PO4, H2PO4−, HPO42−, and PO43−. At a pH range of 4–6, the dominant form is H2PO4−; as the pH increases above 7, H2PO4− transitions to HPO42−, and beyond pH 12, it finally converts to PO43−.
Given this pH-dependent ion speciation, the pH of the solution becomes a critical factor in determining the adsorption capacity of the material. Figure 4 illustrates the adsorption efficiency of Zr@ATP across a pH range of 4 to 9. At an initial phosphate concentration of 10.00 mg/L, the adsorption efficiency of Zr@ATP peaked at 92.70% at pH 5 but declined as the pH increased beyond this point. Overall, the Zr@ATP exhibited superior adsorption performance in acidic conditions compared to alkaline environments.
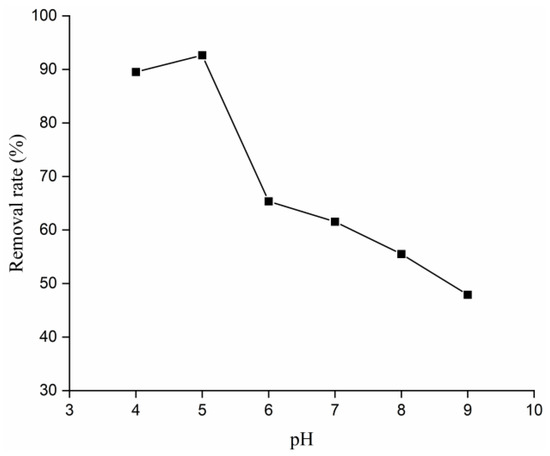
Figure 4.
Effects of different loading pH on phosphorus-removal effect of Zr@ATP.
As shown in Figure 3 and in Supplementary Materials Figure S3, the adsorption mechanism of Zr@ATP is primarily driven by ion exchange, which is strongly influenced by the pH of the solution. This mechanism can be explained by the interaction between phosphate ions and hydrated zirconium oxide. The corresponding chemical reactions indicate that acidic conditions favor the adsorption process. Specifically, at pH 5, phosphate ions predominantly exist as H2PO4−, which readily reacts with the hydrated zirconium oxide, resulting in enhanced phosphate-removal efficiency. In contrast, as the pH increases, the equilibrium shifts to the left, which hinders the adsorption process and results in a decrease in adsorption capacity. Hence, acidic conditions are more favorable for effective phosphate removal by Zr@ATP.
In practical water treatment processes, it is advisable to avoid treating highly alkaline water directly. The alkalinity of the wastewater can be adjusted via pH modification prior to treatment, which ensures the effective removal of phosphorus.
3.3. Application in Phosphorus Removal in Actual Lake Water
The tests were further conducted to evaluate the performance of Zr@ATP in removing phosphorus as well as reducing chlorophyll-a and algae concentrations, simulating conditions for practical application.
3.3.1. Phosphorus Removal in Sediment-Free Water
The phosphorus-removal efficiency of Zr@ATP in sediment-free lake water was evaluated using three groups: 0.00 g (control), 1.50 g (Zr@ATP-1.5), and 3.00 g (Zr@ATP-3.0) of Zr@ATP in 800 mL lake water, incubated at 25 °C for 25 days. As shown in Figure 5, total phosphorus concentrations decreased in all groups during the first 5 days. Besides, Zr@ATP-1.5 and Zr@ATP-3.0 exhibited a more significant reduction in phosphorus compared to the control group. Post day 5, a divergence in trends occurred. In the control group, total phosphorus concentration on the fifth day reached its lowest point at 0.25 mg/L, then the phosphorus levels gradually rose due to the decomposition of dead algae, which released phosphorus back into the water. Conversely, in the Zr@ATP-1.5 and Zr@ATP-3.0 groups, phosphorus concentrations continued to decline and stabilized after 15 days. This demonstrated that Zr@ATP effectively removed phosphorus from the lake water and prevented its re-release, highlighting its potential as a reliable adsorbent for phosphorus removal in aquatic systems.
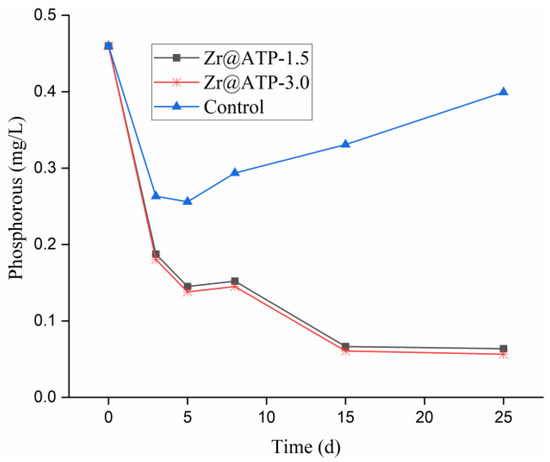
Figure 5.
Adsorption of phosphorus by Zr@ATP in sediment-free lake water.
As displayed in Figure 6A,B, the total phosphorus concentrations in the Zr@ATP-1.5 and Zr@ATP-3.0 groups exhibited a rapid reduction during the initial stages of the experiment, and the phosphorus-removal rates respectively reached 59.23% and 60.77% on the third day. The removal efficiency continued to increase steadily over time, eventually stabilizing after 15 days. On day 15, both groups reached a maximum phosphorus-removal efficiency of 86.15%, reducing the TP concentration to 0.0637 mg/L, meeting the Class IV standard for total phosphorus in lakes and reservoirs according to the environmental quality standards for surface water. Given the comparable phosphorus-removal performance between the two groups, the dosage of 1.5 g Zr@ATP is recommended for practical applications targeting phosphorus removal in overlying water-sediment systems.
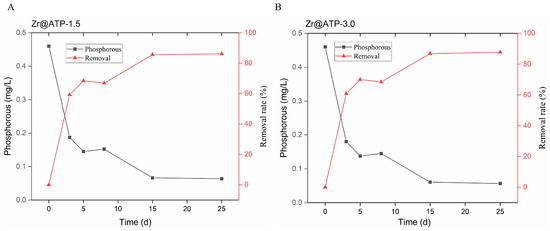
Figure 6.
Effects of different initial adsorbent dosage on phosphorus-removal effect of Zr@ATP in sediment-free lake water. (A) The dosage of Zr@ATP was 1.5 g. (B) The dosage of Zr@ATP was 3.0 g.
In addition, the concentrations of chlorophyll A and algal density were monitored over time in three experimental groups. As displayed in Figure 7A,B, both chlorophyll A concentration and algal density exhibited similar trends. During the initial 3 days, algal density declined rapidly across all groups, which corresponded with the decrease in total phosphorus levels observed in Figure 5. The Zr@ATP-treated groups demonstrated a more pronounced reduction in algal density compared to the control group, suggesting that Zr@ATP effectively adsorbed phosphorus from the water. This phosphorus depletion, a critical nutrient for algal growth, led to accelerated algal mortality. After 5 days, the control group continued to experience a decline in algal density due to sustained phosphorus deficiency, while the Zr@ATP-treated groups, having already significantly reduced the algal population, showed minimal further change. These findings highlight the effectiveness of Zr@ATP in removing phosphorus from water and thereby mitigating algal blooms by restricting the essential nutrients required for algal proliferation.
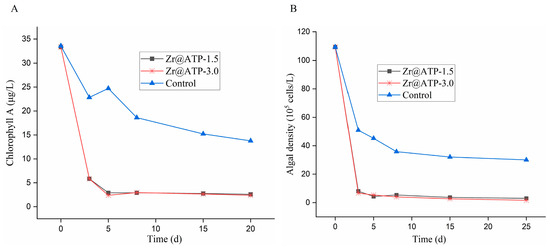
Figure 7.
The concentrations of (A) chlorophyll A and (B) algal density by Zr@ATP in sediment-free lake water.
3.3.2. Phosphorus Removal in Overlying Water-Sediment
Due to the significant influence of sediment on phosphorus levels in the overlying water, an overlying water-sediment system was employed to evaluate the effectiveness of Zr@ATP in phosphorus removal. In this test, 1 cm of river sediment (100 g) was added to the bottom of the reactor to simulate lake conditions, and 1.50 g of Zr@ATP was uniformly distributed on the sediment surface. Subsequently, 800 mL of lake water or deionized water was added, and the system was incubated at 25 °C with a 12:12 light/dark cycle for 25 days.
As shown in Figure 8A, the total phosphorus concentration of control group of lake water-sediment exhibited a slight decline during the first 5 days, followed by a minor increase, resulting in relatively minimal overall variation. Nevertheless, the phosphorus concentration of the Zr@ATP treatment group decreased to 0.082 mg/L, and the phosphorus-removal efficiency of was up to 83.6% (Figure 8B), confirming that Zr@ATP efficiently removed phosphorus from the overlying water.
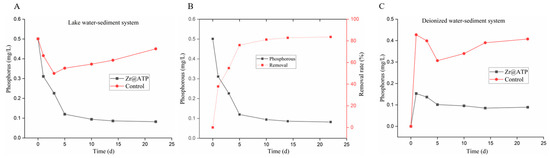
Figure 8.
Adsorption of phosphorus by Zr@ATP in overlying water-sediment. (A) The total phosphorus concentration. (B) Phosphorus-removal effect. (C) Phosphorus-removal effect of Zr@ATP in the deionized water-sediment system.
To further investigate the impact of sediment on the phosphorus-removal capacity of Zr@ATP, a deionized water-sediment system was employed. As depicted in Figure 8C, the total phosphorus concentration in the overlying water initially exhibited a rapid increase on the first day due to phosphorus release from the sediment. But, the phosphorus concentration of the Zr@ATP treatment group was significantly lower than that control group, and continued to decrease, reaching equilibrium by day 15.
Additionally, the concentrations of chlorophyll A and algal density were monitored over time. As illustrated in Figure 9A,B, the control group exhibited a decrease in chlorophyll A concentration from 32.12 μg/L to 20.23 μg/L, while the algal density declined from 99.98 × 105 cells/L to 45.61 × 105 cells/L. In contrast, in the experimental group treated with Zr@ATP, the chlorophyll A concentration significantly decreased from 32.33 μg/L to 8.56 μg/L, and the algal density decreased by 84.6% (dropped from 99.98 × 105 cells/L to 15.37 × 105 cells/L). This indicates that the addition of Zr@ATP effectively reduced both chlorophyll A concentration and algal density by phosphorus removal.
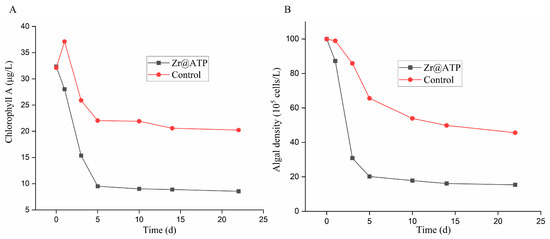
Figure 9.
The concentrations of (A) chlorophyll A and (B) algal density by Zr@ATP in overlying water-sediment.
These findings further demonstrate the effectiveness of Zr@ATP in removing phosphorus in the overlying water-sediment system, indicating its promising potential for practical applications in aquatic environments.
4. Conclusions
In this study, an innovative Zr@ATP composite material was successfully synthesized, incorporating Zr into the attapulgite matrix. The obtained Zr@ATP composite benefits from the enhanced adsorption capacity of Zr while maintaining the structural integrity and low cost of the attapulgite framework. The modification process typically involves the impregnation of Zr onto the surface of attapulgite, creating active sites for phosphate adsorption without compromising the mechanical strength of the material. Studies have shown that Zr@ATP improved the efficiency of phosphate removal, achieving a total phosphorus-removal rate of up to 83.6%. Moreover, the composite successfully reduced algal proliferation from by limiting phosphorus availability in the water. The algal density dropped from 99.98 × 105 cells/L to 15.37 × 105 cells/L, and the chlorophyll concentration significantly decreased from 32.33 μg/L to 8.56 μg/L. These results indicate Zr@ATP’s potential for large-scale application in eutrophic water treatment, particularly in waterbodies where phosphorus control is crucial for preventing algal blooms. To advance Zr@ATP toward widespread application, its long-term stability and regeneration potential under field conditions would be evaluated in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16223233/s1, Figure S1: (A) XRD patterns of ATP and Zr@ATP; (B) FTIR of ATP and Zr@ATP; Figure S2: Results of adsorption isotherm fitting. (A) Langmuir isotherm model fitting results; (B) Freundlich isotherm model fitting result, (C) Redlich-Peterson isotherm model. Figure S3: Adsorption kinetics fitting (A) Pseudo-first-order kinetic model, (B) Pseudo-second-order kinetic model, (C) W-M internal diffusion model, (D) Elovich model. Table S1: Equilibrium isotherm model parameters obtained from model fitting to experimental data; Table S2: Kinetic model parameters obtained from model fitting to experimental data; Table S3: Comparison of phosphate adsorption capacity of magnetic iron oxide nanoparticles reported in the literature.
Author Contributions
Conceptualization, W.S.; Methodology, W.W.; Software, W.W.; Validation, W.W.; Formal analysis, W.S.; Investigation, C.-Y.L. and W.W.; Data curation, C.-Y.L.; Writing—original draft preparation, C.-Y.L.; Writing—review and editing, W.S.; Visualization, W.W.; Supervision, W.S.; Project administration, W.S.; Funding acquisition, C.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Natural Science Foundation of Fujian Province, China (Grant No. 2021J011026).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
Author Wenliang Wu was employed by the company Zhejiang jitai New Materials. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, X.; Wu, L.; Liu, F.; Luo, P.; Zhuang, X.L.; Wu, J.S.; Zhu, Z.K.; Xu, S.J.; Xie, G.X. Performance and mechanisms of thermally treated bentonite for enhanced phosphate removal from wastewater. Environ. Sci. Pollut. Res. 2018, 25, 15980–15989. [Google Scholar] [CrossRef] [PubMed]
- Lucena-Silva, D.; Molozzi, J.; Severiano, J.D.; Becker, V.; Barbosa, J.E.D. Removal efficiency of phosphorus, cyanobacteria and cyanotoxins by the “flock & sink” mitigation technique in semi-arid eutrophic waters. Water Res. 2019, 159, 262–273. [Google Scholar] [PubMed]
- Deng, L.C.; Zhang, Z.Y.; Wang, J.F.; Cui, J.; Zhang, Y.F. Phosphorus removal by Magnesium-based Cementitious Material: Performance and mechanisms. J. Environ. Chem. Eng. 2024, 12, 112172. [Google Scholar] [CrossRef]
- Gao, D.G.; Ji, H.D.; Li, R.L.; Munir, M.T.; Wu, X.F.; Huang, Y.F.; Li, B. Advancing sustainable phosphorus removal and recovery with Metal-Organic frameworks (MOFs). Chem. Eng. J. 2023, 475, 145949. [Google Scholar] [CrossRef]
- Gao, M.C.; Sun, S.F.; Qiu, Q.; Zhou, W.W.; Qiu, L.P. Enrichment denitrifying phosphorus-accumulating organisms in alternating anoxic-anaerobic/aerobic biofilter for advanced nitrogen and phosphorus removal from municipal wastewater. J. Water Process Eng. 2023, 55, 104089. [Google Scholar] [CrossRef]
- Ghosh, S.; Lobanov, S.; Lo, V.K. An overview of technologies to recover phosphorus as struvite from wastewater: Advantages and shortcomings. Environ. Sci. Pollut. Res. 2019, 26, 19063–19077. [Google Scholar] [CrossRef]
- Gu, K.Y.; Yang, X.W.; Yan, X.; He, C.G.; Mao, W.C.; Xiao, F.K.; Wei, X.M.; Fu, X.X.; Jiang, Y.L. Effectiveness of a novel composite filler to enhance phosphorus removal in constructed wetlands. Environ. Sci. Pollut. Res. 2024, 31, 17124–17139. [Google Scholar] [CrossRef]
- Gubernat, S.; Maslon, A.; Czarnota, J.; Koszelnik, P. Phosphorus removal from wastewater using marl and travertine and their thermal modifications. Desalin. Water Treat. 2022, 275, 35–46. [Google Scholar] [CrossRef]
- Li, J.W.; Yu, W.Q.; Lin, Y.H.; Li, J.C.; Ban, B.Y.; Chen, J.; Guo, F.L.; Shi, C.W.; Tang, W.M. Mechanism of phosphorus removal from Si-Al melt by hydrogen. Int. J. Hydrogen Energy 2024, 71, 683–690. [Google Scholar] [CrossRef]
- Li, S.J.; Jiang, F.; Lei, T.; Ren, Z.X.; Wang, S.X.; Yang, X.J. Phosphorus removal by in situ sprayed ferric chloride in Dianchi Lake: Efficiency, stability, and mechanism. Process Saf. Environ. Prot. 2019, 131, 320–328. [Google Scholar] [CrossRef]
- Liao, Y.W.; Chen, S.; Zheng, Q.; Huang, B.Y.; Zhang, J.; Fu, H.Q.; Gao, H.J. Removal and recovery of phosphorus from solution by bifunctional biochar. Inorg. Chem. Commun. 2022, 139, 109341. [Google Scholar] [CrossRef]
- Liu, W.L.; Zhang, L.J.; Zhang, J.B.; Liu, X.; Huang, W.; Huang, D.Y.; Zheng, Z. Effects of modified sediments from a eutrophic lake in removing phosphorus and inhibiting phosphatase activity. Environ. Sci. Pollut. Res. 2019, 26, 1723–1732. [Google Scholar] [CrossRef]
- Liu, X.N.; Shen, F.; Qi, X.H. Adsorption recovery of phosphate from aqueous solution by CaO-biochar composites prepared from eggshell and rice straw. Sci. Total Environ. 2019, 666, 694–702. [Google Scholar] [CrossRef]
- Liu, Y.; Su, J.J. Experimental Study on Enhanced Phosphorus Removal Using Zirconium Oxychloride Octahydrate-Modified Efficient Phosphorus Removal Composite. Appl. Sci. 2023, 13, 12578. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, L.F. Experimental investigation of lanthanum-modified reinforced composite material for phosphorus removal. Appl. Sci. 2024, 14, 135. [Google Scholar] [CrossRef]
- Quang, M.N.; Rogers, T.; Hofman, J.; Lanham, A.B. New framework for automated article selection applied to a literature review of Enhanced Biological Phosphorus Removal. PLoS ONE 2019, 14, e0216126. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Y.; Fu, X.R.; Zhou, Z.K.; Zhang, W.; Qian, D.; Zeng, G.L.; Lyu, S.G. An innovative material for simultaneous removal of phosphorus and ammonia nitrogen in river water: Preparation and application. Water Air Soil Pollut. 2022, 233, 353. [Google Scholar] [CrossRef]
- Xu, Q.L.; Wang, L.; Tan, M.X.; Wang, X.L.; Li, J.J.; Geng, H.J. Phosphorus removal from aqueous solution by adsorption using wetland-based biochar: Batch experiment. Green Process. Synth. 2022, 11, 555–562. [Google Scholar] [CrossRef]
- Yu, B.; Li, X.N.; Yan, H.; Zhang, M.; Ma, J.; Lian, K. Recycling of sludge residue as a coagulant for phosphorus removal from aqueous solutions. Environ. Monit. Assess. 2024, 196, 576. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, B.; Wang, Z.; Gan, Y.H.; Chen, Z.H.; Zhang, Z.H.; Jiang, K.X.; Han, Z.L.; Zhang, K.G.; Yang, W.Z. Efficient phosphorus removal from ultra-low concentration wastewater by flow-electrode capacitive deionization. Sep. Purif. Technol. 2024, 341, 126973. [Google Scholar] [CrossRef]
- Zhang, T.T.; Wu, D.; Wang, Z.L.; Song, G.S.; Fan, G.Z.; Cai, X.Y.; Zhao, Y.L. Highly stable removal of low concentration phosphorus by mechanically activated FeCO3: In situ synergistic mechanisms. Sep. Purif. Technol. 2024, 344, 127291. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Hu, Z.Q.; Xiao, Q.Q.; Wu, Y. Capturing and recovering phosphorus in water via composite material: Research progress, future directions, and challenges. Sep. Purif. Technol. 2025, 353, 128453. [Google Scholar] [CrossRef]
- Zhi, Y.; Paterson, A.R.; Call, D.F.; Jones, J.L.; Hesterberg, D.; Duckworth, O.W.; Poitras, E.P.; Knappe, D.R.U. Mechanisms of orthophosphate removal from water by lanthanum carbonate and other lanthanum-containing materials. Sci. Total Environ. 2022, 820, 153153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.J.; Xu, Q.L.; Wu, Z.J.; Fang, X.T.; Zhong, Q.L.; Yang, J.L.; Yan, J.; Li, Q.G. Preparation and characterization of clay-oyster shell composite adsorption material and its application in phosphorus removal from wastewater. Sustain. Chem. Pharm. 2023, 32, 101023. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Yuan, R.F.; Wang, S.N.; Chen, H.L.; Zhou, B.H.; Cui, Z.X.; Zhang, C.Y. Iron-based materials for nitrogen and phosphorus removal from wastewater: A review. J. Water Process Eng. 2024, 59, 104952. [Google Scholar] [CrossRef]
- Deng, C.; Xue, J.; Wu, Y. Using magnetite/zirconium-comodified attapulgite as a novel phosphorus (P) sorbent for the efficient removal of P and the adsorption mechanism allowing this effect. Appl. Water Sci. 2023, 13, 12. [Google Scholar] [CrossRef]
- Parolo, M.E.; Pettinari, G.R.; Musso, T.B.; Sánchez-Izquierdo, M.P.; Fernández, L.G. Characterization of organo-modified bentonite sorbents: The effect of modification conditions on adsorption performance. Appl. Surf. Sci. 2014, 320, 356–363. [Google Scholar] [CrossRef]
- Yoon, S.-Y.; Lee, C.-G.; Park, J.-A.; Kim, J.-H.; Kim, S.-B.; Lee, S.-H.; Choi, J.-W. Kinetic, equilibrium and thermodynamic studies for phosphate adsorption to magnetic iron oxide nanoparticles. Chem. Eng. J. 2014, 236, 341–347. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X. Kinetics and Thermodynamics of Efficient Phosphorus Removal by a Composite Fiber. Appl. Sci. 2019, 9, 2220. [Google Scholar] [CrossRef]
- Long, F.; Gong, J.-L.; Zeng, G.-M.; Chen, L.; Wang, X.-Y.; Deng, J.-H.; Niu, Q.-Y.; Zhang, H.-Y.; Zhang, X.-R. Removal of phosphate from aqueous solution by magnetic Fe-Zr binary oxide. Chem. Eng. J. 2011, 171, 448–455. [Google Scholar] [CrossRef]
- Kuroki, V.; Bosco, G.E.; Fadini, P.S.; Mozeto, A.A.; Cestari, A.R.; Carvalho, W.A. Use of a La(III)-modified bentonite for effective phosphate removal from aqueous media. J. Hazard. Mater. 2014, 274, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Min, Y.; Zhao, X.; Shi, P.; Lu, H. Preparation of Fe-Modified Diatomite-Based Ceramsite for Efficient Phosphate Adsorption: Utilizing Diatomite’s Distinctive Porous Structure and Surface Silanol Groups. Water 2024, 16, 2218. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).