Short-Term Effects of Abrupt Salinity Changes on Aquaculture Biofilter Performance and Microbial Communities
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Biofilter Feeding
2.3. Salinity Adjustment
2.4. Chemical Analysis
2.5. DNA Extraction and Sequencing
2.6. Sequence Data Analysis
3. Results and Discussion
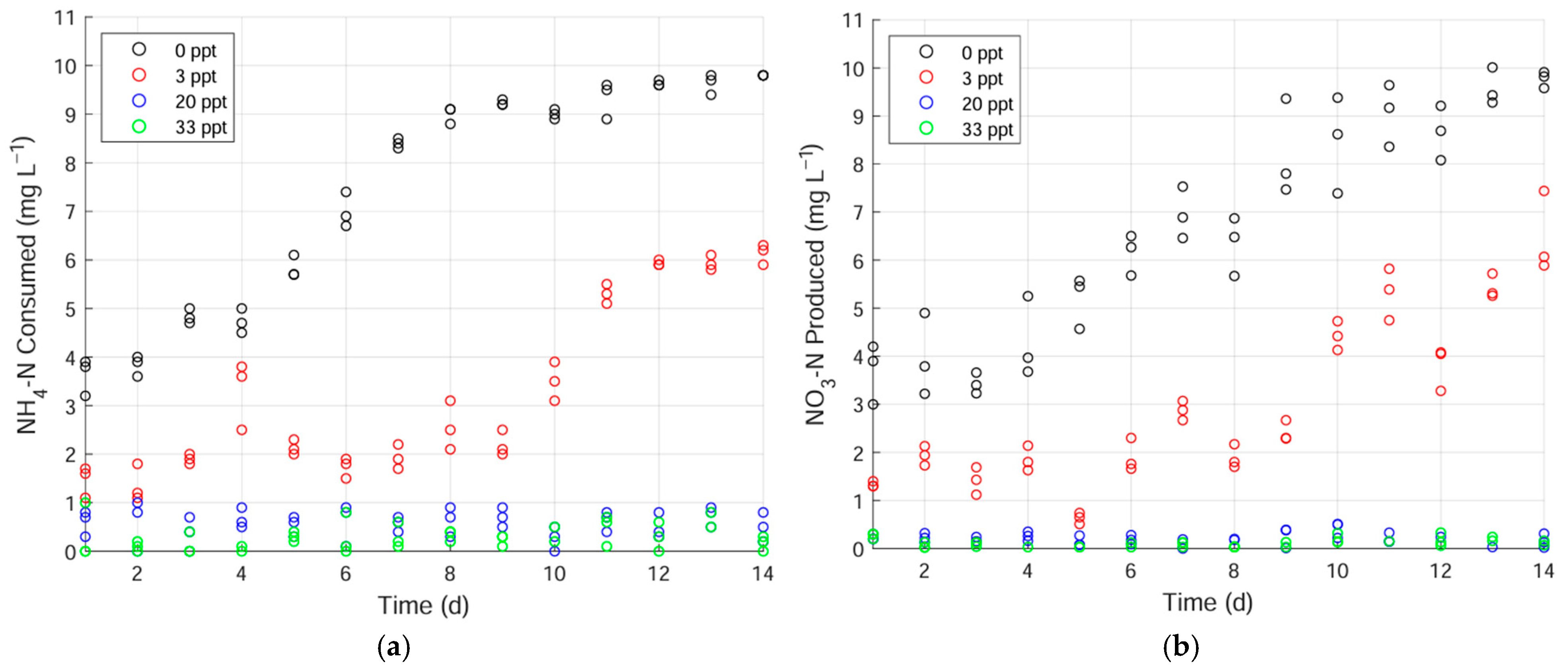
3.1. Nitrification in the Freshwater-Adapted Filters
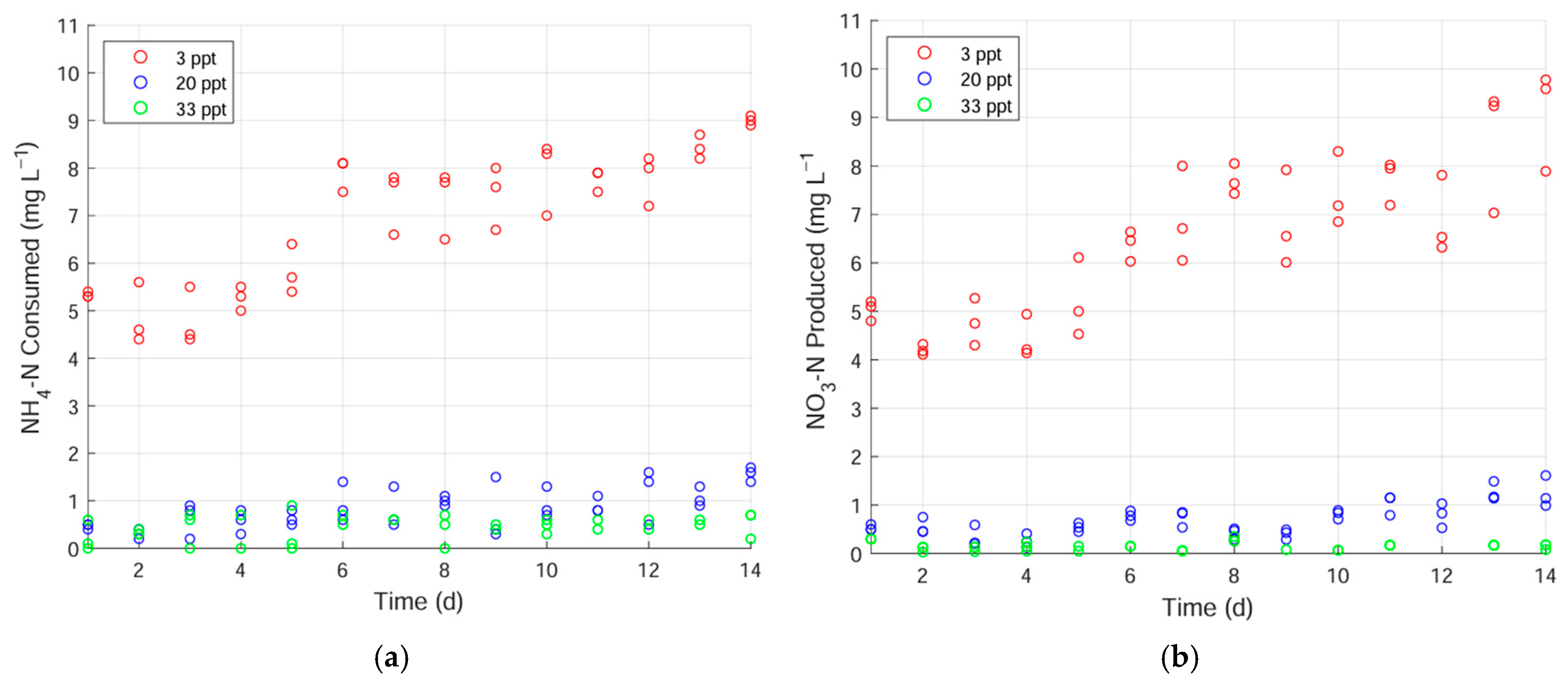
3.2. Nitrification in the Low-Salt-Adapted Biofilters
3.3. Microbial Community Sequencing Results
3.3.1. Bacterial Diversity
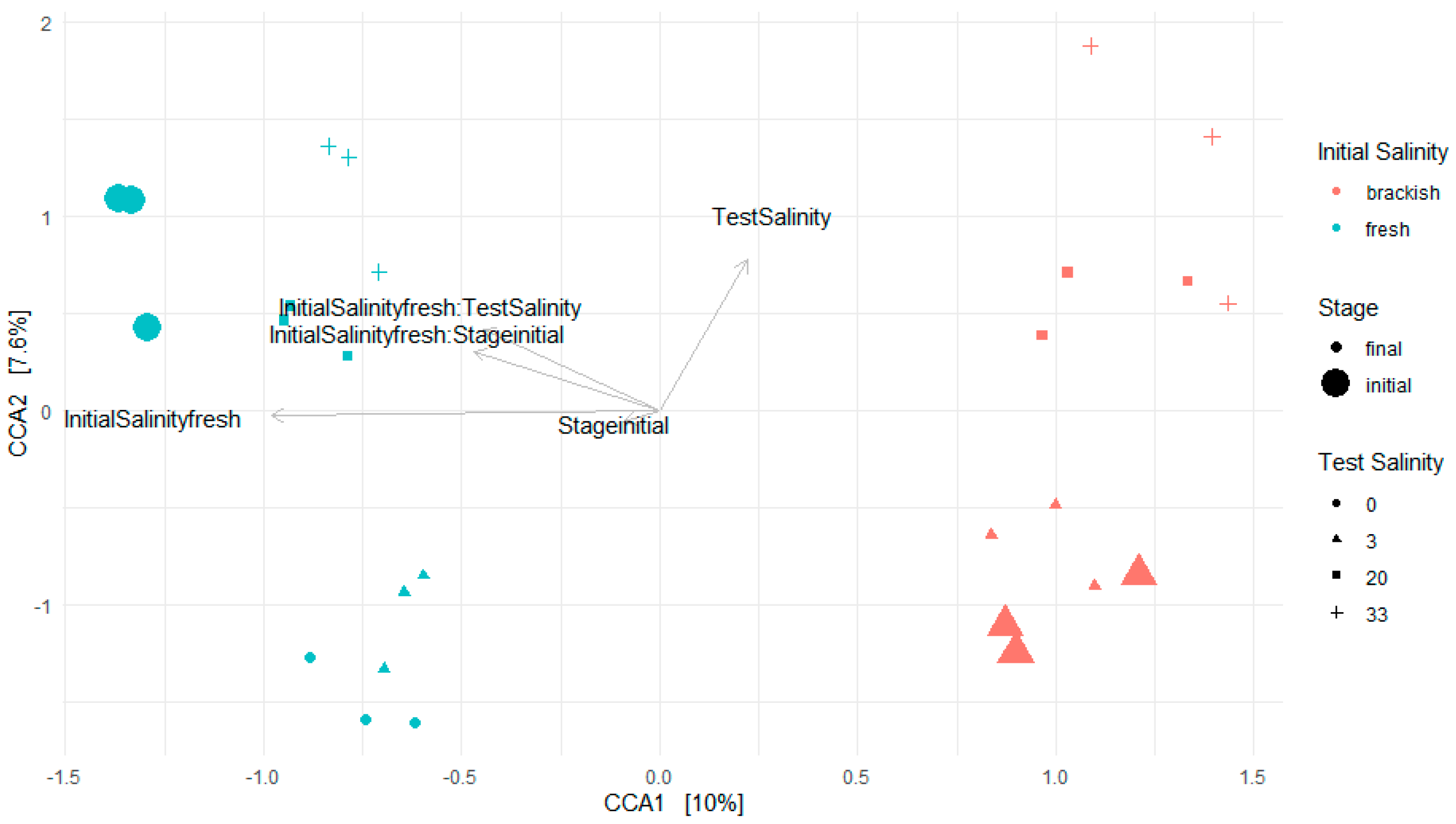
3.3.2. Canonical Coordinate Analysis (CCA)
3.3.3. Influence of Test Conditions and 30-Day Acclimation Period
3.3.4. Nitrifying Bacteria Composition
3.3.5. Influence of Adaptation to Low-Salinity Conditions
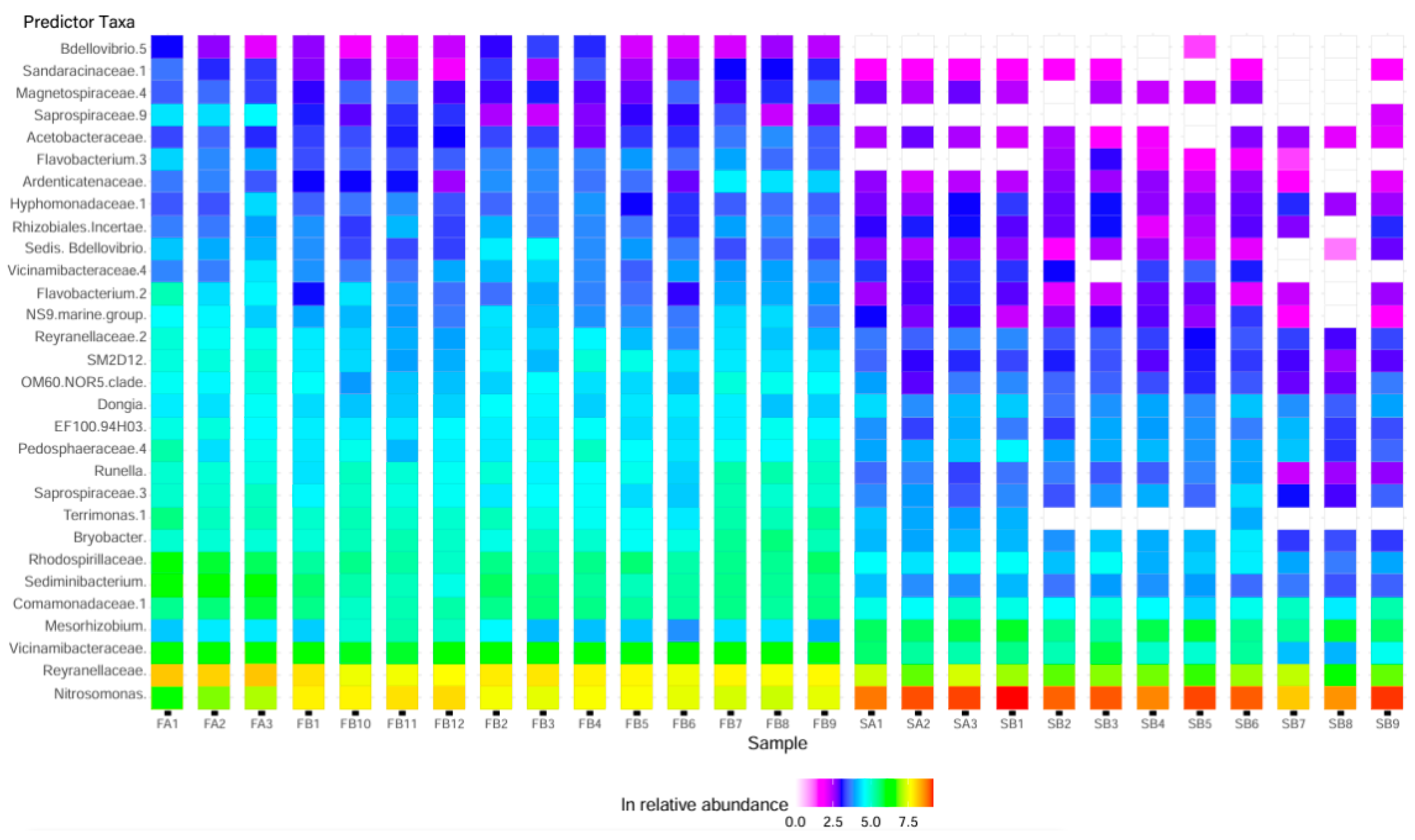
3.3.6. Taxa Predictions for Freshwater versus Low-Salinity Biofilters
3.3.7. Changes in the Most Abundant Nitrifying SVs
3.3.8. Changes in the Overall Most Abundant SVs
4. Conclusions
- (1)
- When biofilters were maintained at 3 ppt before being shifted to 20 ppt, they showed a slight nitrification recovery (11%). Shifting to 33 ppt showed no recovery. When shifted similarly, freshwater biofilters did not recover in either 20 or 33 ppt. Low-level salinity maintenance may not be sufficient to enable traditional biofilters to respond rapidly to abrupt salinity shifts.
- (2)
- Sequencing results showed that heterotrophic bacteria in biofilters may be more sensitive to salinity changes than the nitrifiers in the short-term. Future work could include investigation into salinity shifts on different biofilter compositions with resistance to other environmental factors (such as pH or temperature); this could show whether community resistance to other environmental stressors may better prepare the heterotrophs for salinity shifts and provide faster short-term recoveries.
- (3)
- A longer series of similar tests (upwards of two weeks) could possibly fully characterize the effects of this method of salinity acclimation and help to fully understand the microbial community dynamics in more long-term scenarios.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bregnballe, J. A Guide to Recirculation Aquaculture. FAO Eurofish Rep. 2015, 100. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/7928a883-445d-41e4-9ba2-ff4e464db8a2/content (accessed on 10 December 2021).
- Zimmer, A.M. Ammonia Excretion by the Fish Gill: Discoveries and Ideas That Shaped Our Current Understanding. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2024, 1–19. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, X.; Li, J.; Lee, C.T.; Ong, P.Y.; Zhang, Z.; Li, C. Effect of Aquaculture Salinity on Nitrification and Microbial Community in Moving Bed Bioreactors with Immobilized Microbial Granules. Bioresour. Technol. 2020, 297, 122427. [Google Scholar] [CrossRef] [PubMed]
- Skoyles, A.; Chaganti, S.R.; Mundle, S.O.C.; Weisener, C.G. Nitrification Kinetics and Microbial Community Dynamics of Attached Biofilm in Wastewater Treatment. Water Sci. Technol. 2020, 81, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Kinyage, J.P.H.; Pedersen, P.B.; Pedersen, L.F. Effects of Abrupt Salinity Increase on Nitrification Processes in a Freshwater Moving Bed Biofilter. Aquac. Eng. 2019, 84, 91–98. [Google Scholar] [CrossRef]
- Navada, S.; Vadstein, O. Salinity Acclimation Strategies in Nitrifying Bioreactors. Front. Mar. Sci. 2022, 9, 867592. [Google Scholar] [CrossRef]
- Navada, S.; Vadstein, O.; Gaumet, F.; Tveten, A.K.; Spanu, C.; Mikkelsen, Ø.; Kolarevic, J. Biofilms Remember: Osmotic Stress Priming as a Microbial Management Strategy for Improving Salinity Acclimation in Nitrifying Biofilms. Water Res. 2020, 176, 115732. [Google Scholar] [CrossRef]
- Navada, S.; Sebastianpillai, M.; Kolarevic, J.; Fossmark, R.O.; Tveten, A.K.; Gaumet, F.; Mikkelsen, Ø.; Vadstein, O. A Salty Start: Brackish Water Start-up as a Microbial Management Strategy for Nitrifying Bioreactors with Variable Salinity. Sci. Total Environ. 2020, 739, 139934. [Google Scholar] [CrossRef]
- Breves, J.P.; Shaughnessy, C.A. Endocrine Control of Gill Ionocyte Function in Euryhaline Fishes. J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2024, 1–22. [Google Scholar] [CrossRef]
- Moran, D. Carbon Dioxide Degassing in Fresh and Saline Water. I: Degassing Performance of a Cascade Column. Aquac. Eng. 2010, 43, 29–36. [Google Scholar] [CrossRef]
- Navada, S.; Vadstein, O.; Tveten, A.K.; Verstege, G.C.; Terjesen, B.F.; Mota, V.C.; Venkataraman, V.; Gaumet, F.; Mikkelsen, Ø.; Kamstra, A. Influence of Rate of Salinity Increase on Nitrifying Biofilms. J. Clean. Prod. 2019, 238, 117835. [Google Scholar] [CrossRef]
- Hüpeden, J.; Wemheuer, B.; Indenbirken, D.; Schulz, C.; Spieck, E. Taxonomic and Functional Profiling of Nitrifying Biofilms in Freshwater, Brackish and Marine RAS Biofilters. Aquac. Eng. 2020, 90, 102094. [Google Scholar] [CrossRef]
- Holmes, D.E.; Dang, Y.; Smith, J.A. Nitrogen Cycling during Wastewater Treatment, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 106, ISBN 9780128169759. [Google Scholar]
- Lehtovirta-Morley, L.E. Ammonia Oxidation: Ecology, Physiology, Biochemistry and Why They Must All Come Together. FEMS Microbiol. Lett. 2018, 365, fny058. [Google Scholar] [CrossRef] [PubMed]
- Fumasoli, A.; Bürgmann, H.; Weissbrodt, D.G.; Wells, G.F.; Beck, K.; Mohn, J.; Morgenroth, E.; Udert, K.M. Growth of Nitrosococcus-Related Ammonia Oxidizing Bacteria Coincides with Extremely Low pH Values in Wastewater with High Ammonia Content. Environ. Sci. Technol. 2017, 51, 6857–6866. [Google Scholar] [CrossRef] [PubMed]
- Mehrani, M.J.; Sobotka, D.; Kowal, P.; Ciesielski, S.; Makinia, J. The Occurrence and Role of Nitrospira in Nitrogen Removal Systems. Bioresour. Technol. 2020, 303, 122936. [Google Scholar] [CrossRef]
- Füssel, J.; Lücker, S.; Yilmaz, P.; Nowka, B.; van Kessel, M.A.H.J.; Bourceau, P.; Hach, P.F.; Littmann, S.; Berg, J.; Spieck, E.; et al. Adaptability as the Key to Success for the Ubiquitous Marine Nitrite Oxidizer Nitrococcus. Sci. Adv. 2017, 3, 2–10. [Google Scholar] [CrossRef]
- Huang, J.; Xu, L.; Guo, Y.; Liu, D.; Chen, S.; Tang, Q.; Zheng, H.; Tan, J.; Peng, F. Intermittent Aeration Improving Activated Granular Sludge Granulation for Nitrogen and Phosphorus Removal from Domestic Wastewater. Bioresour. Technol. Rep. 2021, 15, 100739. [Google Scholar] [CrossRef]
- Lücker, S.; Nowka, B.; Rattei, T.; Spieck, E.; Daims, H. The Genome of Nitrospina Gracilis Illuminates the Metabolism and Evolution of the Major Marine Nitrite Oxidizer. Front. Microbiol. 2013, 4, 27. [Google Scholar] [CrossRef]
- Roalkvam, I.; Dronen, K.; Dahle, H.; Wergeland, H.I. A Case Study of Biofilter Activation and Microbial Nitrification in a Marine Recirculation Aquaculture System for Rearing Atlantic Salmon (Salmo salar L.). Aquac. Res. 2020, 52, 94–104. [Google Scholar] [CrossRef]
- Khangembam, C.D.; Sharma, J.G.; Chakrabarti, R. Diversity and Abundance of Ammonia-Oxidizing Bacteria and Archaea in a Freshwater Recirculating Aquaculture System. HAYATI J. Biosci. 2017, 24, 215–220. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Takle, H.; Kolarevic, J.; Calabrese, S.; Timmerhaus, G.; Rosseland, B.O.; Teien, H.C.; Nilsen, T.O.; Handeland, S.O.; Stefansson, S.O.; et al. Performance and Welfare of Atlantic Salmon, Salmo salar L. Post-Smolts in Recirculating Aquaculture Systems: Importance of Salinity and Water Velocity. J. World Aquac. Soc. 2020, 51, 373–392. [Google Scholar] [CrossRef]
- Timmons, M.B.; Guerdat, T.; Vinci, B.J. Recirculating Aquaculture, 4th ed.; Ithaca Publishing Company, LLC.: Ithaca, NY, USA, 2018. [Google Scholar]
- Wang, X.; Wen, X.; Xia, Y.; Hu, M.; Zhao, F.; Ding, K. Ammonia Oxidizing Bacteria Community Dynamics in a Pilot-Scale Wastewater Treatment Plant. PLoS ONE 2012, 7, e36272. [Google Scholar] [CrossRef]
- Morin, L.; Goubet, A.; Madigou, C.; Pernelle, J.J.; Palmier, K.; Labadie, K.; Lemainque, A.; Michot, O.; Astoul, L.; Barbier, P.; et al. Colonization Kinetics and Implantation Follow-up of the Sewage Microbiome in an Urban Wastewater Treatment Plant. Sci. Rep. 2020, 10, 11634. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Wickham, H.; Francois, R.; Henry, L.; Muller, K. dplyr: A Grammar of Data Manipulation. 2022. Available online: https://dplyr.tidyverse.org (accessed on 5 March 2022).
- Emparanza, E.J.M. Problems Affecting Nitrification in Commercial RAS with Fixed-Bed Biofilters for Salmonids in Chile. Aquac. Eng. 2009, 41, 91–96. [Google Scholar] [CrossRef]
- Carvajal-Arroyo, J.M.; Puyol, D.; Li, G.; Lucero-Acuña, A.; Sierra-Álvarez, R.; Field, J.A. Pre-Exposure to Nitrite in the Absence of Ammonium Strongly Inhibits Anammox. Water Res. 2014, 48, 52–60. [Google Scholar] [CrossRef]
- Davidson, J.; Good, C.; Williams, C.; Summerfelt, S.T. Evaluating the Chronic Effects of Nitrate on the Health and Performance of Post-Smolt Atlantic Salmon Salmo Salar in Freshwater Recirculation Aquaculture Systems. Aquac. Eng. 2017, 79, 1–8. [Google Scholar] [CrossRef]
- Schrader, K.K.; Davidson, J.W.; Summerfelt, S.T. Evaluation of the Impact of Nitrate-Nitrogen Levels in Recirculating Aquaculture Systems on Concentrations of the off-Flavor Compounds Geosmin and 2-Methylisoborneol in Water and Rainbow Trout (Oncorhynchus mykiss). Aquac. Eng. 2013, 57, 126–130. [Google Scholar] [CrossRef]
- Gonzalez-Silva, B.M.; Jonassen, K.R.; Bakke, I.; Østgaard, K.; Vadstein, O. Nitrification at Different Salinities: Biofilm Community Composition and Physiological Plasticity. Water Res. 2016, 95, 48–58. [Google Scholar] [CrossRef]
- Glasl, B.; Smith, C.E.; Bourne, D.G.; Webster, N.S. Exploring the Diversity-Stability Paradigm Using Sponge Microbial Communities. Sci. Rep. 2018, 8, 8425. [Google Scholar] [CrossRef] [PubMed]
- Thibaut, L.M.; Connolly, S.R. Understanding Diversity-Stability Relationships: Towards a Unified Model of Portfolio Effects. Ecol. Lett. 2013, 16, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Mikkelson, G.M.; McGill, B.J.; Beaulieu, S.; Beukema, P.L. Multiple Links between Species Diversity and Temporal Stability in Bird Communities across North America. Evol. Ecol. Res. 2011, 13, 361–372. [Google Scholar]
- Sun, D.; Tang, X.; Zhao, M.; Zhang, Z.; Hou, L.; Liu, M.; Wang, B.; Klümper, U.; Han, P. Distribution and Diversity of Comammox Nitrospira in Coastal Wetlands of China. Front. Microbiol. 2020, 11, 589268. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yu, Z.; Du, X.; Zhang, T.; Wang, N.; Tao, W. Characterization of Bacterial Community, Ammonia-Oxidizing Bacteria, and Nitrospira during the Operation of a Commercial-Scale Recirculating Aquaculture System for Culturing Pufferfish Takifugu Rubripes. J. Ocean Univ. China 2020, 19, 1399–1408. [Google Scholar] [CrossRef]
- Bartelme, R.P.; McLellan, S.L.; Newton, R.J. Freshwater Recirculating Aquaculture System Operations Drive Biofilter Bacterial Community Shifts around a Stable Nitrifying Consortium of Ammonia-Oxidizing Archaea and Comammox Nitrospira. Front. Microbiol. 2017, 8, 101. [Google Scholar] [CrossRef]
- Thandar, S.M.; Ushiki, N.; Fujitani, H.; Sekiguchi, Y.; Tsuneda, S. Ecophysiology and Comparative Genomics of Nitrosomonas Mobilis Ms1 Isolated from Autotrophic Nitrifying Granules of Wastewater Treatment Bioreactor. Front. Microbiol. 2016, 7, 1869. [Google Scholar] [CrossRef]
- Fujitani, H.; Momiuchi, K.; Ishii, K.; Nomachi, M.; Kikuchi, S.; Ushiki, N.; Sekiguchi, Y.; Tsuneda, S. Genomic and Physiological Characteristics of a Novel Nitrite-Oxidizing Nitrospira Strain Isolated from a Drinking Water Treatment Plant. Front. Microbiol. 2020, 11, 545190. [Google Scholar] [CrossRef] [PubMed]
- Hobley, L.; King, J.R.; Sockett, R.E. Bdellovibrio Predation in the Presence of Decoys: Three-Way Bacterial Interactions Revealed by Mathematical and Experimental Analyses. Appl. Environ. Microbiol. 2006, 72, 6757–6765. [Google Scholar] [CrossRef][Green Version]
- Shan, J.; Tian, X.; Guan, C.; Zhang, C.; Zhang, Y.; Chen, S. Effect of Copper Ion Sterilization on Bacterial Community in a Freshwater Recirculating Aquaculture System. Curr. Microbiol. 2022, 79, 58. [Google Scholar] [CrossRef]
- de Celis, M.; Belda, I.; Ortiz-Álvarez, R.; Arregui, L.; Marquina, D.; Serrano, S.; Santos, A. Tuning up Microbiome Analysis to Monitor WWTPs’ Biological Reactors Functioning. Sci. Rep. 2020, 10, 4079. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, C.A.; Ross, S.W.; Brooke, S.D. Bacterial Community Diversity of the Deep-Sea Octocoral Paramuricea Placomus. PeerJ 2016, 2016, e2529. [Google Scholar] [CrossRef] [PubMed]
| Stage | Observed # SVs | Shannon’s Diversity | Evenness | |||
|---|---|---|---|---|---|---|
| Mean | St. Dev. | Mean | St. Dev. | Mean | St. Dev | |
| a | ||||||
| Initial | 744 | 50 | 4.811 | 0.036 | 0.728 | 0.005 |
| Final 0 ppt | 836 | 232 | 4.874 | 0.149 | 0.728 | 0.014 |
| Final 3 ppt | 663 | 194 | 4.611 | 0.245 | 0.712 | 0.008 |
| Final 20 ppt | 560 | 65 | 4.635 | 0.202 | 0.733 | 0.019 |
| Final 33 ppt | 739 | 49 | 4.783 | 0.033 | 0.724 | 0.004 |
| b | ||||||
| Initial | 784 | 212 | 4.567 | 0.167 | 0.688 | 0.015 |
| Final 3 ppt | 641 | 224 | 4.305 | 0.235 | 0.670 | 0.008 |
| Final 20 ppt | 488 | 29 | 4.249 | 0.165 | 0.686 | 0.022 |
| Final 33 ppt | 478 | 23 | 4.094 | 0.227 | 0.664 | 0.041 |
| Family | Genus sp. | Freshwater Biofilters (%) | Low-Salinity Biofilters (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | |||||||
| 0 ppt | 3 ppt | 20 ppt | 33 ppt | 3 ppt | 20 ppt | 33 ppt | ||||
| Nitrospiraceae | Nitrospira defluvii | 2.18 | 3.60 | 2.68 | 1.43 | 2.05 | 6.06 | 6.58 | 7.34 | 10.71 |
| Nitrosomonadaceae | Nitrosomonas sp. | 0.64 | 1.19 | 1.31 | 0.98 | 1.87 | 4.49 | 5.40 | 4.09 | 6.66 |
| Nitrospiraceae | Nitrospira sp. | 3.33 | 2.12 | 2.37 | 2.09 | 1.98 | 2.40 | 2.18 | 2.40 | 3.46 |
| Nitrosomonadaceae | Nitrosomonas aestuarii | 1.18 | 1.81 | 3.12 | 3.95 | 1.59 | 1.20 | 1.78 | 1.60 | 1.79 |
| Nitrospiraceae | Nitrospira sp. | 0.55 | 0.63 | 0.71 | 0.59 | 0.56 | 0.61 | 0.57 | 0.57 | 1.09 |
| Nitrosomonadaceae | Nitrosomonas sp. | 0.36 | 0.72 | 0.96 | 0.49 | 0.30 | 0.63 | 0.60 | 0.50 | 0.95 |
| Family | Genus | Freshwater Biofilters (%) | Low-Salinity Biofilters (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Initial | Final | Initial | Final | |||||||
| 0 ppt | 3 ppt | 20 ppt | 33 ppt | 3 ppt | 20 ppt | 33 ppt | ||||
| Microscillaceae | NA | 5.30 | 4.39 | 5.05 | 4.53 | 5.73 | 3.16 | 4.49 | 3.62 | 6.06 |
| Phycisphaeraceae | SM1A02 | 1.95 | 1.71 | 2.82 | 2.55 | 3.16 | 3.23 | 3.88 | 2.34 | 3.91 |
| Hyphomonadaceae | Hirschia | 2.36 | 1.36 | 1.54 | 1.66 | 2.20 | 1.99 | 1.86 | 1.56 | 2.03 |
| Pseudohongiellaceae | Pseudohongiella | 1.79 | 1.16 | 1.37 | 1.40 | 1.50 | 0.76 | 0.92 | 0.88 | 2.00 |
| Pirellulaceae | Pirellula | 0.87 | 0.96 | 1.58 | 1.99 | 0.98 | 0.55 | 0.71 | 0.71 | 0.37 |
| Chitinophagaceae | Terrimonas | 1.12 | 1.17 | 1.03 | 1.45 | 1.21 | 0.41 | 0.47 | 0.42 | 0.41 |
| Microscillaceae | OLB12 | 0.35 | 0.40 | 0.40 | 0.40 | 0.75 | 1.12 | 1.24 | 1.20 | 0.60 |
| Comamonadaceae | Hydrogenophaga | 0.51 | 0.40 | 0.75 | 0.55 | 0.59 | 0.61 | 0.63 | 1.22 | 1.62 |
| Oceanibaculaceae | Oceanibaculum | 0.56 | 0.38 | 0.37 | 0.38 | 0.74 | 0.66 | 0.57 | 0.48 | 1.40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costigan, E.M.; Bouchard, D.A.; Ishaq, S.L.; MacRae, J.D. Short-Term Effects of Abrupt Salinity Changes on Aquaculture Biofilter Performance and Microbial Communities. Water 2024, 16, 2911. https://doi.org/10.3390/w16202911
Costigan EM, Bouchard DA, Ishaq SL, MacRae JD. Short-Term Effects of Abrupt Salinity Changes on Aquaculture Biofilter Performance and Microbial Communities. Water. 2024; 16(20):2911. https://doi.org/10.3390/w16202911
Chicago/Turabian StyleCostigan, Eliza M., Deborah A. Bouchard, Suzanne L. Ishaq, and Jean D. MacRae. 2024. "Short-Term Effects of Abrupt Salinity Changes on Aquaculture Biofilter Performance and Microbial Communities" Water 16, no. 20: 2911. https://doi.org/10.3390/w16202911
APA StyleCostigan, E. M., Bouchard, D. A., Ishaq, S. L., & MacRae, J. D. (2024). Short-Term Effects of Abrupt Salinity Changes on Aquaculture Biofilter Performance and Microbial Communities. Water, 16(20), 2911. https://doi.org/10.3390/w16202911







