Abstract
Pasvik State Nature Reserve is situated in the Arctic zone and its chrysophyte flora is poorly studied. The diversity of the golden-brown algae (chrysophytes) from the Paz river, as well as the peat-bog on its bank, has been investigated using light and scanning electron microscopy. Overall, 34 chrysophyte taxa have been recorded. They represent fifteen genera from five orders. A new species of Synura, S. skaloudiorum, has been described. Morphologically, it resembles S. hibernica, a species restricted in its distribution to Ireland and Newfoundland, but differs mainly in size and structure of the apical scales. Nomenclatural issues on the selected chrysophyte taxa are discussed. The name Kephyrion starmachii was invalidly published, and it was validated. The new nomenclatural combination Chrysothecopsis tubulosus has been proposed. Also, Dinobryon sertularia var. annulatum Shi and Wei has been synonymized with D. annulatum Hilliard and Asmund.
1. Introduction
Golden-brown algae or chrysophytes are a large group of almost exclusively freshwater organisms [1]. According to a recent estimate, it includes 1274 species in 180 genera [2]. Many chrysophytes prefer ponds and small lakes, often with brownish water and with a low content of nutrients. In the temperate zone, the chrysophytes show seasonal occurrence being most abundant in early spring and late autumn [3].
Chrysophytes are characterized by different nutrition modes, namely heterotrophs (e.g., Paraphysomonas), mixotrophs (e.g., Dinobryon), and phototrophs (e.g., Synura). They play an important role in aquatic ecosystems as primary producers and bacteriovores influencing the microbial loop [1,4]. Chrysophytes are widely distributed, but in polar regions, their abundance in freshwater ecosystems may be extremely high [5,6,7]. For instance, in High Arctic lakes, they represented 50–70% of total protist community biomass and 25–50% of total 18S rDNA sequences [5].
Contemporary taxonomy of the chrysophytes is developing rapidly using an integrative approach. Currently, the golden-brown algae are classified within a single class, Chrysophyceae, and nine orders, namely Ochromonadales, Chromulinales, Apoikiales, Chrysosaccales, Hydrurales, Hibberdiales, Segregatospumellales, Synurales, and Paraphysomonadales [3].
The number of studies dealing with the species composition of the golden-brown algae (especially scale-bearing forms) from the (sub-)Arctic has increased in the last decade, e.g., [8,9,10], and several new species have been described [11,12]. Nevertheless, the data on chrysophyte diversity in the waterbodies of the Pasvik State Nature Reserve are still scarce. Sharov [13] studied phytoplankton of the lakes from Kola Peninsula and listed about 20 chrysophyte taxa. Recently, Kapustin and Kapustina [14] have recorded stomatocysts of Chrysococcus furcatus from the Paz River and Kapustin has described the new species of Chrysosphaerella from the peat bog on its bank [15].
The aim of this study is to supplement information on the biodiversity of the golden-brown algae from the Pasvik State Nature Reserve with emphasis on taxonomy and nomenclature of this algal group.
2. Materials and Methods
Pasvik State Nature Reserve is situated in the Pechenga district (Murmansk region) as a narrow zone along the right bank of the Paz (Paatsjoki) River from the Hevoskoski Hydroelectric Station in the south to the Lake Salmiyarvi in the north. Its area is 146.8 km2 [16].
Material for this study was collected in 2017 and 2019. Plankton samples were taken with a plankton net (mesh size 20 μm). A sample from a peat bog was obtained by squeezing water from Sphagnum mosses. Environmental variables (temperature, pH, and conductivity) were measured in situ using a Hanna Combo device (HI 98129, Hanna Instruments, Smithfield, RI, USA).
The samples were taken at the following localities:
- (1)
- The Paz River (N 69°12′46.8″, E 29°18′20.6″); 7 October 2017: environmental variables were not measured;
- (2)
- The Paz River (N 69°00′01.2″, E 28°56′32.0″); 19 June 2019: pH 3.1, temperature 16.5, conductivity 25 µS·cm−1;
- (3)
- A peat bog on the bank of the Paz River (N 69°23.489′, E 29°45.388′); 19 June 2019: environmental variables were not measured.
Microscopical light observations were performed with an Olympus IX71 inverted microscope (Olympus, Hamburg, Germany). For electron-microscopical studies, a few drops of the unpreserved samples were placed on aluminum stubs, air-dried, sputter-coated with 50 nm gold for ten minutes, and observed with a JEOL 6510LV (Papanin Institute for Biology of Inland Waters RAS, Borok, Russia) and a TESCAN Vega III (Borissiak Paleontological Institute RAS, Moscow, Russia) scanning electron microscope, operated at 10 kV and 8 mm distance.
3. Results
A total of 34 taxa of chrysophytes have been recorded from the Pasvik State Nature Reserve (Table 1). They represent fifteen genera from five orders. The most taxon rich genera were Mallomonas (eight taxa), Paraphysomonas, Synura and Dinobryon (four species each). Other genera are represented by one or two species. The number of taxa recorded from the Paz river and from the peat-bog on its bank was almost the same, 18 and 17 taxa, respectively. However, the species compostion was completely different, and only Paraphysomonas aff. vulgaris brevispina was observed at both locations. Only Dinobryon bavaricum, D. divergens, D. sertularia, and Mallomonas akrokomos had previously been recorded in the Paz River [13]. Four taxa (Neotessella laponica, Synura americana, S. macracantha, and Kephyrion starmachii) are reported for the second time in Russia.

Table 1.
List of chrysophyte taxa recorded from the Pasvik State Nature Reserve (“+” means the presence of the taxon).
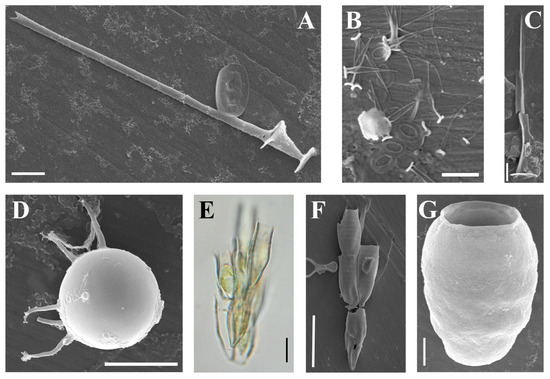
Figure 4.
(A–F) Chrysosphaerella, Spiniferomonas, Chrysastrella, Dinobryon, and Kephyrion taxa from the Pasvik State Nature Reserve. (A) Chrysosphaerella longispina, SEM; (B) Spiniferomonas abei, SEM; (C) Spiniferomonas cf. trioralis, SEM; (D) stomatocyst of Chrysastrella paradoxa, SEM; (E,F) Dinobryon annulatum. (E) LM; (F) SEM; (G) Kephyrion starmachii, SEM. Scale bars: (A,B): 2 µm; (C,G): 1 µm; (D,E): 10 µm; (F): 20 µm.
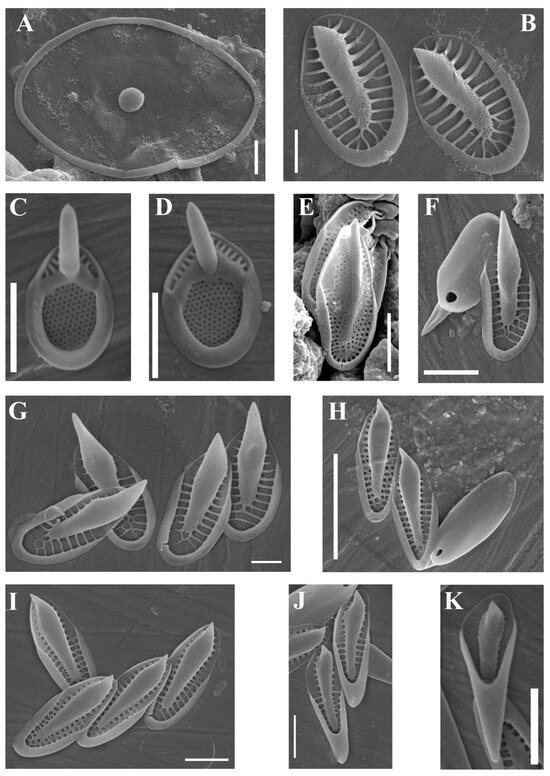
Figure 3.
(A–K) Neotessella and Synura taxa from the Pasvik State Nature Reserve, SEM. (A) Neotessella lapponica; (B) Synura americana; (C,D) Synura echinulata; (E) Synura macracantha; (F–K) Synura skaloudiorum, sp. nov. (F,G) Apical scales; (H,I) body scales; (J,K) rear scales. Scale bars: (A,B,F,G,I,K): 1 µm; (C–E): 2 µm; (H,J): 5 µm.
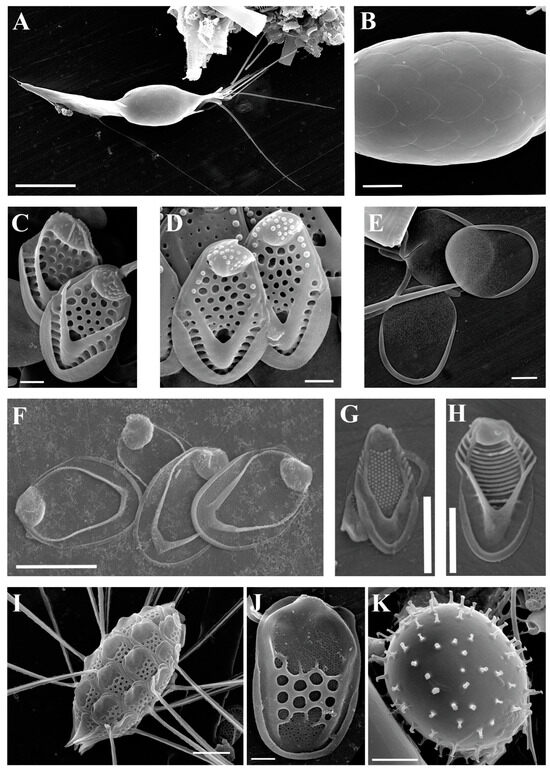
Figure 2.
(A–I) Mallomonas taxa from the Pasvik State Nature Reserve, SEM. (A,B) Mallomonas akrokomos; (A) the whole cell; (B) close-up view of the central part; (C) Mallomonas crassisquama var. crassisquama; (D) Mallomonas crassisquama var. papillosa; (E) Mallomonas caudata; (F) Mallomonas elongata; (G) Mallomonas papillosa; (H) Mallomonas striata; (I,J) Mallomonas punctifera. (I) the whole cell; (J) body scale; (K) stomatocyst of Mallomonas crassisquama. Scale bars: (A): 10 µm; (B,E,G,H): 2 µm; (C,D,J): 1 µm; (F,I,K) 5 µm.
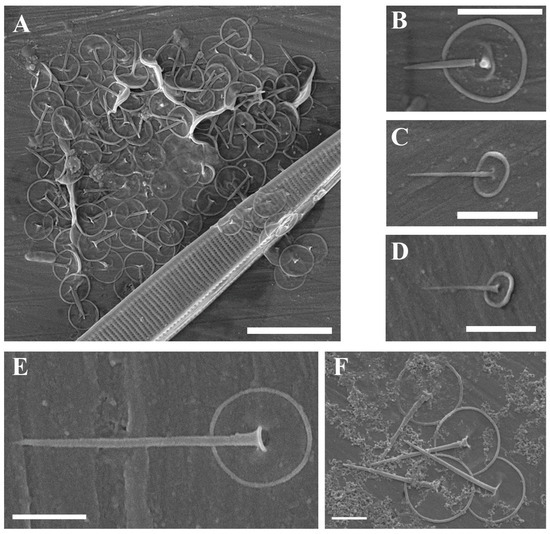
Figure 1.
(A–F) Paraphysomonas taxa from the Pasvik State Nature Reserve, SEM. (A,B) Paraphysomonas aff. vulgaris brevispina; (C,D) Paraphysomonas aff. ovalis; (E) Paraphysomonas cf. acuminata; (F) Paraphysomonas vulgaris. Scale bars: (A): 5 µm; (B–E): 2 µm; (F): 1 µm.
Table 1.
List of chrysophyte taxa recorded from the Pasvik State Nature Reserve (“+” means the presence of the taxon).
| Taxon | Site Number | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Paraphysomonadales | |||
| Paraphysomonas aff. vulgaris brevispina Scoble and Cavalier-Smith (Figure 1A,B) | + | + | |
| Paraphysomonas aff. ovalis Scoble and Cavalier-Smith (Figure 1C,D) | + | ||
| Paraphysomonas cf. acuminata Scoble and Cavalier-Smith (Figure 1E) | + | ||
| Paraphysomonas vulgaris vulgaris Scoble and Cavalier-Smith (Figure 1F) | + | ||
| Synurales | |||
| Mallomonas akrokomos Ruttner (Figure 2A,B) | + | ||
| Mallomonas crassisquama (Asmund) Fott var. crassisquama (Figure 2C) | + | + | |
| Mallomonas crassisquama var. papillosa Siver and Skogstad(Figure 2D) | + | ||
| Mallomonas caudata Iwanoff emend. W. Krieger (Figure 2E) | + | ||
| Mallomonas elongata Reverdin (Figure 2F) | + | + | |
| Mallomonas papillosa K. Harris and D.E. Bradley (Figure 2G) | + | ||
| Mallomonas punctifera Korshikov (Figure 2I,J) | + | ||
| Mallomonas striata Asmund (Figure 2H) | + | ||
| Neotessella lapponica (Skuja) Jo et al. (Figure 3A) | + | ||
| Synura americana Kynčlová and Škaloud (Figure 3B) | + | ||
| Synura echinulata Korshikov (Figure 3C,D) | + | ||
| Synura macracantha (J.B. Petersen and J.B. Hansen) Asmund (Figure 3E) | + | ||
| Synura skaloudiorum Kapustin sp. nov. (Figure 3F–K) | + | ||
| Chromulinales | |||
| Chrysococcus biporus Skuja | + | ||
| Chrysococcus furcatus K.H. Nicholls | + | ||
| Chrysosphaerella longispina Lauterborn (Figure 4A) | + | ||
| Chrysosphaerella septentrionalis Kapustin | + | ||
| Lepochromulina calyx Scherffel | + | ||
| Spiniferomonas abei E. Takahashi (Figure 4B) | + | ||
| Spiniferomonas cf. trioralis E. Takahashi (Figure 4C) | + | + | |
| Chrysosaccales | |||
| Chrysastrella paradoxa Chodat (Figure 4D) | + | ||
| Chrysopyxis bipes F. Stein | + | ||
| Chrysothecopsis tubulosus (Pascher) Kapustin | + | ||
| Ochromonadales | |||
| Dinobryon anulatum D.K. Hilliard and Asmund (Figure 4E,F) | + | ||
| Dinobryon bavaricum O.E. Imhof | + | ||
| Dinobryon divergens O.E. Imhof | + | + | |
| Dinobryon sertularia Ehrenberg | + | ||
| Epipyxis utriculus (Ehrenberg) Ehrenberg | + | ||
| Kephyrion starmachii (Czosnowski) Bourrelly (Figure 4G) | + | ||
| Uroglenopsis botrys (Pascher) Pascher | + | ||
Several Paraphysomonas taxa were observed from the Pasvik State Nature Reserve; however, their exact identification was impossible.
The base plate of the scales is subcircular, 1.88–2.14 × 1.77–2.0 µm, with a dense rim (average width is 0.11 µm); the spines are 1.67–2.0 µm long, uninflated at the base (0.17–0.35 µm wide), and gently tapering to an oblique, dull tip (45–86 nm wide); the S/P ratio is 0.78–1.03.
This species seems to be identical to P. vulgaris brevispina Scoble and Cavalier-Smith [17]. The morphometric data of both taxa overlap. For instance, in P. vulgaris brevispina, the base-plate diameter is 1.7–2.2 µm, whereas, in P. aff. vulgaris brevispina, it is 1.88–2.14 µm. The spines of P. vulgaris brevispina are longer than those of P. aff. vulgaris brevispina (1.8–3.1 µm vs. 1.67–2.0 µm, respectively). However, in P. aff. vulgaris brevispina, the spines are always equal or shorter than base-plate; so, the spine length to base-plate diameter ratio (S/P ratio) is lesser than in P. vulgaris brevispina (0.78–1.03 vs. 1.0–1.6). Scoble and Cavalier-Smith [17] depicted only scales with spines which are longer than the base-plate. Notably, the spine of P. vulgaris brevispina is inflated at the base, whereas in P. aff. vulgaris brevispina it is uninflated.
Interestingly, a specimen similar to my Paraphysomonas aff. vulgaris brevispina was reported from the Boguchany Reservoir (Krasnoyarsk Territory and Irkutsk Region, Russia) under the name P. bandaiensis E. Takahashi [18]. The differences between P. vulgaris brevispina and P. aff. vulgaris brevispina are sufficient enough to describe at least a new subspecies of P. vulgaris or even a new species. However, I would prefer to accumulate additional data before the final decision.
The base plate of the scales is oval, 1.07–1.19 × 0.77–0.84 µm, with an irregularly thickened margin (average width is 0.11 µm); the spines are 2.21–2.22 µm long, and the spine base is 0.15–0.26 µm wide, tapering to rounded tip (29–68 nm wide); the S/P ratio is 1.85–2.07.
Paraphysomonas aff. ovalis resembles P. ovalis Scoble and Cavalier-Smith [17], which is the only known species with oval scales. Unfortunately, Scoble and Cavalier-Smith did not mention base-plate length, so it was measured using depicted scales (Figure 3F, [17]). The base-plates of P. ovalis are smaller than those of P. aff. ovalis (0.7–0.9 × 0.47–0.55 µm vs. 1.07–1.19 × 0.77–0.84 µm, respectively). Also, P. aff. ovalis has scales with much denser margin and longer spines (1.1–1.9 µm vs. 2.21–2.22 µm, respectively).
Also, both taxa differ in their ecological preferences. Paraphysomonas ovalis was isolated from the soil in Harcourt Arboretum, Oxfordshire, UK. P. aff. ovalis is a paludal species, recorded from a peat-bog on the bank of the Paz River. Similar scales have observed from the peatlands in Moscow Region and Kamchatka (Kapustin, unpubl. observ.). Also, the specimens from a mire in the North Caucasus [19] identified as P. ovalis correspond better to P. aff. ovalis than to P. ovalis. Probably, P. aff. ovalis can be segregated into a new species, but further investigations are needed.
The scales of unusual Synura species were observed from a peat-bog on the bank of the Paz River. Once compared with the similar taxa, this Synura represents a new species to science. Its description is given below.
Synura skaloudiorum Kapustin sp. nov. (Figure 3F–K)
Colony dimensions unknown. Body scales 5.2–6.0 µm long and 1.8–2.3 µm wide, consisting of a basal plate with a centrally raised rounded keel protruding into an acute tip (Figure 3H,I). The keel is cylindrical and occasionally slightly widened anteriorly. The basal plate is anteriorly perforated by a rounded or elongated base hole (diameter 0.35–0.52 × 0.23–0.28 µm). Numerous struts (32–40), sometimes interconnected by transverse folds, extend regularly from the keel to the scale perimeter (Figure 3H,I). Apical scales are 4.1–4.5 µm long and 1.9–2.2 µm wide (Figure 3F,G). Struts are less numerous (21–25) without interconnections. The keel of the apical scales is widened in the central part and ends in a long, prominent, and acute tip (Figure 3F,G). Rear scales are 5.0–6.1 µm long and 1.3–1.7 µm wide (Figure 3J,K).
Holotype (here designated): Portion of a single gathering of cells on SEM stub #P32 deposited at the Herbarium of the Papanin Institute for Biology of Inland Waters RAS, Borok (IBIW). The sample was collected on 19 June 2019. Figure 3F–K illustrates the holotype.
Registration: https://phycobank.org/105064
Type locality: peat bog on the bank of the Paz River (69°23.489′ N, 29°45.388′ E), Pasvik Nature Reserve, Murmansk Region (Russia).
Etymology: the species is named after my colleagues from the Charles University in Prague (Czech Republic), Drs. Pavel Škaloud and Magda Škaloudová, for their contribution in the taxonomy of the genus Synura and, particularly, the S. petersenii species complex.
Distribution: this species is distributed in other far-north Russian regions, like the Magadan Region (Figure 10, [20]) and the Republic of Sakha (Yakutia) (Figure 7H, [9]).
3.1. Nomenclatural and Taxonomic Comments on the Selected Taxa
3.1.1. The Correct Authorship of Chrysococcus furcatus
This species was described as a member of euglenoids under the name Trachelomonas furcata Dolgoff based on the empty loricae [21]. Woronichin [22] pointed out that this species is a chrysophycean cyst. Later, Deflandre transferred it into a cyst genus Chrysastrella Chodat under the name Chrysastrella furcata (Dolgoff) Deflandre [23]. In 1981, Nicholls discovered a vegetative stage of this species and redefined it as Chrysococcus furcatus [24]. Although Nicholls stated that he proposed a new combination, he provided a Latin description and designated his material from West Twin Lake, Ontario, Canada as a holotype [24]. Whereas, according to Art. 7.3 of the International Code of Nomenclature for algae, fungi, and plants, a new combination is typified by the type of the basionym [25]. Therefore, Chrysococcus furcatus should be treated as a new species described by Nicholls, and the correct author citation should be Chrysococcus furcatus K.H. Nicholls, not C. furcatus (Dolgoff) K.H. Nicholls.
3.1.2. Validation of Kephyrion starmachii
In 1948, Czosnowski described a new loricate Chrysophyte genus, Cyathochrysis, which differed from Kephyrion mainly by its thick brown lorica [26]. Later, Bourrelly [27] revised the genus Kephyrion and synonymized the genera Cyathochrysis and Stenokalyx with it. However, his combination, K. starmachii (Czosnowski) Bourrelly, was introduced without a full and direct reference to the place of valid publication of basionym, so it is invalid. This combination is validated herein:
Kephyrion starmachii (Czosnowski) Bourrelly, comb. nov.
Basionym: Cyathochrysis starmachii Czosnowski. 1948. Poznań. Towarz. Przyj. Nauk Wydz. Mat.-Przyr., Prace Kom. Biol. 11 (4): 4, pl. I: Figure 20.
Synonym: Kephyrion starmachii (Czosnowski) Bourrelly [27], nom. inval. (Art. 41.5)
Registration: https://phycobank.org/105065
3.1.3. A New Combination in the Genus Chrysothecopsis Conrad
According to Ellis-Adam [28], the name Stephanoporos Conrad and Pascher ex Pascher is a superfluous substitute name for Chrysotheca Scherffel, and it should be rejected. So, a new combination for Stephanoporos tubulosus is proposed:
Chrysothecopsis tubulosus (Pascher) Kapustin, comb. nov.
Basionym: Stephanoporos tubulosus Pascher. 1940. Arch. Protistenk. 93: 344, figs. 7, 8a–d.
Registration: https://phycobank.org/105066
3.1.4. Mallomonas dubia vs. Mallomonas caudata
Recently, Kapustin and Kulikovskiy have proposed to conserve the name Mallomonas caudata Iwanoff emend. W. Krieger against M. fastigata Zacharias ex Lemmermann [29]. However, the Nomenclature Committee for Algae did not recommend the conservation [30] because the name Mallomonas dubia (Seligo) Lemmermann has priority against both M. caudata and M. fastigata.
Molecular evidence shows that several linages exist within M. caudata species complex (P. Škaloud, pers.comm.) Interestingly, scales of M. caudata from the Paz River were covered with small, scattered papillae (see Figure 2E), and they possibly belong to another pseudocryptic species. Therefore, it is better to continue using the name M. caudata instead of M. dubia until further taxonomic revision.
3.1.5. New Synonyms of Dinobryon annulatum
Dinobryon annulatum (“anulatum”) was described from the sphagnous bogs and Carex marshes in Alaska [31]. This species can easily be recognized by its colonies, which have tight-fitting loricae with thickened ring-like structures. Skuja (Pl. 63, Figures 14–18, [32]) illustrated a colony of Dinobryon sertularia from Abisko in the Swedish Lapland, which actually belongs to D. annulatum.
In 1984, Shi and Wei [33] described a morphologically similar taxon under the name Dinobryon sertularia var. annulatum from Tibet. Quite recently, Pang et al. [34] provided LM and SEM micrographs of this taxon and carried out an elemental analysis of its loricae. Since both D. annulatum and D. sertularia var. annulatum seem to be identical in colony appearance, lorica and stomatocyst structure, and dimensions, the latter taxon should be regarded as a synonym of D. annulatum.
Dinobryon annulatum [“anulatum”] Hilliard and Asmund. 1963. Hydrobiologia 22: 383, text-Figure 31, Pl. 3: Figure 11 [31].
= Dinobryon sertularia apud Skuja [32]
= Dinobryon sertularia var. annulatum Shi and Wei in Shi [33], syn. nov.
4. Discussion
4.1. Chrysophyte Flora of the Pasvik State Nature Reserve with Comments on the Selected Taxa
The chrysophyte flora of the Pasvik Reserve is rather typical for the temperate zone of Europe. For example, in the 1960s, Kristiansen revealed 23 chrysophyte taxa from 13 lakes in the Finnish Lapland [35], and many of them occurred in the Paz River too.
Many taxa from the Pasvik Reserve are widely distributed or cosmopolitan (e.g., Dinobryon divergens, D. sertularia, Mallomonas akrokomos, M. caudata, Spiniferomonas abei, etc.) Nevertheless, the flora contains some taxa with limited distribution. For instance, Neotessella lapponica, formerly known as Synura lapponica, is a rare species restricted in its distribution to the Northern Hemisphere [36]. In Russia, it had previously been reported from Rybinsk Reservoir (Yaroslavl Region) [37,38,39]. The record of N. lapponica in the Paz River seems to be the northernmost one. Dinobryon annulatum is distributed in the Arctic and Subarctic [31,32,40], but it also occurs in the mountain regions of China [33,34].
Mallomonas crassisquma is one of the most widely distributed Mallomonas taxa [41]. In Russia, it is also often encountered [39], but M. crassisquama var. papillosa is less common than M. crassisquama var. crassisquama. Siver and Skogstad [41] believed it was unlikely that the papillae formed in response to ecological conditions. However, since both varieties occurred together in the Paz River, the question arises whether the presence of papillae is a reliable taxonomic feature or not. A stomatocyst of M. crassisquama (Figure 2K), which resembles the one observed by other authors [42], was also revealed.
Synura macracantha and S. americana were recorded for the second time in Russia. Voloshko [43] erroneously included S. macracantha in her monograph and mentioned that it was reported from the Upper Volga and the Bolshezemelskaya tundra. However, Balonov [38], who studied silica-scaled Chrysophytes from the Upper Volga, noted “the species was not found in the USSR” [38] (p. 79). Similarly, this species was not included in the paper by Siver et al. [44] devoted to the scaled Chrysophyte flora of the Bolshezemelskaya tundra. Only recently has it been reported in the Yaroslavl Region [39]. Synura americana had previously been reported from the waterbodies of South Urals [45].
We revealed several epiphytic chrysophytes attached to the filamentous algae, e.g., Chrysothecopsis tubulosus, Epipyxis utriculus, and Lepochromulina calyx. The latter species is a rather common epiphytic chrysophyte, but it is rarely reported [46,47]. In Russia, it has previously been recorded by Wisłouch [48] from the Leningrad Region. This species has a lorica that is attached to the filamentous algae with a gelatinous stalk [46,47].
A mass development of Chrysastrella paradoxa was revealed in a peat-bog on the bank of the Paz River. Together with a cryptophyte, Cryptomonas loricata Chodat emend. Hoef-Emden, they were the most abundant flagellates in this locality [49]. Initially, we identified it as Ochromonas sp. and established several monoclonal cultures, which, unfortunately, died soon after encystment. However, further morphological examination of stomatocysts using SEM allowed us to identify our isolates as Chrysastrella paradoxa (see Figure 4D).
For many years, Chrysastrella Chodat has been treated as a cyst genus, and only recently has it been re-instated and re-defined by Andersen et al. [50]. Based on the similarity of stomatocysts of Ochromonas tuberculata Hibberd with those of Chrysastrella paradoxa, Andersen et al. [50] synonymized the former species. Interestingly, Charvet et al. [5] revealed several clones from the Char Lake (Canadian Arctic Archipelago) which had 96% similarity to O. tuberculata in 18S rDNA sequences. Similarly, Esp24 clones from the lake Esperanza (Antarctic Peninsula) were moderately related to O. tuberculata (96.7% similarity) [7]. These clones from polar regions most likely represent yet undescribed new genera of the Ochromonas-like Chrysophytes. Nevertheless, C. paradoxa (=O. tuberculata) seems to be a rather common species in peatlands. Recently, Kapustin et al. [51] has recorded a mass development of stomatocysts of this species from a bog in Subpolar Urals (Russia).
A rare Chrysophyte Kephyrion starmachii was recorded in Russia only once [52], but without any illustrations that could confirm this record. Our Figure 4G is the first SEM micrograph of this species.
4.2. Morphological Comparison of Synura skaloudiorum with Similar Taxa
Synura skaloudiorum belongs to Synura sect. Petersenianae. Taxa from this section are characterized by scales having a central keel that may end in spine-like projections. Currently, this section includes 26 taxa [12,53,54,55,56,57,58].
Although S. skaloudiorum lacks genetic characterization, based on morphological similarity, we can assume it might belong to the same subclade as Synura hibernica Škaloud and Škaloudová [56] and Synura fluviatilis Škaloud, Škaloudová, and Siver [58]. The body scales of both S. skaloudiorum and S. hibernica are almost identical, but in S. skaloudiorum, they are slightly longer and wider (5.2–6.0 μm × 1.8–2.3 μm vs. 3.4–5.6 μm × 1.2–2.0 μm) with a longer keel tip. The apical scales of S. skaloudiorum are larger than those of S. hibernica (4.1–4.5 μm × 1.9–2.2 μm vs. 2.9–3.3 μm × 1.6–1.9 μm), with a wider keel of completely different shape. The scales of S. fluviatilis can be easily differentiated from those of S. skaloudiorum by their general appearance and lesser dimensions.
Synura hibernica is restricted in its distribution to Ireland and Newfoundland [56,58], and S. fluviatilis is currently only known from Newfoundland [58]. In Russia, the scales of S. skaloudiorum have been previously observed from the Magadan Region as S. petersenii f. kufferathii (Figure 10, [20]) and Republic of Sakha (Yakutia) as Synura sp. 1 (Figure 7H, [9]).
5. Conclusions
In this preliminary study based on a limited number of samples, a rich chrysophyte flora has been found. A total of 34 taxa have been identified, including some rare and new species. The discovery of the new species (Synura skaloudiorum) and putatively new taxa (Paraphysomonas aff. vulgaris brevispina and P. aff. ovalis) shows that the Arctic chrysophytes remain unexplored and require further studies.
Funding
This work was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme No. 122042700045-3).
Data Availability Statement
Data is contained within the article.
Acknowledgments
The author is grateful to the staff of the Interlaboratory Center of Electron Microscopy of the Papanin Institute for Biology of Inland Waters, RAS and Roman Rakitov (Borissiak Paleontological Institute, RAS) for the technical assistance with the SEM. Special thanks to Alexander A. Bobrov (Papanin Institute for Biology of Inland Waters, RAS) and Gennady A. Dmitrenko (Pasvik State Nature Reserve) for help during sampling.
Conflicts of Interest
The author declares no conflict of interest.
References
- Bock, C.; Olefeld, J.L.; Vogt, J.C.; Albach, D.C.; Boenigk, J. Phylogenetic and functional diversity of Chrysophyceae in inland waters. Org. Divers. Evol. 2022, 22, 327–341. [Google Scholar] [CrossRef]
- Guiry, M.D. How many species of algae are there? A reprise. Four kingdoms, 14 phyla, 63 classes and still growing. J. Phycol. 2024, 60, 214–228. [Google Scholar] [CrossRef]
- Kristiansen, J.; Škaloud, P. Chrysophyta. In Handbook of the Protists, 2nd ed.; Archibald, J.M., Simpson, A.G.B., Slamovits, C.H., Eds.; Springer International Publishing AG: Cham, Switzerland, 2017; pp. 331–366. [Google Scholar] [CrossRef]
- Lengyel, E.; Barreto, S.; Padisák, J.; Stenger-Kovács, C.; Lázár, D.; Buczkó, K. Contribution of silica-scaled chrysophytes to ecosystems services: A review. Hydrobiologia 2023, 850, 2735–2756. [Google Scholar] [CrossRef]
- Charvet, S.; Vincent, W.F.; Lovejoy, C. Chrysophytes and other protists in High Arctic lakes: Molecular gene surveys, pigment signatures and microscopy. Polar Biol. 2012, 35, 733–748. [Google Scholar] [CrossRef]
- Boopathi, T.; Faria, D.G.; Lee, M.-D.; Lee, J.; Chang, M.; Ki, J.-S. A molecular survey of freshwater microeukaryotes in an Arctic reservoir (Svalbard, 79° N) in summer by using next-generation sequencing. Polar Biol. 2015, 38, 179–187. [Google Scholar] [CrossRef]
- Izaguirre, I.; Unrein, F.; Schiaffino, M.R.; Lara, E.; Singer, D.; Balagué, V.; Gasol, J.M.; Massana, R. Phylogenetic diversity and dominant ecological traits of freshwater Antarctic Chrysophyceae. Polar Biol. 2021, 44, 941–957. [Google Scholar] [CrossRef]
- Gusev, E.S.; Guseva, E.E.; Gabyshev, V.A. Taxonomic composition of silica-scaled chrysophytes in rivers and lakes of Yakutia and Magadanskaya oblast (Russia). Nova Hedw. Beih. 2018, 147, 105–117. [Google Scholar] [CrossRef]
- Bessudova, A.; Gabyshev, V.; Firsova, A.; Gabysheva, O.; Bukin, Y.; Likhoshway, Y. Diversity Variation of Silica-Scaled Chrysophytes Related to Differences in Physicochemical Variables in Estuaries of Rivers in an Arctic Watershed. Sustainability 2021, 13, 13768. [Google Scholar] [CrossRef]
- Bessudova, A.; Gabyshev, V.; Bukin, Y.; Gabysheva, O.; Likhoshway, Y.V. Species richness of scaled Chrysophytes in arctic waters in the Tiksi Region (Yakutia, Russia). Acta Biol. Sib. 2022, 8, 431–459. [Google Scholar] [CrossRef]
- Bessudova, A.; Firsova, A.; Bukin, Y.; Kopyrina, L.; Zakharova, Y.; Likhoshway, Y. Under-Ice Development of Silica-Scaled Chrysophytes with Different Trophic Mode in Two Ultraoligotrophic Lakes of Yakutia. Diversity 2023, 15, 326. [Google Scholar] [CrossRef]
- Bessudova, A.; Gabyshev, V.; Likhoshway, Y. Record of two rare taxa from Synura genus (Chrysophyceae) with a description of a new species (Synura tiksiensis sp. nov.) near the arctic settlement of Tiksi, Yakutia, Russia. Phytotaxa 2022, 560, 247–253. [Google Scholar] [CrossRef]
- Sharov, A.N. Phytoplankton from the Lakes of Kola Peninsula; Karelian Research Center RAS: Petrozavodsk, Russia, 2004; pp. 1–98. (In Russian) [Google Scholar]
- Kapustin, D.A.; Kapustina, N.V. New records of Chrysococcus furcatus (Chrysophyceae) in Russia. Inland Wat. Biol. 2018, 11, 384–386. [Google Scholar] [CrossRef]
- Kapustin, D.; Kulikovskiy, M. Chrysosphaerella septentrionalis sp. nov. (Chrysophyceae, Chromulinales), a New Species from the Arctic including the Description of Chrysosphaerellaceae, fam. nov. Plants 2022, 11, 3166. [Google Scholar] [CrossRef]
- Borovichev, E.A.; Boychuk, M.A. Bryophytes of the Pasvik State Nature Reserve; Karelian Research Center RAS: Petrozavodsk, Russia, 2018; pp. 1–123. (In Russian) [Google Scholar]
- Scoble, J.M.; Cavalier-Smith, T. Scale evolution in Paraphysomonadida (Chrysophyceae): Sequence phylogeny and revised taxonomy of Paraphysomonas, new genus Clathromonas, and 25 new species. Eur. J. Protistol. 2014, 50, 551–592. [Google Scholar] [CrossRef]
- Bessudova, A.; Likhoshway, Y. Scaled chrysophytes (Chrysophyceae) of the Boguchany Reservoir. Sovrem. Nauka Aktual. Probl. Teor. Praktiki. Ser. Estestv. Tekhnicheskiye Nauk. 2017, 11, 4–11. (In Russian) [Google Scholar]
- Prokina, K.I.; Philippov, D.A. Heterotrophic Flagellates from Mires of the North Caucasus, Russia. Inland Water Biol. 2021, 14, 500–516. [Google Scholar] [CrossRef]
- Kuzmin, G.V.; Kuzmina, V.A. Armored representatives of the golden algae from the Magadan Region. Novosti Sistem. Nizsh. Rast. 1987, 24, 40–42. (In Russian) [Google Scholar]
- Dolgoff, G.I. Zur Systematik von Trachelomonas Ehrb. Russ. Hydrobiol. Zeit. 1922, 1, 289–291. (In Russian) [Google Scholar]
- Woronichin, N.N. Notiz über Chrysomonadencysten in Plankton der Flüsse Newa und Boljschaja Newka. Acta Inst. Bot. Acad. Sci. USSR 1933, 1, 7. (In Russian) [Google Scholar]
- Deflandre, P. Sur l’abus de l’emploi en paléontologie du nom de genre Trachelomonas. Ann. Protistol. 1934, 4, 151–165. [Google Scholar]
- Nicholls, K.H. Chrysococcus furcatus (Dolg.) comb. nov.: A new name for Chrysastrella furcata (Dolg.) Defl. based on the discovery of the vegetative stage. Phycologia 1981, 20, 16–21. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. (Eds.) International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code); Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Koeltz Botanical Books: Glashütten, Germany, 2018; 254p, (Regnum Vegetabile 159). [Google Scholar] [CrossRef]
- Czosnowski, J. Materiały do flory wiciowców Polski. Poznań. Towarz. Przyj. Nauk Wydz. Mat.-Przyr. Prace Kom. Biol. 1948, 11, 363–402. [Google Scholar]
- Bourrelly, P. Recherches sur les Chrysophycées. Morphologie, Phylogénie, Systématique. Rev. Algol. Mém. Hors-Sér. 1957, 1, 1–412. [Google Scholar]
- Ellis-Adam, A.C. Some new and interesting benthic Chrysophyceae from a Dutch moorland pool complex. Acta Bot. Neerl. 1983, 32, 1–23. [Google Scholar] [CrossRef]
- Kapustin, D.A.; Kulikovskiy, M.S. (2743) Proposal to conserve the name Mallomonas caudata against M. fastigata (Chrysophyceae: Mallomonadaceae). Taxon 2020, 69, 614–615. [Google Scholar] [CrossRef]
- Andersen, R.A. Report of the Nomenclature Committee for Algae 24. Taxon 2023, 72, 652–653. [Google Scholar] [CrossRef]
- Hilliard, D.K.; Asmund, B. Studies on Chrysophyceae from some ponds and lakes in Alaska. II. Notes on the genera Dinobryon, Hyalobryon and Epipyxis with descriptions of new species. Hydrobiologia 1963, 22, 331–397. [Google Scholar] [CrossRef]
- Skuja, H. Grundzüge der Algenflora und Algenvegetation der Fjeldgegenden um Abisko in Schwedisch-Lappland. Nova Acta Reg. Soc. Sci. Upsal. 1964, 18, 465. [Google Scholar]
- Shi, Z. Some new taxa of Euglenophyta from Xizang (Tibet). Acta Phytotax. Sin. 1984, 22, 337–342. [Google Scholar]
- Pang, W.; Zhuang, J.; Wang, Q. Chrysophytes from the Great Xing’an Mountains, China. Nova Hedw. Beih. 2019, 148, 49–61. [Google Scholar] [CrossRef]
- Kristiansen, J. Flagellates from Finnish Lappland. Bot. Tidsskr. 1964, 59, 315–333. [Google Scholar]
- Němcová, Y.; Pichrtová, M. The rare species Synura lapponica (Synurophyceae) new to the Czech Republic, local vs. global diversity in colonial synurophytes. Biologia 2009, 64, 1070–1075. [Google Scholar] [CrossRef]
- Balonov, I.M.; Kuzmin, G.V. Species of the genus Synura Ehr. (Chrysophyta) in water reservoirs of the Volga cascade. Bot. Zhurn. 1974, 59, 1675–1686. (In Russian) [Google Scholar]
- Balonov, I.M. Genus Synura Ehr. (Chrysophyta). Biology, ecology and systematics. In Biologia, Morphologia i Sistematika Vodnykh Organizmov; Kamshilov, M.M., Ed.; Nauka: Leningrad, Russia, 1976; pp. 61–81. (In Russian) [Google Scholar]
- Kapustin, D.A. An updated checklist of the silica-scaled chrysophytes from Russia. Phytotaxa 2024, in press. [Google Scholar]
- Yermolaev, V.I.; Safonova, T.A. Algae of the genus Dinobryon Ehr. (Chrysophyta) from the water-bodies of Taimyr. Bot. Zhurn. 1974, 59, 556–560. [Google Scholar]
- Siver, P.A.; Skogstad, A. Morphological variation and ecology of Mallomonas crassisquama (Chrysophyceae). Nord. J. Bot. 1988, 7, 99–107. [Google Scholar] [CrossRef]
- Gretz, M.R.; Sommerfeld, M.R.; Wujek, D.E. Scaled Chrysophyceae of Arizona: A Preliminary Survey. J. Arizona-Nevada Acad. Sci. 1979, 14, 75–80. [Google Scholar]
- Voloshko, L.N. Chrysophycean Algae in Water Bodies of the Northern Russia; Renome: St. Petersburg, Russia, 2017; pp. 1–380. (In Russian) [Google Scholar]
- Siver, P.A.; Voloshko, L.N.; Gavrilova, O.V.; Getsen, M.V. The scaled chrysophyte flora of the Bolshezemelskaya tundra (Russia). Nova Hedw. Beih. 2005, 128, 125–150. [Google Scholar]
- Snitko, L.V.; Safronova, T.V.; Snitko, V.P. Chrysophycean algae (Chrysophyceae) in waterbodies of the South Urals and Transural Plateau. Genus Synura (Synuraceae) section Petersenianae. Bot. Zhurn. 2022, 107, 333–349. [Google Scholar]
- Kapustin, D. New and rare species of the loricate golden algae (Chrysophyceae) for the Ukrainian flora from the Polessian Nature Reserve. Novosti Sist. Nizsh. Rast. 2015, 49, 32–46. [Google Scholar] [CrossRef]
- Andersen, R.A. Lepochromulina bursa Scherffel (Chrysophyceae) from Michigan, with ultrastructural observations on the flagellar apparatus and lorica. Nova Hedw. Beih. 2021, 151, 9–26. [Google Scholar] [CrossRef]
- Wislouch, S. Sur les Chrysomonadines des environs de Petrograd. Zhurn. Mikrobiol. 1914, 1, 251–278. [Google Scholar]
- Kulizin, P.V.; Martynenko, N.A.; Gusev, E.S.; Kapustin, D.A.; Vodeneeva, E.L.; Kulikovskiy, M.S. New Species of the Genus Cryptomonas (Cryptophyceae) in the Flora of Russia. Inl. Wat. Biol. 2022, 15, 227–237. [Google Scholar] [CrossRef]
- Andersen, R.A.; Graf, L.; Malakhov, Y.; Yoon, H.S. Rediscovery of the Ochromonas type species Ochromonas triangulata (Chrysophyceae) from its type locality (Lake Veysove, Donetsk region, Ukraine). Phycologia 2017, 56, 591–604. [Google Scholar] [CrossRef]
- Kapustin, D.; Sterlyagova, I.; Patova, E. Morphology of Chrysastrella paradoxa stomatocysts from the Subpolar Urals (Russia) with comments on related morphotypes. Phytotaxa 2019, 402, 295–300. [Google Scholar] [CrossRef]
- Naumenko, Y.V.; Gidora, O.Y. The genus Kephyrion Pascher in the basin of the Sabun River (Western Siberia, Russia). Contemp. Probl. Ecol. 2014, 7, 569–573. [Google Scholar] [CrossRef]
- Němcová, Y.; Nováková, S.; Řezáčová-Škaloudová, M. Synura obesa sp. nov. (Synurophyceae) and other silica-scaled chrysophytes from Abisko (Swedish Lapland). Nova Hedw. 2008, 86, 243–254. [Google Scholar] [CrossRef]
- Siver, P.A.; Lott, A.M. Descriptions of two new species of Synurophyceae from a bog in Newfoundland, Canada: Mallomonas baskettii sp. nov. and Synura kristiansenii sp. nov. Nova Hedw. 2016, 102, 501–511. [Google Scholar] [CrossRef]
- Škaloud, P.; Kynčlová, A.; Benada, O.; Kofroňová, O.; Škaloudová, M. Toward a revision of the genus Synura, section Petersenianae (Synurophyceae, Heterokontophyta): Morphological characterization of six pseudocryptic species. Phycologia 2012, 51, 303–329. [Google Scholar] [CrossRef]
- Škaloud, P.; Škaloudová, M.; Procházková, A.; Němcová, Y. Morphological delineation and distribution patterns of four newly described species within the Synura petersenii species complex (Chrysophyceae, Stramenopiles). Eur. J. Phycol. 2014, 49, 213–229. [Google Scholar] [CrossRef]
- Jo, B.Y.; Kim, J.I.; Škaloud, P.; Siver, P.A.; Shin, W. Multigene phylogeny of Synura (Synurophyceae) and descriptions of four new species based on morphological and DNA evidence. Eur. J. Phycol. 2016, 51, 413–430. [Google Scholar] [CrossRef]
- Škaloud, P.; Škaloudová, M.; Jadrná, I.; Bestová, H.; Pusztai, M.; Kapustin, D.; Siver, P.A. Comparing morphological and molecular estimates of species diversity in the freshwater genus Synura (Stramenopiles): A model for understanding diversity of eukaryotic microorganisms. J. Phycol. 2020, 56, 574–591. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).