Competition of Cladocerans for Natural Seston from a Tropical Shallow Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. The Lake
2.2. Algae Culture
2.3. Sampling, Cultivation, and Acclimation of Cladocerans
2.4. Experiment 1: Competition between Ceriodaphnia richardi and Daphnia gessneri for Limnetic Seston
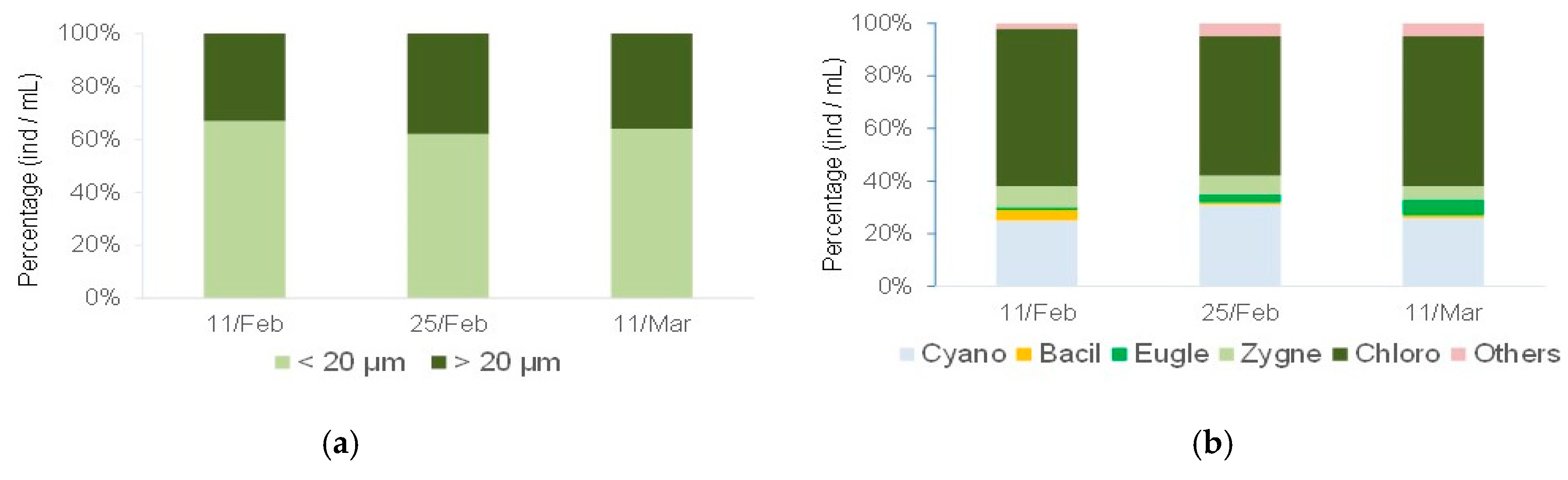
2.4.1. Physical and Chemical Factors, Algal Carbon, and Phytoplankton Composition and Size in the Lake during the Experimental Period
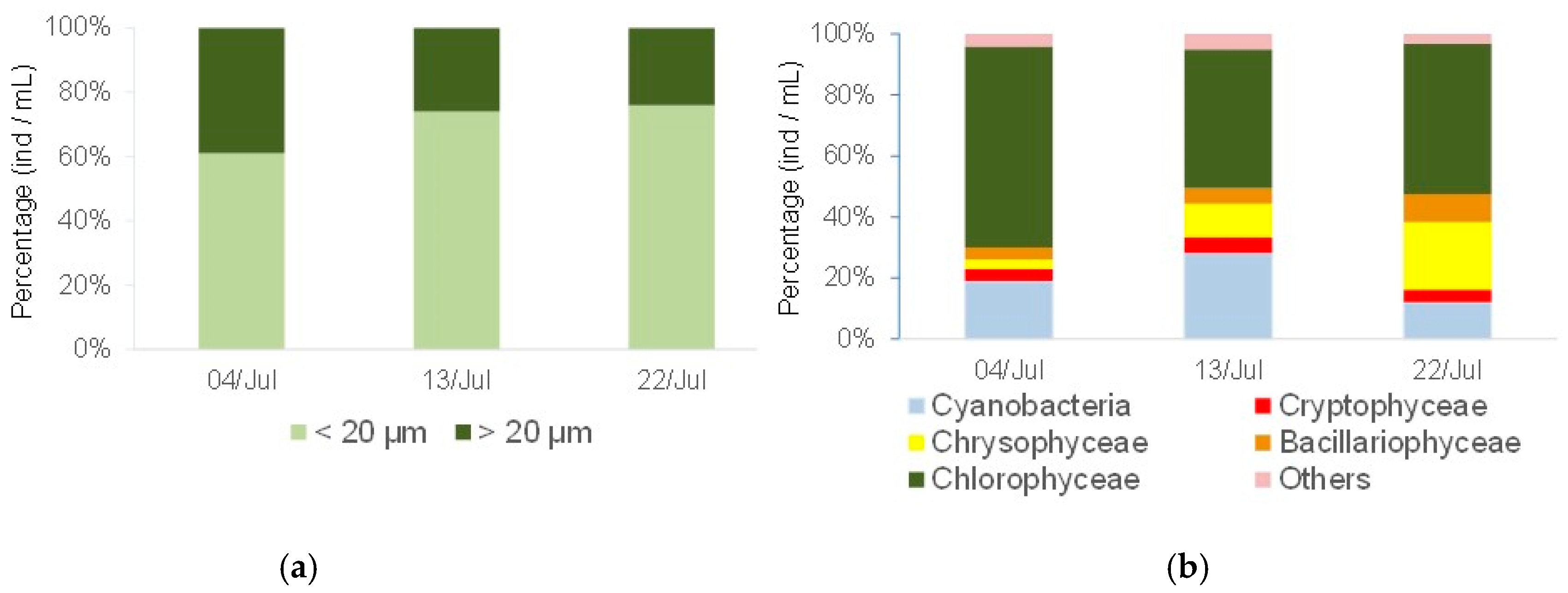
2.5. Experiment 2: Competition between Ceriodaphnia richardi and Diaphanosoma birgei for Littoral Seston
Physical and Chemical Factors, Algal Carbon, and Phytoplankton Composition and Size in the Lake during the Experimental Period
2.6. Data Analysis
3. Results
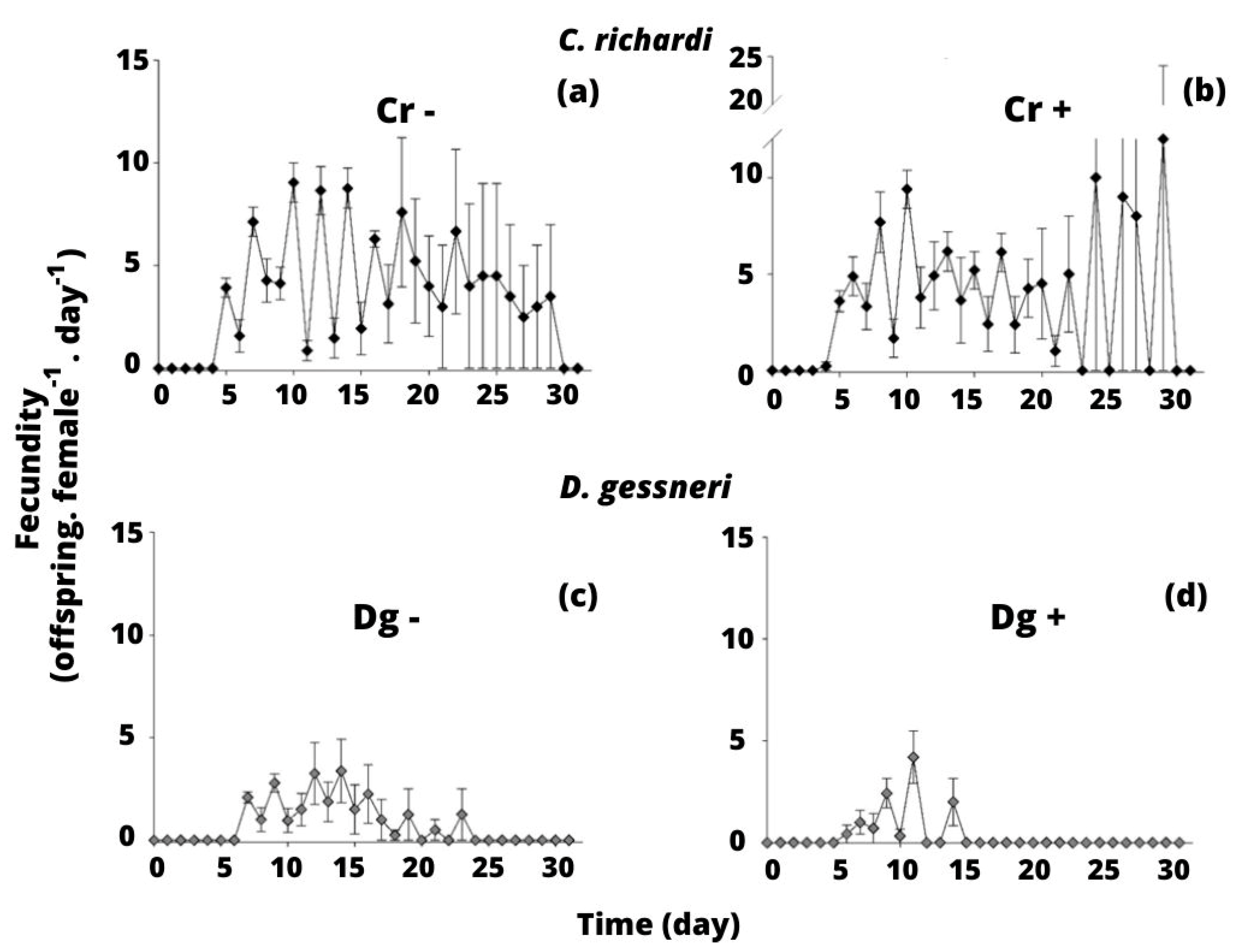
3.1. Experiment 1: Competition between C. richardi and D. gessneri for Limnetic Seston
Physical and Chemical Factors, Algal Carbon, and Phytoplankton Composition and Size in the Lake during the Experimental Period
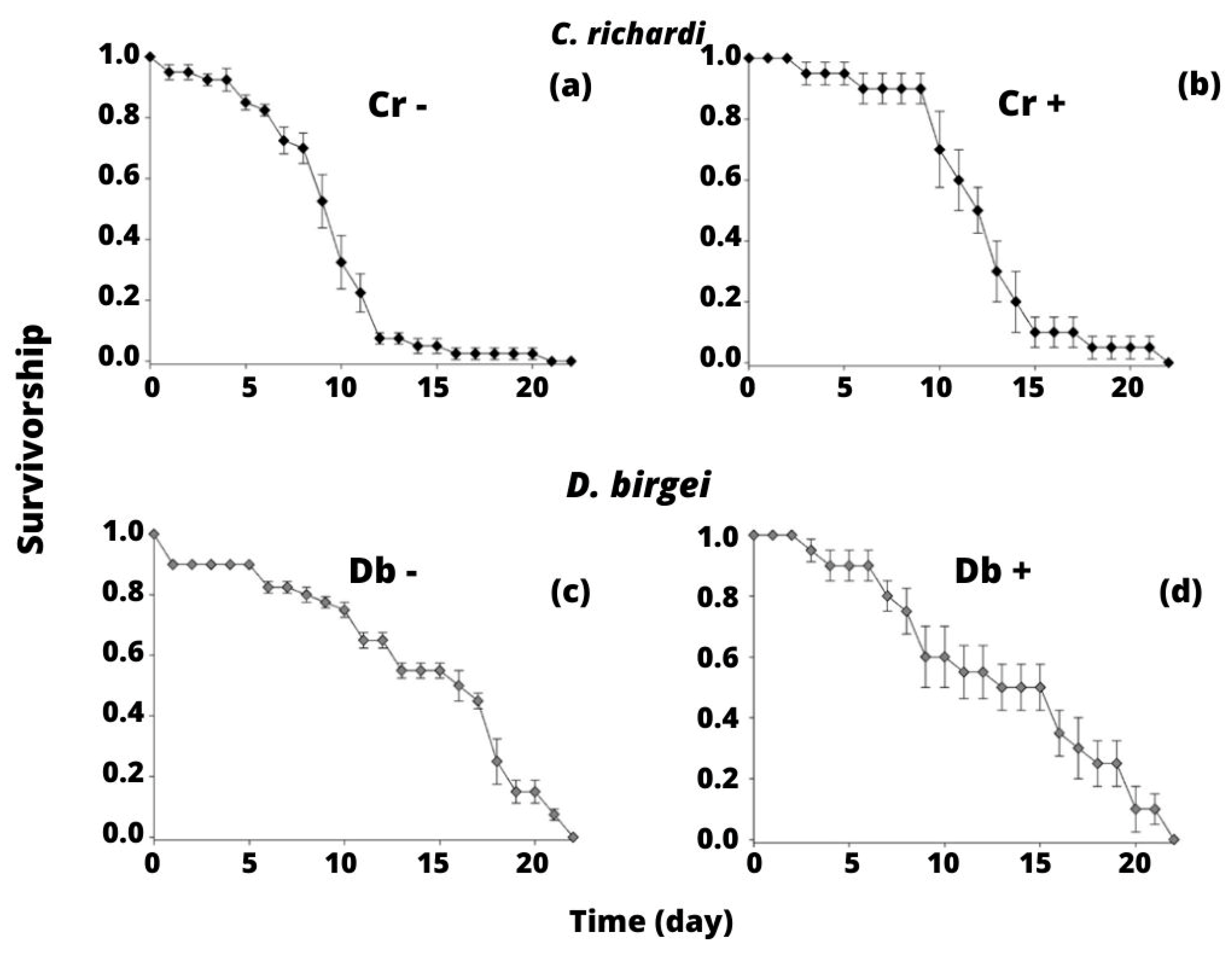
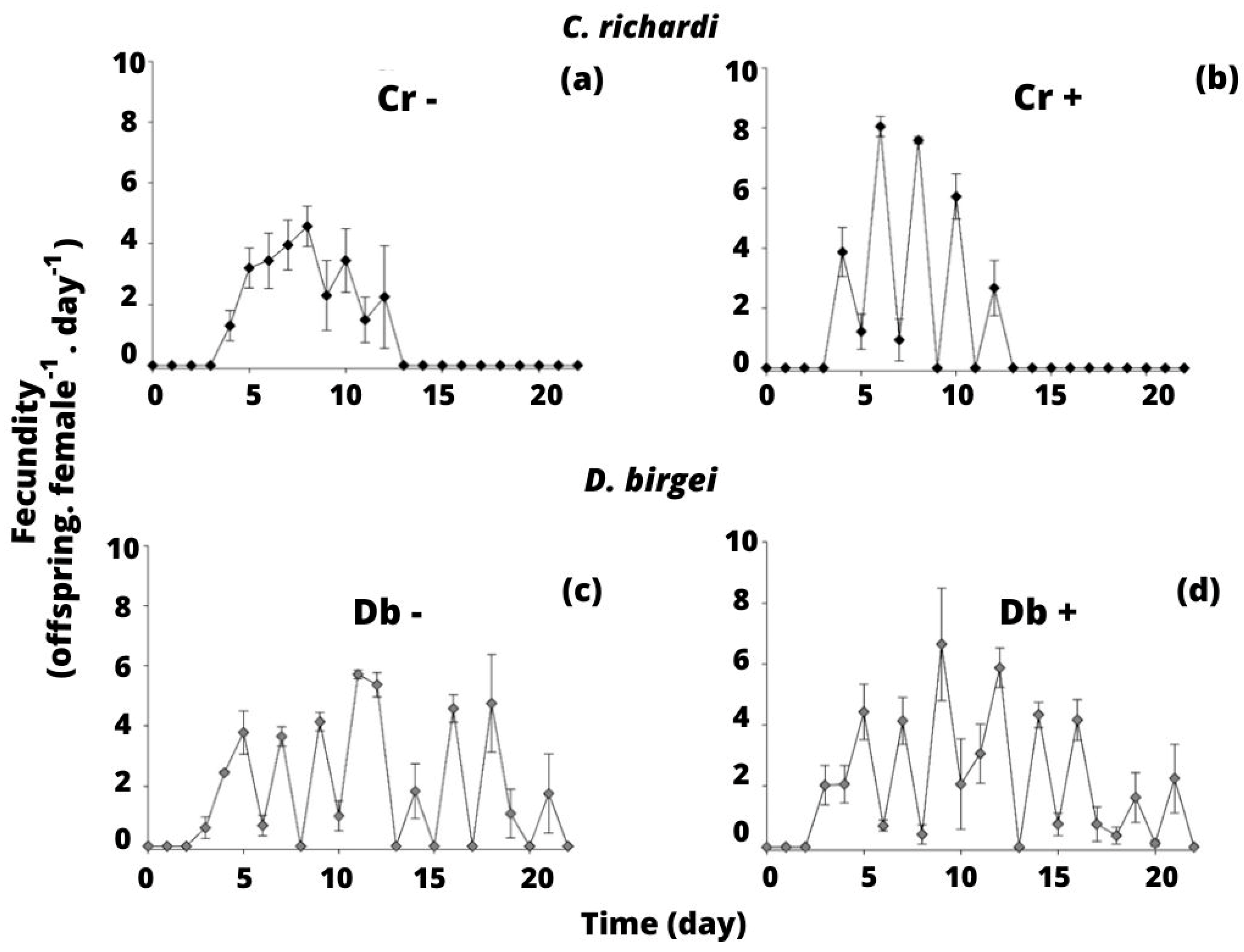
3.2. Experiment 2: Competition between C. richardi and D. birgei for Littoral Seston
Physical and Chemical Factors, Algal Carbon, and Phytoplankton Composition and Size in the Lake during the Experimental Period
4. Discussion
4.1. Experiment 1: Competition between C. richardi and D. gessneri for Limnetic Seston
4.2. Experiment 2: Competition between C. richardi and D. birgei for Littoral Seston
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gliwicz, Z.M. Studies on the feeding of pelagic zooplankton in lakes with varying trophy. Ekol. Polska 1969, 12, 663–708. [Google Scholar]
- Neill, W.E. Experimental studies of microcrustacean competition, community composition and efficiency of resource utilization. Ecology 1975, 56, 809–826. [Google Scholar] [CrossRef]
- Zaret, T.M. Predation and Freshwater Communities; Yale University Press: New Haven, CT, USA, 1980; 187p. [Google Scholar]
- Nandini, S.; Sarma, S.S.S. Competition between Moina macrocopa and Ceriodaphnia dubia: A life table demography study. Int. Rev. Hydrobiol. 2002, 87, 85–95. [Google Scholar] [CrossRef]
- Lynch, M. Complex interactions between natural co-exploiters Daphnia and Ceriodaphnia. Ecology 1978, 59, 552–564. Available online: http://links.jstor.org/sici?sici=0012-9658%28197821%3A3%3C552%3ACIBNCA%3E2.O.CO%3B2-Z (accessed on 5 September 2023). [CrossRef]
- Romanovsky, Y.E.; Feniova, I.Y. Competition among Cladocera: Effect of different levels of food supply. Oikos 1985, 44, 243–252. [Google Scholar] [CrossRef]
- Milbrink, G.; Kruse, M.L.; Bengtsson, J. Competitive ability and life history strategies in four species of Daphnia: D. obtusa, D. magna, D. pulex and D. longispina. Arch. Hydrobiol. 2003, 157, 433–453. [Google Scholar] [CrossRef]
- Gnauck, A.; Matveev, V. Estimating competition in cladocerans using data on dynamics of clutch size and population density. Int. Rev. Hydrobiol. 1983, 68, 785–798. [Google Scholar] [CrossRef]
- Nandini, S.; Sarma, S.S.S. Life table demography of four cladoceran species in relation to algal food (Chlorella vulgaris) density. Hydrobiologia 2000, 435, 117–126. [Google Scholar] [CrossRef]
- Ferreira, T.C.S.; Arcifa, M.S.; Domingos, A.R. Is competition an interaction as relevant as predation for tropical planktonic cladocerans? Acta Limnol. Brasil. 2018, 30, e1. [Google Scholar] [CrossRef]
- Bengtsson, J. Competitive dominance among Cladocera: Are single-factor explanations enough? Hydrobiologia 1987, 145, 245–257. [Google Scholar] [CrossRef]
- Lampert, W. Studies on the carbon balance of Daphnia pulex de Geer as related to environmental conditions. IV. Determination of ‘‘threshold’’ concentration as a factor controlling the abundance of zooplankton species. Arch. Hydrobiol. (Suppl.) 1977, 48, 361–368. [Google Scholar]
- Romanovsky, Y.E. Food limitation and life history strategies in cladoceran crustaceans. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1985, 21, 363–372. [Google Scholar]
- Gliwicz, Z.M. Food thresholds and body size in cladocerans. Nature 1990, 343, 638–640. [Google Scholar] [CrossRef]
- Kreutzer, C.; Lampert, W. Exploitative competition in differently sized Daphnia species: A mechanistic explanation. Ecology 1999, 80, 348–2357. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Fernández-Araiza, M.A.; Nandini, S. Competition between Brachionus calyciflorus Pallas and Brachionus patulus (Müller) (Rotifera) in relation to algal food concentration and initial population density. Aquat. Ecol. 1999, 33, 339–345. [Google Scholar] [CrossRef]
- DeMott, W.R. The role of competition in zooplankton succession. In Plankton Ecology: Succession in Plankton Communities; Sommer, U., Ed.; Springer: Berlin, Germany, 1989; pp. 195–252. [Google Scholar] [CrossRef]
- Goulden, C.E.; Hornig, L.L.P.; Wilson, C. Why do large zooplankton species dominate? Verh. Internat. Ver. Theor. Angew. Limnol. 1978, 20, 2457–2460. [Google Scholar] [CrossRef]
- DeMott, W.R.; Kerfoot, W.C. Competition among cladocerans: Nature of the interactions between Bosmina and Daphnia. Ecology 1982, 63, 1949–1966. [Google Scholar] [CrossRef]
- Stachowicz, J.J. Mutualism, facilitation, and the structure of ecological communities. BioScience 2001, 51, 235–246. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Nandini, S.; Enríquez-Garcia, C.; Sarma, S.S.S. A laboratory study on the demography and competition of three species of littoral cladocerans from Lake Huetzalin, Xochimilco, Mexico. Aquat. Ecol. 2007, 41, 547–556. [Google Scholar] [CrossRef]
- Steiner, C.F.; Darcy-Hall, T.L.; Dorn, N.J.; Garcia, E.A.; Mittelbach, G.G.; Wojdak, J.M. The influence of consumer diversity and indirect facilitation on trophic level biomass and stability. Oikos 2005, 110, 556–566. [Google Scholar] [CrossRef]
- Smith, D.W.; Cooper, S.D. Competition among Cladocera. Ecology 1982, 63, 1004–1015. [Google Scholar] [CrossRef]
- Ferreira, T.C.S.; Arcifa, M.S. Competition and the life table of cladocerans from a tropical shallow lake. Austral Ecol. 2023, 48, 461–475. [Google Scholar] [CrossRef]
- Stemberger, R.S. A general approach to the culture of planktonic rotifers. Can. J. Fish. Aquat. Sci. 1981, 38, 721–724. [Google Scholar] [CrossRef]
- Haney, J.F.; Hall, D.J. Sugar-coated Daphnia: A preservation technique for Cladocera. Limnol. Oceanogr. 1973, 18, 331–333. [Google Scholar] [CrossRef]
- Krebs, C.J. Ecology: The Experimental Analysis of Distribution and Abundance; Harper and Row: New York, NY, USA, 1985; 800p. [Google Scholar]
- Cole, G.A. Textbook of Limnology, 4th ed.; Waveland Press Inc.: Prospect Heights, IL, USA, 1994; 412p. [Google Scholar]
- Jeffrey, S.W.; Humphrey, G.F. New spectrophotometric equations for determining chlorophylls a, b, c1 and c2 in higher plants, algae, and natural phytoplankton. Biochem. Physiol. Pflanzen 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Reynolds, C.S. The Ecology of Freshwater Phytoplankton; Cambridge University Press: Cambridge, UK, 1984; 384p. [Google Scholar]
- Uehlinger, V. Étude statistique des méthodes de dénobrement planctonique. Archiv. Sci. 1964, 17, 121–223. [Google Scholar]
- Utermöhl, H. Zur vervollkommnung der quantitativen phytoplankton-methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 1958, 9, 1–38. [Google Scholar] [CrossRef]
- Hoek, C.V.D.; Mann, D.G.; Jahns, H.M. Algae: An introduction to Phycology; Cambridge University Press: Cambridge, UK, 1997; 627p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. I. Teil Chroococcales. In Süsswasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer, Jena: Stutgart, Germany, 1999; Volume 19, 548p. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprocaryota. II. Teil Oscillatoriales. In Subwasserflora von Mittleuropa; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Gustav Fisher, Jena: Stutgart, Germany, 2005; Volume 19, 759p. [Google Scholar]
- Komárek, J. Cyanoprokaryota. 3. Teil/3rd Heterocytous Genera. In Süsswasserflora von Mitteleuropa; Ettl, H., Gärtner, G., Schagerl, M., Budel, B., Krientitz, L., Eds.; Springer: Berlin, Germany, 2013; Volume 19, p. 1131. [Google Scholar]
- Round, F.E.; Crawford, R.M.; Mann, D.G. The Diatoms—Biology and Morphology of the Genera; Cambridge University Press: Cambridge, UK, 1990; 747p. [Google Scholar]
- Souza, B.B.; Arcifa, M.S.; Ferreira, T.C.S.; Silva, L.H.S.; Fileto, C.; Domingos, A.R. Are the littoral zone conditions suitable for tropical planktonic microcrustaceans? Int. J. Limnol. 2017, 53, 281–291. [Google Scholar] [CrossRef][Green Version]
- Rauschert, E.S.J. Survivorship Curves. Nat. Educ. Knowl. 2010, 3, 18. [Google Scholar]
- Romanovsky, Y.E. Individual growth rate as a measure of competitive advantages in cladoceran crustaceans. Int. Rev. Ges. Hydrobiol. Hydrogr. 1984, 69, 613–632. [Google Scholar] [CrossRef]
- Bunioto, T.C.; Arcifa, M.S. Effects of food limitation and temperature on cladocerans from a tropical Brazilian lake. Aquat. Ecol. 2007, 41, 569–578. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.S.; Arcifa, M.S.; Fileto, C. Resource limitation and food quality for cladocerans in a tropical Brazilian lake. Hydrobiologia 2003, 491, 201–210. [Google Scholar] [CrossRef]
- Threlkeld, S.T. Starvation and the size structure of zooplankton communities. Freshw. Biol. 1976, 6, 489–496. [Google Scholar] [CrossRef]
- Tessier, A.J.; Henry, L.L.; Goulden, C.E.; Durand, M.W. Starvation in Daphnia: Energy reserves and reproductive allocation. Limnol. Oceanogr. 1983, 28, 667–676. [Google Scholar] [CrossRef]
- Matveev, V.; Gabriel, W. Competitive exclusion in Cladocera through elevated mortality of adults. J. Plankton Res. 1994, 16, 1083–1094. [Google Scholar] [CrossRef]
- Lampert, W.; Sommer, U. Limnoecology: The Ecology of Lakes and Streams. Oxford University Press: New York, NY, USA, 1997; 382p. [Google Scholar]
- Case, T.D.; Gilpin, M.E. Interference competition and niche theory. Proc. Natl. Acad. Sci. USA 1974, 71, 3073–3077. [Google Scholar] [CrossRef]
- Lynch, M. The evolution of cladoceran life histories. Q. Rev. Biol. 1980, 55, 23–42. [Google Scholar] [CrossRef]
- Fileto, C.; Arcifa, M.S.; Ferrão-Filho, A.S.; Silva, L.H.S. Influence of phytoplankton fractions on growth and reproduction of tropical cladocerans. Aquat. Ecol. 2004, 38, 503–514. [Google Scholar] [CrossRef]
- Ferrão-Filho, A.S.; Arcifa, M.S.; Fileto, C. Influence of seasonal variation of seston on growth of tropical cladocerans. Brazil. J. Biol. 2005, 65, 77–89. [Google Scholar] [CrossRef]
- Sarma, S.S.S.; Nandini, S.; Gulati, R.D. Life history strategies of cladocerans: Comparisons of tropical and temperate taxa. Hydrobiologia 2005, 542, 315–333. [Google Scholar] [CrossRef]
- Arcifa, M.S.; Ferreira, T.C.S.; Fileto, C.; Castilho-Noll, M.S.M.; Bunioto, T.C.; Minto, W.J. A long-term study on crustacean plankton of a shallow tropical lake: The role of invertebrate predation. J. Limnol. 2015, 74, 606–617. [Google Scholar] [CrossRef]
- DeMott, W.R. Relation between filter mesh-size, feeding mode, and capture efficiency for cladocerans feeding on ultrafine particles. Arch. Hydrobiol. Beih. Ergebn. Limnol. 1985, 21, 125–135. [Google Scholar]
- Geller, W.; Müller, H. The filtration apparatus of Cladocera: Filter mesh-sizes and their implications on food selectivity. Oecologia 1981, 49, 316–321. [Google Scholar] [CrossRef]
- Lazareva, V.I. The distribution of species of genus Diaphanosoma (Crustacea, Cladocera) in reservoirs of Volga and Sheksna Rivers: Impact of environmental factors. Inland Water Biol. 2012, 5, 257–265. [Google Scholar] [CrossRef]
- Hardy, E.R.; Duncan, A. Food concentration and temperature effects on life cycle characteristics of tropical cladocera (Daphnia gessneri Herbst, Diaphanosoma sarsi Richard, Moina reticulata (Daday): 1. Development time. Acta Amaz. 1994, 24, 119–134. [Google Scholar] [CrossRef][Green Version]
- Melão, M.G.G. Desenvolvimento e aspectos reprodutivos de cladóceros e copépodos de águas continentais brasileiras. In Perspectivas da Limnologia no Brasil; Pompêo, M.L.M., Ed.; Gráfica e Editora União: São Luiz, Brazil, 1999; pp. 45–57. [Google Scholar]
- Ferreira, T.C.S. Competição, predação e variação temporal de microcrustáceos planctônicos no Lago Monte Alegre. Master’s Thesis, Universidade de São Paulo, Ribeirão Preto, Brazil, 2013. [Google Scholar]
- Ferreira, T.C.S.; Arcifa, M.S. Experiments on invertebrate predation on cladocerans and its relationships with lake data. J. Limnol. 2022, 81, 1987. [Google Scholar] [CrossRef]

| Species | Average Lifespan | Gross Reproductive Rate | Net Reproductive Rate | Generation Time | Population Growth Rate | Reproductive Effort |
|---|---|---|---|---|---|---|
| Cr− | 23 ± 2.7 | 113.2 ± 38.0 | 43.5 ± 18.3 | 12.8 ± 1.0 | 0.4 ± 0.0 | 2.4 ± 0.4 |
| Cr+ | 23 ± 2.1 ns | 119.1 ± 42.4 ns | 60.9 ± 21.1 ns | 12.6 ± 1.6 ns | 0.5 ± 0.0 ns | 3.2 ± 0.9 ns |
| Dg− | 21 ± 1.4 | 24.8 ± 4.5 | 4.3 ± 1.1 | 11.9 ± 0.6 | 0.1 ± 0.0 | 0.3 ± 0.0 |
| Dg+ p | 15 ± 0.3 0.004 | 11.1 ± 3.4 0.05 | 3.2 ± 1.2 ns | 10.1 ± 0.8 ns | 0.1 ± 0.0 ns | 0.4 ± 0.1 ns |
| Cr− | 23 ± 2.7 | 113.2 ± 38.0 | 43.5 ± 18.3 | 12.8 ± 1.0 | 0.4 ± 0.0 | 2.4 ± 0.4 |
| Dg− p | 21 ± 1.4 ns | 24.8 ± 4.5 0.03 | 4.3 ± 1.1 0.04 | 11.9 ± 0.6 ns | 0.1 ± 0.0 0.000 | 0.3 ± 0.0 0.01 |
| Factors | Depth/Date | 11/Feb | 25/Feb | 11/Feb |
|---|---|---|---|---|
| Temperature (°C) | Surface | 28.60 | 27.60 | 28.20 |
| 3.5 m | 27.40 | 26.40 | 27.10 | |
| Dissolved oxygen (mg L−1) | Surface | 5.80 | 10.82 | 12.60 |
| 3.5 m | 2.17 | 8.50 | 5.70 | |
| pH | Surface | 7.06 | 6.03 | 7.02 |
| 3.5 m | 5.40 | 5.90 | 6.42 | |
| Conductivity (µS cm−1) | Surface | 63.70 | 65.10 | 64.70 |
| 3.5 m | 65.90 | 63.30 | 65.10 | |
| Algal carbon (mg C L−1) | Euphotic zone | 1.00 | 1.10 | 1.29 |
| Species | Average Lifespan | Gross Reproductive Rate | Net Reproductive Rate | Generation Time | Population Growth Rate | Reproductive Effort |
|---|---|---|---|---|---|---|
| Cr− | 15.0 ± 1.4 | 26.0 ± 2.2 | 16.0 ± 1.5 | 7.0 ± 0.1 | 0.4 ± 0.01 | 2.1 ± 0.3 |
| Cr+ p | 16.0 ± 1.6 ns | 30.0 ± 1.7 ns | 25.3 ± 2.5 0.02 | 7.1 ± 0.1 ns | 0.5 ± 0.02 ns | 3.0 ± 0.2 0.01 |
| Db− | 21.0 ± 0.2 | 41.4 ± 2.4 | 25.4 ± 2.5 | 9.2 ± 0.2 | 0.4 ± 0.02 | 1.6 ± 0.1 |
| Db+ p | 19.0 ± 0.9 ns | 45.8 ± 5.2 ns | 25.9 ± 2.0 ns | 9.2 ± 0.5 ns | 0.5 ± 0.04 ns | 1.5 ± 0.1 ns |
| Cr− | 15.0 ± 1.4 | 26.0 ± 2.2 | 16.0 ± 1.5 | 7.0 ± 0.1 | 0.4 ± 0.01 | 2.1 ± 0.3 |
| Db− p | 21.0 ± 0.2 0.01 | 41.4 ± 2.4 0.03 | 25.4 ± 2.5 0.02 | 9.2 ± 0.2 0.003 | 0.4 ± 0.02 ns | 1.6 ± 0.1 ns |
| Factors | Depth/Date | 04/Jul | 13/Jul | 22/Jul |
|---|---|---|---|---|
| Temperature (°C) | Surface | 20.7 | 20.3 | 20.1 |
| 1 m | 20.7 | 20.3 | 20.1 | |
| Dissolved oxygen (mg L−1) | Surface | 10.5 | 9.5 | 10.4 |
| 1 m | 10.5 | 9.4 | 9.8 | |
| pH | Surface | 6.4 | --- | --- |
| 1 m | 6.4 | --- | --- | |
| Conductivity (µS cm−1) | Surface | 54.0 | 55.0 | 54.6 |
| 1 m | 54.0 | 55.0 | 56.5 | |
| Turbidity (NTU) | Water column (0–1 m) | 7.3 | 7.4 | 7.6 |
| Algal carbon (mg C L−1) | Water column (0–1 m) | 0.96 | 0.96 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, T.C.d.S.; Silva, L.H.S.d.; Arcifa, M.S. Competition of Cladocerans for Natural Seston from a Tropical Shallow Lake. Water 2023, 15, 3373. https://doi.org/10.3390/w15193373
Ferreira TCdS, Silva LHSd, Arcifa MS. Competition of Cladocerans for Natural Seston from a Tropical Shallow Lake. Water. 2023; 15(19):3373. https://doi.org/10.3390/w15193373
Chicago/Turabian StyleFerreira, Tânia Cristina dos Santos, Lúcia Helena Sampaio da Silva, and Marlene Sofia Arcifa. 2023. "Competition of Cladocerans for Natural Seston from a Tropical Shallow Lake" Water 15, no. 19: 3373. https://doi.org/10.3390/w15193373
APA StyleFerreira, T. C. d. S., Silva, L. H. S. d., & Arcifa, M. S. (2023). Competition of Cladocerans for Natural Seston from a Tropical Shallow Lake. Water, 15(19), 3373. https://doi.org/10.3390/w15193373




