Abstract
Glycyrrhiza uralensis is an important plant in desert ecology, where low rainfall and water scarcity limit its growth. In order to explore harvested rainwater and use for Glycyrrhiza uralensis growth and to reduce water scarcity in Northwest China’s arid area, this study was conducted in April and July of 2023. Five types of slope micro rainwater collection measures, including horizontal terraces, square ridges, and contour ridges, were utilized to monitor rainfall and runoff. Glycyrrhiza uralensis seedlings were utilized as test subjects for supplementary irrigation in pots utilizing the natural drought method. The results show that supplemental irrigation boosts Glycyrrhiza uralensis root growth and water uptake capacity in short-term drought conditions. Irrigation with 42.97 mm enhanced Glycyrrhiza uralensis root length, belowground dry weight, and water use efficiency by 104.5%, 39.54%, and 4.18%. Supplemental irrigation under prolonged drought stress shifted Glycyrrhiza uralensis development from below- to aboveground, resulting in decreased activity of osmotic adjustment material activity in leaves. After 31 days of continuous drought following supplemental irrigation, plant height and aboveground fresh weight increased by 58.16% and 20.03%, respectively, whereas the superoxide dismutase activity was reduced by 63.16% in the 42.97 mm irrigated treatment. Furthermore, under short-term drought stress following supplemental irrigation, leaf water use efficiency was primarily influenced by osmoregulatory substances and plant growth characteristics. Under long-term drought stress, it was influenced by osmoregulatory substances and photosynthetic properties. This research is critical for preventing soil erosion and restoring grassland ecological health in the Ili River Valley.
1. Introduction
Rainwater collection technology is critical for increasing water use and crop yield in agroecosystems in arid and semi-arid areas [1]. The research location is located in the Ili River Valley of the Ili Kazakh Autonomous Prefecture in the Xinjiang Uygur Autonomous Region, a water-rich region in Xinjiang with a complicated topography and an average annual precipitation of 200–800 mm [2]. In recent years, under the influence of global climate change, extreme weather phenomena, such as droughts, floods, and short-duration rainstorms, occur regularly [3]. Warming accelerates the loss of soil moisture, resulting in a serious soil moisture deficit in the region [4]. In addition, due to the region’s unique natural geological structure, when floods and rainstorms occur, a huge volume of slope runoff has a significant impact on local ecological safety and agricultural sustainability [5,6]. As a result, how to collect and use short-term heavy rainfall to improve effective soil water content is a critical scientific topic that must be solved for soil and water conservation and ecological restoration in this region.
Rainwater collection and utilization include direct use in situ and timely irrigation [7]. Slope micro-collection is a land management technology that directly uses rainwater in situ [8] by changing the surface morphology to promote rainfall infiltration [9], and the water source used is entirely dependent upon natural rainfall [10], reducing the losses caused by evaporation and runoff during crop growth [11,12]. Unlike water conservation technologies, such as standard drip, sprinkler, and micro-irrigation systems, which use water management to conduct precise irrigation for plants [13], the source of water is wholly dependent upon external water sources [14,15]. In contrast, using natural rainfall to supply soil moisture during growth can efficiently balance the link between water conservation and plant hydration [16]. This technology is widely used in nations including Djibouti, Burkina Faso, India, and Syria [17,18,19]. In Djibouti, tiny dams and infiltration ponds are utilized to collect rainwater and boost the soil water storage capacity to improve agricultural output [20]. In Burkina Faso, dismantled constructions such as meniscus are utilized to collect rainwater and boost crop water penetration capacity, promoting natural vegetation restoration [21], In the dry and semi-arid regions of Gansu, Ningxia, and Qinghai in China, researchers have used fish-scale pits, horizontal ditches, and terraces to intercept rainwater runoff, boost rainfall penetration [22,23], and minimize runoff and sand generation, so creating the conditions for successful soil moisture conservation, plant growth, and erosion management [24,25].
Natural rainfall in the dry region of Northwest China is unpredictable, and there are significant variances in soil moisture changes caused by varying degrees of rainfall [26]. Soil water provides the majority of the water required for plant growth and development, as well as transpiration dissipation [27]. When there is no rain for an extended period of time or when temperatures are consistently high, soil moisture becomes a crucial element in restricting plant growth and development, as well as in agricultural productivity [28]. Glycyrrhiza uralensis Fisch. is primarily found in Northwest China’s arid region, and as a desert pioneer plant, it is not only valuable for grassland ecological restoration but also plays an important role in agriculture, drug production, and regional economic development [29,30]. Drought primarily impacts the exterior morphological, structural, and physiological alterations of Glycyrrhiza uralensis [31,32]. Drought stress causes water loss and wilting in Glycyrrhiza uralensis, which restricts root, stem, and leaf growth [33,34], causing the stem–leaf biomass allocation ratio to drop and flow to the roots [35,36,37]. Furthermore, in agricultural production, changing water regulations have a significant impact on the growth and water usage efficiency of Glycyrrhiza uralensis [38,39]. Under moderate water stress, Glycyrrhiza uralensis can lower its own water dissipation by boosting the activity of antioxidant enzymes to minimize membrane damage [40]. Under extreme water stress, the balance of its reactive oxygen metabolism is interrupted, and the peroxidation of membrane lipids intensifies, which increases the loss of its own water. Glycyrrhiza uralensis can only resist drought stress damage by reducing chlorophyll content and modifying photosynthetic properties [41]. At present, domestic and international research on artificially grown Glycyrrhiza uralensis focuses on the impact of different irrigation gradients on Glycyrrhiza uralensis growth, photosynthesis, and main components [42,43], while little research has been conducted to investigate the growth characteristics and water consumption efficiency of Glycyrrhiza uralensis following supplemental irrigation with rainwater collection technologies. Therefore, in the circumstance of limited water resources, improving the water use efficiency of Glycyrrhiza uralensis can help reduce its dependence upon water resources, enhance its drought resistance, and provide stability [44,45].
In summary, this paper investigates the effects of photosynthesis and plant growth on the water use efficiency of Glycyrrhiza uralensis leaves by analyzing the changing patterns of the growth characteristics, photosynthetic characteristics, and photosynthetic physiological indexes of Glycyrrhiza uralensis seedlings under various amounts of supplemental irrigation. It investigates the impact of rainwater collection and usage strategies on drought stress in Glycyrrhiza uralensis, with the goal of clarifying the effects of rainwater harvesting and supplemental irrigation on the growth and water use efficiency of Glycyrrhiza uralensis.
2. Materials and Methods
2.1. Study Area
The rainfall-runoff monitoring points were located in the northern region of the Ili River Basin within the Xinjiang Uygur Autonomous Region, specifically at the Tiechanggou National Soil and Water Conservation Runoff Observation Site in Bayandai Town, Yining City, Xinjiang (81°10′44″ E, 43°58′53″ N), at an elevation of 657 m above sea level. This area is characterized by a north temperate continental climate, featuring low mountainous and hilly landscapes. The climate is notably humid, with considerable diurnal temperature variations. The average annual precipitation is approximately 260 mm, with the majority occurring between April and July [46]. The average annual temperature is around 8.5 °C, while the average annual evaporation rate is approximately 1.6 × 103 mm. The region benefits from roughly 2.5 × 103 h of sunshine each year, and the frost-free period extends for 178 days [47]. The predominant soil type in this area is grey calcareous soil, and the grassland is classified as temperate desert grassland, primarily composed of perennial dry herbs and shrubs. Key plant species in this ecosystem include Seriphidium transillense (Poljakov) Poljakov, Sophora alopecuroides L., and Zygophyllum xanthoxylon (Bunge) Maxim. [48,49]; The rainwater-utilization experiment is located in Urumqi City, Xinjiang Uygur Autonomous Region (86°37′33″ E~88°58′24″ E, 42°45′32″ N~44°08′00″ N), with an altitude of 800 m. The area has a continental temperate climate, with an average annual temperature of about 8.1 °C and an average annual rainfall of about 236 mm. The evaporation is much larger than the rainfall, and the vegetation is sparse [50] (Figure 1).
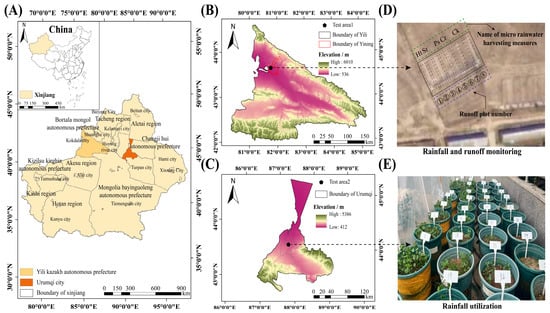
Figure 1.
Overview map of the test area. (A) Xinjiang Uygur Autonomous Region; (B) Yining city; (C) Urumqi city; (D) rainfall and runoff monitoring; (E) rainfall utilization. Ht—horizontal terrace; Sr—square ridge; Ps—pits; Cr—contour ridge; Ck—control.
2.2. Experimental Design
2.2.1. Rainfall and Runoff Monitoring
The experiment was conducted from 18 April to 22 July 2023 at the Tiechanggou National Soil and Water Conservation Runoff Observation Field. A total of five runoff plots, each measuring 20 m × 5 m, were established to evaluate four micro-collection methods alongside control measures. These methods included horizontal steps, square peduncles, pits, and horizontal ditches. A tipping-bucket rain gauge was installed outside the plots to measure the daily rainfall in the area. An iron partition was placed at the lower end of the plots to prevent external rainwater and sediment from entering the catchment troughs below. Additionally, two runoff collection devices were positioned at the lower end of the catchment troughs, with a catchment bucket placed inside each device to collect the runoff volume from each micro-collection method after rainfall events (Figure 2A) [48]. During the observation period, a total of 23 rainfall events occurred, resulting in an overall rainfall of 33.8 mm. Three significant rainfall-runoff events were recorded, with maximum daily rainfalls of 6.7 mm on 22 May, 3.6 mm on 15 June, and 7.4 mm on 22 June. No runoff was observed during the remaining 20 rainfall events (Figure 2B). While monitoring the runoff volume, the ring knife method was employed to obtain samples from the 0–30 cm soil layer of in situ soil within each measure for assessing the soil mass water content [51]. The soil mass water content for each method was then used to characterize the amount of rainfall collected within each measure following rainfall events.
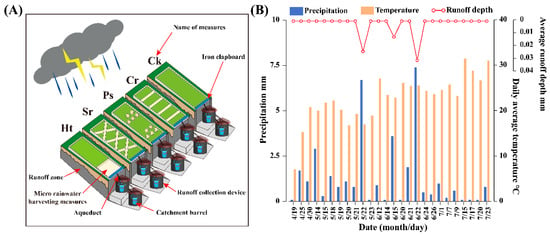
Figure 2.
The layout of micro-rainwater harvesting measures in the study area (A) and the monitoring of rainfall and runoff from April to August 2023 (B). Ht—horizontal terrace; Sr—square ridge; Ps—pits; Cr—contour ridge; Ck—control.
2.2.2. Rainfall Utilization
This experiment was conducted on the campus of Xinjiang Agricultural University from 10 April to 25 July 2023. During this period, the average maximum temperature ranged from 15 to 32 °C, while the average minimum temperature varied from 3 to 19 °C, and the average daily potential evapotranspiration was approximately 3.01 mm day−1 [52]. The test material was Ural red bark Glycyrrhiza uralensis provided by Xinjiang Tianbo Pratacultural Co., Ltd., Urumqi, China, Soil physicochemical properties [53] are soil organic matter content was 15.54 g kg−1, total nitrogen 1.02 g kg−1, alkaline dissolved nitrogen 40.27 mg kg−1, total phosphorus 2.88 g kg−1, effective phosphorus 13.45 mg kg−1, total potassium 1.37 g kg−1, and quick-acting potassium 278.71 mg kg−1, respectively. Glycyrrhiza uralensis seeds were sown in pots on 10 April. The pots had a height of 30 cm and an inner diameter of 26 cm. The bottoms of the pots were sealed, and the pots were filled with 10 kg of dry soil each. The pots were uniformly sown with 10–15 holes with 6–8 seeds per hole. A plastic tray was placed under each pot to avoid water loss, and the periphery of the pots was constructed with a rain shield using plastic sheeting to prevent rainfall from interfering with the experimental results [54]. The experiment was conducted one month after the emergence of Glycyrrhiza uralensis seedlings, and five treatments were set up with three replicates per treatment, totaling 15 pots. The volume of replenishment irrigation was the product of the soil mass water content within each micro-catchment measure and the soil weight of the pots after each rainfall yield; three replenishment irrigations were carried out during the trial period, which took place on 24 May, 17 June, and 24 June, respectively (Table 1). Sampling was carried out on the days before the replenishment, which were 16 June, 23 June, and 25 July, respectively, as there was no flow production from any of the rain catchment measures in the study area in July. The stomatal conductance, transpiration rate, net photosynthetic rate, and intercellular Co2 concentration indexes of Glycyrrhiza uralensis leaves were measured using a portable photosynthesis measurement system Li-6400 (WALZ, Germany, Nuremberg City) from 10:00 a.m. to 12:00 p.m. In the meantime, 6–9 intact seedlings were randomly selected from each treatment for the determination of plant height, root length, aboveground and belowground parts of the fresh weights and dry weights, and according to the aboveground ratio of the dry weight of the underground part to the aboveground part was used to calculate the root–crown ratio [55].

Table 1.
Runoff, rainfall collection, and supplementary irrigation of different micro-rainwater harvesting measures.
2.3. Indicator Monitoring
2.3.1. Chlorophyll Content
Collected from each pot were 0.2 g of fresh leaves, which were placed in a 10 mL test tube of 95% ethanol and placed under darkness for 24 h until the leaves turned white. The absorbance was measured at wavelengths of 663 nm and 645 nm for the calculation of Glycyrrhiza uralensis chlorophyll content using 95% ethanol as a blank control [56], which was calculated using the following formula:
where Ca is the chlorophyll a concentration (mg L−1), Cb is the chlorophyll b concentration (mg L−1), and CT is the total chlorophyll concentration (mg L−1). The total chlorophyll content was calculated by bringing the above total chlorophyll concentration into the following equation.
where ChlT is the total chlorophyll content (mg g−1. FW), CT is the total chlorophyll concentration (mg L−1), V is the volume of the extract (mL), and W is the weight of the leaf sample (g).
2.3.2. Leaf Osmotic Adjustment Substances
The Glycyrrhiza uralensis leaf peroxidase, malondialdehyde, superoxide dismutase, proline, and plant-soluble sugars were determined by the micro-method [57,58], and the catalase was determined by the ammonium molybdate colorimetric method [59].
2.3.3. Leaf Water Use Efficiency
The water use efficiency of the Glycyrrhiza uralensis leaves, with reference to de Santana et al. [60] was measured using the ratio of the net photosynthetic rate to the transpiration rate. The unit is μmol·mmol−1 [61], which was calculated using the following formula:
where A represents the leaf net photosynthetic rate (μmol m−2 S−1), and E indicates the transpiration rate (mmol m−2 S−1).
2.4. Data Processing
Microsoft Excel 2021 software was used to preprocess the experimental data. The psych package in R language 4.2.3 conducted descriptive statistics on the data. The bartlett.test() function analyzed the variability among the indicators of irrigation replenishment. The ‘agricolae’ package performed multiple comparisons and visualizations [62]. The LSD.test() function in the ‘agricolae’ package facilitated multiple comparisons, while the ‘ggplot2’ package assisted with visualization [62]. The water use efficiency of Glycyrrhiza uralensis leaves served as the response variable, with the indicators of Glycyrrhiza uralensis growth, photosynthesis, and osmoregulatory substances as explanatory variables. Redundancy analysis was performed using Canoco 5.0 software [63]. The data were standardized before analysis, and the Monte Carlo replacement test assessed the significance of the explanatory variables. Based on the redundancy analysis, partial redundancy analysis and variance decomposition were applied [64] to derive the contributions of each explanatory variable to leaf water use efficiency, thus determining the primary factors affecting leaf water use efficiency.
3. Results
3.1. Effects of Different Supplemental Irrigation Amounts on Growth Characteristics of Glycyrrhiza uralensis Seedlings
The growth characteristics of Glycyrrhiza uralensis seedlings under different supplemental irrigation conditions appear in Figure 3. From the figure, we observe that the effects of supplemental irrigation on Glycyrrhiza uralensis plant height, root length, fresh weight, and dry weight on 17 June and 24 June were the most significant (p < 0.001). Each index tended to increase with more frequent and heavier supplemental irrigation. The changes in each supplemental irrigation amount highlighted that 42.97 mm significantly exceeded 32.60 mm (p < 0.05). After the replenishment on 17 June, each replenishment volume notably affected the root length, underground fresh weight, aboveground dry weight, and underground dry weight of Glycyrrhiza uralensis. The treatment with 42.97 mm surpassed the 32.60 mm treatment by 104.5%, 39.61%, 36.06%, and 39.54%, respectively (Figure 3B,D–F). Additionally, after the replenishment on 24 June, the replenishment volumes significantly influenced the same attributes of Glycyrrhiza uralensis, with the 42.97 mm treatment outperforming the 32.60 mm treatment. Ground fresh weight improved markedly, as the 42.97 mm treatment exceeded the 32.60 mm treatment by 58.16% and 20.03%, respectively (Figure 3A,C). The root–crown ratio exhibited a decreasing, then an increasing, trend relative to the number of supplemental irrigations. The irrigation on 24 May proved to be significantly higher than that on 17 June and 24 June (p < 0.001). The various supplemental irrigation amounts generated a notable promotional effect when comparing the 35.99 mm treatment on 24 June, which was significantly higher than the 32.60 mm treatment by 9.25% (Figure 3G).
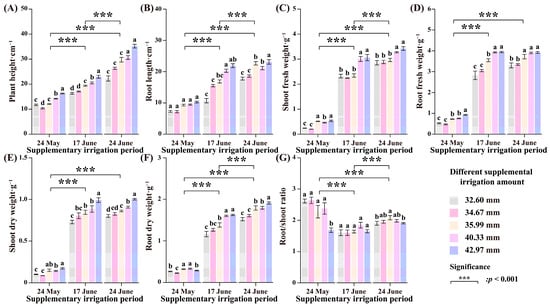
Figure 3.
The growth characteristics of Glycyrrhiza uralensis seedlings under different treatment supplemental irrigation amounts and different supplemental irrigation times were changed. (A) plant height; (B) root length; (C) shoot fresh weight; (D) root fresh weight; (E) shoot dry weight; (F) root dry weight; (G) root/shoot ratio. Different lowercase letters indicate significant differences at the 0.05 level for each supplemental irrigation.
3.2. Effects of Different Supplemental Irrigation Amounts on Photosynthesis of Glycyrrhiza uralensis Leaves
3.2.1. Effects of Different Supplemental Irrigation Amounts on Photosynthetic Characteristics of Glycyrrhiza uralensis Leaves
Figure 4 shows the variation characteristics of photosynthetic properties of Glycyrrhiza uralensis seedlings with different supplemental irrigation levels. As shown in the figure, supplemental irrigation significantly affected the net photosynthetic rate, transpiration rate, stomatal conductance, intercellular Co2 concentration, and chlorophyll content of Glycyrrhiza uralensis leaves on 24 May (p < 0.001). Each index exhibited a decreasing trend, with more instances of supplemental irrigation, and an increasing trend, with a higher amount of supplemental irrigation. The impacts of various supplemental irrigations on the transpiration rate, stomatal conductance, and intercellular Co2 concentration were most pronounced on 17 June (p < 0.001). The treatment of 42.97 mm showed significantly higher increases of 39.85%, 63.78%, and 63.78% compared to the 32.60 mm treatment, respectively. Following the supplemental irrigation on June 17, both the stomatal conductance and the intercellular Co2 concentration were significantly greater in the 42.97 mm treatment than in the 32.60 mm treatment (p < 0.05) (Figure 4B–D). After the supplemental irrigation on 24 June, the 42.97 mm treatment had a more substantial effect on the net photosynthetic rate of Glycyrrhiza uralensis leaves, which was significantly higher by 40.02% (p < 0.05) compared to the 32.60 mm treatment. The 40.33 mm supplemental irrigation amount significantly enhanced the chlorophyll content, surpassing the 32.60 mm treatment by 25.10% (p < 0.05) (Figure 4A,E). The water use efficiency of Glycyrrhiza uralensis leaves displayed a trend of increasing and then decreasing with the number of supplemental irrigation events. After the irrigation on 17 June, the water use efficiency for each leaf significantly exceeded that of the other two periods (p < 0.001). The 40.33 mm treatment yielded a significantly higher efficiency, surpassing the 32.60 mm treatment by 4.18% (p < 0.05) (Figure 4F).
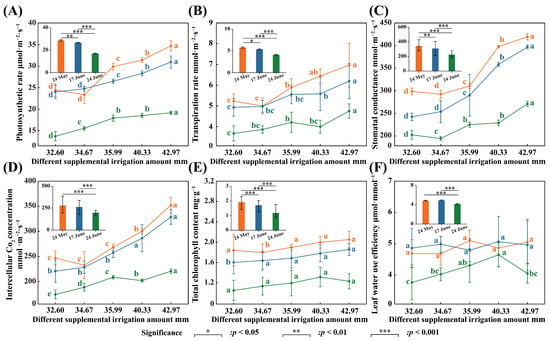
Figure 4.
The variation characteristics of photosynthetic characteristics of Glycyrrhiza uralensis leaves under different treatment supplemental irrigation amounts and different supplemental irrigation times. (A) photosynthetic rate; (B) transpiration rate; (C) stomatal conductance; (D) intercellular Co2 concentration; (E) total chlorophyll content; (F) leaf water use efficiency. Different lowercase letters indicate significant differences at the 0.05 level for each supplemental irrigation.
3.2.2. Effects of Different Supplemental Irrigation Amounts on Osmotic Adjustment Substances in Glycyrrhiza uralensis Leaves
The changes in the osmoregulatory substances in seedling leaves with different supplemental irrigation times appear in Figure 5. The figure indicates that osmoregulatory substances increased with the number of supplemental irrigations but decreased with the amount of irrigation. The changes for each level of supplemental irrigation suggest that the treatment with 32.60 mm of irrigation was significantly higher than the treatment with 42.97 mm (p < 0.05). During the supplemental irrigation period, the effect on osmoregulatory substances was significantly greater on 24 June than on 24 May (p < 0.001). The indicators of osmoregulatory substances were most significant in alleviating drought stress in Glycyrrhiza uralensis after the supplemental irrigation on 17 June. Specifically, after replenishment on 24 May, the treatment with 42.97 mm of irrigation had a greater mitigating effect on peroxidase, catalase, and proline, reducing them by 62.58%, 77.91%, and 80.03%, respectively, compared to the 32.60 mm treatment (Figure 5A,C,E). Following the replenishment on 17 June, the treatment of 42.97 mm demonstrated a greater mitigating effect on malondialdehyde and plant-soluble sugar, which decreased by 43.49% and 37.27%, respectively, compared to the replenishment of 32.60 mm (Figure 5D,F). After the replenishment on 24 June, the treatment of 42.97 mm showed a greater mitigating effect on superoxide dismutase, which decreased by 63.16% compared to the replenishment of 32.60 mm (Figure 5B).
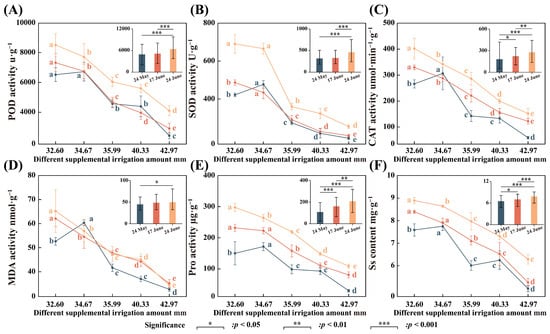
Figure 5.
The variation characteristics of osmotic adjustment substances in Glycyrrhiza uralensis leaves under different treatment supplemental irrigation amounts and different supplemental irrigation times. (A) POD activity; (B) SOD activity; (C) CAT activity; (D) MDA activity; (E) Pro activity; (F) Ss content. Different lowercase letters indicate significant differences at the 0.05 level for each supplemental irrigation.
3.3. Effects of Photosynthesis and Growth Characteristics on Leaf Water Use Efficiency of Glycyrrhiza uralensis
Table 2 presents the results of the redundancy analysis of the water use efficiency in relation to the photosynthesis and growth characteristics of Glycyrrhiza uralensis leaves during various periods of supplemental irrigation. As indicated in the table, the correlations of the explanatory variables with the first two axes were 0.9860 and 0.9907, respectively, following the supplemental irrigation on 24 May. The aboveground fresh weight demonstrated a significant positive correlation with the first ordering axis (p < 0.05). Additionally, plant height and stomatal conductance exhibited a highly significant positive correlation with the second ordering axis (p < 0.01), while catalase, malondialdehyde, catalase, and proline showed a highly significant negative correlation with the second ordering axis (p < 0.01). After the supplemental irrigation on 17 June, the correlations of each explanatory variable with the first two axes were 0.9760 and 0.8338, respectively. The intercellular Co2 concentration, peroxidase, malondialdehyde, catalase, proline, and plant-soluble sugar indicated significant positive correlations (p < 0.05) with the first sorting axis. In contrast, the plant height, root length, belowground fresh weight, belowground dry weight, aboveground dry weight, and stomatal conductance were significantly and negatively correlated with the first sorting axis (p < 0.05). The correlations of the explanatory variables with the first two axes were 0.9766 and 0.9626 after the supplemental irrigation on 24 June, respectively. Plant height, aboveground, and belowground fresh weight showed significant positive correlations with the first sorting axis (p < 0.05). Conversely, peroxidase, malondialdehyde, catalase, proline, and plant-soluble sugar were significantly and negatively correlated with the first sorting axis (p < 0.05). Finally, aboveground dry weight and stomatal conductance were significantly positively correlated with the second sorting axis (p < 0.05) (Figure 6A).

Table 2.
The results of correlation coefficient, eigenvalue, and cumulative explained variance between water use efficiency of Glycyrrhiza uralensis leaves and influencing factors were analyzed.
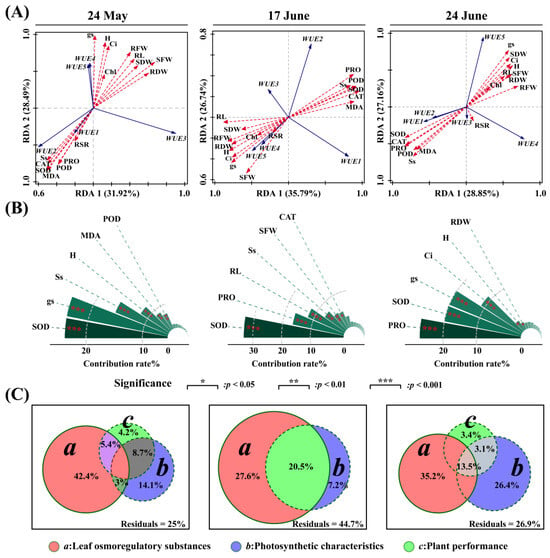
Figure 6.
The two-dimensional ordination diagram (A), factor significance test results (B), and variation decomposition results (C) of response variables and explanatory variables under different supplementary irrigation times. Factors: H—plant height; RL—root length; RFW—root fresh weight; SFW—shoot fresh weight; RDW—root dry weight; SDW—shoot dry weight; RSR—root–shoot ratio; Chl—chlorophyll content; gs—stomatal conductance; Ci—intercellular Co2 concentration; POD—peroxidase; MDA—malondialdehyde; CAT—catalase; SOD—superoxide dismutase; PRO—proline; Ss—soluble sugars.
A partial redundancy analysis showed that superoxide dismutase (29%), stomatal conductance (29%), plant-soluble sugar (16.1%), plant height (9.6%), malondialdehyde (5.8%), and catalase (4.7%) significantly affected leaf water use efficiency after the 24 May supplementation. After the 17 June supplementation, superoxide dismutase (34.7%), proline (18.1%), root length (13.7%), plant-soluble sugars (8.4%), aboveground fresh weight (6.1%), and catalase (5.6%) had significant effects on leaf water use efficiency. Following supplemental irrigation on 24 June, proline (28%), superoxide dismutase (21.7%), stomatal conductance (21.6%), intercellular Co2 concentration (13.6%), plant height (3.1%), and belowground dry weight (2.1%) significantly affected leaf water use efficiency (Figure 6B). Based on the composition of the total variance of the response variables, the leaf osmoregulatory substances, photosynthetic characteristics, and plant growth traits explained 75% of the total variance after the supplemental irrigation on 24 May. Photosynthetic physiology and photosynthetic characteristics indices explained 3%, while photosynthetic physiology and plant growth traits explained 5.4%. Additionally, photosynthetic characteristics and plant growth traits explained 8.7% of the total variance together. After the supplemental irrigation on 17 June, the leaf osmoregulatory substances and plant growth traits explained 55.3% of the total variance, with the leaf osmoregulatory substances and plant growth characteristics together explaining 20.5% of the total variance. After the supplemental irrigation on 24 June, leaf osmoregulatory substances, photosynthesis, and plant growth traits explained 63.1% of the total variance, with leaf osmoregulatory substances and photosynthesis characteristics indices together explaining 13.5% of the total variance. Leaf osmoregulatory substances and photosynthetic characteristics together explained 13.5% of the total variation, while photosynthetic characteristics and plant growth characteristics together explained 3.1% of the total variation (Figure 6C).
4. Discussion
Precipitation is the only source of soil moisture recharge, and temperature is the primary factor influencing grassland evapotranspiration [65]. The average annual precipitation in the Ili River Valley region is around 200–800 mm, but the average annual evapotranspiration is 1200–1900 mm, demonstrating that natural rainfall has a smaller role in recharging soil and vegetation when rainfall is low [66]. The interception rate of rainfall runoff can be raised by modifying the slope microtopography, which enhances water penetration and increases water recharge to the soil and vegetation [67]. However, different microtopographies’ ability to intercept rainwater runoff varies depending on connectivity and habitat [68]. Flow paths with a higher connectedness generate more runoff but recharge comparatively less soil moisture, whereas flow channels with lower connectivity generate less runoff but recharge relatively more soil moisture [69]. The results of this study showed that the Pits did not experience rainfall-produced flow in all rainfall events and, thus, had the best interception of rainfall runoff. The reason for this could be related to the habitat and stand type of the microtopography itself, which increases the moisture content due to gravity, causing rainfall to collect in the pits while also prolonging the time of soil moisture infiltration [70]. Throughout the procedure, the pits and contour ridge received more than 40 mm of water, demonstrating that rainwater runoff plays a larger role in pit and contour ridge water replenishment. Pan et al. [48] planted safflower using several micro-collection measures in grassland in the Ili River Valley and found that the quantity of safflower surviving and the growth of safflower within the pit measure were the best compared to other measures. Su et al. [71] analyzed sealed grassland and contour ridge in the loess hilly area of Ningxia and discovered that contour ridge furrows considerably increased soil permeability and water retention capacity, which is consistent with the current study’s findings.
Plant development and biomass allocation are highly sensitive to variations in soil moisture [72]. The results of this study showed that Glycyrrhiza uralensis plant height, root length, and aboveground and belowground biomass were all highly dependent upon soil moisture, and there was a significant increase in these indexes with increased recharge irrigation, indicating that moisture directly affects plant growth and development [73]. However, when the length of drought stress altered, photosynthesis impeded Glycyrrhiza uralensis growth at varying degrees of supplemental watering. According to research, water stress is the most significant limiting factor for photosynthesis in plants [74], and photosynthesis is the first critical feature of plant development and metabolism to be affected by water [75]. Photosynthesis in plants is controlled by both stomatal and non-stomatal elements. Drought stress inhibits stomatal opening and Co2 input, resulting in a lower photosynthetic rate. It also inhibits primary processes, electron transport, photosynthetic phosphorylation, and carbon assimilation [76]. Glycyrrhiza uralensis was subjected to drought stress for 21, 7, and 31 days in this study. The leaf photosynthetic rate, transpiration rate, stomatal conductance, and intercellular Co2 concentration showed a decreasing trend with an increasing duration of drought, which was attributed to the gradual weakening of drought tolerance of Glycyrrhiza uralensis due to its own damage caused by long-term drought stress [77]. However, when drought persisted for 7 days after supplemental irrigation, the water use efficiency of Glycyrrhiza uralensis leaves reached the highest value under the effect of high supplemental irrigation, which was due to the longer drought stress period in the pre-infusion period, and the effect of high supplementary irrigation on Glycyrrhiza uralensis gradually shifted from the belowground to the aboveground level, which could improve the water use efficiency of leaves by changing the transpiration rate, stomatal conductance, and concentration of intercellular Co2 to reduce the inhibition of photosynthesis [78]. Furthermore, osmoregulatory chemicals have a direct impact on plant growth during dry conditions [79]. The results show that the leaves had the highest index of osmoregulatory chemicals after 31 days of extended drought with supplemental irrigation, which was related to the fact that, when plants are exposed to drought stress, they disturb the osmoregulatory and protective enzyme systems through oxidative stress to impair the plant’s growth. Plants boost the activity of enzymes, such as SOD, POD, and CAT, in the body to adjust to the external environment [80]. In addition, the SOD, POD, and CAT activities of Glycyrrhiza uralensis leaves were gradually increased by replenishment irrigation, in the order of pits > contour ridge > square ridge > horizontal terrace > control. This was due to the accumulation of large amounts of reactive oxygen species in Glycyrrhiza uralensis leaves under low refreshing irrigation, which resulted in a continuous increase in MDA content that damaged the cells and, thus, decreased the activities of SOD and CAT [81].
5. Conclusions
Under short-term drought conditions, high supplementary irrigation amounts greatly enhanced leaf transpiration rates, stomatal conductance, and the intercellular Co2 concentration to reduce the inhibition of photosynthesis and increase root water absorption capacity, hastening belowground development and biomass buildup. Following a 7-day persistent drought, 42.97 mm of supplemental watering increased the water consumption efficiency of Glycyrrhiza uralensis leaves by 4.18%. At the same time, root length, shoot dry weight, and root dry weight increased by 104.5%, 36.06%, and 39.54%, respectively. As to long-term drought stress, the effect of high supplemental irrigation gradually shifted from belowground to aboveground, and the treatment of 42.97 mm supplemental irrigation increased the plant height and aboveground fresh weight of Glycyrrhiza uralensis by 58.16% and 20.03%, respectively, after 31 days of continuous drought. At the same time, it alleviated the damage of the long-term drought stress by decreasing its own peroxidase, catalase, and superoxide dismutase. According to a redundancy analysis, osmoregulatory chemicals have the greatest impact on the water consumption efficiency of Glycyrrhiza uralensis leaves.
Author Contributions
X.L.: conceptualization, methodology, software, validation, investigation, visualization, data curation, writing—review and editing, writing—original draft; W.L.: conceptualization, investigation, formal analysis, data curation; W.Z. and G.H.: conceptualization, methodology, investigation, resources, supervision, project administration, founding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported by the Open Project of Xinjiang Key Laboratory of Soil and Plant Ecological Processes (Grant No. XJKL202309) and the Technology Innovation Guidance Plan of Shandong Province (Grant No. YDZX2023010).
Data Availability Statement
The data used are confidential and are presented in aggregate in the article.
Conflicts of Interest
We declare that we have no financial or personal relationships with other people or organizations that can inappropriately influence our work and that there is no professional or other personal interest of any nature in any product, service, and/or company that could be construed as influencing the position presented in, or the review of, this manuscript.
References
- Karpouzoglou, T.; Barron, J. A global and regional perspective of rainwater harvesting in sub-Saharan Africa’s rainfed farming systems. Phys Chem Earth. Parts A/B/C 2014, 72–75, 43–53. [Google Scholar] [CrossRef]
- Zhou, L.; Zhu, H.; Zhong, H.; Yang, H.; Suo, F.; Shao, X.; Zhou, X. Spatial analysis of soil bulk density in Yili, Xinjiang Uygur Autonomous Region, China. Acta Prataculturae Sin. 2016, 25, 64–75. [Google Scholar]
- Pizzorni, M.; Innocenti, A.; Tollin, N. Droughts and floods in a changing climate and implications for multi-hazard urban planning: A review. City Environ. Interact. 2024, 24, 100169. [Google Scholar] [CrossRef]
- Qing, Y.; Wang, S.; Yang, Z.; Gentine, P.; Zhang, B.; Alexander, J. Accelerated soil drying linked to increasing evaporative demand in wet regions. NPJ Clim. Atmos. Sci. 2023, 6, 205. [Google Scholar] [CrossRef]
- Dai, X.; Wang, P.; Zhang, K. A study on precipitation trend and fluctuation mechanism in northwestern China over the past 60 years. Acta Phys. Sin. Ed. 2013, 62, 129201. [Google Scholar]
- Duan, J.; Liu, Y.; Tang, C.; Shi, Z.; Yang, J. Efficacy of orchard terrace measures to minimize water erosion caused by extreme rainfall in the hilly region of China: Long-term continuous in situ observation. J. Environ. Manag. 2021, 278, 111537. [Google Scholar] [CrossRef]
- Ahmed, S.; Jesson, M.; Sharifi, S. Selection Frameworks for Potential Rainwater Harvesting Sites in Arid and Semi-Arid Regions: A Systematic Literature Review. Water 2023, 15, 2782. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, H.; Wang, J.; Zhao, H.; Zhang, H.; Wu, S.; Chen, Y.; Yang, T.; Deng, H.; Batool, A.; et al. Development and application of micro-field rain-harvesting technologies. Trans. Chin. Soc. Agric. Eng. 2013, 29, 1–17. [Google Scholar]
- Wang, Y.; Li, Q.; Chen, L.; Song, Z.; Wang, L. The Sustainable development of rain-fed agriculture in arid northwest China. Sustain. Dev. 2016, 6, 237–242. [Google Scholar] [CrossRef]
- Duan, C.; Chen, G.; Hu, Y.; Wu, S.; Feng, H.; Dong, Q. Alternating wide ridges and narrow furrows with film mulching improves soil hydrothermal conditions and maize water use efficiency in dry sub-humid regions. Agric. Water Manag. 2021, 245, 106559. [Google Scholar] [CrossRef]
- Zheng, S.; Ren, S.; Zhang, J.; Zhao, J.; Tang, S.; Wang, J.; Pan, Z.; Pan, X.; Hu, Q. Effects of furrow rainwater harvesting measures on growth and water use efficiency of crop based on meta analysis. J. Shanxi Agric. Sci. 2022, 50, 1079–1087. [Google Scholar]
- Komariah; Senge, M. The development of water harvesting research for agriculture. Rev. Agric. Sci. 2013, 1, 31–42. [Google Scholar] [CrossRef]
- Chen, Y.; Leng, Y.; Zhu, F.; Li, S.; Song, T.; Zhang, J. Water-saving techniques: Physiological responses and regulatory mechanisms of crops. Adv. Biotechnol. 2023, 1, 3. [Google Scholar] [CrossRef]
- Taylor, R.; Zilberman, D. Diffusion of Drip Irrigation: The Case of California. Appl. Econ. Perspect. Policy 2017, 39, 16–40. [Google Scholar] [CrossRef]
- Pronti, A.; Auci, S.; Berbel, J. Water conservation and saving technologies for irrigation. A structured literature review of econometric studies on the determinants of adoption. Agric. Water Manag. 2024, 299, 108838. [Google Scholar] [CrossRef]
- Hui, X.; Chen, Y.; Shoukat, M.R.; Yang, H.; Zheng, Y. Sprinkler irrigation on sloping land: Distribution characteristics of droplet impact angle and shear stress. Water 2023, 16, 60. [Google Scholar] [CrossRef]
- Zdruli, P.; Lamaddalena, N. Mediterranean region: Too many people too little land. In Terre et Mer, Ressources Vitales pour la Méditerranée; Lacirignola, C., Ed.; L’Harmattan: Paris, France, 2015; pp. 13–22. [Google Scholar]
- Yadav, B.; Patidar, N.; Sharma, A.; Panigrahi, N.; Sharma, R.; Loganathan, V.; Krishan, G.; Singh, J.; Kumar, S.; Parker, A. Assessment of traditional rainwater harvesting system in barren lands of a semi-arid region: A case study of Rajasthan (India). J. Hydrol. Reg. Stud. 2022, 42, 101149. [Google Scholar] [CrossRef]
- Marques, M.J.; Schwilch, G.; Lauterburg, N.; Crittenden, S.; Tesfai, M.; Stolte, J.; Zdruli, P.; Zucca, C.; Petursdottir, T.; Evelpidou, N.; et al. Multifaceted impacts of sustainable land management in drylands: A review. Sustainability 2016, 8, 177. [Google Scholar] [CrossRef]
- Mouhoumed, R.M.; Ekmekcioğlu, Ö.; Özger, M. A holistic multi-tiered decision framework for evaluating rainwater harvesting potential in arid regions: A case study of the southeastern basin of Djibouti. Groundw. Sustain. Dev. 2024, 25, 101090. [Google Scholar] [CrossRef]
- Zougmoré, R.; Zida, Z.; Kambou, N. Role of nutrient amendments in the success of half-moon soil and water conservation practice in semiarid Burkina Faso. Soil Tillage Res. 2003, 71, 143–149. [Google Scholar] [CrossRef]
- Cheng, J.; Wan, H.; Wang, J.; Yong, S. Soil Water Regulation of the Natural Grassland of Semi-Arid Loess Hilly Region. Acta Agrestia Sin. 2003, 11, 296–300. [Google Scholar]
- Wang, Z.; Jiao, J.; Su, Y.; Chen, Y. The efficiency of large-scale afforestation with fish-scale pits for revegetation and soil erosion control in the steppe zone on the hill-gully Loess Plateau. Catena 2013, 115, 159–167. [Google Scholar] [CrossRef]
- Ye, W.; Ma, E.; Liao, L.; Hui, Y.; Liang, S.; Ji, Y.; Yu, S. Applicability of photovoltaic panel rainwater harvesting system in improving water-energy-food nexus performance in semi-arid areas. Sci. Total Environ. 2023, 896, 164938. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Fan, W.; Li, Y.; Yi, Y. The influence of changes in land use and landscape patterns on soil erosion in a watershed. Sci. Total Environ. 2017, 574, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kendy, E.; Yu, Q.; Liu, C.; Shen, Y.; Sun, H. Effect of soil water deficit on evapotranspiration, crop yield, and water use efficiency in the North China Plain. Agric. Water Manag. 2004, 64, 107–122. [Google Scholar] [CrossRef]
- Jin, X.; Zhao, W.; Li, M.; Ju, W. Effects of soil moisture on the stoichiometric characteristics of aboveground parts of plants following conversion of farmland to grassland on the Loess Plateau. Res. Soil Water Conserv. 2022, 29, 57–63. [Google Scholar]
- Li, X.; Wu, J.; Lei, T.; Zhou, H. Analysis on the critical period of winter wheat yield impacted by water deficit caused by precipitation. Water Resour. Hydropower Eng. 2020, 51, 209–217. [Google Scholar]
- Lang, T.; Pan, L.; Liu, B.; Guo, T.; Hou, X. Vegetation characteristics and response to the soil properties of three medicinal plant communities in Altay Prefecture, China. Sustainability 2020, 12, 10306. [Google Scholar] [CrossRef]
- Husain, I.; Bala, K.; Khan, I.A.; Khan, S.I. A review on phytochemicals, pharmacological activities, drug interactions, and associated toxicities of licorice (Glycyrrhiza sp.). Food Front. 2021, 2, 449–485. [Google Scholar] [CrossRef]
- Cao, Y.J.; Zhao, M.C.; Zheng, C.Y.; Zhu, F. Rhizosphere microorganisms-mediated plant responses to drought stress. Chin. J. Eco-Agric. 2023, 31, 1330–1342. [Google Scholar]
- Xiang, H.; Liu, Y. The research progress of drought stress response of morphological and anatomical structure of Glycyrrhiza uralensis. J. North Agric. 2016, 44, 117–122. [Google Scholar]
- Liu, C.; Wang, W.; Li, S.; Cui, J. Effect of drought stress on growth of Glycyrrhiza uralensis. China J. Chin. Mater. Med. 2004, 29, 931–934. [Google Scholar]
- Li, M.; Wang, G. Effect of Drought Stress on Activities of Cell Defense Enzymes and Lipid Peroxidation in Glycyrrhiza uralensis Seedlings. Acta Ecol. Sin. 2011, 31, 2259–2264. [Google Scholar]
- Yan, H.; Fang, H.; Huang, D. Effects of drought stress on the biomass distribution and photosynthetic characteristics of cluster mulberry. Chin. J. Appl. Ecol. 2011, 22, 3365–3370. [Google Scholar]
- Shi, C.; Chen, T.; Wang, C.; Qing, X.; Liao, Y. Effect of Drought Stress on Seed Germination and Biomass Allocation of Root and Shoot of Different Drought Resistant Wheat Cultivars. J. Triticeae Crops 2016, 36, 483–490. [Google Scholar]
- Zhang, D.; Yang, Z.; Song, X.; Zhang, F.; Liu, Y. TMT-based proteomic analysis of liquorice root in response to drought stress. BMC Genom. 2022, 23, 524. [Google Scholar] [CrossRef]
- Pinnamaneni, S.R.; Anapalli, S.S.; Fisher, D.K.; Reddy, K.N. Water use efficiencies of different maturity group soybean cultivars in the humid Mississippi Delta. Water 2021, 13, 1496. [Google Scholar] [CrossRef]
- Gago, J.; Douthe, C.; Florez-Sarasa, I.; Escalona, J.M.; Galmes, J.; Fernie, A.R.; Flexas, J.; Medrano, H. Opportunities for improving leaf water use efficiency under climate change conditions. Plant Sci. 2014, 226, 108–119. [Google Scholar] [CrossRef]
- An, Y.; Zhang, Q.; Li, S.; Liu, H. Effects of Drought Stress and Rehydration on Antioxidant Enzyme Activity and Photosynthetic Characteristic of Glycyrrhiza uralensis Fisch. Ningxia J. Agric. For. Sci. Technol. 2021, 62, 679. [Google Scholar]
- Liu, Y.; Cai, G.; Chem, G. Effects of Drought Stress on Active Oxygen Metabolism in Glycyrrhiza uralensis Seedlings. Chin. J. Grassl. 2012, 34, 93–98. [Google Scholar]
- Abudurezike, A.; Tuerhong, T.; Aikebaier, G.; Zang, Y.; Shawuer, A. Effects of Diffeent Drip Irrigation RateAmounts on Growth of Glycyrrhiza uralensis during Seedling Stage in Desert Areas. Xinjiang Agric. Sci. 2022, 59, 1945. [Google Scholar]
- Abudurezike, A.; Liu, X.; Aikebaier, G.; Shawuer, A.; Tian, X. Effect of different irrigation and fertilizer coupling on the liquiritin contents of the licorice in Xinjiang arid area. Ecol. Indic. 2024, 158, 111451. [Google Scholar] [CrossRef]
- Li, F.; Gao, P.; Duan, T. Response and mechanism of arbuscular mycorrhizal fungi to abiotic stress. Acta Agrestia Sin. 2016, 24, 491–500. [Google Scholar]
- Lin, S.; Wang, G.; Hu, Z.; Huang, K.; Sun, J.; Sun, X. Spatiotemporal Variability and Driving Factors of Tibetan Plateau Water Use Efficiency. J. Geophys. Res. Atmos. 2020, 125, e2020JD032642. [Google Scholar] [CrossRef]
- Li, Z.; Hu, G.; Qu, T.; Zhang, H.; Zhang, W.; Li, Y.; Aikebaier, Y. Responses of Runoff and Sediment Yield to Rainfall, Soil Types Under Different Managed Grasslands in Yili Valley. Res. Soil Water Conserv. 2022, 29, 62–69. [Google Scholar]
- Zhang, J. Study on the soil development rule in Yili river basin. J. Shihezi Univ. (Nat. Sci.) 2005, 23, 583–587. [Google Scholar]
- Pan, Y.; Zhang, W.; Hu, G.; Li, Y. Effects of Different Micro Rainwater Harvesting Measures on Safflower Growth. Bull. Soil Water Conserv. 2023, 43, 104–110. [Google Scholar]
- Li, Y.; Hu, G.; Zhang, W.; Liu, X.; Sun, G. Characteristics of soil and water loss of natural and artificial vegetation under different rainfall patterns in ILi River Valley. Southwest China J. Agric. Sci. 2024, 37, 404–411. [Google Scholar]
- Tang, X.; Liu, F.; Hu, X. Urban growth simulation and scenario projection for the arid regions using heuristic cellular automata. Sci. Rep. 2024, 14, 21106. [Google Scholar] [CrossRef]
- Ling, Q.; Xu, Z. Study of the Water Infiltration Processes using ERT and TDR in Layered Soils. Acta Pedol. Sin. 2023, 60, 390–398. [Google Scholar]
- European Centre for Medium-Range Weather Forecasts (ECMWF). (1979–Present). ERA5 Hourly Data on Single Levels from 1979 to Present. Copernicus Climate Data Store. Available online: https://cds.climate.copernicus.eu/datasets/reanalysis-era5-single-levels?tab=overview (accessed on 8 August 2023).
- Bao, S.; Qing, H.; Lao, J. Soil Agrochemical Analysis, Title of Presentation; China Agriculture Press: Beijing, China, 1988; pp. 22–111. [Google Scholar]
- Zhang, J.; Dong, X.; Xin, Z.; Liu, M.; Zhang, R.; Huang, Y.; Sun, F. Effects of Artificial Simulated Precipitation on Seed Characters and Germination of Nitraria tangutorum. Southwest China J. Agric. Sci. 2019, 32, 1181–1186. [Google Scholar]
- Yang, B.; Shan, L.; Ma, J.; Xie, T.; Yang, J.; Wei, C. Response of growth and root morphological characteristics of Reaumuria soongorica seedlings to drought-rehydration. Arid Zone Res. 2021, 38, 10. [Google Scholar]
- Zhang, D.; Liu, Y.; Zhang, H.; Wang, Y.; Zhang, Z.; Liu, M. Response of Osmotic Regulators and Sucrose Metabolization related Enzymes to Drought Stress in Glycyrrhiza uralensis. Acta Bot. Boreali-Occident. Sin. 2020, 40, 819–827. [Google Scholar]
- Szőllősi, R. Superoxide dismutase (SOD) and abiotic stress tolerance in plants: An overview. In Oxidative Damage to Plants; Academic Press: London, UK, 2014; pp. 89–129. [Google Scholar]
- Yu, D.; Li, X.; Li, Y.; Ali, F.; Li, F.; Wang, Z. Dynamic roles and intricate mechanisms of ethylene in epidermal hair development in Arabidopsis and cotton. New Phytol. 2022, 234, 375–391. [Google Scholar] [CrossRef] [PubMed]
- Ruan, K.; Wang, T.; Bi, N.; Shi, S.; Li, S.; Liu, Z. Allelopathic effects of aqueous extracts of fallen leaves of Acer truncatum on three medicinal plants. Acta Prataculturae Sin. 2024, 33, 151–159. [Google Scholar]
- De Santana, T.A.; Oliveira, P.S.; Silva, L.D.; Laviola, B.G.; De Almeida, A.F.; Gomes, F.P. Water use efficiency and consumption in different Brazilian genotypes of Jatropha curcas L. subjected to soil water deficit. Biomass Bioenergy 2015, 75, 119–125. [Google Scholar] [CrossRef]
- Rawson, H.; Turner, N.; Begg, J. Agronomic and physiological responses of soybean and sorghum crops to water deficits. IV. Photosynthesis, transpiration and water use efficiency in leaves. Funct. Plant Biol. 1978, 5, 195. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Yu, Y.; Zhang, H.; Zhang, Z.; Wang, C.; Wang, K.; Jiang, R. Effects of different plastic film mulching treatments on soil microbial communities and enzyme activities in the Weibei drylands of the Loess Plateau. J. Agric. Environ. Sci. 2020, 39, 2578–2586. [Google Scholar]
- Van den Brink, P.J.; Van den Brink, N.W.; Ter Braak, C.J.F. Multivariate analysis of ecotoxicological data using ordination: Demonstrations of utility on the basis of various examples. Australas. J. Ecotoxicol. 2003, 9, 141–156. [Google Scholar]
- Zhang, X.; Huang, Y.; Li, Z. Multi factor combined action and main controlling factors of soil water in deep loess profiles in Northern Shaanxi. Res. Soil Water Conserv. 2023, 30, 173–180. [Google Scholar]
- Liu, T.; Cheng, J.; Li, H.; Zhu, F. Coupling Relationship between Vegetation Restoration Measure and Soil Factor of Highway Slope in Ili. J. Highw. Transp. Res. Dev. 2021, 38, 28–35. [Google Scholar]
- Qing, Y.; Chen, X.; Chen, G.; Wu, M. Runoff Reduction Effect of Low Impact Development (LID) Reconstruction under Different Rainfall Intensities. China Water Wastewater 2020, 36, 104–108. [Google Scholar]
- Si, M.; Cao, J.; Yang, H. Advances in research on the effects of micro-topography changes on surface hydrological processes. J. Agro-Environ. Sci. 2019, 27, 1587–1595. [Google Scholar]
- Asadi, H.; Shahedi, K.; Jarihani, B.; Sidle, R. Rainfall-runoff modelling using hydrological connectivity index and artificial neural network approach. Water 2019, 11, 212. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, Y.; Huo, Z.; Chen, S.; Zhang, Q. Study on the effect of runoff control in the concave herbaceous field based on numerical simulation. J. Tianjin Agric. Univ. 2024, 31, 69–77. [Google Scholar]
- Su, T.; Han, B.; Ma, H.; Ma, F.; Zhao, F.; Zhou, Y.; Jia, X. Effects of contour trenches engineering measures on soil moisture dynamics and balance of typical steppe in Loess Hilly region. Trans. Chin. Soc. Agric. Eng. 2019, 35, 125–134. [Google Scholar]
- Li, S.; Zhao, G.; Xu, W.; Gao, Z.; Wu, A.; Xu, B. Responses of old world bluestem root systems to changes in soil water conditions. Acta Prataculturae Sin. 2016, 25, 169–177. [Google Scholar]
- Ramos-Scharrón, C.E. Land disturbance effects of roads in runoff and sediment production on dry-tropical settings. Geoderma 2018, 310, 107–119. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Yu, F.; Hu, B.; Yan, J.; Sha, H.; Zhao, H. Differential activity of the antioxidant defence system and alterations in the accumulation of osmolyte and reactive oxygen species under drought stress and recovery in rice (Oryza sativa L.) tillering. Sci. Rep. 2019, 9, 8543. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, L.; Shen, Q.; Yang, J.; Han, X.; Tian, F.; Wu, J. Effects of water stress on photosynthesis, yield, and water use efficiency in winter wheat. Water 2020, 12, 2127. [Google Scholar] [CrossRef]
- Flexas, J.; Baron, M.; Bota, J.; Ducruet, J.M.; Galle, A.; Galmes, J.; Jiménez, M.; Pou, A.; Ribas-Carbó, M.; Sajnani, C.; et al. Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri×V. rupestris). J. Exp. Bot. 2009, 60, 2361–2377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, Y.; Zhang, H.; Zhang, Z.; Wang, Y.; Liu, M. Response of Photosynthesis and Leaf Morphological Characteristics to Drought Stress in Glycyrrhiza uralensis. Bull. Bot. Res. 2021, 41, 449–457. [Google Scholar]
- Liu, C.; Wang, W.; Cui, J.; Li, S. Effects of Drought Stress on Photosynthesis Characteristics and Biomass Allocation of Glycyrrhiza uralensis. J. Desert Res. 2006, 26, 142–145. [Google Scholar]
- Keren, C.; Farrant, J.M. Recovery of the resurrection plant Craterostigma wilmsii from desiccation: Protection versus repair. J. Exp. Bot. 2002, 53, 1805–1813. [Google Scholar]
- Lozano-Parra, J.; Schnabel, S.; Pulido, M.; Gómez-Gutiérrez, Á.; Lavado-Contador, F. Effects of soil moisture and vegetation cover on biomass growth in water-limited environments. Land Degrad. Dev. 2018, 25, 28460–28470. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, A.; Zhang, Y.; Yang, X.; Yang, S.; Zhao, K. Effects of Progressive Drought Stress on the Growth, Ornamental Values, and Physiological Properties of Begonia semperflorens. Horticulturae 2024, 10, 405. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).