Abstract
Biological treatment is currently a favorable option to treat wastewater due to its environmentally friendly methods and minimal toxic by-products. The majority of biological wastewater treatment uses bacteria as treatment agents, which are known to have excellent capabilities for removing various pollutants. Researchers have extensively explored the use of extracellular polymeric substances (EPSs) generated by bacteria in wastewater treatment. This review focuses on the sources of EPSs, factors influencing their production, and their role in wastewater treatment. Bacterial species, nutrient availability, pH, temperatures, and the presence of toxins were mentioned to be the factors influencing EPS production by bacteria in wastewater treatment. Produced EPSs by bacteria may promote the aggregation, adsorption, decolorization, and degradation of pollutants. This review highlights the challenges of discovering new potential bacterial species and complex EPS extraction methods, as well as the importance of mass production for larger-scale applications.
1. Introduction
An extracellular polymeric substance (EPS) comprises a high-molecular-weight natural polymer produced by microorganisms through different processes. Researchers have classified EPSs into soluble/loosely bound EPSs (dissolved macromolecules, slimes, and colloids) and tightly bound EPS structures (capsular polymers, loosely bound polymers, concentrated gel, and attached organic substances) (Figure 1) [1,2]. Three methods are defined in the EPS separation process. First, by using chemicals (enhanced electronegativity, for example, formaldehyde or sodium hydroxide); second, by physical methods (sonication, cation exchange resin, heating, or centrifugation); and third, by physicochemical processes [3]. The main composition of EPSs consists of polysaccharides, proteins, lipids, uronic acid, DNA, humic substances, and other polymeric compounds. Having a unique composition, EPSs show excellent adsorption properties, biodegradability, and hydrophilicity/hydrophobicity [3,4]. However, EPS composition may differ depending on a variety of factors, including culture species, growth profile, extraction method, types of growth medium, environmental conditions, and operational parameters [5].
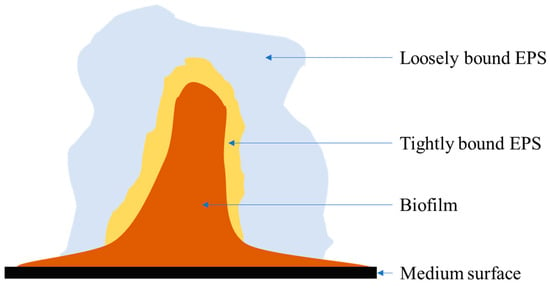
Figure 1.
EPS produced by biofilm.
EPS utilization is currently emerging in various industrial applications, including thickening, flocculants, emulsifiers, gelling, antitumor agents, and stabilizers [6]. Depending on the intended use, EPSs can take various forms, including powder, fiber, pellets, and high-viscosity solutions [7]. The cosmetic industry introduced EPSs as an ingredient in sunscreen cream and moisturizers for antioxidants and hydration [8]. The food industry manipulates EPSs, acting as a thickener, texturizer, stabilizer, and emulsifying agent to enhance food quality [9]. Their agricultural applications include improving soil quality, soil properties, and fertility to increase crop productivity [10].
In previous studies, the use of EPSs in water and wastewater treatment has attracted the attention of many researchers. EPSs play an essential role in wastewater treatment because it forms a protective layer for the cells against harmful substances (phenol, heavy metals, and antibiotics). Moreover, it provides carbon and energy sources needed for metabolic functions during starvation. They can also rapidly form microbial aggregations by attaching cells closely [3]. In the field of wastewater treatment, various EPS applications have been studied. These applications include the removal of heavy metals [11,12], removal of toxins [13], decolorization of dyes [14], removal of suspended solids [15], and sludge dewatering [16]. Most of the recent review papers have focused on the type of unit to be used in biological wastewater treatment, while the review articles focused on the EPSs produced by bacteria, and the mechanisms involved during the application itself are currently limited.
This review aims to discuss EPS sources and their application in wastewater treatment. A comparative study of EPS roles in wastewater treatment from previous studies has been summarized using the SCOPUS database. The main keywords of EPS and wastewater treatment were used to access the related articles from 2010 to 2023. Related articles were then assessed and refined to only 120 articles. Six sections are then developed in this review to understand more about this topic, focusing on EPS production and properties, EPS roles in wastewater treatment, mechanisms involved during the treatment, natural bioproducts produced by EPSs from wastewater treatment, limitations of the current study, and future research directions.
2. EPS Properties and Production
2.1. Properties and Function of EPSs
Microorganisms produce EPSs, also known as extracellular polysaccharides, exopolymers, or exopolysaccharides, which are primarily composed of polysaccharides and proteins with a small amount of other compounds. Sugars, lipids, polymers, extracellular DNA, and humic substances constitute a small portion of the EPS [17,18]. The primary function of an EPS in interaction with another microorganism is to perform adhesion, cohesion, and genetic material transfer. The EPS handles the cohesion of microorganisms and the adhesion of biofilm to surfaces, allowing interaction among microorganisms and acting as an adhesive between the cells [19]. Beside the aforementioned functions, the EPS also functions in creating a habitable environment for the microbial community, including the function of keeping the concentration gradient on the surface to facilitate diffusion, facilitating sorption, enzyme retention, cooperation between communities, competition against competing species or communities, and providing tolerance/resistance toward toxins (Figure 2).
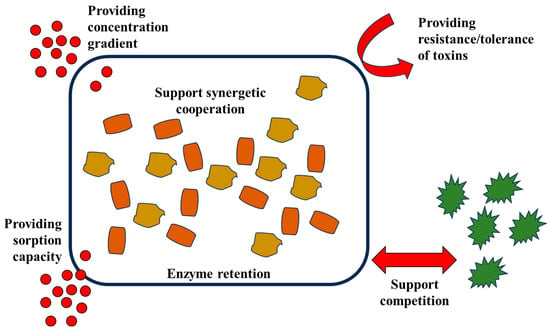
Figure 2.
Functions of EPS in creating habitable environments for the bacterial community.
2.2. Production of EPSs
Various types of bacteria [20], cyanobacteria [21], microalgae [22], yeasts [23], and fungi [24] can produce EPSs. For example, Zhou et al. [25] mentioned that some microalgae species such as Scenedesmus obliquus and Chlorella sp. produce EPSs, which enhances the flocculating activity and promotes the efficiencies of wastewater treatment. In another study, Sun et al. [26] mentioned the function of EPSs produced by fungal species of Phoma sp. ZJ6 in providing essential nutrients for denitrifying bacteria in wastewater treatment units. This review paper is focused on the bacterial EPS, which is mostly used in wastewater treatment.
Bacteria primarily produce EPSs as a protective and adaptive response to environmental stresses, such as nutrient limitation [27], temperature changes [23], pH fluctuations [28], and the presence of toxic substances [29]. The production of EPSs was reported to be high under slightly non-favorable environmental conditions for bacteria to grow [30]. Before delving into the environmental conditions, it is important to note that bacterial species significantly influence the production of EPSs. Some environmental factors, such as nutrient availability, temperature, oxygen levels, the presence of signaling molecules, and the presence of toxins, can modulate the expression of these genes and influence EPS production.
Different species within the same genus may produce EPSs differently. Bacillus subtilis and Bacillus polyfermenticus showed significant differences in EPS production up to 3% [31] under the same incubation conditions. Under a different genus, for example, Azotobacter beijreinckii and B. subtilis, up to 30 mg differences in EPSs under the same growth conditions and incubation periods [32]. These findings clearly showed that bacterial species played an important role in the production of EPSs, as it is related to its specific metabolism that corresponds to how the species react to the environmental conditions.
The availability of nutrients and food sources, such as carbon, nitrogen, and phosphorus, has a substantial influence on EPS production [33]. Bacteria require these nutrients in order to produce the EPS building blocks [34]. As bacteria react to nutritional stress by making more EPSs to scavenge and store critical nutrients, limited nutrient availability can boost EPS production [35].
Environmental conditions such as temperature, pH, oxygen levels, and salinity play a role in EPS production. In addition to those, the design of the bioreactor also affects the total EPS production by bacteria. Different bacterial species exhibit specific environmental preferences for EPS production. For example, some bacteria produce more EPSs at lower temperatures or under specific pH conditions [35]. Most bacteria prefer low salinity levels, while slight increases in salinity levels may force bacteria to adapt, in which case they may produce more EPSs as a response mechanism [36]. Oxygen levels also impact EPS production, as low oxygen levels may help aerobic-facultative bacteria make more EPSs [37].
In addition to the previously mentioned factors, the presence of signaling molecules may also enhance or inhibit the production of EPSs by bacteria. Some chemical compounds and also enzymes may trigger the starting mechanism by bacteria to produce EPSs, such as rhizobial lipochitin oligosaccharides (nod factors) [38]. Compounds like UDP-glucose, UDP-galactose, and dTDP rhamnose, along with enzymes like phosphoglucomutase and UDP-glucose phosphorylase, are known to enhance the production of EPSs by bacteria [39]. Fluctuations or limitations of nod factors or the aforementioned enzyme activities may lead to a reduction in EPS production.
The presence of toxins may also interfere with EPS production. As previously mentioned, bacteria’s production of EPSs was enhanced by slightly unfavorable growth conditions. The presence of small concentrations of toxins such as metal may boost the production of EPSs as bacteria are forced to survive [35]. Physical and mechanical forces, such as shear stress, surface roughness, and fluid flow, can also influence EPS production. Bacteria respond to these forces by adjusting their EPS production to enhance attachment, colonization, and stability within biofilms [18,40]. For example, increased shear stress can induce higher EPS production to reinforce biofilm structures [41].
In addition to the environmental factors, bacterial species and strain variability may differentiate the EPS production capability. Some bacteria are known to produce copious amounts of EPSs, while others may produce relatively low quantities [35]. Moreover, variations in EPS composition and structural features can occur between bacterial species, influencing their adhesive properties, biofilm formation, and overall functionality. Several publications mentioning the production of EPSs by various species are summarized in Table 1.

Table 1.
Production of EPSs by several bacteria species.
2.3. Bioreactors for EPS Production
When considering bioreactor design, the use of different setups may affect bacteria’s production of EPSs. For example, Alcaligenes faecalis and Agrobacterium radiobacter showed a higher EPS production in low-shear mixing through low-shear axial flow impeller reactors, while most industries use stirred tank reactors for the mass production of EPSs [49]. Table 2 tabulates several designated bioreactors for EPS production. Table 2 reveals that the design of the bioreactor significantly influences EPS production. EPS production went up by 11% when a membrane bioreactor was used instead of aerobic granular sludge with the same inoculum of bacteria. With the same activated sludge design, different states of nitrification vs. nitritation conditions showed a drastic difference in EPS production (396.9 mg/g MLVSS vs. 82.5 mg/g MLVSS). This summary demonstrated that the use of different bioreactor designs to produce EPSs resulted in significant differences in yield.

Table 2.
Bioreactor designs for EPS production.
3. EPS Roles in Wastewater Treatment
The most well-known application of EPSs in wastewater treatment is the bioflocculation process. The presence of negatively charged particles in wastewater, mainly due to the carbohydrate and protein composition, enhances the flocculation mechanism, while the inclusion of cations in the process triggers the charge neutralization processes [54]. Mixed-culture EPSs are reported to perform better than pure-culture EPSs because of the higher quantity of EPSs produced, better adhesive properties, and relatively lower surface charges [55,56]. An EPS with a higher molecular weight (MW) has a longer polymer chain and more places where it can stick to things to help them clump together [57].
Adsorption, decolorization, and degradation as additional applications of EPSs in wastewater treatment are mentioned in previous studies. Positive-charged metal ions and negative-charged EPSs interact to contribute to the adsorption of metal by EPSs [58,59]. The presence of carboxylics, phenolics, carbonyls, and hydroxyls in the EPS corona aids in metal adsorption via electrostatic attraction or the formation of nanoparticles in the nanoparticles–EPS–metal complex [60]. In terms of decolorization, the process involved cell dye absorption, followed by degradation by co-metabolism and special enzymes to break the azo linkage and naphthalene ring [59]. Table 3 summarizes more current applications and details of EPSs in wastewater treatment.

Table 3.
Variability of EPS application in wastewater treatment.
4. Mechanisms Involving EPSs in Wastewater Treatment
4.1. EPSs Promoting Aggregation and Flocculation
One of EPSs’ main qualities for biopolymer applications has been its flocculation ability. Several studies have been conducted to determine the critical structure–function link between EPS functional composition and flocculation abilities [74,75]. Various methods have been used to predict flocculation activity, and the bridging creation model has been used to explain the flocculant activity of high-molecular-weight EPSs [76,77]. In the patch flocculation model, the flocculation of bacteria with the negatively charged cell surface is caused by positively charged macromolecules binding to the surface of particles [78,79]. Coulomb forces have the ability to neutralize a portion of the surface charge in the patch model. Reduced electrostatic repulsion causes particulate matter agglomeration, as well as the production of flocs via bridges between negatively charged particles [79] (Figure 3). The use of EPSs was proven to improve the aggregation of suspended solids by changing the formation of microflocs (<200 µm) into macroflocs (>200 µm) [80].
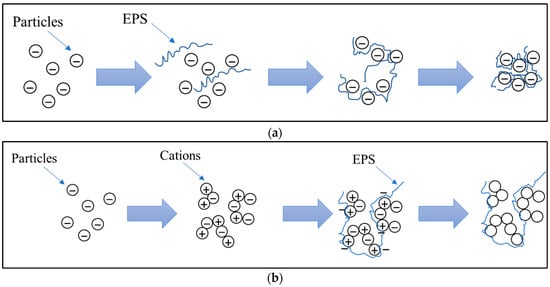
Figure 3.
Mechanism of flocculation in wastewater treatment promoted by EPSs via (a) bridging creation and (b) patch flocculation.
4.2. EPSs Promoting Adsorption
An anionic EPS is made up of negatively charged ionizable phosphates, carboxylates, acetates, amines, and sometimes sulfate groups that react with positively charged metal ions [81]. To figure out how an EPS binds to metals, we need to look at the different properties of the functional groups that are on the EPS from different microbes. Heavy metal binding is aided by amino functional groups in polysaccharides, which aid in heavy metal binding [82]. Carboxyl or hydroxyl groups form coordination bonds that enhance the stability of metal–ion polymer complexes [83,84,85]. Electrostatic interactions help cations stick to negatively charged functional groups like uronic acid and phosphoryl groups that are part of membranes, as well as amino acid carboxylic groups (Figure 4). Polymers that are positively charged may bind or coordinate with hydroxyl groups [82]. In addition to electrostatic interactions, some other mechanisms, such as hydrophobic, covalent, and polymer–polymer interactions, were also mentioned to occur in the adsorption by EPSs [86].

Figure 4.
Mechanism of EPSs binding metal inside their matrix by electrostatic interaction.
4.3. EPSs Promoting Decolorization
Reduction–oxidation is the most common mechanism occurring in decolorization by EPSs [87] (Figure 5). This process can occur intracellularly (inside bacterial cells) or extracellularly (involving EPSs) and involves the transfer of electrons, with electrons transferred from the dye resulting in decolorization [88]. Some species, such as Bacillus megaterium [65] and Aliiglaciecola lipolytica [58], were mentioned to produce EPSs with decolorization capabilities. The decolorization process may involve membrane-bound enzymes and extracellular enzymes, also known as exoenzymes, which are also part of EPSs [89,90]. In addition to that, the mechanism of protonation and deprotonation of the surface functional groups may affect the electrostatic charge of dye [91], which can also promote decolorization by EPSs. Azoreductases, laccases, and peroxidases are enzyme families that have already been discovered as being capable of this reduction [58,92,93,94]. Bacteria that have the capability to produce these enzymes may secrete it into their EPSs in certain conditions. Mechanisms of dye decolorization by EPSs are depicted in Figure 5.
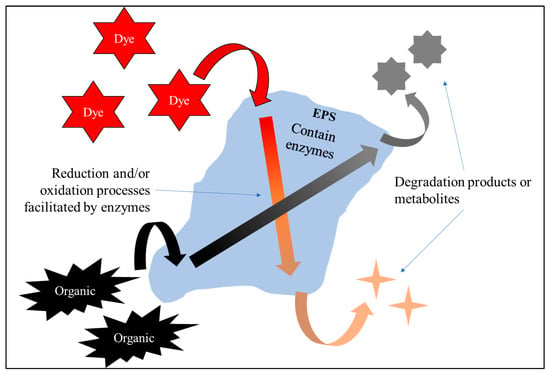
Figure 5.
Mechanism of decolorization and degradation by EPSs.
4.4. EPSs Promoting Degradation
Enzymes inside EPSs facilitate the degradation of organic compounds. Previous research has proven that the EPS adsorption of organic materials occurs at the first stage of degradation. The binding of EPSs and organic materials was then taking place inside the EPS matrix [95]. Some bacteria families, such as Rhodobacteraceae, Flavobacteriaceae, Sphingomonadaceae, Methylococcaceae, and Comamonadaceae, were mentioned to produce EPSs with the capability of organic degradation [70]. Extracellular electron transfer was mentioned as the most prominent mechanism during organic degradation by EPSs [96]. There are many enzymes involved in the degradation of various organic pollutants by EPSs. Hydrolase, oxidoreductase, amylase, cellulase, proteinase, DNase, and polygalacturonase were mentioned to be involved during the process [96,97]. Complex organic materials require a series of processes to be totally degraded, resulting in the production of intermediates or metabolites after degradation by EPSs [98]. It is worth mentioning that the complete degradation of organic pollutants is rarely completed by a single species’ EPSs. In addition to that, some intermediates or metabolic products may also possess higher toxicity as compared to the original compound, which needs to be considered during the process [99].
5. Configuration of Bioreactors Used in Wastewater Treatment Involving EPSs
5.1. Membrane Bioreactors
Membrane bioreactors have been recognized as an effective biotechnology for the elimination and/or recovery of pollutants from water. It primarily involves the disintegration of biological waste constituents through physical separation and a biofilm-based system utilizing a membrane module that substitutes secondary settling. It is utilized for the treatment of various urban and industrial wastes, exhibiting the exceptional removal of both inorganic and organic substances [100]. It possesses distinct advantages compared to alternative treatment techniques [101].
In membrane bioreactors, microorganisms produce EPSs due to their metabolic activities, introduction of shear stress, and/or long solid retention time [102,103]. EPSs help to strengthen the biofilm formation and contribute to floc formation, while also facilitating nutrient exchange.
5.2. Moving Bed Bioreactors
Moving bed bioreactors are biofilm-based wastewater treatment techniques employed in more than fifty nations. It was conceived in Norway throughout the 1980s and 1990s [104,105]. This configuration system has achieved significant success and is utilized for municipal and industrial wastewater treatment. This method integrates the optimal features of both activated sludge and biofiltration procedures. This reactor can facilitate aerobic, anoxic, and anaerobic activities [104,106,107]. In this procedure, the carriers that serve as substrates for biofilm formation move freely inside the tank’s capacity. Similar to membrane bioreactors, EPSs were produced due to the consumption of organic materials and dynamic shear stress. In moving bed bioreactors, EPSs facilitate the attachment of biofilm onto carriers, keeping its structure and regulating the stability of nutrients, while also preventing washout [104,106].
5.3. Fluidized Bed Bioreactors
Fluidized bed bioreactors utilize small carriers to create a bed within a column, which is kept in a fluidized state by the flow of wastewater, resulting in an increase in the bed’s volume [108]. A recycling line is employed within this system to maintain the vertical hydraulic velocity of the continuously delivered wastewater [109]. Aeration transpires when influent wastewater merges with effluent extracted from the upper portion of the bed during the recycling process. Typically, medium particles in this configuration exhibit a size gradient from the top to the bottom of the bioreactor [110,111]. Microorganisms in this configuration produce EPSs as part of their biofilm matrix and because of the extended solid retention period. EPSs function similarly in this system, as mentioned above in moving bed bioreactors; however, EPS formation needs to be considered in this configuration to avoid overly thick biofilms to maintain its performance.
5.4. Trickling Filter
A variety of trickling filters have been utilized for wastewater treatment. The system comprises a three-phase biofilm reactor, a pump station for recirculating influent, a clarifying unit, and a trickle filter unit. The elimination of suspended solids from wastewater treated with this unit requires additional liquid–solid separation, as trickling filter generates total suspended solids [112]. Secondary clarifiers, whether circular or rectangular, are frequently employed at this phase [113]. EPSs are produced in this system as the result of the organic degradation by microorganisms, shear stress, and long solid retention times. Overly thick biofilms due to excessive EPS production also occur in this setup and need to be regulated to avoid the system’s clogging.
6. Natural Bioproducts from EPSs for Wastewater Treatment
6.1. Bioflocculant
EPSs produced by wastewater has potential as a natural bioflocculant that is harmless and biodegradable [62]. This natural bioflocculant can be used as a stabilizing, emulsifying, and viscosifying agent [114]. The site-blocking effects were believed to have governed the flocculation mechanism in the EPS mixture. Ajao et al. [61] explain the high flocculation performance of mixed EPSs (low, medium, and high MW) at the lowest dosage. The low-molecular-weight (5.3 × 104 Da) bioflocculant was extracted from Bacillus subtilis F9 isolated from wastewater sludge and tested with high solubility, which is appropriate in flocculating small particles [115].
The bioflocculant-producing strain isolated the bioflocculant (MBF83) from ethanol wastewater treatment sludge for the purpose of removing arsenic. The bioflocculant comprises 74.1% polysaccharide and 24.2% protein. An 86.1% of arsenite removal was recorded [116]. The bridging mechanism was observed for arsenic removal using bioflocculant from swine wastewater [117]. The bioflocculant produced by microalgae (Cyanothece sp.) was discovered as a new agent in aggregating nano- and microplastics, especially in low concentrations [66].
6.2. Coating Material
In the municipal wastewater treatment plant, the EPSs recovered from aerobic granular sludge contained two major components, which were granular and alginate-like exopolymers (ALEs) [118,119]. Both components were believed to contribute to the gel-forming properties of the EPS granules. Because of its similar functional characteristics, scientists described EPSs in aerobic granular sludge (AGS) as having gel formation properties similar to sodium alginate [120]. The alginate-like exopolysaccharides (ALEs) contributed to forming a stable hydrogel [121]. The composition of ALEs includes humic substances, proteins, and sugars [122].
In aerobic granular sludge, only some of the EPSs’ polymers have a gel-forming characteristic, categorized as structural polymers [123]. EPSs from granular sludge contained a high amount of ALEs compared to floc sludge [124]. Different metal ions were studied and showed other effects on the hydrogel stiffness. Felz et al. [121] concluded that the hydrogels’ stiffness was less when alkaline earth metals were utilized compared to transition metal ions.
The amphiphilic properties of lipids and polysaccharides could be used as waterproofing and grease resistance agents [125,126]. This biopolymer property has potential applications in the agriculture, horticulture, construction, medical, and paper industries [127]. In EPSs, the hydrogel structure can store water, making it useful as a drug carrier [128]. Moreover, the EPSs’ ability to retain moisture is beneficial in curing cement to prevent cement cracking due to the drying process—this ability to maintain moisture is due to the hydrophilic character of the polymer that is similar to alginate [129].
Furthermore, research revealed that the thermal decomposition characteristics of EPSs recovered from the aerobic granular and activated sludge of domestic wastewater were similar to those of ammonium polyphosphate. This EPS granule and EPS sludge demonstrated self-extinguishing properties. Flax fabric was coated with this EPS-based material, and results showed the flame retardancy meets the standards of the US Federal Aviation Regulation [130].
7. Limitations of the Current Study and Future Research Directions
Research on EPS production and application for wastewater treatment is still far from perfect. Current studies still have some limitations, which may lead to new challenges for further improvements, as summarized in Figure 6.
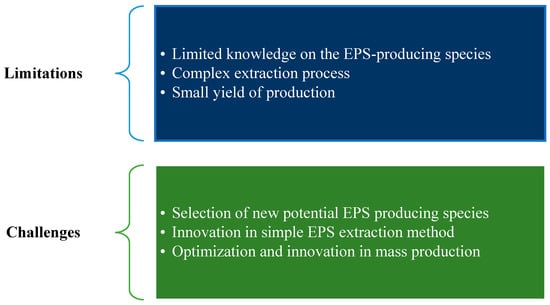
Figure 6.
Limitations and challenges in research on EPS production and application.
7.1. Limitations of the Current Study
7.1.1. Limited Knowledge of Potential Species for EPS Production
Not all microorganism species can produce EPSs, and not all produced EPSs functioned well in assisting the wastewater treatment [4]. Some genera like Bacillus sp. [131,132], Lactobacillus sp. [55], and Pseudomonas sp. [133] were EPS-producing species which showed potential roles in wastewater treatment. Researchers discovered that the production of EPSs varies depending on the species [134], which implies that discovering more species could open up new options and alternatives for EPS production. Currently identified EPS-producing species and EPS characteristics functioning in wastewater treatment are very limited compared to the number of microorganism species [135]. Numerous species remain unexplored [136].
7.1.2. EPS Extraction
The extraction of EPSs after cultivation includes several complex stages that also utilize chemicals [4,137]. For example, the extraction process of EPSs used cold ethanolic precipitation [43], while another method even utilizes membrane filtration [103] and even electroseparation [138]. One of the limitations in enhancing the use of EPSs in wastewater treatment is the complex extraction process. In cold ethanolic precipitation, a massive amount of alcohol is needed to extract bacterial EPSs (ratio of 1 EPS to 3 alcohol v/v). This method requires the use of a centrifuge to separate an EPS while also taking a minimum of 24 h contact time before the EPS can be harvested [139]. Before performing membrane filtration, bacterial suspension needs to be preconditioned by centrifugation, and a series of chemicals (NaOH and HCl) were used to extract the EPS. Dialysis then needs to be performed to purify the obtained EPS before membrane filtration [103]. In electroseparation, the EPS needs to be pre-conditioned with formaldehyde before performing extraction [138]. Researchers suggest that the use of abundant chemicals could pose a challenge to the achievement of sustainable and green wastewater treatment through EPSs [140]. Previously mentioned extraction protocols required very complex procedures and sophisticated equipment with a long time requirement, which makes them difficult to conduct or replicate by other researchers [141]. The currently available techniques for EPS extraction are still considered to be costly and time-consuming.
7.1.3. Mass Production
Current research on EPS applications in wastewater treatment is still and mostly limited to the laboratory scale [137]. Some industrial-scale applications of EPSs are texture improvements for bakeries [142], emulsifiers [143], and aiding antioxidant production [144]. Real-scale applications, especially for industrial wastewater treatment, is currently rare [145]. Zeng et al. [146] mentioned that the use of EPSs produced by bacteria in activated sludge promotes sludge aggregation in real municipal wastewater treatment. In another study, EPSs were reported to serve as a protective barrier for real-scale aerobic granule sludge reactors treating urban wastewater [147]. It is suggested that upscaling the obtained result to a pilot scale and even an accurate industrial scale is suggested to escalate this research topic. The real-scale application of EPSs to wastewater treatment must take into account several previously mentioned limitations in order to optimize the treatment outcome. Additionally, the produced EPSs by microorganisms is very little, while industrial-scale utilization requires large and continuous supplies [148]. The mass production of EPSs reported an average yield of 20 to 100 mg of EPS per liter substrate [134,149]. The production of EPSs was reported to have an average yield of 20 to 100 mg of EPS per liter substrate in mass production [134]. The utilization of research optimization was applied to scale up the production of EPSs by Bacillus velezensis KY498625, but it only achieved 7.6 g/L as the maximum production [149].
7.2. Future Research Directions
7.2.1. Selection of New Potential EPS-Producing Species
Understanding this potential, research in seeking new EPS-producing species and analyzing its characteristics will enhance and strengthen the knowledge of the roles of EPSs in wastewater treatment. Certain EPS compounds may perform well as coagulation agents [55,134], while others perform well as degradation agents or enzymes to boost the reaction kinetics [133,150]. In addition to the identification of potential species, its EPS production yield and the role of the produced EPS in wastewater treatment will highly contribute to this research topic.
7.2.2. Innovation in Simple EPS Extraction Method
Developing a simple extraction technique for EPSs is considered to advance this topic. The currently available EPS extraction techniques are divided into two major categories, namely physical and chemical methods [140]. Physical extraction still relies on energy consumption [123], while chemical extraction utilizes chemical reagents, which may cause further pollution [7]. Proposing a minimum chemical- and energy-required method with high EPS recovery will support this technique’s continued sustainable and environmentally friendly application.
7.2.3. Optimization and Innovation in Mass Production
The production of EPSs by microorganisms requires the optimum growing medium to obtain the highest yield of EPSs. The currently used growing medium is mostly synthesized in the laboratory, thus still relying on the related chemical industries [141,149]. The optimization of this condition for EPS production still awaits to be explored in depth. Optimization studies related to carbon sources [80], nutrient sources [128], environmental conditions [58], and reaction catalysts [129] need to be explored in detail to obtain the highest yield.
The utilization of local resources as a growing medium of microorganisms is also currently rare and has much potential to be unlocked further [151,152]. Local resources are considered to be abundant and easy to obtain. The cultivation of microorganisms and the production of EPSs are suggested to utilize waste products. Brevibacillus parabrevis was successfully cultivated in municipal wastewater as a growing medium. This species produced EPSs, which have high flocculating activity, reaching >90% [55]. Lukwambe et al. [153] reported the success of cultivating a microbial community, mostly from the Proteobacteria and Bacteroidetes families, using aquaculture wastewater. This microbial community produced EPSs that improved the water recirculation quality in biofloc aquaculture cultivation systems. The cultivation of potential EPS-producing species from wastewater with characteristics similar to the requirement for optimum EPS production is highly recommended and awaits to be explored. The utilization of waste products as a medium or added material in cultivation processes may reduce the amount of waste or wastewater while also producing valuable compounds that can be used to treat wastewater.
8. Conclusions
The use and involvement of EPSs in wastewater treatment showed a favorable potential for future improvement. The wastewater treatment by EPSs primarily involved flocculation, adsorption, decolorization, and degradation. Flocculation by bacterial EPSs demonstrated activity ranging from 60 to >90%, with bridge creation and patch flocculation shown as working mechanisms. The adsorption of metals also showed an efficiency of >90%, with matrix entrapment being the main mechanism. The presence of enzymes in the EPS also facilitated decolorization and degradation. The degradation mechanisms involved reductase, laccase, and peroxidase. Despite its promising potential, the minimum development of the simpler rapid extraction of EPSs is the current limitation, with innovation for larger-scale applications being the current challenge for further application.
Author Contributions
H.A.H.: supervision, finding acquisition, conceptualization, writing—original draft; N.F.M.R.: conceptualization, writing—original draft; J.A. (Jahira Alias): writing—original draft; J.A. (Jamilah Ahmad): writing—original draft; N.S.M.S.: writing—original draft; N.N.R.: writing—original draft; J.B.: writing—original draft; S.R.S.A.: supervision, funding acquisition; A.R.O.: supervision, funding acquisition; H.H.W.J.: writing—review and editing; H.J.: writing—review and editing; S.B.K.: writing—original draft, writing—review and editing, visualization, conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
Universiti Kebangsaan Malaysia, Geran Universiti Penyelidikan no. GUP-2022-028.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
Author (Setyo Budi Kurniawan) would like to acknowledge Modal Insan Penyelidikan (RJA2) from Universiti Kebangsaan Malaysia for the Postdoctoral Researcher Scheme 2024.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
References
- Liu, Z.; Smith, S.R. Enzyme Recovery from Biological Wastewater Treatment. Waste Biomass Valor. 2021, 12, 4185–4211. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Gu, Y.; Chen, Y.; Hu, Y.; Tang, B.; Zhou, J.; Yang, Y.; Shi, L. Exploiting Extracellular Polymeric Substances (EPS) Controlling Strategies for Performance Enhancement of Biological Wastewater Treatments: An Overview. Chemosphere 2017, 180, 396–411. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Shen, Z.; Han, Z.; Zhou, Y. The Effect of Extracellular Polymeric Substances on Exogenous Highly Toxic Compounds in Biological Wastewater Treatment: An Overview. Bioresour. Technol. Rep. 2019, 5, 28–42. [Google Scholar] [CrossRef]
- Sheng, G.P.; Yu, H.Q.; Li, X.Y. Extracellular Polymeric Substances (EPS) of Microbial Aggregates in Biological Wastewater Treatment Systems: A Review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, Structure, and Function of Extracellular Polymeric Substances (EPS) of Microbial Biofilm in Biological Wastewater Treatment Systems: A Review. Desalinat. Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- Elnahas, M.O.; Amin, M.A.; Hussein, M.M.D.; Shanbhag, V.C.; Ali, A.E.; Wall, J.D. Isolation, Characterization and Bioactivities of an Extracellular Polysaccharide Produced from Streptomyces Sp. MOE6. Molecules 2017, 22, 1396. [Google Scholar] [CrossRef]
- Vasilieva, Z.; Gaponenkov, I.; Vasekha, M.; Ivanova, T. Extraction of Extracellular Polymeric Substances of Activated Sludge and Their Application for Wastewater Treatment. IOP Conf. Ser. Earth Environ. Sci. 2019, 302, 012018. [Google Scholar] [CrossRef]
- Brunt, E.G.; Burgess, J.G. The Promise of Marine Molecules as Cosmetic Active Ingredients. Int. J. Cosmet. Sci. 2018, 40, 1–15. [Google Scholar] [CrossRef]
- Zannini, E.; Waters, D.M.; Coffey, A.; Arendt, E.K. Production, Properties, and Industrial Food Application of Lactic Acid Bacteria-Derived Exopolysaccharides. Appl. Microbiol. Biotechnol. 2016, 100, 1121–1135. [Google Scholar] [CrossRef]
- Awasthi, S. Application of EPS in Agriculture: An Important Natural Resource for Crop Improvement. Agric. Res. Technol. 2017, 8, 22–24. [Google Scholar] [CrossRef]
- Nouha, K.; Kumar, R.S.; Tyagi, R.D. Heavy Metals Removal from Wastewater Using Extracellular Polymeric Substances Produced by Cloacibacterium Normanense in Wastewater Sludge Supplemented with Crude Glycerol and Study of Extracellular Polymeric Substances Extraction by Different Methods. Bioresour. Technol. 2016, 212, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolski, R.; Szcześ, A.; Czemierska, M.; Jarosz-Wikołazka, A. Studies of Cadmium(II), Lead(II), Nickel(II), Cobalt(II) and Chromium(VI) Sorption on Extracellular Polymeric Substances Produced by Rhodococcus Opacus and Rhodococcus Rhodochrous. Bioresour. Technol. 2017, 225, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Wang, Y.; Wang, X.; Li, M.; Han, F.; Ju, L.; Zhang, G.; Shi, L.; Li, K.; Wang, B.; et al. Toxicity Assessment of 4-Chlorophenol to Aerobic Granular Sludge and Its Interaction with Extracellular Polymeric Substances. J. Hazard. Mater. 2015, 289, 101–107. [Google Scholar] [CrossRef]
- Li, R.; Ning, X.a.; Sun, J.; Wang, Y.; Liang, J.; Lin, M.; Zhang, Y. Decolorization and Biodegradation of the Congo Red by Acinetobacter Baumannii YNWH 226 and Its Polymer Production’s Flocculation and Dewatering Potential. Bioresour. Technol. 2015, 194, 233–239. [Google Scholar] [CrossRef]
- Agunbiade, M.; Pohl, C.; Ashafa, O. Bioflocculant Production from Streptomyces Platensis and Its Potential for River and Waste Water Treatment. Braz. J. Microbiol. 2018, 49, 731–741. [Google Scholar] [CrossRef]
- Wang, L.F.; Qian, C.; Jiang, J.K.; Ye, X.D.; Yu, H.Q. Response of Extracellular Polymeric Substances to Thermal Treatment in Sludge Dewatering Process. Environ. Pollut. 2017, 231, 1388–1392. [Google Scholar] [CrossRef] [PubMed]
- Żur, J.; Wojcieszyńska, D.; Guzik, U. Metabolic Responses of Bacterial Cells to Immobilization. Molecules 2016, 21, 958. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular Polymeric Substances, a Key Element in Understanding Biofilm Phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef]
- Flemming, H.-C. EPS—Then and Now. Microorganisms 2016, 4, 41. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Tufail, M.A.; Asghar, H.N.; Nazli, F.; Zahir, Z.A. Appraising the Potential of EPS-producing Rhizobacteria with ACC-deaminase Activity to Improve Growth and Physiology of Maize under Drought Stress. Physiol. Plant 2020, 172, 463–476. [Google Scholar] [CrossRef]
- Nishanth, S.; Bharti, A.; Gupta, H.; Gupta, K.; Gulia, U.; Prasanna, R. Cyanobacterial Extracellular Polymeric Substances (EPS): Biosynthesis and Their Potential Applications. In Microbial and Natural Macromolecules; Academic Press: Cambridge, MA, USA, 2021; pp. 349–369. [Google Scholar]
- Udaiyappan, A.F.M.; Hasan, H.A.; Takriff, M.S.; Abdullah, S.R.S.; Maeda, T.; Mustapha, N.A.; Mohd Yasin, N.H.; Nazashida Mohd Hakimi, N.I. Microalgae-Bacteria Interaction in Palm Oil Mill Effluent Treatment. J. Water Process Eng. 2020, 35, 101203. [Google Scholar] [CrossRef]
- Gientka, I.; Blazejak, S.; Stasiak-Rózanska, L.; Chlebowska-Smigiel, A. Exopolysaccharides from Yeast: Insight into Optimal Conditions for Biosynthesis, Chemical Composition and Functional Properties—Review. Acta Sci. Pol. Technol. Aliment. 2015, 14, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, MBI.S10957. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Cui, X.; Wu, B.; Wang, Z.; Liu, Y.; Ren, T.; Xia, S.; Rittmann, B.E. Microalgal Extracellular Polymeric Substances (EPS) and Their Roles in Cultivation, Biomass Harvesting, and Bioproducts Extraction. Bioresour. Technol. 2024, 406, 131054. [Google Scholar] [CrossRef]
- Sun, Y.; Su, J.; Ali, A.; Zhang, S.; Zheng, Z.; Min, Y. Effect of Fungal Pellets on Denitrifying Bacteria at Low Carbon to Nitrogen Ratio: Nitrate Removal, Extracellular Polymeric Substances, and Potential Functions. Sci. Total Environ. 2022, 847, 157591. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Ismail, N.I.; Abdullah, S.R.S. Future Challenges in Diesel Biodegradation by Bacteria Isolates: A Review. J. Clean. Prod. 2020, 251, 119716. [Google Scholar] [CrossRef]
- Chug, R.; Mathur, S.; Kothari, S.L.; Harish; Gour, V.S. Maximizing EPS Production from Pseudomonas Aeruginosa and Its Application in Cr and Ni Sequestration. Biochem. Biophys. Rep. 2021, 26, 100972. [Google Scholar] [CrossRef]
- Biswas, J.; Paul, A.K. Optimization of Factors Influencing Exopolysaccharide Production by Halomonas Xianhensis Sur308 under Batch Culture. AIMS Microbiol. 2017, 3, 564–579. [Google Scholar] [CrossRef]
- Prete, R.; Alam, M.K.; Perpetuini, G.; Perla, C.; Pittia, P.; Corsetti, A. Lactic Acid Bacteria Exopolysaccharides Producers: A Sustainable Tool for Functional Foods. Foods 2021, 10, 1653. [Google Scholar] [CrossRef]
- Park, Y.J.; Kim, Y.J.; Yu, H.H.; Lee, N.-K.; Paik, H.-D. Cell-Free Supernatants of Bacillus Subtilis and Bacillus Polyfermenticus Inhibit Listeria Monocytogenes Biofilm Formation. Food Control 2023, 144, 109387. [Google Scholar] [CrossRef]
- Chug, R.; Gour, V.S.; Mathur, S.; Kothari, S.L. Optimization of Extracellular Polymeric Substances Production Using Azotobacter Beijreinckii and Bacillus Subtilis and Its Application in Chromium (VI) Removal. Bioresour. Technol. 2016, 214, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Delbarre-Ladrat, C.; Sinquin, C.; Marchand, L.; Bonnetot, S.; Zykwinska, A.; Verrez-Bagnis, V.; Colliec-Jouault, S. Influence of the Carbon and Nitrogen Sources on Diabolican Production by the Marine Vibrio Diabolicus Strain CNCM I-1629. Polym. 2022, 14, 1994. [Google Scholar] [CrossRef] [PubMed]
- Costa, O.Y.A.; Raaijmakers, J.M.; Kuramae, E.E. Microbial Extracellular Polymeric Substances: Ecological Function and Impact on Soil Aggregation. Front. Microbiol. 2018, 9, 337094. [Google Scholar] [CrossRef]
- Nguyen, P.; Nguyen, T.; Bui, D.; Hong, P. Exopolysaccharide Production by Lactic Acid Bacteria: The Manipulation of Environmental Stresses for Industrial Applications. AIMS Microbiol. 2020, 6, 451–469. [Google Scholar] [CrossRef]
- Morcillo, R.; Manzanera, M. The Effects of Plant-Associated Bacterial Exopolysaccharides on Plant Abiotic Stress Tolerance. Metabolites 2021, 11, 337. [Google Scholar] [CrossRef]
- Yan, M.; Wang, B.; Xu, X.; der Meister, T.; Tabγač, H.; Hwang, F.; Liu, Z. Extrusion of Dissolved Oxygen by Exopolysaccharide from Leuconostoc Mesenteroides and Its Implications in Relief of the Oxygen Stress. Front. Microbiol. 2018, 9, 2467. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Maiti, T.K. Structure of Extracellular Polysaccharides (EPS) Produced by Rhizobia and Their Functions in Legume–Bacteria Symbiosis—A Review. Achiev. Life Sci. 2016, 10, 136–143. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Yellamma, K. Genetic and Metabolic Engineering of Microorganisms for the Production of Various Food Products. In Recent Developments in Applied Microbiology and Biochemistry; Academic Press: Cambridge, MA, USA, 2019; pp. 167–182. [Google Scholar]
- Buhari, J.; Hasan, H.A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R. Future and Challenges of Co-Biofilm Treatment on Ammonia and Bisphenol A Removal from Wastewater. J. Water Process Eng. 2023, 54, 103969. [Google Scholar] [CrossRef]
- Pechaud, Y.; Peyre Lavigne, M.; Bessiere, Y.; Ochoa, J.C.; Queinnec, I.; Paul, E. Influence of Shear Stress, Organic Loading Rate and Hydraulic Retention Time on the Biofilm Structure and on the Competition between Different Biological Aggregate Morphotypes. J. Environ. Chem. Eng. 2022, 10, 107597. [Google Scholar] [CrossRef]
- Gangalla, R.; Sampath, G.; Beduru, S.; Sarika, K.; Kaveriyappan Govindarajan, R.; Ameen, F.; Alwakeel, S.; Thampu, R.K. Optimization and Characterization of Exopolysaccharide Produced by Bacillus Aerophilus Rk1 and Its in Vitro Antioxidant Activities. J. King Saud. Univ. Sci. 2021, 33, 101470. [Google Scholar] [CrossRef]
- Hasan, H.A.; Ezril Hafiz, R.; Muhamad, M.H.; Sheikh Abdullah, S.R.; Hasan, H.A.; Ezril Hafiz, R.; Muhamad, M.H.; Sheikh Abdullah, S.R.; Hassimi, A.H.; Ezril Hafiz, R.; et al. Bioflocculant Production Using Palm Oil Mill and Sago Mill Effluent as a Fermentation Feedstock: Characterization and Mechanism of Flocculation. J. Environ. Manag. 2020, 260, 110046. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, Y.; Han, C.; Liu, H.; Chen, Y.; Zhou, J.; Su, S. Production of a Bioflocculant Using Old Polyester Fibre as a Fermentation Feedstock and Its Use in Treatment of Polyester Alkali-Peeling Wastewater. J. Environ. Chem. Eng. 2021, 9, 105455. [Google Scholar] [CrossRef]
- Sayyed, R.Z.; Jamadar, D.D.; Patel, P.R. Production of Exo-Polysaccharide by Rhizobium Sp. Indian. J. Microbiol. 2011, 51, 294–300. [Google Scholar] [CrossRef]
- Ghosh, P.K.; Ganguly, J.; Maji, P.; Maiti, T.K. Production and Composition of Extracellular Polysaccharide Synthesized by Rhizobium Undicola Isolated from Aquatic Legume, Neptunia Oleracea Lour. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 581–590. [Google Scholar] [CrossRef]
- Yan, Z.-R.; Meng, H.-S.; Yang, X.-Y.; Zhu, Y.-Y.; Li, X.-Y.; Xu, J.; Sheng, G.-P. Insights into the Interactions between Triclosan (TCS) and Extracellular Polymeric Substance (EPS) of Activated Sludge. J. Environ. Manag. 2019, 232, 219–225. [Google Scholar] [CrossRef]
- Kurniawan, S.B.S.B.S.B.; Imron, M.F.M.F.; Sługocki, Ł.; Nowakowski, K.; Ahmad, A.; Najiya, D.; Abdullah, S.R.S.S.R.S.; Othman, A.R.A.R.; Purwanti, I.F.I.F.; Hasan, H.A.H.A.; et al. Assessing the Effect of Multiple Variables on the Production of Bioflocculant by Serratia Marcescens: Flocculating Activity, Kinetics, Toxicity, and Flocculation Mechanism. Sci. Total Environ. 2022, 836, 155564. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Panesar, P.S.; Mehariya, S. Microbial Exopolysaccharides; CRC Press: Boca Raton, FL, USA, 2024; ISBN 9781003342687. [Google Scholar]
- Singh, N.K.; Pandey, S.; Singh, R.P.; Dahiya, S.; Gautam, S.; Kazmi, A.A. Effect of Intermittent Aeration Cycles on EPS Production and Sludge Characteristics in a Field Scale IFAS Reactor. J. Water Process Eng. 2018, 23, 230–238. [Google Scholar] [CrossRef]
- Miqueleto, A.P.; Dolosic, C.C.; Pozzi, E.; Foresti, E.; Zaiat, M. Influence of Carbon Sources and C/N Ratio on EPS Production in Anaerobic Sequencing Batch Biofilm Reactors for Wastewater Treatment. Bioresour. Technol. 2010, 101, 1324–1330. [Google Scholar] [CrossRef]
- Traina, F.; Capodici, M.; Torregrossa, M.; Viviani, G.; Corsino, S.F. PHA and EPS Production from Industrial Wastewater by Conventional Activated Sludge, Membrane Bioreactor and Aerobic Granular Sludge Technologies: A Comprehensive Comparison. Chemosphere 2024, 355, 141768. [Google Scholar] [CrossRef]
- Shao, Y.; Zhang, H.; Buchanan, I.; Mohammed, A.; Liu, Y. Comparison of Extracellular Polymeric Substance (EPS) in Nitrification and Nitritation Bioreactors. Int. Biodeterior. Biodegrad. 2019, 143, 104713. [Google Scholar] [CrossRef]
- More, T.T.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Biopolymers Production by Mixed Culture and Their Applications in Water and Wastewater Treatment. Water Environ. Res. 2015, 87, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Nouha, K. EPS Producing Microorganisms from Municipal Wastewater Activated Sludge. J. Pet. Environ. Biotechnol. 2015, 7, 1. [Google Scholar] [CrossRef]
- Ajao, V.; Bruning, H.; Rijnaarts, H.; Temmink, H. Natural Flocculants from Fresh and Saline Wastewater: Comparative Properties and Flocculation Performances. Chem. Eng. J. 2018, 349, 622–632. [Google Scholar] [CrossRef]
- Al-Dhabi, N.A.; Esmail, G.A.; Valan Arasu, M. Sustainable Conversion of Palm Juice Wastewater into Extracellular Polysaccharides for Absorption of Heavy Metals from Saudi Arabian Wastewater. J. Clean. Prod. 2020, 277, 124252. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Shang, H.; Li, Q.; Zhou, W. Treatment of Azo Dye Wastewater by the Self-Flocculating Marine Bacterium Aliiglaciecola Lipolytica. Environ. Technol. Innov. 2020, 19, 100810. [Google Scholar] [CrossRef]
- Zhang, B.; Ning, D.; Yang, Y.; Van Nostrand, J.D.; Zhou, J.; Wen, X. Biodegradability of Wastewater Determines Microbial Assembly Mechanisms in Full-Scale Wastewater Treatment Plants. Water Res. 2020, 169, 115276. [Google Scholar] [CrossRef]
- Bezawada, J.; Hoang, N.V.; More, T.T.; Yan, S.; Tyagi, N.; Tyagi, R.D.; Surampalli, R.Y. Production of Extracellular Polymeric Substances (EPS) by Serratia Sp.1 Using Wastewater Sludge as Raw Material and Flocculation Activity of the EPS Produced. J. Environ. Manag. 2013, 128, 83–91. [Google Scholar] [CrossRef]
- Ajao, V.; Fokkink, R.; Leermakers, F.; Bruning, H.; Rijnaarts, H.; Temmink, H. Bioflocculants from Wastewater: Insights into Adsorption Affinity, Flocculation Mechanisms and Mixed Particle Flocculation Based on Biopolymer Size-Fractionation. J. Colloid. Interface Sci. 2021, 581, 533–544. [Google Scholar] [CrossRef]
- Yellapu, S.K.; Klai, N.; Kaur, R.; Tyagi, R.D.; Surampalli, R.Y. Oleaginous Yeast Biomass Flocculation Using Bioflocculant Produced in Wastewater Sludge and Transesterification Using Petroleum Diesel as a Co-Solvent. Renew. Energy 2019, 131, 217–228. [Google Scholar] [CrossRef]
- Li, Z.; Lu, P.; Zhang, D.; Chen, G.; Zeng, S.; He, Q. Population Balance Modeling of Activated Sludge Flocculation: Investigating the Influence of Extracellular Polymeric Substances (EPS) Content and Zeta Potential on Flocculation Dynamics. Sep. Purif. Technol. 2016, 162, 91–100. [Google Scholar] [CrossRef]
- Kyung, O.; Hendren, Z.; Dong, G.; Dong, D.; Woo, J. In Fl Uence of Activated Sludge Derived-Extracellular Polymeric Substance (ASD-EPS) as Bio- Fl Occulation of Microalgae for Biofuel Recovery. Algal Res. 2020, 45, 101736. [Google Scholar] [CrossRef]
- Pu, L.; Zeng, Y.-J.; Xu, P.; Li, F.-Z.; Zong, M.-H.; Yang, J.-G.; Lou, W.-Y. Using a Novel Polysaccharide BM2 Produced by Bacillus Megaterium Strain PL8 as an Efficient Bioflocculant for Wastewater Treatment. Int. J. Biol. Macromol. 2020, 162, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Silva, L.; Paulo, J.; Faria, M.; Nogueira, N.; Cordeiro, N. Microalgal-Based Biopolymer for Nano- and Microplastic Removal: A Possible Biosolution for Wastewater Treatment. Environ. Pollut. 2020, 263, 114385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Xu, X.-Y.; Zhang, X.-L.; Zou, K.; Liu, B.-Z.; Qing, T.-P.; Feng, B. Nanoparticles-EPS Corona Increases the Accumulation of Heavy Metals and Biotoxicity of Nanoparticles. J. Hazard. Mater. 2020, 409, 124526. [Google Scholar] [CrossRef]
- Tyagi, B.; Gupta, B.; Thakur, I.S. Biosorption of Cr (VI) from Aqueous Solution by Extracellular Polymeric Substances (EPS) Produced by Parapedobacter sp. ISTM3 Strain Isolated from Mawsmai cave, Meghalaya, India. Environ. Res. 2020, 191, 110064. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K. Flocculation efficiency of the Sinorhizobium meliloti 1021 exopolysaccharide relative to mineral oxide suspensions—A preliminary study for wastewater treatment. Sep. Purif. Technol. 2018, 201, 51–59. [Google Scholar] [CrossRef]
- Paulo, A.M.S.; Amorim, C.L.; Costa, J.; Mesquita, D.P.; Ferreira, E.C.; Castro, P.M.L. Science of the Total Environment Long-Term Stability of a Non-Adapted Aerobic Granular Sludge Process Treating Fi Sh Canning Wastewater Associated to EPS Producers in the Core Microbiome. Sci. Total Environ. 2020, 756, 144007. [Google Scholar] [CrossRef]
- Hu, Y.Q.; Wei, W.; Gao, M.; Zhou, Y.; Wang, G.X.; Zhang, Y. Effect of Pure Oxygen Aeration on Extracellular Polymeric Substances (EPS) of Activated Sludge Treating Saline Wastewater. Process Saf. Environ. Prot. 2019, 123, 344–350. [Google Scholar] [CrossRef]
- Govarthanan, M.; Jeon, C.; Jeon, Y.; Kwon, J.-H.; Bae, H.; Kim, W. Non-toxic nano approach for wastewater treatment using Chlorella vulgaris exopolysaccharides immobilized in iron-magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 162, 1241–1249. [Google Scholar] [CrossRef]
- Cui, Y.-W.; Huang, J.-L.; Alam, F. Fast Granulation of Halophilic Activated Sludge Treating Low-Strength Organic Saline Wastewater via Addition of Divalent Cations. Chemosphere 2021, 264, 128396. [Google Scholar] [CrossRef]
- Alias, J.; Hasan, H.A.; Abdullah, S.R.S.; Othman, A.R. Properties of Bioflocculant-Producing Bacteria for High Flocculating Activity Efficiency. Environ. Technol. Innov. 2022, 27, 102529. [Google Scholar] [CrossRef]
- Almansoory, A.F.; Al-Baldawi, I.A.; Hazaimeh, M. Optimization of the EPS Production of a Bacterial Floc Consortium Using Different Parameters. Biocatal. Agric. Biotechnol. 2020, 23, 101466. [Google Scholar] [CrossRef]
- Lapointe, M.; Barbeau, B. Understanding the Roles and Characterizing the Intrinsic Properties of Synthetic vs. Natural Polymers to Improve Clarification through Interparticle Bridging: A Review. Sep. Purif. Technol. 2020, 231, 115893. [Google Scholar] [CrossRef]
- Choy, S.Y.; Prasad, K.M.N.; Wu, T.Y.; Raghunandan, M.E.; Phang, S.M.; Juan, J.C.; Ramanan, R.N. Starch-Based Flocculant Outperformed Aluminium Sulfate Hydrate and Polyaluminium Chloride through Effective Bridging for Harvesting Acicular Microalga Ankistrodesmus. Algal Res. 2018, 29, 343–353. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Kurniawan, S.B.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R. Performance of Water-Extracted Leucaena Leucocephala Seeds as Coagulant and Alum in Treating Aquaculture Effluent: Effect of Dosage, Rapid Mixing Speed, and Settling Time. Int. J. Environ. Sci. Technol. 2023, 20, 9981–9994. [Google Scholar] [CrossRef]
- Alnawajha, M.M.; Kurniawan, S.B.; Imron, M.F.; Abdullah, S.R.S.; Hasan, H.A.; Othman, A.R. Plant-Based Coagulants/Flocculants: Characteristics, Mechanisms, and Possible Utilization in Treating Aquaculture Effluent and Benefiting from the Recovered Nutrients. Environ. Sci. Pollut. Res. 2022, 29, 58430–58453. [Google Scholar] [CrossRef]
- Ye, L.; Wu, J.; Huang, M.; Yan, J. The Role of Suspended Extracellular Polymeric Substance (EPS) on Equilibrium Flocculation of Clay Minerals in High Salinity Water. Water Res. 2023, 244, 120451. [Google Scholar] [CrossRef]
- Mohite, B.V.; Koli, S.H.; Narkhede, C.P.; Patil, S.N.; Patil, S.V. Prospective of Microbial Exopolysaccharide for Heavy Metal Exclusion. Appl. Biochem. Biotechnol. 2017, 183, 582–600. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-Based Biopolymer Hydrogels for Heavy Metal Detection and Adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef]
- Fouda-Mbanga, B.G.; Prabakaran, E.; Pillay, K. Carbohydrate Biopolymers, Lignin Based Adsorbents for Removal of Heavy Metals (Cd2+, Pb2+, Zn2+) from Wastewater, Regeneration and Reuse for Spent Adsorbents Including Latent Fingerprint Detection: A Review. Biotechnol. Rep. 2021, 30, e00609. [Google Scholar] [CrossRef]
- Ramli, N.N.; Kurniawan, S.B.; Ighalo, J.O.; Mohd Said, N.S.; Marsidi, N.; Buhari, J.; Ramli Shah, R.A.; Zulkifli, M.; Alias, J.; Daud, N.M.; et al. A Review of the Treatment Technologies for Hexavalent Chromium Contaminated Water. BioMetals 2023, 36, 1189–1219. [Google Scholar] [CrossRef] [PubMed]
- Ramli, N.N.N.N.; Othman, A.R.A.R.; Kurniawan, S.B.S.B.S.B.; Abdullah, S.R.S.S.R.S.; Hasan, H.A.H.A. Metabolic Pathway of Cr(VI) Reduction by Bacteria: A Review. Microbiol. Res. 2023, 268, 127288. [Google Scholar] [CrossRef]
- Ren, L.; Hong, Z.; Qian, W.; Li, J.; Xu, R. Adsorption Mechanism of Extracellular Polymeric Substances from Two Bacteria on Ultisol and Alfisol. Environ. Pollut. 2018, 237, 39–49. [Google Scholar] [CrossRef]
- Khatri, A.; White, M. Sustainable Dyeing Technologies. In Sustainable Apparel; Woodhead Publishing: Sawston, UK, 2015; pp. 135–160. [Google Scholar]
- Brigé, A.; Motte, B.; Borloo, J.; Buysschaert, G.; Devreese, B.; Van Beeumen, J.J. Bacterial Decolorization of Textile Dyes Is an Extracellular Process Requiring a Multicomponent Electron Transfer Pathway. Microb. Biotechnol. 2008, 1, 40–52. [Google Scholar] [CrossRef]
- Li, Y.; Sun, L.-L.; Sun, Y.-Y.; Cha, Q.-Q.; Li, C.-Y.; Zhao, D.-L.; Song, X.-Y.; Wang, M.; McMinn, A.; Chen, X.-L.; et al. Extracellular Enzyme Activity and Its Implications for Organic Matter Cycling in Northern Chinese Marginal Seas. Front. Microbiol. 2019, 10, 2137. [Google Scholar] [CrossRef]
- Bozelli, J.C.; Epand, R.M. Determinants of Lipids Acyl Chain Specificity: A Tale of Two Enzymes. Biophys. Chem. 2020, 265, 106431. [Google Scholar] [CrossRef]
- Martínez, R.J.; Godínez, L.A.; Robles, I. Waste Resources Utilization for Biosorbent Preparation, Sorption Studies, and Electrocatalytic Applications. In Valorization of Wastes for Sustainable Development; Elsevier: Amsterdam, The Netherlands, 2023; pp. 395–418. [Google Scholar]
- Ikram, M.; Zahoor, M.; Naeem, M.; Islam, N.U.; Shah, A.B.; Shahzad, B. Bacterial Oxidoreductive Enzymes as Molecular Weapons for the Degradation and Metabolism of the Toxic Azo Dyes in Wastewater: A Review. Z. Phys. Chem. 2023, 237, 187–209. [Google Scholar] [CrossRef]
- Debnath, R.; Saha, T. An Insight into the Production Strategies and Applications of the Ligninolytic Enzyme Laccase from Bacteria and Fungi. Biocatal. Agric. Biotechnol. 2020, 26, 101645. [Google Scholar] [CrossRef]
- Chandanshive, V.V.; Rane, N.R.; Gholave, A.R.; Patil, S.M.; Jeon, B.H.; Govindwar, S.P. Efficient Decolorization and Detoxification of Textile Industry Effluent by Salvinia Molesta in Lagoon Treatment. Environ. Res. 2016, 150, 88–96. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, Y.; Khanal, S.K.; Lu, H.; Fang, H.; Zhao, Q. Understanding the Role of Extracellular Polymeric Substances on Ciprofloxacin Adsorption in Aerobic Sludge, Anaerobic Sludge, and Sulfate-Reducing Bacteria Sludge Systems. Environ. Sci. Technol. 2018, 52, 6476–6486. [Google Scholar] [CrossRef]
- Wei, Z.; Niu, S.; Wei, Y.; Liu, Y.; Xu, Y.; Yang, Y.; Zhang, P.; Zhou, Q.; Wang, J.J. The Role of Extracellular Polymeric Substances (EPS) in Chemical-Degradation of Persistent Organic Pollutants in Soil: A Review. Sci. Total Environ. 2024, 912, 168877. [Google Scholar] [CrossRef] [PubMed]
- Lü, F.; Wang, J.; Shao, L.; He, P. Enzyme Disintegration with Spatial Resolution Reveals Different Distributions of Sludge Extracellular Polymer Substances. Biotechnol. Biofuels 2016, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-M.; Wu, H.-Z.; Wang, Y.-X.; Zhu, S.; Wei, C.-H. Enhancement of Phenol Biodegradation: Metabolic Division of Labor in Co-Culture of Stenotrophomonas Sp. N5 and Advenella Sp. B9. J. Hazard. Mater. 2020, 400, 123214. [Google Scholar] [CrossRef] [PubMed]
- Köhler, A.; Hellweg, S.; Escher, B.I.; Hungerbühler, K. Organic Pollutant Removal versus Toxicity Reduction in Industrial Wastewater Treatment: The Example of Wastewater from Fluorescent Whitening Agent Production. Environ. Sci. Technol. 2006, 40, 3395–3401. [Google Scholar] [CrossRef]
- De Sotto, R.; Bae, S. Nutrient Removal Performance and Microbiome of an Energy-Efficient Reciprocation MLE-MBR Operated under Hypoxic Conditions. Water Res. 2020, 182, 115991. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Gu, J.; Liu, H.; Wang, S.; Zhang, M.; Liu, Y. An Innovative Anaerobic MBR-Reverse Osmosis-Ion Exchange Process for Energy-Efficient Reclamation of Municipal Wastewater to NEWater-like Product Water. J. Clean. Prod. 2019, 230, 1287–1293. [Google Scholar] [CrossRef]
- Bin, Z.; Baosheng, S.; Min, J.; Taishi, G.; Zhenghong, G. Extraction and Analysis of Extracellular Polymeric Substances in Membrane Fouling in Submerged MBR. Desalination 2008, 227, 286–294. [Google Scholar] [CrossRef]
- Huang, H.; Fan, X.; Peng, P.; Peng, C.; Gao, Y.; Zhang, X.; Ren, H. Two Birds with One Stone: Simultaneous Improvement of Biofilm Formation and Nitrogen Transformation in MBBR Treating High Ammonia Nitrogen Wastewater via Exogenous N-Acyl Homoserine Lactones. Chem. Eng. J. 2020, 386, 124001. [Google Scholar] [CrossRef]
- Li, C.; Liang, J.; Lin, X.; Xu, H.; Tadda, M.A.; Lan, L.; Liu, D. Fast Start-up Strategies of MBBR for Mariculture Wastewater Treatment. J. Environ. Manag. 2019, 248, 109267. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Q.; Zhu, Y.; Zhao, T. Response of Wastewater Treatment Performance, Microbial Composition and Functional Genes to Different C/N Ratios and Carrier Types in MBBR Inoculated with Heterotrophic Nitrification-Aerobic Denitrification Bacteria. Bioresour. Technol. 2021, 336, 125339. [Google Scholar] [CrossRef] [PubMed]
- Shitu, A.; Zhu, S.; Qi, W.; Tadda, M.A.; Liu, D.; Ye, Z. Performance of Novel Sponge Biocarrier in MBBR Treating Recirculating Aquaculture Systems Wastewater: Microbial Community and Kinetic Study. J. Environ. Manag. 2020, 275, 111264. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Pakshirajan, K. Continuous Removal and Recovery of Metals from Wastewater Using Inverse Fluidized Bed Sulfidogenic Bioreactor. J. Clean. Prod. 2021, 284, 124769. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.A.; Kamika, I.; Momba, M.N.B. Data on the Degradation of Pharmaceuticals and Their Metabolites by a Fungal Consortium in a Non-Sterile Stirred Fluidized Bioreactor. Data Brief. 2020, 28, 105057. [Google Scholar] [CrossRef]
- Aboabboud, M.; Ibrahim, H.; Awad, A. Biological Ammonia Removal from Drinking Water in Fluidized Bed Reactors. WIT Trans. Ecol. Environ. 2008, 111, 453–463. [Google Scholar] [CrossRef]
- Gao, D.W.; Hu, Q.; Yao, C.; Ren, N.Q.; Wu, W.M. Integrated Anaerobic Fluidized-Bed Membrane Bioreactor for Domestic Wastewater Treatment. Chem. Eng. J. 2014, 240, 362–368. [Google Scholar] [CrossRef]
- Tekerlekopoulou, A.G.; Vayenas, D.V. Ammonia, Iron and Manganese Removal from Potable Water Using Trickling Filters. Desalination 2007, 210, 225–235. [Google Scholar] [CrossRef]
- Tang, W.; Li, X.; Liu, H.; Wu, S.; Zhou, Q.; Du, C.; Teng, Q.; Zhong, Y.; Yang, C. Sequential Vertical Flow Trickling Filter and Horizontal Flow Multi-Soil-Layering Reactor for Treatment of Decentralized Domestic Wastewater with Sodium Dodecyl Benzene Sulfonate. Bioresour. Technol. 2020, 300, 122634. [Google Scholar] [CrossRef]
- Giri, S.S.; Ryu, E.C.; Park, S.C. Characterization of the Antioxidant and Anti-Inflammatory Properties of a Polysaccharide-Based Bioflocculant from Bacillus Subtilis F9. Microb. Pathog. 2019, 136, 103642. [Google Scholar] [CrossRef]
- Giri, S.S.; Harshiny, M.; Sen, S.S.; Sukumaran, V.; Park, S.C. Production and Characterization of a Thermostable Bioflocculant from Bacillus Subtilis F9, Isolated from Wastewater Sludge. Ecotoxicol. Environ. Saf. 2015, 121, 45–50. [Google Scholar] [CrossRef]
- Cao, G.; Zhang, Y.; Chen, L.; Liu, J.; Mao, K.; Li, K.; Zhou, J. Production of a Bioflocculant from Methanol Wastewater and Its Application in Arsenite Removal. Chemosphere 2015, 141, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, C. Removal of Arsenite by a Microbial Bioflocculant Produced from Swine Wastewater. Chemosphere 2017, 181, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Nancharaiah, Y.V.; Sarvajith, M. Aerobic Granular Sludge Process: A Fast Growing Biological Treatment for Sustainable Wastewater Treatment. Curr. Opin. Environ. Sci. Health 2019, 12, 57–65. [Google Scholar] [CrossRef]
- Seviour, T.; Yuan, Z.; van Loosdrecht, M.C.M.; Lin, Y. Aerobic Sludge Granulation: A Tale of Two Polysaccharides? Water Res. 2012, 46, 4803–4813. [Google Scholar] [CrossRef]
- Karakas, I.; Sam, S.B.; Cetin, E.; Dulekgurgen, E.; Yilmaz, G. Resource Recovery from an Aerobic Granular Sludge Process Treating Domestic Wastewater. J. Water Process Eng. 2020, 34, 101148. [Google Scholar] [CrossRef]
- Felz, S.; Kleikamp, H.; Zlopasa, J.; van Loosdrecht, M.C.M.; Lin, Y. Impact of Metal Ions on Structural EPS Hydrogels from Aerobic Granular Sludge. Biofilm 2020, 2, 100011. [Google Scholar] [CrossRef]
- Felz, S.; Vermeulen, P.; van Loosdrecht, M.C.M.; Lin, Y.M. Chemical Characterization Methods for the Analysis of Structural Extracellular Polymeric Substances (EPS). Water Res. 2019, 157, 201–208. [Google Scholar] [CrossRef]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of Structural Extracellular Polymeric Substances from Aerobic Granular Sludge. J. Vis. Exp. 2016, 115, 54534. [Google Scholar] [CrossRef]
- Schambeck, C.M.; Magnus, B.S.; de Souza, L.C.R.; Leite, W.R.M.; Derlon, N.; Guimarães, L.B.; da Costa, R.H.R. Biopolymers Recovery: Dynamics and Characterization of Alginate-like Exopolymers in an Aerobic Granular Sludge System Treating Municipal Wastewater without Sludge Inoculum. J. Environ. Manag. 2020, 263, 110394. [Google Scholar] [CrossRef]
- Nancharaiah, Y.V.; Kiran Kumar Reddy, G. Aerobic Granular Sludge Technology: Mechanisms of Granulation and Biotechnological Applications. Bioresour. Technol. 2018, 247, 1128–1143. [Google Scholar] [CrossRef]
- Lin, Y.M.; Nierop, K.G.J.; Girbal-Neuhauser, E.; Adriaanse, M.; van Loosdrecht, M.C.M. Sustainable Polysaccharide-Based Biomaterial Recovered from Waste Aerobic Granular Sludge as a Surface Coating Material. Sustain. Mater. Technol. 2015, 4, 24–29. [Google Scholar] [CrossRef]
- Van Leeuwen, K.; de Vries, E.; Koop, S.; Roest, K. The Energy & Raw Materials Factory: Role and Potential Contribution to the Circular Economy of the Netherlands. Environ. Manag. 2018, 61, 786–795. [Google Scholar] [CrossRef]
- Shukla, A.; Mehta, K.; Parmar, J.; Pandya, J.; Saraf, M. Depicting the Exemplary Knowledge of Microbial Exopolysaccharides in a Nutshell. Eur. Polym. J. 2019, 119, 298–310. [Google Scholar] [CrossRef]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular Biopolymers Recovered as Raw Biomaterials from Waste Granular Sludge and Potential Applications: A Critical Review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame Retardant Property of Flax Fabrics Coated by Extracellular Polymeric Substances Recovered from Both Activated Sludge and Aerobic Granular Sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef]
- Gupta, A.; Thakur, I.S. Study of Optimization of Wastewater Contaminant Removal along with Extracellular Polymeric Substances (EPS) Production by a Thermotolerant Bacillus Sp. ISTVK1 Isolated from Heat Shocked Sewage Sludge. Bioresour. Technol. 2016, 213, 21–30. [Google Scholar] [CrossRef]
- Xia, L.; Tan, J.; Wu, P.; He, Q.; Song, S.; Li, Y. Biopolymers Extracted from Klebsiella Sp. and Bacillus Sp. in Wastewater Sludge as Superb Adsorbents for Aqueous Hg(II) Removal from Water. Chem. Phys. Lett. 2020, 754, 137689. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, K.; Jin, H.; Lei, L.; Zhang, H.; Gan, H. Removal of Acenaphthene from Wastewater by Pseudomonas Sp. in Anaerobic Conditions: The Effects of Extra and Intracellular Substances. Environ. Technol. 2020, 41, 1298–1306. [Google Scholar] [CrossRef]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M.; Swings, J.; De Vuyst, L. Biodiversity of Exopolysaccharides Produced by Streptococcus Thermophilus Strains Is Reflected in Their Production and Their Molecular and Functional Characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef]
- Daba, G.M.; Elnahas, M.O.; Elkhateeb, W.A. Contributions of Exopolysaccharides from Lactic Acid Bacteria as Biotechnological Tools in Food, Pharmaceutical, and Medical Applications. Int. J. Biol. Macromol. 2021, 173, 79–89. [Google Scholar] [CrossRef]
- Gupta, J.; Rathour, R.; Dupont, C.L.; Kaul, D.; Thakur, I.S. Genomic Insights into Waste Valorized Extracellular Polymeric Substances (EPS) Produced by Bacillus Sp. ISTL8. Environ. Res. 2021, 192, 110277. [Google Scholar] [CrossRef]
- Nagaraj, V.; Skillman, L.; Li, D.; Ho, G. Review—Bacteria and Their Extracellular Polymeric Substances Causing Biofouling on Seawater Reverse Osmosis Desalination Membranes. J. Environ. Manag. 2018, 223, 586–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Fang, H.H.P. Extraction of Extracellular Polymeric Substances (EPS) of Sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Purwanti, I.F.; Imron, M.F.; Ismail, N.I.; Ahmad, A.; Hasan, H.A. Isolation and Characterisation of Bioflocculant-Producing Bacteria from Aquaculture Effluent and Its Performance in Treating High Turbid Water. J. Water Process Eng. 2021, 42, 102194. [Google Scholar] [CrossRef]
- Abu Bakar, S.N.H.; Abu Hasan, H.; Abdullah, S.R.S.; Kasan, N.A.; Muhamad, M.H.; Kurniawan, S.B. A Review of the Production Process of Bacteria-Based Polymeric Flocculants. J. Water Process Eng. 2021, 40, 101915. [Google Scholar] [CrossRef]
- Ahmad, A.; Kurniawan, S.B.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Exploring the Extraction Methods for Plant-Based Coagulants and Their Future Approaches. Sci. Total Environ. 2022, 818, 151668. [Google Scholar] [CrossRef]
- Angelin, J.; Kavitha, M. Exopolysaccharides from Probiotic Bacteria and Their Health Potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Li, W.; Xia, X.; Tang, W.; Ji, J.; Rui, X.; Chen, X.; Jiang, M.; Zhou, J.; Zhang, Q.; Dong, M. Structural Characterization and Anticancer Activity of Cell-Bound Exopolysaccharide from Lactobacillus Helveticus MB2-1. J. Agric. Food Chem. 2015, 63, 3454–3463. [Google Scholar] [CrossRef]
- Xu, D.; Hu, Y.; Wu, F.; Jin, Y.; Xu, X.; Gänzle, M.G. Comparison of the Functionality of Exopolysaccharides Produced by Sourdough Lactic Acid Bacteria in Bread and Steamed Bread. J. Agric. Food Chem. 2020, 68, 8907–8914. [Google Scholar] [CrossRef]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of Extracellular Polymeric Substances (EPS) Production in Bioaggregation: Application to Wastewater Treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905. [Google Scholar] [CrossRef]
- Zeng, J.; Gao, J.-M.; Chen, Y.-P.; Yan, P.; Dong, Y.; Shen, Y.; Guo, J.-S.; Zeng, N.; Zhang, P. Composition and Aggregation of Extracellular Polymeric Substances (EPS) in Hyperhaline and Municipal Wastewater Treatment Plants. Sci. Rep. 2016, 6, 26721. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Amorim, C.L.; Ramos, M.A.; Mesquita, D.P.; Inocêncio, P.; Ferreira, E.C.; van Loosdrecht, M.; Castro, P.M.L. Variability in the Composition of Extracellular Polymeric Substances from a Full-Scale Aerobic Granular Sludge Reactor Treating Urban Wastewater. J. Environ. Chem. Eng. 2020, 8, 104156. [Google Scholar] [CrossRef]
- Samer, M. Biological and Chemical Wastewater Treatment Processes. Wastewater Treat. Eng. 2015, 247, 1128–1143. [Google Scholar] [CrossRef]
- Moghannem, S.A.M.; Farag, M.M.S.; Shehab, A.M.; Azab, M.S. Exopolysaccharide Production from Bacillus Velezensis KY471306 Using Statistical Experimental Design. Braz. J. Microbiol. 2018, 49, 452–462. [Google Scholar] [CrossRef]
- Hua, X.; Wu, Z.; Zhang, H.; Lu, D.; Wang, M.; Liu, Y.; Liu, Z. Degradation of Hexadecane by Enterobacter Cloacae Strain TU That Secretes an Exopolysaccharide as a Bioemulsifier. Chemosphere 2010, 80, 951–956. [Google Scholar] [CrossRef]
- Uthayasooriyan, M.; Pathmanathan, S.; Ravimannan, N.; Sathyaruban, S. Formulation of Alternative Culture Media for Bacterial and Fungal Growth. Pharm. Lett. 2016, 8, 431–436. [Google Scholar]
- Arulanantham, R.; Pathmanathan, S.; Ravimannan, N.; Niranjan, K. Alternative Culture Media for Bacterial Growth Using Different Formulation of Protein Sources. J. Nat. Prod. Plant Resour 2012, 2, 697–700. [Google Scholar]
- Lukwambe, B.; Yang, W.; Zheng, Y.; Nicholaus, R.; Zhu, J.; Zheng, Z. Bioturbation by the Razor Clam (Sinonovacula Constricta) on the Microbial Community and Enzymatic Activities in the Sediment of an Ecological Aquaculture Wastewater Treatment System. Sci. Total Environ. 2018, 643, 1098–1107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).