Chironomid Pupal Exuviae Technique in Ecological Research of Man-Made Water Bodies
Abstract
1. Introduction
2. Materials and Methods
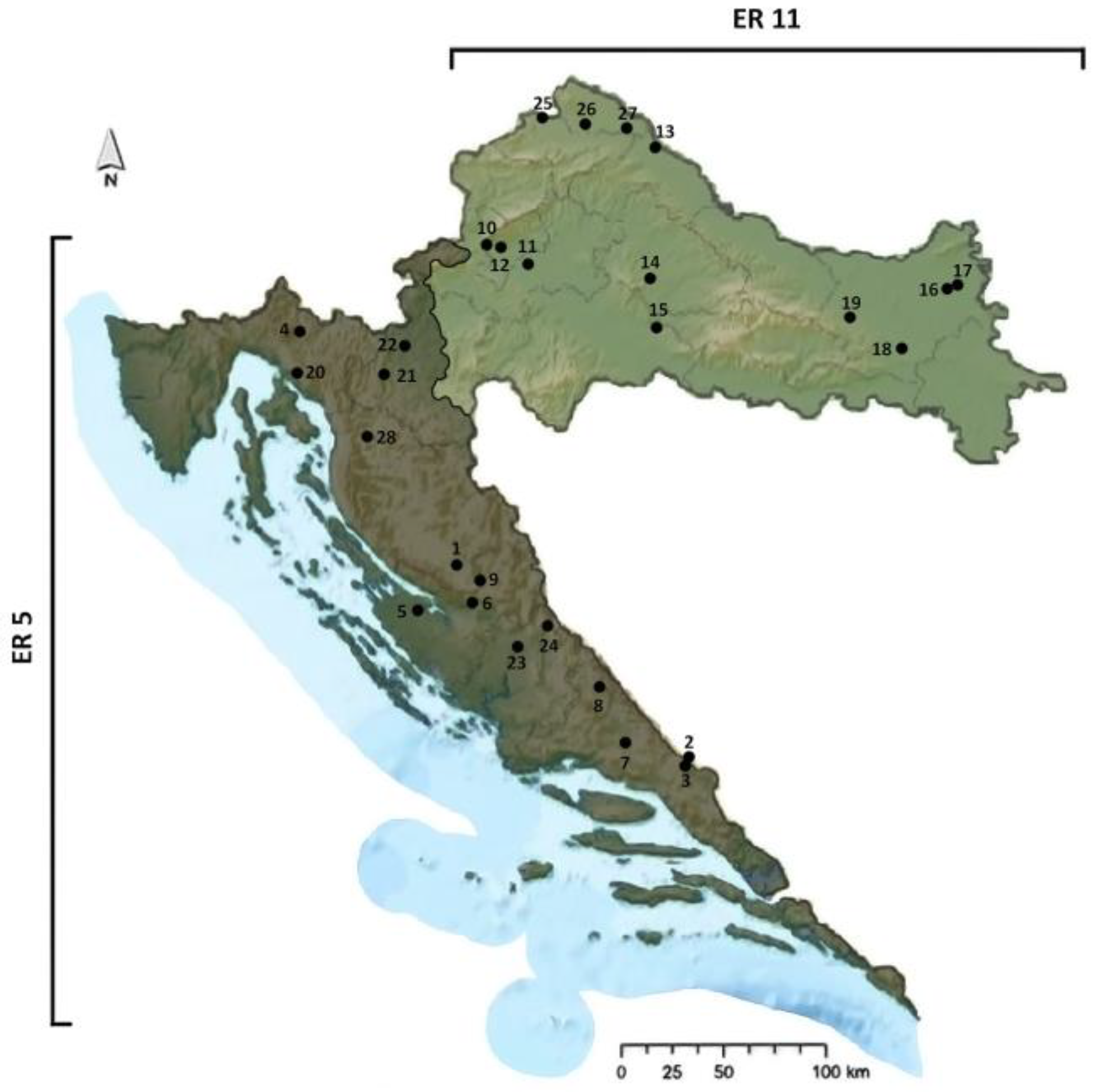
2.1. Study Area
2.2. Sampling Protocol
2.3. Environmental Parameters
2.4. Data Analyses
3. Results
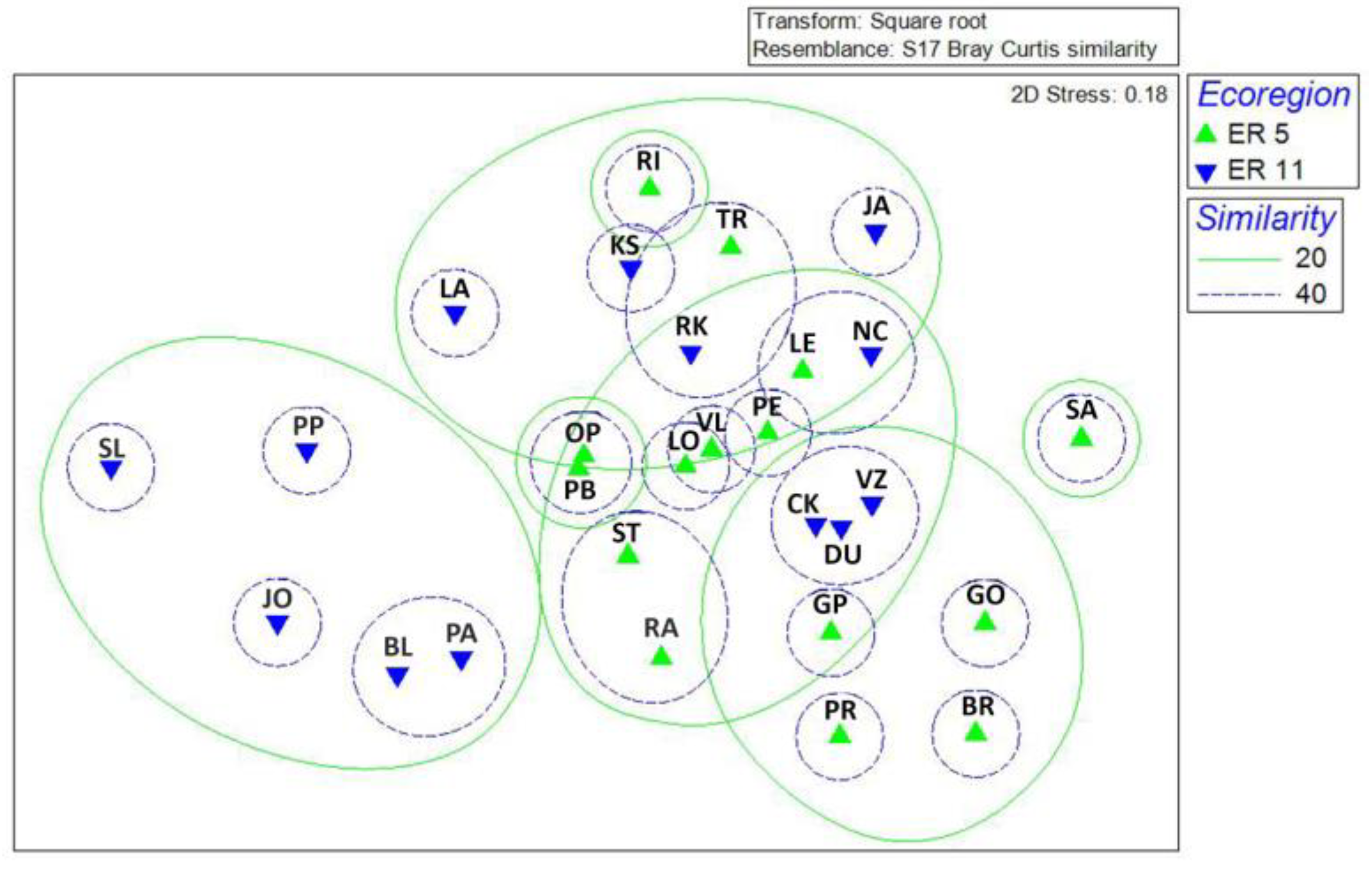
3.1. Chironomidae Assemblages
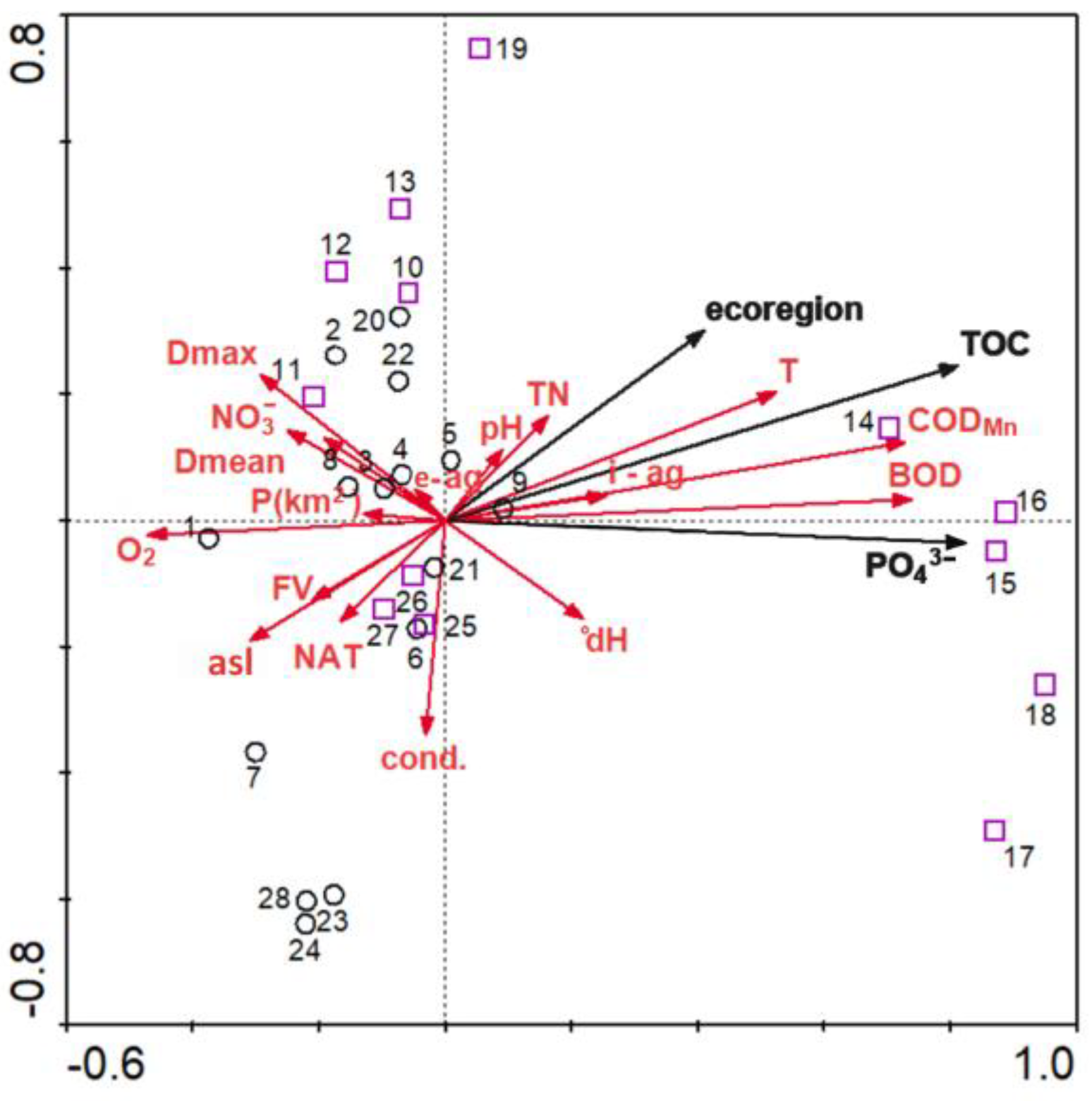
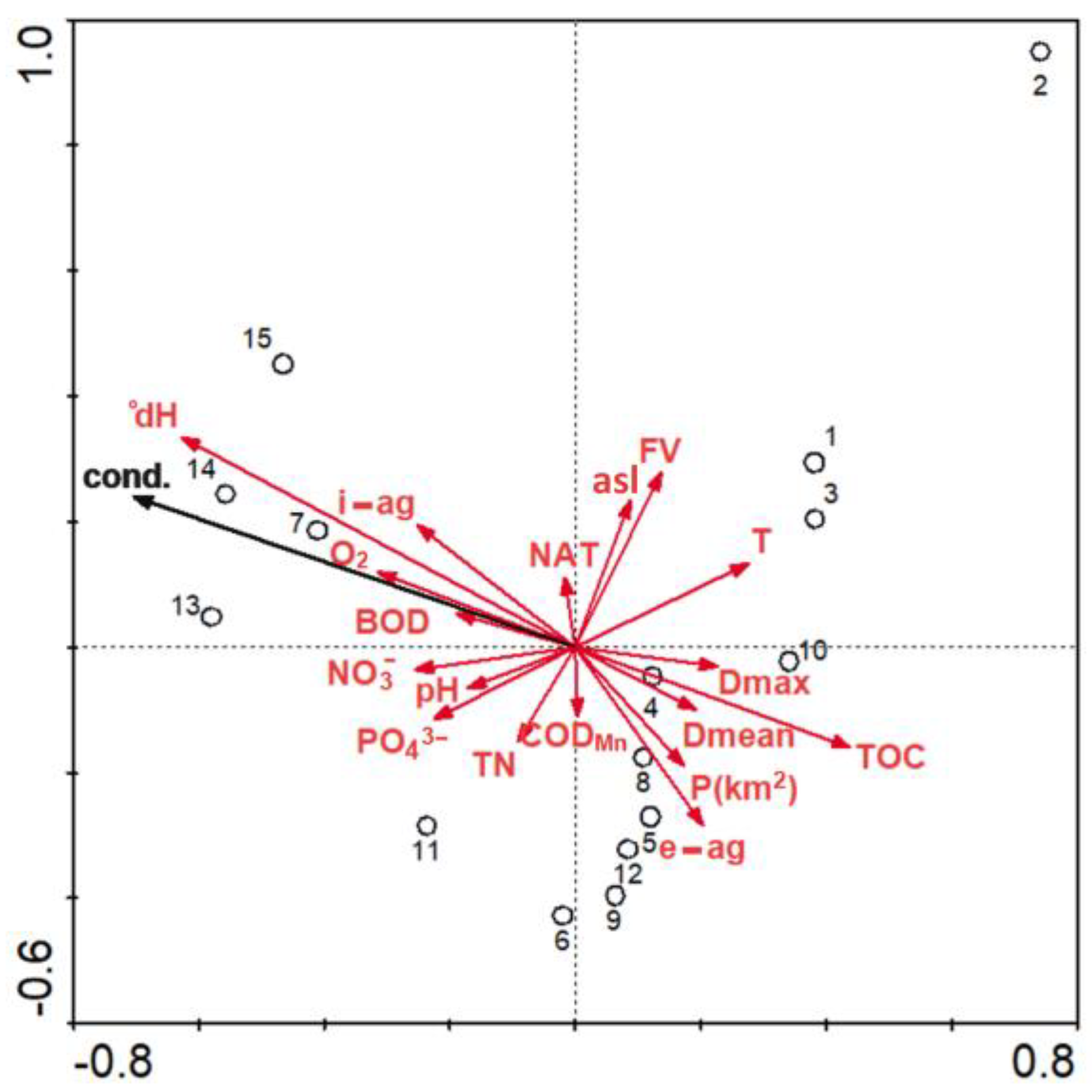
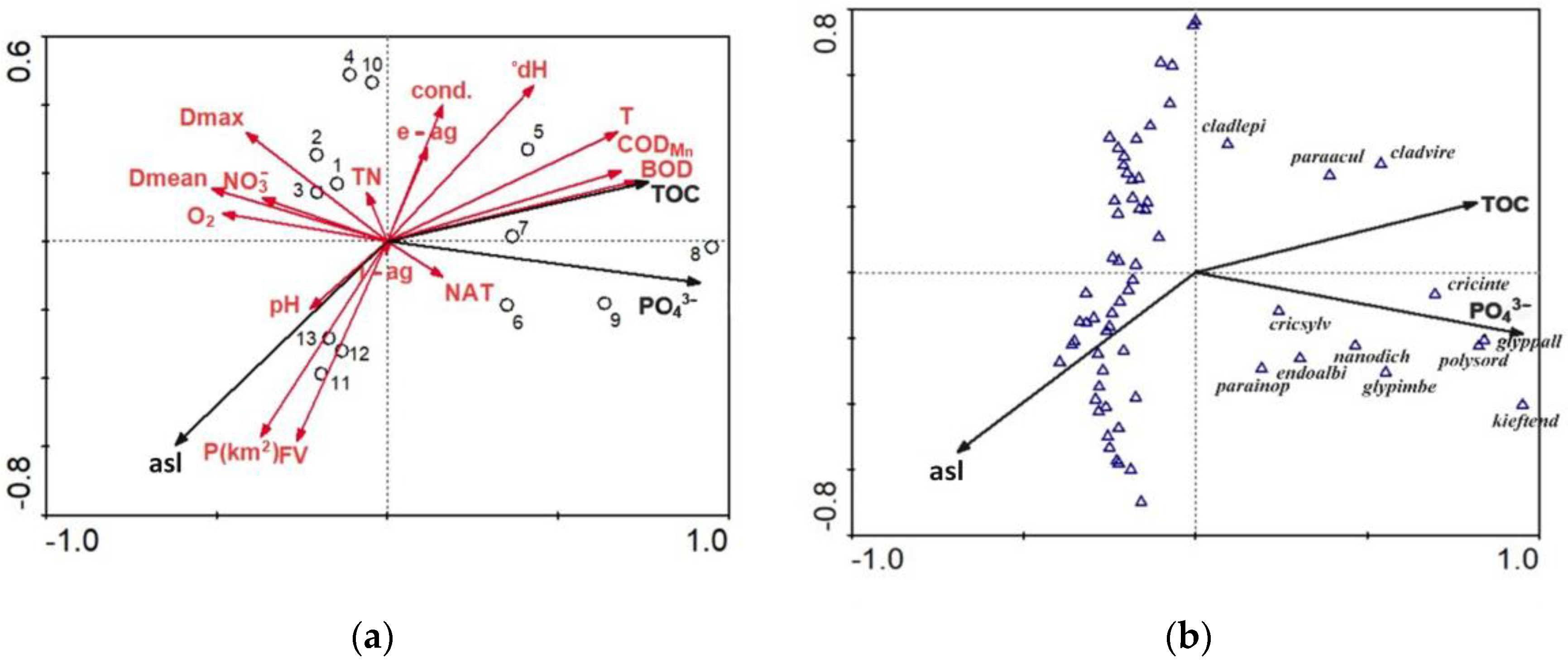
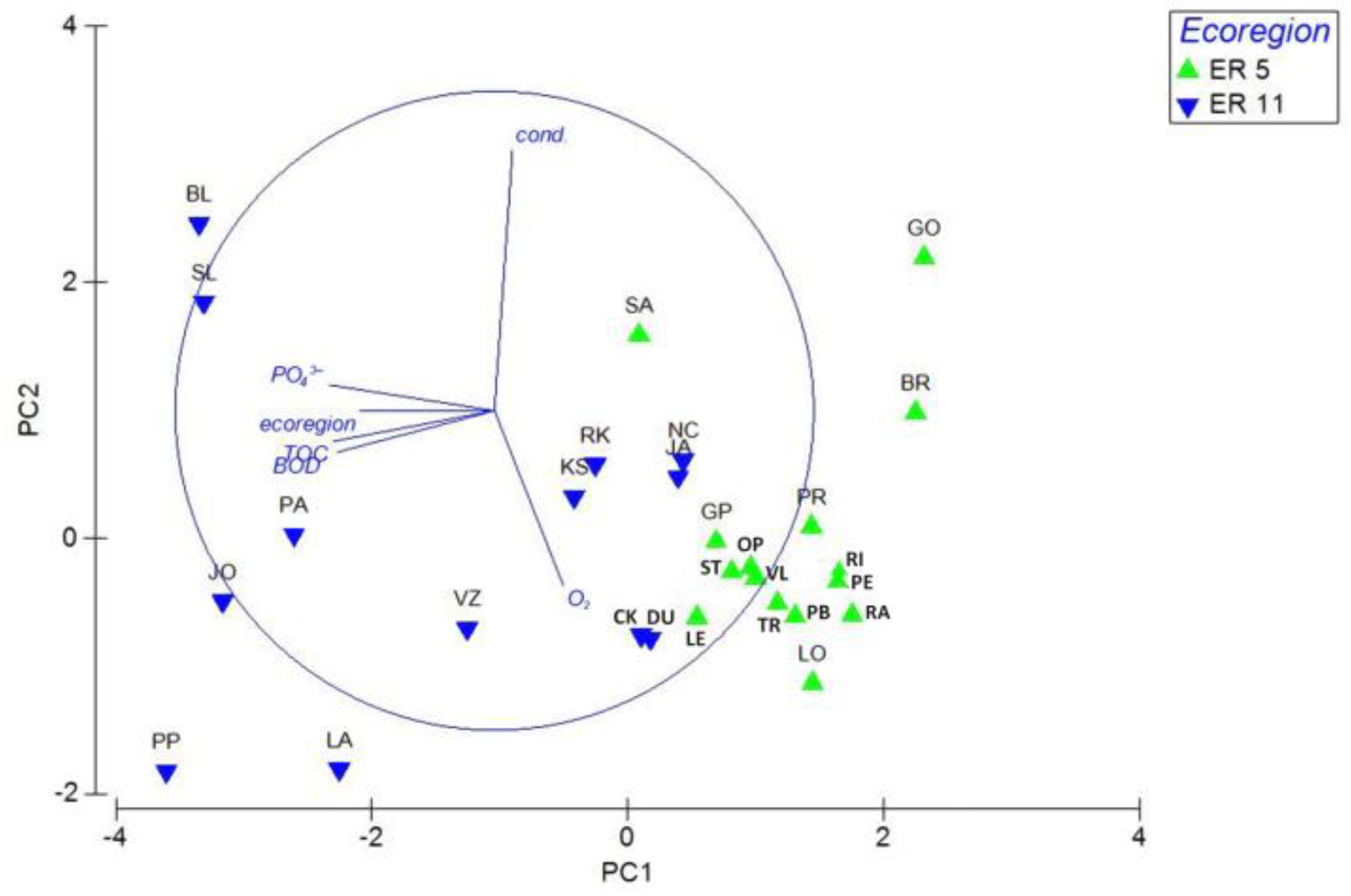
3.2. Environmental and Spatial Drivers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seaman, W., Jr.; Sprague, L.M. Artificial Habitats for Marine and Freshwater Fisheries, 1st ed.; Academic Press: San Diego, CA, USA, 1991; pp. 1–267. ISBN 9780080571171. [Google Scholar]
- Petrere, M., Jr. Fisheries in Large Tropical Reservoirs in South America. Lakes Reserv. Res. Manag. 1996, 2, 111–133. [Google Scholar] [CrossRef]
- Habdija, I.; Primc-Habdija, B. Limnologija—Ekologija Slatkih Voda; Alfa: Zagreb, Croatia, 2019; pp. 1–352. ISBN 9789533641683. [Google Scholar]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and Flow Regulation of the World’s Large River Systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Petts, G.E. Impounded Rivers: Perspectives for Ecological Management; John Wiley: Chichester, UK, 1984; pp. 1–326. ISBN 0471103063. [Google Scholar]
- Wang, Z.; Hu, C. Strategies for Managing Reservoir Sedimentation. Int. J. Sediment Res. 2009, 24, 369–384. [Google Scholar] [CrossRef]
- Carmignani, J.R.; Roy, A.H. Ecological Impacts of Winter Water Level Drawdowns on Lake Littoral Zones: A Review. Aquat. Sci. 2017, 79, 803–824. [Google Scholar] [CrossRef]
- Wiatkowski, M. Influence of Słup Dam Reservoir on Flow and Quality of Water in the Nysa Szalona River. Pol. J. Environ. Stud. 2011, 20, 469–478. [Google Scholar]
- Feng, L.; Hou, X.; Zheng, Y. Monitoring and Understanding the Water Transparency Changes of Fifty Large Lakes on the Yangtze Plain Based on Long-Term MODIS Observations. Remote Sens. Environ. 2019, 221, 675–686. [Google Scholar] [CrossRef]
- Tundisi, J.G.; Matsumura-Tundisi, T. Integration of Research and Management in Optimizing Multiple Uses of Reservoirs: The Experience in South America and Brazilian Case Studies. Hydrobiologia 2003, 500, 231–242. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Tang, Q.; Naselli-Flores, L.; Jeppesen, E.; Han, B.P. The Relationship Between Phytoplankton Diversity and Ecosystem Functioning Changes with Disturbance Regimes in Tropical Reservoirs. Ecosystems 2023, 26, 752–767. [Google Scholar] [CrossRef]
- Davies, S.P.; Jackson, S.K. The Biological Condition Gradient: A Descriptive Model for Interpreting Change in Aquatic Ecosystems. Ecol. Appl. 2006, 16, 1251–1266. [Google Scholar] [CrossRef]
- King, R.S.; Baker, M.E. Use, Misuse, and Limitations of Threshold Indicator Taxa Analysis (TITAN) for Natural Resource Management. In Application of Threshold Concepts in Natural Resource Decision Making; Springer: New York, NY, USA, 2014; pp. 231–254. ISBN 978-1-4899-8040-3. [Google Scholar]
- Vilenica, M.; Previšić, A.; Ivković, M.; Popijač, A.; Vučković, I.; Kučinić, M.; Kerovec, M.; Gattolliat, J.L.; Sartori, M.; Mihaljević, Z. Mayfly (Insecta: Ephemeroptera) Assemblages of a Regulated Perennial Mediterranean River System in the Western Balkans. Biologia 2016, 71, 1038–1048. [Google Scholar] [CrossRef]
- Vilenica, M.; Vučković, N.; Mihaljević, Z. Littoral Mayfly Assemblages in South-East European Man-Made Lakes. J. Limnol. 2019, 78, 47–59. [Google Scholar] [CrossRef]
- Vilenica, M.; Pozojević, I.; Vučković, N.; Mihaljević, Z. How Suitable Are Man-Made Water Bodies as Habitats for Odonata? Knowl. Manag. Aquat. Ecosyst. 2020, 421, 13. [Google Scholar] [CrossRef]
- Armitage, P.D.; Cranston, P.S.; Pinder, L.C.V. The Chironomidae: Biology and Ecology of Non-biting Midges; Chapman and Hall: London, UK, 1995; pp. 1–572. ISBN 041245260X. [Google Scholar]
- Moog, O. Fauna Aquatica Austriaca. Katalog zur Autökologischen Einstufung Aquatischer Organismen Österreichs, 2nd ed.; Bundesministerium für Land- und Forstwirtschaft, Umwelt und Wasserwirtschaft Wasserwirtschaftskataster: Wien, Austria, 2002; pp. 1–670. ISBN 3-85-174-044-0. [Google Scholar]
- Fraterrigo, J.M.; Downing, J.A.; Fraterrigo, J.M.; Downing, J.A. The Influence of Land Use on Lake Nutrients Varies with Watershed Transport Capacity. Ecosystems 2008, 11, 1021–1034. [Google Scholar] [CrossRef]
- Motta, L.; Massaferro, J. Climate and Site-Specific Factors Shape Chironomid Taxonomic and Functional Diversity Patterns in Northern Patagonia. Hydrobiologia 2019, 839, 131–143. [Google Scholar] [CrossRef]
- Belle, S.; Goedkoop, W. Functional Diversity of Chironomid Communities in Subarctic Lakes across Gradients in Temperature and Catchment Characteristics. Limnology 2021, 22, 5–16. [Google Scholar] [CrossRef]
- Cranston, P.S. Introduction. In The Chironomidae: Biology and Ecology of Nonbiting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman and Hall: London, UK, 1995; pp. 1–5. [Google Scholar]
- Hazra, N.; Saha, G.K.; Mazumdar, A.; Chaudhuri, P.K. Records of Chironomids of the Tribe Pentaneurini (Diptera: Chironomidae) in the Eastern Himalayas of India. Ann. Soc. Entomol. Fr. 2011, 47, 330–339. [Google Scholar] [CrossRef]
- Lindegaard, C. Classification of Water-Bodies and Pollution. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman and Hall: London, UK, 1995; pp. 385–404. ISBN 041245260X. [Google Scholar]
- Schiffels, S. Commensal and Parasitic Chironomidae. Denisia 2014, 33, 393–407. [Google Scholar]
- Vilenica, M.; Ergović, V.; Mihaljević, Z. Mayfly (Ephemeroptera) Assemblages of a Pannonian Lowland Mountain, with First Records of the Parasite Symbiocladius Rhithrogenae (Zavrel, 1924) (Diptera: Chironomidae). Ann. Limnol. 2018, 54, 31. [Google Scholar] [CrossRef]
- Berg, M.B. Larval Food and Feeding Behaviour. In The Chironomidae. Biology and Ecology of Nonbiting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman and Hall: London, UK, 1995; pp. 136–168. ISBN 041245260X. [Google Scholar]
- Vallenduuk, H.J. Chironomini Larvae of Western European Lowlands (Diptera: Chironomidae). Keys with Notes to the Species. In With a Redescription of Glyptotendipes (Caulochironomus) Nagorskayae and a First Description of Glyptotendipes (Caulochironomus) Kaluginae New Species, 1st ed.; Erik Mauch Verlag: Dinkelscherben, Germany, 2017; pp. 1–216. ISBN 0935-333X. [Google Scholar]
- EC Parliament and Council. Directive 2000/60/EC of the European Parliament and of the Council Establishing a Framework for Community Action in the Field of Water Policy. OJEU, 22 December 2000; pp. 1–72. [Google Scholar]
- Milošević, D.; Čerba, D.; Szekeres, J.; Csányi, B.; Tubić, B.; Simić, V.; Paunović, M. Artificial Neural Networks as an Indicator Search Engine: The Visualization of Natural and Man-Caused Taxa Variability. Ecol. Indic. 2016, 61, 777–789. [Google Scholar] [CrossRef]
- Ruse, L. Chironomid Pupal Exuviae as Indicators of Lake Status. Arch. Hydrobiol. 2002, 3, 367–390. [Google Scholar] [CrossRef]
- Raunio, J.; Muotka, T. The Use of Chironomid Pupal Exuviae in River Biomonitoring: The Importance of Sampling Strategy. Arch. Hydrobiol. 2005, 164, 529–545. [Google Scholar] [CrossRef]
- Calle-Martínez, D.; Casas, J.J. Chironomid Species, Stream Classification, and Water-Quality Assessment: The Case of 2 Iberian Mediterranean Mountain Regions. J. N. Am. Benthol. Soc. 2006, 25, 465–476. [Google Scholar] [CrossRef]
- Raunio, J.; Paasivirta, L. Emergence Patterns of Lotic Chironomidae (Diptera: Nematocera) in Southern Finland and the Use of Their Pupal Exuviae in River Biomonitoring. Fundam. Appl. Limnol. 2008, 170, 291–301. [Google Scholar] [CrossRef]
- Wilson, R.S.; Bright, P.L. The Use of Chironomid Pupal Exuviae for Characterising Streams. Freshw. Biol. 1973, 3, 283–302. [Google Scholar] [CrossRef]
- Wilson, R.S.; Ruse, L.P. A Guide to the Identification of Genera of Chironomid Pupal Exuviae Occurring in Britain and Ireland (Including Common Genera from Northern Europe) and Their Use in Monitoring Lotic and Lentic Fresh Waters; Freshwater Biological Association: Ambleside, UK, 2005; pp. 1–176. ISBN 978-0-900386-73-2. [Google Scholar]
- Wilson, R.S.; McGill, J.D. A New Method of Monitoring Water Quality in a Stream Receiving Sewage Effluent, Using Chironomid Pupal Exuvia. Water Res. 1977, 11, 959–962. [Google Scholar] [CrossRef]
- Bitušík, P.; Svitok, M.; Kološta, P.; Hubková, M. Classification of the Tatra Mountain Lakes (Slovakia) Using Chironomids (Diptera, Chironomidae). Biologia 2006, 61, 191–201. [Google Scholar] [CrossRef]
- Ferrington, L.C.; Blackwood, M.A.; Wright, C.A.; Crisp, N.H.; Kavanaugh, J.L.; Schmidt, F.J. A Protocol for Using Surface-Floating Pupal Exuviae of Chironomidae for Rapid Bioassessment of Changing Water Quality; International Association of Hydrological Sciences: Wien, Austria, 1991; pp. 181–190. ISBN 0947571086. [Google Scholar]
- Anderson, A.M.; Ferrington, L.C. Resistance and Resilience of Winter-Emerging Chironomidae (Diptera) to a Flood Event: Implications for Minnesota Trout Streams. Hydrobiologia 2013, 707, 59–71. [Google Scholar] [CrossRef]
- Saulino, H.H.L.; Cañedo-Argüelles, M.; Trivinho-Strixino, S.; Gorni, G.R.; Corbi, J.J. Chironomid Pupal Exuviae Communities Support the “Field of Dreams” Hypothesis after the Riparian Vegetation Recovery in Headwater Urban Streams. Ecol. Indic. 2021, 127, 107766. [Google Scholar] [CrossRef]
- Downes, B.; Barmuta, L.A.; Fairweather, P.G.; Faith, D.P.; Keough, M.J.; Lake, P.S.; Mapstone, B.D.; Quinn, G.P. Monitoring Ecological Impacts. Concepts and Practice in Flowing Waters; Cambridge University Press: Cambridge, UK, 2002; pp. 1–421. ISBN 9780521771573. [Google Scholar]
- Ruse, L.P. Chironomid (Diptera) species recorded from UK lakes as pupal exuviae. J. Entomol. Acarol. Res. 2013, 45, e13. [Google Scholar] [CrossRef]
- Illies, J. Limnofauna Europaea. Eine Zusammenstellung Aller Die Europäische Binnengewässer Bewohnenden Mehrzelligen Tierarten Mit Angaben Über Ihre Verbreitung Und Ökologie, 2nd ed.; Gustav Fischer Verlag: Stuttgart, Germany, 1978; pp. 1–532. ISBN 0013-2969. [Google Scholar]
- Wiederholm, T. Chironomidae of the Holarctic Region, Keys and Diagnoses; Publishing House of the Swedish Research Councils: Stockholm, Sweden, 1983; pp. 1–457. ISBN 25459. [Google Scholar]
- Langton, P.H. A Key to Pupal Exuviae of West Palearctic Chironomidae; Private Publishing: Huntingdon, UK, 1991; pp. 1–386. [Google Scholar]
- CLC Hrvatska. CORINE Land Cover Hrvatska; Hrvatska Agencija za Okoliš i Prirodu: Zagreb, Croatia, 2013. [Google Scholar]
- McCune, B.; Grace, J.B. Analysis of Ecological Communities, 2nd ed.; MjM Software Design: Gleneden Beach, OR, USA, 2002; pp. 1–300. ISBN 0-9721290-0-6. [Google Scholar]
- Pollesch, N.L.; Dale, V.H. Normalization in Sustainability Assessment: Methods and Implications. Ecol. Econ. 2016, 130, 195–208. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. Primer V6: User Manual—Tutorial; PRIMER-E Limited: Plymouth, UK, 2006; pp. 1–190. [Google Scholar]
- ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and User’s Guide to Canoco for Windows: Software for Canonical Community Ordination (Version 4); Microcomputer Power: Ithaca, NY, USA, 1998; pp. 1–500. [Google Scholar]
- Mihaljević, Z.; Vučković, N.; Čerba, D. Littoral Chironomid Assemblages in the Reservoirs along the Eastern Adriatic Coast. In Book of Abstracts, Proceedings of the 20th International Symposium on Chironomidae, Trento, Italy, 2–8 July 2017; Trident: Barnala, India, 2017. [Google Scholar]
- Čerba, D.; Mihaljević, Z.; Vidaković, J. Colonisation of Temporary Macrophyte Substratum by Midges (Chironomidae: Diptera). Ann. Limnol.-Int. J. Lim. 2010, 46, 181–190. [Google Scholar] [CrossRef]
- Čerba, D.; Mihaljević, Z.; Vidaković, J. Colonisation Trends, Community and Trophic Structure of Chironomid Larvae (Chironomidae: Diptera) in a Temporal Phytophylous Assemblage. Fundam. Appl. Limnol. 2011, 179, 203–214. [Google Scholar] [CrossRef]
- Vilenica, M.; Brigić, A.; Ergović, V.; Koh, M.; Alegro, A.; Šegota, V.; Rimac, A.; Rumišek, M.; Mihaljević, Z. Taxonomic and Functional Odonata Assemblage Metrics: Macrophyte–Driven Changes in Anthropogenically Disturbed Floodplain Habitats. Hydrobiologia 2024, 851, 3787–3807. [Google Scholar] [CrossRef]
- Tarkowska-Kukuryk, M. Water Soldier Stratiotes aloides L. (Hydrocharitaceae) as a Substratum for Macroinvertebrates in a Shallow Eutrophic Lake. Pol. J. Ecol. 2006, 3, 441–451. [Google Scholar]
- Čerba, D.; Koh, M.; Ergović, V.; Mihaljević, Z.; Milošević, D.; Hamerlík, L. Chironomidae (Diptera) of Croatia with Notes on the Diversity and Distribution in Various Habitat Types. Zootaxa 2020, 4780, 259–274. [Google Scholar] [CrossRef]
- Grzybkowska, M. Diel Drift of Chironomidae in a Large Lowland River (Central Poland). Netherland J. Aquat. Ecol. 1992, 26, 355–360. [Google Scholar] [CrossRef]
- Malo, J.; Morais, M.; Pinto, P.A. Contribution to the Knowledge of the Chironomid Fauna (Diptera) in Southern Portugal. Ann. Limnol.-Int. J. Lim. 1998, 34, 165–170. [Google Scholar] [CrossRef]
- Saether, O.A. Chironomid Communities as Water Quality Indicators. Holarct. Ecol. 1979, 2, 65–74. [Google Scholar] [CrossRef]
- Čerba, D.; Vlaičević, B.; Davidović, R.A.; Koh, M.; Ergović, V.; Turković Čakalić, I. Chironomidae in Shallow Water Bodies of a Protected Lowland Freshwater Floodplain Ecosystem. Sci. Prog. 2023, 106, 00368504231172653. [Google Scholar] [CrossRef]
- Moller Pillot, H.K.M. Chironomidae Larvae, Biology and Ecology of the Chironomini; KNNV Publishing: Zeist, The Netherlands, 2009; pp. 1–270. ISBN 9789004284418. [Google Scholar]
- Raunio, J.; Paavola, R.; Muotka, T. Effects of Emergence Phenology, Taxa Tolerances and Taxonomic Resolution on the Use of the Chironomid Pupal Exuvial Technique in River Biomonitoring. Freshw. Biol. 2007, 52, 165–176. [Google Scholar] [CrossRef]
- Ruse, L. Classification of Nutrient Impact on Lakes Using the Chironomid Pupal Exuvial Technique. Ecol. Indic. 2010, 10, 594–601. [Google Scholar] [CrossRef]
- Kranzfelder, P.; Anderson, A.M.; Egan, A.T.; Mazack, J.E.; Bouchard, R.W.; Rufer, M.M.; Ferrington, L.C. Use of Chironomidae (Diptera) Surface-Floating Pupal Exuviae as a Rapid Bioassessment Protocol for Water Bodies. J. Vis. Exp. 2015, 101, e52558. [Google Scholar] [CrossRef]
- Bouchard, R.W.; Ferrington, L.C. The Effects of Subsampling and Sampling Frequency on the Use of Surface-Floating Pupal Exuviae to Measure Chironomidae (Diptera) Communities in Wadeable Temperate Streams. Environ. Monit. Assess. 2011, 181, 205–223. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Bonacci, O.; Roje-Bonacci, T. Drastic Hydrological Changes Caused by Hydroelectrical Development in Karst: A Case of the Karst River Zrmanja (Croatia). Environ. Earth Sci. 2015, 74, 6767–6777. [Google Scholar] [CrossRef]
- Seekell, D.; Cael, B.; Norman, S.; Byström, P. Patterns and Variation of Littoral Habitat Size Among Lakes. Geophys. Res. Lett. 2021, 48, e2021GL095046. [Google Scholar] [CrossRef]
- Thorp, J.H. Functional Relationships of Freshwater Invertebrates. In Thorp and Covich’s Freshwater Invertebrates, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 65–82. ISBN 978-0-12-385026-3. [Google Scholar]
- Griffiths, H.I.; Krystufek, B.; Reed, J.M. Balkan Biodiversity: Pattern and Process in the European Hotspot; Kluwer Academic Publishers: London, UK, 2004; pp. 1–332. ISBN 978-90-481-6732-6. [Google Scholar]
- Popijač, A.; Sivec, I. First Records of the Alpine Stonefly Species Protonemura julia Nicolai, 1983 (Insecta, Plecoptera) in Croatia. Nat. Croat. 2009, 18, 83–89. [Google Scholar]
- Tockner, K.; Zarfl, C.; Robinson, C.T. Rivers of Europe, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–942. ISBN 978-0-08-102612-0. [Google Scholar]
- Kron, W.; Müller, O. Efficiency of Flood Protection Measures: Selected Examples. Water Policy 2019, 21, 449–467. [Google Scholar] [CrossRef]
- Moss, B. Ecology of Freshwater: Man and Medium, Past to Future; Blackwell Science: Oxford, UK, 1998; pp. 1–584. ISBN 0632035129. [Google Scholar]
- Sternberg, R. Damming the River: A Changing Perspective on Altering Nature. Renew. Sustain. Energy Rev. 2006, 10, 165–197. [Google Scholar] [CrossRef]
- Allan, J.D. Landscapes and Riverscapes: The Influence of Land Use on Stream Ecosystems. Source Annu. Rev. Ecol. Evol. Syst. 2004, 35, 257–284. [Google Scholar] [CrossRef]
- Brasil, L.S.; Juen, L.; Cabette, H.S.R. The Effects of Environmental Integrity on the Diversity of Mayflies, Leptophlebiidae (Ephemeroptera), in Tropical Streams of the Brazilian Cerrado. Ann. Limnol.-Int. J. Lim. 2014, 50, 325–334. [Google Scholar] [CrossRef]

| Ecoregion | Study Site | N | E | Altitude (m asl) | Surface Area (km2) | Depth Max (m) | Depth Mean (m) |
|---|---|---|---|---|---|---|---|
| ER 5 | OP | 45.36745 | 15.66164 | 575 | 0.89 | 6.7 | 2.9 |
| RI | 43.49671 | 17.13342 | 387 | 1.9 | 39.6 | 16 | |
| PB | 43.47466 | 17.12161 | 269 | 2.11 | 5.3 | 4 | |
| LO | 45.36859 | 14.70618 | 767 | 2.1 | 44.7 | 33.8 | |
| VL | 44.15676 | 15.42684 | 122 | 0.28 | 10 | 4 | |
| RA | 45.20495 | 15.74683 | 9 | 0.65 | 7.5 | 5.8 | |
| PR | 43.56273 | 16.71913 | 284 | 0.65 | 20 | 6.3 | |
| PE | 43.82191 | 16.55311 | 330 | 20.09 | 31 | 20.8 | |
| ST | 44.29232 | 15.81408 | 553 | 3.34 | 6.5 | 4.3 | |
| TR | 45.22876 | 14.66736 | 60 | 0.41 | 4 | 2.7 | |
| SA | 45.22878 | 15.22602 | 320 | 1.7 | 6.2 | 3 | |
| LE | 45.35769 | 15.30444 | 182 | 1.46 | 42.5 | 21 | |
| BR | 44.00897 | 16.03684 | 187 | 0.03 | 18 | 10 | |
| GO | 44.09888 | 16.22131 | 307 | 0.17 | 6 | 3 | |
| GP | 44.94508 | 15.11866 | 430 | 0.43 | 6.5 | 5.4 | |
| ER 11 | VZ | 46.39212 | 16.16303 | 191 | 2.85 | 8.7 | 5 |
| CK | 46.31263 | 16.37021 | 168 | 10.5 | 13.2 | 7 | |
| DU | 46.32488 | 16.60154 | 150 | 16.6 | 13.4 | 8 | |
| RK | 45.78997 | 15.8441 | 120 | 2 | 5 | 3 | |
| NC | 45.71174 | 16.10337 | 103 | 0.9 | 40 | 15 | |
| JA | 45.7816 | 15.92521 | 115 | 6.4 | 7 | 4 | |
| KS | 46.23603 | 16.90369 | 128 | 1.5 | 20 | 8 | |
| PP | 45.6377 | 16.87405 | 96 | 0.75 | 6.5 | 2 | |
| PA | 45.43809 | 16.89871 | 104 | 2.73 | 6.3 | 3 | |
| BL | 45.59153 | 18.73839 | 80 | 1.25 | 5 | 3 | |
| SL | 45.60828 | 18.80041 | 79 | 0.12 | 7 | 4 | |
| JO | 45.32281 | 18.45246 | 93 | 0.79 | 1.4 | 1 | |
| LA | 45.48027 | 18.11297 | 123 | 0.5 | 11 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ergović, V.; Čerba, D.; Vučković, N.; Mihaljević, Z. Chironomid Pupal Exuviae Technique in Ecological Research of Man-Made Water Bodies. Water 2024, 16, 2917. https://doi.org/10.3390/w16202917
Ergović V, Čerba D, Vučković N, Mihaljević Z. Chironomid Pupal Exuviae Technique in Ecological Research of Man-Made Water Bodies. Water. 2024; 16(20):2917. https://doi.org/10.3390/w16202917
Chicago/Turabian StyleErgović, Viktorija, Dubravka Čerba, Natalija Vučković, and Zlatko Mihaljević. 2024. "Chironomid Pupal Exuviae Technique in Ecological Research of Man-Made Water Bodies" Water 16, no. 20: 2917. https://doi.org/10.3390/w16202917
APA StyleErgović, V., Čerba, D., Vučković, N., & Mihaljević, Z. (2024). Chironomid Pupal Exuviae Technique in Ecological Research of Man-Made Water Bodies. Water, 16(20), 2917. https://doi.org/10.3390/w16202917








