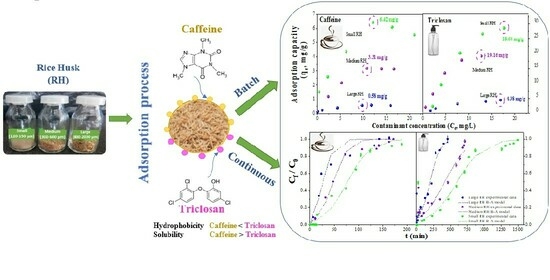
Efficient Decontamination: Caffeine/Triclosan Removal using Rice Husk in Batch and Fixed-Bed Columns
Abstract
1. Introduction
2. Materials and Methods
2.1. Rice Husk Material Conditioning
2.2. Experimental Model
2.2.1. Batch Adsorption Tests
2.2.2. Fixed-Bed Columns
2.3. Analytical/Instrumental Methods
2.3.1. Material Characterization
2.3.2. Caffeine/Triclosan Quantification before/after the Adsorption Tests
2.4. Data Analysis
2.4.1. Isotherm and Kinetic Models
2.4.2. Breakthrough Curve
2.5. Statistical Analysis
3. Results and Discussion
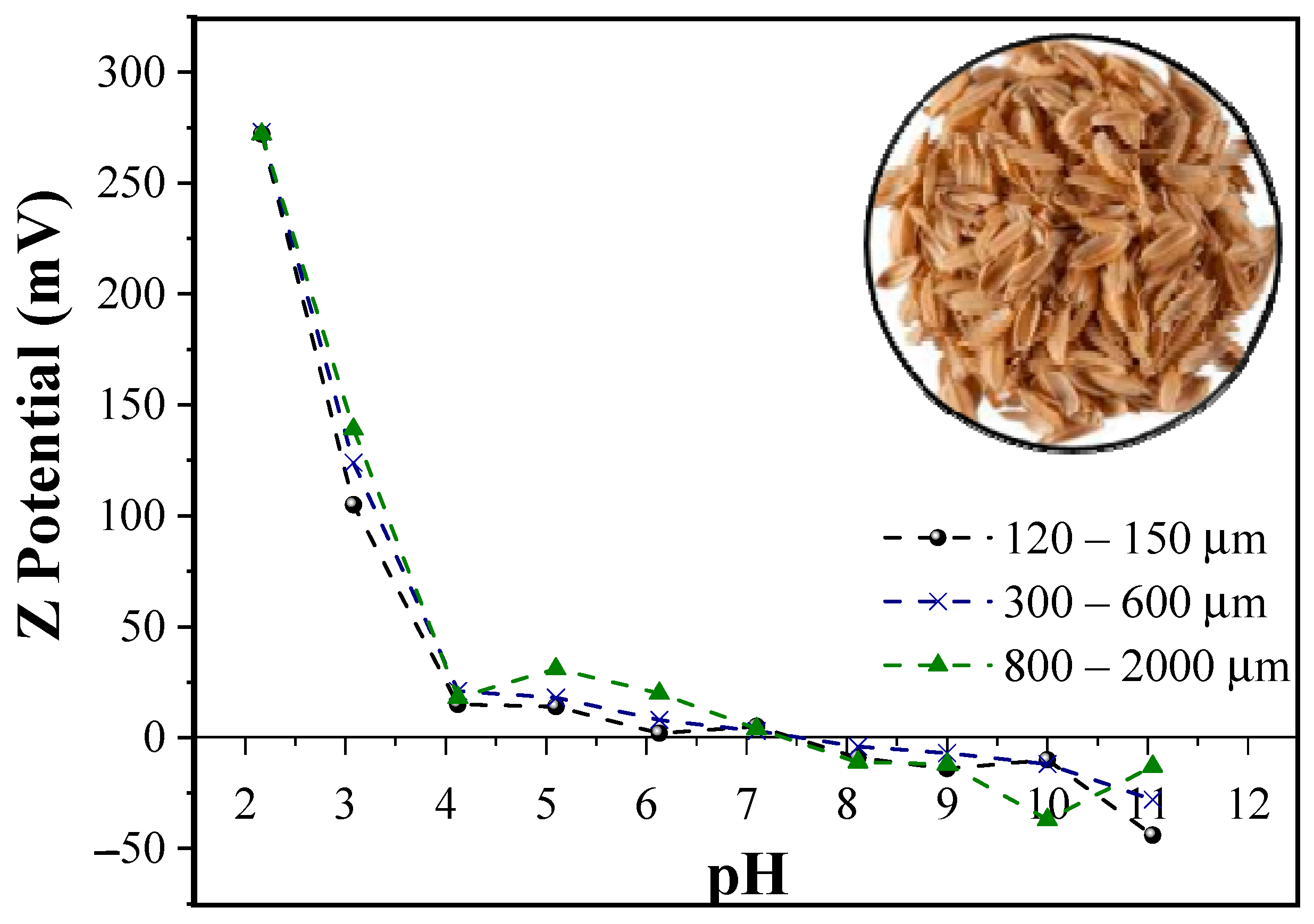
3.1. Point of Zero Charge
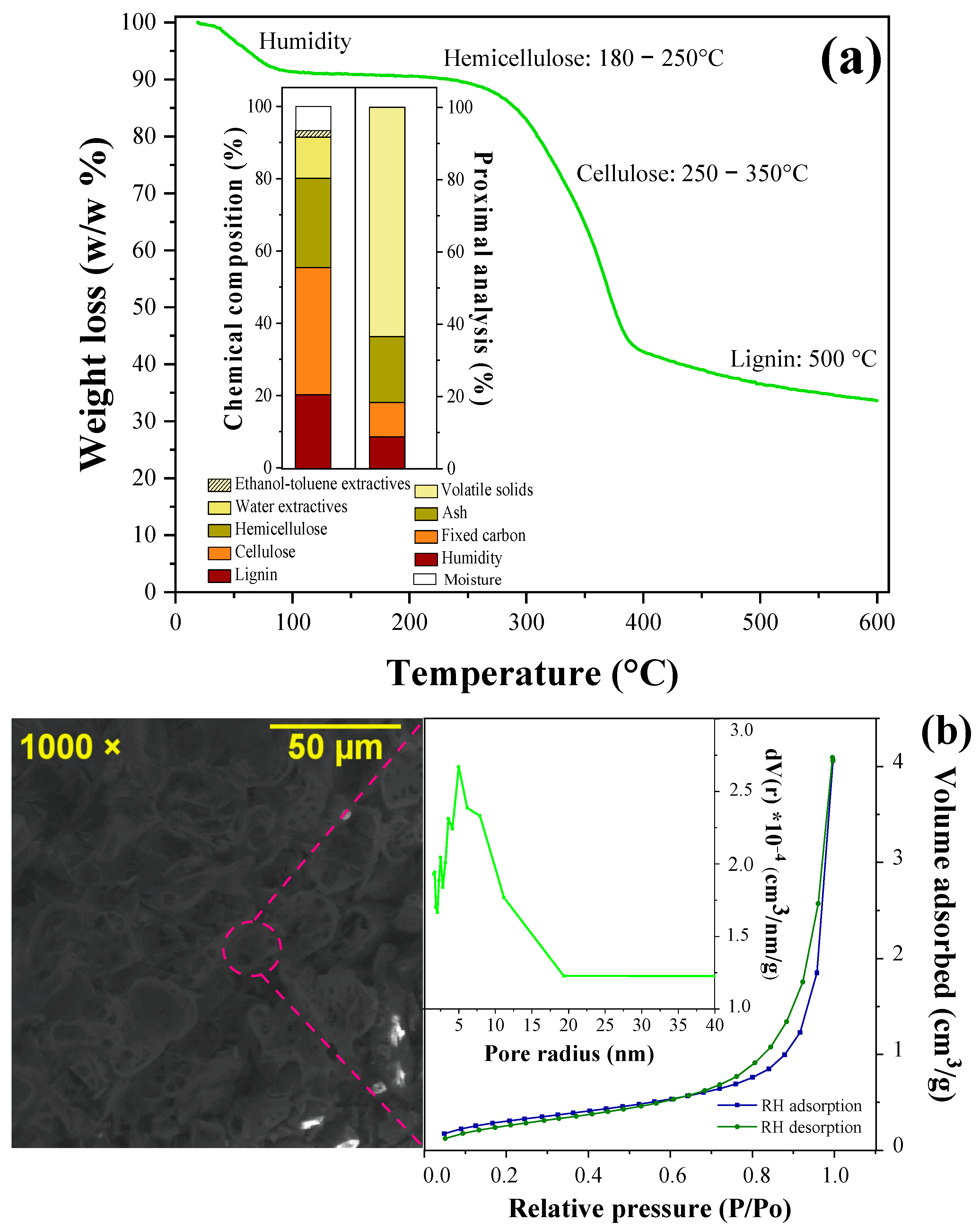
3.2. Physical–Chemical Characterization and TGA Analysis
3.3. Morphological Characterization of RH
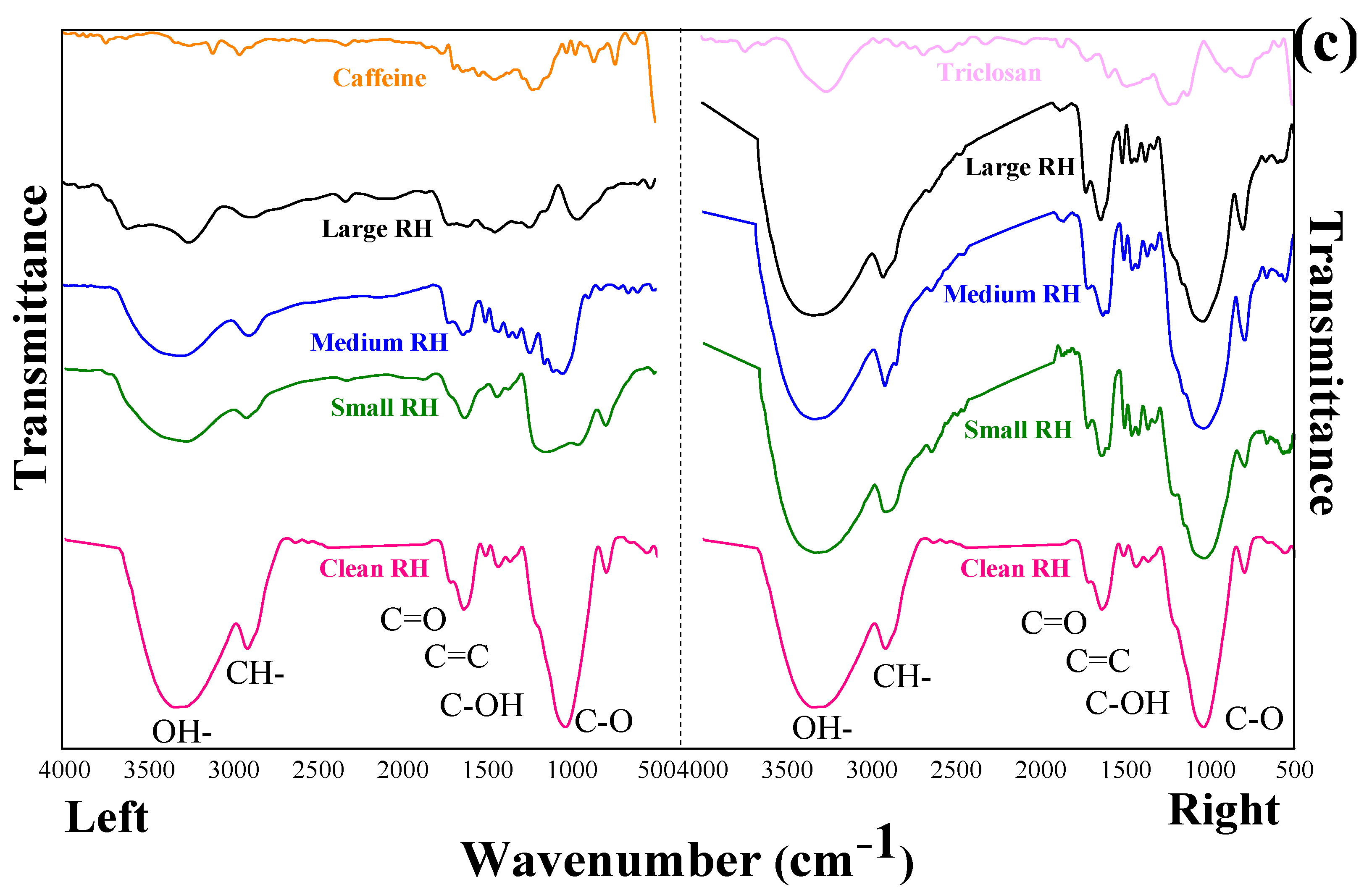
3.4. FTIR Spectroscopy Analysis
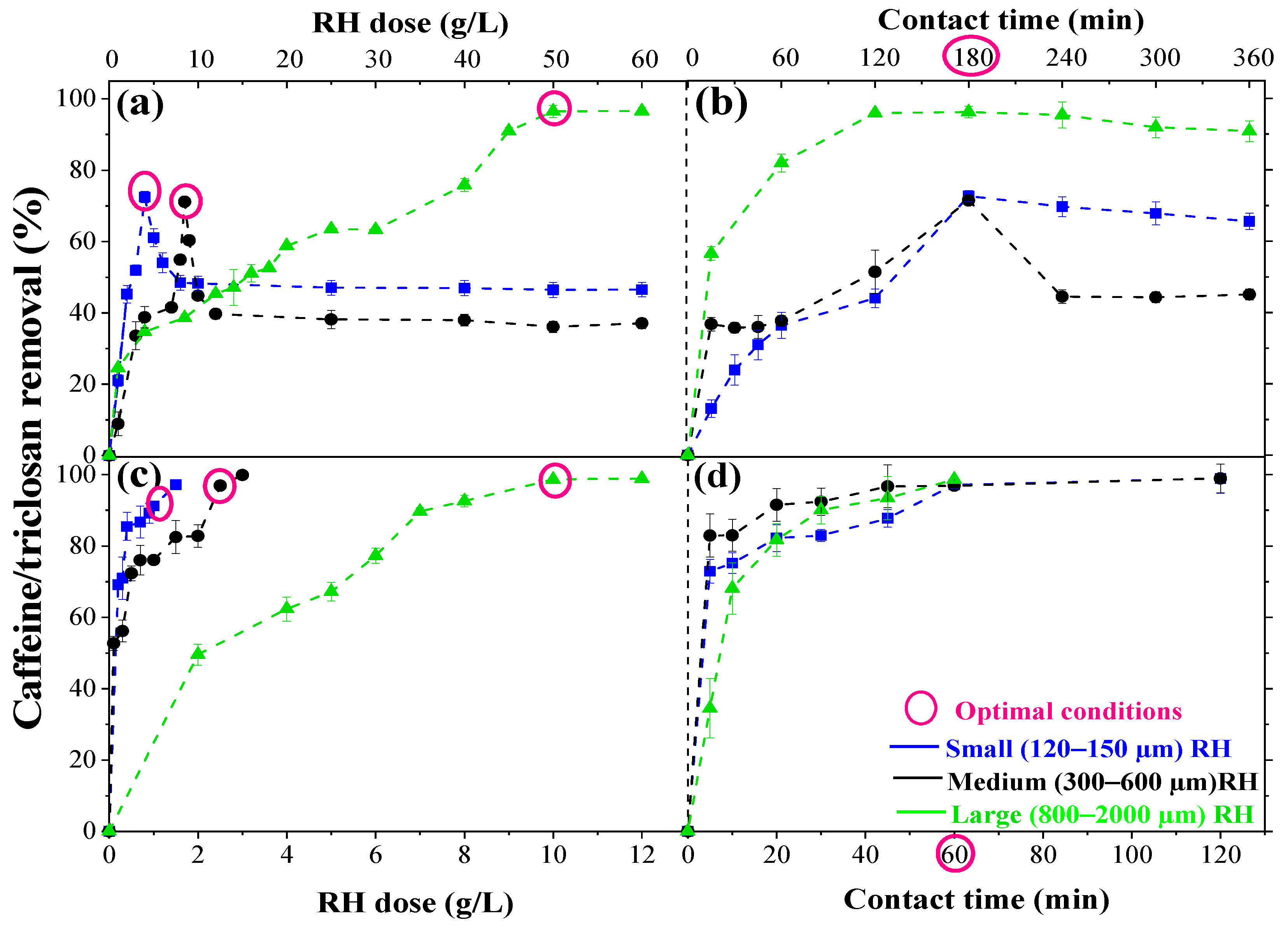
3.5. Optimal Adsorption Parameters
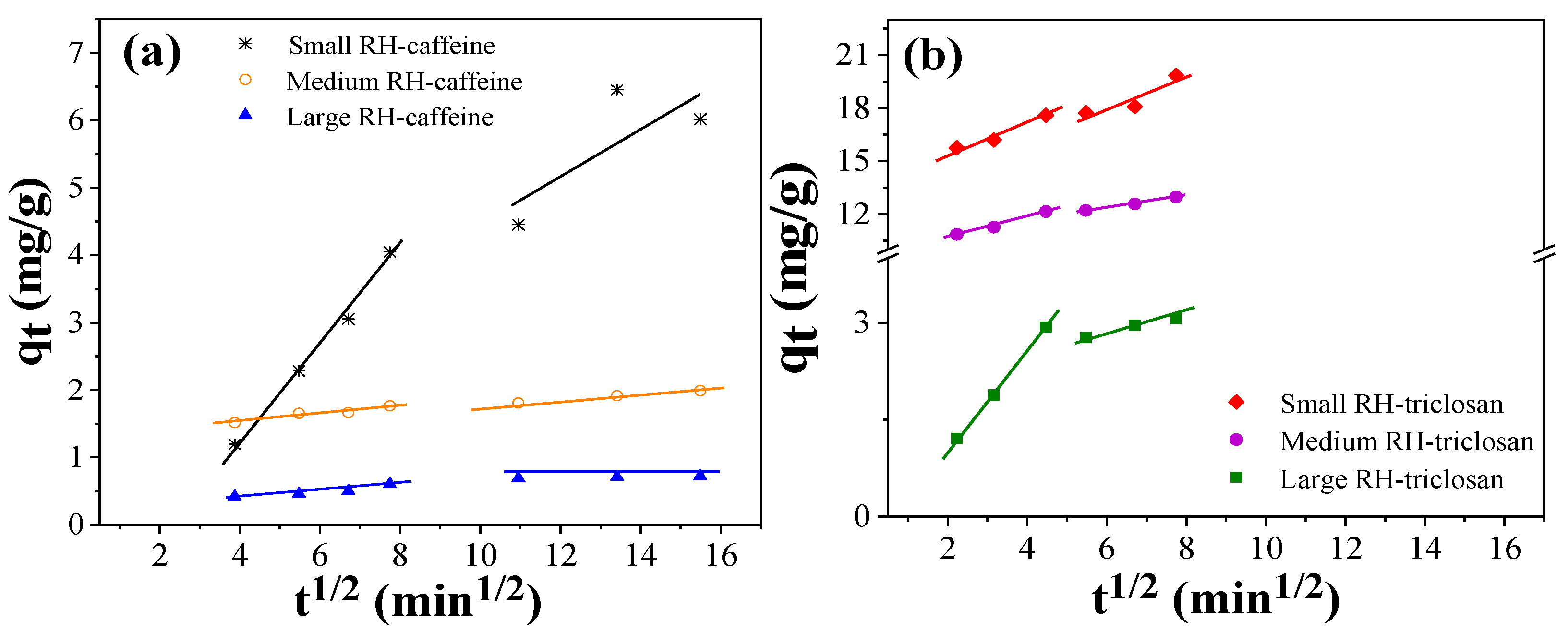
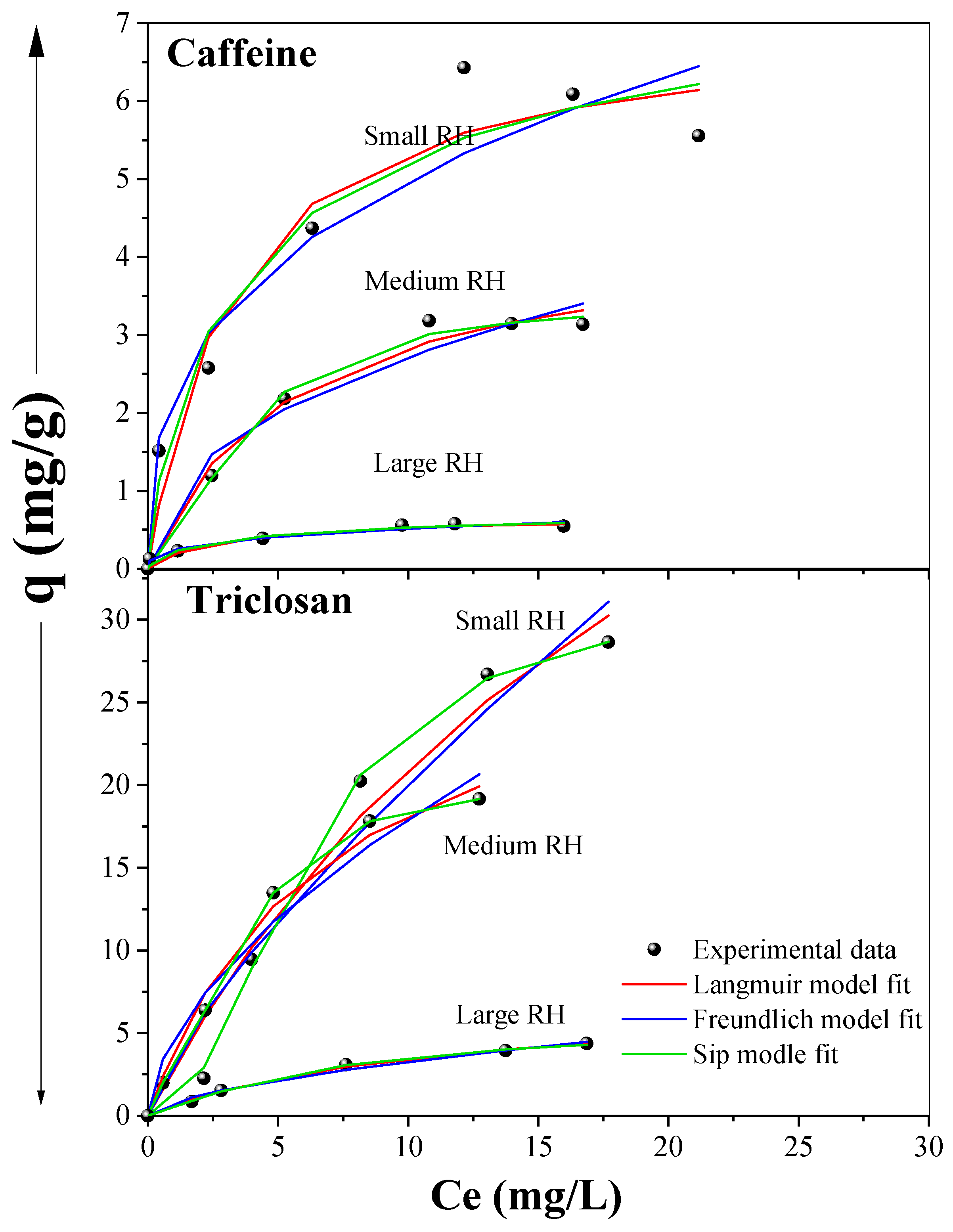
3.6. Adsorption Isotherms and Kinetics
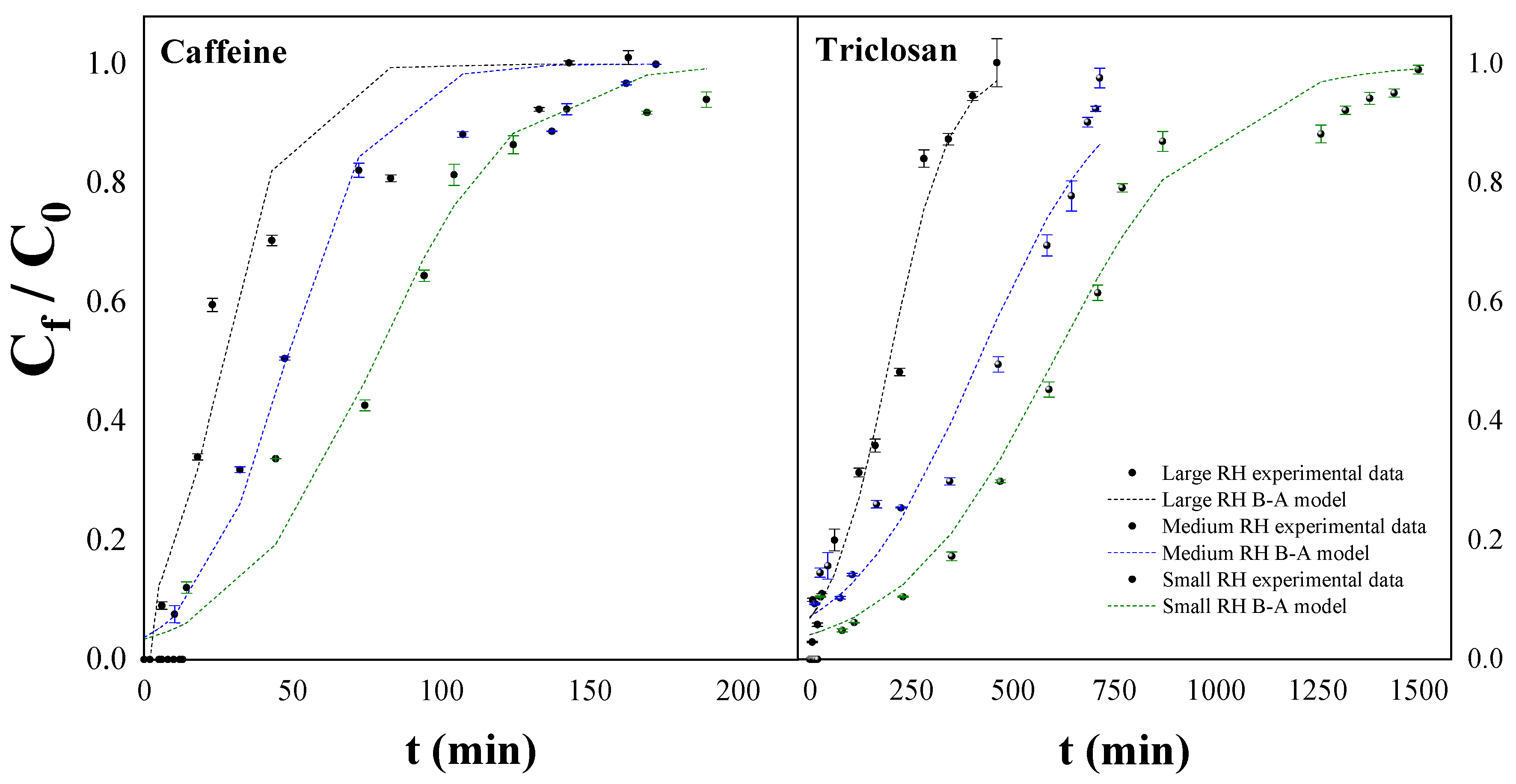
3.7. Fixed-Bed Columns
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Almeida-Naranjo, C.E.; Guerrero, V.H.; Villamar-Ayala, C.A. Emerging Contaminants and Their Removal from Aqueous Media Using Conventional/Non-Conventional Adsorbents: A Glance at the Relationship between Materials, Processes and Technologies. Water 2023, 15, 1626. [Google Scholar] [CrossRef]
- Chen, Z.; Guo, J.; Jiang, Y.; Shao, Y. High Concentration and High Dose of Disinfectants and Antibiotics Used during the COVID-19 Pandemic Threaten Human Health. Environ. Sci. Eur. 2021, 33, 11. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Taoufik, N.; García, A.M.; Korili, S.A. Comparative Removal of Emerging Contaminants from Aqueous Solution by Adsorption on an Activated Carbon. Environ. Technol. 2018, 40, 3017–3030. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Wang, X.; Yin, F.; Xu, G. Characterizing the Removal Routes of Seven Pharmaceuticals in the Activated Sludge Process. Sci. Total Environ. 2019, 650, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Rigueto, C.V.T.; Nazari, M.T.; De Souza, C.F.; Cadore, J.S.; Brião, V.B.; Piccin, J.S. Alternative Techniques for Caffeine Removal from Wastewater: An Overview of Opportunities and Challenges. J. Water Process Eng. 2020, 35, 101231. [Google Scholar] [CrossRef]
- Rodríguez-Gil, J.L.; Cáceres, N.; Dafouz, R.; Valcárcel, Y. Caffeine and Paraxanthine in Aquatic Systems: Global Exposure Distributions and Probabilistic Risk Assessment. Sci. Total Environ. 2018, 612, 1058–1071. [Google Scholar] [CrossRef]
- Dafouz, R.; Cáceres, N.; Rodríguez-gil, J.L.; Mastroianni, N.; López, M.; Alda, D.; Barceló, D.; Gil, Á.; Miguel, D.; Valcárcel, Y. Science of the Total Environment Does the Presence of Caffeine in the Marine Environment Represent an Environmental Risk? A Regional and Global Study. Sci. Total Environ. 2018, 615, 632–642. [Google Scholar] [CrossRef]
- Triwiswara, M.; Kang, J.K.; Moon, J.K.; Lee, C.G.; Park, S.J. Removal of Triclosan from Aqueous Solution Using Thermally Treated Rice Husks. Desalination Water Treat. 2020, 202, 317–326. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Li, Z.; Yang, L.; Liu, X. Effects of Particle Size and Solution Chemistry on Triclosan Sorption on Polystyrene Microplastic. Chemosphere 2019, 231, 308–314. [Google Scholar] [CrossRef]
- Kaur, H.; Hippargi, G.; Pophali, G.R.; Bansiwal, A. Biomimetic Lipophilic Activated Carbon for Enhanced Removal of Triclosan from Water. J. Colloid. Interface Sci. 2019, 535, 111–121. [Google Scholar] [CrossRef]
- Luo, Z.; He, Y.; Zhi, D.; Luo, L.; Sun, Y.; Khan, E.; Wang, L.; Peng, Y.; Zhou, Y.; Tsang, D.C.W. Current Progress in Treatment Techniques of Triclosan from Wastewater: A Review. Sci. Total Environ. 2019, 696, 133990. [Google Scholar] [CrossRef]
- Chaukura, N.; Gwenzi, W.; Tavengwa, N.; Manyuchi, M.M. Biosorbents for the Removal of Synthetic Organics and Emerging Pollutants: Opportunities and Challenges for Developing Countries. Environ. Dev. 2016, 19, 84–89. [Google Scholar] [CrossRef]
- Santaeufemia, S.; Abalde, J.; Torres, E. Eco-Friendly Rapid Removal of Triclosan from Seawater Using Biomass of a Microalgal Species: Kinetic and Equilibrium Studies. J. Hazard. Mater. 2019, 369, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Ijanu, E.M.; Kamaruddin, M.A.; Norashiddin, F.A. Coffee Processing Wastewater Treatment: A Critical Review on Current Treatment Technologies with a Proposed Alternative. Appl. Water Sci. 2020, 10, 1–11. [Google Scholar] [CrossRef]
- Parida, V.K.; Saidulu, D.; Majumder, A.; Srivastava, A.; Gupta, B.; Gupta, A.K. Emerging Contaminants in Wastewater: A Critical Review on Occurrence, Existing Legislations, Risk Assessment, and Sustainable Treatment Alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Mushtaq, S.; Aslam, Z.; Ali, R.; Aslam, U.; Naseem, S.; Ashraf, M.; Bello, M.M. Surfactant Modified Waste Ash for the Removal of Chloro and Nitro Group Substituted Benzene from Wastewater. Water Sci. Technol. 2022, 86, 1969–1980. [Google Scholar] [CrossRef]
- Zahir, A.; Aslam, Z.; Aslam, U.; Abdullah, A.; Ali, R.; Bello, M.M. Paspalum Notatum Grass-Waste-Based Adsorbent for Rhodamine B Removal from Polluted Water. Chem. Biochem. Eng. Q. 2020, 34, 93–104. [Google Scholar] [CrossRef]
- Barka, N.; Abdennouri, M.; El, M.; Qourzal, S. Biosorption Characteristics of Cadmium and Lead onto Eco-Friendly Dried Cactus (Opuntia Ficus Indica) Cladodes. J. Environ. Chem. Eng. 2013, 1, 144–149. [Google Scholar] [CrossRef]
- Shamsollahi, Z.; Partovinia, A. Recent Advances on Pollutants Removal by Rice Husk as a Bio-Based Adsorbent: A Critical Review. J. Environ. Manag. 2019, 246, 314–323. [Google Scholar] [CrossRef]
- Aslam, U.; Ramzan, N.; Aslam, Z.; Iqbal, T.; Sharif, S.; Hasan, S.W.U.; Malik, A. Enhancement of Fuel Characteristics of Rice Husk via Torrefaction Process. Waste Manag. Res. 2019, 37, 737–745. [Google Scholar] [CrossRef]
- Khoriha, N.; Mohd, E.; Hadibarata, T. Triclosan Removal by Adsorption Using Activated Carbon Derived from Waste Biomass : Isotherms and Kinetic Studies. J. Chem. Soc. Dalton Trans. 2018, 65, 951–959. [Google Scholar] [CrossRef]
- Portinho, R.; Zanella, O.; Féris, L.A. Grape Stalk Application for Caffeine Removal through Adsorption. J. Environ. Manag. 2017, 202, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Balarak, D.; Mostafapour, F.K.; Lee, S.M.; Jeon, C. Adsorption of Bisphenol A Using Dried Rice Husk: Equilibrium, Kinetic and Thermodynamic Studies. Appl. Chem. Eng. 2019, 30, 316–323. [Google Scholar]
- N’diaye, A.D.; Kankou, M.S. Sorption of Aspirin from Aqueous Solutions Using Rice Husk as Low Cost Sorbent. J. Environ. Treat. 2020, 8, 1–5. [Google Scholar]
- Triwiswara, M.; Lee, C.G.; Moon, J.K.; Park, S.J. Adsorption of Triclosan from Aqueous Solution onto Char Derived from Palm Kernel Shell. Desalin. Water Treat. 2020, 177, 71–79. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Pashalidis, I.; Orfanos, A.G.; Manariotis, I.D.; Tatarchuk, T.; Sellaoui, L.; Bonilla-Petriciolet, A.; Mittal, A.; Núñez-Delgado, A. Removal of Caffeine, Nicotine and Amoxicillin from (Waste)Waters by Various Adsorbents. A Review. J. Environ. Manag. 2020, 261, 110236. [Google Scholar] [CrossRef]
- Panneerselvam, P.; Morad, N.; Tan, K.A. Magnetic Nanoparticle (F3O4) Impregnated onto Tea Waste for the Removal of Nickel(II) from Aqueous Solution. J. Hazard. Mater. 2011, 186, 160–168. [Google Scholar] [CrossRef]
- Quan, B.; Li, X.; Zhang, H.; Zhang, C.; Ming, Y.; Huang, Y.; Xi, Y.; Weihua, X.; Yunguo, L.; Tang, Y. Technology and Principle of Removing Triclosan from Aqueous Media: A Review. Chem. Eng. J. 2019, 378, 122185. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile Dye Wastewater Characteristics and Constituents of Synthetic Effluents: A Critical Review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Sandoval, R.; Cooper, A.M.; Aymar, K.; Jain, A.; Hristovski, K. Removal of Arsenic and Methylene Blue from Water by Granular Activated Carbon Media Impregnated with Zirconium Dioxide Nanoparticles. J. Hazard. Mater. 2011, 193, 296–303. [Google Scholar] [CrossRef]
- ASTM D4442-2020; Standard Test Methods for Direct Moisture Content Measurement of Wood and Wood-Based Materials. American Society for Testing Materials: West Conshohocken, PA, USA, 2020.
- ASTM D 1107-2021; Standard Test Method for Ethanol-Toluene Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D1110-21; Standard Test Methods for Water Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D 1106-2021; Standard Test Method for Acid-Insoluble Lignin in Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM-D1109–84(2021); Standard Test Method for 1% Sodium Hydroxide Solubility of Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM D1102-84(2021); Standard Test Method for Ash in Wood. American Society for Testing Materials: West Conshohocken, PA, USA, 2021.
- ASTM E 872-19; Standard Test Method for Volatile Matter in the Analysis of Particulate Wood Fuels. American Society for Testing Materials: West Conshohocken, PA, USA, 2019.
- Almeida-Naranjo, C.E.; Aldás, M.B.; Cabrera, G.; Guerrero, V.H. Caffeine Removal from Synthetic Wastewater Using Magnetic Fruit Peel Composites: Material Characterization, Isotherm and Kinetic Studies. Environ. Chall. 2021, 5, 100343. [Google Scholar] [CrossRef]
- Peñafiel, M.E.; Matesanz, J.M.; Vanegas, E.; Bermejo, D.; Mosteo, R.; Ormad, M.P. Comparative Adsorption of Ciprofloxacin on Sugarcane Bagasse from Ecuador and on Commercial Powdered Activated Carbon. Sci. Total Environ. 2021, 750, 141498. [Google Scholar] [CrossRef] [PubMed]
- Sotelo, J.L.; Rodríguez, A.; Álvarez, S.; García, J. Removal of Caffeine and Diclofenac on Activated Carbon in Fixed Bed Column. Chem. Eng. Res. Des. 2012, 90, 967–974. [Google Scholar] [CrossRef]
- Fakioğlu, M.; Kalpaklı, Y. Mechanism and Behavior of Caffeine Sorption: Affecting Factors. RSC Adv. 2022, 12, 26504–26513. [Google Scholar] [CrossRef]
- Ali, I.; Imanova, G.T.; Albishri, H.M.; Alshitari, W.H.; Locatelli, M.; Siddiqui, M.N.; Hameed, A.M. An Ionic-Liquid-Imprinted Nanocomposite Adsorbent: Simulation, Kinetics and Thermodynamic Studies of Triclosan Endocrine Disturbing Water Contaminant Removal. Molecules 2022, 27, 5358. [Google Scholar] [CrossRef]
- Cho, E.J.; Kang, J.K.; Moon, J.K.; Um, B.H.; Lee, C.G.; Jeong, S.; Park, S.J. Removal of Triclosan from Aqueous Solution via Adsorption by Kenaf-derived Biochar: Its Adsorption Mechanism Study via Spectroscopic and Experimental Approaches. J. Environ. Chem. Eng. 2021, 9, 106343. [Google Scholar] [CrossRef]
- Degermenci, G.; Degermenci, N.; Ayvaoglu, V.; Durmaz, E.; Cakir, D.; Akan, E. Adsorption of Reactive Dyes on Lignocellulosic Waste; Characterization, Equilibrium, Kinetic and Thermodynamic Studies. J. Clean. Prod. 2019, 225, 1220–1229. [Google Scholar] [CrossRef]
- Chakraborty, S.; Chowdhury, S.; Das Saha, P. Adsorption of Crystal Violet from Aqueous Solution Onto. Carbohydr. Polym. 2011, 86, 1533–1541. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, P.; Kaur, K. Adsorptive Removal of Imazethapyr and Imazamox from Aqueous Solution Using Modified Rice Husk. J. Clean. Prod. 2020, 244, 118699. [Google Scholar] [CrossRef]
- Gajera, Z.R.; Verma, K.; Tekade, S.P.; Sawarkar, A.N. Kinetics of Co-Gasification of Rice Husk Biomass and High Sulphur Petroleum Coke with Oxygen as Gasifying Medium via TGA. Bioresour. Technol. Rep. 2020, 11, 100479. [Google Scholar] [CrossRef]
- Daffalla, S.B.; Mukhtar, H.; Shaharun, M.S. Preparation and Characterization of Rice Husk Adsorbents for Phenol Removal from Aqueous Systems. PLoS ONE 2020, 15, e0243540. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin, S.; Siddiqui, M.T.H.; Baloch, H.A.; Mubarak, N.M.; Griffin, G.; Madapusi, S.; Tanksale, A. Upgradation of Chemical, Fuel, Thermal, and Structural Properties of Rice Husk through Microwave-Assisted Hydrothermal Carbonization. Environ. Sci. Pollut. Res. 2018, 25, 17529–17539. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Li, X.; Zhang, J.; Hu, B.; Zhu, W.; Liang, W.; Sun, K. Full-Size Pore Structure Characterization of Deep-Buried Coals and Its Impact on Methane Adsorption Capacity: A Case Study of the Shihezi Formation Coals from the Panji Deep Area in Huainan Coalfield, Southern North China. J. Pet. Sci. Eng. 2019, 173, 975–989. [Google Scholar] [CrossRef]
- Ata, A.; Nalcaci, O.O.; Ovez, B. Macro Algae Gracilaria Verrucosa as a Biosorbent: A Study of Sorption Mechanisms. Algal Res. 2012, 1, 194–204. [Google Scholar] [CrossRef]
- Noreen, S.; Bhatti, H.N.; Nausheen, S.; Sadaf, S.; Ashfaq, M. Batch and Fixed Bed Adsorption Study for the Removal of Drimarine Black CL-B Dye from Aqueous Solution Using a Lignocellulosic Waste: A Cost Affective Adsorbent. Ind. Crops Prod. 2013, 50, 568–579. [Google Scholar] [CrossRef]
- Yao, N.; Li, C.; Yu, J.; Xu, Q.; Wei, S.; Tian, Z.; Yang, Z.; Yang, W.; Shen, J. Insight into Adsorption of Combined Antibiotic-Heavy Metal Contaminants on Graphene Oxide in Water. Sep. Purif. Technol. 2020, 236, 116278. [Google Scholar] [CrossRef]
- Montaseri, H.; Forbes, P.B.C. A Review of Monitoring Methods for Triclosan and Its Occurrence in Aquatic Environments. TrAC Trends Anal. Chem. 2016, 85, 221–231. [Google Scholar] [CrossRef]
- Yokoyama, J.T.C.; Cazetta, A.L.; Bedin, K.C.; Spessato, L.; Fonseca, J.M.; Carraro, P.S.; Ronix, A.; Silva, M.C.; Silva, T.L. Stevia Residue as New Precursor of CO2-Activated Carbon : Optimization of Preparation Condition and Adsorption Study of Triclosan. Ecotoxicol. Environ. Saf. 2019, 172, 403–410. [Google Scholar] [CrossRef]
- Shirvanimoghaddam, K.; Trojanowska, E. Sorption of Pharmaceuticals and Personal Care Products (PPCPs) onto a Sustainable Cotton Based Adsorbent. Sustain. Chem. Pharm. 2020, 18, 100324. [Google Scholar] [CrossRef]
- Anastopoulos, I.; Katsouromalli, A.; Pashalidis, I. Oxidized Biochar Obtained from Pine Needles as a Novel Adsorbent to Remove Caffeine from Aqueous Solutions. J. Mol. Liq. 2020, 304, 112661. [Google Scholar] [CrossRef]
- Tejedor, J.; Guerrero, V.H.; Vizuete, K.; Debut, A. Environmentally Friendly Synthesis of Silicon Dioxide Nanoparticles and Their Application for the Removal of Emerging Contaminants in Aqueous Media. J. Phys. Conf. Ser. 2022, 2238, 012005. [Google Scholar] [CrossRef]
- Keerthanan, S.; Rajapaksha, S.M.; Trakal, L.; Vithanage, M. Caffeine Removal by Gliricidia Sepium Biochar: Influence of Pyrolysis Temperature and Physicochemical Properties. Environ. Res. 2020, 189, 109865. [Google Scholar] [CrossRef] [PubMed]
- Keerthanan, S.; Bhatnagar, A.; Mahatantila, K.; Jayasinghe, C. Engineered Tea-Waste Biochar for the Removal of Caffeine, a Model Compound in Pharmaceuticals and Personal Care Products (PPCPs), from Aqueous Media. Environ. Technol. Innov. 2020, 19, 100847. [Google Scholar] [CrossRef]
- Ma, J.; Zhao, J.; Zhu, Z.; Li, L.; Yu, F. Effect of Microplastic Size on the Adsorption Behavior and Mechanism of Triclosan on Polyvinyl Chloride. Environ. Pollut. 2019, 254, 113104. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.N.; You, S.; Hosseini-bandegharaei, A.; Chao, H. Mistakes and Inconsistencies Regarding Adsorption of Contaminants from Aqueous Solutions: A Critical Review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Gin, K.Y.H. Occurrence and Removal of Pharmaceuticals, Hormones, Personal Care Products, and Endocrine Disrupters in a Full-Scale Water Reclamation Plant. Sci. Total Environ. 2017, 599–600, 1503–1516. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Van, H.T.; Chu, T.H.H.; Nguyen, T.H.V.; Nguyen, T.D.; Hoang, L.P.; Hoang, V.H. Paper Waste Sludge-Derived Hydrochar Modified by Iron (III) Chloride for Enhancement of Ammonium Adsorption: An Adsorption Mechanism Study. Environ. Technol. Innov. 2021, 21, 101223. [Google Scholar] [CrossRef]
- Castillo, N.E.T.; Sierra, J.S.O.; Oyervides-muñoz, M.A.; Sosa-hernández, J.E.; Iqbal, H.M.N.; Parra-saldívar, R.; Elda, M. Exploring the Potential of Coffee Husk as Caffeine Bio-Adsorbent—A Mini-Review. Case Stud. Chem. Environ. Eng. 2020, 3, 100070. [Google Scholar] [CrossRef]
- N’diaye, A.D.; Kankou, M.S. Modeling of Adsorption Isotherms of Caffeine onto Groundnut Shell as a Low Cost Adsorbent. J. Environ. Treat. Tech. 2020, 8, 1191–1195. [Google Scholar] [CrossRef]
- N’diaye, A.D.; Ahmed Kankou, M.S. Valorization of Balanites Aegyptiaca Seeds from Mauritania : Modeling of Adsorption Isotherms of Caffeine from Aqueous Solution. J. Environ. Treat. Tech. 2019, 7, 450–455. [Google Scholar]
- Raninga, M.; Mudgal, A.; Patel, V.K.; Patel, J.; Sinha, M.K. Modification of activated carbon-based adsorbent for removal of industrial dyes and heavy metals: A review. Mat. Today Proc. 2023, 77, 286–294. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M.; Ahmed, M.J.K.; Begum, S. Remediation of Eriochrome Black T-Contaminated Aqueous Solutions Utilizing H3PO4-Modified Berry Leaves as a Non-Conventional Adsorbent. Desal. Water Treat. 2015, 56, 1507–1519. [Google Scholar] [CrossRef]
- Sivarajasekar, N.; Mohanraj, N.; Baskar, R.; Sivamani, S. Fixed-Bed Adsorption of Ranitidine Hydrochloride Onto Microwave Assisted—Activated Aegle Marmelos Correa Fruit Shell: Statistical Optimization and Breakthrough Modelling. Arab. J. Sci. Eng. 2018, 43, 2205–2215. [Google Scholar] [CrossRef]
- Gupta, A.; Garg, A. Adsorption and Oxidation of Ciprofloxacin in a Fixed Bed Column Using Activated Sludge Derived Activated Carbon. J. Environ. Manag. 2019, 250, 109474. [Google Scholar] [CrossRef]
- Al-shawabkeh, A.F.; Omar, W.; Hasseine, A.; Al-amayreh, M. Experimental Study of the Application of Date Palm Trunk Fiber as Biosorbent for Removal Cadmium Using a Fixed Bed Column : Investigation of the Influence of Particle Size Experimental Study of the Application of Date Palm Trunk Fiber as Biosorbent for Re. Desal. Water Treat. 2021, 223, 328–334. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Hameed, B.H. Removal of Emerging Pharmaceutical Contaminants by Adsorption in a Fixed- Bed Column: A Review. Ecotoxicol. Environ. Saf. 2018, 149, 257–266. [Google Scholar] [CrossRef]
- de Franco, M.A.E.; de Carvalho, C.B.; Bonetto, M.M.; Soares, R.d.P.; Féris, L.A. Removal of Amoxicillin from Water by Adsorption onto Activated Carbon in Batch Process and Fixed Bed Column: Kinetics, Isotherms, Experimental Design and Breakthrough Curves Modelling. J. Clean. Prod. 2017, 161, 947–956. [Google Scholar] [CrossRef]


| Model | Parameter | Caffeine | Triclosan | |||||
|---|---|---|---|---|---|---|---|---|
| Small RH | Medium RH | Large RH | Small RH | Medium RH | Large RH | |||
| Kinetics | qe experimental [mg/g] | 5.242 | 1.787 | 0.648 | 18.311 | 12.466 | 2.865 | |
| Pseudo-first-order | qe [mg/g] | 6.329 | 1.824 | 0.695 | 18.057 | 12.314 | 3.038 | |
| k1 [min−1] | 0.015 | 0.107 | 0.039 | 0.375 | 0.397 | 0.105 | ||
| Pseudo-second-order | qe[mg/g] | 8.271 | 1.938 | 0.770 | 18.951 | 12.826 | 3.591 | |
| k2 [g/(mg min)] | 0.002 | 0.106 | 0.075 | 0.043 | 0.075 | 0.034 | ||
| Elovich | α [mg/(g min)] | 0.137 | 13.613 | 0.189 | 110.139 | 66.888 | 0.844 | |
| β [mg/g] | 0.420 | 0.622 | 7.891 | 0.682 | 1.194 | 1.285 | ||
| Intra particle diffusion | Kp1 [mg/(g min1/2)] | 0.721 | 0.060 | 0.046 | 0.840 | 0.581 | 0.773 | |
| C1 [mg/g] | −1.643 | 1.295 | 0.223 | 13.743 | 9.502 | −0.535 | ||
| R2 | 0.989 | 0.934 | 0.895 | 0.967 | 0.988 | 0.999 | ||
| SSE | 0.033 | 0.032 | 0.019 | 0.062 | 0.010 | 8.436 × 10−4 | ||
| Kp2 [mg/(g min1/2)] | 0.358 | 0.041 | 0.007 | 0.915 | 0.330 | 0.131 | ||
| C2 [mg/g] | 0.878 | 1.363 | 0.621 | 12.473 | 10.383 | 2.063 | ||
| R2 | 0.601 | 0.996 | 0.996 | 0.835 | 0.996 | 0.984 | ||
| SSE | 0.880 | 6.500 × 10−5 | 1.760 × 10−6 | 0.426 | 0.001 | 6.944 × 10−4 | ||
| Isotherms | Langmuir | qe experimental [mg/g] | 6.425 | 3.182 | 0.578 | 28.635 | 19.164 | 4.378 |
| qm [mg/g] | 7.083 | 4.433 | 0.668 | 70.772 | 30.613 | 7.012 | ||
| KL[L/mg] | 0.309 | 0.178 | 0.382 | 0.042 | 0.147 | 0.097 | ||
| R2 | 0.941 | 0.958 | 0.899 | 0.955 | 0.985 | 0.996 | ||
| χ2 | 0.363 | 0.032 | 0.007 | 0.257 | 0.054 | 0.003 | ||
| SSE | 1.816 | 0.129 | 0.018 | 22.995 | 3.216 | 0.036 | ||
| Freundlich | KF [(mg/g)1−1/n] | 2.264 | 0.991 | 0.249 | 3.419 | 4.723 | 0.819 | |
| 1/n | 0.343 | 0.438 | 0.316 | 0.768 | 0.580 | 0.601 | ||
| R2 | 0.925 | 0.956 | 0.956 | 0.932 | 0.952 | 0.980 | ||
| χ2 | 0.457 | 0.075 | 0.003 | 0.394 | 0.177 | 0.013 | ||
| SSE | 2.283 | 0.300 | 0.008 | 35.027 | 10.545 | 0.180 | ||
| Sips | qm[mg/g] | 8.147 | 3.450 | 0.810 | 31.400 | 22.579 | 5.747 | |
| KL [L/mg] | 0.218 | 0.276 | 0.248 | 0.165 | 0.264 | 0.148 | ||
| 1/n | 0.766 | 1.760 | 0.707 | 2.196 | 1.513 | 1.202 | ||
| R2 | 0.931 | 0.991 | 0.927 | 0.998 | 0.995 | 0.998 | ||
| χ2 | 0.423 | 0.016 | 0.013 | 0.011 | 0.018 | 0.001 | ||
| SSE | 1.694 | 0.048 | 0.005 | 0.943 | 1.062 | 0.019 | ||
| EC | Particle Size (µm) | Mass (g) | Vc (L) | EBTC (d) | FBU (%) | hMTZ (cm) | C/C0 = 0.1 | C/C0 = 0.9 | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| tb (min) | Vb (mL) | qb (mg/g) | ts (min) | qs (mg/g) | |||||||
| Caffeine | 120–150 | 1.741 | 0.003 | 0.436 | 91.04 | 0.36 | 14 | 7.15 | 0.12 | 169 | 0.13 |
| 300–600 | 1.689 | 88.07 | 0.47 | 10 | 5.15 | 0.10 | 142 | 0.12 | |||
| 800–2000 | 1.545 | 76.83 | 0.93 | 6 | 3.00 | 0.08 | 133 | 0.10 | |||
| Triclosan | 120–150 | 1.741 | 24.00 | 3.04 | 26 | 13.15 | 0.30 | 1259 | 1.26 | ||
| 300–600 | 1.489 | 16.62 | 3.34 | 12 | 6.15 | 0.17 | 684 | 1.01 | |||
| 800–2000 | 1.145 | 14.60 | 3.42 | 7 | 3.50 | 0.12 | 341 | 0.84 | |||
| EC | Particle Size (µm) | KAB [L/(minxmg)] | N0 (mg/L) | R2 | χ2 | SSE |
|---|---|---|---|---|---|---|
| Caffeine | 120–150 | 1.340 × 10−3 | 352.706 | 0.993 | 2.685 × 10−3 | 2.706 × 10−3 |
| 300–600 | 2.114 × 10−3 | 216.708 | 0.981 | 6.151 × 10−3 | 0.042 | |
| 800–2000 | 2.828 × 10−3 | 119.931 | 0.943 | 0.017 | 0.101 | |
| Triclosan | 120–150 | 1.172 × 10−4 | 3797.217 | 0.929 | 0.028 | 0.245 |
| 300–600 | 1.379 × 10−4 | 2628.774 | 0.910 | 0.032 | 0.210 | |
| 800–2000 | 2.962 × 10−4 | 1240.89 | 0.937 | 0.024 | 0.133 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida-Naranjo, C.E.; Cuestas, J.; Guerrero, V.H.; Villamar-Ayala, C.A. Efficient Decontamination: Caffeine/Triclosan Removal using Rice Husk in Batch and Fixed-Bed Columns. Water 2024, 16, 197. https://doi.org/10.3390/w16020197
Almeida-Naranjo CE, Cuestas J, Guerrero VH, Villamar-Ayala CA. Efficient Decontamination: Caffeine/Triclosan Removal using Rice Husk in Batch and Fixed-Bed Columns. Water. 2024; 16(2):197. https://doi.org/10.3390/w16020197
Chicago/Turabian StyleAlmeida-Naranjo, Cristina E., Jeniffer Cuestas, Victor H. Guerrero, and Cristina A. Villamar-Ayala. 2024. "Efficient Decontamination: Caffeine/Triclosan Removal using Rice Husk in Batch and Fixed-Bed Columns" Water 16, no. 2: 197. https://doi.org/10.3390/w16020197
APA StyleAlmeida-Naranjo, C. E., Cuestas, J., Guerrero, V. H., & Villamar-Ayala, C. A. (2024). Efficient Decontamination: Caffeine/Triclosan Removal using Rice Husk in Batch and Fixed-Bed Columns. Water, 16(2), 197. https://doi.org/10.3390/w16020197