Abstract
Textile industry wastewater (WW) has intense color, high chemical oxygen demand (COD), pH, and salinity, making it challenging for conventional treatment. Soda lakes, with high alkalinity and salinity, host diverse microbes capable of textile dye degradation. This study evaluated anaerobic/aerobic reactors using alkaliphilic microbial consortia from Lake Chitu, an Ethiopian soda lake, for treating synthetic and real textile WW. The experimental setup consisted of a first-stage anaerobic reactor followed by a second-stage aerobic reactor, operating continuously with a predetermined flow rate and hydraulic residence time. After evaluating synthetic WW, real textile WW was collected in two batches (rounds I and II). The treatment setup removed 99% of the dye color for synthetic WW, 98% for round I, and 96% for round II. COD removal was 87% for synthetic WW, 86% for round I, and 93.37% for round II. TKN removal reached 90% for synthetic WW, 91% for round I, and 96% for round II at a steady state. Residual COD and TKN values met the final effluent discharge standards. GC–MS and IR analyses revealed that dyes were broken down into intermediate organic compounds under anaerobic conditions and further degraded into smaller molecules under aerobic conditions. This integrated reactor approach effectively removes dyes and enhances COD and TKN removal. The study’s novelty lies in evaluating both synthetic and real textile WW using integrated reactors under alkaline conditions in a continuous process, inoculating alkaliphilic consortia, without pre-enrichment or external nutrient addition to real WW. The study provides insights into the effectiveness of alkaliphilic microbial consortia derived from soda lakes for treating textile WW using integrated reactor conditions. Reactor microbiome characterization is needed to further explore microbial diversity and community structure.
1. Introduction
Textile manufacturing is known to result in the release of large quantities of wastewater characterized by high chemical oxygen demand (COD), high pH, high salinity, and high concentration of dye [1]. Therefore, the release of untreated textile wastewater into receiving water bodies can cause severe environmental pollution [2]. Textile industry wastewater is especially dangerous due to its high dye concentration and high COD, which cause environmental pollution and are considered to be the most challenging to remove [3]. The high COD causes the rapid depletion of dissolved oxygen, affecting aquatic life. Additionally, the different dyes released by the textile industry are toxic and carcinogenic, affecting aquatic life and human health [4,5]. In dyeing processes, up to 20% of the WW containing these dyes is discharged into the environment [6]. These dyes are photolytically, chemically, and biologically highly stable and highly persistent [7,8].
Physicochemical and biological methods have been used for the treatment of textile dye WW. Many physicochemical methods have limitations, including not being environmentally friendly, the production of sludge, and inefficient dye color removal [9]. The huge volumes of WW generated by the textile industry incur high chemical costs owing to its high organic matter content [10]. Hence, biological treatment is environmentally friendly, cost-effective, and efficient compared with physicochemical methods [11]. However, conventional biological treatment of textile WW has several limitations, including low efficiency for high-strength WW, sensitivity to toxic compounds, inability to handle extreme conditions, and incomplete degradation of complex compounds [12]. Therefore, advanced biological treatment techniques for textile WW are needed because conventional systems struggle with the high concentrations of pollutants contained in the WW, such as dyes, COD, and specific toxic compounds. Conventional processes employed for textile WW treatment are mostly aerobic processes such as activated sludge and trickling filters, which can be ineffective in reducing complex chemicals in the WW.
One of the currently appealing advanced techniques for treating textile WW is the use of integrated reactor conditions, specifically anaerobic processes followed by aerobic ones. This approach is important for effectively reducing complex dye structures and further oxidizing dye transformation products. In contrast, single reactor conditions have not been as effective for complete dye degradation [13,14]. High molecular weight pollutants, such as dyes, can first be reduced by anaerobic microorganisms into simple, assimilable compounds, while subsequent aerobic conditions enhance complete dye degradation [15,16,17,18,19]. Anaerobic/aerobic treatment processes have recently been recognized as more effective approaches to enhance the treatment efficiency of textile WW. However, efficiency still needs improvement by operating the system under alkaline conditions using alkaliphilic microorganisms.
Non-alkaliphilic microbial consortia are ineffective in treating textile WW due to their alkaline and saline nature, as well as their complex composition, which includes toxic chemicals. Therefore, using alkaliphilic or alkaline-tolerant microbial consortia is critical for effectively treating textile WW. Soda lake-derived alkaliphilic microbial consortia are expected to be effective in this context. The hypothesis is that these consortia, adapted to conditions similar to those encountered in textile WW, can effectively degrade textile dyes. These microorganisms are resistant to harsh conditions, such as alkaline pH and high salinity, and possess enhanced enzymatic capabilities, high tolerance to toxic compounds, metabolic diversity, and adaptability. Additionally, they have several adaptive response mechanisms that promote their survival in the challenging environment of textile WW [20].
This study evaluated the treatment performance of a laboratory-scale continuous anaerobic/aerobic integrated process using alkaliphilic microbial consortia derived from soda lakes on both synthetic and real textile WW. The research aimed to answer two questions: (i) Do soda lake microbial consortia effectively adapt to and remove dyes from synthetic and real textile WW? (ii) Is the integrated anaerobic/aerobic process effective in enhancing organic matter removal?
2. Material and Methods
2.1. Experimental Design, and Description
A continuous anaerobic/aerobic integrated treatment process was constructed (Figure 1), with a working volume of 2 L for the anaerobic reactor and 4 L for the aerobic reactor. Both reactors were continuously mixed on magnetic plates by using a magnetic stirrer. Dissolved oxygen (DO) was delivered using an adjustable air pump (HAILEA: ACO-6603) with an output capacity of 7.0 L/min and distributed uniformly through the submerged tube containing a diffuser. To maintain an adequate population of microbes for the treatment of WW, activated sludge return (SR) from the clarifier to the anaerobic reactor was performed at approximately 20–40% of the reactor volumes. A portion of the effluent from the aerobic reactor was occasionally recycled back into the anaerobic reactor to reduce nutrient concentrations, such as nitrate, in the final discharge water, as anaerobic or facultative anaerobic bacteria can use these compounds as terminal electron acceptors. The process was operated at a constant flux and defined hydraulic retention time (HRT) based on predetermined and optimized test parameter values. Aeration in the aerobic reactor was controlled by the delivery flow and occasional monitoring of DO concentration. The DO concentration was maintained at approximately 4.0 ± 0.5 mg/L at ambient temperature. Because the treatment process is integrated, DO might be transferred to the anaerobic reactor via the pipeline if the concentration is saturated in the aerobic reactor. Thus, the design had a ventilation system that could vent out of the reactor, resulting in negligible DO values in the anaerobic reactor. All tests for the analyzed parameters were conducted at room temperature. Operating parameters, such as reactor volume, HRT, and inoculum size were designed and maintained as shown in Table 1. The optimized flow rate (Q) and HRT were previously determined.
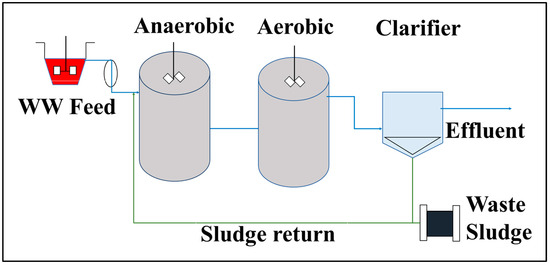
Figure 1.
Experimental setup flow layouts for the continuous anaerobic/aerobic integrated treatment process.

Table 1.
Design and experimental condition used for this study.
2.2. WW Source and Synthetic WW Preparation
First, a single dye (Direct Red 28) containing synthetic WW was evaluated. A range of dye concentrations (50–800 mg/L) was prepared and tested to evaluate the consortia degradation capacity (Table S1). The synthetic WW was prepared using mineral salt media (MSM) compositions (Table S2). Direct red 28 (DR28) dye containing synthetic WW was adapted and prepared using a mineral salt medium (MSM) (Table S2) [21]. Glucose (0.5% w/v) and yeast extract (0.1% w/v) were used as carbon and nitrogen sources, respectively. A 1% sodium carbonate solution was used to adjust pH. After a synthetic WW treatment evaluation was completed, the treatment process was evaluated for real textile WW. The experimental conditions identified during the synthetic WW treatment were used for the real WW treatment. Textile WW was sampled from a wet-processing Bahir Dar textile factory. Owing to variations in WW composition, dye concentration and colors, and COD concentration at different times, samples were collected at two different times, referred to as rounds I and II, to evaluate the potential of these microbes for dye removal with different dye colors and COD loads. Carbon and nitrogen sources were not supplied for the real WW treatment.
2.3. Inoculum Source
There are five soda lakes in Ethiopia; for this study, Lake Chitu was selected as an inoculum source of alkaliphilic microbial consortia. The lake is found 285 km south of Addis Ababa, Ethiopia. Surface water and sediment samples were collected from Lake Chitu (Figure S1). The pH and salinity of the lake during the sampling time were measured in situ and were 10.2 ± 0.3, and 6.5 ± 0.5%, respectively. Other chemical characteristics, such as ammonia, nitrate, nitrite, phosphate, and sulfate, were determined off-site at the laboratory.
2.4. Inoculum Preparation and Culturing Conditions
The collected samples were transported on ice to the microbial biotechnology laboratory at the Institute of Biotechnology, Addis Ababa University. The water and sediment samples were immediately mixed using a sterile Erlenmeyer flask and left to settle overnight to remove heavier solid particles. The clear supernatant was added directly as an inoculum for the treatment setup, and a 20% v/v inoculum was used. The prepared inoculum was added to the reactors containing dye WW. As anaerobic conditions require some time to start up owing to the slow growth of anaerobes, the adaptation time for these microbes was provided by using both reactors under anaerobic conditions before the continuous flow experimental phase started, during which the sludge gradually grew on the surface of the reactors. One decolorization was observed, and the continuous flow of feed WW and oxygen supplied to the aerobic reactor was started.
2.5. Treatment Performance Evaluation
Dye removal was evaluated by measuring the absorbance of dye colors at the predetermined maximum absorbance, Lambda max, (λmax), using a UV-Vis spectrophotometer, Lambda 35 (PerkinElmer Inc, Waltham, US). Real textile WW can contain a mixture of dyes, and it is difficult to determine its maximum absorbance precisely; therefore, UV-Vis spectrophotometer scanning was conducted to determine the maximum absorbance. The wavelengths at which the absorbance was measured were 483 nm, 532 nm, and 575 nm and 420 nm, for synthetic WW, round I collected, and round II collected real textile WW, respectively. Every 5 days, samples from the clarifier were withdrawn, aseptically centrifuged, and examined for color removal. The percentage decolorization was calculated by measuring the absorbance before and after the treatment using Equation (1).
The chemical oxygen demand (COD), biological oxygen demand (BOD), and total Kjeldahl nitrogen (TKN) were determined after the process reached a steady state and percentage removal was calculated by taking the differences of the initial and final concentrations using Equations (2) and (3). The COD, BOD, and TKN concentrations were determined in the anaerobic, aerobic, and final clarifier. These test parameters were measured according to American Public Health Association (APHA) standard methods for water and wastewater examination [22,23]. Briefly, untreated WW and treated effluents were collected in a clear container for COD measurement. The samples were added to a COD reagent vial containing an oxidizing agent, sulfuric acid, and silver sulfate. After thorough mixing, the vials were placed on a heat block and digestion took place for 2 h. Thereafter, the digested solutions were cooled to room temperature, concentration measurements were carried out using a COD colorimeter, and the COD value was directly recorded in mg/L. Similarly, untreated and treated WW samples were collected in a clean, airtight container for BOD measurement. The BOD bottles were filled with samples, leaving no air bubbles, and tested immediately. The initial DO concentration (before incubation) was measured using a DO meter and subjected to 5 days of incubation at 20 °C using a BOD incubator. After a 5-day incubation period, the final DO concentration was measured. TKN tests were conducted by modifying the digestion temperature to 500 °C using the standard macro-Kjeldahl method [23] to determine total nitrogen. This is because, while some textile azo dyes are degradable from 300 °C to 400 °C, others may be thermochemically stable at 500 °C [24]. In addition to sulfuric acid, 30% hydrogen peroxide was used to completely oxidize the organic nitrogen in WW.
Total ammonia, nitrite, nitrate, phosphate, and sulfate were tested at a steady state, which was reached using the tablet photometer technique (palintest photometer, model: 75) according to the manufacturer’s protocols for each parameter. These test parameters were used for real textile WW, but not for synthetic WW.
where: = initial value; and = final value.
2.6. Metabolite Analysis Using FTIR and GC–MS
The metabolites were characterized using Fourier transform infrared (FTIR) spectroscopy (JASCO-6600: JASCO International Co., Ltd., Tokyo, Japan) with a scanning range of 400–4000 cm−1. Five milliliters of untreated and treated samples were centrifuged at 6000 rpm for 10 min. One drop of supernatant and 100 mg of IR grade (purity ≥ 99%) potassium bromide (KBr) powder were ground with a mortar and pestle to form pellets. The analysis mode was set as percentage transmittance (%T). The instrumental parameters, such as resolution and scan rate, were set to default values.
Dye-transformed chemicals (metabolites) were conducted using gas chromatography-mass spectrometry (GC–MS). The sample preparation and analysis procedures were adapted from Jain et al. (2012) and Khan and Malik (2018) [25,26]. Briefly, the samples were centrifuged at 6000 rpm for 10 min, and the clear supernatant was extracted using a 1:1 volume of HPLC-grade ethyl acetate. The analysis was performed according to the manufacturer’s protocol (Agilent Technologies Inc., Santa Clara, CA, USA). Nitrogen gas (purity 99.99%) was used as the carrier gas at a flow rate of 1 min−1. The starting temperature was set to 60 °C for 2 min, and the injection temperature was maintained at 280 °C, followed by an increase at a rate of 10 °C/minute. The chromatogram was subjected to mass spectrometry, and compounds were identified based on m/z using the National Institute of Standards and Technology (NIST) library search. The components eluted at different retention times were subjected to mass spectrometry and identified based on their molecular weights and mass spectra.
3. Result and Discussion
3.1. Treatment Performance of the Integrated Treatment Process
3.1.1. Dye Decolorization
To assess the ability of microbial consortia to remove dyes, the decolorization efficiency was evaluated by comparing the absorbance of untreated and treated WW effluents taken from the clarifier. The decolorization efficiency increased with treatment time for both synthetic and real textile WWs (Figure 2). In the first experiment for synthetic WW, a decolorization efficiency of 99% was achieved after 25 days of continuous treatment, whereas in real textile WW, a steady state was reached after 35 days of treatment, although a similar trend was observed, with a removal efficiency of >96%. Dye biodegradation in real textile WW is expected to take longer than in synthetic WW for several reasons. Real textile WW has a complex composition that includes a mixture of dyes, along with other organic and inorganic chemicals such as surfactants, heavy metals, and salts, whereas synthetic WW typically contains a controlled concentration of specific dyes and minimal other components. The complexity of the composition of real textile WW can inhibit microbial activity and slow down the overall process. In addition, the dye structure present in the real WW may be more complex or resistant to biodegradation, requiring a longer time, whereas easily biodegradable dyes are mostly chosen to study microbial activity. Therefore, it can be deduced that the controlled and simplified nature of synthetic WW allows for faster dye biodegradation compared to the complex WW composition of real textile industry WW.
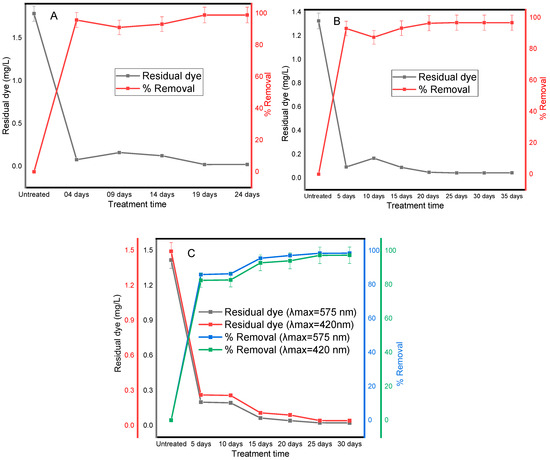
Figure 2.
Decolorization trends in the integrated anaerobic/aerobic continuous treatment process for synthetic WW (A) and real textile WW collected for rounds I and II (B,C). The y-axis shows the residual dyes and percentage of color removal, while the x-axis shows the treatment time. For round II collected WW (C), two peaks were detected, and the color removal was evaluated for the two λmax (575 nm and 420 nm).
The removal efficiency after 5 days of acclimatization was calculated to be 91.03% for synthetic WW. Its efficacy increased to 93.09% after 15 days of treatment, and then to 98.86% and 98.84% after 20 and 25 days of treatment, respectively, suggesting that the gradual improvement is a result of growth and adaptation of the inoculum as the time course increases. As demonstrated by percentage dye decolorization, minor variations were observed from day 14 to 24 for synthetic WW and from day 15 to 35 for real WW, and especially, the results for the last three sampling days were nearly identical, and it is assumed that the system reached a steady state. Anaerobic microbes have been shown to play a key role in the biological breakdown of dyes when treating complex textile WW [27]. Anaerobic alkaliphilic microbial consortia have been shown to function differently depending on the color of the dye present in textile WW because of their ability to degrade and detoxify persistent organic pollutants [28]. Anaerobic conditions are needed because the dyes must first be degraded into aromatic amines and other intermediate organic substances, followed by an aerobic phase, which continues to further degrade into smaller organics and mineralize [18]. As these smaller molecules were not detected in the decolorization measurements, it is important to note that while these measurements show the removal of the dye during the anaerobic step, an aerobic step is still needed for complete mineralization. In integrated bioreactors, anaerobic microbes can maximize color removal efficiency, further oxidize intermediate organic substances, and reduce the organic load under subsequent aerobic conditions. In addition, under anaerobic conditions, high-molecular-weight substances can be reduced to small molecules, which aerobic microbes can easily utilize as energy and carbon sources [29]. Dye removal suggests that this integrated treatment process can be applied to treating real WW-containing dyes. As reported previously, the dye concentration from textile WW can vary over a wide range of values [30].
To further evaluate how the degradation impacted the dye molecules, untreated WW and treated water were scanned using UV/Vis spectroscopy (Figure 3). The maximum absorbance (λmax) peak for synthetic WW was 485 nm, while different absorbance values were obtained for the real textile WW samples collected in rounds I and II. For real textile WW, more than one maximum absorbance peak suggested that it could contain different dye mixtures. Furthermore, the different colors of dye collected at different times suggest that textile dye concentrations may vary over time. The disappearance of the absorption peaks at 485 nm (for synthetic WW) and at 575, 532, 506, 420, 384, and 361 nm (for real textile WW) supports the conclusion that dye decolorization occurred as a result of these alkaliphilic microbial consortia. However, one limitation of UV-Vis absorption spectra is that they cannot provide information regarding the dye-degraded chemicals present in the final effluent. Dyes can be decolorized and transformed into different colorless chemicals, such as aromatic amines, which may be produced but cannot be detected by UV-Vis absorption within the defined scanning range [31]. Thus, FTIR and GC–MS analysis are needed to further evaluate the degradation processes and identify chemicals present in the effluent.

Figure 3.
UV–Vis spectral analysis of before and after treatment of (A) synthetic WW and (B,C) real textile WW round I and II collected samples.
3.1.2. Chemical and Biological Oxygen Demand
Chemical oxygen demand (COD) and biological oxygen demand (BOD) are critical for assessing WW treatment processes. The COD concentrations for the untreated WW and treated (steady state) effluent were determined in the anaerobic reactor, aerobic reactor, and clarifier after the treatment process reached a steady state as shown in Table 2. The COD removal efficiency was recorded differently under different reactor conditions. This could be due to the action of different groups of microorganisms responsible for organic matter degradation under each condition and the loads of organic matter in each reactor [32]. In addition, the residence time of the permeate in each reactor is different. In the aerobic reactor, the COD removal efficacy was high compared to the anaerobic reactor, and the maximum removal was calculated to be 81.73%, 85.69%, and 91.88% for synthetic WW, round I, and II collected real textile WW samples, respectively. One possible explanation for this is the further degradation of intermediate organic substances by aerobic microorganisms. In the effluent from the clarifier, the COD removal efficiencies were 82.75%, 86.32%, and 93.37% for the synthetic WW, round I, and round II collected real WW, respectively. Similarly, the optimum removal efficiencies of BOD at steady state were 53.98%, 57.1%, and 73.9% for synthetic, round I, and round II collected real WW, respectively.

Table 2.
Trends of COD and BOD removal and their ratio for synthetic and real textile WW collected in both rounds under an anaerobic/aerobic treatment process.
Previous studies have reported that a sequential anaerobic/aerobic process can enhance COD removal. Shoukat et al. (2019) reported COD removal of approximately 87% at a hydraulic retention time (HRT) of 48 h [18]. In the current study, the optimum removal efficiency of COD was recorded at an HRT of 18, 36, and 36 h for the anaerobic reactor, aerobic reactor, and clarifier, respectively, with initial COD concentrations of 684 mg/L, 496 mg/L, and 1417 mg/L for synthetic, round I, and round II collected real WW, respectively. Thus, the combination of reactor conditions in conventional biological treatment techniques is one strategy to increase COD removal efficiency. Also, this can minimize operational costs and footprint compared to a single reaction condition [33]. In addition, an integrated treatment process can not only reduce high organic matter in the reactors but also reduce the overall treatment cost [34].
The BOD/COD ratios in the untreated WW were 0.11, 0.113, and 0.229 for synthetic, round I, and round II collected from real WW, respectively. Though the ratio in the anaerobic reactor is low, it subsequently increased in the sequential aerobic treatment and reached 0.44, 0.352, and 0.901 for synthetic, round I, and II collected real WW, respectively, at the final effluent. This value is within the required standard of the BOD/COD ratio (minimum value = 0.40) for what is considered easily biodegradable WW [35]. This indicates that biodegradability was enhanced under the integrated reactor conditions. The BOD/COD ratio for the round I collected real WW is slightly below the standard. This could be due to the BOD concentrations for this sample being 56 mg/L for the untreated WW and 24 mg/L for treated effluent, which is very low compared to the BOD concentrations for the synthetic WW and round II WW. Untreated textile WW can generally have a BOD/COD ratio of 0.1 to 0.20 depending on the WW composition [36]. This suggests that it is necessary to pretreat WW, including anaerobic acclimatization time, so that complex organic dye structures can be decomposed, generating simpler and more easily assimilable nutrients for microbes to degrade under subsequent reactor conditions. Thus, it can be deduced that a sequential aerobic condition is crucial for the enhanced removal of organic substances, ensuring that the final effluent meets discharge standard limits.
3.1.3. Total Kjeldahl Nitrogen (TKN)
TKN is one of the key WW engineering parameters during the design, modeling, and upscaling of hybrid technologies in the treatment process [37]. The residual TKN value was high (summation of organic nitrogen and ammonia concentration), and its removal efficiency was lower under anaerobic conditions than under aerobic conditions (Figure 4A,B). The average TKN removal rates in the integrated treatment process were 90%, 91%, and 96% for synthetic WW, round I, and round II collected real textile WW, respectively (Figure 4B). The removal efficiency of TKN was low in the anaerobic reactor, but its efficiency increased in the aerobic reactor and final clarifier, suggesting that aerobes assimilated ammonia and organic nitrogen, which can be produced in a series of reactions, as the nutrient source [18]. The degradation starts with the breakdown of azo bonds, a process that can be facilitated by azoreductase under anaerobic conditions, leading to the formation of aromatic amines. In the azo dye reduction reaction, substances containing amino and amine groups are produced as intermediate compounds [18,38,39]. Anaerobes can remove the amine groups, leading to the release of ammonia, usually involved in deaminase enzymatic action. This ammonia can then be oxidized into nitrate by ammonia-oxidizing microorganisms under aerobic conditions, thereby greatly reducing TKN concentration [3]. This suggests that subsequent aerobic conditions, where organic nitrogen and ammonia are converted into nitrite/nitrate, are required to increase TKN removal rates in the treatment process. The residual TKN in the aerobic reactor and in the final clarifier was below the discharge standard limits for both synthetic and real WW. Thus, the integrated anaerobic/aerobic process plays a critical role in the removal of TKN and can improve textile WW treatment efficiency. Moreover, the TKN removal efficiency was highest for round II collected WW samples as compared with synthetic and round I collected real WW. The TKN removal rate in real textile WW can vary over different sampling times, even when taken from the same source, due to several factors such as variability in WW composition and microbial activity, which may be affected by the presence of inhibitory compounds. The textile production process can vary significantly over time, with different dyes, chemicals, and finishing agents being used depending on the products being manufactured, leading to fluctuations in WW composition and, consequently, influencing the microbial degradation of organic nitrogen compounds. Additionally, the efficiency of microbial activity can vary based on the microorganisms’ ability to adapt to different conditions, such as changes in nutrient availability or the presence of toxic compounds, which can impact microbial performance at different times.

Figure 4.
TKN determination in untreated, anaerobic, and aerobic reactors and clarifiers in the anaerobic/aerobic integrated treatment process. Residual TKN value in mg/L (A). Percentage removal of TKN (B).
3.2. Inorganic Nitrogens, Sulfur Compounds, and Phosphate
Nitrogenous (total ammonia, nitrite, and nitrate), sulfurous (sulfate and sulfide), and phosphate were tested for untreated WW at two points during the treatment process (5 and 10 days), and when the process reached a steady state (Table 3). The concentrations of ammonia and nitrate found in the reactors during the treatment process indicate that organic nitrogen decomposition, nitrification, and denitrification processes are carried out. For round I and II collected samples, 0.6 mg/L and 2.8 mg/L of total ammonia (TA) were detected, respectively. However, after a 5-day acclimatization, the TA concentration increased to 9.73 mg/L and 6.79 mg/L for round I and II collected samples, respectively. This could be due to the azo bonds in dyes, which anaerobically degrade to amino- and amine-containing transformed chemicals, followed by deamination [40]. One explanation for this is that the disappearance of the dye color suggests that the azo bond was broken down to colorless organic matter, such as aromatic amine, after which further deamination could occur. After a 10-day treatment time (with aerobic conditions starting after 5 days), the TA concentration decreased significantly from 5.02 mg/L and 6.48 mg/L, for round I and round II, respectively. At steady state, the concentration of TA decreased substantially, suggesting that ammonia-oxidizing microbes could oxidize it, resulting in a reduction of ammonia and an increased concentration of nitrate [41]. This makes sequential aerobic reactor conditions crucial for enhancing ammonia removal in the biological WW treatment process because ammonia oxidizers are primarily aerobes [42].

Table 3.
Test parameters evaluation of samples taken at the untreated WW with both reactors under anaerobic conditions at 5 days, 10 days (with aerobic conditions starting after 5 days), and after steady-state treatment.
Moderate nitrate concentrations were recorded for the inoculum source sample (8.83 mg/L) and the WW (18.27 mg/L and 3.75 mg/L) for rounds I and II collected WW, respectively). After a 5-day anaerobic treatment, nitrate concentration decreased significantly to 6.81 mg/L, and 1.64 mg/L for rounds I and II collected WW, respectively. This could be because anaerobes or facultative anaerobes of the microbial consortia could utilize them as a final electron acceptor [43]. These microbes are mostly denitrifiers, which potentially use nitrate as a final electron acceptor instead of an oxygen molecule in cellular respiration under anaerobic conditions. However, the nitrate concentration increased back to 25.40 mg/L in the 10-day treatment duration (with aerobic conditions starting after 5 days). This is likely because microbes in the aerobic reactor frequently oxidize ammonia (fed from the anaerobic reactor) to nitrates and flow to the clarifier. Later, the nitrate concentration decreased significantly and reached 2.29 mg/L at a steady state due to internal effluent recycling being employed from the aerobic reactor to the anaerobic reactor. Therefore, internal effluent recycling in integrated reactor conditions is essential to reduce the high nitrate concentration in the final discharge water. The findings suggest the presence of denitrifying microorganisms in Ethiopian soda lakes, particularly Lake Chitu, where the inoculum was used in this study. Previous studies support that diverse groups of denitrifying bacteria have been identified in Lake Chitu [44].
The concentration of phosphate was 6.67 mg/L, and 9.65 mg/L for rounds I and II collected WW, respectively. After 5 days of acclimatization, a high amount of phosphate was removed. Its concentration was completely removed for round I collected WW and reduced to 1.133 mg/L for round II collected WW. The inability to completely remove phosphate concentrations from round II collected real WW can be attributed to its high concentration in the untreated WW compared to round I. High concentrations of phosphate initially present in the WW may not be completely removed by microorganisms due to several factors, including saturation of microbial uptake capacity, nutrient imbalance, and the formation of insoluble phosphate compounds with other chemicals that are not readily available for uptake by microbes. Additionally, the complexity of the WW composition in round II may contain inhibitory compounds that can interfere with the microbial efficiency of phosphate removal. Furthermore, microbial activities responsible for phosphate uptake in round II could be limited by the availability of nutrients. Similarly, sulfate concentrations decreased after 5 days of acclimatization, suggesting that sulfate-reducing bacteria are present and can use sulfate as a terminal electron acceptor. For instance, sulfate-reducing chemolithotrophic bacteria are common in soda lakes [45,46]. Generally, having internal effluent recycling from the aerobic reactor back to the anaerobic reactor can greatly decrease the nutrient concentrations, such as nitrate, sulfate, and phosphate in the final discharge water, thus improving the water quality [47]. It is thus important to employ a moderate amount of internal effluent recycling to reduce nutrient concentrations and to be used as a final electron acceptor for anaerobes [48]. Thus, determining the most feasible approach for managing nutrients in the WW treatment process is a critical issue. The cultivation of algae in nitrate-, sulfate-, and phosphate-rich treated WW could be used as an alternative approach to utilize nutrients for their growth [49], and the application of algae-based post-treatment could increase nutrient recovery and offset economic challenges.
3.3. Degraded Chemical Products Analysis
3.3.1. FTIR Spectral Analysis
Fourier transform infrared spectroscopy (FTIR) can be used to study the degradation of dyes and the chemicals they produce in the degradation process. The IR spectra and the assigned chemical bonds for the untreated textile WW, treated after 5 days of acclimatization, and the final effluent at a steady state were analyzed as shown in Figure 5, Table S3. The apparent difference observed in the FTIR spectra of untreated, 5-day treated and final day treated effluents are indicators of the biodegradation that occurred. The peaks in the untreated textile WW disappeared and/or decreased in peak intensity, and new peaks were also identified, suggesting the breakdown of dye and the formation of new chemicals in the treatment process [50,51]. The characteristic bands at 3441 cm−1 for the untreated and treated (steady-state) samples confirm the presence of organics with asymmetric intramolecular bonded hydroxyl in alcohols and/or phenolic compounds [52,53]. However, the peak at 3441 cm−1 disappeared after a 5-day anaerobically treated sample. The appearance of N-H stretching for the primary aromatic amine (-NH2) can be produced by the breakdown of azo bonds (-N=N-), resulting in an overlap with the hydroxyl functional groups [54,55]. The peak intensity of approximately 3453 cm−1 for the stretching vibration of hydroxyl (-OH) in the final effluent was higher than that of the untreated WW, which could be due to an increase in the number of -OH-containing molecules, such as benzoic acid, phenol, and hexanoic acid. [14]. The presence of broad peaks at around 2080 cm−1 for the 5-day sample and the final effluent is assigned to the carbon-carbon triple-bond (−C≡C−) stretching of alkynes, suggesting the presence of small molecules of organic acids [56]. A sharp peak at approximately 1635 cm−1 in the treated WW, which is assigned to the vibration of the carbonyl carbon (C=O), indicates the presence of an amide (R-CONR2) stretching vibration [57], or the C=C stretching vibration of the aromatic ring [58]. Therefore, in the treatment process, different organic substances are produced. The peak at approximately 1562 cm−1 in the untreated WW could be assigned to the azo bond (-N=N-) but disappeared after treatment, suggesting that azo bond cleavage had occurred. This is an indication of decolorization because one of the chromophore groups responsible for dye color is azo bond [59]. In the fingerprint region (1500–500 cm−1), various absorption bands were observed. These are mainly due to bending vibration within the molecules, such as carbon-carbon in aromatic compounds [60], sulfur-oxygen in sulfonyl compounds [29], carbon-chloride in halogenated dyes [61], and amine deformation in acyclic compounds [62]. In conclusion, the IR spectra ensure the presence of organic substances and dye in the untreated textile WW. In the final effluent sample, the disappearance of many compounds was observed, suggesting that they were utilized by microorganisms for growth and metabolism.
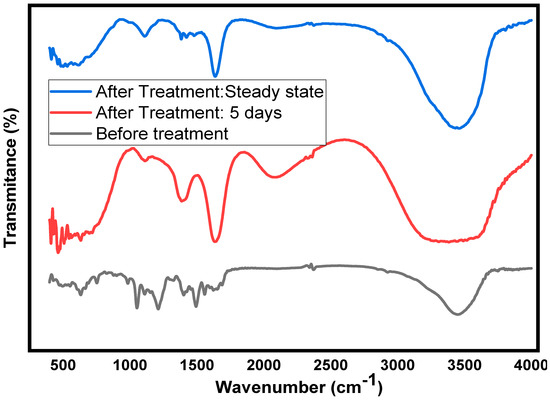
Figure 5.
FTIR spectra of untreated textile WW, a 5-day anaerobically acclimatized sample, and the final effluent.
3.3.2. Identification of Degraded Organic Substances Using GC–MS
Identification of chemicals using GC–MS techniques is required to evaluate organic substances that are produced in the treatment process. The chromatographic spectra of degraded organic chemicals produced in the treated effluent (Figure 6) were identified (Table S4) using GC–MS and compared with those of the untreated influent. GC–MS analysis supports the biodegradation of dyes and dye-transformed intermediate chemicals in the treatment process. In untreated WW, the chromatogram revealed 11 peaks, and 9 were identified at retention times of 3.745–13.352 min. After 5 days of treatment, 30 peaks, many of which were not observed in the untreated water, appeared at retention times ranging from 3.745 to 18.239 min. The clear and visible peaks are indicated in Figure 6. In total, 15 out of 30 substances were matched and identified using the NIST library (Table S4). This strongly suggests that the dyes from textile WW were partially degraded into several intermediate chemicals during anaerobic conditions. However, many of the peaks were not detected in the final effluent sample, indicating that aerobes further degraded these intermediate organic chemicals. Further degradation into small organic molecules by aerobic microbes is required for the detoxification of toxic intermediates [63].
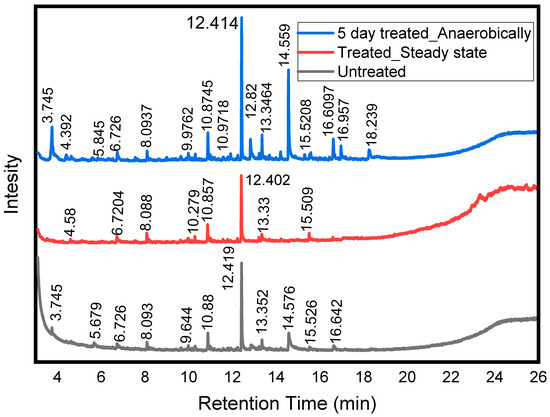
Figure 6.
Chromatograms for untreated, 5-day anaerobically acclimatized, and treated textile industry WW samples.
The dye biodegradation process occurs through the formation of intermediate chemicals which could be found to be more toxic than the parent dyes; thus, the identification of these intermediates is critical [64]. Mass spectrometry is an important analytical technique used to identify, by molecular weight determination, the chemicals produced during degradation [65]. Many of the intermediate products in the anaerobic treatment were phenolics, heterocyclic compounds, and organic acids/ketones/esters including 2-Propenoic acid, butyl ester, cyclodecane, cyclopropane, and 1,1′-ethenylidenebis- (Table S4). The mass spectra only identified a small number of substances in the treated WW, compared with untreated and 5-day treated samples. This is likely due to mineralization and/or decreased peak intensity (below the detection limit) [53]. Several intermediate substances identified in the anaerobically treated sample disappeared, and only six were identified in the final effluent. Most of the detected chemicals were organic acids, esters, and phenolics. Aromatic amines were not detected, indicating that the integrated reactor conditions further oxidized this intermediate chemical into other chemical products or resulted in mineralization. Studies have reported that the reduction of azo bonds can occur by azo reductases, after which the produced aromatic amines can be further oxidized by using NAD(P)H and NADH as cofactors [50,66]. However, further oxidation by laccases can catalyze several aromatic substances with a highly non-specific free radical mechanism to avoid the formation of toxic aromatic amines. Similar results to those of the present study were reported by Jayapal et al. (2018), who found a mixture of organic acids such as benzoic acid and nitrobenzene as the final degraded products from the anaerobic/aerobic degradation of a single dye (Methyl Red) [14]. Similar results were obtained by Selim et al. (2021), who performed GC–MS analysis of the degraded products of real textile WW [67]. Furthermore, Mohanty and Kumar (2021) reported a dye degradation study using different microorganisms in which several different organic compounds were detected [68].
In general, many of the compounds identified through GC–MS analysis in the present study could be due to the real WW composition; most studies were performed using a single dye at the time of collection and used a long incubation time. This could result in multiple enzymatic degradation steps to produce more intermediates of various molecular weights. In the present study, low-molecular-weight aliphatic chain hydrocarbons and organic acid substances were observed, confirming that alkaliphilic microbial consortia are efficient for the breakdown of dyes in textile WW into non-/less toxic small molecules. However, the study was conducted in a continuous anaerobic/aerobic integrated process, and some of the peaks in the untreated WW were also observed in the treated WW, indicating that these compounds could persist without being utilized by microbial consortia and accumulate in the final effluent.
In summary, integrated anaerobic/aerobic dye degradation processes operated under alkaline conditions and using microbial consortia can reduce high molecular weight organic matter including dyes. A dye cannot initially be degraded under aerobic conditions. Aerobic microbes may not use most textile dyes as the final electron acceptors because of their high toxicity and because oxygen is a stronger electron acceptor than azo dyes [69]. Thus, an anaerobic condition as a first-stage reactor followed by an aerobic reactor usually results in high treatment efficiency for textile WW.
4. Conclusions
Alkaliphilic microbial consortia derived from a soda lake were used in this study, and the integrated anaerobic/aerobic reactor, operating under alkaline conditions, effectively removed over 97% of the dyes and 86% to 93% of the COD from different WW samples. COD and TKN removal were enhanced by sequential aerobic conditions, resulting in low concentrations in the final discharge water and meeting discharge standard limits. Furthermore, GC–MS analysis revealed that mixtures of dyes and other organic substances in real textile WW were degraded into non-toxic, low molecular weight compounds, such as organic acids, esters, and ketones. Real WW samples collected at different times showed varying dye compositions and COD concentrations, necessitating different treatment durations to effectively remove dyes and achieve a steady state in the system. The ability of alkaliphilic microbial consortia to decolorize both synthetic and real WW makes them highly suitable for the biological treatment of textile WW under integrated reactor conditions. Scaling up to the pilot scale is crucial for validating system performance, optimizing operational parameters, and demonstrating the technology. Additionally, characterizing the reactor microbiome is important for understanding microbial diversity and community structure, allowing for the exploration of these microbes’ potential in the future.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/w16202937/s1. Table S1. A series of dye concentration removal efficiency evaluation; Table S2. Mineral salts medium (MSM) composition; Table S3. FTIR spectra of untreated and treated (steady state) textile WW with the corresponding assigned functional group; Table S4. library identified substances with percentage peak area resulting from GC-MS of textile WW before and after alkaliphilic microbial consortia treated samples; Figure S1. (a) Lake Chitu, (b) Mud sampling at Lake Chitu as alkaliphilic microbial sources; Figure S2. Chemical structure of direct red 28 dye (DR28) and picture of powder dyes; Figure S3. Photograph representation for the before and after treatment for (a) synthetic WW, real textile WW (b & c) round I and II collected samples.
Author Contributions
T.A.A.: Conceptualization, methodology, validation, formal analysis, investigation, data curation, writing—original draft, writing—review and editing. A.S.: conceptualization, resources, writing—review and editing, supervision, project administration, funding acquisition. C.J.P.: conceptualization, writing—review and editing, supervision, project administration. C.S.: conceptualization, writing—review and editing, supervision. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the Swedish International Development Cooperation Agency (SIDA) through a research and training grant awarded (Project No. 51080124) to Addis Ababa University and the Swedish University of Agricultural Sciences (AAU-SLU) PhD program, https://sida.aau.edu.et/index.php/international-comparative-education-phd-program/ (accessed on 16 June 2024).
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
We thank the Wildlife Conservation Authority for providing access to samples from Lake Chitu, the Bahir Dar Textile Share Company for allowing us access to textile wastewater from its treatment plants, and Amare Gessesse for his valuable support for this study.
Conflicts of Interest
The authors declare that they have no known competing interests in financial/personal relationships that could influence the work reported in this study.
References
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Saratale, R.G.; Cho, S.; Saratale, G.D.; Kadam, A.A.; Varjani, S.; Palem, R.R.; Mulla, S.I.; Kim, D.; Shin, H. Lignin-Mediated Silver Nanoparticle Synthesis for Photocatalytic Degradation of Reactive Yellow 4G and In Vitro Assessment of Antioxidant, Antidiabetic, and Antibacterial Activities. Polymers 2022, 14, 648. [Google Scholar] [CrossRef] [PubMed]
- Tizazu, S.; Tesfaye, G.; Andualem, B.; Wang, A.; Guadie, A. Evaluating the Potential of Thermo-Alkaliphilic Microbial Consortia for Azo Dye Biodegradation under Anaerobic-Aerobic Conditions: Optimization and Microbial Diversity Analysis. J. Environ. Manag. 2022, 323, 116235. [Google Scholar] [CrossRef]
- Garg, S.K.; Tripathi, M. Microbial Strategies for Discoloration and Detoxification of Azo Dyes from Textile Effluents. Res. J. Microbiol. 2017, 12, 1–9. [Google Scholar] [CrossRef]
- Parisi, M.L.; Fatarella, E.; Spinelli, D.; Pogni, R.; Basosi, R. Environmental Impact Assessment of an Eco-Efficient Production for Coloured Textiles. J. Clean. Prod. 2015, 108, 514–524. [Google Scholar] [CrossRef]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of Textile Dyes on Health and Ecosystem: A Review of Structure, Causes, and Potential Solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An Overview on the Removal of Synthetic Dyes from Water by Electrochemical Advanced Oxidation Processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial Degradation of Dyes: An Overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Khan, R.; Bhawana, P.; Fulekar, M.H. Microbial Decolorization and Degradation of Synthetic Dyes: A Review. Rev. Environ. Sci. Biotechnol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced Oxidation Processes for In-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Textile Wastewater Treatment: A Review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Azanaw, A.; Birlie, B.; Teshome, B.; Jemberie, M. Case Studies in Chemical and Environmental Engineering Textile Effluent Treatment Methods and Eco-Friendly Resolution of Textile Wastewater. Case Stud. Chem. Environ. Eng. 2022, 6, 100230. [Google Scholar] [CrossRef]
- Azimi, B.; Abdollahzadeh-Sharghi, E.; Bonakdarpour, B. Anaerobic-Aerobic Processes for the Treatment of Textile Dyeing Wastewater Containing Three Commercial Reactive Azo Dyes: Effect of Number of Stages and Bioreactor Type. Chin. J. Chem. Eng. 2021, 39, 228–239. [Google Scholar] [CrossRef]
- Baêta, B.E.L.; Lima, D.R.S.; Queiroz Silva, S.; Aquino, S.F. Influence of the Applied Organic Load (OLR) on Textile Wastewater Treatment Using Submerged Anaerobic Membrane Bioreactors (SAMBR) in the Presence of Redox Mediator and Powdered Activated Carbon (PAC). Braz. J. Chem. Eng. 2016, 33, 817–825. [Google Scholar] [CrossRef]
- Jayapal, M.; Jagadeesan, H.; Shanmugam, M.; Danisha, J.P.; Murugesan, S. Sequential Anaerobic-Aerobic Treatment Using Plant Microbe Integrated System for Degradation of Azo Dyes and Their Aromatic Amines by-Products. J. Hazard. Mater. 2018, 354, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, I.H.; Basheer, F. Treatment of Adsorbable Organic Halide (AOX) from Pulp and Paper Industry Wastewater Using Aerobic Granules in Pilot Scale SBR. J. Water Process Eng. 2017, 19, 60–66. [Google Scholar] [CrossRef]
- Bogale, F.M.; Teffera, B.; Aragaw, T.A. Recent Developments in Integrated Anaerobic/Aerobic (A/O) Process for Textile Industry Wastewater Treatment: A Review. J. Hazard. Mater. Adv. 2024, 14, 100438. [Google Scholar] [CrossRef]
- Işik, M.; Sponza, D.T. Anaerobic/Aerobic Treatment of a Simulated Textile Wastewater. Sep. Purif. Technol. 2008, 60, 64–72. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, S.J.; Jamal, Y. Hybrid Anaerobic-Aerobic Biological Treatment for Real Textile Wastewater. J. Water Process Eng. 2019, 29, 100804. [Google Scholar] [CrossRef]
- Gadow, S.I.; Li, Y.Y. Development of an Integrated Anaerobic/Aerobic Bioreactor for Biodegradation of Recalcitrant Azo Dye and Bioenergy Recovery: HRT Effects and Functional Resilience. Bioresour. Technol. Rep. 2020, 9, 100388. [Google Scholar] [CrossRef]
- Aragaw, T.A.; Bogale, F.M.; Gessesse, A. Adaptive Response of Thermophiles to Redox Stress and Their Role in the Process of Dye Degradation From Textile Industry Wastewater. Front. Physiol. 2022, 13, 908370. [Google Scholar] [CrossRef]
- Guadie, A.; Gessesse, A.; Xia, S. Halomonas sp. Strain A55, a Novel Dye Decolorizing Bacterium from Dye-Uncontaminated Rift Valley Soda Lake. Chemosphere 2018, 206, 59–69. [Google Scholar] [CrossRef] [PubMed]
- APHA Standard. Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA, 1998. [Google Scholar]
- Bremner, M. Nitrogen-Total. In Methods of Soil Analysis Part 3. Chemical Methods-SSSA Book Series 5; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Nguyen, T.L.; Saleh, M.A. Thermal Degradation of Azobenzene Dyes. Results Chem. 2020, 2, 100085. [Google Scholar] [CrossRef]
- Jain, K.; Shah, V.; Chapla, D.; Madamwar, D. Decolorization and Degradation of Azo Dye—Reactive Violet 5R by an Acclimatized Indigenous Bacterial Mixed Cultures-SB4 Isolated from Anthropogenic Dye Contaminated Soil. J. Hazard. Mater. 2012, 213–214, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Malik, A. Toxicity Evaluation of Textile Effluents and Role of Native Soil Bacterium in Biodegradation of a Textile Dye. Environ. Sci. Pollut. Res. 2018, 25, 4446–4458. [Google Scholar] [CrossRef]
- Deng, Z.; Fung, K.Y.; Ng, K.M.; Wei, C. Design of Anaerobic Fluidized Bed Bioreactor—Dyeing Effluents. Chem. Eng. Sci. 2016, 139, 273–284. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Zafar, S.; Bukhari, D.A.; Rehman, A. Azo Dyes Degradation by Microorganisms—An Efficient and Sustainable Approach. Saudi J. Biol. Sci. 2022, 29, 103437. [Google Scholar] [CrossRef]
- Barathi, S.; Aruljothi, K.N.; Karthik, C.; Padikasan, I.A.; Ashokkumar, V. Biofilm Mediated Decolorization and Degradation of Reactive Red 170 Dye by the Bacterial Consortium Isolated from the Dyeing Industry Wastewater Sediments. Chemosphere 2022, 286, 131914. [Google Scholar] [CrossRef]
- Baêta, B.E.L.; Lima, D.R.S.; Silva, S.Q.; Aquino, S.F. Evaluation of Soluble Microbial Products and Aromatic Amines Accumulation during a Combined Anaerobic/Aerobic Treatment of a Model Azo Dye. Chem. Eng. J. 2015, 259, 936–944. [Google Scholar] [CrossRef]
- Zieliński, M.; Kazimierowicz, J.; Dębowski, M. Advantages and Limitations of Anaerobic Wastewater Treatment—Technological Basics, Development Directions, and Technological Innovations. Energies 2023, 16, 83. [Google Scholar] [CrossRef]
- Plascencia-Jatomea, R.; González, I.; Gómez, J.; Monroy, O. Operation and Dynamic Modeling of a Novel Integrated Anaerobic-Aerobic-Anoxic Reactor for Sewage Treatment. Chem. Eng. Sci. 2015, 138, 31–40. [Google Scholar] [CrossRef]
- Mareai, B.M.; Fayed, M.; Aly, S.A.; Elbarki, W.I. Performance Comparison of Phenol Removal in Pharmaceutical Wastewater by Activated Sludge and Extended Aeration Augmented with Activated Carbon. Alexandria Eng. J. 2020, 59, 5187–5196. [Google Scholar] [CrossRef]
- Andrio, D.; Asmura, J.; Yenie, E.; Putri, K. Enhancing BOD 5 /COD Ratio Co-Substrate Tofu Wastewater and Cow Dung during Ozone Pretreatment. MATEC Web Conf. 2019, 276, 06027. [Google Scholar] [CrossRef]
- Afanga, H.; Zazou, H.; Titchou, F.E.; Rakhila, Y.; Akbour, R.A.; Elmchaouri, A.; Ghanbaja, J.; Hamdani, M. Integrated Electrochemical Processes for Textile Industry Wastewater Treatment: System Performances and Sludge Settling Characteristics. Sustain. Environ. Res. 2020, 30, 2. [Google Scholar] [CrossRef]
- Narayanan, C.M.; Narayan, V. Biological Wastewater Treatment and Bioreactor Design: A Review. Sustain. Environ. Res. 2019, 29, 33. [Google Scholar] [CrossRef]
- Punzi, M.; Nilsson, F.; Anbalagan, A.; Svensson, B.M.; Jönsson, K.; Mattiasson, B.; Jonstrup, M. Combined Anaerobic-Ozonation Process for Treatment of Textile Wastewater: Removal of Acute Toxicity and Mutagenicity. J. Hazard. Mater. 2015, 292, 52–60. [Google Scholar] [CrossRef]
- Paździor, K.; Wrębiak, J.; Klepacz-Smółka, A.; Gmurek, M.; Bilińska, L.; Kos, L.; Sójka-Ledakowicz, J.; Ledakowicz, S. Influence of Ozonation and Biodegradation on Toxicity of Industrial Textile Wastewater. J. Environ. Manag. 2017, 195, 166–173. [Google Scholar] [CrossRef]
- Awolusi, O.O.; Kumari, S.; Bux, F. Evaluation of Ammonia Oxidizing Bacterial Community Structure of a Municipal Activated Sludge Plant by 454 High-Throughput Pyrosequencing. Environ. Process. 2018, 5, 43–57. [Google Scholar] [CrossRef]
- Pan, K.L.; Gao, J.F.; Li, H.Y.; Fan, X.Y.; Li, D.C.; Jiang, H. Ammonia-Oxidizing Bacteria Dominate Ammonia Oxidation in a Full-Scale Wastewater Treatment Plant Revealed by DNA-Based Stable Isotope Probing. Bioresour. Technol. 2018, 256, 152–159. [Google Scholar] [CrossRef]
- Zhu, B.; Friedrich, S.; Wang, Z.; Táncsics, A.; Lueders, T. Availability of Nitrite and Nitrate as Electron Acceptors Modulates Anaerobic Toluene-Degrading Communities in Aquifer Sediments. Front. Microbiol. 2020, 11, 1867. [Google Scholar] [CrossRef]
- Ali, Y.; Simachew, A.; Gessesse, A. Diversity of Culturable Alkaliphilic Nitrogen-Fixing Bacteria from a Soda Lake in the East African Rift Valley. Microorganisms 2022, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Ersoy Omeroglu, E.; Sudagidan, M.; Yurt, M.N.Z.; Tasbasi, B.B.; Acar, E.E.; Ozalp, V.C. Microbial Community of Soda Lake Van as Obtained from Direct and Enriched Water, Sediment and Fish Samples. Sci. Rep. 2021, 11, 18364. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lan, S.M.; Zhu, Z.P.; Zhang, C.; Zeng, G.M.; Liu, Y.G.; Cao, W.C.; Song, B.; Yang, H.; Wang, S.F.; et al. The Bioenergetics Mechanisms and Applications of Sulfate-Reducing Bacteria in Remediation of Pollutants in Drainage: A Review. Ecotoxicol. Environ. Saf. 2018, 158, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, B.; Ye, L.; Peng, Y. The Combined Effects of COD/N Ratio and Nitrate Recycling Ratio on Nitrogen and Phosphorus Removal in Anaerobic/Anoxic/Aerobic (A2/O)-Biological Aerated Filter (BAF) Systems. Biochem. Eng. J. 2015, 93, 235–242. [Google Scholar] [CrossRef]
- Assefa, R.; Bai, R.; Leta, S.; Kloos, H. Nitrogen Removal in Integrated Anaerobic–Aerobic Sequencing Batch Reactors and Constructed Wetland System: A Field Experimental Study. Appl. Water Sci. 2019, 9, 136. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Aditya, L.; Vu, H.P.; Johir, A.H.; Bennar, L.; Ralph, P.; Hoang, N.B.; Zdarta, J.; Nghiem, L.D. Nutrient Removal by Algae-Based Wastewater Treatment. Curr. Pollut. Rep. 2022, 8, 369–383. [Google Scholar] [CrossRef]
- Ogunlaja, A.; Nwankwo, I.N.; Omaliko, M.E.; Olukanni, O.D. Biodegradation of Methylene Blue as an Evidence of Synthetic Dyes Mineralization during Textile Effluent Biotreatment by Acinetobacter pittii. Environ. Process. 2020, 7, 931–947. [Google Scholar] [CrossRef]
- Adebajo, S.O.; Ojo, A.E.; Bankole, P.O.; Oladotun, A.O.; Akintokun, P.O.; Ogunbiyi, E.O.; Bada, A. Degradation of Paint and Textile Industrial Effluents by Indigenous Bacterial Isolates. Bioremediat. J. 2023, 27, 412–421. [Google Scholar] [CrossRef]
- Patil, P.; Desai, N.; Govindwar, S.; Jadhav, J.P.; Bapat, V. Degradation Analysis of Reactive Red 198 by Hairy Roots of Tagetes patula L. (Marigold). Planta 2009, 230, 725–735. [Google Scholar] [CrossRef]
- Ikram, M.; Naeem, M.; Zahoor, M.; Hanafiah, M.M.; Oyekanmi, A.A.; Islam, N.U.; Ullah, M.; Mahnashi, M.H.; Al Ali, A.; Jalal, N.A.; et al. Bacillus Subtilis: As an Efficient Bacterial Strain for the Reclamation of Water Loaded with Textile Azo Dye, Orange II. Int. J. Mol. Sci. 2022, 23, 10637. [Google Scholar] [CrossRef]
- Amin, S.; Rastogi, R.P.; Chaubey, M.G.; Jain, K.; Divecha, J.; Desai, C.; Madamwar, D. Degradation and Toxicity Analysis of a Reactive Textile Diazo Dye-Direct Red 81 by Newly Isolated Bacillus Sp. DMS2. Front. Microbiol. 2020, 11, 576680. [Google Scholar] [CrossRef] [PubMed]
- Kishor, R.; Purchase, D.; Saratale, G.D.; Ferreira, L.F.R.; Bilal, M.; Iqbal, H.M.N.; Bharagava, R.N. Environment Friendly Degradation and Detoxification of Congo Red Dye and Textile Industry Wastewater by a Newly Isolated Bacillus Cohnni (RKS9). Environ. Technol. Innov. 2021, 22, 101425. [Google Scholar] [CrossRef]
- Guo, G.; Tian, F.; Zhang, C.; Liu, T.; Yang, F.; Hu, Z.; Liu, C.; Wang, S.; Ding, K. Performance of a Newly Enriched Bacterial Consortium for Degrading and Detoxifying Azo Dyes. Water Sci. Technol. 2019, 79, 2036–2045. [Google Scholar] [CrossRef] [PubMed]
- Su, W.T.; Lin, C.H. Fungal-Bacterial Synergism Enhanced Decolorization of Reactive Red 120 by Response Surface Methodology. Int. Biodeterior. Biodegrad. 2013, 82, 1–8. [Google Scholar] [CrossRef]
- Bankole, P.O.; Adekunle, A.A.; Obidi, O.F. Mycodecolorization of Reactive Red HE7B Dye by Achaetomium Strumarium and Aspergillus Flavus and Shelf Life Determination. Cogent Environ. Sci. 2017, 3, 1278646. [Google Scholar] [CrossRef]
- Ahmed, F.; Dewani, R.; Pervez, M.K.; Mahboob, S.J.; Soomro, S.A. Non-Destructive FT-IR Analysis of Mono Azo Dyes. Bulg. Chem. Commun. 2016, 48, 71–77. [Google Scholar]
- Li, H.X.; Xu, B.; Tang, L.; Zhang, J.H.; Mao, Z.G. Reductive Decolorization of Indigo Carmine Dye with Bacillus Sp. MZS10. Int. Biodeterior. Biodegrad. 2015, 103, 30–37. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Öztürk, A.; Bayol, E. Biosorption of Astrazon Red Dye by the Bacterium Rhodopseudomonas Sp. Strain 51ATA. Environ. Earth Sci. 2021, 80, 49. [Google Scholar] [CrossRef]
- Kurade, M.B.; Waghmode, T.R.; Jadhav, M.U.; Jeon, B.H.; Govindwar, S.P. Bacterial-Yeast Consortium as an Effective Biocatalyst for Biodegradation of Sulphonated Azo Dye Reactive Red 198. RSC Adv. 2015, 5, 23046–23056. [Google Scholar] [CrossRef]
- Ghobadi Nejad, Z.; Borghei, S.M.; Yaghmaei, S. Biodegradation of Synthetic Dye Using Partially Purified and Characterized Laccase and Its Proposed Mechanism. Int. J. Environ. Sci. Technol. 2019, 16, 7805–7816. [Google Scholar] [CrossRef]
- Saravanan, S.; Carolin, C.F.; Kumar, P.S.; Chitra, B.; Rangasamy, G. Biodegradation of Textile Dye Rhodamine-B by Brevundimonas Diminuta and Screening of Their Breakdown Metabolites. Chemosphere 2022, 308, 136266. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, S.; Bankole, P.O.; Sadasivam, S.K. Microbial Degradation of Azo Dyes by Textile Effluent Adapted, Enterobacter Hormaechei under Microaerophilic Condition. Microbiol. Res. 2021, 250, 126805. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.U.; Hinsu, A.T.; Kotadiya, R.J.; Rank, J.K.; Andharia, K.N.; Kothari, R.K. Decolorization and Biodegradation of Textile Di-Azo Dye Acid Blue 113 by Pseudomonas Stutzeri AK6. 3 Biotech 2020, 10, 214. [Google Scholar] [CrossRef] [PubMed]
- Selim, M.T.; Salem, S.S.; Mohamed, A.A.; El-Gamal, M.S.; Awad, M.F.; Fouda, A. Biological Treatment of Real Textile Effluent Using Aspergillus Flavus and Fusarium Oxysporium and Their Consortium along with the Evaluation of Their Phytotoxicity. J. Fungi 2021, 7, 193. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Kumar, A. Enhanced Degradation of Anthraquinone Dyes by Microbial Monoculture and Developed Consortium through the Production of Specific Enzymes. Sci. Rep. 2021, 11, 7678. [Google Scholar] [CrossRef]
- Franca, R.D.G.; Vieira, A.; Carvalho, G.; Oehmen, A.; Pinheiro, H.M.; Barreto Crespo, M.T.; Lourenço, N.D. Oerskovia Paurometabola Can Efficiently Decolorize Azo Dye Acid Red 14 and Remove Its Recalcitrant Metabolite. Ecotoxicol. Environ. Saf. 2020, 191, 110007. [Google Scholar] [CrossRef]
- Singh, R.L.; Singh, P.K.; Singh, R.P. Enzymatic Decolorization and Degradation of Azo Dyes—A Review. Int. Biodeterior. Biodegrad. 2015, 104, 21–31. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).