Low Strength Wastewater Treatment Using a Combined Biological Aerated Filter/Anammox Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Wastewater Composition and Reactors
2.2. Parameters of Operation Conditions
2.3. Measurements and Methods
3. Results and Discussion
3.1. The Operation Strategy of Removing COD While Retaining Ammonia Nitrogen in the BAF Process
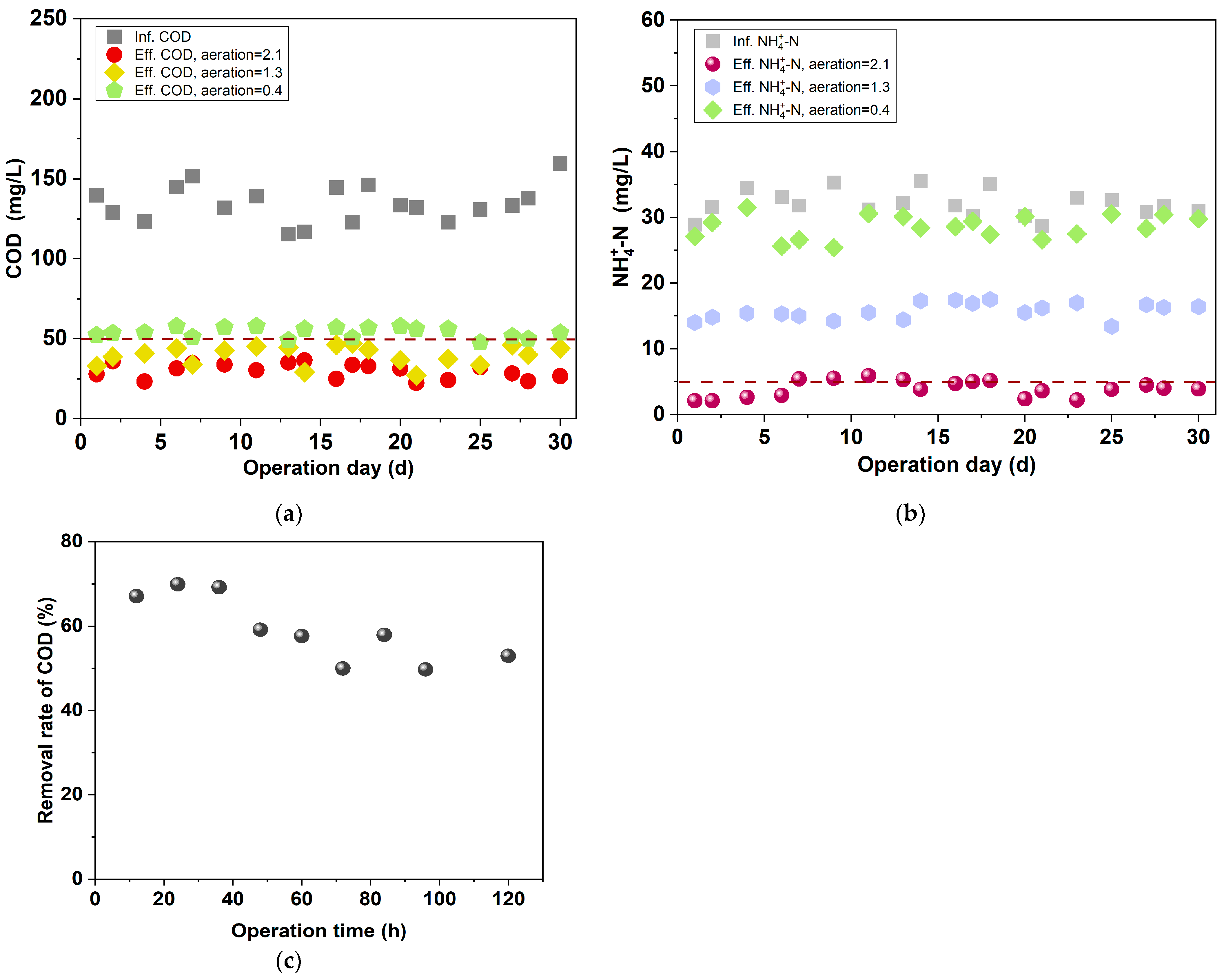
3.1.1. Effect of Reducing Aeration Intensity on the Treating Performance of BAF
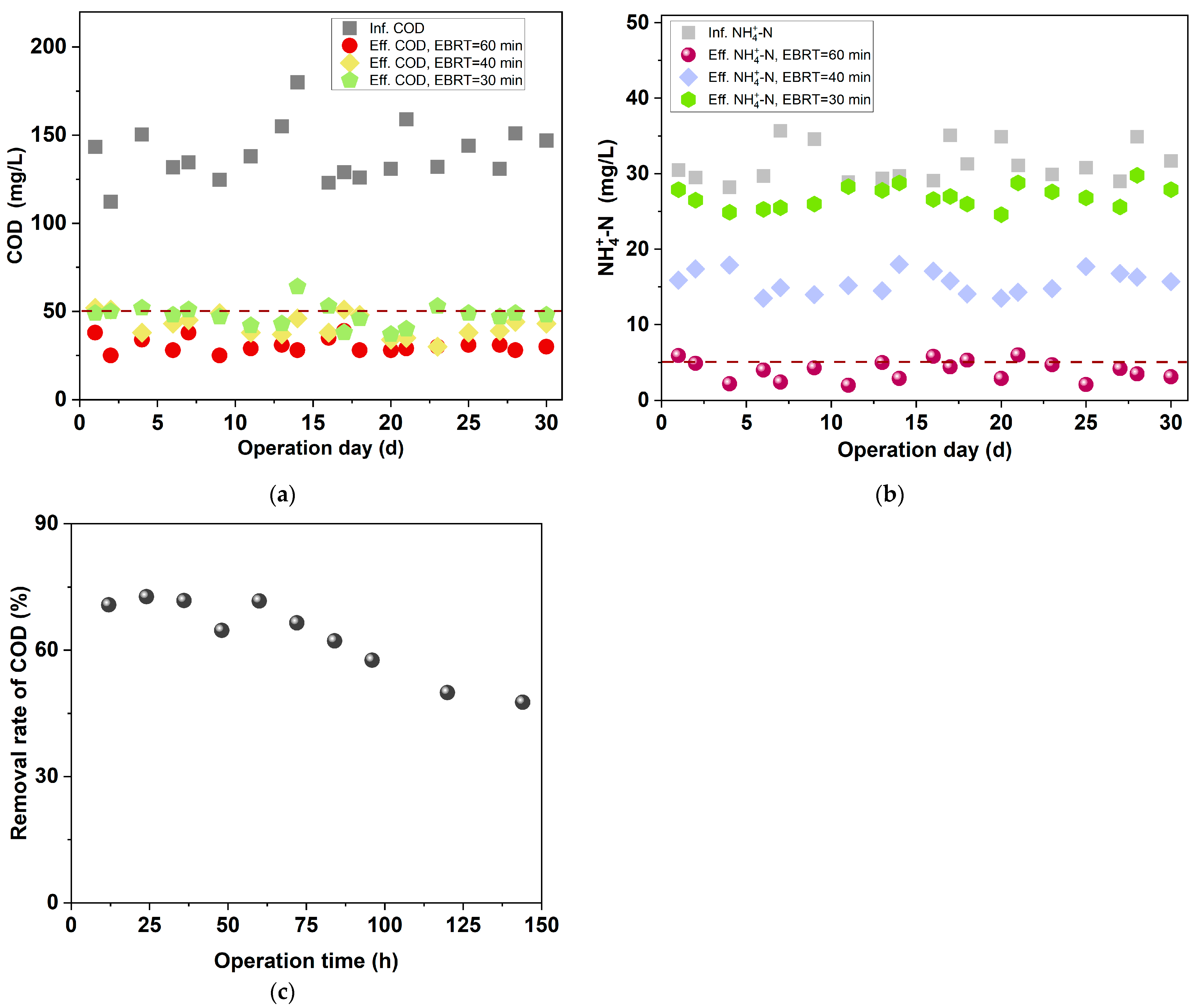
3.1.2. Effect of Reducing EBRT on the Treating Performance of BAF
3.2. Influence of Carrier Types on the Nitrogen Removal Performance of ANAMMOX Reactors during the Start-Up Period
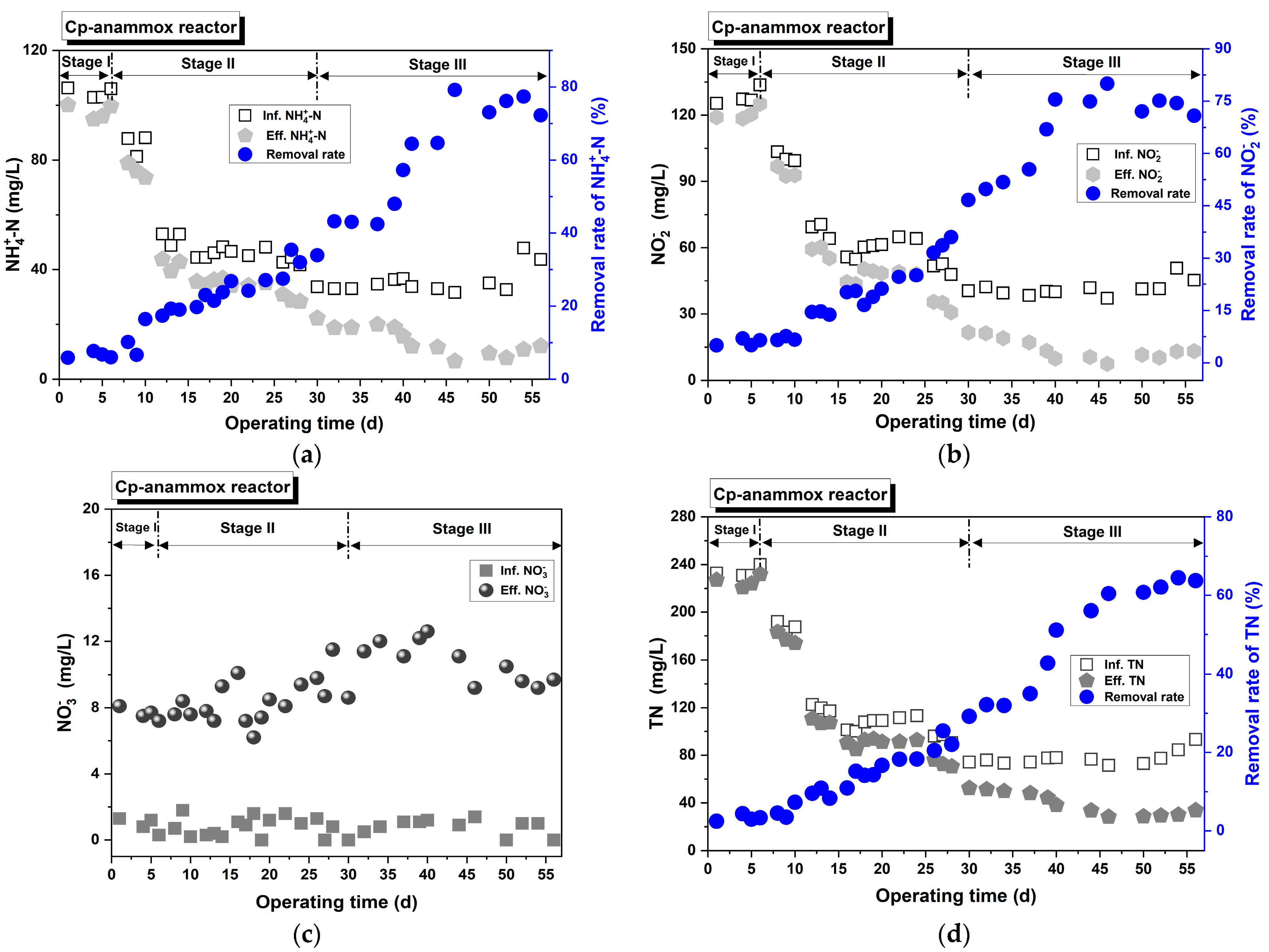
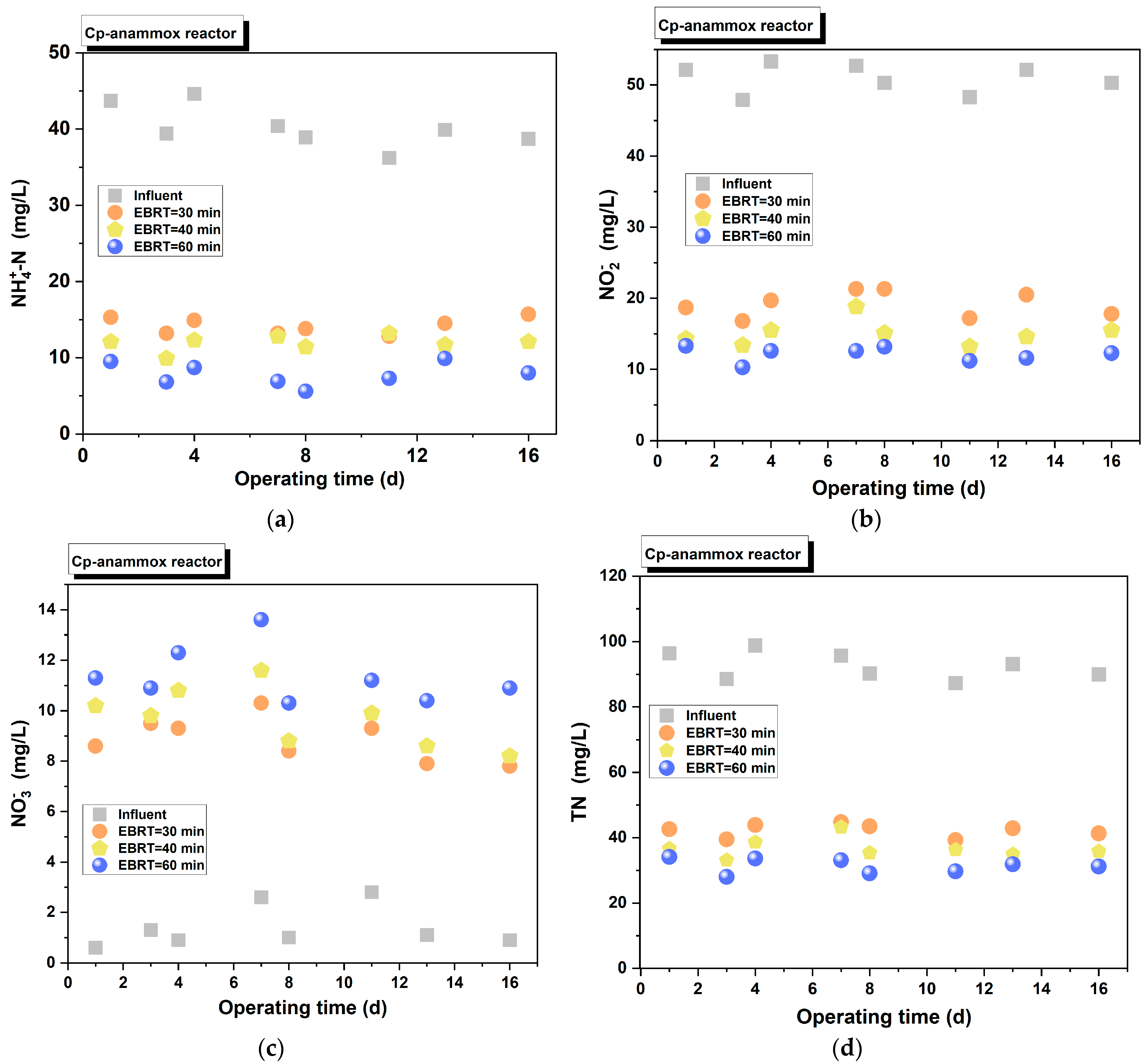
3.2.1. Cp-Anammox
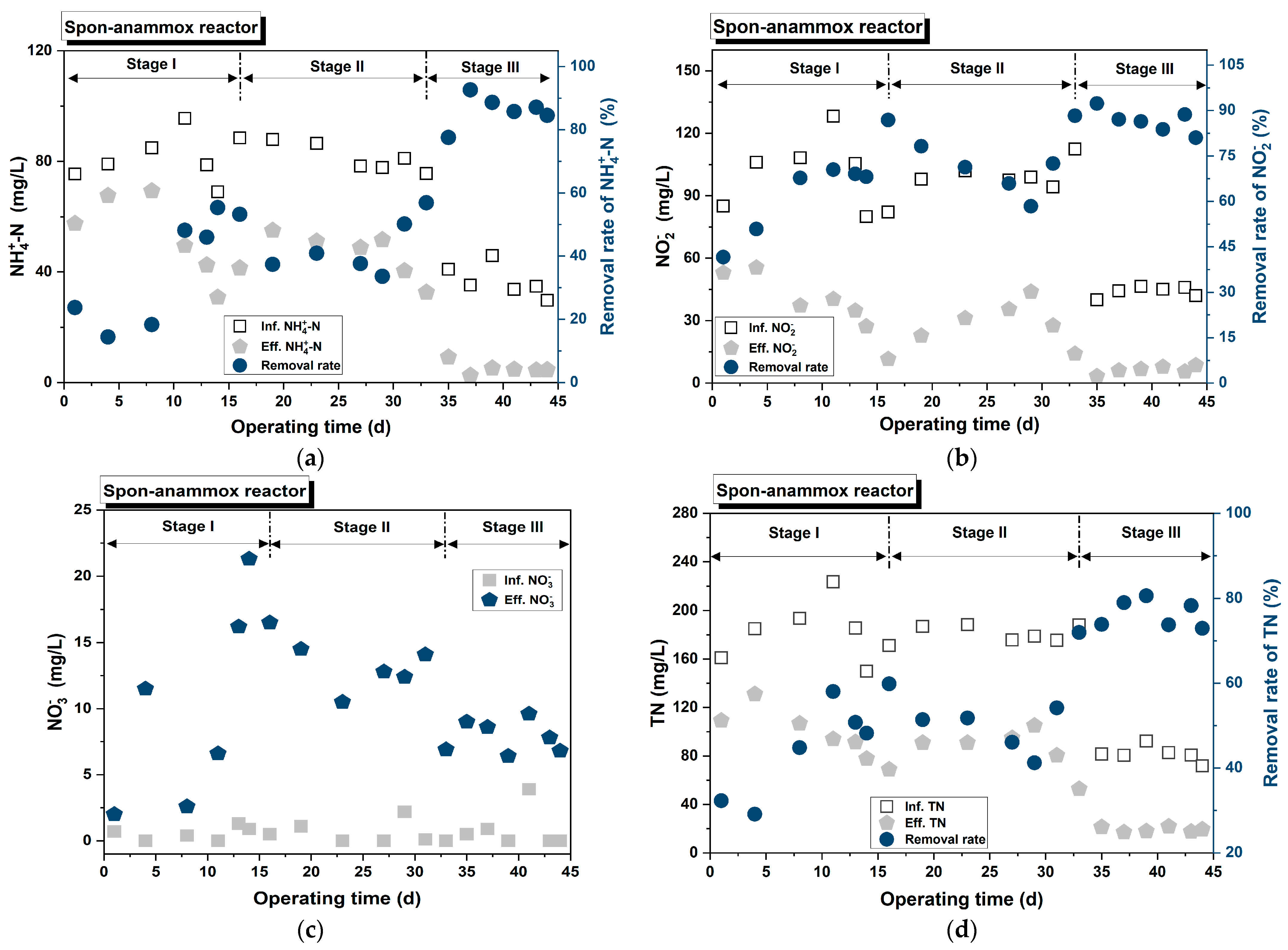
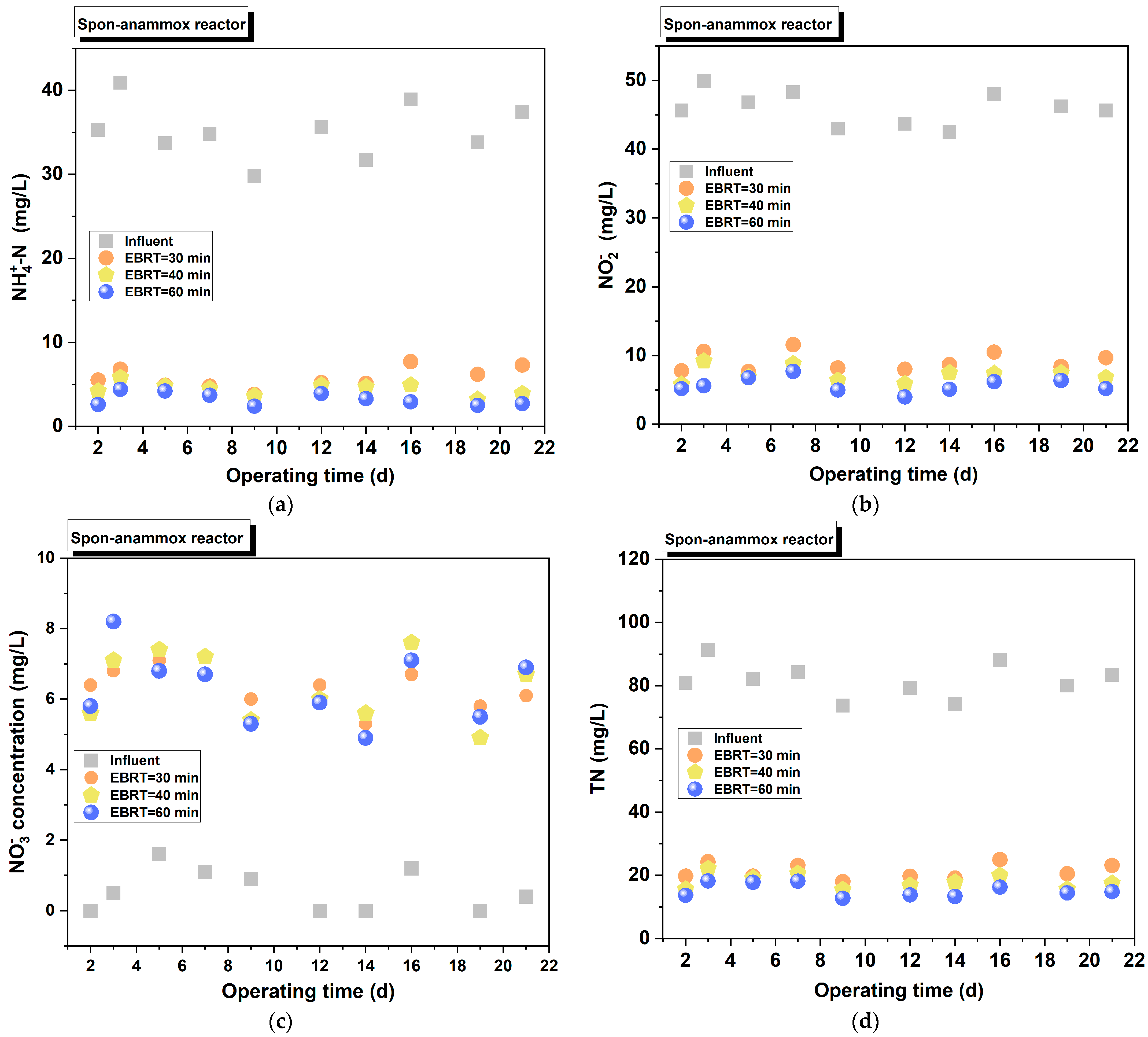
3.2.2. Spon-ANAMMOX
3.3. Influence of Operating Conditions on Nitrogen Removal Performance of Anammox Reactor during Stable Operation Period
3.3.1. Impact of EBRT
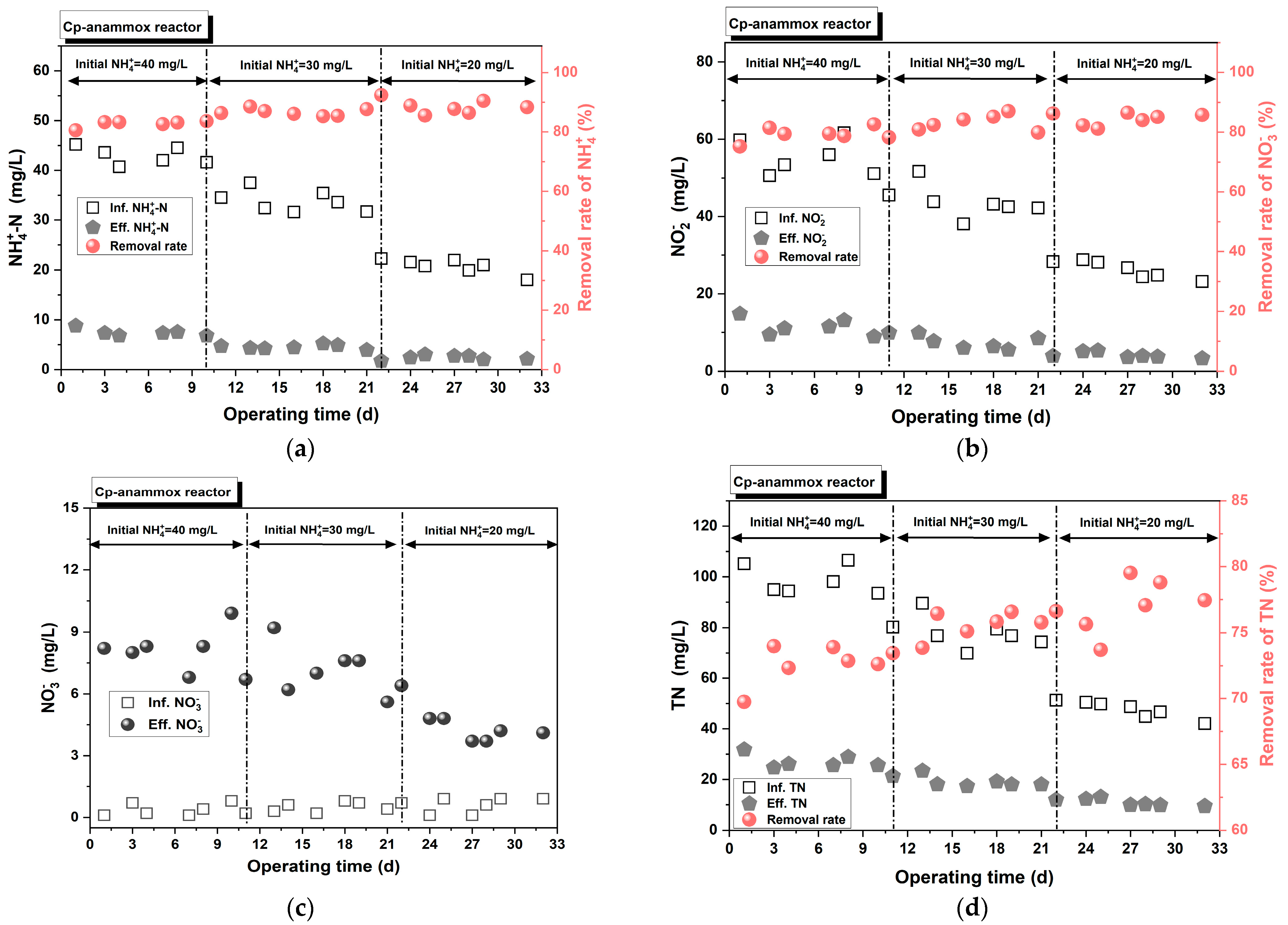
3.3.2. Impact of Influent Concentration
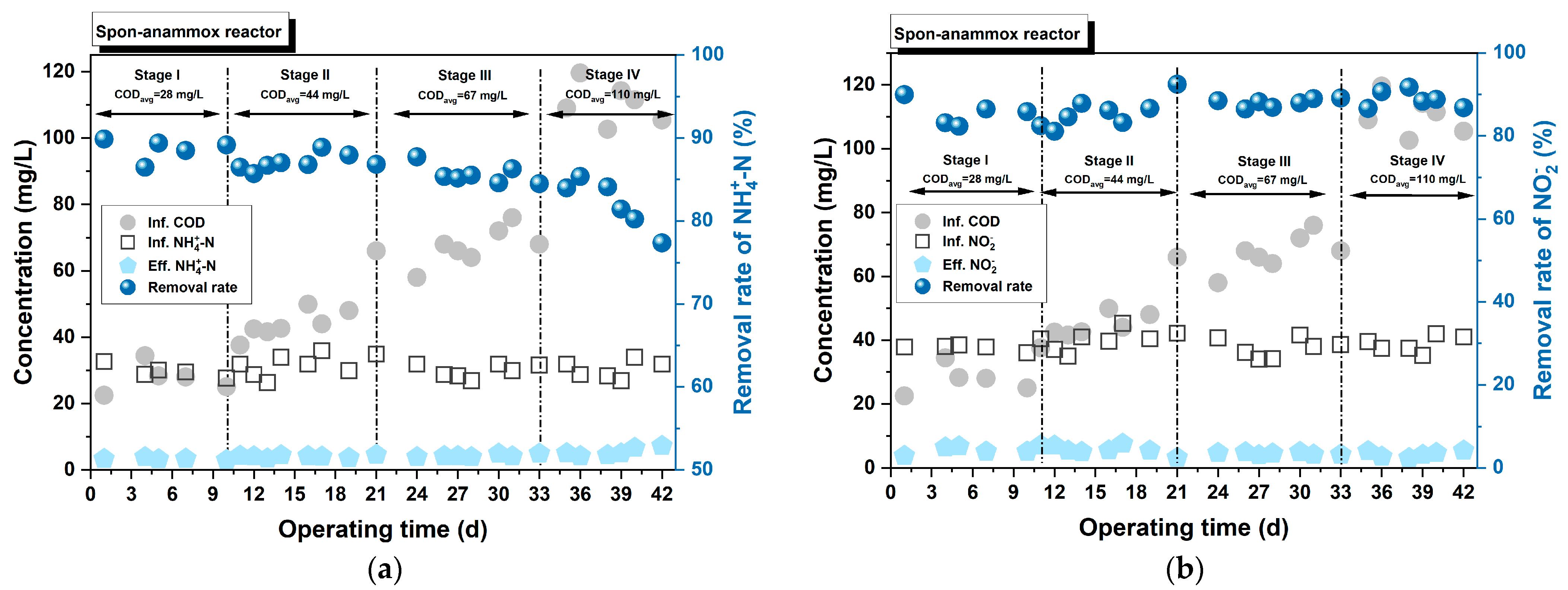
3.3.3. Impact of Influent COD Concentration
3.4. Evaluation of BAF/AX Combined Process
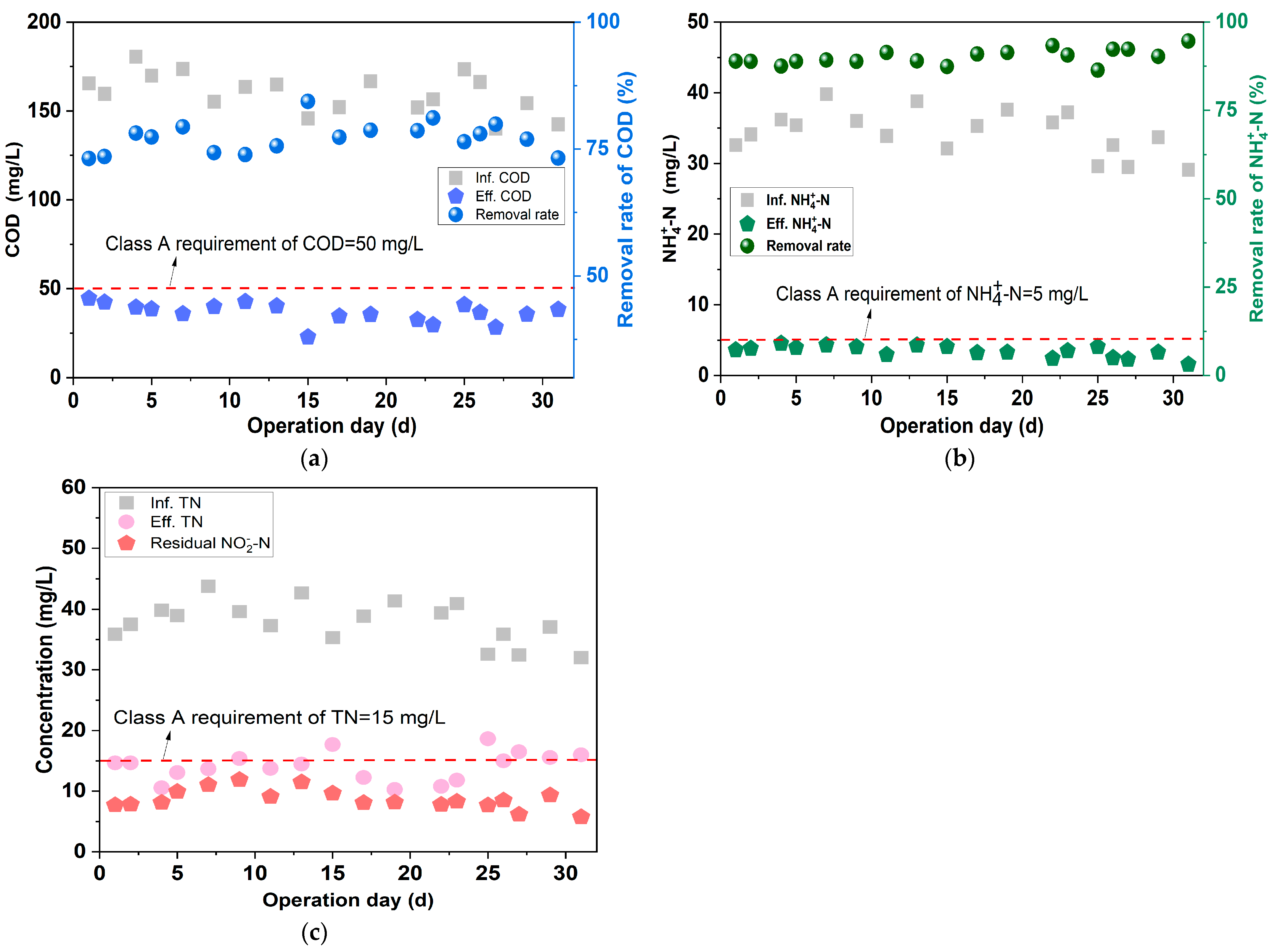
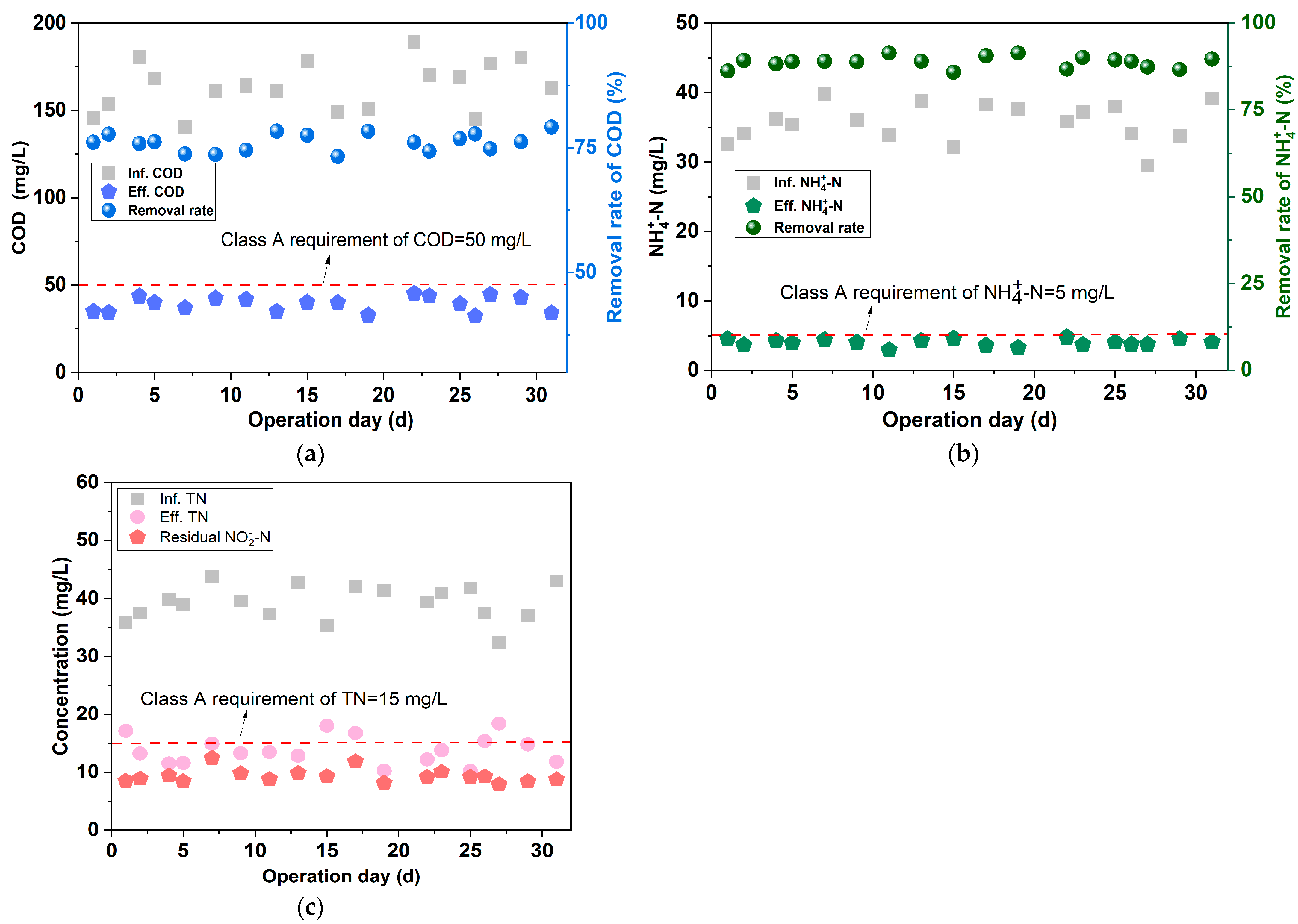
3.4.1. Treating Performance
3.4.2. Economic Cost and Engineering Potential Analysis
4. Conclusions
- (1)
- To provide suitable influent quality for the anammox unit, the treatment performance of the BAF was investigated under various aeration intensities and EBRTs. In the BAF unit, when the aeration intensity was 0.4 m3·m−2·h−1 or the EBRT was 30 min, the effluent COD was ≤55 mg/L, and the removal efficiency of was as low as 4~12%, which provided ammonia nitrogen substrate for the subsequent anammox units.
- (2)
- The anammox process was successfully launched and operated using two carriers: ceramic particles and sponges. Furthermore, the Spon-anammox reactor displayed superior nitrogen removal capability under the following conditions: a shorter EBRT of 40 min, an initial ≤ 30 mg/L, or an influent COD ≤ 67 mg/L.
- (3)
- When the BAF was operated under the aforementioned condition as the control strategy, the effluent from the combined BAF/AX process met the Class A requirement. Compared to low aeration intensity, a control strategy such as low EBRT in the BAF demonstrated greater advantages for wastewater treatment in the combined BAF/AX process. This was because the treating capacity was expanded without the need for additional treatment units or land, and also reduced costs.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAF | Biological aerated filter |
| anammox | Anaerobic ammonia oxidation process |
| Ammonia nitrogen | |
| EBRT | Empty bed residence time |
| COD | Chemical oxygen demand |
| TN | Total nitrogen |
| TP | Total phosphorus |
| DN | Denitrification |
| / | The ratio of and |
| AnAOB | Anaerobic ammonia oxidation bacteria |
| DO | Dissolved oxygen |
| // | The ratio of (removal concentration)/ (removal concentration)/ (generated concentration) |
| FA | Free ammonia |
| AnGS | ANAMMOX granular sludge |
| TVL | Treatment volume load |
| BAF/AX | BAF/ANAMMOX process |
| lowAI | Low aeration intensity |
| Inf. | Influent |
| Eff. | Effluent |
References
- Dong, Y.; Cheng, X.; Li, C.; Xu, L. Spatially eutrophication potential and policy implication of nitrogen emission for surface water: A case study in Guangzhou city, China. Environ. Manag. 2023, 342, 118336. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cheng, X.; Li, C.; Xu, L.; Lin, W. Characterization of nitrogen emissions for freshwater eutrophication modelling in life cycle impact assessment at the damage level and urban scale. Ecol. Indic. 2023, 154, 110598. [Google Scholar] [CrossRef]
- Galoppo, S.; Fenti, A.; Falco, G.; Huang, Q.; Chianese, S. Efficient electrochemical removal of ammoniacal nitrogen from livestock wastewater: The role of the electrode material. Heliyon 2024, 10, e36803. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Zhang, M.; Chen, M.; Wu, Q.; Yang, L.; Yang, L. Klebsiella oxytoca (EN-B2): A novel type of simultaneous nitrification and denitrification strain for excellent total nitrogen removal during multiple nitrogen pollution wastewater treatment. Bioresour. Technol. 2023, 367, 128236. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhang, B.; Zhao, Z.W. Metagenomic analysis reveals the responses of microbial communities and nitrogen metabolic pathways to polystyrene micro(nano)plastics in activated sludge systems. Water Res. 2023, 241, 120161. [Google Scholar] [CrossRef]
- Biase, A.; Kowalski, S.M.; Devlin, T.R.; Oleszkiewicz, J.A. Moving bed biofilm reactor technology in municipal wastewater treatment: A review. J. Environ. Manag. 2019, 247, 849–889. [Google Scholar] [CrossRef]
- Hu, H.; Han, H.; Ma, W.; Wang, W.; Wang, B. Research on a pre-denitrification double-layer media biological aerated filter in municipal wastewater treatment. Desalination Water Treat. 2011, 31, 366–371. [Google Scholar] [CrossRef]
- Wang, H.; Dong, W.; Li, T.; Liu, T. A modified BAF system configuring synergistic denitrification and chemical phosphorus precipitation: Examination on pollutants removal and clogging development. Bioresour. Technol. 2015, 189, 44–52. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, G. Biofilm Characteristics, Microbial Community Structure and Function of an Up-flow Anaerobic Filter-Biological Aerated Filter (UAF-BAF) Driven by COD/N Ratio. Sci. Total Environ. 2020, 708, 134422. [Google Scholar] [CrossRef]
- Meng, X.M.; Fan, F.F.; Wu, L.F. Prediction of Major Pollutants Discharge from Wastewater in 31 Cities of China. Sustain. Prod. Consum. 2021, 26, 54–64. [Google Scholar]
- Miao, Y.; Zhang, J.; Peng, Y.; Wang, S. An improved start-up strategy for mainstream Anammox process through inoculating ordinary nitrification sludge and a small amount of Anammox sludge. J. Hazard. Mater. 2020, 384, 121325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Yu, D.; Li, J.; Zhao, X.; Ma, G.; Zhi, J.; Dong, G.; Miao, Y. Migration of microorganisms between nitrification–denitrification flocs, anammox biofilms and blank carriers during mainstream anammox start-up. Bioresour. Technol. 2024, 393, 130129. [Google Scholar] [CrossRef] [PubMed]
- Xing, B.S.; Tang, X.F.; Li, L.H.; Fu, Y.L.; Liu, J.Y.; Wang, Y.G.; Sun, X.X.; Li, Y.Y.; Chen, R.; Jin, R.C. A new substrate equalization method for optimizing the influent conditions and fluid flow patterns of a multifed upflow anaerobic sludge blanket reactor with mature anammox granules. Bioresour. Technol. 2024, 400, 130700. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, X.R.; Sun, Y.J.; Xi, Z.H.; Liu, J.Z.; Feng, Z.T.; Zhou, J.M.; Liu, X.T.; Wang, Y.; Jin, R.C.; et al. Reason and control strategy for denitrification and anammox sludge flotation in nitrogen removal process: Mechanisms, strategies and perspectives. Environ. Res. 2024, 258, 119456. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, Y.; Wang, X.; Chen, F.; Lin, L.; Ruan, Q.; Wang, Y.; Wang, F.; Cao, W.; Chiang, P. Optimizing of operation strategies of the single-stage partial nitrification-Anammox process. J. Cleaner Prod. 2020, 256, 120667. [Google Scholar] [CrossRef]
- Sui, Q.; Wang, Y.; Wang, H.; Yue, W.; Chen, Y.; Yu, D.; Chen, M.; Wei, Y. Roles of Hydroxylamine and Hydrazine in the In-situ Recovery of One-stage Partial Nitritation-Anammox Process: Characteristics and Mechanisms. Sci. Total Environ. 2020, 707, 135648. [Google Scholar] [CrossRef]
- Cui, B.; Yang, Q.; Liu, X.; Wu, W.; Liu, Z.; Gu, P. Achieving Partial Denitrification-Anammox in Biofilter for Advanced Wastewater Treatment. Environ. Int. 2020, 138, 105612. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Q.P.; Zhang, J.S.; An, N.; Yu, H.Y.; Fu, X.; Li, Z.H. Enhanced nitrogen removal for low C/N wastewater via preventing futile carbon oxidation and augmenting anammox. Water Res. X 2024, 25, 100253. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association, American Water Works Association, Water Environment Federation. APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- GB18918-2002; Discharge Standard of Pollutants for Municipal Wastewater Treatment Plant. National Environmental Protection Administration, General Administration of Quality Supervision, Inspection and Quarantine of China: Beijing, China, 2002.
- Wu, Q.; Chen, C.; Zhang, Y.; Tang, P.; Ren, X.; Shu, J.; Liu, X.; Cheng, X.; Tiraferri, A.; Liu, B. Safe purification of rural drinking water by biological aerated filter coupled with ultrafiltration. Sci. Total Environ. 2023, 868, 161632. [Google Scholar] [CrossRef]
- Wang, K.; Zhou, Z.; Qiang, J.; Yu, S.; Wang, X.; Yuan, Y.; Zhao, X.; Qin, Y.; Xiao, K. Emerging wastewater treatment strategy for efficient nitrogen removal and compact footprint by coupling mainstream nitrogen separation with chemical coagulation and biological aerated filter. Bioresour. Technol. 2021, 320, 124389. [Google Scholar] [CrossRef]
- Hong, H.; Lin, Y.; Ou, Y.; Lin, X.; Chen, B.; Qin, Y. Fast start-up of anammox reactor under high-loading rate shock. Tech. Water Treat. 2023, 49, 97–101. (In Chinese) [Google Scholar]
- Zhang, L.; Yang, J.; Hira, D.; Fujii, T.; Zhang, W.; Furukawa, K. High-rate nitrogen removal from anaerobic digester liquor using an up-flow anammox reactor with polyethylene sponge as a biomass carrier. J. Biosci. Bioeng. 2011, 111, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, N.; He, A.; Wang, C.; Li, Z.; Zhang, G.; Xue, R. Carrier type affects anammox community assembly, species interactions and nitrogen conversion. Bioresour. Technol. 2023, 369, 128422. [Google Scholar] [CrossRef]
- Wang, S.; Gong, H.; Ding, J.; Xu, E.; Cui, R.; Yang, D.; Gu, G.; Dai, X. Unbalanced inhibition on granular and mixed anammox sludge by different molecular weight fractions of unbiodegradable proportion of sludge anaerobic digestion reject water. J. Water Process. Eng. 2021, 42, 102197. [Google Scholar] [CrossRef]
- Yan, Y.; Li, X.; Ren, S.; Zhang, Q.; Wu, D.; Zhou, J.; Peng, Y. Efficient nitrogen removal and robustness enhancement of a two-stage partial nitrification-anammox (PN/A) process with low sludge concentration for mature landfill leachate. Bioresour. Technol. 2023, 387, 129573. [Google Scholar] [CrossRef]
- Fernández, I.; Dosta, J.; Fajardo, C.; Campos, J.L.; Mosquera-Corral, A.; Méndez, R. Short- and long-term effects of ammonium and nitrite on the Anammox process. J. Environ. Manag. 2012, 95, S170–S174. [Google Scholar] [CrossRef]
- Cigdem, K.A.; Kozet, Y.; Bulent, M. Inhibitory effects of free ammonia on Anammox bacteria. Biodegrad. 2012, 23, 751–762. [Google Scholar]
- Dosta, J.; Fernández, I.; Vázquez-Padín, J.R.; Mosquera-Corral, A.; Campos, J.L.; Mata-Alvarez, J.; Méndez, R. Short- and long-term effects of temperature on the Anammox process. J. Hazard. Mater. 2008, 154, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, L.; Hira, D.; Fukuzaki, Y.; Furukawa, K. Highrate nitrogen removal by the Anammox process at ambient temperature. Bioresour. Technol. 2011, 102, 672–676. [Google Scholar] [CrossRef]
- Tang, C.J.; Zheng, P.; Mahmood, Q.; Chen, J.W. Start-up and inhibition analysis of the Anammox process seeded with anaerobic granular sludge. J. Ind. Microbiol. Biotechnol. 2009, 36, 1093–1100. [Google Scholar] [CrossRef]
- Leal, C.D.; Pereira, A.D.; Nunes, F.T.; Ferreira, L.O.; Coelho, A.C.C.; Bicalho, S.K.; Mac Conell, E.F.A.; Ribeiro, T.B.; de Lemos Chernicharo, C.A.; de Araújo, J.C. Anammox for nitrogen removal from anaerobically pre-treated municipal wastewater: Effect of COD/N ratios on process performance and bacterial community structure. Bioresour. Technol. 2016, 211, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, R.; Guo, Y.; Zhang, Z.; Kao, C.M.; Chen, S. Investigation of COD and COD/N ratio for the dominance of anammox pathway for nitrogen removal via isotope labelling technique and the relevant bacteria. J. Hazard. Mater. 2018, 366, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Li, Z.R.; Zhang, J.; Chen, Y.; Wang, Q. Impact of COD/N on anammox granular sludge with different biological carriers. Sci. Total Environ. 2020, 728, 138557. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]





| Composition | Chemicals | Concentration |
|---|---|---|
| COD | C2H3NaO2 | 120~160 mg/L |
| NH4Cl | 30~40 mg/L | |
| Total phosphorus (TP) | KH2PO4 | 2~3 mg/L |
| Calcium ion | Ca2+ | 10 mg/L |
| Magnesium ion | Mg2+ | 25 mg/L |
| Alkalinity | CaCO3 | 600 mg/L |
| Actual domestic sewage | / | 5~10% |
| Operation Unit | Influent | Other Condition |
|---|---|---|
| BAF | , 30~40 mg/L | Aeration intensity, 0.4/1.3/2.1 m3·m−2·h−1; EBRT, 30/40/60 min; |
| TN, 30~40 mg/L | ||
| COD, 120~160 mg/L | ||
| TP, 2~3 mg/L |
| Reactor | Startup Period | EBRT | ||
|---|---|---|---|---|
| Cp-anammox | Stage I | 4 h | 100 mg/L | 130 mg/L |
| Stage II | 2 h | 100~40 mg/L | 130~55 mg/L | |
| Stage III | 1 h | 40~30 mg/L | 50~40 mg/L | |
| Spon-anammox | Stage I | 4 h | 80 mg/L | 100 mg/L |
| Stage II | 2 h | 80 mg/L | 100 mg/L | |
| Stage III | 1 h | 80 mg/L | 100 mg/L | |
| Stage I | 4 h | 30~40 mg/L | 40~50 mg/L | |
| Stage II | 2 h | 30~40 mg/L | 40~50 mg/L | |
| Stage III | 1 h | 30~40 mg/L | 40~50 mg/L |
| Reactor | Influence Factor | EBRT | Influent | Influent |
|---|---|---|---|---|
| Cp-anammox | EBRT (30/40/60 min) | / | 35~45 mg/L | 45~55 mg/L |
| Influent (40/30/20 mg/L) | 60 min | / | / = 1:1.32 | |
| Spon-anammox | EBRT (30/40/60 min) | / | 30~45 mg/L | 43~50 mg/L |
| Influent (40/30/20 mg/L) | 40 min | / | / = 1:1.32 | |
| Influent COD (20~110 mg/L) | 40 min | 30~40 mg/L | / = 1:1.32 |
| Operation Condition | Operation Unit | Influent | Other Condition |
|---|---|---|---|
| BAF/anammox under low aeration intensity | BAF | = 30~40 mg/L TN = 30~40 mg/L COD = 120~160 mg/L TP = 2~3 mg/L | Aeration intensity = 0.4 m3·m−2·h−1 EBRT = 60 min |
| Spon-anammox reactor | = 45 mg/L | EBRT = 60 min | |
| BAF/anammox under low EBRT | BAF | = 30~40 mg/L TN = 30~40 mg/L COD = 120~160 mg/L TP = 2~3 mg/L | Aeration intensity = 2.1 m3·m−2·h−1 EBRT = 30 min |
| Spon-anammox reactor | = 45 mg/L | EBRT = 40 min |
| Parameters | Conventional BAF Operation (kg·m−3·d−1) | BAF Operated under Low Aeration Intensity (kg·m−3·d−1) | BAF Operated under Low EBRT (kg·m−3·d−1) |
|---|---|---|---|
| COD | 2.68 | 2.27 | 2.34 |
| 0.73 | 0.08 | 0.11 |
| Process | Cost of Chemicalss for Nitrogen Removal (yuan/m3) | Electricity Costs from Aeration (yuan/m3) | Depreciation Costs of BAF (yuan/m3) | Total Cost (yuan/m3) |
|---|---|---|---|---|
| BAF-DN | 0.280 | 0.288 | 0 | 0.568 |
| BAF(lowAI)-anammox | 0.616 | 0.055 | 0 | 0.671 |
| BAF(lowEBRT)-anammox | 0.616 | 0.144 | 0.347 | 0.413 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Li, J.; Song, T.; Li, Y.; Wang, Z.; Zhang, X. Low Strength Wastewater Treatment Using a Combined Biological Aerated Filter/Anammox Process. Water 2024, 16, 2821. https://doi.org/10.3390/w16192821
Xie W, Li J, Song T, Li Y, Wang Z, Zhang X. Low Strength Wastewater Treatment Using a Combined Biological Aerated Filter/Anammox Process. Water. 2024; 16(19):2821. https://doi.org/10.3390/w16192821
Chicago/Turabian StyleXie, Wanying, Ji Li, Tao Song, Yong Li, Zhenlin Wang, and Xiaolei Zhang. 2024. "Low Strength Wastewater Treatment Using a Combined Biological Aerated Filter/Anammox Process" Water 16, no. 19: 2821. https://doi.org/10.3390/w16192821
APA StyleXie, W., Li, J., Song, T., Li, Y., Wang, Z., & Zhang, X. (2024). Low Strength Wastewater Treatment Using a Combined Biological Aerated Filter/Anammox Process. Water, 16(19), 2821. https://doi.org/10.3390/w16192821






