Abstract
This study aimed to treat sewage sludge through microwave irradiation at a laboratory scale. The objective was to investigate the effect of microwave irradiation on microorganisms, water content, organic matter, and agronomic nutrients present in sewage sludge. Three types of sewage sludges obtained from a full-scale wastewater treatment plant were considered: Sludge A (raw sludge), Sludge B (subjected to 15 days of solar exposure, achieving 48% dryness), and Sludge C (exposed to solar conditions and left open to the air for 23 months, reaching 94% dryness). These diverse sludges were exposed to microwave irradiation at various power levels (analysed variables: ε (Watts/g), θ (°C), T (min)). The specific exposure powers and temperature levels for the water reduction analysis were: 555, 955, 1355, and 1500 Watts/g and 55, 75, 95, and 105 °C, respectively. On the other hand, microbiological and agronomic nutrient analyses were conducted at 75 °C–1355 W and 95 °C–1355 W. After microwave exposure experiments, the results demonstrated the high effectiveness of microwave technology in eradicating indicator microorganisms of faecal contamination and reducing sludge volume while not affecting trace elements of significant agricultural value. The reduction in Escherichia Coli revealed that 4 min of irradiation was necessary to completely eliminate it to 0 ulog, indicating a 100% reduction, in Sludge A. In Sludges B and C, an additional 1 min was needed under conditions of 75 °C and 1355 W for a mass of 50 g. Moreover, Sludge A (46.27 × 105 or 4.80 ulog of dry matter), Sludge B (1.29 × 106 or 6.11 ulog of dry matter), and Sludge C (8.77 × 104 or 4.94 ulog of dry matter) were heavily contaminated with faecal coliforms. It took 6 min to reduce faecal coliforms to below the detection threshold.
1. Introduction
According to the report from the Joint Monitoring Program in 2019, WHO/UNICEF [1], 4.2 billion people, or 55% of the global population, lack access to safely managed sanitation services while 2 billion people still lack basic sanitation facilities (such as toilets, latrines, septic tanks…). Population growth and urbanisation have prompted the world to reorganise the sanitation system safely through an appropriate value chain for managing sludge [2]. Drinking water resources are increasingly contaminated by wastewater [3]. In order to face this challenge, several conventional options for wastewater and sludge treatment (sludge drying beds, anaerobic digestion, composting with organic solid waste) are available [4,5,6]. These treatment processes have shown their limitations in addressing environmental pollution [4], as well as the contamination of natural resources (surface water, groundwater, aquifers…) [7]. Due to the lack of funds and considering the risks to public health, it is necessary to develop environmentally and economically safe technologies [8]. In wastewater treatment plants, solid-phase treatment is of paramount importance due to the large volume of raw sludge produced [9]. This product can contain a wide variety of potential contaminants for the environment, such as pathogens, heavy metals, and organic compounds [10]. Therefore, the appropriate management of raw sludge in wastewater treatment plants (WWTPs) is truly crucial. Sludge dehydration is currently carried out in most wastewater treatment plants worldwide to minimise the space occupied by the sludge and ensure a safe disposal in the environment [9,10,11,12]. Apart from minimising, the dewatering process can facilitate transportation, increase the efficiency of energy utilisation, and even reduce the leachate production in sludge landfill sites [13]. However, dewatering sewage sludge is a complex task in most WWTPs due to the highly colloidal structures of microbial aggregates [13]. This is why most sewage sludge is currently not properly treated or incinerated [14].
Sludge management in most African cities is a clear example of this environmental issue. Sludge undergoes no treatment and it is discharged into the environment, resulting in the contamination of soils, surface water, and groundwater [15] with pathogens and other hazardous pollutants, such as nitrates. Eutrophication is also one of the current issues, caused by the excessive release of phosphorus into the environment. For example, the sludge deposited in Kara is used as fertiliser for vegetable farming; however, these sludges are highly contaminated with faecal coliforms, which ultimately results in the contamination of the cultivated vegetables [16]. In this context, Castro-Rosas et al. [17] researched the presence of faecal coliforms and E. coli in salads and they demonstrated that 99% TC and 85% E. coli were found in ready-to-eat salads. This study was carried out in the city of Pachuca, where most of the vegetables consumed locally are fertilised with sewage sludge.
In this sense, thermal drying proves to be an effective method for treating large quantities of sludge [7]. As an alternative to these environmentally controversial waste disposal methods, a new approach based on MW heating has emerged. MW technology has demonstrated its effectiveness in various fields, such as the food industry, industrial processes, and sewage sludge treatment, where previous mechanical dehydration has been employed. However, as far as we know, there has not been previous research on pilot experiments using MW irradiation with sludge that was previously dehydrated using natural methods like solar drying on a drying bed, which is widespread in developing areas. MW irradiation technology found broad applications in various environmental contexts, such as hazardous and radioactive waste remediation, sludge wastewater treatment (Mawioo, 2020 [18]; Remya and Lin, 2011 [19]), and other disciplines, like advanced oxidation processes, such as catalytic and photochemical oxidation systems [20]. This novel MW irradiation technology holds the potential to enhance product quality and make a breakthrough in this field [21]. It has been proposed as an effective and efficient method for sludge drying [7]. Consequently, there is a growing interest in employing this technology to recover a stable and hygienic value-added product (Tyagi and Lo, 2013 [22]), without compromising the organic matter. MW-assisted wastewater treatment is appealing due to its reduced reactor size, short reaction time, minimal use of chemical additives, and low energy consumption [3,23]. Human faecal sludge (septage) is more than just waste, it is resource-rich material teeming with organic matter suitable for agricultural fertilisation or as an energy source. However, its high water content (over 90%) makes septage unusable and requires prior dewatering before any potential application, owing to its highly hydrophilic nature [5]. Recovering sludge resulting from MW irradiation, devoid of any pathogenic microorganisms, poses a critical and fundamental challenge in faecal sludge management. These pathogenic microorganisms, commonly termed faecal indicator bacteria (FIB), should be reduced to safe levels (for instance, E. coli ≤ 1000 CFU/g TS [24]) to mitigate the risks of contamination. Thus, the aim of this study was to assess MW technology for treating sun-dehydrated sewage sludge to obtain a value-added product with an acceptable dryness level and devoid of any pathogenic agents. Various experiments were conducted to determine the optimal combination of time, temperature, and energy to achieve stable and hygienic sludge after MW treatment. These trials encompassed diverse exposure times, temperatures, and energy levels to establish the necessary duration and obtain a stable and hygienic product.
2. Materials and Methods
2.1. Research Design
In this study, three types of sewage sludge samples from a municipal full-scale WWTP were examined: Sludge A (raw sludge), Sludge B (sludge dehydrated for 15 days with a dryness of 48%), and Sludge C (sludge dehydrated and exposed to open air for 23 months with a dryness of approximately 94%). The WWTP, located in Huétor Santillán, a small town in Granada (Spain), receives urban wastewater as an influent and employs a biological treatment based on trickling filters, primarily designed for small populations. Notably, the studied WWTP utilises a sludge drying system based on solar exposure in drying beds, in contrast to conventional mechanical methods used in most WWTPs. Therefore, the objective was to replicate, as closely as possible, the conditions found in developing countries where solar drying constitutes the primary system for sewage sludge dehydration. Following sampling, the collected sludge samples were transported to the Environmental Engineering Laboratory of the Water Research Institute (Granada, Spain), where they were stored at 4 °C. Subsequently, the physical, chemical, and biological characteristics were assessed within 48 h. Table 1 shows the parameters considered in this investigation.

Table 1.
Physico-bio-chemical parameters of sludge before and after treatment.
2.2. Description of the MW Apparatus
The ETHOS X MW, sourced from the Japanese company Thermo Fisher Scientific (Waltham, MA, USA), was utilised for treating the sludge samples. This particular MW model is equipped with two 1800-watt magnetrons responsible for generating MW irradiation and operates at a frequency of 2.45 GHz. It boasts a touchscreen computer that leverages all device functionalities, enabling convenient control and precise integration of temperature, energy, and time parameters. Additionally, it functions as an exhaust hood and incorporates a ventilation system that aids in cooling the reactors. The reactor itself comprises fourteen cavities designed for inserting samples.
2.3. MW Treatment Protocol
The MW experiments were conducted at the laboratory of the Water and Microbiology Institute of the University of Granada (Spain). The primary goal was to evaluate the MW’s ability to eradicate pathogenic microorganisms, like E. coli, TC, and Faecal Enterococcus, while also reducing the weight/volume to an acceptable dryness level for agricultural or energy applications. For each experiment, 50 g was placed into the MW reactor cavity and subjected to various combinations of power, temperature, and irradiation time.
Following the specifications of the ETHOS X MW and insights from the relevant literature on this subject [5,7,24,25,26], four distinct power levels were applied: 555 Watts/g, 955 Watts/g, 1355 Watts/g, and 1500 Watts/g, all operating at a consistent frequency of 2.45 GHz across durations ranging from 1 to 6 min. In terms of temperature, four different levels were employed: 55, 75, 95, and 105 °C. These operational ranges were established after preliminary tests to ascertain their appropriateness for this study. However, specific power and temperature combinations for the microbiological and agronomic nutrient analysis were limited to 75 °C–1355 Watts/g and 95 °C–1355 Watts/g. This selection was based on the organic matter’s resistance to destruction at these values, yielding the most significant outcomes. For water content analysis, all aforementioned power and temperature levels were used.
The temperature and power changes were tracked using an acquisition software connected to the ETHOS X MW via an optical fibre. Following the MW exposure, the samples were taken out from the MW cavity for both microbiological analysis and to assess the reduction in water content within the raw sludge.
2.4. Physico-Chemical Analysis of Sludge
The physical and chemical parameters of the various sludge samples before MW treatment underwent measurement and analysis. Initially, pH and conductivity were determined using a benchtop pH meter and conductivity meter, both from the HACH SesiON brand. Subsequently, moisture content and dry matter were assessed by initially weighing the samples and, then, subjecting them to an oven at 105 °C, before reweighing.
Additionally, trace elements were examined utilising inductively coupled plasma optical emission spectroscopy (ICP-OES). Moreover, heavy metals, total nitrogen, total phosphorus, and total organic carbon were analysed through inductively coupled plasma mass spectrometry (ICP-MS). Both the ICP-OES and ICP-MS analyses were conducted at the Scientific Instrumentation Center (CIC) of Granada University.
2.5. Microbiological Analysis and Culture Media
Faecal contamination indicator organisms, such as E. coli, TC, and faecal Enterococcus, were assessed before and after treatment to measure the reduction in microbial load and to evaluate the effectiveness of MW treatment on sludge.
MacConkey Agar (MAC), a selective and differential culture medium designed to isolate Gram-negative enteric bacteria, like E. coli and TC, was prepared by dissolving 51 g in 1000 mL of distilled water (or 25.5 in 500 mL). The preparation process was the same as for Tryptic Soy Agar (TSA). Slanetz Bartley Agar (SBA), utilised for faecal Enterococcus isolation, consisted of 41.5 g per litre of distilled water. E. coli samples were incubated at 44 °C while TC and faecal Enterococcus were incubated at 37 °C.
2.6. Dilution Methodology Prior to Inoculation
One gram (1 g) of each type of sludge was introduced into 9 mL of 0.9% sodium chloride (NaCl) saline solution in a sterile tube and agitated to obtain a homogeneous solution. After complete dissolution, a stock solution with a concentration of 10−1 was obtained. This stock solution was further diluted to 10−7 for the sludge samples before treatment and to 10−5 for the samples after MW treatment using the following procedure: 1 mL of the stock solution (10−1) was introduced into a sterile tube containing 9 mL of 0.9% sodium chloride (NaCl) saline solution to achieve a dilution of 10−2 (0.01). The same dilution steps were repeated until reaching a dilution of 10−7 for the raw sludge samples and 10−5 for the MW-treated samples (Figure 1).
Figure 1.
Dilution of the various samples.
2.7. Measurement of Weight/Volume
The weight of the samples was measured before and after treatment using a benchtop balance from the American company the Itin Scale Company, specifically, the ACCULAR ATILON model, with a sensitivity of 0.01 g. After treatment, the samples were cooled in a desiccator before weighing. The difference between the two measurements determined the moisture content in the sludge samples.
3. Results and Discussion
3.1. Microbiological Composition of Sludge before MW Treatment
3.1.1. E. coli Resistance
The evaluations of E. coli, TC, faecal Enterococcus, and faecal Streptococcus contents in raw and dried sludge are detailed in Table 2. The results illustrate that Sludges A, B, and C are loaded with E. coli at levels of ± 3.2 × 104 ± 40 CFU/mL, 1 × 104 ± 147 CFU/g, and 4.5 × 103 ± 13.5 CFU/g, respectively. Interestingly, when comparing the concentration of E. coli in Sludges B and C, it was demonstrated that the reduction in E. coli was greater in the initial 15 days of drying than over a period of 23 months. Al-Gheeti et al. [27] suggest that E. coli is exceptionally persistent and capable of surviving in the environment for extended periods without a host. Even after more than a year of storage following drying, Sludge C showed only a reduction of 0.35 ulog in E. coli. These findings highlight the necessity for additional treatment before considering energy or agricultural utilisation.

Table 2.
Microbiological composition of different types of sludge before treatment.
3.1.2. Total Coliforms
Coliforms are bacteria commonly found in sludge and can be attributed to various diseases. The results obtained revealed that in raw sludge (Sludge A), over 3.5 × 105 CFU/g of TCs was detected. After 23 months of storage, only a reduction of 0.71 ulog was observed. Following 15 days of drying, a reduction of 0.37 ulog of coliforms was noted. Similar to E. coli, TCs have exhibited a remarkable capacity to persist in the environment for extended durations. These findings underscore the significance of post-treatment for dried sludge to remove more pathogenic microorganisms. However, the pattern of reduction in TCs concerning Sludges B and C was totally opposite in relation to E. coli. Specifically, the reduction in total coliforms was greater over the 23-month period (0.71 ulog) compared to the initial 15 days of drying (0.37 ulog). This variance could be linked to the preceding biological treatment in the WWTP under study, which consists of a biotrickling filter system. The efficiency of the filter bed is influenced by the distinct characteristics of its layers (sand, gravel, and chippings), significantly impacting the microbiological quality of the sludge. In this scenario, coliforms thrived in the biofilm environment, contributing to the higher concentration in Sludge A and the lowest reduction within 15 days compared to other bacterial groups. However, during the sludge drying phase, they might not adapt as effectively as E. coli.
3.1.3. Faecal Enterococcus and Streptococcus
In relation to the removal of faecal Enterococcus and Streptococcus, there seems to be a reduction during the dehydration process; however, considerable amounts persist, requiring further elimination. According to the bacterial culture results, concentrations of faecal Enterococcus bacteria ranged from 3.8 × 104 ± 100 CFU/g, to 1 × 104 ± 17.3 CFU/g, to 1.2 × 103 ± 45.4 CFU/g for Sludge A, Sludge B, and Sludge C, respectively. Similarly, faecal Streptococcus in the same types of sludge showed concentrations ranging from 1 × 104 ± 54.1 CFU/g, to 1.5 × 103 ± 36.3, to 4.09 × 103 ± 49 CFU/g. Both bacterial genera exhibit remarkable resilience in harsh environments due to their inherent resistance to adverse conditions, ability to enter latency states, and the presence of nutrients. Specifically, Enterococcus species display notable persistence, surviving in environments with temperatures ranging from 10 to 45 °C, high pH levels of 9, and a certain NaCl content of 6.5% [27]. Additionally, it should be noted that faecal Streptococcus even showed proliferation during the 23 months of drying, demonstrating its ability to adapt to the environment better than E. coli.
3.2. Physical Composition of Sludge before MW Treatment
Table 3 displays the results of the physical parameters of the sludge. The pH ranges between 6.88 and 8 while conductivity varies from 0.98 mS/cm to 25.32 mS/cm. The initial free water content was recorded as 85 for Sludge A, 51.8 for Sludge B, and 5.47 for Sludge C.

Table 3.
Physical composition of sludge before MW treatment.
3.3. Reduction in Pathogenic Microorganisms by MW during Experiment 1 (75 °C–1355 W)
It is noteworthy that even after solar drying, the sludge retains its unhygienic nature. After 23 months, significant amounts of microorganisms, approximately 106 CFU/g, still persist in the sludge, signifying that MW treatment acts as a supplementary approach to solar treatment. The World Health Organization (WHO) recommends a contamination level below 1000 CFU/100 mL, equivalent to a 3 ulog reduction in coliforms and E. coli. These microorganisms contribute to various diseases, including frequent occurrences of diarrhoea. Presently, the valorisation of sewage sludge is increasingly feasible.
In this context, Figure 2a illustrates the logarithmic base 10 (log10) reduction in E. coli in the three sludge types. The results demonstrated that it took 4 min of MW irradiation to completely destroy 0 ulog or 100% of the E. coli in Sludge A; whereas, in Sludges B and C, 5 min is the optimum contact time for their reduction to 75 °C under a power of 1355 W for a mass of 50 g. These results align well with Mawioo et al.’s findings [25], which focused on various sludge types: activated centrifuged sludge (C-WAS), activated noncentrifuged sludge (WAS), faecal sludge (FS), and septic tank sludge (SS). Their research showed that elimination was below the detection threshold (500 CFU/g TS), which corresponds to approximately 5.90 ulog. A specific study on E. coli was carried out by Mawioo et al. [25]. It was found that reduction below the detection limit (1000 CFU/g TS) was achieved with a contact time of 1 min at a temperature of 71 °C under a power of 800 W. Reinthaler et al. [28] documented the initial median E. coli values in the treatment inflow from Plant A as 2.0 × 10−4 CFU/mL, and 6.1 × 10−4 CFU/mL for Plant B, with a reduction of 2 ulog. Therefore, this confirms the hypothesis of Zhang et al. [29] that “all faecal sludge contains enormous potential pathogens and deserves proper treatment before use”.
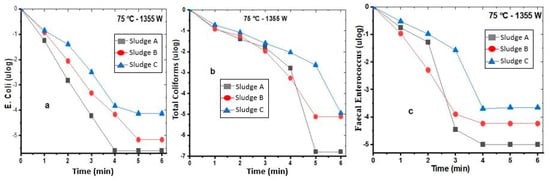
Figure 2.
Effect of the 75 °C–1355 W combination on the reduction in E. coli (a), CF (b), and faecal Enterococcus (c) in Sludge A, Sludge B, and Sludge C.
Sludge A (46.27 × 105 or 4.80 ulog MS), Sludge B (1.29 × 106 or 6.11 ulog MS), and Sludge C (8.77 × 104 or 4.94 ulog MS) exhibited high levels of faecal coliforms. According to Figure 2b, it took 6 min under the same experimental conditions to completely eliminate the coliforms. The thermal effect induces the destruction of microbial cells as the contact time increases [25]. Park et al. [30] demonstrated that 70 °C for 5 min is necessary to reduce strains, such as coliforms and E. coli, to 0 ulog. They concluded that power, temperature, and solids content significantly impact the quality of treated sludge. Pino-Jelcic et al. [31] reported a reduction of 4.2 ± 0.4 ulog in faecal coliforms in sludge through the MW effect under the conditions of 1000 W and 60 °C.
Faecal Enterococcus bacteria are among the pathogens commonly found in sewage sludge. At a temperature of 70 °C and a power of 1355 W, the reduction in Enterococcus to 0 ulog was observed after a contact time of 4 min. Figure 2c illustrates this variation perfectly.
3.4. Reduction in Pathogenic Microorganisms during Experiment 2 (95 °C–1355 W)
The same experiments as the previous ones were repeated but with a change in temperature from 75 °C to 95 °C in order to see its effect on the reduction in target microorganisms. According to Figure 3, it took 2 to 3 min to completely destroy TCs, faecal Enterococcus, and E. coli in all types of sludge, except for coliforms in Sludge B, which reached 0 ulog after 4 min. These results are very promising because they achieve a complete removal of pathogenic microorganisms in a very short period of time. In comparison with other conventional technologies, which use electrothermal treatment, microwave treatment stands out for its thermal effect, which is volumetric and does not involve thermal diffusion from the surface into the material, such as in the case of electrically assisted thermal drying or incineration technologies. This difference accounts for its notably rapid reaction rates during thermosetting [21].
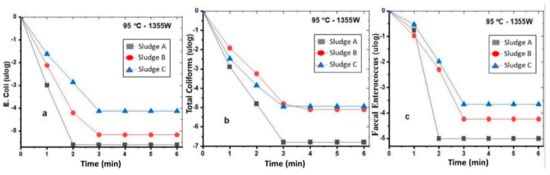
Figure 3.
Effect of 95 °C–1355 W combination on the reduction in E. coli (a), faecal coliforms (b), and faecal Enterococcus (c) in Sludge A, Sludge B, and Sludge C.
3.5. Effect of Energy on Water Content at Different Temperatures
One of the objectives of sludge treatment is to reduce weight or volume for ease of transportation. Figure 4 shows the drying kinetics of sludge at different temperatures. As expected, they indicate a gradual decrease in the water content of Sludge A (95%) over time. Water content decreases faster with increasing temperature and energy. According to the results obtained, exposing the sludge to 1500 W/g at different temperatures is two times as effective as exposing it to 555 W/g at the same temperatures. The drying process can be divided into two major phases based on the regression of water content at different energies. In the first phase, the water content in the sludge exponentially decreases until it reaches a maximum rate and remains relatively constant in the second phase. When the power is set at 555 W/g, the times required to reach equilibrium (80%) are 30, 24, 15, and 12 min, respectively, for temperatures of 55 °C, 75 °C, 95 °C, and 105 °C. On the other hand, to reach 80% reduction when the energy is set at 955 W/g, at temperatures of 55 °C, 75 °C, 95 °C, and 105 °C, the required times to achieve the maximum reduction rate are 21, 15, 12, and 9 min. Analysis of these results shows that the time is halved when transitioning from 555 W/g to 955 W/g at 105 °C, demonstrating the effect of energy on the reduction in sludge weight/volume. For sludge exposed to MW at 955 W and 1500 W, the contact times required to achieve 90% water removal are 18, 12, 9, and 6 min and 15, 10, 7, and 4 min, respectively.
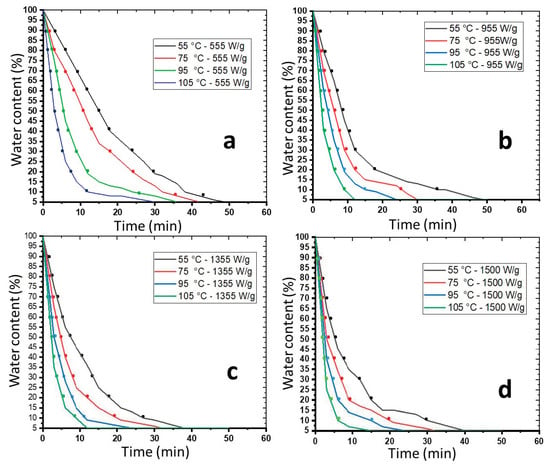
Figure 4.
Evolution of the water content of Sludge A in f(t); at 555 W/g, at different temperatures (a); at 955 W/g, at different temperatures (b); at 1355 W/g, at different temperatures (c); and at 1500 W/g, at different temperatures (d).
Similar findings were documented by Mawioo et al. [5], indicating a reduction in weight of over 70% following MW treatment. The same author’s studies also indicated higher reductions in SS sludge, followed by WAS, C-WAS, and FS sludge, with respective reductions of approximately 93%, 90%, 80%, and 63%. In line with our results, Kocbek et al. [26] demonstrated that as MW power increased, the required exposure time decreased. Specifically, with a power increase from 1 to 6 kW, the maximum average drying rates of the sludge surged from 0.03 to 0.28 kg of water per 1 kg of TS per minute. The literature denotes slightly lower drying rates for MW at the pilot scale. For instance, Mawioo et al. [25] reported drying rates ranging from 0.01 to 0.040 kg of water per 1 kg of dry solids per second per minute for a laboratory-scale MW system. Bennamoun et al.’s [32] study on the MW drying of wastewater sludge unveiled that at different power levels (480, 840, and 1080 W), the dried sludge manifested diverse forms at varying masses. Additionally, they demonstrated that the drying rate declined with an increase in the initial mass (from 0.45 kg.kg−1.min−1 for 90 g to 0.25 kg.kg−1.min−1 for 150 g) and, simultaneously, increased with a rise in the power level (from 0.15 kg.kg−1.min−1 for 480 W to 0.45 kg.kg−1.min−1 for 1080 W).
3.6. Impact of the MW Treatment on the Agronomic Nutrients
Table 4 outlines the average concentration of agronomic nutrients in the three types of sludge both before and after MW treatment. The data indicate minimal variation between the two conditions, suggesting that microwave treatment has an insignificant impact on these elements. The standard deviation across each parameter before and after treatment remains relatively low, ranging from 3.828% to 9.700% for N, C, P, Ca, Fe, Mg, K, Na, Al, S, and Si, respectively. The impact of MW treatment on organic matter is crucial and is influenced by the temperature achieved through MW irradiation. It is important to note that when temperatures exceed 110 °C at higher power levels, the organic matter starts to degrade, leading to the destruction of C, N, P, and S. In our study, the MW treatment temperatures reached 105 °C, significantly lower than the ignition temperature for volatile solids (550 °C). This finding highlights a key limitation of MW treatment in significantly affecting organic matter. Similar results were observed by Mawioo et al. [25], indicating minimal reduction in organic matter. However, their study involved a different type of sewage sludge treated by centrifugation rather than solar drying. This suggests that MW treatment shows consistent effectiveness in reducing organic matter, regardless of the method of sludge drying.

Table 4.
Agronomic nutrient composition before (BF) and after (AT) MW treatment.
In addition, sewage sludge contains various metals that serve as crucial nutrients for plants, such as K, Ca, Mg, Na, and Fe. The results obtained from our study are highly satisfactory as the concentrations of these metals remained unaffected by MW irradiation. Hence, the agronomic value of the solar-dried sludge remains intact, despite being stored for an extended period on drying beds. The significant presence of organic matter and nutrient content could potentially improve soil quality. This enhancement could occur through the improvement of the clay–humus complex, thereby enhancing soil structure. Consequently, it may lead to increased water retention and reduced erosive effects [33]. These advantages are particularly beneficial for depleted soils, which are widespread in African regions [33].
However, an excessive presence of these elements could pose a threat to the natural environment, potentially leading to pollution episodes like eutrophication or the accumulation of heavy metals. Considering this concern, the European Union (EU) instituted Regulation (EU) 2019/1009, which establishes guidelines on the availability of fertilising products in the market. Regarding solid organic fertilisers [34], N stands out for being the most important primary nutrient, with a specified limit value of 2.5%. Upon examination, it is evident that the nitrogen content in our dried sludge slightly surpasses this defined limit after MW treatment (2.53%). Despite this, it remains suitable for use as an organic fertiliser in agricultural applications.
4. Conclusions
This study evaluated the efficacy of MW treatment on three distinct types of sewage sludge based on their exposure duration to sunlight. Initially, analyses conducted before treatment revealed high concentrations of pathogenic organisms in the sludge, suggesting that solar treatment alone is insufficient to stabilise the sludge for agricultural use. Subsequently, following MW treatment, pathogenic microorganisms, like E. coli, TCs, and Enterococcus, were entirely eradicated, registering a 0% presence across all three sewage sludge types. Achieving this required only 6 min in Experiment 1 (75 °C–1355 W) and 2–3 min in Experiment 2 (95 °C–1355 W). Additionally, water content saw a remarkable reduction of 90% within 4–15 min, depending on the selected temperature and power. These observations demonstrated a clear correlation between MW power intensity, sludge decomposition rate, and the impact of power and exposure duration. These findings underscore the critical need for the precise adjustment of these parameters to optimise treatment efficiency, especially for larger-scale applications. Furthermore, no significant impact was observed on organic matter and agronomic nutrients while the concentrations of heavy metals remained below acceptable standards. This allows for the utilisation of the final sludge-derived product. The preservation of beneficial elements for agricultural soils, coupled with the effective removal of pathogenic microorganisms and moisture by MW technology, proves highly advantageous for agronomic purposes. Therefore, MW technology presents a promising solution, particularly in developing regions where sludge dehydration primarily occurs through natural solar drying methods. Nonetheless, further research is imperative to compare the efficiency of MW technology with conventional dehydration methods in wastewater treatment plants (WWTPs). Consequently, the next step of this research will focus on evaluating the practical feasibility of this technology on a larger scale.
Author Contributions
P.K.: Conceptualisation, Methodology, Investigation, Data curation, Writing—original draft, A.J.A.-B.: Investigation, Writing—original draft, E.K.K.: Software, K.S.: Software, J.G.-L.: Methodology, Investigation, Supervision, F.O.: Coordination, Supervision, Writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors upon request.
Acknowledgments
The results of this research were obtained through a collaboration between the University of Kara (Togo) and the University of Granada (Spain). Lastly, we extend our sincere appreciation to Manuel Gallardo Altamirano for his significant contribution to the success of this research.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
Microwave (MW), total coliforms (TC), Escherichia Coli (E. coli), faecal indicator bacteria (FIB), indicator microorganisms of faecal contamination (IMFC), MacConkey Agar (MAC), Tryptic Soy Agar (TSA), Slanetz Bartley Agar (SBA), CFU (colony-forming unit), Eh (redox potential), TS (total solids), Ulog (logarithmic reduction).
References
- WHO. Guidelines on Sanitation and Health; World Health Organization: Geneva, Switzerland, 2018.
- Samal, K.; Moulick, S.; Mohapatra, B.G.; Samanta, S.; Sasidharan, S.; Prakash, B.; Sarangi, S. Design of faecal sludge treatment plant (FSTP) and availability of its treatment technologies. Energy Nexus 2022, 7, 100091. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Q.; Clark, J.H.; Graham, N.J.; Hou, D.; Ok, Y.S.; Tsang, D.C. Tailoring wood waste biochar as a reusable microwave absorbent for pollutant removal: Structure-property-performance relationship and iron-carbon interaction. Bioresour. Technol. 2022, 362, 127838. [Google Scholar] [CrossRef]
- Katukiza, A.Y.; Ronteltap, M.; Niwagaba, C.B.; Foppen, J.W.A.; Kansiime, F.; Lens, P.N.L. Sustainable sanitation technology options for urban slums. Biotechnol. Adv. 2012, 30, 964–978. [Google Scholar] [CrossRef] [PubMed]
- Mawioo, P.M.; Hooijmans, C.M.; Garcia, H.A.; Brdjanovic, D. Microwave treatment of faecal sludge from intensively used toilets in the slums of Nairobi, Kenya. J. Environ. Manag. 2016, 184, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Katukiza, A.Y.; Ronteltap, M.; van der Steen, P.; Foppen, J.W.A.; Lens, P.N.L. Quantification of microbial risks to human health caused by waterborne viruses and bacteria in an urban slum. J. Appl. Microbiol. 2014, 116, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Kržišnik, D.; Humar, M.; Brdjanovic, D. Effects of the sludge physical-chemical properties on its microwave drying performance. Sci. Total Environ. 2022, 828, 154142. [Google Scholar] [CrossRef]
- Kumar, T.; Rajpal, A.; Bhargava, R.; Prasad, K.S.H. Performance evaluation of vermifilter at different hydraulic loading rate using river bed material. Ecol. Eng. 2014, 62, 77–82. [Google Scholar] [CrossRef]
- Gholipour, A.; Fragoso, R.; Duarte, E.; Galvão, A. Sludge Treatment Reed Bed under different climates: A review using meta-analysis. Sci. Total Environ. 2022, 843, 156953. [Google Scholar] [CrossRef]
- El-Qanni, A.; Alsayed, M.; Alsurakji, I.H.; Najjar, M.; Odeh, D.; Najjar, S.; Hmoudah, M.; Zubair, M.; Russo, V.; Di Serio, M. A technoeconomic assessment of biological sludge dewatering using a thermal rotary dryer: A case study of design applicability, economics, and managerial feasibility. Biomass Convers. Biorefin. 2022, 13, 16297–16583. [Google Scholar] [CrossRef]
- Yoshida, H.; Christensen, T.H.; Scheutz, C. Life cycle assessment of sewage sludge management: A review. Waste Manag. Res. 2013, 31, 1083–1101. [Google Scholar] [CrossRef]
- Cies, B.M.; Namies, J.; Konieczka, P. Review of sewage sludge management: Standards, regulations and analytical methods. J. Clean. Prod. 2015, 90, 1–15. [Google Scholar] [CrossRef]
- Wu, B.; Dai, X.; Chai, X. Critical review on dewatering of sewage sludge: Influential mechanism, conditioning technologies and implications to sludge re-utilizations. Water Res. 2020, 180, 115912. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Inguanzo, M.; Pis, J.J. Microwave-induced pyrolysis of sewage sludge. Water Res. 2002, 36, 3261–3264. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Mohan, R.R.; Rathi, S.; Raju, N.J. Technology options for faecal sludge management in developing countries: Benefits and revenue from reuse. Environ. Technol. Innov. 2017, 7, 203–218. [Google Scholar] [CrossRef]
- Piyabalo, K.; Edem, K.K.; Nitale, M.K.; Kwamivi, N.S.; Bouwèdèo, T.B. Quantification and characterization of faecal sludge produced in Kara. Afr. J. Biotechnol. 2021, 20, 194–201. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Cerna-Cortés, J.F.; Méndez-Reyes, E.; Lopez-Hernandez, D.; Gómez-Aldapa, C.A.; Estrada-Garcia, T. Presence of faecal coliforms, Escherichia coli and diarrheagenic E. coli pathotypes in ready-to-eat salads, from an area where crops are irrigated with untreated sewage water. Int. J. Food Microbiol. 2012, 156, 176–180. [Google Scholar] [CrossRef]
- Mawioo, P.M. Novel Concepts, Systems and Technology for Sludge Management in Emergency and Slum Settings. In Novel Concepts, Systems and Technology for Sludge Management in Emergency and Slum Settings; CRC Press: London, UK, 2020. [Google Scholar] [CrossRef]
- Remya, N.; Lin, J.G. Current status of microwave application in wastewater treatment—A review. Chem. Eng. J. 2011, 166, 797–813. [Google Scholar] [CrossRef]
- Xia, H.; Li, C.; Yang, G.; Shi, Z.; Jin, C.; He, W.; Xu, J.; Li, G. A review of microwave-assisted advanced oxidation processes for wastewater treatment. Chemosphere 2022, 287, 131981. [Google Scholar] [CrossRef]
- Vialkova, E.; Obukhova, M.; Belova, L. Microwave Irradiation in Technologies of Wastewater and Wastewater Sludge Treatment: A Review. Water 2021, 13, 1784. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Microwave irradiation: A sustainable way for sludge treatment and resource recovery. Renew. Sustain. Energy Rev. 2013, 18, 288–305. [Google Scholar] [CrossRef]
- Wei, R.; Wang, P.; Zhang, G.; Wang, N.; Zheng, T. Microwave-responsive catalysts for wastewater treatment: A review. Chem. Eng. J. 2020, 382, 122781. [Google Scholar] [CrossRef]
- Mawioo, P.M.; Rweyemamu, A.; Garcia, H.A.; Hooijmans, C.M.; Brdjanovic, D. Evaluation of a microwave based reactor for the treatment of blackwater sludge. Sci. Total Environ. 2016, 548–549, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Mawioo, P.M.; Garcia, H.A.; Hooijmans, C.M.; Velkushanova, K.; Simonič, M.; Mijatović, I.; Brdjanovic, D. A pilot-scale microwave technology for sludge sanitization and drying. Sci. Total Environ. 2017, 601–602, 1437–1448. [Google Scholar] [CrossRef]
- Kocbek, E.; Garcia, H.A.; Hooijmans, C.M.; Mijatović, I.; Lah, B.; Brdjanovic, D. Microwave treatment of municipal sewage sludge: Evaluation of the drying performance and energy demand of a pilot-scale microwave drying system. Sci. Total Environ. 2020, 742, 140541. [Google Scholar] [CrossRef] [PubMed]
- Al-Gheethi, A.A.; Efaq, A.N.; Bala, J.D.; Norli, I.; Abdel-Monem, M.O.; Kadir, M.O.A. Removal of pathogenic bacteria from sewage-treated effluent and biosolids for agricultural purposes. Appl. Water Sci. 2018, 8, 74. [Google Scholar] [CrossRef]
- Reinthaler, F.F.; Posch, J.; Feierl, G.; Wüst, G.; Haas, D.; Ruckenbauer, G.; Mascher, F.; Marth, E. Antibiotic resistance of E. coli in sewage and sludge. Water Res. 2003, 37, 1685–1690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Fu, Y.; Wang, F.; Yang, J.; Pan, Z.; Huang, M.; Shen, K.; Shen, C. Multidrug-resistant enteroaggregative Escherichia coli (EAEC) enters dormant state during heat treatment: A potential hazard in municipal sludge. Environ. Pollut. 2022, 305, 119312. [Google Scholar] [CrossRef]
- Park, W.J.; Ahn, J.H.; Hwang, S.; Lee, C.K. Effect of output power, target temperature, and solid concentration on the solubilization of waste activated sludge using microwave irradiation. Bioresour. Technol. 2010, 101, S13–S16. [Google Scholar] [CrossRef]
- Pino-Jelcic, S.A.; Hong, S.M.; Park, J.K. Enhanced anaerobic biodegradability and inactivation of fecal coliforms and Salmonella spin wastewater sludge by using microwaves. Water Environ. Res. 2006, 78, 209–216. [Google Scholar] [CrossRef]
- Bennamoun, L.; Chen, Z.; Afzal, M.T. Microwave drying of wastewater sludge: Experimental and modeling study. Dry. Technol. 2016, 34, 235–243. [Google Scholar] [CrossRef]
- An-nori, A.; Ezzariai, A.; El Mejahed, K.; El Fels, L.; El Gharous, M.; Hafidi, M. Solar Drying as an Eco-Friendly Technology for Sewage Sludge Stabilization: Assessment of Micropollutant Behavior, Pathogen Removal, and Agronomic Value. Front. Environ. Sci. 2022, 10, 814590. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation No 2003/2003. Off. J. Eur. Union 2003, 1–114. Available online: http://data.europa.eu/eli/reg/2019/1009/oj (accessed on 15 December 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).