Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs)
Abstract
1. Introduction
2. Materials and Methods
3. Causes of Algal Blooms
4. Methods to Control HABs
4.1. Chemical Methods
4.2. Physical Methods
4.3. Challenges, Monitoring, and Considerations
4.4. Biological Management of HABs
4.4.1. Biomanipulation
Longevity of Biomanipulation
Determinants of Biomanipulation Efficacy
Environmental Conditions and Weather
4.4.2. The Role of Submerged Macrophytes in Eutrophication Control
Utilization of Natural and Engineered Macrophytes
The Impact of Macrophyte Density on Algal Suppression
Allelopathic Dynamics in Aquatic Ecosystems
4.4.3. Utilizing Straw to Combat Harmful Algal Blooms
Mechanism of Action
Barley Straw
Rice Straw and Ragi Straw
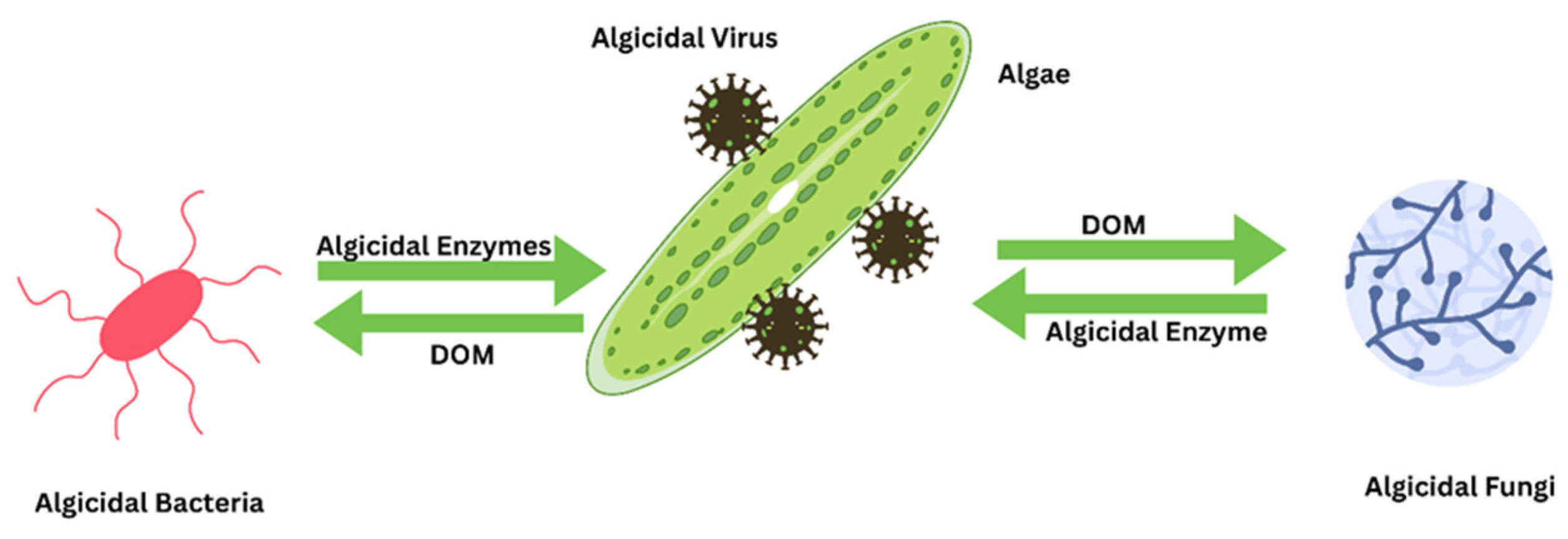
4.4.4. Bacterial Use for Controlling HABs
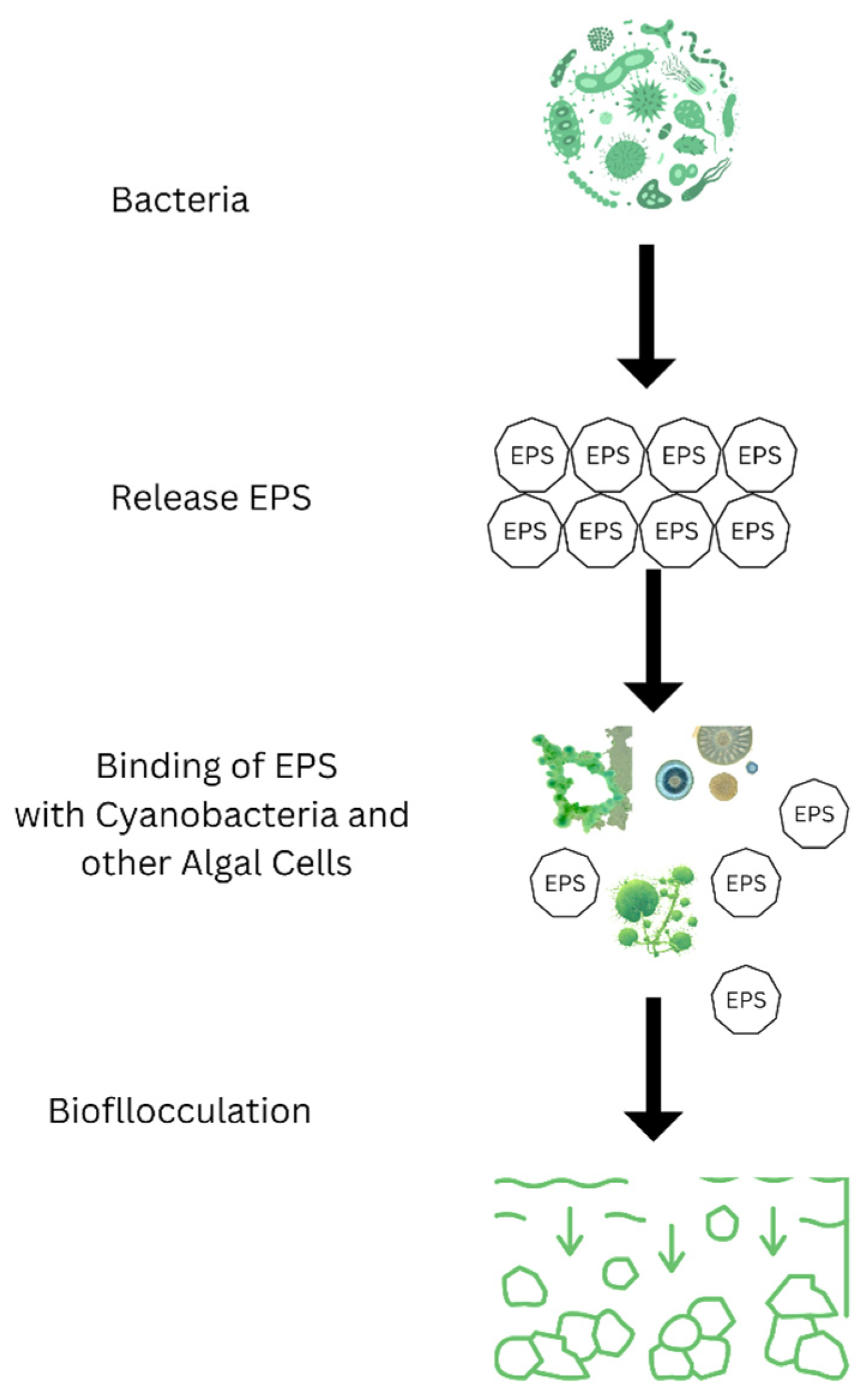
Bioflocculation
Inhibition of Harmful Algal Growth
Actinomycetes for Inhibiting HABs
4.4.5. Fungi for Algal Control
Fungi-Based Pelletization
4.4.6. Seaweeds for HABs Control
4.4.7. Viruses for HABs Control
4.4.8. Use of Biological Approaches at Lake
4.5. Future Prospect
4.6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, J.; Lao, Y.-M.; Song, J.-T.; Jin, H.; Zhu, J.-M.; Cai, Z.-H. Temporal heterogeneity of microbial communities and metabolic activities during a natural algal bloom. Water Res. 2020, 183, 116020. [Google Scholar] [CrossRef] [PubMed]
- Berdalet, E.; Fleming, L.E.; Gowen, R.; Davidson, K.; Hess, P.; Backer, L.C.; Moore, S.K.; Hoagland, P.; Enevoldsen, H. Marine harmful algal blooms, human health and wellbeing: Challenges and opportunities in the 21st century. J. Mar. Biol. Assoc. UK 2016, 96, 61–91. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Klaiber, H.A. Bloom and bust: Toxic algae’s impact on nearby property values. Ecol. Econ. 2017, 135, 209–221. [Google Scholar] [CrossRef]
- Zingone, A.; Enevoldsen, H.O. The diversity of harmful algal blooms: A challenge for science and management. Ocean. Coast. Manag. 2000, 43, 725–748. [Google Scholar] [CrossRef]
- Anderson, D.M. Turning back the harmful red tide. Nature 1997, 388, 513–514. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, G.; Zhu, X.; Chen, L.; Cai, Z. A review of the relationship between algae and bacteria in harmful algal blooms. Acta Ecol. Sin. 2014, 34, 269–281. [Google Scholar]
- Kramer, B.J.; Davis, T.W.; Meyer, K.A.; Rosen, B.H.; Goleski, J.A.; Dick, G.J.; Oh, G.; Gobler, C.J. Nitrogen limitation, toxin synthesis potential, and toxicity of cyanobacterial populations in Lake Okeechobee and the St. Lucie River Estuary, Florida, during the 2016 state of emergency event. PLoS ONE 2018, 13, e0196278. [Google Scholar] [CrossRef]
- Plaas, H.E.; Paerl, H.W. Toxic cyanobacteria: A growing threat to water and air quality. Environ. Sci. Technol. 2020, 55, 44–64. [Google Scholar] [CrossRef]
- Hallegraeff, G. Harmful algal blooms: A global overview. Man. Harmful Mar. Microalgae 2003, 33, 1–22. [Google Scholar]
- Wells, M.L.; Karlson, B.; Wulff, A.; Kudela, R.; Trick, C.; Asnaghi, V.; Berdalet, E.; Cochlan, W.; Davidson, K.; De Rijcke, M.; et al. Future HAB science: Directions and challenges in a changing climate. Harmful Algae 2020, 91, 101632. [Google Scholar] [CrossRef]
- Balaji-Prasath, B.; Wang, Y.; Su, Y.P.; Hamilton, D.P.; Lin, H.; Zheng, L.; Zhang, Y. Methods to control harmful algal blooms: A review. Environ. Chem. Lett. 2022, 20, 3133–3152. [Google Scholar] [CrossRef]
- Alayande, A.B.; Lim, J.; Kim, J.; Hong, S.; Al-Amoudi, A.S.; Park, B. Fouling control in SWRO desalination during harmful algal blooms: A historical review and future developments. Desalination 2022, 543, 116094. [Google Scholar] [CrossRef]
- Koreivienė, J.; Anne, O.; Kasperovičienė, J.; Burškytė, V. Cyanotoxin management and human health risk mitigation in recreational waters. Environ. Monit. Assess. 2014, 186, 4443–4459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; He, Y.; Li, H.; Wei, Y.; Zhao, L.; Yang, G.; Chen, X. Using flocculation and subsequent biomanipulation to control microcystis blooms: A laboratory study. Harmful Algae 2020, 99, 101917. [Google Scholar] [CrossRef] [PubMed]
- Demeke, A. Cyanobacteria blooms and biological control methods. Int. J. Fauna Biol. Stud. 2016, 3, 32–38. [Google Scholar]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic control of harmful algal blooms (HABs): A brief review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Cunha, M.Â. Assessment of the microbiological quality of recreational waters: Indicators and methods. Euro-Mediterr. J. Environ. Integr. 2017, 2, 25. [Google Scholar] [CrossRef]
- Monsalve, E.R.; Quiroga, E. Farmed shrimp aquaculture in coastal wetlands of Latin America—A review of environmental issues. Mar. Pollut. Bull. 2022, 183, 113956. [Google Scholar] [CrossRef]
- Karlson, B.; Andersen, P.; Arneborg, L.; Cembella, A.; Eikrem, W.; John, U.; West, J.J.; Klemm, K.; Kobos, J.; Lehtinen, S.; et al. Harmful algal blooms and their effects in coastal seas of Northern Europe. Harmful Algae 2021, 102, 101989. [Google Scholar] [CrossRef]
- Jacquet, S.; Miki, T.; Noble, R.; Peduzzi, P.; Wilhelm, S. Viruses in aquatic ecosystems: Important advancements of the last 20 years and prospects for the future in the field of microbial oceanography and limnology. Adv. Oceanogr. Limnol. 2010, 1, 97–141. [Google Scholar] [CrossRef]
- Wojewódka, M.; Hruševar, D. The role of paleolimnology in climate and environment reconstruction and lake restoration in light of research on selected bioindicators. Holist. Approach Environ. 2020, 10, 16–28. [Google Scholar] [CrossRef]
- Gogoi, N.; Paul, B.; Purkayastha, K.D. Eco-bioengineering Tools in Ecohydrological Assessment of Eutrophic Water Bodies. Ecotoxicology 2022, 31, 581–601. [Google Scholar]
- Silverstein, M.R.; Segrè, D.; Bhatnagar, J.M. Environmental microbiome engineering for the mitigation of climate change. Glob. Chang. Biol. 2023, 29, 2050–2066. [Google Scholar] [CrossRef] [PubMed]
- Sha, J.; Xiong, H.; Li, C.; Lu, Z.; Zhang, J.; Zhong, H.; Zhang, W.; Yan, B. Harmful algal blooms and their eco-environmental indication. Chemosphere 2021, 274, 129912. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Church, J.; Son, Y.; Kim, K.-T.; Lee, W.H. Recent advances in ultrasonic treatment: Challenges and field applications for controlling harmful algal blooms (HABs). Ultrason. Sonochemistry 2017, 38, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, S.S.U.H.; Yapa, N.; Karunarathna, S.C.; Suwannarach, N. Perceived intensification in harmful algal blooms is a wave of cumulative threat to the aquatic ecosystems. Biology 2022, 11, 852. [Google Scholar] [CrossRef]
- Chakraborty, S.; Moorthi, S.D.; Karnatak, R.; Feudel, U. Irregular harmful algal blooms triggered by feedback between toxin production and zooplankton feeding. Ecol. Model. 2022, 473, 110120. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G. Harmful cyanobacterial blooms: Causes, consequences, and controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Paerl, H.W. Mitigating harmful cyanobacterial blooms in a human-and climatically-impacted world. Life 2014, 4, 988–1012. [Google Scholar] [CrossRef]
- Paerl, H.W.; Fulton, R.S.; Moisander, P.H.; Dyble, J. Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci. World J. 2001, 1, 76–113. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.; Johnson, M.-V.V.; Morton, S.L.; Perkins, D.A.; Reavie, E.D.; Scott, G.I.; Smith, S.A.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Burford, M.A. Globally changing nutrient loads and harmful algal blooms: Recent advances, new paradigms, and continuing challenges. Oceanography 2017, 30, 58–69. [Google Scholar] [CrossRef]
- Zhan, M.-M.; Liu, P.-R.; Liu, X.-Y.; Hong, Y.; Xie, X. Inactivation and removal technologies for algal-bloom control: Advances and challenges. Curr. Pollut. Rep. 2021, 7, 392–406. [Google Scholar] [CrossRef]
- Anderson, D.M. Prevention, control and mitigation of harmful algal blooms: Multiple approaches to HAB management. Harmful Algae Manag. Mitig. 2004, 123–130. Available online: https://www.researchgate.net/publication/255649174_Prevention_control_and_mitigation_of_harmful_algal_blooms_multiple_approaches_to_HAB_management (accessed on 6 January 2024).
- McPartlin, D.A.; Loftus, J.H.; Crawley, A.S.; Silke, J.; Murphy, C.S.; O’Kennedy, R.J. Biosensors for the monitoring of harmful algal blooms. Curr. Opin. Biotechnol. 2017, 45, 164–169. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-based methods for harmful algal blooms control: A review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef]
- Dyble, J.; Bienfang, P.; Dusek, E.; Hitchcock, G.; Holland, F.; Laws, E.; Lerczak, J.; McGillicuddy, D.J., Jr.; Minnett, P.; Moore, S.K.; et al. Environmental controls, oceanography and population dynamics of pathogens and harmful algal blooms: Connecting sources to human exposure. In Environmental Health; Springer: Berlin/Heidelberg, Germany, 2008; Volume 7, pp. 1–13. [Google Scholar]
- Li, S.; Tao, Y.; Zhan, X.-M.; Dao, G.-H.; Hu, H.-Y. UV-C irradiation for harmful algal blooms control: A literature review on effectiveness, mechanisms, influencing factors and facilities. Sci. Total Environ. 2020, 723, 137986. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A critical review on control methods for harmful algal blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar] [CrossRef]
- Sierp, M.T.; Qin, J.G.; Recknagel, F. Biomanipulation: A review of biological control measures in eutrophic waters and the potential for Murray cod Maccullochella peelii peelii to promote water quality in temperate Australia. Rev. Fish Biol. Fish. 2009, 19, 143–165. [Google Scholar] [CrossRef]
- Visser, P.M.; Ibelings, B.W.; Bormans, M.; Huisman, J. Artificial mixing to control cyanobacterial blooms: A review. Aquat. Ecol. 2016, 50, 423–441. [Google Scholar] [CrossRef]
- Kibuye, F.A.; Zamyadi, A.; Wert, E.C. A critical review on operation and performance of source water control strategies for cyanobacterial blooms: Part II-mechanical and biological control methods. Harmful Algae 2021, 109, 102119. [Google Scholar] [CrossRef] [PubMed]
- Donaghay, P.L.; Osborn, T.R. Toward a theory of biological-physical control of harmful algal bloom dynamics and impacts. Limnol. Oceanogr. 1997, 42 Pt 2, 1283–1296. [Google Scholar] [CrossRef]
- Sanseverino, I.; Conduto, D.; Pozzoli, L.; Dobricic, S.; Lettieri, T. Algal bloom and its economic impact. Eur. Comm. Jt. Res. Cent. Inst. Environ. Sustain. 2016. Available online: https://data.europa.eu/doi/10.2788/660478 (accessed on 6 January 2024).
- Anderson, D.M. Approaches to monitoring, control and management of harmful algal blooms (HABs). Ocean. Coast. Manag. 2009, 52, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C. Present and future opportunities in the use of atomic force microscopy to address the physico-chemical properties of aquatic ecosystems at the nanoscale level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar]
- Badagian, N.; Schirmer, M.P.; Parada, A.P.; Gonzalez-Sapienza, G.; Brena, B.M. Determination of Microcystins in Fish Tissue by ELISA and MALDI-TOF MS Using a Highly Specific Single Domain Antibody. Toxins 2023, 15, 84. [Google Scholar] [CrossRef]
- Stumpf, R.; Culver, M.; Tester, P.; Tomlinson, M.; Kirkpatrick, G.; Pederson, B.; Truby, E.; Ransibrahmanakul, V.; Soracco, M. Monitoring Karenia brevis blooms in the Gulf of Mexico using satellite ocean color imagery and other data. Harmful Algae 2003, 2, 147–160. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M.; Pahlevan, N. Widespread global increase in intense lake phytoplankton blooms since the 1980s. Nature 2019, 574, 667–670. [Google Scholar] [CrossRef]
- Baek, S.H.; Son, M.; Jung, S.W.; Na, D.H.; Cho, H.; Yamaguchi, M.; Kim, S.W.; Kim, Y.O. Enhanced species-specific chemical control of harmful and non-harmful algal bloom species by the thiazolidinedione derivative TD49. J. Appl. Phycol. 2014, 26, 311–321. [Google Scholar] [CrossRef]
- Liu, B.; Gai, S.; Lan, Y.; Cheng, K.; Yang, F. Metal-based adsorbents for water eutrophication remediation: A review of performances and mechanisms. Environ. Res. 2022, 212, 113353. [Google Scholar] [CrossRef] [PubMed]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Gil-Izquierdo, A.; Pedreño, M.; Montoro-García, S.; Tárraga-Martínez, M.; Iglesias, P.; Ferreres, F.; Barceló, D.; Núñez-Delicado, E.; Gabaldón, J. A sustainable approach by using microalgae to minimize the eutrophication process of Mar Menor lagoon. Sci. Total Environ. 2021, 758, 143613. [Google Scholar] [CrossRef] [PubMed]
- Maris, J. Evaluating Rice Straw as a Substitute for Barley Straw in Inhibiting Algal Growth in Farm Ponds. Bachelor’s Thesis, University of Arkansa, Fayetteville, AR, USA, 2019. [Google Scholar]
- Iredale, R.S.; McDonald, A.T.; Adams, D.G. A series of experiments aimed at clarifying the mode of action of barley straw in cyanobacterial growth control. Water Res. 2012, 46, 6095–6103. [Google Scholar] [CrossRef] [PubMed]
- Eladel, H.; Battah, M.; Dawa, A.; Abd-Elhay, R.; Anees, D. Effect of rice straw extracts on growth of two phytoplankton isolated from a fish pond. J. Appl. Phycol. 2019, 31, 3557–3563. [Google Scholar] [CrossRef]
- Shumway, S.E.; Burkholder, J.M.; Morton, S.L. Harmful Algal Blooms: A Compendium Desk Reference; John Wiley & Sons: Hoboken, NJ, USA, 2018. [Google Scholar]
- Gedalanga, P.; Kotay, S.M.; Sales, C.M.; Butler, C.S.; Goel, R.; Mahendra, S. Novel applications of molecular biological and microscopic tools in environmental engineering. Water Environ. Res. 2013, 85, 917–950. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.; Burkholder, J.; Anderson, D.; Cochlan, W.; Dennison, W.; Dortch, Q.; Gobler, C.; Heil, C.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Wells, M.L.; Trainer, V.L.; Smayda, T.J.; Karlson, B.S.; Trick, C.G.; Kudela, R.M.; Ishikawa, A.; Bernard, S.; Wulff, A.; Anderson, D.M.; et al. Harmful algal blooms and climate change: Learning from the past and present to forecast the future. Harmful Algae 2015, 49, 68–93. [Google Scholar] [CrossRef]
- Theiling, C.H. A Review of Algal Phytoremediation Potential to Sequester Nutrients from Eutrophic Surface Water. 2023. Available online: https://erdc-library.erdc.dren.mil/jspui/handle/11681/47720 (accessed on 6 January 2024).
- Hoagland, P.; Scatasta, S. The economic effects of harmful algal blooms. Ecol. Harmful Algae 2006, 189, 391–402. [Google Scholar]
- Nicholls, K. A limnological basis for a Lake Simcoe phosphorus loading objective. Lake Reserv. Manag. 1997, 13, 189–198. [Google Scholar] [CrossRef]
- Salonen, K.; Sarvala, J.; Horppila, J.; Keto, J.; Malin, I.; Malinen, T.; Niemistö, J.; Ruuhijärvi, J. Development of Lake Vesijärvi through four decades of remediation efforts. Hydrobiologia 2020, 847, 4601–4619. [Google Scholar] [CrossRef]
- Wang, C.; Lu, Y.; Sun, B.; Zhang, M.; Mao, R.; Li, X.; Song, S.; Zhao, J.; Yu, M.; Shi, Y.; et al. Biomanipulation impacts on per-and polyfluoroalkyl substances accumulation and trophic transfer in an eutrophic lake. Environ. Int. 2022, 160, 107057. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, K.H.; Hwang, S.J.; Lee, T.K. Role of algal community stability in harmful algal blooms in river-connected lakes. Microb. Ecol. 2021, 82, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, E.; Søndergaard, M.; Lauridsen, T.L.; Davidson, T.A.; Liu, Z.; Mazzeo, N.; Trochine, C.; Özkan, K.; Jensen, H.S.; Trolle, D.; et al. Biomanipulation as a restoration tool to combat eutrophication: Recent advances and future challenges. Adv. Ecol. Res. 2012, 47, 411–488. [Google Scholar]
- Ren, L.; Gao, Y.; Hu, Z.; Jiang, X.; Yang, L. The Growth of Vallisneria natans and Its Epiphytic Biofilm in Simulated Nutrient-Rich Flowing Water. Water 2022, 14, 2236. [Google Scholar] [CrossRef]
- Ekvall, M.K.; Urrutia-Cordero, P.; Hansson, L.-A. Linking cascading effects of fish predation and zooplankton grazing to reduced cyanobacterial biomass and toxin levels following biomanipulation. PLoS ONE 2014, 9, e112956. [Google Scholar] [CrossRef] [PubMed]
- Urrutia-Cordero, P.; Ekvall, M.K.; Hansson, L.-A. Controlling harmful cyanobacteria: Taxa-specific responses of cyanobacteria to grazing by large-bodied Daphnia in a biomanipulation scenario. PLoS ONE 2016, 11, e0153032. [Google Scholar] [CrossRef]
- Jůza, T.; Duras, J.; Blabolil, P.; Sajdlová, Z.; Hess, J.; Chocholoušková, Z.; Kubečka, J. Recovery of the Velky Bolevecky pond (Plzen, Czech Republic) via biomanipulation–key study for management. Ecol. Eng. 2019, 136, 167–176. [Google Scholar] [CrossRef]
- Triest, L.; Stiers, I.; Van Onsem, S. Biomanipulation as a nature-based solution to reduce cyanobacterial blooms. Aquat. Ecol. 2016, 50, 461–483. [Google Scholar] [CrossRef]
- De Backer, S.; Teissier, S.; Triest, L. Stabilizing the clear-water state in eutrophic ponds after biomanipulation: Submerged vegetation versus fish recolonization. Hydrobiologia 2012, 689, 161–176. [Google Scholar] [CrossRef]
- Jeppesen, E.; Meerhoff, M.; Jacobsen, B.A.; Hansen, R.S.; Søndergaard, M.; Jensen, J.P.; Lauridsen, T.L.; Mazzeo, N. Restoration of shallow lakes by nutrient control and biomanipulation—The successful strategy varies with lake size and climate. Hydrobiologia 2007, 581, 269–285. [Google Scholar] [CrossRef]
- Van Colen, W.; Portilla, K.; Oña, T.; Wyseure, G.; Goethals, P.; Velarde, E.; Muylaert, K. Limnology of the neotropical high elevation shallow lake Yahuarcocha (Ecuador) and challenges for managing eutrophication using biomanipulation. Limnologica 2017, 67, 37–44. [Google Scholar] [CrossRef]
- Sayer, C.D.; Burgess, A.M.Y.; Kari, K.; Davidson, T.A.; Peglar, S.; Yang, H.; Rose, N. Long-term dynamics of submerged macrophytes and algae in a small and shallow, eutrophic lake: Implications for the stability of macrophyte-dominance. Freshw. Biol. 2010, 55, 565–583. [Google Scholar] [CrossRef]
- Gao, H.; Qian, X.; Wu, H.; Li, H.; Pan, H.; Han, C. Combined effects of submerged macrophytes and aquatic animals on the restoration of a eutrophic water body—A case study of Gonghu Bay, Lake Taihu. Ecol. Eng. 2017, 102, 15–23. [Google Scholar] [CrossRef]
- Wang, F.; Nishijima, W.; Uchida, Y.; Umehara, A.; Nakai, S.; Kasamo, K.; Shiraki, Y. Impact of eelgrass bed recovery and expansion on phytoplankton growth through nutrient competition. J. Environ. Manag. 2020, 260, 109898. [Google Scholar] [CrossRef] [PubMed]
- Declerck, S.; Vanderstukken, M.; Pals, A.; Muylaert, K.; Meester, L.D. Plankton biodiversity along a gradient of productivity and its mediation by macrophytes. Ecology 2007, 88, 2199–2210. [Google Scholar] [CrossRef] [PubMed]
- Švanys, A.; Paškauskas, R.; Hilt, S. Effects of the allelopathically active macrophyte Myriophyllum spicatum on a natural phytoplankton community: A mesocosm study. Hydrobiologia 2014, 737, 57–66. [Google Scholar] [CrossRef]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A review on control of harmful algal blooms by plant-derived allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Boylan, J.D.; Morris, J.E. Limited effects of barley straw on algae and zooplankton in a midwestern pond. Lake Reserv. Manag. 2003, 19, 265–271. [Google Scholar] [CrossRef][Green Version]
- West, M.; Fenner, N.; Gough, R.; Freeman, C. Evaluation of algal bloom mitigation and nutrient removal in floating constructed wetlands with different macrophyte species. Ecol. Eng. 2017, 108, 581–588. [Google Scholar] [CrossRef]
- Szabó, S.; Peeters, E.; Várbíró, G.; Borics, G.; Lukács, B.A. Phenotypic plasticity as a clue for invasion success of the submerged aquatic plant Elodea nuttallii. Plant Biol. 2019, 21, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Mowe, M.A.; Song, Y.; Sim, D.Z.; Lu, J.; Mitrovic, S.M.; Tan, H.T.; Yeo, D.C. Comparative study of six emergent macrophyte species for controlling cyanobacterial blooms in a tropical reservoir. Ecol. Eng. 2019, 129, 11–21. [Google Scholar] [CrossRef]
- Dai, Y.; Jia, C.; Liang, W.; Hu, S.; Wu, Z. Effects of the submerged macrophyte Ceratophyllum demersum L. on restoration of a eutrophic waterbody and its optimal coverage. Ecol. Eng. 2012, 40, 113–116. [Google Scholar] [CrossRef]
- Haggard, K.G.; Geiger, N.S.; Hayes, P.M.; Milligan, A.J. Suppression of cyanobacterial growth of Aphanizomenon flos-aquae by vascular plant decomposition products in Upper Klamath Lake, Oregon. Lake Reserv. Manag. 2013, 29, 13–22. [Google Scholar] [CrossRef]
- Cooke, G.D.; Welch, E.B.; Peterson, S.; Nichols, S.A. Restoration and Management of Lakes and Reservoirs; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Hongying, H.; Yu, H. Algal-bloom control by allelopathy of aquatic macrophytes-A review. Front. Environ. Sci. Eng. 2008, 2, 421–438. [Google Scholar]
- Ferrier, M.; Sr, B.B.; Terlizzi, D.; Lacouture, R. The effects of barley straw (Hordeum vulgare) on the growth of freshwater algae. Bioresour. Technol. 2005, 96, 1788–1795. [Google Scholar] [CrossRef]
- Hu, J.; Kokoette, E.; Xu, C.; Huang, S.; Tang, T.; Zhang, Y.; Liu, M.; Huang, Y.; Yu, S.; Zhu, J.; et al. Natural Algaecide Sphingosines Identified in Hybrid Straw Decomposition Driven by White-Rot Fungi. Adv. Sci. 2023, 10, 2300569. [Google Scholar] [CrossRef]
- Mohan, S.M. Comparative study of rice straw and ragi straw for the inhibition of algal bloom in fresh water. Int. Res. J. Biol. Sci. 2012, 1, 31–37. [Google Scholar]
- Alam, M.A.; Vandamme, D.; Chun, W.; Zhao, X.; Foubert, I.; Wang, Z.; Muylaert, K.; Yuan, Z. Bioflocculation as an innovative harvesting strategy for microalgae. Rev. Environ. Sci. Bio/Technol. 2016, 15, 573–583. [Google Scholar] [CrossRef]
- Chen, H.; Zhong, C.; Berkhouse, H.; Zhang, Y.; Lv, Y.; Lu, W.; Yang, Y.; Zhou, J. Removal of cadmium by bioflocculant produced by Stenotrophomonas maltophilia using phenol-containing wastewater. Chemosphere 2016, 155, 163–169. [Google Scholar] [CrossRef]
- Powell, R.J.; Hill, R.T. Mechanism of algal aggregation by Bacillus sp. strain RP1137. Appl. Environ. Microbiol. 2014, 80, 4042–4050. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.-F.; Lin, H.; Wang, G.; Lu, L.-L.; Zhao, Y.-H. Preparation of a new-style composite containing a key bioflocculant produced by Pseudomonas aeruginosa ZJU1 and its flocculating effect on harmful algal blooms. J. Hazard. Mater. 2015, 284, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, H.; Fu, L.; An, X.; Zhang, B.; Li, Y.; Chen, Z.; Zheng, W.; Yi, L.; Zheng, T. A novel algicide: Evidence of the effect of a fatty acid compound from the marine bacterium, Vibrio sp. BS02 on the harmful dinoflagellate, Alexandrium tamarense. PLoS ONE 2014, 9, e91201. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Huo, M.; Sun, C.; Cui, X.; Zhou, D.; Crittenden, J.C.; Yang, W. Bioresources inner-recycling between bioflocculation of Microcystis aeruginosa and its reutilization as a substrate for bioflocculant production. Sci. Rep. 2017, 7, 43784. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lei, X.; Zhu, H.; Zhang, H.; Guan, C.; Chen, Z.; Zheng, W.; Fu, L.; Zheng, T. Chitinase producing bacteria with direct algicidal activity on marine diatoms. Sci. Rep. 2016, 6, 21984. [Google Scholar] [CrossRef]
- Shilo, M. Lysis of blue-green algae by myxobacter. J. Bacteriol. 1970, 104, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, X.; Zhou, Y.; Zheng, W.; Yu, C.; Zheng, T. Novel insights into the algicidal bacterium DH77-1 killing the toxic dinoflagellate Alexandrium tamarense. Sci. Total Environ. 2014, 482, 116–124. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Ishida, K.; Ito, Y.; Okada, S.; Murakami, M. Bacillamide, a novel algicide from the marine bacterium, Bacillus sp. SY-1, against the harmful dinoflagellate, Cochlodinium polykrikoides. Tetrahedron Lett. 2003, 44, 8005–8007. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Lei, X.; Zhang, H.; Guan, C.; Chen, Z.; Zheng, W.; Xu, H.; Tian, Y.; Yu, Z.; et al. The first evidence of deinoxanthin from Deinococcus sp. Y35 with strong algicidal effect on the toxic dinoflagellate Alexandrium tamarense. J. Hazard. Mater. 2015, 290, 87–95. [Google Scholar] [CrossRef]
- Seong, K.A.; Jeong, H.J. Interactions between marine bacteria and red tide organisms in Korean waters. Algae 2013, 28, 297–305. [Google Scholar] [CrossRef]
- Inaba, N.; Watanabe, T.; Sakami, T.; Nishi, H.; Tahara, Y.; Imai, I. Temporal and spatial distribution of algicidal and growth-inhibiting bacteria in the coastal sea of southwest Japan. J. Plankton Res. 2014, 36, 388–397. [Google Scholar] [CrossRef]
- Scherer, P.I.; Millard, A.D.; Miller, A.; Schoen, R.; Raeder, U.; Geist, J.; Zwirglmaier, K. Temporal dynamics of the microbial community composition with a focus on toxic cyanobacteria and toxin presence during harmful algal blooms in two South German lakes. Front. Microbiol. 2017, 8, 2387. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhai, C.; Chen, R.; Liu, C.; Xue, Y.; Jiang, J. The dynamics of the water bloom-forming Microcystis aeruginosa and its relationship with biotic and abiotic factors in Lake Taihu, China. Ecol. Eng. 2012, 47, 274–277. [Google Scholar] [CrossRef]
- Gumbo, R.J.; Ross, G.; Cloete, E.T. Biological control of Microcystis dominated harmful algal blooms. Afr. J. Biotechnol. 2008, 7. Available online: https://www.researchgate.net/publication/237465093_Biological_control_of_Microcystis_dominated_harmful_algal_blooms (accessed on 6 January 2024).
- Alamri, S.A.; Mohamed, Z.A. Selective inhibition of toxic cyanobacteria by β-carboline-containing bacterium Bacillus flexus isolated from Saudi freshwaters. Saudi J. Biol. Sci. 2013, 20, 357–363. [Google Scholar] [CrossRef]
- Zhang, J.-T.; Yang, F.; Du, K.; Li, W.-F.; Chen, Y.; Jiang, Y.-L.; Li, Q.; Zhou, C.-Z. Structure and assembly pattern of a freshwater short-tailed cyanophage Pam1. Structure 2022, 30, 240–251.e4. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Ma, H.; Ren, S.; Gao, X.; He, X.; Zhu, S.; Deng, R.; Zhang, S. Insights into the mechanism of cyanobacteria removal by the algicidal fungi Bjerkandera adusta and Trametes versicolor. MicrobiologyOpen 2020, 9, e1042. [Google Scholar] [CrossRef]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Effect of lignocellulose related compounds on microalgae growth and product biosynthesis: A review. Energies 2014, 7, 4446–4481. [Google Scholar] [CrossRef]
- Dai, W.; Chen, X.; Wang, X.; Xu, Z.; Gao, X.; Jiang, C.; Deng, R.; Han, G. The algicidal fungus Trametes versicolor F21a eliminating blue algae via genes encoding degradation enzymes and metabolic pathways revealed by transcriptomic analysis. Front. Microbiol. 2018, 9, 826. [Google Scholar] [CrossRef]
- Wang, Q.; Su, M.; Zhu, W.; Li, X.; Jia, Y.; Guo, P.; Chen, Z.; Jiang, W.; Tian, X. Growth inhibition of Microcystis aeruginosa by white-rot fungus Lopharia spadicea. Water Sci. Technol. 2010, 62, 317–323. [Google Scholar] [CrossRef]
- Zeng, G.; Zhang, M.; Wang, P.; Li, X.; Wu, P.; Sun, D. Genotoxicity effects of Phanerochaete chrysosporium against harmful algal bloom species by micronucleus test and comet assay. Chemosphere 2019, 218, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Miao, F.; Zuo, J.; Liu, X.; Ji, N. Algicidal activities of secondary metabolites of marine macroalgal-derived endophytic fungi. J. Oceanol. Limnol. 2019, 37, 112–121. [Google Scholar] [CrossRef]
- Tortella, G.; Durán, N.; Rubilar, O.; Parada, M.; Diez, M. Are white-rot fungi a real biotechnological option for the improvement of environmental health? Crit. Rev. Biotechnol. 2015, 35, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.W. Structure and action mechanism of ligninolytic enzymes. Appl. Biochem. Biotechnol. 2009, 157, 174–209. [Google Scholar] [CrossRef] [PubMed]
- Heitner, C.; Dimmel, D.; Schmidt, J. Lignin and Lignans: Advances in Chemistry; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Grimm, L.; Kelly, S.; Völkerding, I.; Krull, R.; Hempel, D. Influence of mechanical stress and surface interaction on the aggregation of Aspergillus niger conidia. Biotechnol. Bioeng. 2005, 92, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cheng, Y.; Li, Y.; Wan, Y.; Liu, Y.; Lin, X.; Ruan, R. Novel fungal pelletization-assisted technology for algae harvesting and wastewater treatment. Appl. Biochem. Biotechnol. 2012, 167, 214–228. [Google Scholar] [CrossRef]
- Tyagi, Y.R.; Surampalli, R. Isolation of extra cellular biopolymer producing microorganisms from wastewater sludge for sludge settling and dewatering. In Proceedings of the WEFTEC 2006, Dallas, TX, USA, 21–25 October 2006; Water Environment Federation: Alexandria, VA, USA, 2006; pp. 473–489. [Google Scholar]
- Xie, S.; Sun, S.; Dai, S.Y.; Yuan, J.S. Efficient coagulation of microalgae in cultures with filamentous fungi. Algal Res. 2013, 2, 28–33. [Google Scholar] [CrossRef]
- Aljuboori, A.H.R.; Idris, A.; Abdullah, N.; Mohamad, R. Production and characterization of a bioflocculant produced by Aspergillus flavus. Bioresour. Technol. 2013, 127, 489–493. [Google Scholar] [CrossRef]
- Zheng, Y.; Jin, R.; Zhang, X.; Wang, Q.; Wu, J. The considerable environmental benefits of seaweed aquaculture in China. Stoch. Environ. Res. Risk Assess. 2019, 33, 1203–1221. [Google Scholar] [CrossRef]
- Xu, S.; Yu, Z.; Zhou, Y.; Yue, S.; Liang, J.; Zhang, X. The potential for large-scale kelp aquaculture to counteract marine eutrophication by nutrient removal. Mar. Pollut. Bull. 2023, 187, 114513. [Google Scholar] [CrossRef]
- Fei, X. Solving the coastal eutrophication problem by large scale seaweed cultivation. In Asian Pacific Phycology in the 21st Century: Prospects and Challenges: Proceeding of The Second Asian Pacific Phycological Forum, held in Hong Kong, China, 21–25 June 1999; Springer: Berlin/Heidelberg, Germany, 2004; pp. 145–151. [Google Scholar]
- Claret, M.; Galbraith, E.D.; Palter, J.B.; Bianchi, D.; Fennel, K.; Gilbert, D.; Dunne, J.P. Rapid coastal deoxygenation due to ocean circulation shift in the northwest Atlantic. Nat. Clim. Chang. 2018, 8, 868–872. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Gao, L.; Jiang, M.; Jian, A.; He, L. The potential of seaweed cultivation to achieve carbon neutrality and mitigate deoxygenation and eutrophication. Environ. Res. Lett. 2021, 17, 014018. [Google Scholar] [CrossRef]
- He, P.; Xu, S.; Zhang, H.; Wen, S.; Dai, Y.; Lin, S.; Yarish, C. Bioremediation efficiency in the removal of dissolved inorganic nutrients by the red seaweed, Porphyra yezoensis, cultivated in the open sea. Water Res. 2008, 42, 1281–1289. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Agusti, S.; Lin, F.; Li, K.; Pan, Y.; Yu, Y.; Zheng, Y.; Wu, J.; Duarte, C.M. Nutrient removal from Chinese coastal waters by large-scale seaweed aquaculture. Sci. Rep. 2017, 7, 46613. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Murase, J.; Asakawa, S.; Kimura, M. Novel cyanophage photosynthetic gene psbA in the floodwater of a Japanese rice field. FEMS Microbiol. Ecol. 2009, 70, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.W.; Bullerjahn, G.S.; Eldridge, M.L.; Rinta-Kanto, J.M.; Poorvin, L.; Bourbonniere, R.A. Seasonal hypoxia and the genetic diversity of prokaryote populations in the central basin hypolimnion of Lake Erie: Evidence for abundant cyanobacteria and photosynthesis. J. Great Lakes Res. 2006, 32, 657–671. [Google Scholar] [CrossRef]
- Zhang, D.; He, Y.; Gin, K.Y.-H. Novel freshwater cyanophages provide new insights into evolutionary relationships between freshwater and marine cyanophages. Microbiol. Spectr. 2021, 9, e00593-21. [Google Scholar] [CrossRef]
- Waajen, G.W.; Van Bruggen, N.C.; Pires, L.M.D.; Lengkeek, W.; Lürling, M. Biomanipulation with quagga mussels (Dreissena rostriformis bugensis) to control harmful algal blooms in eutrophic urban ponds. Ecol. Eng. 2016, 90, 141–150. [Google Scholar] [CrossRef]
- Rong, C.; Zhou, K.; Li, S.; Xiao, K.; Xu, Y.; Zhang, R.; Yang, Y.; Zhang, Y. Isolation and characterization of a novel cyanophage encoding multiple auxiliary metabolic genes. Viruses 2022, 14, 887. [Google Scholar] [CrossRef] [PubMed]
- Mankiewicz-Boczek, J.; Jaskulska, A.; Pawełczyk, J.; Gągała, I.; Serwecińska, L.; Dziadek, J. Cyanophages infection of Microcystis bloom in lowland dam reservoir of Sulejów, Poland. Microb. Ecol. 2016, 71, 315–325. [Google Scholar] [CrossRef]
- Mann, N.H. Phages of the marine cyanobacterial picophytoplankton. FEMS Microbiol. Rev. 2003, 27, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Takashima, Y.; Tomaru, Y.; Shirai, Y.; Takao, Y.; Hiroishi, S.; Nagasaki, K. Isolation and characterization of a cyanophage infecting the toxic cyanobacterium Microcystis aeruginosa. Appl. Environ. Microbiol. 2006, 72, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Yoshida-Takashima, Y.; Yoshida, M.; Ogata, H.; Nagasaki, K.; Hiroishi, S.; Yoshida, T. Cyanophage infection in the bloom-forming cyanobacteria Microcystis aeruginosa in surface freshwater. Microbes Environ. 2012, 27, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Engel, B.A.; Reuhs, M.; Simsek, H. Cyanophage technology in removal of cyanobacteria mediated harmful algal blooms: A novel and eco-friendly method. Chemosphere 2023, 315, 137769. [Google Scholar] [CrossRef] [PubMed]
- Coloma, S.; Dienstbier, A.; Bamford, D.; Sivonen, K.; Roine, E.; Hiltunen, T. Newly isolated Nodularia phage influences cyanobacterial community dynamics. Environ. Microbiol. 2017, 19, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Antosiak, A.; Šulčius, S.; Malec, P.; Tokodi, N.; Łobodzińska, A.; Dziga, D. Cyanophage infections reduce photosynthetic activity and expression of CO2 fixation genes in the freshwater bloom-forming cyanobacterium Aphanizomenon flos-aquae. Harmful Algae 2022, 116, 102215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, P.; Zhao, S.; Kang, S.; Wang, P.; Zhou, M.; Lyu, J. Control and remediation methods for eutrophic lakes in the past 30 years. Water Sci. Technol. 2020, 81, 1099–1113. [Google Scholar] [CrossRef]
- Waajen, G.; van Oosterhout, F.; Douglas, G.; Lürling, M. Geo-engineering experiments in two urban ponds to control eutrophication. Water Res. 2016, 97, 69–82. [Google Scholar] [CrossRef]
- Yudianto, D.; Yuebo, X. Numerical modeling and practical experience of Xuxi River’s natural restoration using biological treatment. Water Environ. Res. 2011, 83, 2087–2098. [Google Scholar] [CrossRef]
- Hashim, S.; Yuebo, X.; Saifullah, M.; Jan, R.N.; Muhetaer, A. Integrated evaluation of urban water bodies for pollution abatement based on fuzzy multicriteria decision approach. BioMed Res. Int. 2015, 2015, 327280. [Google Scholar] [CrossRef]
- Yu, S.; Miao, C.; Song, H.; Huang, Y.; Chen, W.; He, X. Efficiency of nitrogen and phosphorus removal by six macrophytes from eutrophic water. Int. J. Phytoremediation 2019, 21, 643–651. [Google Scholar] [CrossRef] [PubMed]
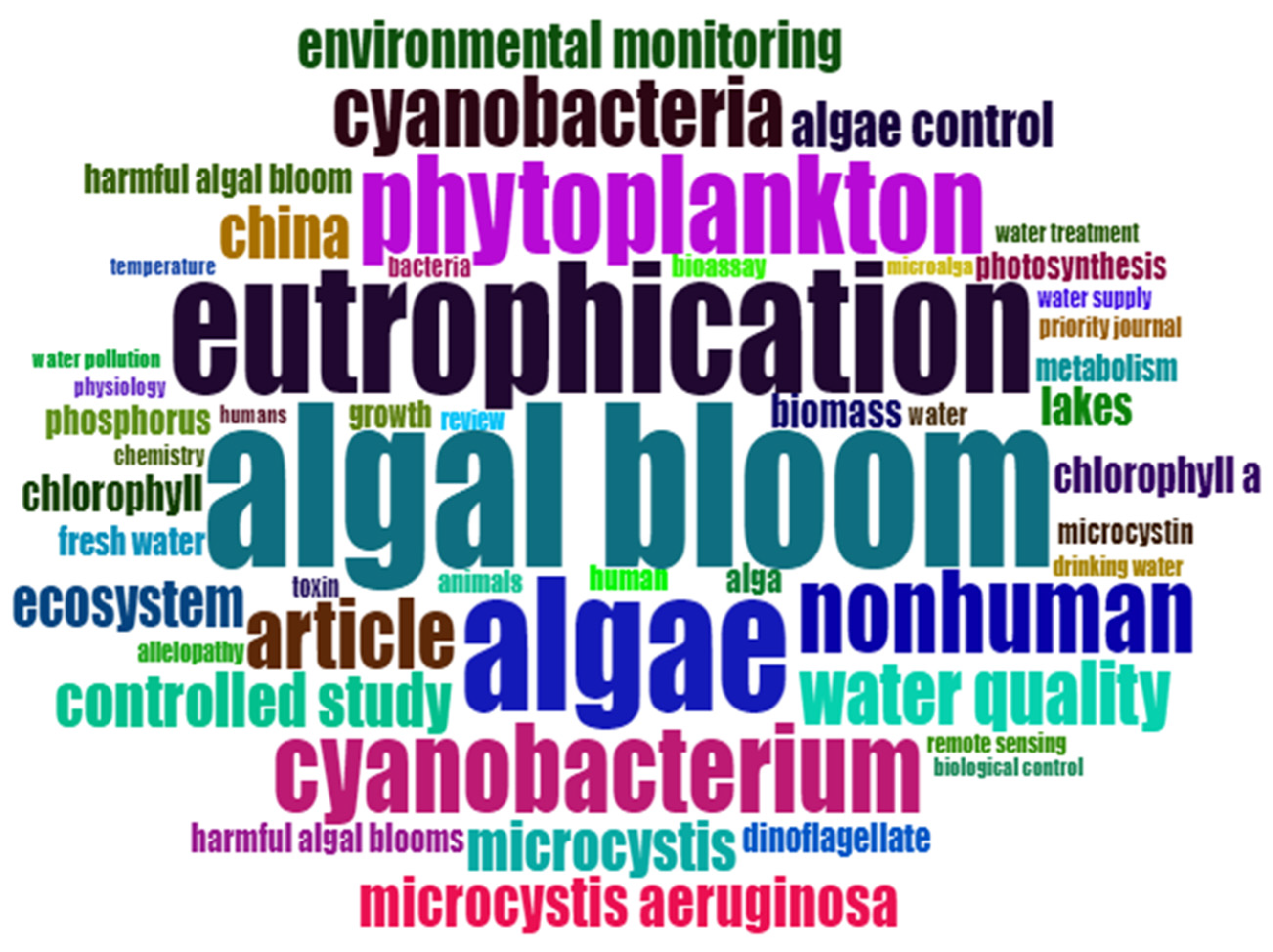
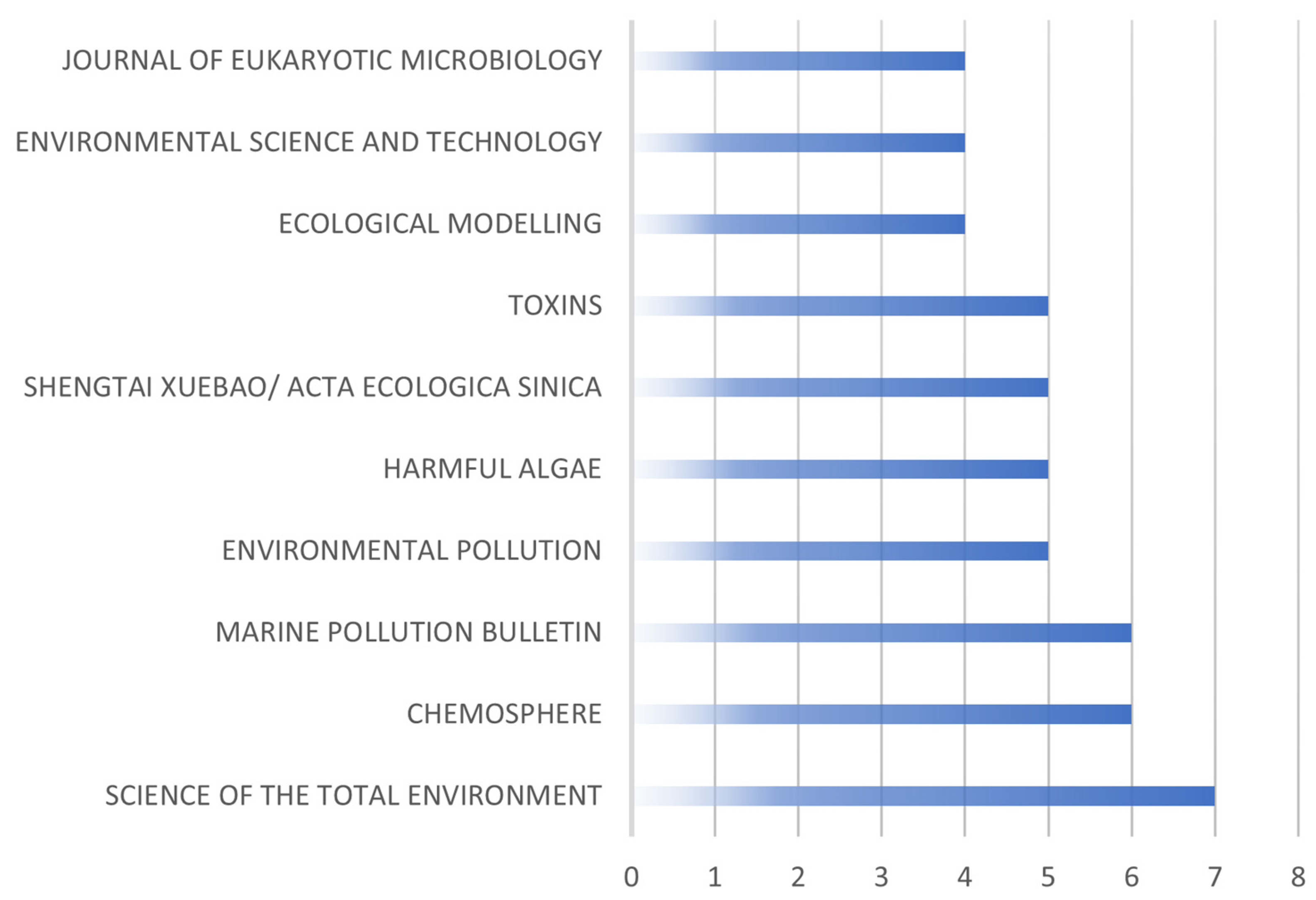
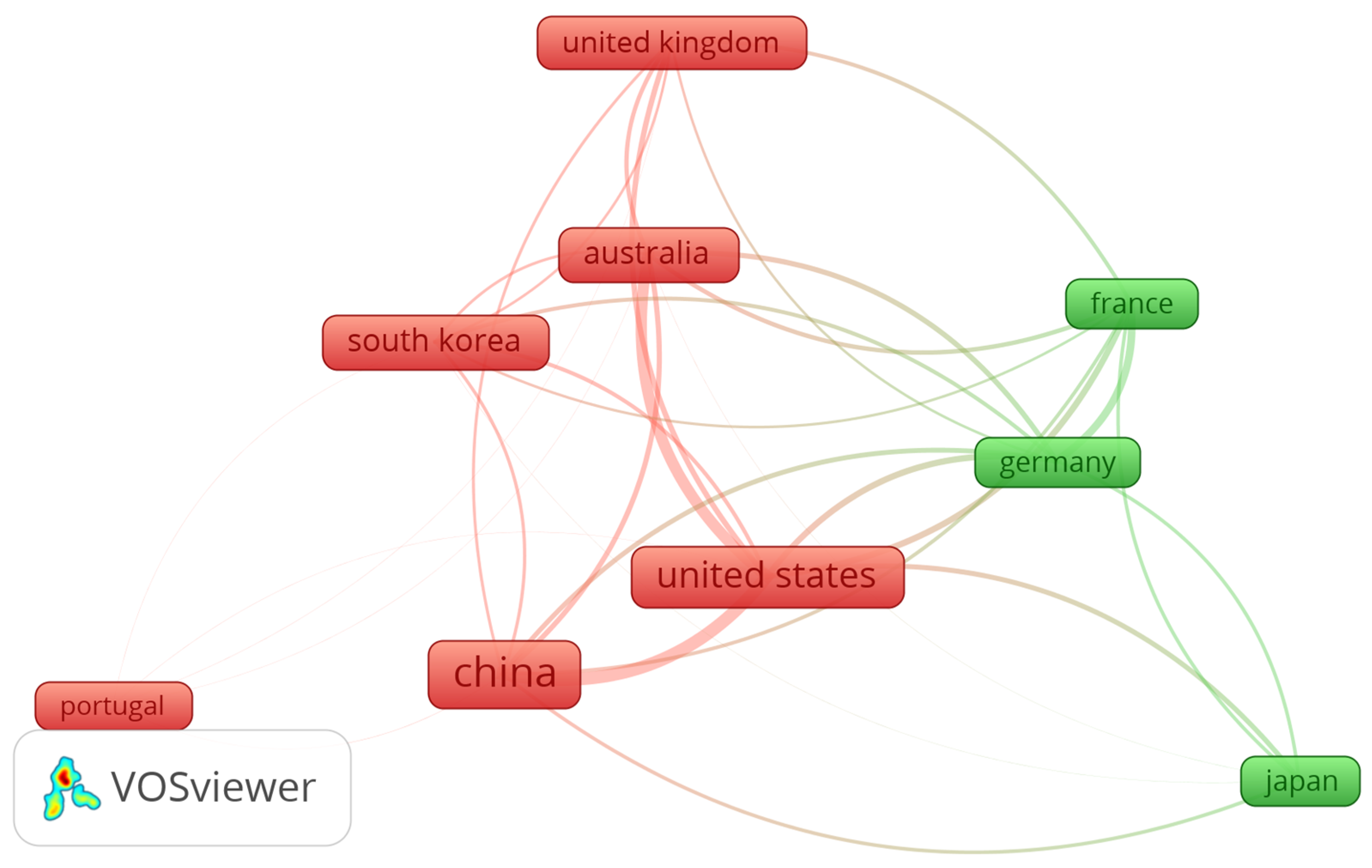
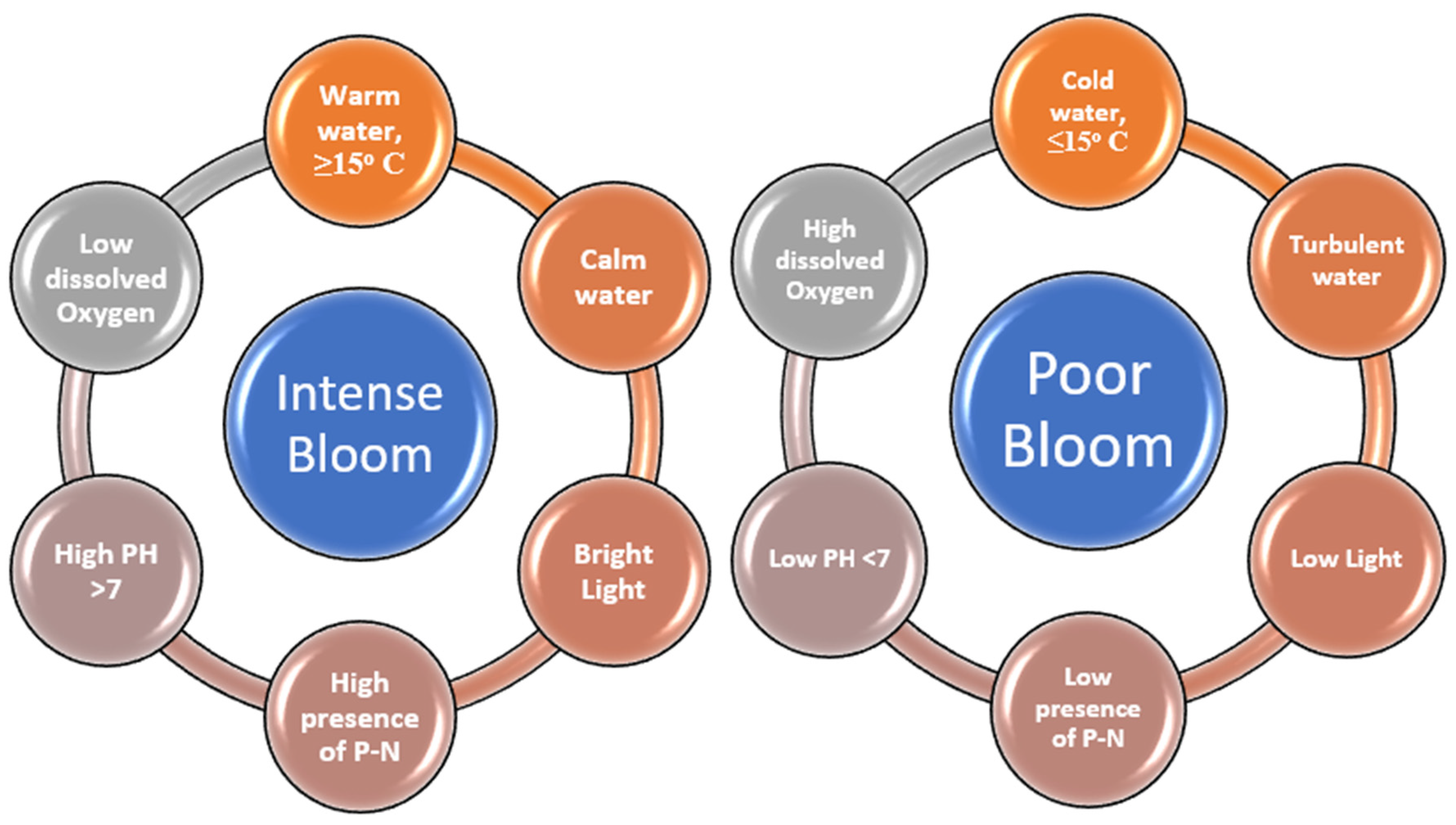

| Factors | Chemical | Physical | Biological |
|---|---|---|---|
| Action Mechanism | Utilizing metals like copper, iron, and aluminum disrupts algal cells and interferes with their processes. Employing herbicides (e.g., atrazine, simazine) and photosensitizers (e.g., hydrogen peroxide) against algal growth | Limiting essential nutrients like nitrogen and phosphorus for algal growth. Applying UV-C irradiation and methods like soil modification, hydrodynamic interventions, aeration, dredging, and filtration. | Employing microorganisms (e.g., macrophytes, microalgae, bacteria, viruses) and allelopathic chemicals. Using crop straw (e.g., rice, barley) for HABs control. |
| Challenges | Risk of environmental toxin release, leading to unintended ecological impacts. | High energy demands, impracticality in large-scale applications, and financial constraints. | Challenges due to complex ecological interactions and potential for unintended outcomes. |
| References | [50,51,52] | [25,39,43,53,54,55] | [43,55,56,57,58] |
| Type of Straw | Type of Action | Description | Reference |
|---|---|---|---|
| Barley Straw | Cyanobacteria Growth Inhibition | Barley straw is recognized for its ability to control cyanobacterial growth by releasing allelopathic substances during aerobic decomposition, which hinders cyanobacteria. | [40] |
| Rice Straw | Cyanobacteria Growth Inhibition | Extracts from rice straw significantly inhibit cyanobacteria (Anabaena sp.) growth, depending on extract concentration, while promoting microalgae growth (Chlorella sp.) in a concentration-dependent manner. | [57] |
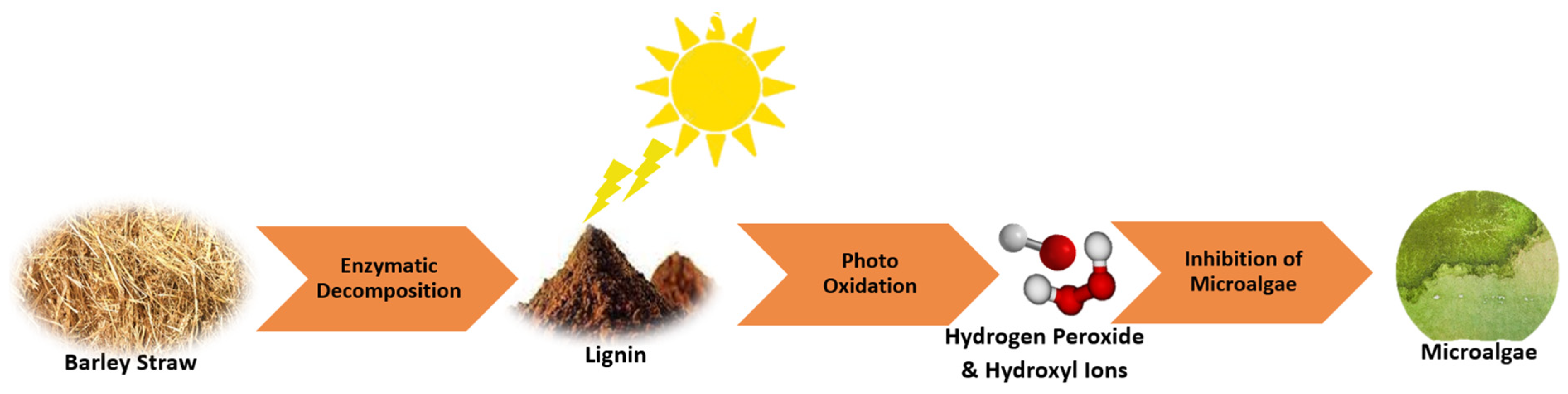
| Barley Straw | Hydrogen Peroxide Emission | The decomposition of barley straw may emit hydrogen peroxide (H2O2) through the photooxidation of lignin and quinone, though further study is needed to understand this mechanism. | [68] |
| Barley Straw | Oxygenation Necessity | Effective algae control using barley straw requires sufficient oxygenation, as oxygen consumption increases during the straw’s decomposition process in water. | [83] |
| Barley Straw | Release of Phenolic Compounds | Decomposing barley straws emit phenolic compounds, which are toxic to algae, supporting the theory that allelopathic interactions affect cyanobacterial populations. | [83] |
| Barley Straw | Algal Biomass Reduction | Deploying barley straws in reservoirs has been observed to significantly lower algal counts, likely due to the production of organic compounds with potential algal toxicity during decomposition. | [89] |
| Wheat Straw | Increase in Algal Biomass | Introducing chopped wheat straw unexpectedly increased HABs growth, with a notable rise in chlorophyll-a levels. | [91] |
| Rice Straw | Algae Growth Inhibition | Rice and ragi straws show inhibitory effects on algae due to the combined action of various compounds released during decomposition. | [92] |
| Bacteria | Type of Action | Description | References |
|---|---|---|---|
| Bacillus amyloliquefaciens | Bio-flocculation | Creates bioflocculants that help cluster and remove algal cells, playing a role in controlling HABs. | [37] |
| Streptomyces neyagawaensis | Algicidal | Generates allelopathic substances that suppress the growth of various algae, including harmful species, affecting specific species and strains. | [57] |
| Streptomyces sp. | Anti-algal | Produces metabolites that suppress algal growth, showing effectiveness against harmful algal blooms. | [94] |
| Myxobacteria | Algicidal | Eliminates algae through direct contact, effectively removing them through close interaction. | [101] |
| Streptococcus thermophilus | Algicidal | Demonstrates chemotaxis towards algal cells and produces enzymes that break down algal cell walls, leading to cell destruction. | [102] |
| Pseudomonas aeruginosa | Algicidal | EIt emits compounds that directly disrupt algal cells, ending harmful algal blooms. | [103] |
| Bacillus Fluxus | Algicidal | Releases harmine and norharmane, which are highly toxic to cyanobacteria and help inhibit the growth of HABs. | [110] |
| Citrobacter sp. AzoR-1 | Algicidal | PIt produces a bio-flucculant that clusters M. aeruginosa cells via interparticle bridging and biosorption, aiding HABs control. | [111] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anabtawi, H.M.; Lee, W.H.; Al-Anazi, A.; Mohamed, M.M.; Aly Hassan, A. Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water 2024, 16, 224. https://doi.org/10.3390/w16020224
Anabtawi HM, Lee WH, Al-Anazi A, Mohamed MM, Aly Hassan A. Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water. 2024; 16(2):224. https://doi.org/10.3390/w16020224
Chicago/Turabian StyleAnabtawi, Hassan Mohamad, Woo Hyoung Lee, Abdulaziz Al-Anazi, Mohamed Mostafa Mohamed, and Ashraf Aly Hassan. 2024. "Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs)" Water 16, no. 2: 224. https://doi.org/10.3390/w16020224
APA StyleAnabtawi, H. M., Lee, W. H., Al-Anazi, A., Mohamed, M. M., & Aly Hassan, A. (2024). Advancements in Biological Strategies for Controlling Harmful Algal Blooms (HABs). Water, 16(2), 224. https://doi.org/10.3390/w16020224








