Hydrochemical Characteristics and Formation Mechanism of Geothermal Fluids in Zuogong County, Southeastern Tibet
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
3.1. Hydrochemical Analysis
3.2. Hydrogeochemical Geothermometer
| Geothermometer | Calculation Formula | Reference |
|---|---|---|
| a. Chalcedony (no loss of steam) | Fournier (1977) | |
| b. Chalcedony (maximum steam loss) | Arnórsson et al. (1983) | |
| c. Quartz (no loss of steam) | Fournier (1977) | |
| d. Quartz (maximum steam loss) | Fournier (1977) | |
| e.Na-K | Fournier and Potter (1979) | |
| f.K-Mg | Giggenbach (1988) | |
| g.Na-K-Ca | β = 4/3(when t < 100 °C) or β = 1/3(when t > 100 °C) | Fournier and Truesdell (1973) |
| h.Na-Li | Kharaka et al. (1982) |
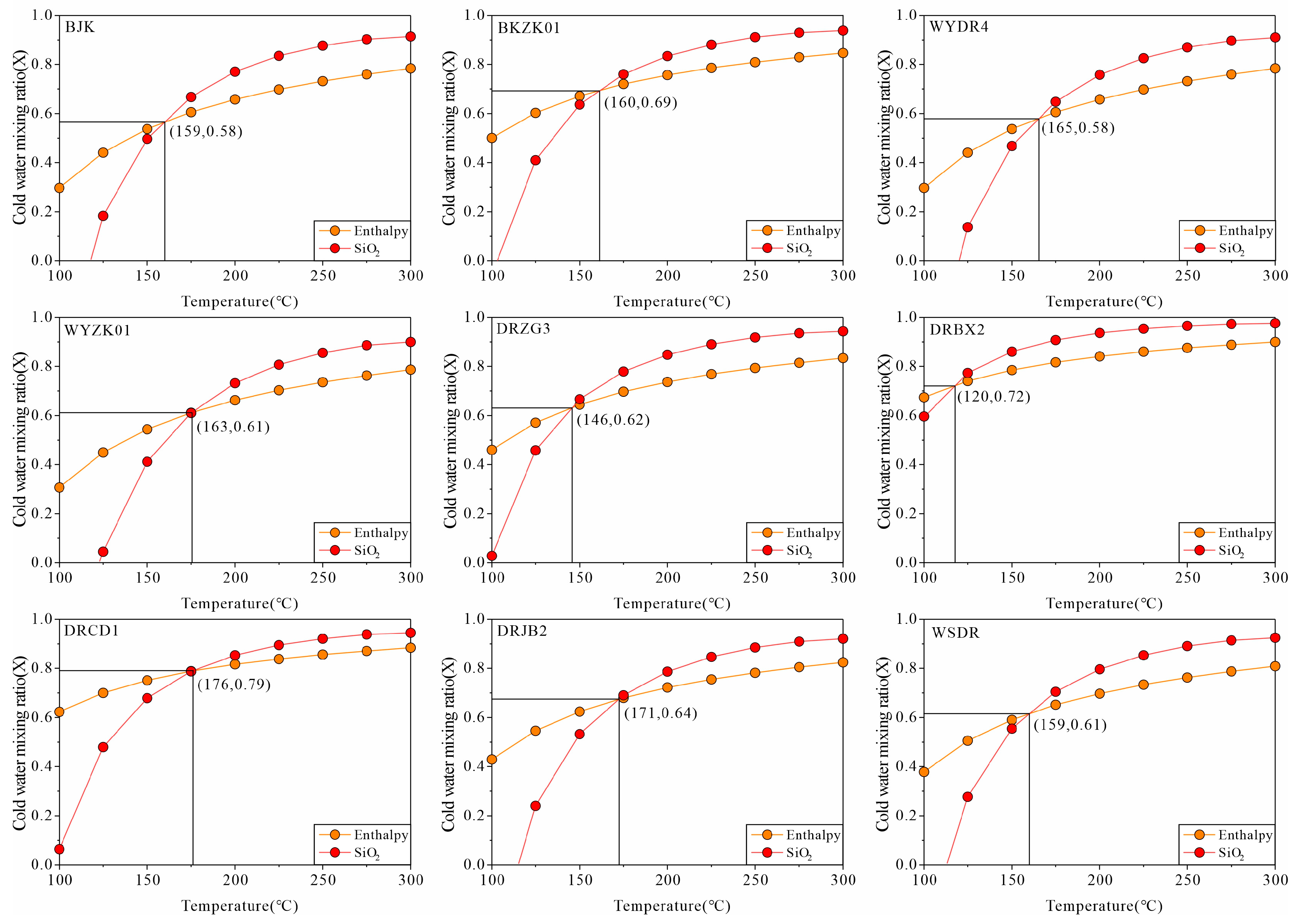
3.3. Hydrogeochemical Modeling
| Temperature (°C) | Enthalpy (J/g) | SiO2 (mg/L) | Temperature (°C) | Enthalpy (J/g) | SiO2 (mg/L) |
|---|---|---|---|---|---|
| 50 | 50.0 | 13.5 | 200 | 203.6 | 265.0 |
| 75 | 75.0 | 26.6 | 225 | 230.9 | 365.0 |
| 100 | 100.1 | 48.0 | 250 | 259.2 | 486.0 |
| 125 | 125.1 | 80.0 | 275 | 289.0 | 614.0 |
| 150 | 151.0 | 125.0 | 300 | 321 | 692 |
| 175 | 177.0 | 185.0 |
4. Results
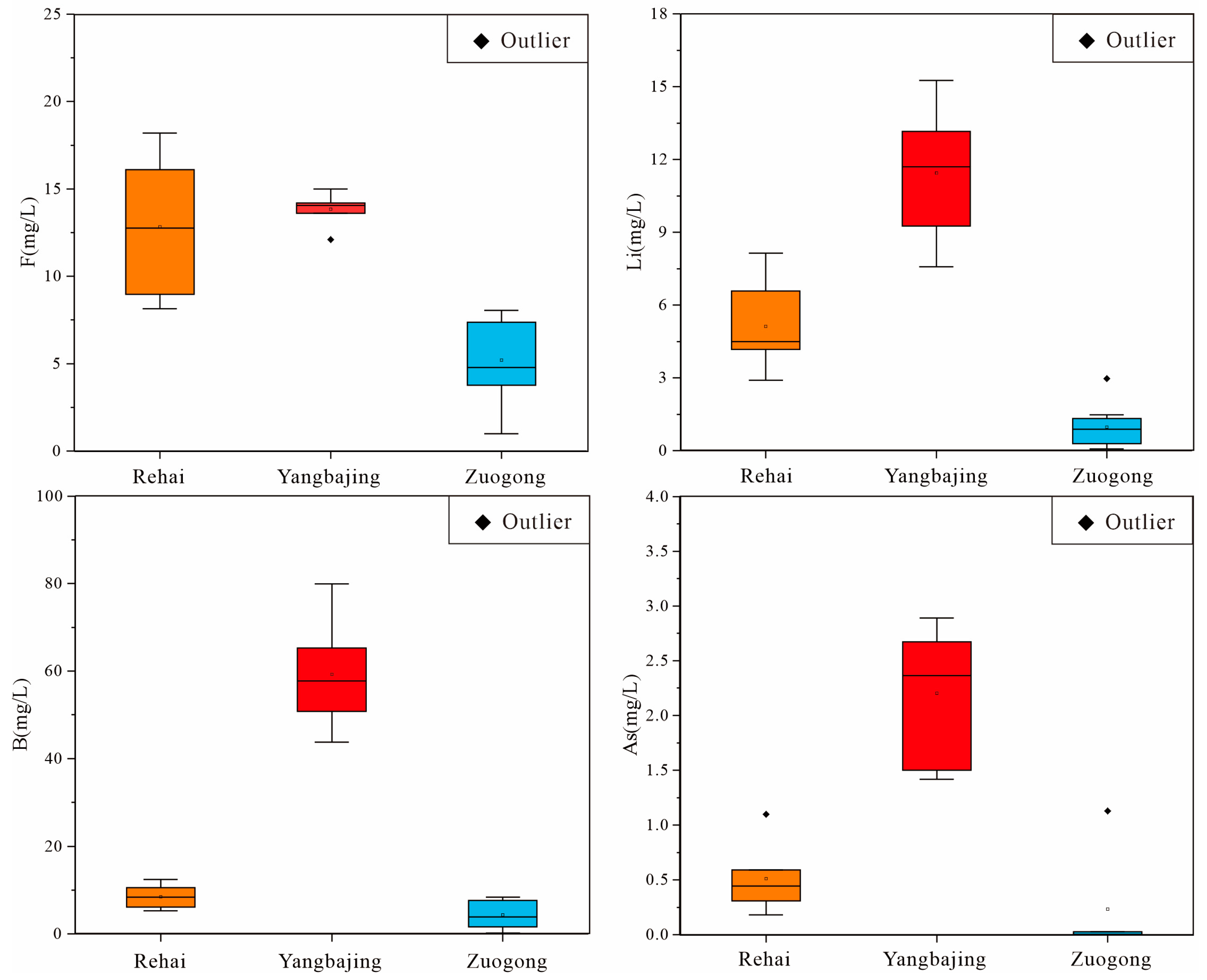
4.1. Water Chemistry
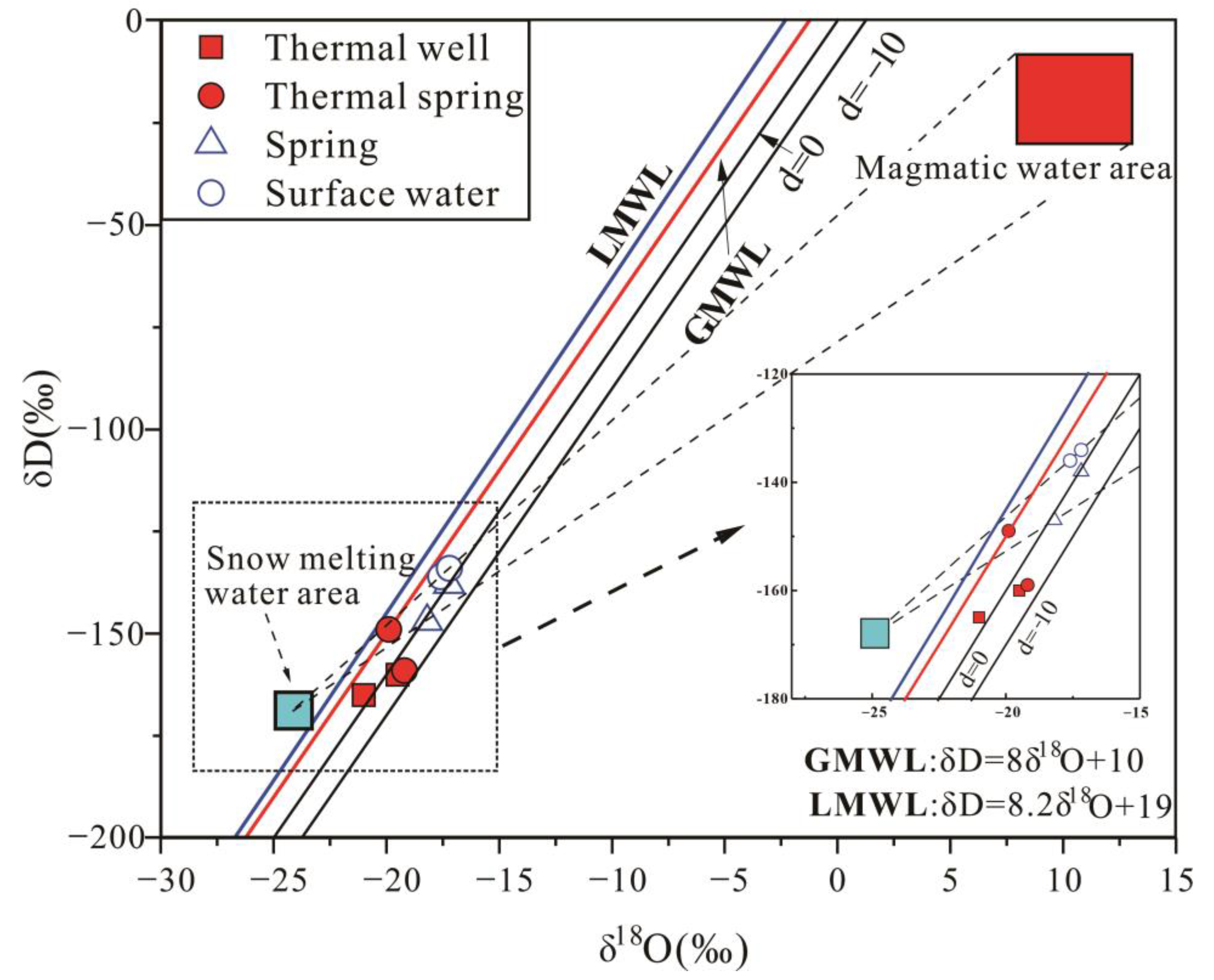
4.2. Hydrogen and Oxygen Isotope Compositions
5. Discussion
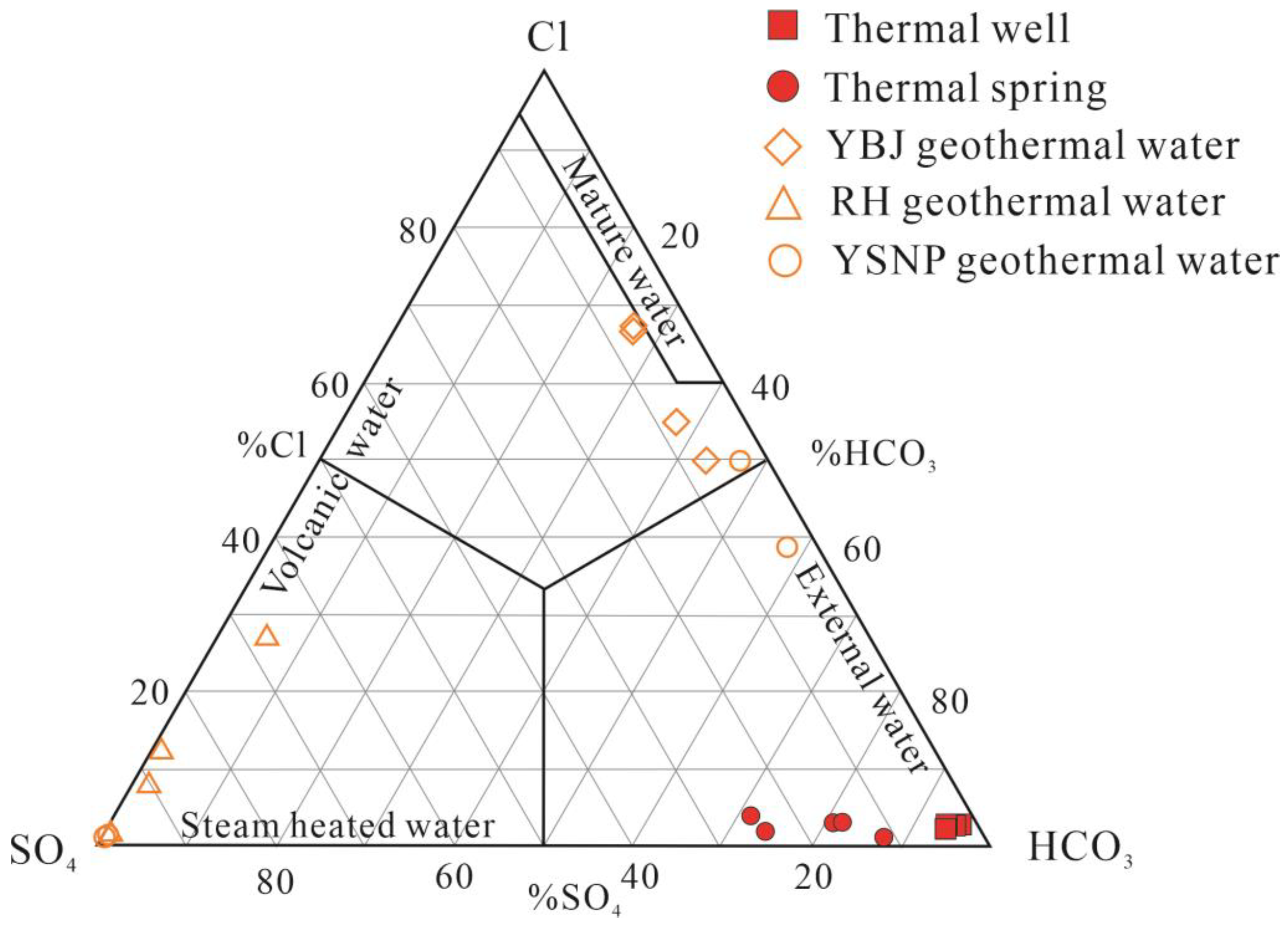
5.1. Hydrochemical Characteristics of Thermal Groundwater
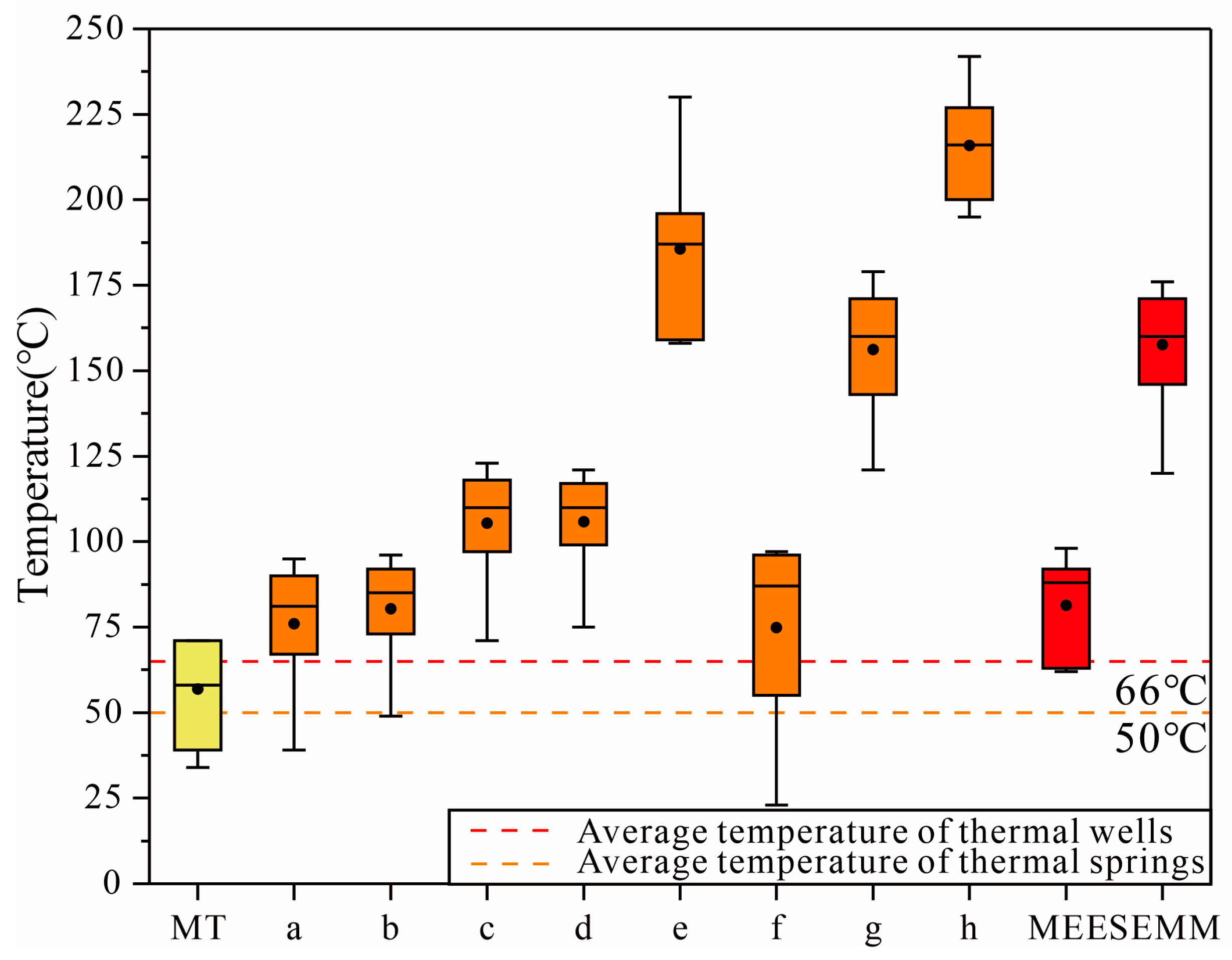
5.2. Estimation of Circulation Temperature and Depth for Thermal Groundwater
5.3. Recharge Source of Geothermal Water
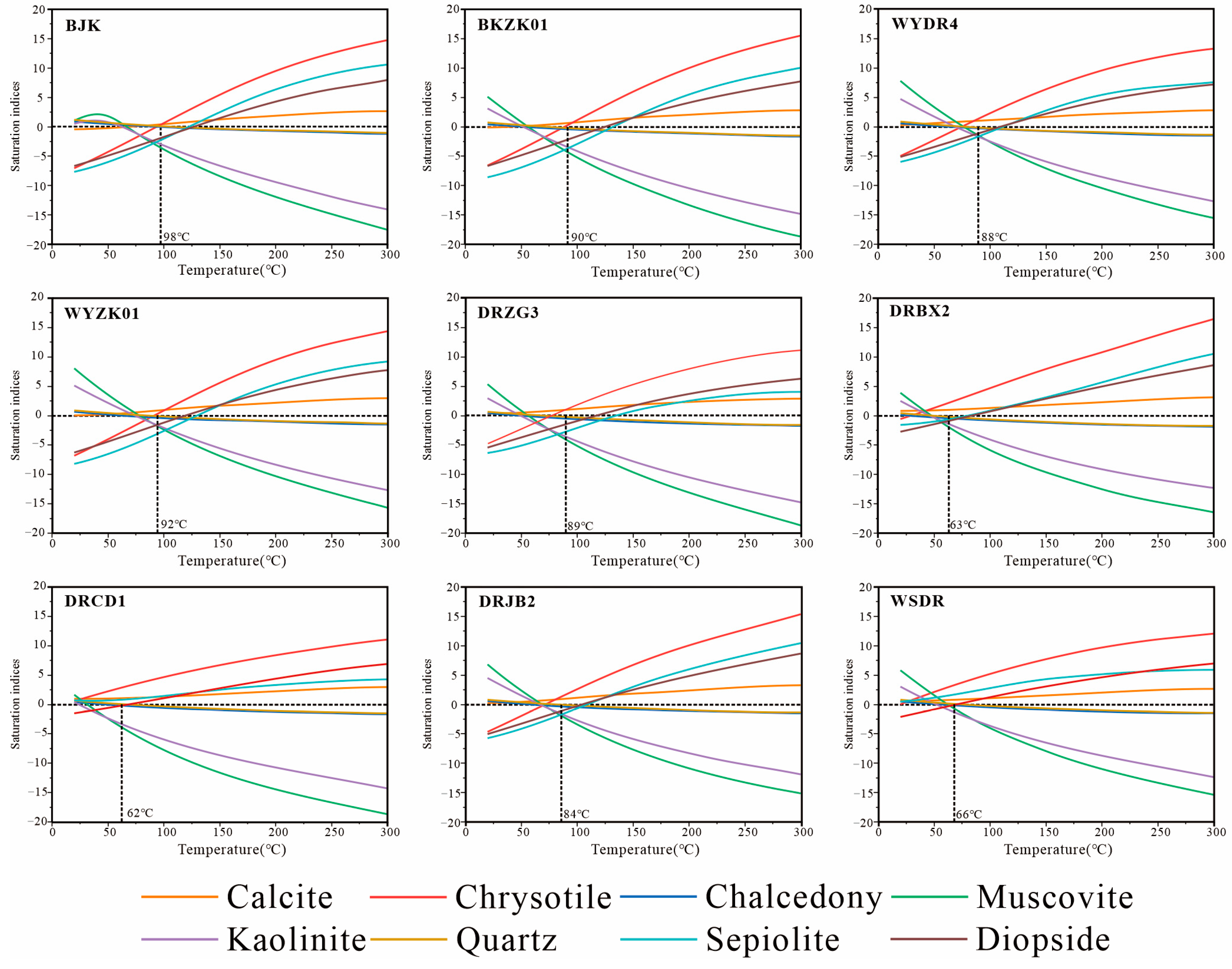
5.4. Water–Rock Interaction Modeling
5.5. Geothermal Conceptual Model
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, G.L.; Lin, W.J. Main hydro-geothermal systems and their genetic models in China. Acta Geol. Sin. 2020, 94, 1923–1937. (In Chinese) [Google Scholar]
- Yang, B.; Li, W.; Xiong, J.; Yang, J.; Huang, R.; Xie, P. Health Risk Assessment of Heavy Metals in Soil of Lalu Wetland Based on Monte Carlo Simulation and ACPS-MLR. Water 2023, 15, 4223. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, T.; Feng, B.; Yuan, Y.; Li, F.; Feng, G.; Jiang, Z. Thermal and Fluid Processes in a Closed-Loop Geothermal System Using CO2 as a Working Fluid. Renew. Energy 2020, 154, 351–367. [Google Scholar] [CrossRef]
- Wang, J.; Pang, Z.; Cheng, Y.; Huang, Y.; Jiang, G.; Lu, Z.; Kong, Y. Current state, utilization and prospective of global geothermal energy. Sci. Technol. Rev. 2023, 41, 5–11. [Google Scholar]
- Tong, W.; Zhang, M.T.; Zhang, Z.F.; Liao, Z.J.; You, M.Z.; Zhu, M.X.; Guo, J.Y.; Liu, S.B. Geothermy in Xizang; Science Press: Beijing, China, 1981. [Google Scholar]
- Zheng, S.H.; Zhang, Z.F.; Ni, B.L.; Hou, F.G.; Shen, M.Z. Hydrogen-oxygen stable isotope study of geothermal water in Xizang. Acta Sci. Nat. Univ. Pekin. 1982, 1, 99–106. (In Chinese) [Google Scholar]
- Li, Z.Q. Present Hydrothermal Activities during Collisional Orogenics of the Tibetan Plateau; Chinese Academy of Geologecal Sciences: Beijing, China, 2002. [Google Scholar]
- Zhao, P.; Xie, E.J.; Dor, J.; Jin, J.; Hu, X.C.; Du, S.P.; Yao, Z.H. Geochemical characteristics of geothermal gases and their geological implications in Tibet. Acta Petrol. Sin. 2002, 18, 539–550. [Google Scholar]
- Klemperer, S.L.; Zhao, P.; Whyte, C.J.; Thomas, H.; Laura, J.; Karl, E.; Liu, T.Z.; Carmen, W.; David, R.; Ding, L. Limited underthrusting of India below Tibet: 3He/4He analysis of thermal springs locates the mantle suture in continental collision. Proc. Natl. Acad. Sci. USA 2022, 119, e2113877119. [Google Scholar] [CrossRef]
- Hao, Y.L.; Kuang, X.X.; Feng, Y.Q.; Wang, Y.C.; Zhou, H.; Zheng, C.M. Discovery and genesis of helium-rich geothermal fluids along the India–Asia continental convergent margin. Geochim. Cosmochim. Acta 2023, 360, 175–191. [Google Scholar] [CrossRef]
- Mao, X.M.; Zhu, D.B.; Innocent, N.; He, Y.Y.; Shi, Z.D. The mechanism of high-salinity thermal groundwater in Xinzhou geothermal field, South China: Insight from water chemistry and stable isotopes. J. Hydrol. 2021, 593, 125889. [Google Scholar] [CrossRef]
- Davraz, A.; Nalbantçılar, M.; Varol, S.; Önden, İ. Hydrogeochemistry and reservoir characterization of the Konya geothermal fields, Central Anatolia/Turkey. Geochemistry 2022, 82, 125867. [Google Scholar] [CrossRef]
- Saibi, H.; Joseph, F.B.; Carlos, P. Hydrochemistry and geothermometry of thermal waters from UAE and their energetic potential assessment. Geothermics 2021, 92, 102061. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, R.; Deng, C.; Huang, X.; Lv, G.; Li, X.; Wang, Y. Genetic mechanism of geothermal water in the sandstone reservoir of western Sichuan, SW China: Evidence from hydrochemistry and δD−δ18O isotopes. Ore Geol. Rev. 2024, 167, 105994. [Google Scholar] [CrossRef]
- Craig, H. Standards for reporting concentrations of deuterium and oxygen-18 in natural waters. Science 1961, 133, 1833–1834. [Google Scholar] [CrossRef]
- Li, Y.M.; Luo, J.; Chen, K.; Huang, T.M.; Tian, J.; Cheng, Y.Z. Genesis of geothermal fluid with high fluorine content and reservoir temperature assessment in Fengliang geothermal field, eastern Guangdong. Geol. Rev. 2023, 69, 1337–1348. (In Chinese) [Google Scholar]
- Fournier, R.O. Chemical geothermometers and mixing models for geothermal systems. Geothermics 1977, 5, 41–50. [Google Scholar] [CrossRef]
- Arnórsson, S.; Gunnlaugss, E.; Svavarsson, H. The chemistry of geothermal waters in Iceland. III. Chemical geothermometry in geothermal investigations. Geochim. Cosmochim. Acta 1983, 47, 567–577. [Google Scholar] [CrossRef]
- Fournier, R.O.; Potter, R.W. Magnesium correction to the Na-K-Ca chemical geothermometer. Geochim. Cosmochim. Acta 1979, 43, 1543–1550. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Geothermal solute equilibria-derivation of Na-K-Mg-Ca geoindicators. Geochim. Cosmochim. Acta 1988, 52, 2749–2765. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Lico, M.S.; Law, L.M. Chemical geothermometers applied to formation waters, Gulf of Mexico and California basins. AAPG Bull. 1982, 66, 588. [Google Scholar]
- Fournier, R.O.; Truesdell, A.H. Empirical Na-K-Ca geothermometer for natural waters. Geochim. Cosmochim. Acta 1973, 37, 1255–1275. [Google Scholar] [CrossRef]
- Lang, X.J.; Lin, W.J.; Liu, Z.M.; Xing, L.X.; Wang, G.L. Hydrochemical Characteristics of Geothermal Water in Guide Basin. Earth Sci. 2016, 41, 1723–1734. (In Chinese) [Google Scholar]
- Luo, M.; Ren, R.; Yuan, W. Type, distribution and genesis of geothermal resource in Sichuan. Acta Geol. Sichuan 2016, 361, 47–50. (In Chinese) [Google Scholar]
- Guo, N.; Liu, Z.; Nan, D.W.; Sun, H.X.; Li, H.T.; Zhao, H.H. Characteristics and reservoir temperatures of hot springs in Jueyong, Chamdo, Xizang (Tibet). Geol. Rev. 2020, 66, 499–509. [Google Scholar]
- Cao, R.; Dor, J.; Cai, Y.Q.; Chen, X.L.; Mao, X.; Meng, H. Geochemical and H–O–Sr–B isotope signatures of Yangyi geothermal fields: Implications for the evolution of thermal fluids in fracture-controlled type geothermal system, Tibet, China. Geotherm. Energy 2023, 11, 23. [Google Scholar] [CrossRef]
- Dai, W. The Hydrogeochemical Characteristics and the Evolution of Geothermal Water in Guide Area, Qinghai. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2020. [Google Scholar]
- Zhang, H.X.; Wang, G.L.; Zhang, W.; Ma, F.; Zhu, X.; Yue, G.F.; Yu, M.X. Characteristics of the Rongcheng Bulge Geothermal Field and the Evolution of Geothermal Fluids, Xiong’an New Area, China. Water 2022, 14, 2468. [Google Scholar] [CrossRef]
- Yu, J.S. Research on Isotopic Geochemistry in China; Science Press: Beijing, China, 1997. [Google Scholar]
- Zheng, X.H.; Duan, C.Y.; Xia, B.R.; Jiang, Y.; Wen, J. Hydrogeochemical modeling of the shallow thermal water evolution in Yangbajing geothermal field, Tibet. J. Earth Sci. 2019, 30, 870–878. [Google Scholar] [CrossRef]
- Wang, X.; Wang, G.L.; Lu, C.; Gan, H.N.; Liu, Z. Evolution of deep parent fluids of geothermal fields in the Nimu–Nagchu geothermal belt, Tibet, China. Geothermics 2018, 71, 118–131. [Google Scholar] [CrossRef]
- Yuan, J.; Guo, Q.; Wang, Y. Geochemical behaviors of boron and its isotopes in aqueous environment of the Yangbajing and Yangyi geothermal fields, Tibet, China. J. Geochem. Explor. 2014, 140, 11–22. [Google Scholar] [CrossRef]
- Guo, Q.H.; Wang, Y.X.; Liu, W. Major hydrogeochemical processes in the two reservoirs of the Yangbajing geothermal field, Tibet, China. J. Volcanol. Geotherm. Res. 2007, 166, 255–268. [Google Scholar] [CrossRef]
- Liao, Z.J.; Zhao, P. Yunnan−Tibet Geothermal Belt−Geothermal Resources and Case Histories; Science Press: Beijing, China, 1999. [Google Scholar]
- Wang, G.L.; Liu, F.; Lin, W.J.; Zhang, W.; Yuan, R.X.; Xi, Y.F.; Wei, S.C.; Liao, Y.Z.; Wang, Y.R. The crustal heat production rate and crustal and mantle heat flow distribution in the land areas of China. Chin. J. Feophysics 2023, 66, 5041–5056. (In Chinese) [Google Scholar]
- Guo, Q.H.; Liu, M.L.; Li, J.X.; Zhang, A.B.; Wang, Y.X. Acid hot springs discharged from the Rehai hydrothermal system of the Tengchong volcanic area (China): Formed via magmatic fluid absorption or geothermal steam heating? Bull. Volcanol. 2014, 76, 868. [Google Scholar] [CrossRef]
- Guo, Q.H.; Nordstrom, D.K.; McCleskey, R.B. Towards understanding the puzzling lack of acid geothermal springs in Tibet (China): Insight from a comparison with Yellowstone (USA) and some active volcanic hydrothermal systems. J. Volcanol. Geotherm. Res. 2014, 288, 94–104. [Google Scholar] [CrossRef]
- Tong, W. Thermal Springs in Tibet; Science Press: Beijing, China, 2000. [Google Scholar]
- Tian, J.; Pang, Z.; Guo, Q.; Wang, Y.C.; Huang, T.M.; Kong, Y.L. Geochemistry of geothermal fluids with implications on the sources of water and heat recharge to the Rekeng high-temperature geothermal system in the Eastern Himalayan Syntax. Geothermics 2018, 74, 92–105. [Google Scholar] [CrossRef]
- Fan, Y.F.; Pang, Z.H.; Liao, D.W.; Tian, J.; Hao, Y.L.; Huang, T.M.; Li, Y.M. Hydrogeochemical Characteristics and Genesis of Geothermal Water from the Ganzi Geothermal Field, Eastern Tibetan Plateau. Water 2019, 11, 1631. [Google Scholar] [CrossRef]
- Fournier, R.O. Water geothermometers applied to geothermal energy. In Application of Geochemistry in Geothermal Reservoir Development; Amore, F.D., Ed.; UNITAR/UNDP: Rome, Italy, 1991. [Google Scholar]
- Hai, K. Using Several Methods to Estimate the Temperatures of Deep Geothermal Reservoirs. Master’s Thesis, China University of Geosciences (Beijing), Beijing, China, 2019. [Google Scholar]
- Hou, Z.Y.; Xu, T.F.; Li, S.T.; Jiang, Z.J.; Feng, B.; Cao, Y.Q.; Feng, G.H.; Yuan, Y.L.; Hu, Z.X. Reconstruction of different original water chemical compositions and estimation of reservoir temperature from mixed geothermal water using the method of integrated multicomponent geothermometry: A case study of the Gonghe Basin, northeastern Tibetan Plateau, China. Appl. Geochem. 2019, 108, 104389. [Google Scholar]
- Wang, C.G.; Zheng, M.P.; Zhang, X.F.; Xing, E.Y.; Ye, C.Y.; Ren, J.H.; Li, M.M.; He, J.T.; Wang, F.X. Hydrochemical characteristics and origins of geothermal fluids in the Gudui high-temperature geothermal system in Comei County, southern Tibet. Acta Geol. Sin. 2024, 98, 558–578. (In Chinese) [Google Scholar]
- Sun, H.L.; Ma, F.; Lin, W.J.; Liu, Z.; Wang, G.L.; Nan, D.W. Geochemical Characteristics and Geothermometer Application in High Temperature Geothermal Field in Tibet. Geol. Sci. Technol. Inf. 2015, 34, 171–177. (In Chinese) [Google Scholar]
- Pang, Z.H.; Reed, M. Theoretical chemical thermometry on geothermal waters: Problems and methods. Geochim. Cosmochim. Acta 1998, 62, 1083–1091. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Wang, G.L.; Zhang, C.Y.; Xing, L.X.; Li, M.; Zhang, W. Genesis of geothermal fluid in typical geothermal fields in Western Sichuan, China. Acta Geol. Sin.-Engl. Ed. 2021, 95, 873–882. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Li, X.; Xu, M.; Duo, J.; Wu, X.Y.; Xiao, Y.; Huang, X. Hydrogeochemical Characteristies of Geothermal Waters in the DaofuArea of the Xianshuihe Geothermal Belt. Saf. Environ. Eng. 2021, 28, 42–51. (In Chinese) [Google Scholar]
- Zhang, W.; Wang, G.L.; Zhao, J.Y.; Liu, F. Geochemical Characteristics of Medium-high Temperature Geothermal Fluids in West Sichuan and Their Geological Implications. Geoscinece 2021, 35, 188–198. (In Chinese) [Google Scholar]
- Cui, Y.; Kang, F.X.; Zhong, Z.N.; Yang, X.C.; Sui, H.P.; Zhao, Q. Gas Isotope Constraints on the Geothermal Heat Source Mechanism in Northwest Shandong Plain, China. Acta Geosci. Sin. 2022, 44, 113–121. [Google Scholar]
- Ma, Z.Y.; Yu, J.; Li, Q.; Wang, X.G.; Li, F.; Mu, G.X.; Hu, Y.; Jia, X.B.; Li, W.L. Environmental Isotope Distribution and Hydrologic Geologic Sense of Guanzhong Basin Geothermal Water. J. Earth Sci. Environ. 2008, 30, 396–401. (In Chinese) [Google Scholar]
- Wang, S.Q. Hydrogeochemical Processes and Genesis Mechanism of High-Temperature Geothermal System in Gudui, Tibet. Ph.D. Thesis, China University of Geosciences (Beijing), Beijing, China, 2017. [Google Scholar]
- Wang, X.; Wang, G.L.; Gan, H.N.; Liu, N.; Nan, D.W.; Liu, Z. Genetic mechanisms of sinter deposit zones in the Yangyi geothermal field, Tibet: Evidence from the hydrochemistry of geothermal fluid. Geothermics 2022, 103, 102408. [Google Scholar] [CrossRef]
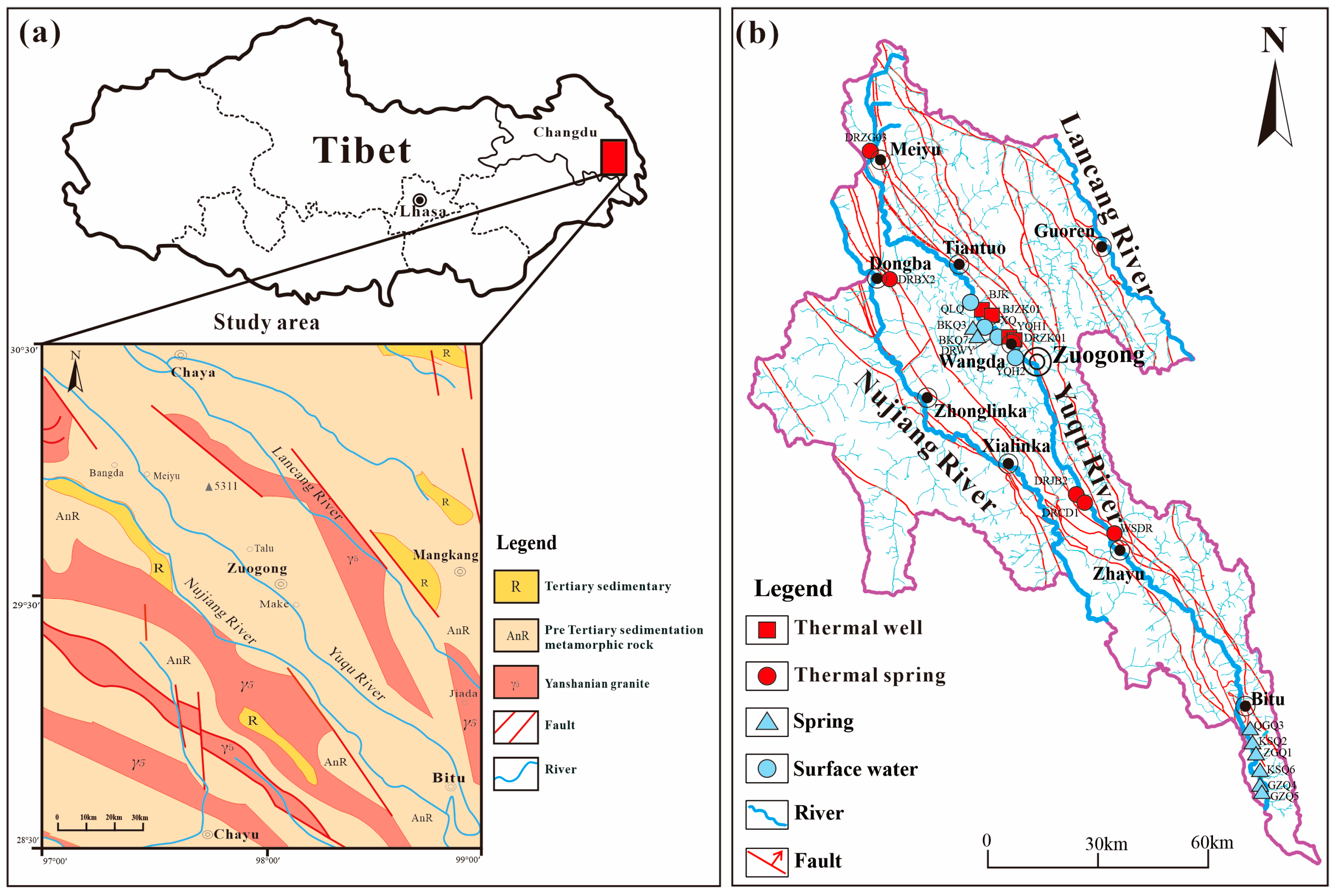
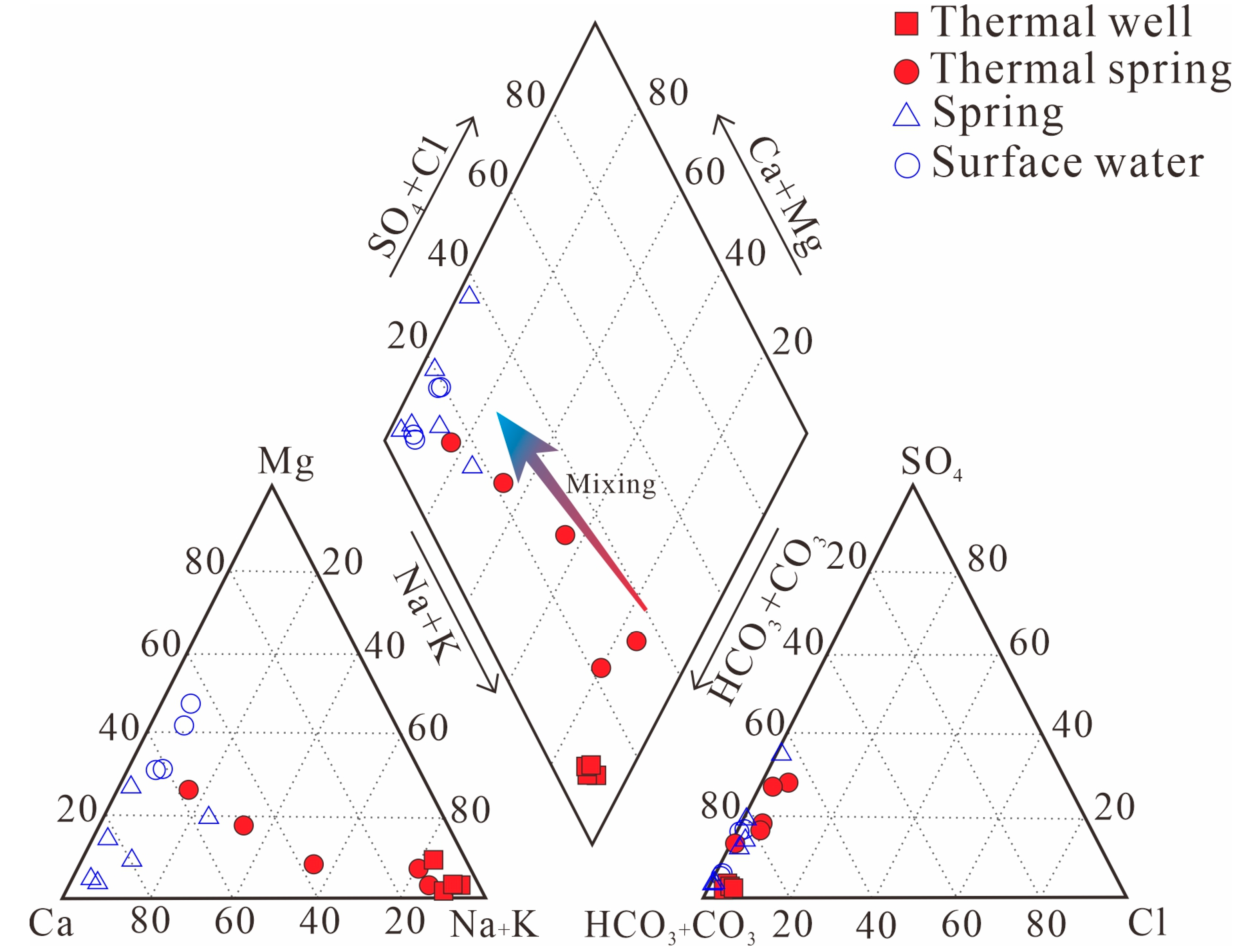
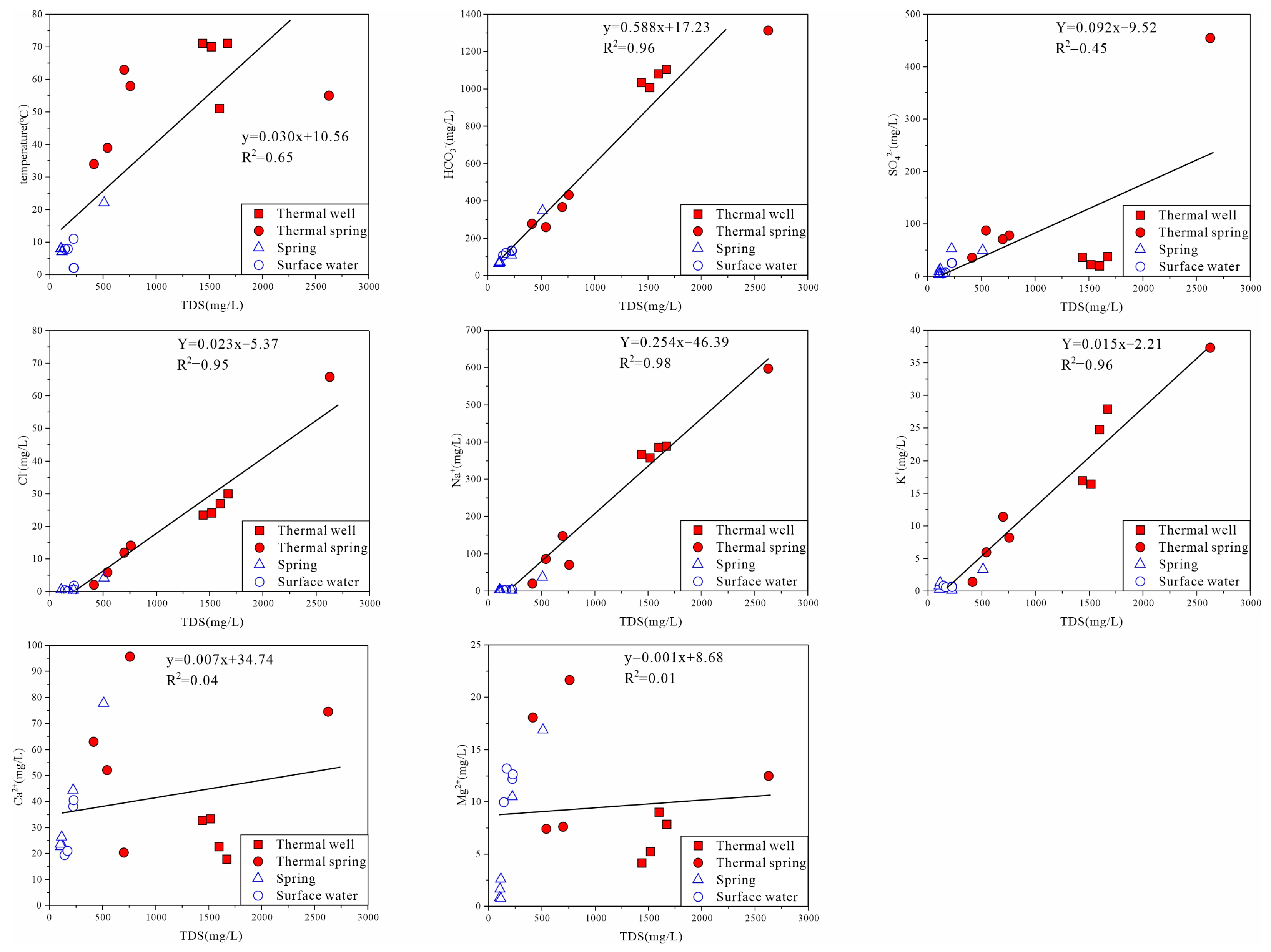
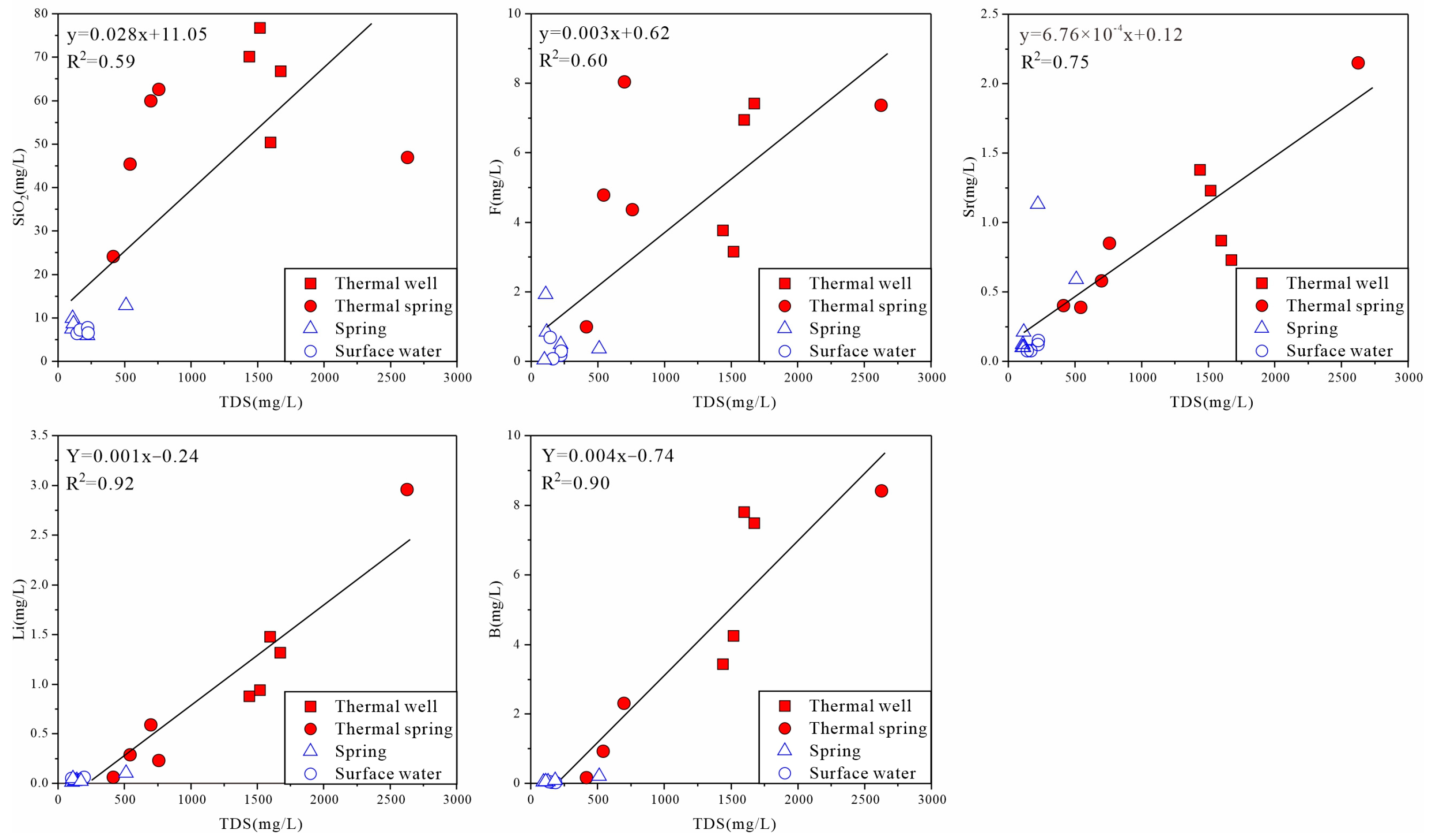


| Sample ID | Elevation | Temperature | pH | Na | K | Ca | Mg | HCO3 | CO3 | Cl | SO4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m | °C | mg/L | ||||||||||
| BKJ | thermal well | 3866 | 71 | 6.85 | 388.1 | 27.9 | 17.85 | 7.85 | 1105.00 | 0 | 29.97 | BKJ |
| BKZK01 | thermal well | 3899 | 51 | 7.04 | 385.4 | 24.78 | 22.65 | 9.01 | 1080.00 | 0 | 26.87 | BKZK01 |
| WYDR4 | thermal well | 3826 | 71 | 7.41 | 365.8 | 16.92 | 32.68 | 4.13 | 1034.00 | 0 | 23.38 | WYDR4 |
| WYZK01 | thermal well | 70 | 7.06 | 357.7 | 16.4 | 33.38 | 5.24 | 1007.00 | 0 | 24.09 | 22.63 | |
| DRZG3 | thermal spring | 4091 | 55 | 7.34 | 596.8 | 37.32 | 74.56 | 12.48 | 1313 | 0 | 65.84 | DRZG3 |
| DRBX2 | thermal spring | 3359 | 34 | 7.95 | 20.09 | 1.4 | 63.05 | 18.06 | 277.10 | 0 | 1.99 | DRBX2 |
| DRCD1 | thermal spring | 3558 | 39 | 8.18 | 85.95 | 5.93 | 52.06 | 7.41 | 268.15 | 8.65 | 5.94 | DRCD1 |
| DBJB2 | thermal spring | 58 | 7.1 | 70.55 | 8.2 | 95.65 | 21.67 | 432.20 | 0 | 14.05 | 78.26 | |
| WSDR | thermal spring | 3424 | 63 | 7.95 | 147.73 | 11.39 | 20.3 | 7.62 | 365.96 | 0 | 11.89 | WSDR |
| ZGQ1 | spring | 3850 | 22 | 7.68 | 37.37 | 3.32 | 77.75 | 16.86 | 346.80 | 0 | 4.05 | ZGQ1 |
| KSQ2 | spring | 3013 | 8 | 8.29 | 3.73 | 0.28 | 22.63 | 1.65 | 68.83 | 5.05 | 0.53 | KSQ2 |
| QGQ3 | spring | 3094 | 7 | 8.02 | 1.26 | 0 | 23.55 | 2.6 | 71.11 | 0 | 0.01 | QGQ3 |
| GZQ4 | spring | 2697 | 8 | 8.3 | 0.55 | 0.8 | 23.58 | 0.8 | 71.02 | 5.77 | 0.01 | GZQ4 |
| GZQ5 | spring | 3818 | 7 | 8.2 | 0.76 | 1.26 | 26.3 | 0.71 | 75.43 | 5.05 | 0.01 | GZQ5 |
| KSQ6 | spring | 2932 | 11 | 8.24 | 2.12 | 0.15 | 44.35 | 10.5 | 112.88 | 2.88 | 0.39 | KSQ6 |
| BKQ7 | spring | 4058 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | BKQ7 |
| BKQ3 | spring | 3905 | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | BKQ3 |
| QLQ | surface water | 3890 | 8 | 7.8 | 2.75 | 0.84 | 19.36 | 9.97 | 108.40 | 0 | 0.41 | QLQ |
| XQ | surface water | 3880 | 8 | 8.14 | 3.19 | 0.55 | 20.96 | 13.18 | 123.99 | 3.59 | 0.16 | XQ |
| YQH1 | surface water | 3818 | 2 | 8.55 | 5.04 | 0.73 | 38.18 | 12.19 | 144.29 | 10.09 | 0.66 | YQH1 |
| YQH2 | surface water | 3815 | 2 | 8.58 | 4.66 | 0.64 | 40.54 | 12.62 | 143.54 | 11.54 | 1.8 | YQH2 |
| Sample ID | NO3 | SiO2 | B | F | Li | Sr | As | TDS | δD | δ18O | Types of hydrochemistry | |
| mg/L | ug/L | mg/L | ‰ | |||||||||
| BKJ | thermal well | 1.12 | 66.79 | 7.49 | 7.42 | 1.32 | 0.73 | n.d | 1673 | −160 | −19.5 | BKJ |
| BKZK01 | thermal well | n.d | 50.37 | 7.80 | 6.95 | 1.48 | 0.87 | n.d | 1598 | n.a | n.a | BKZK01 |
| WYDR4 | thermal well | 2.3 | 70.14 | 3.44 | 3.77 | 0.88 | 1.38 | 0.7 | 1439 | −165 | −21 | WYDR4 |
| WYZK01 | thermal well | n.d | 76.72 | 4.25 | 3.15 | 0.94 | 1.23 | n.d | 1519 | n.a | n.a | WYZK01 |
| DRZG3 | thermal spring | 2.42 | 46.89 | 8.41 | 7.37 | 2.96 | 2.15 | 1130 | 2627 | −159 | −19.2 | DRZG3 |
| DRBX2 | thermal spring | 1.45 | 24.15 | 0.17 | 0.99 | 0.06 | 0.4 | 26 | 415.6 | −149 | −19.9 | DRBX2 |
| DRCD1 | thermal spring | 1.09 | 45.39 | 0.93 | 4.78 | 0.29 | 0.39 | 2.7 | 542.3 | n.a | n.a | DRCD1 |
| DBJB2 | thermal spring | n.d | 62.63 | n.d | 4.37 | 0.23 | 0.85 | n.d | 758.1 | n.a | n.a | DBJB2 |
| WSDR | thermal spring | 0.77 | 59.97 | 2.31 | 8.04 | 0.59 | 0.581 | 1.2 | 699 | n.a | n.a | WSDR |
| ZGQ1 | spring | 2.04 | 12.81 | 0.2 | 0.35 | 0.10 | 0.59 | 1.6 | 511.9 | n.a | n.a | ZGQ1 |
| KSQ2 | spring | 0.74 | 9.88 | 0.0375 | 1.92 | 0.0074 | 0.12 | 12 | 108.4 | n.a | n.a | KSQ2 |
| QGQ3 | spring | 0.92 | 7.73 | n.d | 0.84 | 0.0065 | 0.21 | 13 | 116.1 | n.a | n.a | QGQ3 |
| GZQ4 | spring | 1.35 | 7.4 | 0.00375 | n.d | 0.0058 | 0.098 | 3.8 | 105.1 | n.a | n.a | GZQ4 |
| GZQ5 | spring | 1.77 | 8.7 | 0.00275 | 0.035 | 0.0064 | 0.1 | 2.5 | 115.2 | n.a | n.a | GZQ5 |
| KSQ6 | spring | 1.29 | 5.93 | 0.00725 | 0.48 | 0.016 | 1.13 | 6 | 223 | n.a | n.a | KSQ6 |
| BKQ7 | spring | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | −138 | −17.2 | BKQ7 |
| BKQ3 | spring | n.a | n.a | n.a | n.a | n.a | n.a | n.a | n.a | −147 | −18.2 | BKQ3 |
| QLQ | surface water | 4.08 | 6.45 | 0.0215 | 0.69 | 0.0038 | 0.075 | n.d | 142.7 | −136 | −17.6 | QLQ |
| XQ | surface water | 0.94 | 7.26 | 0.01625 | 0.095 | 0.0046 | 0.078 | n.d | 167.7 | −134 | −17.2 | XQ |
| YQH1 | surface water | 1.74 | 7.75 | 0.016 | 0.18 | 0.015 | 0.12 | 1.2 | 223.6 | n.a | n.a | YQH1 |
| YQH2 | surface water | 2.52 | 6.5 | 0.0165 | 0.29 | 0.018 | 0.15 | 1.1 | 226 | n.a | n.a | YQH2 |
| Sample | Measured Temperature | a | b | c | d | e | f | g | h | MEE | SEMM | Thermal Circulation Depth | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | After Mixing (m) | Before Mixing (m) | |||||||||||
| BKJ | 71 | 87 | 90 | 116 | 115 | 190 | 96 | 179 | 216 | 98 | 158 | 2687 | 4401 |
| BKZK01 | 51 | 72 | 77 | 102 | 103 | 182 | 91 | 171 | 225 | 90 | 160 | 2459 | 4459 |
| WYDR4 | 71 | 90 | 92 | 118 | 117 | 159 | 91 | 150 | 195 | 88 | 165 | 2401 | 4601 |
| WYZK01 | 70 | 95 | 96 | 123 | 121 | 158 | 87 | 150 | 200 | 92 | 163 | 2516 | 4544 |
| DRZG3 | 55 | 69 | 74 | 99 | 100 | 180 | 97 | 167 | 242 | 89 | 146 | 2430 | 4059 |
| DRBX2 | 34 | 39 | 49 | 71 | 75 | 188 | 23 | 121 | 208 | 63 | 120 | 1687 | 3316 |
| DRCD1 | 39 | 67 | 73 | 97 | 99 | 187 | 59 | 143 | 216 | 62 | 176 | 1659 | 4916 |
| DBJB2 | 58 | 84 | 87 | 113 | 112 | 230 | 55 | 160 | 214 | 84 | 171 | 2287 | 4773 |
| WSDR | 63 | 81 | 85 | 110 | 110 | 196 | 74 | 165 | 227 | 66 | 159 | 1773 | 4430 |
| Sample | Type | Elevation (m) | δD (‰) | Recharge Altitude (m) |
|---|---|---|---|---|
| BKJ | thermal well | 3866 | −160 | 4828 |
| WYDR4 | thermal well | 3826 | −165 | 4980 |
| DRZG3 | thermal spring | 4091 | −159 | 5014 |
| DRBX2 | thermal spring | 3359 | −149 | 3897 |
| Flow Path | Sample | Na | K | Ca | Mg | HCO3 + CO3 | Cl | SO4 | Si | F | Sr |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Initial | XQ | 2.75 | 0.84 | 19.36 | 9.97 | 108.40 | 0.41 | 5.69 | 6.45 | 0.095 | 0.078 |
| Final | BKZK01 | 1237.10 | 78.07 | 29.97 | 6.87 | 3242.59 | 85.76 | 52.82 | 61.49 | 22.21 | 2.633 |
| Initial | XQ | 2.75 | 0.84 | 19.36 | 9.97 | 108.40 | 0.41 | 5.69 | 6.45 | 0.095 | 0.078 |
| Final | WSDR | 374.49 | 27.89 | 21.77 | 3.95 | 768.81 | 29.85 | 173.13 | 61.66 | 20.47 | 1.368 |
| Flow Path | Mode Reactant | Mole Transfer | Flow Path | Mode Reactant | Mole Transfer |
|---|---|---|---|---|---|
| Thermal well (XQ-BKZK01) | Albite | 4.73 × 10−2 | Thermal spring (XQ-WSDR) | Albite | 1.51 × 10−2 |
| Calcite | −3.88 × 10−3 | Calcite | −2.18 × 10−3 | ||
| Celestite | 2.22 × 10−5 | Celestite | 1.48 × 10−5 | ||
| Clinochlore | −6.78 × 10−4 | Clinochlore | −4.94 × 10−5 | ||
| Fluorite | 5.65 × 10−4 | Fluorite | 5.37 × 10−4 | ||
| Halite | 2.47 × 10−3 | Gypsum | 1.73 × 10−3 | ||
| Kaolinite | −4.42 × 102 | Halite | 8.32 × 10−4 | ||
| K-Feldspar | −4.42 × 102 | Kaolinite | 5.90 × 10−2 | ||
| Muscovite | 4.42 × 102 | K-Feldspar | 6.75 × 10−2 | ||
| Quartz | 8.83 × 102 | Muscovite | −6.68 × 10−2 | ||
| CO2(g) | 4.43 × 10−2 | Quartz | −1.64 × 10−1 | ||
| CO2(g) | 1.30 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.; Nan, D.; Liu, Z.; Gesang, N.; Bianma, C.; Zhao, H.; Zheng, Y.; Xiao, P. Hydrochemical Characteristics and Formation Mechanism of Geothermal Fluids in Zuogong County, Southeastern Tibet. Water 2024, 16, 2852. https://doi.org/10.3390/w16192852
Han S, Nan D, Liu Z, Gesang N, Bianma C, Zhao H, Zheng Y, Xiao P. Hydrochemical Characteristics and Formation Mechanism of Geothermal Fluids in Zuogong County, Southeastern Tibet. Water. 2024; 16(19):2852. https://doi.org/10.3390/w16192852
Chicago/Turabian StyleHan, Sihang, Dawa Nan, Zhao Liu, Nima Gesang, Chengcuo Bianma, Haihua Zhao, Yadong Zheng, and Peng Xiao. 2024. "Hydrochemical Characteristics and Formation Mechanism of Geothermal Fluids in Zuogong County, Southeastern Tibet" Water 16, no. 19: 2852. https://doi.org/10.3390/w16192852
APA StyleHan, S., Nan, D., Liu, Z., Gesang, N., Bianma, C., Zhao, H., Zheng, Y., & Xiao, P. (2024). Hydrochemical Characteristics and Formation Mechanism of Geothermal Fluids in Zuogong County, Southeastern Tibet. Water, 16(19), 2852. https://doi.org/10.3390/w16192852






