Use of Brushite as Adsorbent for the Removal of Anionic and Cationic Dyes Present in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Development of the Bioadsorbent
2.3. Obtaining the PR and GV Adsorption Isotherms
2.4. Thermodynamic Analysis
2.5. Dye Removal Kinetics
2.6. Brushite Characterization
3. Results and Discussion
3.1. Effect of Initial PR and GV Concentration on the Equilibrium Adsorption Process
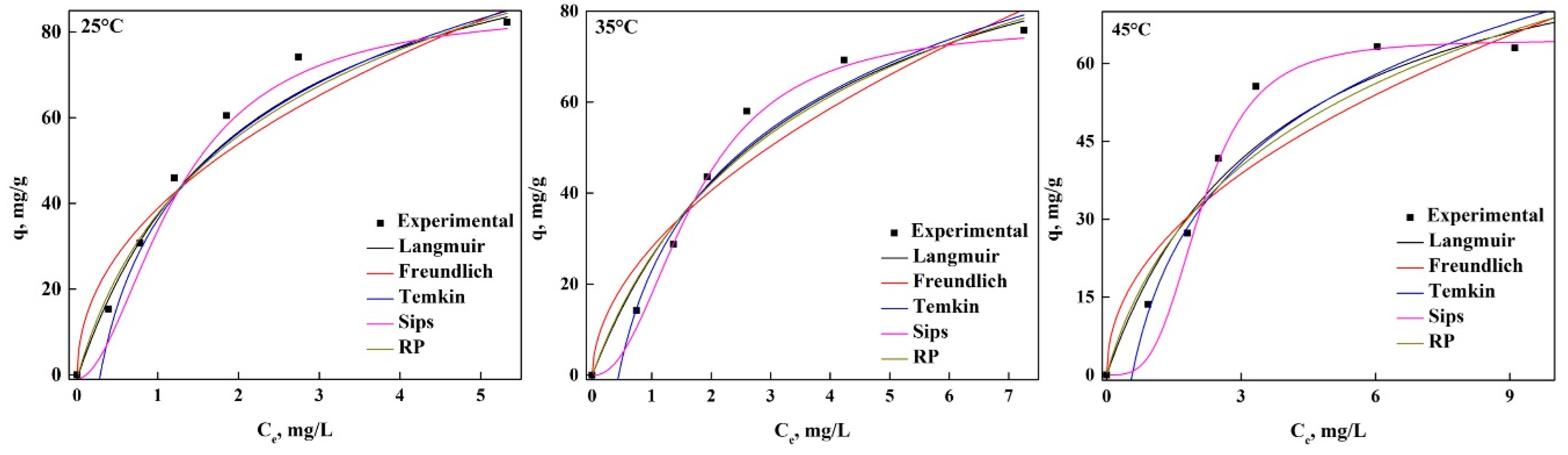
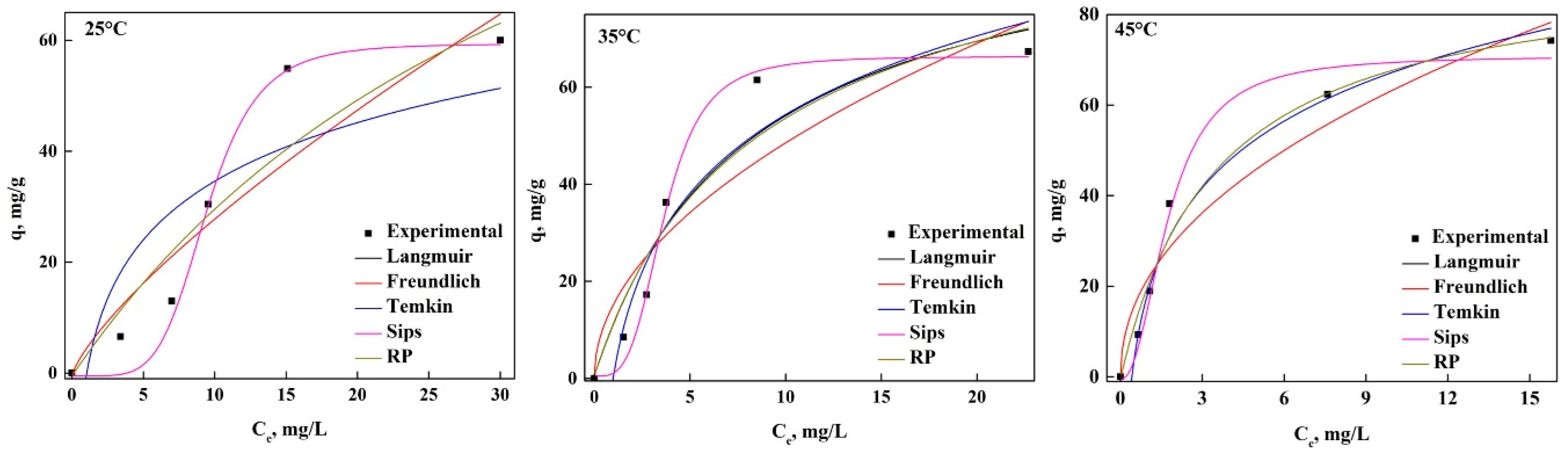
3.2. Adsorption of GV and PR in Equilibrium
3.3. Thermodynamic Analysis of the Adsorption Process of Both Dyes in nDCPD
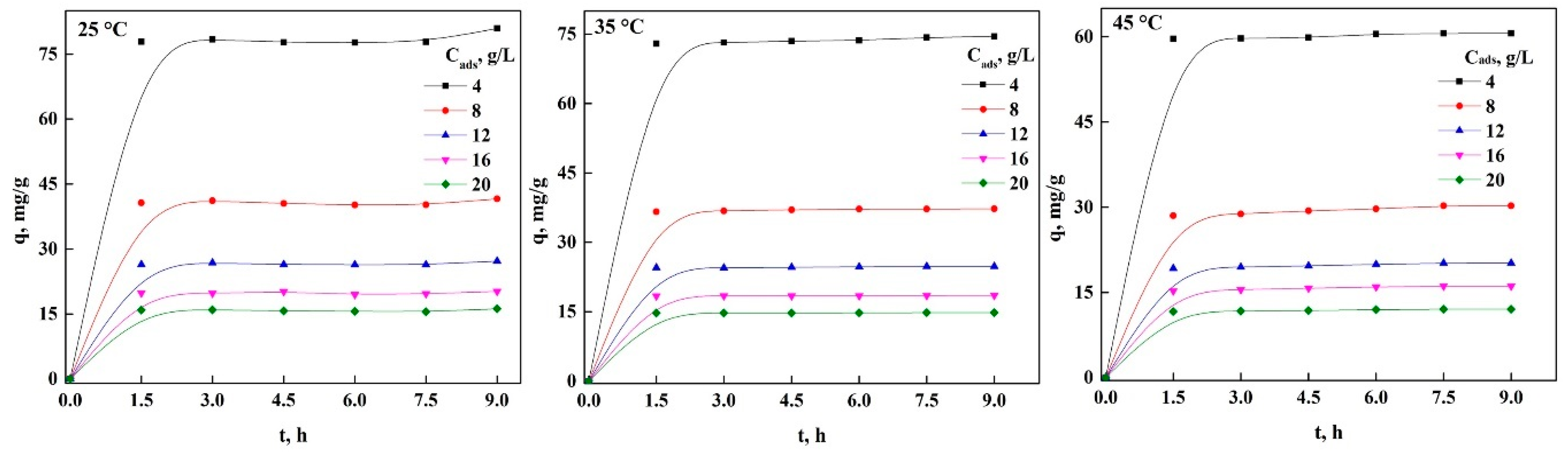
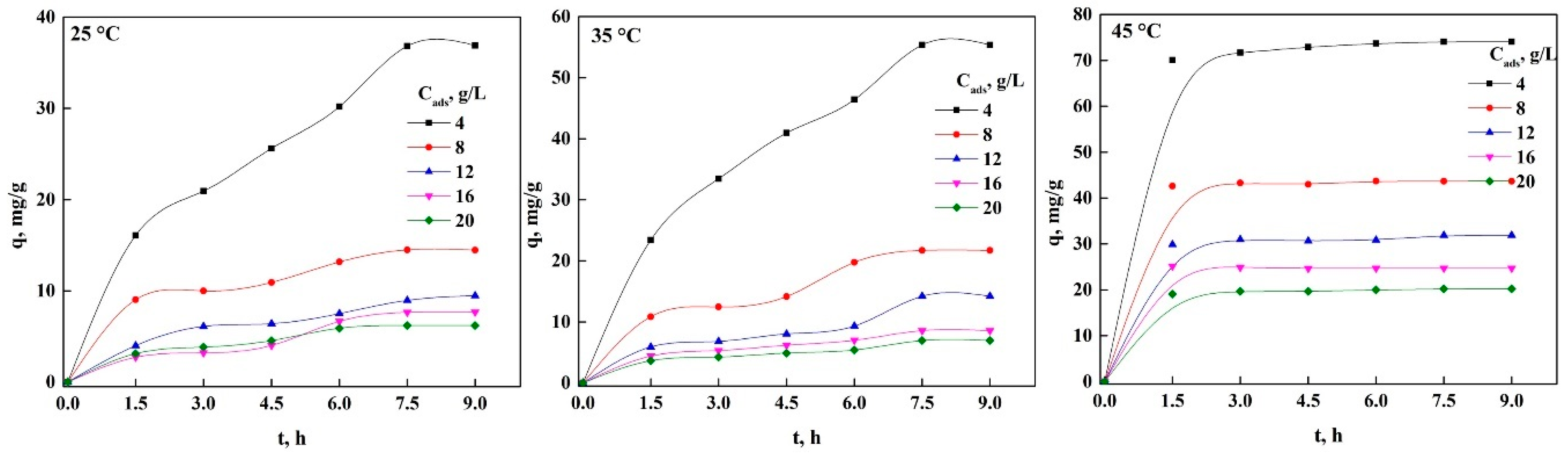
3.4. Effect of Bioadsorbent Concentration on the Adsorption Process of Both Dyes in nDCPD
3.5. Adsorption Kinetics of PR and GV on nDCPD
3.6. Mass Transfer Limitation Analysis
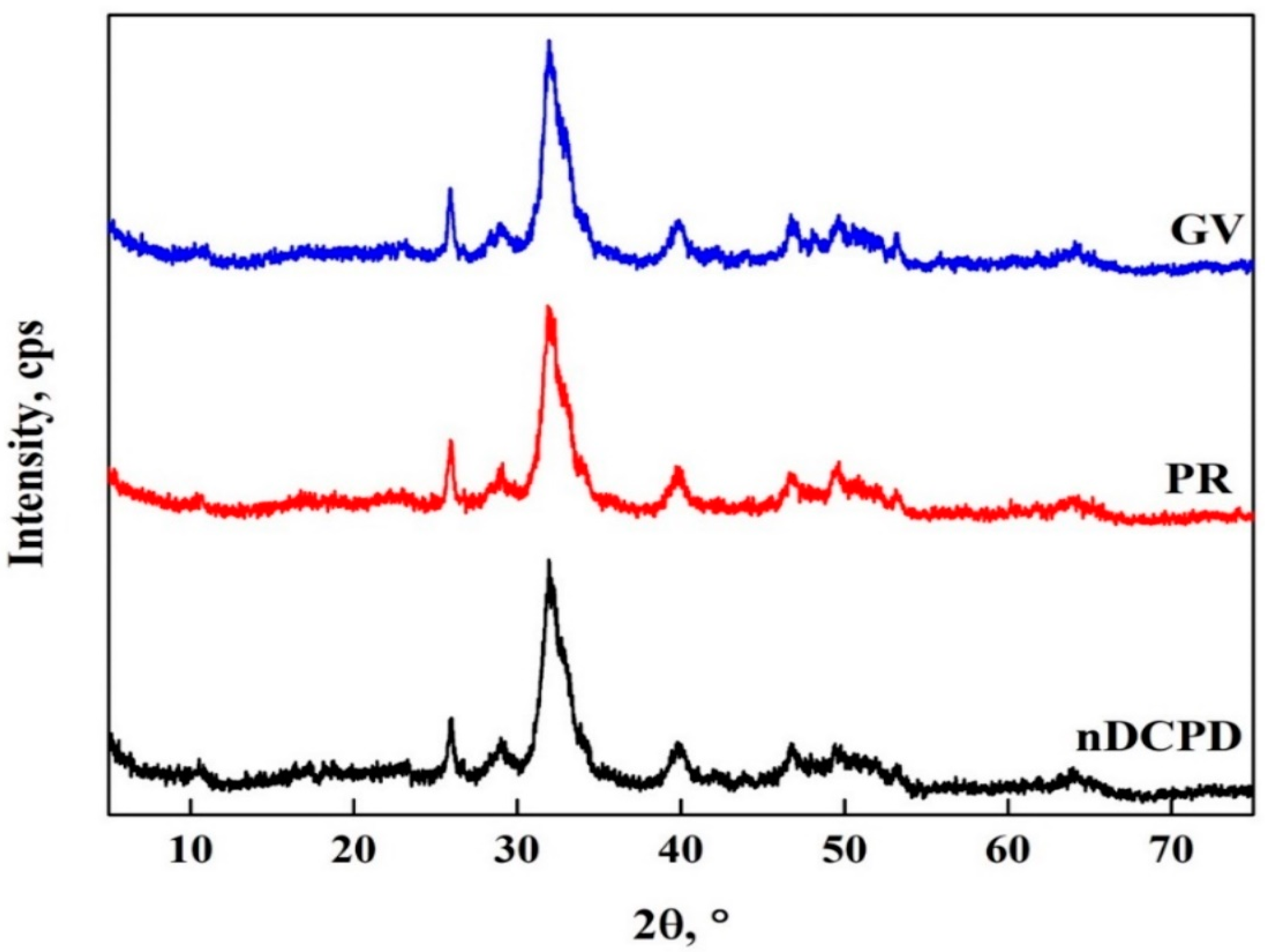
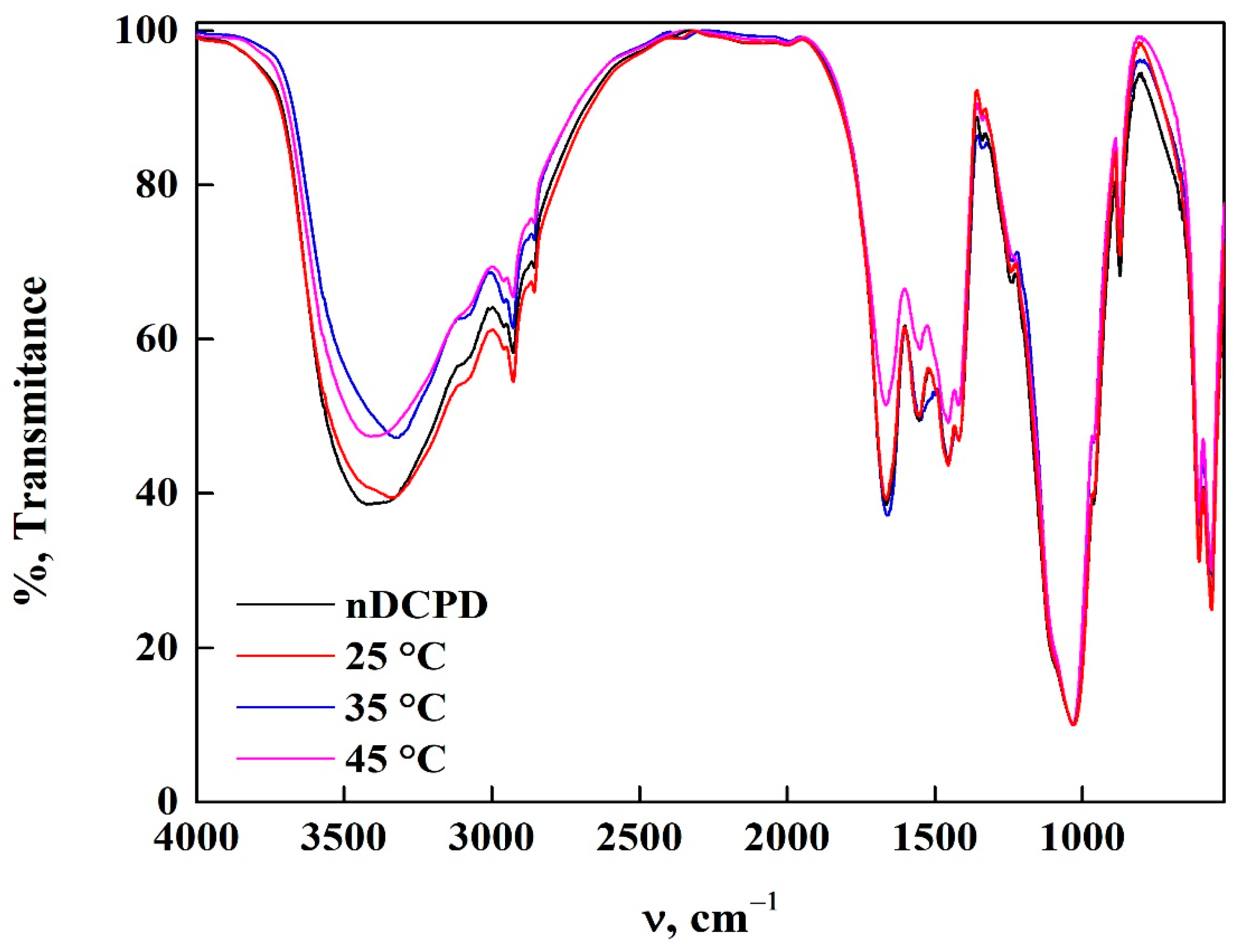
3.7. Characterization of nDCPD in PR and GV Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ning, W.; Jie, C.; Jianan, W.; Jiangtao, F.; Wei, Y. Removal of phenol red by polyaniline/TiO2 hydrate: Adsorption Kinect, isotherm, and mechanism studies. Powder Technol. 2019, 347, 93–102. [Google Scholar] [CrossRef]
- Chong, S.N.; Hadibarata, T. Adsorption of Phenol Red and Remazol Brilliant Blue R by Coconut Shells (Cocos nucifera) and Ambarella Peels (Spondias dulcis). Biointerface Res. Appl. Chem. 2021, 11, 8564–8576. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef] [PubMed]
- Nayagam, J.O.P.; Prasanna, K. Utilization of shell-based agricultural waste adsorbents for removing dyes: A review. Chemosphere 2022, 291, 132737. [Google Scholar] [CrossRef]
- Ayach, J.; Duma, L.; Badran, A.; Hijazi, A.; Martinez, A.; Bechelany, M.; Baydoun, E.; Hamad, H. Enhancing Wastewater Depollution: Sustainable Biosorption Using Chemically Modified Chitosan Derivatives for Efficient Removal of Heavy Metals and Dyes. Materials 2024, 17, 2724. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L. Preparation of straw activated carbon and its application in wastewater treatment: A review. J. Clean. Prod. 2021, 283, 124671. [Google Scholar] [CrossRef]
- Mayet, A.M.; Hijji, M.; Saleh, E.A.M.; Reza, A.; Kadhim, S.I.; Abdullaev, S.S.; Alsalamy, A.; Hassan, Z.F.; Gomez, C.V.; Tene, T. The Role of Biocomposites and Nanocomposites in Eliminating Organic Contaminants from Effluents. Water 2023, 15, 3093. [Google Scholar] [CrossRef]
- Rani, S.; Chaudhary, S. Adsorption of methylene blue and crystal violet dye from waste water using Citrus limetta peel as an adsorbent. Mater. Today Proc. 2022, 60, 336–344. [Google Scholar] [CrossRef]
- Bukhari, A.; Javed, T.; Haider, M.N. Adsorptive exclusion of crystal violet dye from wastewater by using fish scales as an adsorbent. J. Dispers. Sci. Technol. 2022, 44, 2081–2092. [Google Scholar] [CrossRef]
- Mahmood, Z.A.; Farhan, A.M.; Kadhim, N.J.; Hade, M.S. Kinetic and Theoretical Study of Removal Gentian Violet from Aqueous Solution Using Stachy Plant. Baghdad Sci. J. 2023, 20, 1283–1296. [Google Scholar] [CrossRef]
- Asghari, E.; Saraji, M. Evaluating cottonwood seeds as a low-cost biosorbent for crystal violet removal from aqueous matrics. Int. J. Phytoremediation 2023, 25, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Priyadharsini, P.; SundarRajan, P.; Pavithra, K.G.; Naveen, S.; SanjayKumar, S.; Gnanaprakash, D.; Arun, J.; Pugazhendhi, A. Nanohybrid photocatalysts in dye (Colorants) wastewater treatment: Recent trends in simultaneous dye degradation, hydrogen production, storage and transport feasibility. J. Clean. Prod. 2023, 426, 139180. [Google Scholar] [CrossRef]
- Ali, D.A.; Saad, F.A.; Elsawy, H.A. Kinetics and Isotherm Studies for Adsorption of Gentian Violet Dye from Aqueous Solutions Using Synthesized Hydroxyapatite. J. Environ. Public Health 2023, 2023, 7418770. [Google Scholar] [CrossRef]
- Phuong, N.T.; Nam, N.H.; Hong, C.T.; Dac, D.V.Q.; Thu, L.P.; Hai, D.T.; Osial, M.; Giersig, M.; Thanh, D.T.M. Apatite Ore-based Nanostructures: Novel and Eco-friendly Sorbent for Eicient Removal of Wastewater Containing Pb2+ and Fe3+. Water Air Soil Pollut. 2023, 234, 550. [Google Scholar] [CrossRef]
- Akram, M.; Nazir, H.; Salman, M.; Rehman, R.; Farooq, U.; Tahir, S. Kinetic and Isothermal Investigations of Cost-Effective Sorptive Elimination of Gentian Violet Dye from Water Using Haplophragma adenophyllum Biowaste. J. Chem. 2021, 2021, 5549536. [Google Scholar] [CrossRef]
- Athira, T.M.; Sumi, S. Agro-based Adsorbents for Dye Removal from Aqueous Solutions: A Review. Water Air Soil Pollut. 2024, 235, 120. [Google Scholar] [CrossRef]
- Filice, S.; Bongiorno, C.; Libertino, S.; Compagnini, C.; Gradon, L.; Iannazzo, D.; La Magna, A.; Scalese, S. Structural Characterization and Adsorption Properties of Dunino Raw Halloysite Mineral for Dye Removal from Water. Materials 2021, 14, 3676. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Kini, M.S.; Selvaraj, R. A review on adsorptive removal of dyes from wastewater by hydroxyapatite nanocomposites. Environ. Sci. Pollut. Res. 2021, 28, 11835–11849. [Google Scholar] [CrossRef]
- Chahinez, H.-O.; Abdelkader, O.; Leila, Y.; Tran, H.N. One-stage preparation of palm petiole-derived biochar: Characterization and application for adsorption of crystal violet dye in water. Environ. Technol. Innov. 2020, 19, 100872. [Google Scholar] [CrossRef]
- Márquez, C.O.; García, V.J.; Guaypatin, J.R.; Fernández-Martínez, F.; Ríos, A.C. Cationic and Anionic Dye Adsorption on a Natural Clayey Composite. Appl. Sci. 2021, 11, 5127. [Google Scholar] [CrossRef]
- Badhai, P.; Kashyap, S.; Behera, S.H. Adsorption of phenol red onto GO-Fe3O4 hybrids in aqueous media. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100282. [Google Scholar] [CrossRef]
- Patil, S.R.; Sutar, S.S.; Jadhav, J.P. Sorption of crystal violet from aqueous solution using live roots of Eichhornia crassipes: Kinetic, isotherm, phyto and cyto-genotoxicity studies. Environ. Technol. Innov. 2020, 18, 100648. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Yuniarto, A.; Hadibarata, T. Adsorption of Basic Dyes Crystal Violet on Agricultural Biomass: Characterization, Isotherm and Kinetic Studies. Int. J. Integr. Eng. 2022, 14, 269–275. Available online: http://penerbit.uthm.edu.my/ojs/index.php/ijie (accessed on 3 February 2023). [CrossRef]
- Amalraj, R.; Ramsenthil, R.; Durai, G.; Jayakumar, R.; Palaniraj, R. Dyes Removal Using Novel Sorbents—A Review. J. Pharm. Res. Int. 2021, 33, 355–382. [Google Scholar] [CrossRef]
- Alguacil, F.J.; López, F.A. Organic Dyes versus Adsorption Processing. Molecules 2021, 26, 5440. [Google Scholar] [CrossRef]
- Wazir, M.B.; Daud, M.; Ali, F.; Al-Harthi, M.A. Dendrimer assisted dye-removal: A critical review of adsorption and catalytic degradation for wastewater treatment. J. Mol. Liq. 2020, 315, 113775. [Google Scholar] [CrossRef]
- Latif, S.; Alanazi, K.D.; Alshammari, B.H.; Al-Ahmed, A.; Alanazi, A.M. Utilization of river tamarind stem driven biochar for eicient removal of phenol dye from polluted water: Insights from adsorption studies. Biomass Convers. Biorefinery 2024, 14. [Google Scholar] [CrossRef]
- Abbas, M.; Harrache, Z.; Trari, M. Removal of gentian violet in aqueous solution by activated carbon equilibrium, kinetics, and thermodynamic study. Adsorpt. Sci. Technol. 2019, 37, 566–589. [Google Scholar] [CrossRef]
- Sboui, N.; Agougui, H.; Jabli, M.; Boughzala, K. Synthesis, physico-chemical, and structural properties of silicate apatites: Effect of synthetic methods on apatite structure and dye removal. Inorg. Chem. Commun. 2022, 142, 109628. [Google Scholar] [CrossRef]
- Linh, N.L.M.; Lieu, P.K.; Duong, T.; Hung, N.V.; Duc, H.V.; Hoa, L.T.; Thu, N.T.A.; Khieu, D.Q. Phenol Red Adsorption from Aqueous Solution on the Modified Bentonite. J. Chem. 2020, 2020, 1504805. [Google Scholar] [CrossRef]
- Wang, R.-F.; Deng, L.-G.; Li, K.; Fan, X.-J.; Li, W.; Lu, H.-Q. Fabrication and characterization of sugarcane bagasse–calcium carbonate composite for the efficient removal of crystal violet dye from wastewater. Ceram. Int. 2020, 46, 27484–27492. [Google Scholar] [CrossRef]
- Bożęcka, A.; Orlof-Naturalna, M.; Kopeć, M. Methods of Dyes Removal from Aqueous Environment. J. Ecol. Eng. 2021, 22, 111–118. [Google Scholar] [CrossRef]
- Wakkel, M.; Khiari, B.; Zagrouba, F. Textile wastewater treatment by agro-industrial waste: Equilibrium modelling, thermodynamics and mass transfer mechanisms of cationic dyes adsorption onto low-cost lignocellulosic adsorbent. J. Taiwan Inst. Chem. Eng. 2019, 96, 439–452. [Google Scholar] [CrossRef]
- Rojas, J.; Suarez, D.; Torres-Palma, R.A.; Moreno, A.; Silva-Agredo, J. Kinetics, Isotherms and Thermodynamic Modeling of Liquid Phase Adsorption of Crystal Violet Dye onto Shrimp-Waste in Its Raw, Pyrolyzed Material and Activated Charcoals. Appl. Sci. 2019, 9, 5337. [Google Scholar] [CrossRef]
- Haripriyan, U.; Arun, J.; Gopinath, K.P.; Mythili, R.; Kim, W.; Govarthanan, M. A mini-review on innovative strategies for simultaneous microbial bioremediation of toxic heavy metals and dyes from wastewater. Arch. Microbiol. 2023, 205, 29–35. [Google Scholar] [CrossRef]
- Pandey, D.; Daverey, A.; Dutta, K.; Arunachalam, K. Dye removal from simulated and real textile effluent using laccase immobilized on pine needle biochar. J. Water Process Eng. 2023, 53, 103710. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, Y.; Zhang, Z.; Wang, X.; Shen, J. Adsorption of cationic dyes from aqueous solution using hydrophilic silica aerogel via ambient pressure drying. Chin. J. Chem. Eng. 2020, 28, 2467–2473. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and removal of crystal violet dye from aqueous solution by modified rice husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Meraji, S.H.; Sanati, A.M.; Ramavandi, B. Phenol red dye removal from wastewater using TiO2-FSM-16 and Ni-FSM-16 photocatalysts. Heliyon 2023, 9, e14488. [Google Scholar] [CrossRef]
- Nanthamathee, C.; Dechatiwongse, P. Kinetic and thermodynamic studies of neutral dye removal from water using zirconium metal-organic framework analogues. Mater. Chem. Phys. 2021, 258, 123924. [Google Scholar] [CrossRef]
- Mansouri, F.E.; Farissi, H.E.; Zerrouk, M.H.; Cacciola, F.; Bakkali, C.; Brigui, J.; Lovillo, M.P.; Esteves da Silva, J.C.G. Dye Removal from Colored Textile Wastewater Using Seeds and Biochar of Barley (Hordeum vulgare L.). Appl. Sci. 2021, 11, 5125. [Google Scholar] [CrossRef]
- Pérez, S.; Giraldo, S.; Forgionny, A.; Floréz, E.; Acelas, N. Eco-friendly reuse of agricultural wastes to produce biocomposites with high potential in water treatment and fertilizers. Biomass Convers. Biorefinery 2022, 14, 8537–8547. [Google Scholar] [CrossRef]
- Yassine, I.; Joudi, M.; Hafdi, H.; Hayimi, B.; Moudlar, J.; Bensemlai, M.; Nasrellah, H.; Abderrahim, M.; Bakasse, M. Synthesis of Brushite from Phophogypsum Industrial Waste. Biointerface Res. Appl. Chem. 2022, 12, 6580–6588. [Google Scholar] [CrossRef]
- Rojas-Montoya, I.D.; Fosado-Esquivel, P.; Henao-Holguín, L.V.; Esperanza-Villegas, A.E.; Bernad-Bernad, M.; Gracia-Mora, J. Adsorption/desorption studies of norfloxacin on brushite nanoparticles from reverse microemulsions. Adsorption 2020, 26, 825–834. [Google Scholar] [CrossRef]
- Hernández Maldonado, J.A.; Torres García, F.A.; Salazar Hernández, M.M.; Hernández Soto, R. Removal of chromium from contaminated liquid effluents using natural brushite obtained from bovine bone. Desalin. Water Treat. 2017, 95, 262–273. [Google Scholar] [CrossRef]
- Gallo, M.; Tadier, S.; Meille, S.; Gremillard, L.; Chevalier, J. The in vitro evolution of resorbable brushite cements: A physico-chemical, micro-structural and mechanical study. Acta Biomater. 2017, 53, 515–525. [Google Scholar] [CrossRef]
- López-Ahumada, E.; Salazar-Hernández, M.; Talavera-López, A.; Solis-Marcial, O.J.; Hernández-Soto, R.; Ruelas-Leyva, J.P.; Hernández, J.A. Removal of Anionic and Cationic Dyes Present in Solution Using Biomass of Eichhornia crassipes as Bioadsorbent. Molecules 2022, 27, 6442. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N.; Mitropoulos, A.C. Chitosan adsorbents for dye removal: A review. Polym. Int. 2017, 66, 1800–1811. [Google Scholar] [CrossRef]
- Nirmala, N.; Shriniti, V.; Aasresha, K.; Arun, J.; Gopinath, K.P.; Dawn, S.S.; Sheeladevi, A.; Priyadharsini, P.; Birindhadevi, K.; Chi, N.T.L.; et al. Removal of toxic metals from wastewater environment by graphene-base composites: A review on isotherm and kinetic models, recent trends, challenges and future directions. Sci. Total Environ. 2022, 840, 156564. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhou, Y.; Jiang, Y.; Sun, C. The removal of basic dyes from aqueous solutions using agricultural by-products. J. Hazard. Mater. 2008, 157, 374–385. [Google Scholar] [CrossRef]
- Prasad, R.; Sharma, D.; Yadav, K.D.; Ibrahim, H. Eichhornia crassipes as biosorbent for industrial wastewater treatment: Equilibrium and kinetic studies. Can. J. Chem. Eng. 2021, 100, 439–450. [Google Scholar] [CrossRef]
- Alebachew, N.; Yadav, O.P.; Lokesh. Removal of Phenol Red Dye from Contaminated Water Using Barley (Hordeum vulgare L.) Husk-Derived Activated Carbon. Sci. Int. 2021, 5, 7–16. [Google Scholar] [CrossRef]
- Georgin, J.; Franco, D.S.P.; Drumm, F.C.; Grassi, P.; Netto, M.S.; Allasia, D.; Dotto, G.L. Paddle cactus (Tacinga palmadora) as potential low-cost adsorbent to treat textile effluents containing crystal violet. Chem. Eng. Commun. 2020, 207, 1368–1379. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N. Adsorption of toxic crystal violet dye using coffee husks: Equilibrium, kinetics and thermodynamics study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Chen, H.; Lu, J.; Yu, G.; Möslang, M.; Zhou, Y. Superior adsorption capacity of functionalised straw adsorbent for dyes and heavymetal ions. J. Hazard. Mater. 2020, 382, 121040. [Google Scholar] [CrossRef]
- Fan, H.; Ma, Y.; Wan, J.; Wang, Y. Removal of gentian violet and rhodamine B using banyan aerial roots after modification and mechanism studies of differential adsorption behaviors. Environ. Sci. Pollut. Res. 2020, 27, 9152–9266. [Google Scholar] [CrossRef]
- Mancilla-Sanchez, E.; Gómez-Gutiérrez, C.M.; Vargas, E.; Guerra-Rivas, G.; Soto-Robles, C.A.; Vilchis-Nestor, A.R.; Luque, P.A. Obtaining hydroxyapatite from the exoskeleton and spines of the purple sea urchin Strongylocentrotus purpuratus. Int. J. Appl. Ceram. Technol. 2018, 16, 438–443. [Google Scholar] [CrossRef]
- Erol, M.; Özyuguran, A.; Çelebican, Ö. Synthesis, Characterization, and In Vitro Bioactivity of Sol-Gel-Derived Zn, Mg, and Zn-Mg Co-Doped Bioactive Glasses. Chem. Eng. Technol. 2010, 33, 1066–1074. [Google Scholar] [CrossRef]





| Model | Equation | Parameters |
|---|---|---|
| Langmuir | qm = maximum adsorbed capacity KL = Langmuir constant | |
| Freundlich | n = adsorption intensity measurement KF = Freundlich constant related to adsorption capacity | |
| Temkin | A = constant related to the heat of adsorption B = bonding equilibrium constant (maximum binding energy) | |
| Dubinin–Radushkevich (DR) | qm = maximum adsorbed capacity kDR = rate constant ε = DR model parameter | |
| Sips | KS = constant related to adsorption energy ns = dimensionless parameter of SIPS model | |
| Redlich–Peterson (RP) | KR, aR, β = Redlich–Peterson constant |
| Model | Equation | Parameters |
|---|---|---|
| Pseudo First Orden (PFO) | qmax = maximum adsorbed capacity k1 = PPO rate constant | |
| Pseudo Second Orden (PSO) | k2 = PSO rate constant | |
| Elovich | α = initial adsorption rate β = It is related to the covered surface and activation energy by chemiadsorption | |
| Intraparticular Diffusion (ID) | kint = ID rate constant C = indicate the diffusional phenomena control the sorption rate | |
| External Diffusion (ED) | kExt = ED rate constant C0 = initial concentration of dye in solution V = dye solution volume m = adsorbent mass | |
| Avrami | kA = cinetic constante nA = Avrami of exponent |
| GV | PR | |||||
|---|---|---|---|---|---|---|
| Models | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C |
| Langmuir | ||||||
| q, mg/g | 147.22 | 98.451 | 94.915 | 121.39 | 121.265 | 93.382 |
| KL, L/mg | 0.2863 | 0.1151 | 0.0464 | 0.0833 | 0.2956 | 0.8870 |
| RL | 0.68–0.19 | 0.46–0.09 | 0.26–0.04 | 0.70–0.29 | 0.40–0.10 | 0.18–0.09 |
| R2 | 0.8646 | 0.9038 | 0.9709 | 0.9679 | 0.9515 | 0.9003 |
| %∆q | 59.372 | 18.6051 | 10.5370 | 19.432 | 11.946 | 9.4557 |
| Freundlich | ||||||
| KF, mg/g*(L/mg)1/n | 5.0144 | 15.037 | 22.819 | 41.1945 | 29.858 | 22.931 |
| n | 1.3143 | 1.9495 | 2.1727 | 2.1616 | 1.8854 | 2.0943 |
| R2 | 0.8376 | 0.8361 | 0.9179 | 0.9356 | 0.9064 | 0.8313 |
| %∆q | 36.0772 | 38.1780 | 50.2946 | 3.4831 | 5.1723 | 4.4106 |
| Temkin | ||||||
| A, L/mg | 0.0752 | 0.0981 | 19.5284 | 38.4691 | 24.4304 | 13.8894 |
| B, kJ/mol | 15.6876 | 23.8596 | 25.8703 | 30.1609 | 30.1140 | 24.5567 |
| R2 | 0.7469 | 0.5244 | 0.6726 | 0.4666 | 0.6629 | 0.5164 |
| %∆q | 4.4532 | 32.5152 | 21.1038 | 7.0570 | 4.4712 | 4.1241 |
| Sips | ||||||
| q, mg/g | 61.207 | 67.138 | 73.592 | 90.033 | 81.973 | 64.485 |
| Ks, L/mg | 0.1057 | 0.2732 | 0.5676 | 0.8041 | 0.5809 | 0.4723 |
| nS | 5.2506 | 3.5830 | 2.2223 | 1.8911 | 2.2110 | 3.4634 |
| R2 | 0.9935 | 0.9960 | 0.9988 | 0.9881 | 0.9921 | 0.9950 |
| %∆q | 0.7052 | 8.8839 | 5.1608 | 3.8651 | 3.1381 | 3.2333 |
| RP | ||||||
| KR, L/g | 3.8825 | 12.7834 | 27.1742 | 71.8030 | 38.8517 | 32.1360 |
| aR, (L/mg)β | 0.0264 | 0.1515 | 0.2863 | 0.8018 | 0.3942 | 0.5797 |
| β | 1.0000 | 0.9530 | 0.9235 | 0.8510 | 0.9100 | 0.9523 |
| R2 | 0.8646 | 0.8989 | 0.9709 | 0.9593 | 0.9425 | 0.8766 |
| %∆q | 3.5108 | 8.9011 | 21.6620 | 6.7514 | 3.9836 | 13.3026 |
| DR | ||||||
| q, mg/g | 70.996 | 71.519 | 73.870 | 88.165 | 82.259 | 64.240 |
| kDR, mol2/J2 | 0.1379 | 0.0203 | 0.0046 | 0.0030 | 0.0048 | 0.0069 |
| E, kJ/mol | 7.3721 | 3.5093 | 1.3446 | 6.0633 | 7.2169 | 9.1287 |
| R2 | 0.9532 | 0.9834 | 0.9955 | 0.9640 | 0.9746 | 0.9717 |
| %∆q | 7.4907 | 9.0599 | 5.4908 | 2.9383 | 3.0269 | 5.9102 |
| Phenol Red | |||
| T, K | −∆G, kJ/mol | ∆H, kJ/mol | ∆S, kJ/mol K |
| 298.15 | −35.50 | −93.2 | 0.174 |
| 308.15 | −39.94 | ||
| 318.15 | −44.14 | ||
| Gentian Violet | |||
| 298.15 | −38.02 | 70.1 | 0.348 |
| 308.15 | −37.01 | ||
| 318.15 | −35.86 | ||
| Cads, g/L | 4 | 8 | 12 | 16 | 20 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C |
| PFO | |||||||||||||||
| Qe, mg/g | 78.526 | 73.841 | 60.258 | 40.733 | 37.107 | 29.697 | 26.652 | 24.689 | 19.888 | 19.859 | 18.474 | 15.893 | 15.824 | 14.776 | 11.916 |
| k1, h−1 | 3.2034 | 2.9505 | 3.0503 | 4.2126 | 2.8987 | 2.1128 | 3.2403 | 3.2645 | 2.2513 | 4.2380 | 3.6972 | 2.0938 | 12699 | 3.6363 | 2.4530 |
| R2 | 0.9985 | 0.9997 | 0.9998 | 0.9989 | 0.9999 | 0.9981 | 0.9991 | 0.9999 | 0.9990 | 0.9987 | 1.0000 | 0.9987 | 0.9985 | 0.9999 | 0.9992 |
| ∆%q | 9.5612 | 7.1489 | 9.5607 | 8.9845 | 9.3256 | 7.6723 | 8.4509 | 8.2306 | 7.4781 | 8.4367 | 8.2145 | 9.5671 | 10.3209 | 6.4231 | 7.6893 |
| PSO | |||||||||||||||
| Qe, mg/g | 78.954 | 74.353 | 60.637 | 40.991 | 37.344 | 30.358 | 26.886 | 24.926 | 20.236 | 20.121 | 18.728 | 16.229 | 16.089 | 15.030 | 12.129 |
| k2, g/mg*h | 0.5024 | 0.4065 | 0.5565 | 1.0000 | 1.0000 | 0.2971 | 1.0000 | 1.0000 | 0.5663 | 1.0000 | 1.0000 | 0.5721 | 1.0000 | 1.0000 | 1.0000 |
| R2 | 0.9986 | 0.9998 | 0.9999 | 0.9988 | 1.0000 | 0.9993 | 0.9990 | 0.9999 | 0.9997 | 0.9982 | 0.9994 | 0.9997 | 0.9995 | 0.9993 | 0.9997 |
| ∆%q | 2.0134 | 1.9832 | 2.7461 | 3.9035 | 2.4531 | 3.0087 | 2.7650 | 2.2561 | 1.8760 | 3.9823 | 2.7690 | 2.1356 | 2.7832 | 4.0021 | 3.0997 |
| Elovich | |||||||||||||||
| β, mg/g*h | 0.2828 | 0.3029 | 0.3893 | 0.5463 | 0.8456 | 0.7800 | 0.8356 | 0.9500 | 1.1810 | 1.1190 | 1.2691 | 1.4762 | 1.4055 | 1.5867 | 1.9709 |
| α, g/mg | 3 × 109 | 4 × 109 | 9 × 109 | 2 × 109 | 3 × 109 | 4 × 109 | 1 × 109 | 3 × 109 | 5 × 109 | 9 × 108 | 2 × 109 | 2 × 109 | 7 × 109 | 2 × 109 | 1 × 106 |
| R2 | 0.9957 | 0.9971 | 0.9973 | 0.9961 | 1.0000 | 0.9996 | 0.9953 | 0.9969 | 0.9993 | 0.9941 | 0.9960 | 0.9997 | 0.9925 | 0.9963 | 0.9988 |
| ∆%q | 32.754 | 35.9823 | 29.8791 | 25.673 | 44.5120 | 23.510 | 37.8932 | 23.189 | 17.8634 | 20.0025 | 25.731 | 23.4876 | 32.8912 | 37.3241 | 29.453 |
| ID | |||||||||||||||
| kID, mg/g*h0.5 | 23.702 | 22.2698 | 18.1502 | 12.1643 | 11.1644 | 9.1538 | 7.9966 | 7.4206 | 6.0854 | 5.9402 | 5.5234 | 4.8837 | 4.7041 | 4.4269 | 3.6283 |
| 22.996 | 20.9595 | 17.1622 | 11.851 | 10.5779 | 7.9209 | 7.6603 | 7.0721 | 5.4137 | 5.7598 | 5.3566 | 4.2642 | 4.6482 | 4.2661 | 3.2938 | |
| C, mg/g | 0.0.6766 | 0.6772 | 0.6751 | 0.6612 | 0.6743 | 0.7127 | 0.6689 | 0.6717 | 0.7013 | 0.6632 | 0.6639 | 0.7090 | 0.6546 | 0.6668 | 0.6927 |
| R2 | 32.875 | 25.6478 | 23.1278 | 22.890 | 25.6721 | 24.614 | 22.1278 | 18.769 | 16.5489 | 17.6532 | 23.675 | 12.6734 | 52.6370 | 33.2178 | 21.4367 |
| ∆%q | |||||||||||||||
| ED | |||||||||||||||
| kext, h−1 | 0.0412 | 0.0382 | 0.0302 | 0.0430 | 0.0384 | 0.0297 | 0.0420 | 0.0384 | 0.0299 | 0.0417 | 0.0382 | 0.0321 | 0.0415 | 0.0382 | 0.0298 |
| R2 | 0.4561 | 0.1469 | 0.1856 | 0.2312 | 0.1563 | 0.1045 | 0.3421 | 0.1612 | 0.1302 | 0.0456 | 0.1783 | 0.1051 | 0.1803 | 0.1719 | 0.1490 |
| ∆%q | 39.567 | 22.9215 | 15.4361 | 19.635 | 26.8320 | 29.870 | 32.1233 | 33.658 | 25.6341 | 16.3490 | 21.492 | 25.9571 | 25.8031 | 31.2256 | 31.6782 |
| Avrami | |||||||||||||||
| qe, mg/g | 78.526 | 73.841 | 60.258 | 40.733 | 37.107 | 29.698 | 26.652 | 24.689 | 19.888 | 19.856 | 18.474 | 15.893 | 15.824 | 14.776 | 11.916 |
| kA, h−1 | 1.7927 | 1.6339 | 1.6658 | 1.9507 | 1.6232 | 1.3569 | 1.7054 | 1.7261 | 1.4093 | 1.9589 | 1.8416 | 1.3529 | 2.4531 | 1.8254 | 1.4790 |
| nA | 1.8980 | 1.8058 | 1.8312 | 2.1595 | 1.7859 | 1.5571 | 1.9004 | 1.8913 | 1.5975 | 2.1635 | 2.0076 | 1.5477 | 2.4523 | 1.9921 | 1.6585 |
| R2 | 0.9985 | 0.9997 | 0.9998 | 0.9989 | 0.9999 | 0.9981 | 0.9991 | 0.9999 | 0.9990 | 0.9987 | 1.0000 | 0.9987 | 0.9985 | 0.9999 | 0.9992 |
| ∆%q | 12.563 | 10.5632 | 11.4532 | 9.0875 | 17.5326 | 22.678 | 21.7854 | 23.760 | 14.2521 | 15.9704 | 9.5480 | 21.4523 | 10.8324 | 11.2562 | 8.7520 |
| Cads, g/L | 4 | 8 | 12 | 16 | 20 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Models | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C | 25 °C | 35 °C | 45 °C |
| PFO | |||||||||||||||
| qe, mg/g | 39.531 | 71.156 | 73.350 | 14.049 | 23.808 | 43.459 | 9.8194 | 20.316 | 31.264 | 13.465 | 8.7534 | 24.824 | 6.6049 | 7.0169 | 19.965 |
| k1, h−1 | 0.2712 | 0.1586 | 2.0481 | 0.4931 | 0.2714 | 2.6024 | 0.2887 | 0.1318 | 2.0602 | 0.1008 | 0.3326 | 0.5964 | 0.3231 | 0.3308 | 2.0607 |
| R2 | 0.9358 | 0.9069 | 0.9992 | 0.9530 | 0.9519 | 0.9997 | 0.9753 | 0.9224 | 0.9983 | 0.9550 | 0.9618 | 0.9997 | 0.9743 | 0.9432 | 0.9992 |
| ∆%q | 2.7166 | 1.4302 | 2.7649 | 0.9832 | 1.2312 | 2.2289 | 3.2217 | 2.1287 | 1.94787 | 2.9532 | 3.8903 | 2.5432 | 1.7245 | 4.0021 | 3.2121 |
| PSO | |||||||||||||||
| qe, mg/g | 51.564 | 100.98 | 74.871 | 16.776 | 31.590 | 43.837 | 13.061 | 30.068 | 31.903 | 22.276 | 11.188 | 25.091 | 8.5879 | 8.9012 | 20.264 |
| k2, g/mg*h | 0.0050 | 0.0013 | 0.1234 | 0.0357 | 0.0079 | 0.5068 | 0.0201 | 0.0032 | 0.2942 | 0.0028 | 0.0298 | 1.0000 | 0.0359 | 0.0381 | 0.4688 |
| R2 | 0.9477 | 0.9127 | 0.9999 | 0.9721 | 0.9593 | 0.9998 | 0.9830 | 0.9259 | 0.9989 | 0.9550 | 0.9738 | 0.9986 | 0.9797 | 0.9583 | 0.9997 |
| ∆%q | 15.1183 | 13.7320 | 3.6107 | 4.2108 | 2.6589 | 20.6711 | 3.68721 | 8.6205 | 5.6218 | 4.7689 | 9.5471 | 4.5521 | 6.7892 | 5.3240 | 6.2314 |
| Elovich | |||||||||||||||
| β, mg/g*h | 0.0915 | 0.0552 | 0.3060 | 0.2967 | 0.1458 | 0.5150 | 0.3362 | 0.2056 | 0.7033 | 0.3166 | 0.4054 | 0.8998 | 0.5161 | 0.5176 | 1.1204 |
| α, g/mg | 31.360 | 33.449 | 33.878 | 26.486 | 17.610 | 20.814 | 7.1366 | 8.0390 | 9.3688 | 3.7827 | 8.2037 | 2.170 | 5.5979 | 6.8414 | 8.578 |
| R2 | 0.9501 | 0.8983 | 0.9996 | 0.9818 | 0.9586 | 0.9974 | 0.9847 | 0.9020 | 0.9986 | 0.9224 | 0.9784 | 0.9922 | 0.9802 | 0.9650 | 0.9994 |
| ∆%q | 37.8945 | 23.5621 | 21.675 | 15.789 | 32.780 | 20.0056 | 56.8921 | 23.541 | 7.9856 | 21.223 | 32.8741 | 19.5632 | 15.6891 | 27.892 | 10.6732 |
| ID | |||||||||||||||
| kID, mg/g*h0.5 | 12.2228 | 17.4541 | 22.485 | 4.7647 | 7.4133 | 13.1152 | 3.387 | 4.4598 | 9.6042 | 2.4640 | 2.9530 | 7.3178 | 2.1504 | 2.2775 | 6.1186 |
| 9.7736 | 0.0000 | 19.749 | 1.2655 | 0.3244 | 12.2672 | 0.1120 | 0.0000 | 8.3831 | 0.0000 | 0.3093 | 7.4088 | 0.1813 | 0.2653 | 5.3810 | |
| C, mg/g | 0.9699 | 0.9275 | 0.9860 | 0.9537 | 0.9727 | 0.9485 | 0.9905 | 0.9305 | 0.9087 | 0.9202 | 0.9836 | 0.9279 | 0.9808 | 0.9737 | 0.9178 |
| R2 | 43.5478 | 33.7651 | 52.895 | 45.327 | 21.225 | 33.4621 | 44.7891 | 22.548 | 43.6781 | 30.378 | 25.6210 | 41.0956 | 32.0867 | 27.980 | 29.0648 |
| ∆%q | |||||||||||||||
| ED | |||||||||||||||
| kext, h−1 | 0.0146 | 0.0210 | 0.0379 | 0.0120 | 0.0178 | 0.0465 | 0.0110 | 0.0158 | 0.0091 | 0.0115 | 0.0138 | 0.0385 | 0.0128 | 0.0138 | 0.0553 |
| R2 | 0.7871 | 0.8630 | 0.0898 | 0.5671 | 0.7986 | 0.1139 | 0.7999 | 0.8888 | 0.9334 | 0.9325 | 0.7436 | 0.8245 | 0.7477 | 0.7368 | 0.7023 |
| ∆%q | 73.2522 | 48.5231 | 54.320 | 33.101 | 42.986 | 41.9571 | 29.0786 | 39.760 | 46.5908 | 22.410 | 29.6741 | 36.7832 | 47.3210 | 20.045 | 56.4310 |
| Avrami | |||||||||||||||
| qe, mg/g | 39.531 | 71.156 | 73.350 | 14.049 | 23.808 | 43.459 | 9.8194 | 20.316 | 31.264 | 13.465 | 8.7534 | 24.824 | 6.6049 | 7.0169 | 19.965 |
| kA, h−1 | 0.3600 | 0.1991 | 1.3384 | 0.5329 | 0.3537 | 1.5311 | 0.3139 | 0.1651 | 1.3355 | 0.1293 | 0.3976 | 0.5453 | 0.3870 | 0.4013 | 1.3407 |
| nA | 0.7535 | 0.7965 | 1.5303 | 0.9254 | 0.7675 | 1.6997 | 0.9198 | 0.7989 | 1.5427 | 0.7804 | 0.8365 | 0.9621 | 0.8347 | 0.8249 | 1.5370 |
| R2 | 0.9358 | 0.9069 | 0.9992 | 0.9530 | 0.9519 | 0.9997 | 0.9753 | 0.9224 | 0.9983 | 0.9550 | 0.9618 | 0.9997 | 0.9743 | 0.9432 | 0.9992 |
| ∆%q | 42.6254 | 38.5478 | 39.674 | 35.783 | 32.786 | 29.6560 | 26.5219 | 22.337 | 19.4389 | 25.674 | 28.6512 | 32.6743 | 33.8901 | 31.974 | 28.3678 |
| Adsorbent | Cads, g/L | kp1, mg/g h0.5 | kp2, mg/g h0.5 | kp3, mg/g h0.5 |
|---|---|---|---|---|
| nDCPD-GV, 25 °C | 4 | 63.59 | ------ | 11.89 |
| 8 | 33.20 | 5.302 | ||
| 12 | 21.59 | 2.482 | ||
| 18 | 16.19 | 2.236 | ||
| 20 | 10.56 | 2.230 | ||
| nDCPD-GV, 35 °C | 4 | 63.50 | 0.977 | |
| 8 | 29.42 | 0.198 | ||
| 12 | 20.01 | 0.114 | ||
| 18 | 15.02 | 0.081 | ||
| 20 | 12.03 | 0.076 | ||
| nDCPD-GV, 45 °C | 4 | 48.70 | 0.996 | |
| 8 | 23.89 | 0.707 | ||
| 12 | 15.71 | 0.553 | ||
| 18 | 12.44 | 0.356 | ||
| 20 | 9.49 | 0.341 | ||
| nDCPD-PR, 25 °C | 4 | 15.56 | 12.73 | 0.990 |
| 8 | 7.37 | 4.36 | 0.302 | |
| 12 | 3.26 | 1.92 | 0.151 | |
| 18 | 2.53 | 2.74 | ‘0.036 | |
| 20 | 2.22 | 4.89 | 0.031 | |
| nDCPD-PR, 35 °C | 4 | 19.12 | 8.43 | 0.765 |
| 8 | 8.86 | 5.96 | 0.383 | |
| 12 | 4.81 | 3.48 | 0.153 | |
| 18 | 3.59 | 2.37 | 0.104 | |
| 20 | 2.98 | 1.61 | 0.038 | |
| nDCPD-PR, 45 °C | 4 | 57.23 | 2.83 | 0.996 |
| 8 | 34.74 | 2.21 | 0.194 | |
| 12 | 24.38 | 0.575 | 0.005 | |
| 18 | 20.56 | 0.144 | 0.006 | |
| 20 | 15.58 | 0.122 | 0.001 |
| Sample | wt, % | |||||
|---|---|---|---|---|---|---|
| C | O | Si | Ca | P | Ca/P | |
| nDCPD | 37.59 | 35.28 | 0.28 | 15.89 | 10.96 | 1.45 |
| RF-nDCPD | 25.13 | 37.92 | 0.34 | 21.67 | 14.94 | 1.44 |
| GV-nDCPD | 20.34 | 34.92 | 0.40 | 26.24 | 18.10 | 1.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talavera-Lopez, A.; Mendes-Salas, A.; Salazar-Hernández, M.; Ardila A., A.N.; Hernandez-Soto, R.; Solis-Marcial, O.J.; Hernández, J.A. Use of Brushite as Adsorbent for the Removal of Anionic and Cationic Dyes Present in Aqueous Solutions. Water 2024, 16, 2810. https://doi.org/10.3390/w16192810
Talavera-Lopez A, Mendes-Salas A, Salazar-Hernández M, Ardila A. AN, Hernandez-Soto R, Solis-Marcial OJ, Hernández JA. Use of Brushite as Adsorbent for the Removal of Anionic and Cationic Dyes Present in Aqueous Solutions. Water. 2024; 16(19):2810. https://doi.org/10.3390/w16192810
Chicago/Turabian StyleTalavera-Lopez, Alfonso, Antonio Mendes-Salas, Mercedes Salazar-Hernández, Alba N. Ardila A., Rosa Hernandez-Soto, Oscar Joaquín Solis-Marcial, and Jose A. Hernández. 2024. "Use of Brushite as Adsorbent for the Removal of Anionic and Cationic Dyes Present in Aqueous Solutions" Water 16, no. 19: 2810. https://doi.org/10.3390/w16192810
APA StyleTalavera-Lopez, A., Mendes-Salas, A., Salazar-Hernández, M., Ardila A., A. N., Hernandez-Soto, R., Solis-Marcial, O. J., & Hernández, J. A. (2024). Use of Brushite as Adsorbent for the Removal of Anionic and Cationic Dyes Present in Aqueous Solutions. Water, 16(19), 2810. https://doi.org/10.3390/w16192810








